Abstract
Caffeine has long been used as a pharmacological probe for studying ryanodine receptor (RyR)-mediated Ca2+ release and cardiac arrhythmias. However, the precise mechanism by which caffeine activates RyRs is elusive. Here we investigated the effects of caffeine on spontaneous Ca2+ release and on the response of single cardiac RyR (RyR2) channels to luminal or cytosolic Ca2+. We found that HEK293 cells expressing RyR2 displayed partial or “quantal” Ca2+ release in response to repetitive additions of submaximal concentrations of caffeine. This quantal Ca2+ release was abolished by ryanodine. Monitoring of endoplasmic reticulum luminal Ca2+ revealed that caffeine reduced the luminal Ca2+ threshold at which spontaneous Ca2+ release occurs. Interestingly, spontaneous Ca2+ release in the form of Ca2+ oscillations persisted in the presence of 10 mM caffeine, and was diminished by ryanodine, demonstrating that unlike ryanodine, caffeine, even at high concentrations, does not hold the channel open. At the single channel level, caffeine markedly reduced the threshold for luminal Ca2+ activation, but had little effect on the threshold for cytosolic Ca2+ activation, indicating that the major action of caffeine is to reduce the luminal, but not the cytosolic, Ca2+ activation threshold. Furthermore, as with caffeine, the clinically relevant, pro-arrhythmic methylxanthines aminophylline and theophylline potentiated luminal Ca2+ activation of RyR2, and increased the propensity for spontaneous Ca2+ release, mimicking the effects of diseased-linked RyR2 mutations. Collectively, our results demonstrate that caffeine triggers Ca2+ release by reducing the threshold for luminal Ca2+ activation of RyR2, and suggest that disease-linked RyR2 mutations and RyR2-interacting pro-arrhythmic agents may share the same arrhythmogenic mechanism.
Keywords: Ryanodine Receptor, Spontaneous Ca2+ release, Quantal Ca2+ release, Cardiac arrhythmias, Methylxanthines
INTRODUCTION
A number of naturally-occurring mutations in the cardiac Ca2+ release channel/ryanodine receptor (RyR2) have been linked to at least two forms of cardiac arrhythmias: catecholaminergic polymorphic ventricular tachycardia (CPVT) and arrhythmogenic right ventricular displaysia type 2 (ARVD2), but their causal mechanisms have not been completely defined [1]. We have recently shown that disease-causing RyR2 mutations enhance the sensitivity of the channel to activation by luminal Ca2+ and reduce the threshold for spontaneous Ca2+ release, also known as store-overload-induced Ca2+ release (SOICR)[2,3]. It is well known that spontaneous Ca2+ release can alter membrane potential by generating delayed afterdepolarizations (DADs), which in turn can lead to triggered arrhythmias [4]. Alternatively, it has also been proposed that disease-linked RyR2 mutations alter protein-protein or inter-domain interactions that are important for stabilizing the closed state of the channel, thus rendering the channel hyperactive and leaky [5,6].
In addition to RyR2 mutations, RyR2-interacting drugs, including caffeine and other methylxanthines (aminophylline and theophylline), have been shown to promote catecholamine-induced arrhythmias, but by itself caffeine is not arrhythmogenic and does not have a sustained impact on stimulated Ca2+ release [7–14]. This pro-arrhythmic characteristic of caffeine resembles that of the RyR2 CPVT mutations, which predispose patients to exercise or stress-induced cardiac arrhythmias, but cause no obvious structural or functional cardiac defects at rest [1]. These observations suggest that caffeine and CPVT mutations may affect the RyR2 channel in a similar manner. Consistent with this hypothesis, caffeine has been shown to reduce the threshold for spontaneous Ca2+ release [2,7,15]. However, exactly how caffeine reduces the threshold for spontaneous Ca2+ release is not well understood.
Caffeine has commonly been used as an RyR agonist for inducing Ca2+ release from intracellular Ca2+ stores [16–20]. A unique feature of caffeine-induced Ca2+ release from RyR-gated Ca2+ stores is its lack of desensitization. Multiple additions of caffeine at submaximal concentrations can each induce a partial and transient Ca2+ release from intracellular Ca2+ stores in cells expressing RyRs or from sarcoplasmic reticulum membrane vesicles [17–20], a phenomenon known as “quantal” Ca2+ release. The partial or quantal Ca2+ release induced by incremental concentrations of caffeine was thought to result from the sequential activation of different populations of RyRs expressed in the same cell with different sensitivities to caffeine in an all-or-none fashion [19,20]. However, it has also been shown that the ability of caffeine to induce Ca2+ release is dependent on the store Ca2+ content [17,21,22]. When the store Ca2+ level is below a threshold level, caffeine is no longer able to induce Ca2+ release despite its continued presence. Hence, the partial or quantal Ca2+ release is believed to result from store-dependent negative feedback regulation of caffeine activation of the channel [17,22]. Similar to the phenomenon of quantal Ca2+ release, caffeine at low concentrations has also been shown to only transiently potentiate stimulated Ca2+ release in cardiac cells [15]. This transient effect of caffeine is believed to be due to the auto-regulation of SR Ca2+ release by the SR Ca2+ content [23]. Furthermore, Ca2+ release studies using SR membrane vesicles have also shown that a certain level of store Ca2+ content must be present before caffeine-induced Ca2+ release can occur [24–26]. Together, these observations clearly indicate that SR luminal Ca2+ plays an important role in the action of caffeine, but the molecular basis of this luminal Ca2+ dependence is unclear.
Caffeine is commonly thought to sensitize the RyR2 channel to activation by cytosolic Ca2+, leading to an increase in the open probability (Po) of the channel [27,28]. An enhanced Po of RyR2 would result in a decreased SR Ca2+ content, which would, in turn, reduce the Po of RyR2. As a result of this counteractive reduction in luminal Ca2+, caffeine, despite its continued presence, only causes a transient effect on SR Ca2+ release [15]. However, recent studies revealed that single RyR2 channels are rather insensitive to caffeine in the absence of luminal Ca2+ [29]. Alternatively, since RyR2 is also regulated by luminal Ca2+ [30–32], caffeine may alter the response of the channel to luminal Ca2+. Based on our observation that CPVT RyR2 mutations reduce the threshold for spontaneous Ca2+ release by increasing the sensitivity of the channel to luminal Ca2+ activation, we reasoned that caffeine, which also reduces the threshold for spontaneous Ca2+ release, might sensitize the channel to luminal Ca2+ activation. To test this hypothesis, in the present study we investigated the impact of caffeine on the sensitivity of single RyR2 channels to activation by cytosolic or luminal Ca2+. We found that caffeine preferentially potentiated the luminal Ca2+ activation of RyR2 at low cytosolic Ca2+ concentrations. Similar effects were also observed with two other methylxanthines, aminophylline and theophylline. These observations suggest that the pro-arrhythmic action of clinically relevant methylxanthines likely results from their luminal Ca2+ activating properties.
EXPERIMENTAL PROCEEDURES
Materials
Soybean phosphatidylcholine, heart phosphatidylethanolamine, and brain phosphatidylserine were obtained from Avanti Polar Lipids, Inc (Alabaster, AL). [3H]ryanodine was from PerkinElmer Life Sciences. CHAPS and other reagents were purchased from Sigma.
Ca2+ release measurements
The free cytosolic Ca2+ concentration in transfected HEK293 cells was measured using the fluorescent Ca2+ indicator dye fluo-3-AMas described previously [33].
Generation of stable, inducible HEK293 cells
HEK293 cells expressing RyR2 (wt) were generated and characterized previously [2].
Single cell Ca2+ imaging (luminal Ca2+)
Luminal Ca2+ transients in HEK293cells expressing RyR2 were measured using single-cell Ca2+ imaging and the Ca2+ sensitive FRET-based cameleon protein D1ER [34]. Stable, inducible HEK293 cells expressing RyR2 were used, but were additionally transfected, using the Ca2+ phosphate precipitation method, with D1ER cDNA 24 hr before RyR2 expression was induced. The cells were continuously perfused with Krebs-Ringer- Hepes (KRH) buffer (125 mM NaCl, 5 mM KCl, 1.2 mM KH2PO4, 6 mM glucose, 1.2 mM MgCl2, 25 mM Hepes, pH 7.4) containing 2 mM CaCl2, various concentrations of caffeine (0, 0.3, 1, 10 mM) and in the presence or absence of 20 μM ryanodine at room temperature. Images were captured with Compix Inc. Simple PCI 6 software at 470 nm and 535 nm emission, with excitation at 430 nm, every 2 s using an inverted microscope (Nikon TE2000-S) equipped with an S-Fluor 20x/0.75 objective. The amount of FRET was determined from the ratio of the emissions at 535 and 470 nm.
Single channel recordings
Single-channel analyses were carried out as described previously [3]. Briefly, RyR2 proteins were partially purified from cell lysate by sucrose density gradient centrifugation. Heart phosphatidylethanolamine and brain phosphatidylserine (Avanti Polar Lipid), dissolved in chloroform, were combined in a 1:1 ratio (w/w), dried under nitrogen gas and suspended in 30 μl of n-decane at a concentration of 12 mg lipid/ml. Bilayers were formed across a 250-μm hole in a Delrin partition separating two chambers. The trans chamber (800 μl) was connected to the head stage input of an Axopatch 200A amplifier (Axon Instruments Inc.). The cis chamber (1.2 ml) was held at virtual ground. A symmetrical solution containing 250 mM KCl and 25 mM Hepes (pH 7.4), was used for all recordings, unless indicated otherwise. A 4 μl aliquot (~1 μg of protein) of the sucrose density gradient-purified RyR2 protein was added to the cis chamber. Spontaneous channel activity was always tested for sensitivity to EGTA and Ca2+. Only those single channels that are EGTA sensitive and display a stable open probability were used for analyses. The chamber to which the addition of EGTA inhibited the activity of the incorporated channel was presumed to correspond to the cytosolic side of the channel. The direction of single channel currents was always measured from the luminal to the cytosolic side of the channel, unless mentioned otherwise. Recordings were filtered at 2,500 Hz. Free Ca2+ concentrations were calculated using the computer program of Fabiato and Fabiato [35]. Data analyses were carried out using the pClamp 8.1 software (Axon Instruments Inc.).
Single cell Ca2+ imaging of HEK293 cells
Intracellular Ca2+ transients in stable, inducible HEK293 cells expressing RyR2 were measured using single-cell Ca2+ imaging and the fluorescent Ca2+ indicator dye fura-2 acetoxymethyl ester (fura-2 AM) as described previously [2]. Cells grown on glass coverslips for 24 hr after induction by 1 μg/ml tetracycline were loaded with 5 μM fura-2 AM in Krebs- Ringer- Hepes (KRH) buffer (125 mM NaCl, 5 mM KCl, 1.2 mM KH2PO4, 6 mM glucose, 1.2 mM, MgCl2, 25 mM Hepes, pH 7.4) plus 0.02% pluronic F-127 (Molecular Probes) and 0.1 mg/ml BSA for 20 min at room temperature. The coverslips were then mounted in a perfusion chamber (Warner Instruments, Hamden, CT) on an inverted microscope (Nikon TE2000-S) equipped with an S-Fluor 20x/0.75 objective. The cells were continuously perfused with KRH buffer containing 0, 0.1, 0.2, 0.3, 0.5, 1 mM CaCl2 and 0.3 mM caffeine, aminophylline or theophylline at room temperature. 10 mM caffeine was applied at the end of each experiment. Time-lapse images (0.25 frames s−1) were captured and analyzed with the Compix Inc. Simple PCI 6 software. Fluorescent intensities were measured from regions of interest centered on individual cells. Only those cells that responded to caffeine were used in analysis (60–80%).
Isolation of adult rat ventricular myocytes
All studies with rats were approved by the Animal Care Committee of the University of Calgary and complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996). Single rat ventricular myocytes were isolated as described previously [36]. Isolated cells were stored at room temperature in a solution containing 20 mM taurine, 5 mg/ml albumin, and 0.5 mM CaCl2, until used for single cell Ca2+ imaging studies.
Single cell Ca2+ imaging of rat ventricular myocytes
Freshly isolated rat ventricular myocytes were placed on glass coverslips coated with 0.02% (w/v) gelatin and 10 μg/ml fibronectin, and loaded with 5 μM fluo-4-AM Ca2+ (Molecular Probes) plus 0.02% pluronic F-127 in Krebs-Ringer-Hepes (KRH) buffer (125 mM NaCl, 5 mM KCl, 6 mM glucose, 1.2 mM MgCl2 and 25 mM Hepes, pH 7.4) (without KH2PO4) in the presence of 1.0 mM Ca2+ for 20 min at room temperature. The coverslips were mounted in a perfusion chamber on an inverted microscope (Nikon TE2000-S) equipped with an S-Fluor 20x/0.75 objective. The [Ca2+] was then stepped to 5 mM for 5 min before further increasing it to 10 mM. The cells were then continuously perfused with KRH buffer containing 10 mM CaCl2 at room temperature in the absence and presence of 0.5 mM caffeine, aminophylline, or theophylline. Time-lapse images were captured every ~1.3 sec, during the excitation periods, and analyzed using Compix Inc. Simple PCI 6 software.
[3H]Ryanodine binding
Equilibrium [3H]ryanodine (NEN Life Science) binding to cell lysate was performed as described previously [33]. Briefly, a binding mixture (300 μl) containing 30 μl of cell lysate (3–5 mg/ml), 25 mM Tris/50 mM Hepes (pH 7.4), 5 nM [3H]ryanodine, a protease inhibitor mix, and various concentrations of CaCl2, KCl and 2.5 mM caffeine, aminophylline or theophylline as indicated, was incubated at 37°C for 2.5–3.5 hr. The binding mixture was diluted with 5 ml of ice-cold washing buffer containing 25 mM Tris (pH 8.0), and 250 mM KCl, and immediately filtered through Whatman GF/B filters presoaked with 1% polyethylenimine. The filters were washed four times with 5 ml of ice-cold washing buffer and the radioactivity associated with the filters was determined by liquid scintillation counting. Nonspecific binding was determined by measuring [3H]ryanodine binding in the presence of 50 μM unlabeled ryanodine. All binding assays were done in duplicate. Data shown are mean ± SEM for n experiments. Statistical significance was evaluated using the unpaired Student’s t test. A P value of 0.05 is considered to be statistically significant.
RESULTS
Caffeine induces “quantal” Ca2+ release in HEK293 cells expressing RyR2
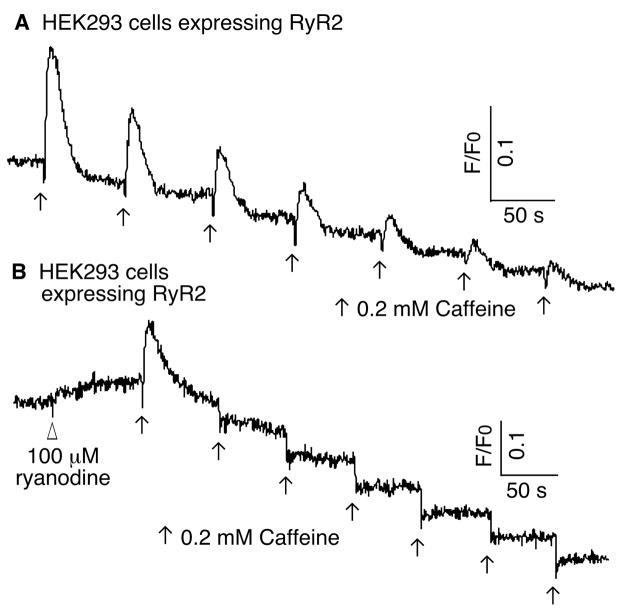
Partial or quantal Ca2+ release in response to incremental concentrations of caffeine has been observed with native cells expressing RyRs and SR vesicles isolated from skeletal muscle [17–20]. To determine whether partial or quantal Ca2+ release occurs in a heterologous expression system, we assessed the response of HEK293 cells transfected with mouse RyR2 cDNA to multiple additions of caffeine. As shown in Fig. 1A, the addition of 0.2 mM caffeine induced a transient Ca2+ release in HEK293 cells expressing RyR2. In the continued presence of caffeine, a second addition of 0.2 mM caffeine was able to trigger another transient Ca2+ release in these cells. This partial Ca2+ release was clearly observed even after the 7th consecutive addition of 0.2 mM caffeine, although the amplitude of each Ca2+ release was progressively reduced. Hence, partial or quantal Ca2+ release also occurs in a heterologous expression system, suggesting that the quantal nature of caffeine-induced Ca2+ release reflects an intrinsic property of its activation of RyR2.
Fig. 1. Caffeine-induced “quantal” Ca2+ release in HEK293 cells expressing RyR2.
HEK293 cells expressing RyR2 were loaded with 5 μM fluo-3 AM. (A) The fluorescent intensity of the cells was monitored continuously before and after repeated additions of 0.2 mM caffeine. (B) The fluorescent signals from the fluo-3-loaded cells were measured before and after the addition of ryanodine (100 μM) followed by the repeated additions of caffeine (0.2 mM). Traces shown are from representative experiments that have been repeated three times with similar results.
The partial Ca2+ release induced by a submaximal concentration of caffeine (Fig. 1A) could be due to the opening of a subpopulation of the RyR2 channels. To test this hypothesis, we pretreated HEK293 cells expressing RyR2 with 100μM ryanodine before multiple additions of caffeine. Since ryanodine only binds to the open channel and the binding of ryanodine converts the channel to a fully open state [37,38], the ryanodine-modified channel is no longer sensitive to caffeine. If the first addition of 0.2 mM caffeine only activates a subpopulation of RyR2, one would expect that the ryanodine pretreatment could only modify that subpopulation of RyR2 that was opened by the first addition of caffeine, and that HEK293 cells pretreated with ryanodine would still respond to multiple additions of caffeine, as each addition of caffeine would activate a new subpopulation of channels. In contrast to this prediction, we found that cells pretreated with ryanodine only responded to the first addition of caffeine.
As shown in Fig. 1B, in the absence of caffeine, the addition of ryanodine caused a slow release of Ca2+. This is likely due to the binding of ryanodine to a small population of RyR2 channels that are open under these conditions and consequently an increase in Po of these channels. This slow release of Ca2+ would be equilibrated at some point with Ca2+ uptake into the endoplasmic reticulum (ER) or Ca2+ extrusion into the extracellular space, leading to a steady-state cytosolic Ca2+ level corresponding to the plateau in the fluorescent signal. The subsequent addition of 0.2 mM caffeine activated the remaining ryanodine-unmodified RyR2 channels and caused a large Ca2+ release. The caffeine-activated channels would then be modified by ryanodine into a fully activated state, leading to a depletion of intracellular Ca2+ store. The released Ca2+ would be extruded into the extracellular space, resulting in a transient Ca2+ release. Importantly, unlike those seen in Fig. 1A, 6 subsequent additions of caffeine yielded little or no Ca2+ release. The overall decline of fluorescent signals throughout the recordings is due to quenching of the fluo-3 fluorescent dye by caffeine. This caffeine-dependent quenching can clearly be seen in Fig. 1B, where every addition of caffeine caused an immediate drop in the fluorescent signal, while the fluorescent signals between two additions of caffeine are relatively constant. Furthermore, due to the difference in the release kinetics, the amplitudes of ryanodine-induced Ca2+ release and caffeine-induced Ca2+ release under these different conditions may not be directly comparable. The difference in the decay kinetics of the caffeine-induced Ca2+ transients in the presence and absence of ryanodine is likely the result of the modification of the RyR2 channel by ryanodine. These observations suggest that the first addition of 0.2 mM caffeine was able to open nearly all the RyR2 channels, which were subsequently converted by ryanodine into a fully open state and thus became unresponsive to further additions of caffeine. Hence, the partial or quantal Ca2+ release in HEK293 cells transfected with a single class of RyR2 cDNA is unlikely to be due to the existence of different populations of RyR2 with various caffeine sensitivities.
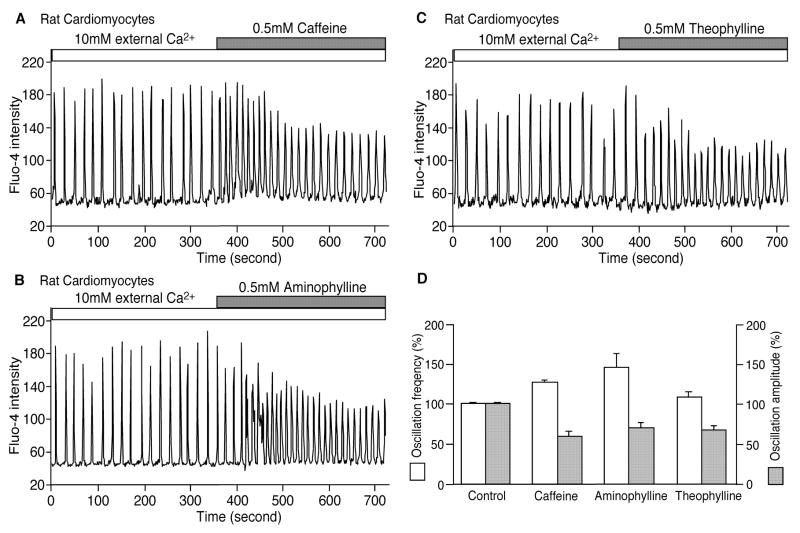
Fig. 6. Methylxanthines enhance spontaneous Ca2+ release in rat cardiomyocytes.
Rat cardiac myocytes attached to gelatin-fibronectin pre-treated glass coverslips were loaded with 5 μM fluo-4 AM. The cells were then continuously perfused with 10 mM [Ca2+]o plus 0.5 mM caffeine (A), aminophylline (B) or theophylline (C). Fluo-4 fluorescent intensities of representative myocytes are shown. (D) The frequency (white) and amplitude (grey) of spontaneous Ca2+ waves (%, mean ± SEM) in rat cardiac myocytes in the absence of methylxanthines (control) or presence of caffeine, aminophylline or theophylline are shown. The frequency and amplitude of spontaneous Ca2+ release observed for the control was normalized to 100%. The total numbers of cardiac myocytes analyzed for spontaneous Ca2+ release were 9 for caffeine, 10 for aminophylline and 16 for theophylline from 3 separate experiments.
Caffeine reduces the threshold at which spontaneous Ca2+-release occurs
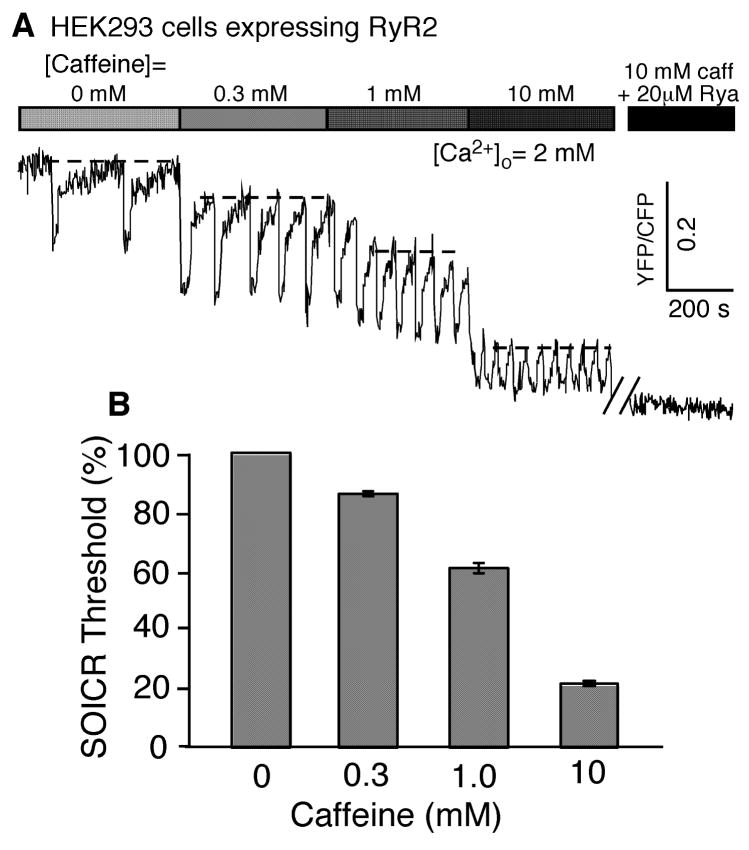
It has been shown that caffeine-induced Ca2+ release is dependent on the ER/SR luminal Ca2+ concentration [17,21,22]. To further investigate the luminal Ca2+ dependence of caffeine activation, we directly monitored the ER luminal Ca2+ dynamics in HEK293 cells expressing RyR2 before and after the additions of various concentrations of caffeine using a luminal Ca2+ indicator protein (D1ER). HEK293 cells expressing RyR2 were transfected with D1ER, a soluble fluorescence resonance energy transfer (FRET) based Ca2+ indicator protein that is expressed within the lumen of the ER due to a KDEL retention motif [34]. As seen in Fig. 2A, in the absence of caffeine and the presence of 2 mM external Ca2+, HEK293 cells expressing RyR2 displayed spontaneous Ca2+ release, which is reflected by the transient downward deflections in the FRET signal, similar to results reported previously [39]. It is worth noting that spontaneous Ca2+ release occurs when the ER Ca2+ reaches a certain level (represented by a dash-line at 0 mM caffeine). We referred to this luminal Ca2+ level as the luminal Ca2+ threshold at which spontaneous Ca2+ release occurs. In the presence of 0.3 mM caffeine, the luminal Ca2+ level (represented by a dash-line at 0.3 mM caffeine) at which spontaneous Ca2+ release occurred was reduced to 86.3 ± 0.9% (n = 34, P < 0.001) of that in the absence of caffeine (Fig. 2B). Similarly, after perfusing the cells with 1 mM caffeine, the luminal Ca2+ threshold (represented by a dash-line at 1mM caffeine) at which spontaneous Ca2+ release occurred was further reduced to 61.1 ± 1.5% (n = 34, P < 0.001) of that in the absence of caffeine (Fig. 2B). Interestingly, even in the presence of 10 mM caffeine, spontaneous Ca2+ release in the form Ca2+ oscillations still persisted despite a markedly reduced luminal Ca2+ threshold (21.4 ± 1.1%, n = 34, P < 0.001) (Fig. 2B). This observation indicates that the RyR2 channel is activated only when the luminal Ca2+ reaches a threshold level, even in the presence of 10 mM caffeine. On the other hand, the addition of 20μM ryanodine abolished Ca2+ oscillations. Ryanodine is known to dramatically sensitize the channel to cytosolic Ca2+ activation and convert the channel to a persistent activated state [37,38]. As a result, the ER Ca2+ store would be completely depleted in the presence of 10 mM caffeine and 20 μM ryanodine. Taken together, these observations demonstrate that unlike ryanodine, caffeine, even at high concentrations, does not always open the RyR2 channel, and that the action of caffeine is to reduce the luminal Ca2+ threshold at which spontaneous Ca2+ release occurs. The fact that the amplitude of spontaneous Ca2+ oscillations is also reduced as the caffeine concentration is increased further supports this view. This is because a reduced threshold at which spontaneous Ca2+ release occurs will reduce the maximal SR Ca2+ loading as Ca2+ will be released from the SR when it reaches the threshold level. A reduced level of SR Ca2+ loading will, in turn, decrease the amount of Ca2+ release and thus the amplitude of Ca2+ oscillations.
Fig. 2. Caffeine reduces the luminal Ca2+ threshold level at which spontaneous Ca2+ release occurs.
HEK293 cells expressing RyR2 were grown on glass coverslips. Cells were transfected with D1ER cDNA 48 hr before imaging and RyR2 expression was induced 24 hr before imaging. The cells were perfused with KRH buffer containing 2 mM Ca2+ and 0, 0.3, 1 or 10 mM caffeine with or without 20μM ryanodine. (A) A representative trace captured using single cell imaging. The dash-lines illustrate the relative luminal Ca2+ threshold for spontaneous Ca2+ release at each concentration of caffeine. (B) The relative luminal Ca2+ threshold for spontaneous Ca2+ release at various caffeine concentrations. The threshold for spontaneous Ca2+ release is expressed as a percentage of the threshold in the absence of caffeine. Data represents the mean ± SEM of 34 cells from 3 separate experiments.
Caffeine preferentially sensitizes the luminal Ca2+ activation of RyR2 at low cytosolic Ca2+ concentrations
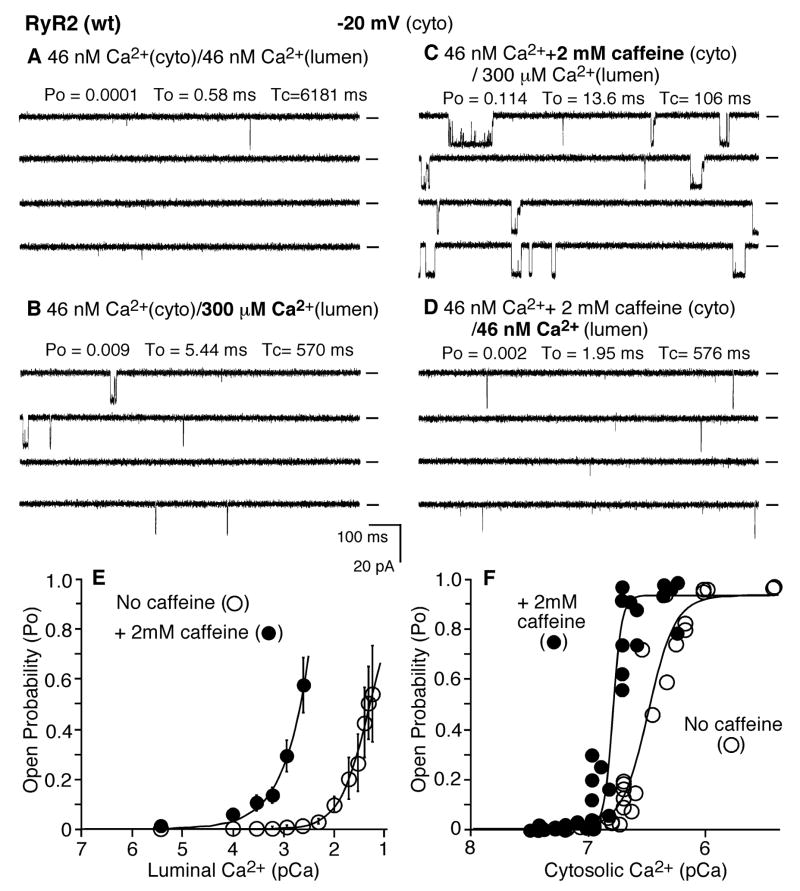
To understand how caffeine reduces the threshold for spontaneous Ca2+ release, we assessed the impact of caffeine on single RyR2 channels. As shown in Fig. 3A, a single RyR2 channel exhibited little activity at low luminal (45 nM) and cytosolic (45 nM) Ca2+ concentrations. Increasing the luminal Ca2+ to 300 μM slightly activated the channel (Fig. 3B). A subsequent addition of 2 mM caffeine to the cytosolic side of the channel markedly increased the channel activity (Fig. 3C). The average Po after the addition of caffeine was 0.094 ± 0.025 in the presence of 300 μM luminal Ca2+, which was significantly greater than that before the addition of caffeine (0.006 ± 0.001) (n = 4) (P < 0.02). Importantly, this caffeine activation was dependent on luminal Ca2+. Reducing the luminal Ca2+ from 300 μM to ~45 nM decreased the activity of the channel to the basal level with Po of 0.002 ± 0.001 (n = 4) (P < 0.05) (Fig. 3D). These data indicate that caffeine preferentially potentiates the luminal Ca2+ response of RyR2 at low cytosolic Ca2+ levels.
Fig. 3. Caffeine enhances the response of single RyR2 channels to luminal Ca2+ activation.
Single-channel activities of RyR2 were recorded in a symmetrical recording solution containing 250 mM KCl and 25mM HEPES (pH 7.4) at a holding potential of −20 mV. EGTA was added to either the cis or trans chamber to determine the orientation of the incorporated channel. The side of the channel to which an addition of EGTA inhibited the activity of the incorporated channel presumably corresponds to the cytosolic face. The Ca2+ concentration on both the cytosolic and luminal sides of the incorporated channel was first adjusted to ~46 nM (A). The channel was activated by 300 μM luminal Ca2+ (B). Caffeine was then added to the cytosolic side of the channel in the presence of 300 μM luminal Ca2+ (C), followed by a decrease of luminal Ca2+ to ~46 nM (D). Openings are downward. Open probability (Po), arithmetic mean open time (To), and arithmetic mean closed time (Tc) are indicated at the top of each panel. A short line to the right of each current trace indicates the baseline. A continuous recording is shown. The average recording time for each condition shown in panels A–D from 4 channels is 103 s. The relationship between Po and luminal Ca2+ concentration is shown in E, and the relationship between Po and cytosolic Ca2+ concentration is shown in F. Data points shown in E are means ± SEM from 5 RyR2 channels in the presence of 2 mM caffeine (solid circles) and 8 RyR2 channels in the absence of caffeine (open circles), and those shown in F are individual measurements obtained from 7 RyR2 channels in the presence of 2 mM caffeine (solid circles) and 5 RyR2 channels in the absence of caffeine. The average recording time is 107 s for E and 93 s for F.
To further characterize the luminal and cytosolic Ca2+ dependence of caffeine activation, we determined the effect of caffeine on the sensitivity of single RyR2 channels to activation by luminal or cytosolic Ca2+. As shown in Fig. 3E, in the absence of caffeine, single RyR2 channels were activated by luminal Ca2+ with a threshold of ~3 mM (n =8), similar to that shown previously [40]. In the presence of 2 mM caffeine, single RyR2 channels were much more sensitive to activation by luminal Ca2+. Caffeine markedly reduced the threshold for luminal Ca2+ activation to ~0.1 mM (n =5) (Fig. 3E). On the other hand, caffeine (2 mM) only slightly reduced the EC50 for activation of the RyR2 channel by cytosolic Ca2+ from 0.31 μM (n=5) to 0.17 μM (n = 7) (Fig. 3F). It should be noted that caffeine has little effect on the threshold for activation by cytosolic Ca2+ (~100 nM in the presence and absence of caffeine) (Fig. 3F). These data suggest that at low cytosolic and high luminal Ca2+ concentrations, a condition resembling that seen in resting cells, caffeine preferentially sensitizes the RyR2 channel to activation by luminal Ca2+.
Other methylxanthines also potentiate the response of RyR2 to luminal Ca2+
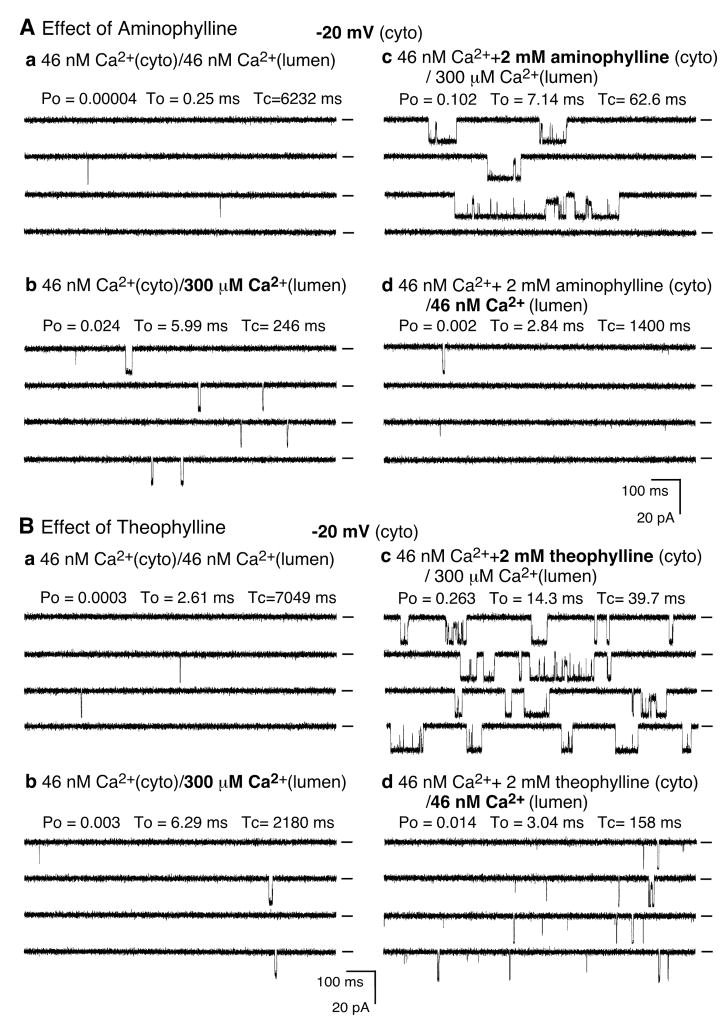
Caffeine is a member of the methylxanthine family of compounds. To determine whether other methylxanthines also preferentially sensitize the luminal Ca2+ activation of RyR2, we assessed the effect of the clinically relevant methylxanthines aminophylline and theophylline on the luminal Ca2+ response of single RyR2 channels. As shown in Fig. 4A, aminophylline (2 mM) markedly activated the RyR2 channel in the presence of 45 nM cytosolic Ca2+ and 300 μM luminal Ca2+. The average Po was significantly increased after the addition of aminophylline, increasing from 0.015 ± 0.008 to 0.102 ± 0.026 (n = 6, P < 0.02). As seen with caffeine, this aminophylline-induced enhancement of channel activity depends on luminal Ca2+. Reducing the luminal Ca2+ concentration to 45 nM abolished the effect of aminophylline by decreasing the Po to 0.002 ± 0.0003 (n = 6, P < 0.02) (Fig. 4Ad). Fig. 4B shows the impact of theophylline. Again and as with caffeine and aminophylline, theophylline activated single RyR2 channels in a luminal Ca2+ dependent manner. The average Po was significantly augmented by theophylline from 0.009 ± 0.003 to 0.241 ± 0.03 (n = 5, P < 0.01), and was reduced to the basal level (Po = 0.005 ± 0.003, n = 5, P < 0.01) when luminal Ca2+ was removed. Therefore, like caffeine, aminophylline and theophylline also preferentially sensitize the RyR2 channel to luminal Ca2+ activation at low cytosolic Ca2+ concentrations.
Fig. 4. Aminophylline and theophylline enhance the luminal Ca2+ activation of RyR2.
The effects of aminophylline (A) and theophylline (B) on single-channel activities of RyR2 were recorded in a symmetrical recording solution as described in Fig. 3. The Ca2+ concentration on both the cytosolic and luminal sides of the incorporated channel was first adjusted to ~46 nM (Aa, Ba). The channel was activated by 300 μM luminal Ca2+ (Ab, Bb). Aminophylline (Ac) or theophylline (Bc) was then added to the cytosolic side the channel in the presence of 300 μM luminal Ca2+ followed by a decrease of luminal Ca2+ to ~46 nM (Ad, Bd). A similar luminal Ca2+ dependence of activation by aminophylline or theophylline was observed in 5–6 RyR2 channels. A continuous recording is shown for each condition. The average recording time is 134 s for panel A and 96 s for panel B.
Methylxanthines increase the propensity for spontaneous Ca2+ release in HEK293 cells expressing RyR2
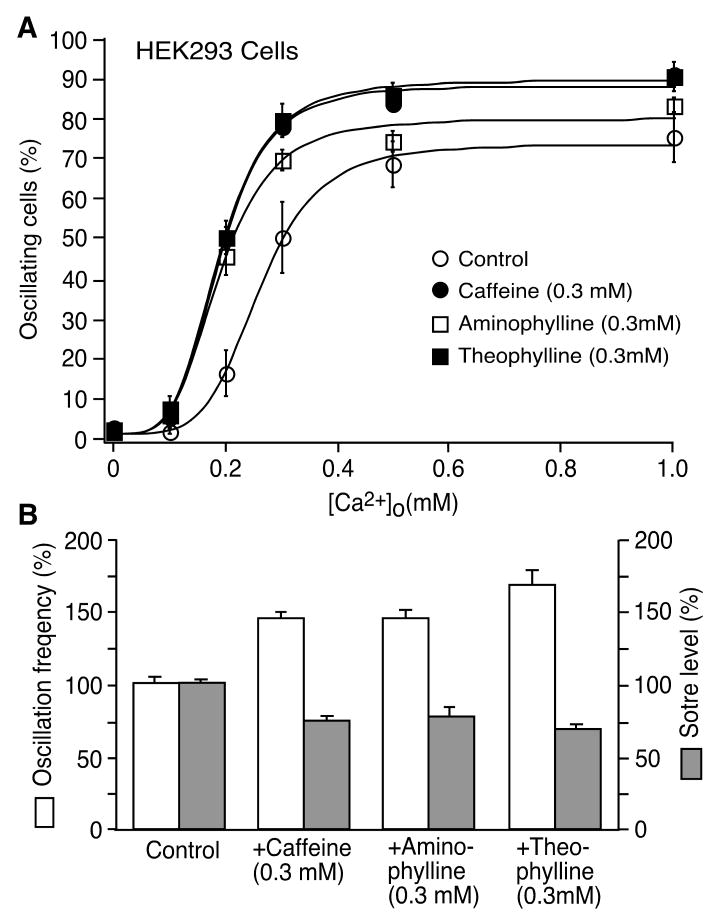
Considering their enhancement of luminal Ca2+ activation, which is closely linked to spontaneous Ca2+ release, it follows that methylxanthines should likewise increase spontaneous Ca2+ release. To test this possibility, we determined the impact of caffeine, aminophylline, and theophylline on spontaneous Ca2+ release in HEK293 cells expressing RyR2. Spontaneous Ca2+ release was induced in these cells by elevating the external Ca2+ concentrations in the absence or presence of caffeine (0.3 mM), aminophylline (0.3 mM), or theophylline (0.3 mM), and was monitored using single cell Ca2+ imaging and the fluorescent Ca2+ indicator, fura-2 AM. Analyzing the fraction of cells that displayed spontaneous Ca2+ release in the form of Ca2+ oscillations at each external Ca2+ concentration showed that all three methylxanthines increased the propensity for spontaneous Ca2+ release (Fig. 5A). For instance, the fraction of oscillating cells was 49.1 ± 4.4% (mean ± SEM) in the presence of 0.3 mM caffeine, 44.6 ± 4.6% in 0.3 mM aminophylline, or 49.3 ± 2.6% in 0.3 mM theophylline, significantly higher than that in the absence of methylxanthines (control, 14.9 ± 5.6%) (P < 0.003). The frequency of spontaneous Ca2+ oscillations was increased to 143.9 ± 4.1% by caffeine (P < 0.001), 145.1 ± 4.4% by aminophylline (P < 0.005), and 166.7 ± 10.2% by theophylline (P < 0.001). The store level was reduced to 74.7 ± 2.0% by caffeine (P < 0.001), 78.0 ± 7.0% by aminophylline (P < 0.05), and 69.9 ± 3.0% by theophylline (P < 0.001) (Fig. 5B). The number of HEK293 cells used for analyses was 377 for the control, 507 for caffeine, 417 for aminophylline, and 370 for theophylline. These results are consistent with the notion that methylxanthines reduce the threshold for spontaneous Ca2+ release.
Fig. 5. Methylxanthines increase the propensity for spontaneous Ca2+ release in HEK293 cells.
(A) The fraction (%, mean ± SEM) of RyR2-expressing cells that display Ca2+ oscillations at various [Ca2+]o is shown in the absence of methylxanthines (control, open circles) and in the presence of 0.3 mM caffeine (filled circles), aminophylline (open squares) or theophylline (filled squares). The total numbers of cells analyzed for Ca2+ oscillations were 377 for the control, 507 for caffeine, 417 for aminophylline, and 370 for theophylline from 4–6 separate experiments. (B) The frequency (white) of spontaneous Ca2+ oscillations and the store Ca2+ level (grey) (%, mean ± SEM) in HEK293 cells in the absence of methylxanthines (control) or presence of caffeine, aminophylline or theophylline are shown. Both the frequency of Ca2+ oscillations and the store Ca2+ content were determined at 1 mM [Ca2+]o. The store Ca2+ levels were estimated by measuring the peak of Ca2+ release induced by 10 mM caffeine. The average frequency and store level observed in the presence of methylxanthines were normalized to the control values (100%).
Methylxanthines enhance the propensity for spontaneous Ca2+ release in isolated rat cardiac myocytes
Methylxanthines have been shown to promote cardiac arrhythmias under some conditions [7–14]. It is possible that their pro-arrhythmic nature is related to their enhancing effect on spontaneous Ca2+ release. To test this possibility, we determined whether methylxanthines are able to enhance spontaneous Ca2+ release in cardiac myocytes. As with HEK293 cells, spontaneous Ca2+ release was induced in rat cardiac cells by increasing the external Ca2+ concentration and was monitored using single cell Ca2+ imaging and the Ca2+ indicator, fluo-4-AM. As shown in Fig. 6, spontaneous Ca2+ release in the form of Ca2+ waves was observed in cardiac cells in the presence of 10 mM external Ca2+. The addition of caffeine (0.5 mM) (Fig. 6A), aminophylline (0.5 mM) (Fig. 6B), or theophylline (0.5 mM) (Fig. 6C) increased the frequency and decreased the amplitude of spontaneous Ca2+ waves. The average frequency was 126.9 ± 2.6% of control (P < 0.0001) for caffeine, 146.1 ± 15.5% (P < 0.01) for aminophylline, and 108.2 ± 6.4 (P < 0.01) for theophylline. The average amplitude was 59.7 ± 6.0% of control (P < 0.0001) for caffeine, 69.8 ± 6.1% (P < 0.0001) for aminophylline, and 66.9 ± 5.3% (P < 0.001) for theophylline. The number of cardiac cells used for analyses was 9 for caffeine, 10 for aminophylline, and 16 for theophylline. These data indicate that, as with HEK293 cells, methylxanthines increase the frequency and decrease the amplitude of Ca2+ waves, which is consistent with the hypothesis that methylxanthines reduce the threshold for spontaneous Ca2+ release in cardiomyocytes.
Methylxanthines increase the basal activity of RyR2, resembling the effect of disease-linked RyR2 mutations
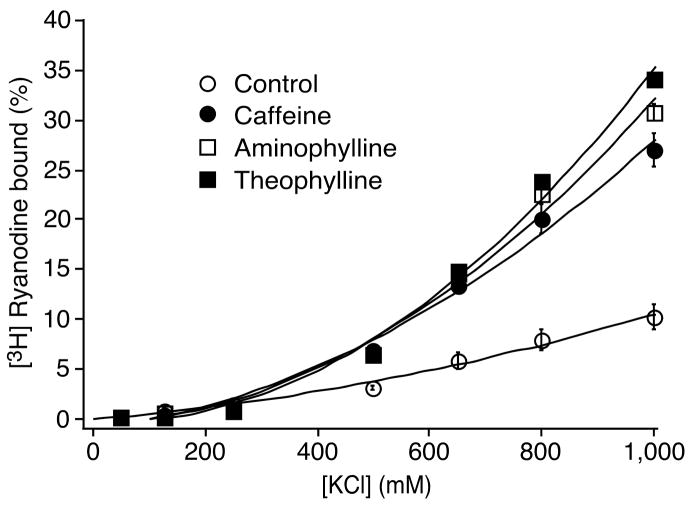
We have shown that disease-linked RyR2 mutations enhance the sensitivity of the channel to luminal Ca2+ activation and reduce the threshold for spontaneous Ca2+ release. These are the same properties shared by methylxanthines. We have also shown that a number of RyR2 mutations linked to cardiac arrhythmias display an increased activity at high KCl concentrations in the near absence of Ca2+ [2,3]. This basal activity likely reflects the stability of the closed state of the channel. To determine whether methylxanthines have any effects on the basal activity of the channel, we performed [3H]ryanodine binding assays. Fig. 7 shows that all three methylxanthines significantly increase the basal activity of the channel. For instance, the basal level of [3H]ryanodine binding at 800 mM KCl was significantly increased from 7.9 ± 1.0 % (control) to 20.2 ± 1.5 % (n = 3, P < 0.05) by caffeine (2.5 mM), 22.5 ± 0.9 % (n = 3, p < 0.001) by aminophylline (2.5 mM), or 23.9 ± 0.4 % (n = 3, P < 0.001) by theophylline (2.5 mM). These data suggest that methylxanthines destabilize the closed state of the channel in a manner similar to those RyR2 mutations known to cause cardiac arrhythmias.
Fig. 7. Effects of methylxanthines on the basal activity of [3H]ryanodine binding.
[3H]ryanodine binding to cell lysate prepared from HEK293 cells expressing RyR2 was carried out at ~3 nM Ca2+ (pCa 8.49), various concentrations of KCl (50–1000 mM), 2.5 mM caffeine (filled circles), aminophylline (open squares) or theophylline (filled squares), and 5 nM [3H]ryanodine. The channel activity in the absence of methylxanthines (control) is also shown (open circles). The amount of [3H]ryanodine binding at various Ca2+ concentrations was normalized to the maximal binding at 1000 mM KCl and pCa 4. Data points shown are mean ± SEM from 3 separate experiments.
DISCUSSION
Caffeine has widely been used as a probe to study the mechanism of RyR2-associated catecholamine-induced cardiac arrhythmias, but the molecular basis of caffeine activation of RyR2 is unclear. Based on our recent finding that disease-linked RyR2 mutations enhance the luminal Ca2+ activation of RyR2 and reduce the threshold for spontaneous Ca2+ release or store-overload-induced Ca2+ release (SOICR) [2,3], we propose that caffeine promotes catecholamine-induced arrhythmias by sensitizing the RyR2 channel to activation by luminal Ca2+. In support of this hypothesis, we have found that caffeine reduces the threshold for luminal, but not cytosolic, Ca2+ activation and the threshold for spontaneous Ca2+ release. In addition, we have found that as with caffeine, two other methylxanthine compounds, aminophylline and theophylline, also potentiate the channel to luminal Ca2+ activation and increase the propensity for spontaneous Ca2+ release. Our results suggest that altered luminal Ca2+ activation of RyR2 underlies a common arrhythmogenic mechanism of inherited and drug-induced arrhythmias associated with RyR2.
How does caffeine trigger Ca2+ release: cytosolic Ca2+ activation vs luminal Ca2+ activation?
Although caffeine has been widely used as an agonist of RyRs to induce Ca2+ release from intracellular stores in various muscle and non-muscle cells, it is not clear how caffeine triggers the opening of RyRs and consequently Ca2+ release. It is commonly believed that caffeine triggers Ca2+ release by sensitizing the channel to cytosolic Ca2+ activation [27,28]. In other words, the RyR channels are activated by the resting cytosolic Ca2+ upon the addition of caffeine. However, if the activation of RyRs by caffeine were mediated by the resting cytosolic Ca2+, one would expect that in a steady state the activation of RyRs would be maintained in the continuing presence of caffeine, as the resting cytosolic Ca2+ after the addition of caffeine would be similar to or greater than that before caffeine stimulation. Such a sustained activation of RyRs by cytosolic Ca2+ in the presence of caffeine would lead to Ca2+ release in an all-or-none fashion and deplete the intracellular Ca2+ stores. In contrast to this prediction, caffeine at submaximal concentrations is able to repetitively trigger partial Ca2+ release in a number of cell types, a phenomenon known as “quantal” Ca2+ release [16–20]. Consistent with these observations, we found that HEK293 cells expressing recombinant RyR2 also displayed partial or quantal Ca2+ release in response to repetitive additions of caffeine (0.2 mM) (Fig. 1A). On the other hand, when these RyR2-expressing HEK293 cells were pretreated with ryanodine, which is known to drastically (>1,000 fold) sensitize the RyR2 channel to activation by cytosolic Ca2+ [37], they only responded to the first addition of caffeine, but not to subsequent additions, in an all-or-none manner (Fig. 1B). It is difficult to reconcile these observations with the idea that caffeine-induced Ca2+ release is mediated via the activation of the RyR channel by cytosolic Ca2+.
However, the RyR channel can also be activated by luminal Ca2+. So if, alternatively, caffeine induces intracellular Ca2+ release by sensitizing the channel to activation by luminal Ca2+, this apparent paradox would be resolved. In this scheme, upon the addition of caffeine the RyR channel is opened by the store luminal Ca2+. Therefore, one would expect that caffeine-induced Ca2+ release would be partial and dependent on luminal Ca2+. This is because a certain concentration of caffeine will sensitize the channel to activation by a certain level of luminal Ca2+. As a result of Ca2+ release, the store luminal Ca2+ level will decrease. The activation of the channel by luminal Ca2+ and thus Ca2+ release would cease when the luminal Ca2+ concentration falls below a threshold level. However, upon increasing the caffeine concentration by a subsequent addition of caffeine, the channel will be further sensitized and again activated by the luminal Ca2+ until the luminal Ca2+ level decreases to a new steady state. Indeed, it has been shown that caffeine decreased the ER luminal Ca2+ level in a concentration dependent manner. The steady state luminal Ca2+ level was progressively decreased with increased concentrations of caffeine [17]. Interestingly, caffeine failed to trigger Ca2+ release if the luminal Ca2+ concentration was lower than the steady state level corresponding to that concentration of caffeine [17]. Similarly, caffeine was found to be unable to trigger Ca2+ release from SR membrane vesicles that were loaded with Ca2+ below a threshold level [24–26]. The failure of caffeine to trigger Ca2+ release in the presence of a normal resting cytosolic Ca2+, but a reduced luminal Ca2+ level, further indicates that luminal Ca2+, but not cytosolic Ca2+, is the major mediator of caffeine induced-Ca2+ release.
We have previously shown that when the luminal Ca2+ level reaches a threshold level, spontaneous Ca2+ release occurs in HEK293 cells expressing RyR2 [2,3,39]. In the present study, we investigated the impact of caffeine on the threshold for spontaneous Ca2+ release. We found that in the presence of 0.3 mM caffeine, spontaneous Ca2+ release occurred at a lower luminal Ca2+ level compared to that in the absence of caffeine (Fig. 2). The threshold for spontaneous Ca2+ release was further reduced after the addition of 1 mM caffeine. Interestingly, despite the markedly reduced threshold and amplitude, spontaneous Ca2+ release in the form of Ca2+ oscillations persisted even in the presence of 10 mM caffeine, and was only abolished by the addition of ryanodine. These observations indicate that, unlike ryanodine, caffeine, even at high concentrations, does not cause a sustained activation of the RyR2 channel. Caffeine only activates the channel when the luminal Ca2+ reaches a certain threshold. Hence, the continued presence of caffeine is not always associated with an increased Po of RyR2. Collectively, the action of caffeine is to reduce the threshold for luminal Ca2+ activation of RyR2, but not necessarily to increase the Po of RyR2.
How does caffeine reduce the threshold for spontaneous Ca2+ release?
Early studies on the effect of caffeine on the cytosolic Ca2+ dependent activation of single RyR2 channels were performed in planar lipid bilayers using Ca2+ as the charge carrier. These studies demonstrated that caffeine markedly enhanced the Po of single RyR2 channels in the presence of submicromolar concentrations of cytosolic Ca2+ and millimolar concentrations of luminal Ca2+ [27,28]. Since both cytosolic and luminal Ca2+ were present, it is not clear whether the activation of RyR2 by caffeine under these conditions resulted from the sensitization of the channel to cytosolic Ca2+ or luminal Ca2+ or both. To distinguish these possibilities, we determined the impact of caffeine on the cytosolic Ca2+ dependence of activation in the near absence of luminal Ca2+ or on the luminal Ca2+ dependence of activation in the near absence of cytosolic Ca2+. We found that at low concentrations of cytosolic Ca2+, caffeine markedly reduced the threshold for luminal Ca2+ activation, whereas, at low concentrations of luminal Ca2+, caffeine had little effect on the threshold for cytosolic Ca2+ activation (Fig. 3). These data indicate that at submicromolar levels of cytosolic Ca2+ and millimolar levels of luminal Ca2+, caffeine preferentially sensitizes the channel to luminal Ca2+ activation. Consistent with this view, it has recently been shown that in the presence of 100 nM cytosolic Ca2+ caffeine readily activated single RyR2 channels using Ca2+ as the charge carrier, but had little effect on single RyR2 channel when Ba2+ was used as the charge carrier [29]. These observations demonstrate that the activation of single RyR2 channels by caffeine at submicromolar levels of cytosolic Ca2+ is dependent on the presence of luminal Ca2+. Taken together, these single channel studies indicate that caffeine reduces the threshold for spontaneous Ca2+ release by decreasing the threshold for luminal Ca2+ activation of the RyR2 channel.
Caffeine mimics the actions of disease-linked RyR2 mutations
We have recently demonstrated that augmented luminal, but not cytosolic, Ca2+ activation of RyR2 is a common feature of a number of disease-linked RyR2 mutations [2,3,39]. Our observation that caffeine reduces the threshold for luminal, but not cytosolic, Ca2+ activation of single RyR2 channels indicates that caffeine mimics the effect of disease-linked RyR2 mutations. Indeed, as with disease-linked RyR2 mutations, caffeine at low concentrations reduces the threshold for spontaneous Ca2+ release, but has no sustained effect on Ca2+-induced Ca2+ release (CICR) [2,7,15,23]. Patients with CPVT RyR2 mutations show no structural or functional cardiac abnormalities at rest, but are predisposed to catecholamine-induced cardiac arrhythmias [1]. Similarly, caffeine alone does not induce spontaneous Ca2+ release, DADs, or cardiac arrhythmia, but promotes catecholamine-induced triggered activities [7–10]. Furthermore, as with disease-linked RyR2 mutations, caffeine increases the basal level of [3H]ryanodine binding (Fig. 7). Hence, caffeine and disease-linked RyR2 mutations alter the properties of the channel in the same manner.
Other methylxanthine compounds aminophylline and theophylline have been used clinically for the treatment of pulmonary diseases. However, their use has recently been limited due largely to their pro-arrhythmic properties [13,14,41,42]. We have found that, like caffeine, both aminophylline and theophylline preferentially potentiate luminal Ca2+ activation of RyR2, reduce the threshold for spontaneous Ca2+ release, and increase the basal activity of RyR2 (Figs. 4–7). These effects likely underlie the arrhythmogenic mechanism of these methylxanthines.
Luminal Ca2+ activation of RyR, a common target for regulation
It has been proposed that under normal SR Ca2+ loading the sensitivity of RyR2 to cytosolic Ca2+ activation is extremely low at resting cytosolic Ca2+ [43]. However, during SR Ca2+ overload, RyR2 becomes much more sensitive to activation [44]. This observation suggests that RyR2 is readily regulated by luminal Ca2+. We have recently shown that the activation of the channel by luminal Ca2+ is distinct from its activation by cytosolic Ca2+ [45]. An increased body of evidence indicates that luminal Ca2+ activation of RyR2 is an important target for regulation by endogenous and exogenous effectors [2,45–47]. In addition to methylxanthines, a number of drugs, such as sulmazole, thymol, doxorubicin, ethanol, and shingosyl phosphorylcholine have been found to induce partial or quantal Ca2+ release from RyR-gated intracellular Ca2+ stores [18]. It is possible that these drugs also induce quantal Ca2+ release by sensitizing the channel to activation by luminal Ca2+. Hence, modulating the sensitivity of the channel to luminal Ca2+ activation may be a common mechanism of regulation of RyRs.
Mechanisms underlying spontaneous Ca2+ release
The phenomenon of spontaneous SR Ca2+ release in cardiac cells has been known for decades. However, the exact mechanism underlying this process has not been completely defined. In early studies using skinned cardiac cells, Fabiato demonstrated that there are two kinds of Ca2+-induced release of Ca2+ from the SR [48,49]. One is termed Ca2+-induced Ca2+ release (CICR), which has a time and Ca2+ dependence of activation and inactivation by cytosolic Ca2+. The other is known as spontaneous SR Ca2+ release, which has no time dependence of activation and is not inactivated by high concentrations of cytosolic Ca2+, but requires SR Ca2+ overload. A key feature of the activation of SR Ca2+ release by cytosolic Ca2+ or CICR is its dependence on the rate of increase in the cytosolic Ca2+ concentration. A high rate of increase in the cytosolic Ca2+ concentration triggers CICR, whereas a low rate of increase in the cytosolic Ca2+ concentration inhibits CICR and causes SR Ca2+ accumulation. Importantly, when the SR Ca2+ content has accumulated to a critical level, spontaneous SR Ca2+ release occurs [48,49].
Consistent with Fabiato’s early observations in skinned cardiac cells, Eisner and his colleagues have shown in intact cardiac myocytes that elevated external Ca2+ concentrations cause a slight increase in the cytosolic Ca2+ level and lead to SR Ca2+ accumulation [50]. Similarly, they found that when the SR Ca2+ content reaches a threshold level, spontaneous SR Ca2+ release occurs in the absence of membrane depolarization. Spontaneous SR Ca2+ release was not observed when the SR Ca2+ content was below this threshold level. After spontaneous SR Ca2+ release occurred, further elevation of external Ca2+ concentration increased the frequency of spontaneous Ca2+ release, but had little effect on its amplitude. In other words, the spontaneous SR Ca2+ release induced by elevated external Ca2+ concentrations occurs only when the SR Ca2+ content reaches a threshold level. Although the cytosolic Ca2+ concentration also increases slightly during external Ca2+ elevation, the rate of increase in the cytosolic Ca2+ concentration may be too slow to trigger CICR. Hence, spontaneous SR Ca2+ release induced by elevated external Ca2+ concentrations is likely the result of SR Ca2+ overload, rather than the consequence of cytosolic Ca2+ activation or CICR.
We have previously demonstrated that elevating the external Ca2+ concentration also increases the store Ca2+ content in HEK293 cells expressing RyR2 [2,3], similar to those observed in cardiac cells. More importantly, and as with cardiac cells, when the store Ca2+ reaches a threshold level, spontaneous Ca2+ oscillations occur in these RyR2-expressing HEK293 cells, but not in RyR2-non-expressing cells. Recently, using an ER Ca2+ sensor, D1ER, we were able to directly show that spontaneous Ca2+ release or Ca2+ oscillations occur in HEK293 cells when the ER Ca2+ reaches a threshold level [39]. Therefore, as with cardiac cells, the spontaneous Ca2+ release observed in HEK293 cells is likely the result of store Ca2+ overload.
What then is the role of cytosolic Ca2+ activation or CICR in spontaneous Ca2+ release induced by elevated external Ca2+? We have recently demonstrated that a disease-associated RyR2 mutation, A4860G, abolishes the luminal Ca2+ activation of RyR2, but has little effect on the sensitivity of the channel to activation by cytosolic Ca2+ [45]. Importantly, this A4860G mutation also abolishes spontaneous Ca2+ oscillations in HEK293 cells, despite its normal sensitivity to cytosolic Ca2+ activation. These observations indicate that spontaneous Ca2+ release is closely linked to the luminal, but not the cytosolic, Ca2+ activation of the RyR2 channel, which is consistent with the fact that spontaneous Ca2+ release occurs only when the SR Ca2+ content reaches a threshold level. Based on these recent observations and those of previous studies, it is likely that spontaneous Ca2+ release is initiated by the luminal Ca2+ activation of the RyR2 channel. However, since CICR is known to be involved in the propagation of Ca2+ waves, spontaneous SR Ca2+ release in the form of propagating Ca2+ waves is likely the combined product of spontaneous Ca2+ release and CICR.
Molecular basis of luminal Ca2+ regulation of RyR2
It has been proposed that luminal Ca2+ activates RyRs by passing through the open channel and acting on the cytosolic Ca2+ activation site (a “feed-through” hypothesis) [31,51]. However, Gyorke et al. and Ching et al. found that RyR2 could still be activated by luminal Ca2+ in the absence of luminal-to-cytosolic Ca2+ flux [52,53]. Furthermore, the application of trypsin to the luminal side of the RyR2 channel diminishes luminal Ca2+ activation, but not Ca2+ fluxes, arguing against the “feed-through” mechanism and suggesting the existence of a luminal Ca2+ activation site distinct from the cytosolic Ca2+ activation site [53]. Recently, a third model incorporating both the feed-through and true luminal Ca2+ activation mechanisms, called the luminal-triggered Ca2+ feed-through mechanism, has been proposed. In this model, luminal-to-cytosolic Ca2+ flux is required for a full activation of the channel by luminal Ca2+ [54]. However, we have recently shown that elevating the luminal Ca2+ concentration to 50 mM did not activate the A4860G mutant channel, despite the presence of luminal-to-cytosolic Ca2+ flux and the normal activation of the channel by cytosolic Ca2+. These observations indicate that luminal-to-cytosolic Ca2+ flux does not activate the RyR2 channel, and suggest that the activation of RyR2 by luminal Ca2+ is mediated by a luminal Ca2+ sensor.
The identity of this putative luminal Ca2+ sensor is also controversial. It has been proposed that calsequestrin, a low affinity, high capacity SR Ca2+ binding protein, acts as a luminal Ca2+ sensor and is responsible for the activation of RyR2 by luminal Ca2+ [55]. According to this theory, the complex of calsequestrin, triadin and junctin confers the sensitivity of RyR2 to luminal Ca2+. At low concentrations of SR luminal Ca2+, calsequestrin binds to the triadin/junctin/RyR2 complex in a Ca2+-sensitive manner and suppresses the stimulatory effect of triadin/junctin on the RyR2 channel. At high SR luminal Ca2+ concentrations, calsequestrin dissociates from triadin/junctin/RyR2, so that triadin/junctin activates RyR2 in the absence of calsequestrin [55]. Hence, calsequestrin, by virtue of its Ca2+ dependent association with and dissociation from the triadin/junctin/RyR2 complex, servers as a luminal Ca2+ sensor for the luminal Ca2+ regulation of RyR2.
Recently, Qin et al. have proposed that RyR2 is regulated by luminal Ca2+ through a calsequestrin-independent and a calsequestrin-dependent mechanism [56]. Different from the mechanism proposed by Gyorke et al., the calsequestrin-dependent mechanism proposed by Qin et al. does not involve the association or dissociation of calsequestrin. Instead, the Ca2+-sensitivity of the interaction between a calsequestrin monomer and the triadin/junctin/RyR2 complex is the key in conferring the responsiveness of RyR2 to luminal Ca2+ activation. The removal of calsequestrin from the triadin/junctin/RyR2 complex completely abolishes the luminal Ca2+ response of RyR2, but does not lead to the activation of RyR2 by luminal Ca2+, as would be expected based on the mechanism proposed by Gyorke et al. [55]. Thus, exactly how calsequestrin is involved in the regulation of RyR2 by luminal Ca2+ is unclear.
The view that calsequestrin serves as the luminal Ca2+ sensor for RyR2 is also apparently inconsistent with the observation that purified native RyRs remain sensitive to luminal Ca2+ activation [31,40,45,57]. Moreover, recent studies have shown that SR Ca2+ release in cardiac myoctyes isolated from calsequestrin knock-out mice remains steeply nonlinear with increasing SR Ca2+ content, indicating that the RyR2 channel can sense luminal Ca2+ in the absence of calsequestrin [58]. Another important observation is that calsequestrin knockout cardiac myocytes display largely unaltered SR Ca2+ release and SR Ca2+ content under basal conditions, suggesting that calsequestrin does not play an essential role in modulating the gating of RyR2 and SR Ca2+ leak at rest or at low SR Ca2+ concentrations [58]. These observations have led to the conclusion that calsequestrin, although it may modulate SR Ca2+ release, is not required for luminal Ca2+ sensing [58]. Consistent with this finding, we found that recombinant RyR2 expressed in HEK293 cells, which lack calsequestrin, is activated by luminal Ca2+ [40,45]. The reasons for these apparently controversial findings regarding the role of calsequestrin in the luminal Ca2+ regulation of RyR2 from different groups are not clear and further studies are needed.
Summary
In summary, our data demonstrate that caffeine triggers Ca2+ release by reducing the threshold for luminal, but not cytosolic, Ca2+ activation of the RyR2 channel. Unlike ryanodine, which induces a full activation of the channel, caffeine, even at high concentrations, does not always hold the channel in the open state. Rather, the action of caffeine is to reduce the luminal Ca2+ threshold at which spontaneous Ca2+ release occurs. As with caffeine, the clinically relevant methylxanthines aminophylline and theophylline preferentially potentiate luminal Ca2+ activation, reduce the threshold for spontaneous Ca2+ release, and increase the basal channel activity, mimicking the actions of disease-linked RyR2 mutations.
Acknowledgments
This work was supported by research grants from NIH, CIHR, and HSFA to SRWC. The authors would like to thank Dr. Jonathan Lytton for helpful discussions and the use of his single cell Ca2+ imaging facility and Jeff Bolstad for critical reading of the manuscript.
ABREVIATIONS USED
- RyR2
Cardiac Ryanodine Receptor
- SOICR
Store Overload Induced Ca2+ Release
- SR
Sarcoplasmic Reticulum
- DAD
Delayed Afterdeplolarization
- CPVT
Catecholaminergic Polymorphic Ventricular Tachycardia
- ARVD2
Arrhythmogenic Right Ventricular Cardiomyopathy type 2
- FRET
Fluorescence resonance energy transfer
- HEK293
Human Embryonic Kidney Cells
References
- 1.Ter Keurs HE, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev. 2007;87:457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H, Chen SRW. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) PNAS. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, Zhang L, Chen SRW. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 4.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Ikemoto N, Yamamoto T. Regulation of calcium release by interdomain interaction within ryanodine receptors. Front Biosci. 2002;7:d671–83. doi: 10.2741/A803. [DOI] [PubMed] [Google Scholar]
- 6.Lehnart SE, Terrenoire C, Reiken S, Wehrens XHT, Song L-, Tillman EJ, Mancarella S, Coromilas J, Lederer WJ, Kass RS, Marks AR. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. PNAS. 2006;103:7906–7910. doi: 10.1073/pnas.0602133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venetucci LA, Trafford AW, Eisner DA. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: Threshold sarcoplasmic reticulum calcium content is required. Circ Res. 2007;100:105–111. doi: 10.1161/01.RES.0000252828.17939.00. [DOI] [PubMed] [Google Scholar]
- 8.Liu N, Colombi B, Memmi M, Zissimopoulos S, Rizzi N, Negri S, Imbriani M, Napolitano C, Lai FA, Priori SG. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: Insights from a RyR2 R4496C knock-in mouse model. Circ Res. 2006;99:292–298. doi: 10.1161/01.RES.0000235869.50747.e1. [DOI] [PubMed] [Google Scholar]
- 9.Kannankeril PJ, Mitchell BM, Goonasekera SA, Chelu MG, Zhang W, Sood S, Kearney DL, Danila CI, De Biasi M, Wehrens XH, Pautler RG, Roden DM, Taffet GE, Dirksen RT, Anderson ME, Hamilton SL. Mice with the R176Q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc Natl Acad Sci USA. 2006;103:12179–12184. doi: 10.1073/pnas.0600268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nam GB, Burashnikov A, Antzelevitch C. Cellular mechanisms underlying the development of catecholaminergic ventricular tachycardia. Circulation. 2005;111:2727–2733. doi: 10.1161/CIRCULATIONAHA.104.479295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balasubramaniam R, Chawla S, Grace AA, Huang CL. Caffeine-induced arrhythmias in murine hearts parallel changes in cellular ca(2+) homeostasis. Am J Physiol Heart Circ Physiol. 2005;289:H1584–93. doi: 10.1152/ajpheart.01250.2004. [DOI] [PubMed] [Google Scholar]
- 12.Bittar G, Friedman H. The arrhythmogenicity of theophylline. A multivariate analysis of clinical determinants. Chest. 1991;99:1415–1420. doi: 10.1378/chest.99.6.1415. [DOI] [PubMed] [Google Scholar]
- 13.Wennmalm A, Wennmalm M. Coffee, catecholamines and cardiac arrhythmia. Clin Physiol. 1989;9:201–206. doi: 10.1111/j.1475-097x.1989.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 14.Joseph X, Whitehurst V, Bloom S, Balazs T. Enhancement of cardiotoxic effects of beta-adrenergic bronchodilators by aminophylline in experimental animals. Fundam Appl Toxicol. 1981;1:443–447. doi: 10.1016/s0272-0590(81)80025-5. [DOI] [PubMed] [Google Scholar]
- 15.Trafford AW, Sibbring GC, Diaz ME, Eisner DA. The effects of low concentrations of caffeine on spontaneous ca release in isolated rat ventricular myocytes. Cell Calcium. 2000;28:269–76. doi: 10.1054/ceca.2000.0156. [DOI] [PubMed] [Google Scholar]
- 16.Herrmann-Frank A, Luttgau HC, Stephenson DG. Caffeine and excitation-contraction coupling in skeletal muscle: A stimulating story. J Muscle Res Cell Motil. 1999;20:223–237. doi: 10.1023/a:1005496708505. [DOI] [PubMed] [Google Scholar]
- 17.Alonso MT, Barrero MJ, Michelena P, Carnicero E, Cuchillo I, Garcia AG, Garcia-Sancho J, Montero M, Alvarez J. Ca2+-induced Ca2+ release in chromaffin cells seen from inside the ER with targeted aequorin. J Cell Biol. 1999;144:241–254. doi: 10.1083/jcb.144.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dettbarn C, Gyorke S, Palade P. Many agonists induce “quantal” Ca2+ release or adaptive behavior in muscle ryanodine receptors. Mol Pharmacol. 1994;46:502–507. [PubMed] [Google Scholar]
- 19.Cheek TR, Berridge MJ, Moreton RB, Stauderman KA, Murawsky MM, Bootman MD. Quantal Ca2+ mobilization by ryanodine receptors is due to all-or-none release from functionally discrete intracellular stores. Biochem J. 1994;301:879–883. doi: 10.1042/bj3010879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheek TR, Moreton RB, Berridge MJ, Stauderman KA, Murawsky MM, Bootman MD. Quantal Ca2+ release from caffeine-sensitive stores in adrenal chromaffin cells. J Biol Chem. 1993;268:27076–27083. [PubMed] [Google Scholar]
- 21.McCarron JG, Olson ML. A single luminally-continuous sarcoplasmic reticulum with apparently separate Ca2+ stores in smooth muscle. J Biol Chem. 2007 doi: 10.1074/jbc.M708923200. [DOI] [PubMed] [Google Scholar]
- 22.Koizumi S, Lipp P, Berridge MJ, Bootman MD. Regulation of ryanodine receptor opening by lumenal ca(2+) underlies quantal ca(2+) release in PC12 cells. J Biol Chem. 1999;274:33327–33333. doi: 10.1074/jbc.274.47.33327. [DOI] [PubMed] [Google Scholar]
- 23.Eisner DA, Trafford AW, Diaz ME, Overend CL, O’Neill SC. The control of ca release from the cardiac sarcoplasmic reticulum: Regulation versus autoregulation. Cardiovasc Res. 1998;38:589–604. doi: 10.1016/s0008-6363(98)00062-5. [DOI] [PubMed] [Google Scholar]
- 24.Nelson TE. Abnormality in calcium release from skeletal sarcoplasmic reticulum of pigs susceptible to malignant hyperthermia. J Clin Invest. 1983;72:862–70. doi: 10.1172/JCI111057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohnishi ST, Taylor S, Gronert GA. Calcium-induced Ca2+ release from sarcoplasmic reticulum of pigs susceptible to malignant hyperthermia. the effects of halothane and dantrolene. FEBS Lett. 1983;161:103–17. doi: 10.1016/0014-5793(83)80739-x. [DOI] [PubMed] [Google Scholar]
- 26.Nelson TE. Malignant hyperthermia: A pharmacogenetic disease of ca++ regulating proteins. Curr Mol Med. 2002;2:347–69. doi: 10.2174/1566524023362429. [DOI] [PubMed] [Google Scholar]
- 27.Rousseau E, Meissner G. Single cardiac sarcoplasmic reticulum Ca2+-release channel: Activation by caffeine. Am J Physiol. 1989;256:H328–33. doi: 10.1152/ajpheart.1989.256.2.H328. [DOI] [PubMed] [Google Scholar]
- 28.Sitsapesan R, Williams AJ. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J Physiol. 1990;423:425–439. doi: 10.1113/jphysiol.1990.sp018031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaburjakova J, Gaburjakova M. Comparison of the effects exerted by luminal Ca2+ on the sensitivity of the cardiac ryanodine receptor to caffeine and cytosolic Ca2+ J Membr Biol. 2006;212:17–28. doi: 10.1007/s00232-006-7018-z. [DOI] [PubMed] [Google Scholar]
- 30.Sitsapesan R, Williams A. Regulation of the gating of the sheep cardiac sarcoplasmic reticulum ca(2+)-release channel by luminal Ca2+ J Membr Biol. 1994;137:215–226. doi: 10.1007/BF00232590. [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Meissner G. Regulation of cardiac muscle Ca2+ release channel by sarcoplasmic reticulum lumenal Ca2+ Biophys J. 1998;75:2302–2312. doi: 10.1016/S0006-3495(98)77674-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gyorke S, Gyorke I, Lukyanenko V, Terentyev D, Viatchenko-Karpinski S, Wiesner TF. Regulation of sarcoplasmic reticulum calcium release by luminal calcium in cardiac muscle. Front Biosci. 2002;7:d1454–63. doi: 10.2741/A852. [DOI] [PubMed] [Google Scholar]
- 33.Li P, Chen SR. Molecular basis of ca(2)+ activation of the mouse cardiac ca(2)+ release channel (ryanodine receptor) J Gen Physiol. 2001;118:33–44. doi: 10.1085/jgp.118.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci USA. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol(Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- 36.Shimoni Y, Chuang M, Abel ED, Severson DL. Gender-dependent attenuation of cardiac potassium currents in type 2 diabetic db/db mice. J Physiol (Lond) 2004;555:345–354. doi: 10.1113/jphysiol.2003.055590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masumiya H, Li P, Zhang L, Chen SR. Ryanodine sensitizes the ca(2+) release channel (ryanodine receptor) to ca(2+) activation. J Biol Chem. 2001;276:39727–3935. doi: 10.1074/jbc.M106557200. [DOI] [PubMed] [Google Scholar]
- 38.Du GG, Guo X, Khanna VK, MacLennan DH. Ryanodine sensitizes the cardiac ca(2+) release channel (ryanodine receptor isoform 2) to ca(2+) activation and dissociates as the channel is closed by ca(2+) depletion. Proc Natl Acad Sci USA. 2001;98:13625–13630. doi: 10.1073/pnas.241516898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones PP, Jiang D, Bolstad J, Hunt DJ, Zhang L, Demaurex N, Chen SR. Endoplasmic reticulum Ca2+ measurements reveal that the cardiac ryanodine receptor mutations linked to cardiac arrhythmia and sudden death alter the threshold for store-overload-induced Ca2+ release. Biochem J. 2008;412:171–178. doi: 10.1042/BJ20071287. [DOI] [PubMed] [Google Scholar]
- 40.Kong H, Wang R, Chen W, Zhang L, Chen K, Shimoni Y, Duff HJ, Chen SR. Skeletal and cardiac ryanodine receptors exhibit different responses to Ca2+ overload and luminal ca2+ Biophys J. 2007;92:2757–2770. doi: 10.1529/biophysj.106.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varriale P, Ramaprasad S. Aminophylline induced atrial fibrillation. Pacing Clin Electrophysiol. 1993;16:1953–1955. doi: 10.1111/j.1540-8159.1993.tb00987.x. [DOI] [PubMed] [Google Scholar]
- 42.Whitehurst VE, Joseph X, Vick JA, Alleva FR, Zhang J, Balazs T. Reversal of acute theophylline toxicity by calcium channel blockers in dogs and rats. Toxicology. 1996;110:113–121. doi: 10.1016/0300-483x(96)03343-4. [DOI] [PubMed] [Google Scholar]
- 43.Cheng H, Lederer MR, Xiao RP, Gomez AM, Zhou YY, Ziman B, Spurgeon H, Lakatta EG, Lederer WJ. Excitation-contraction coupling in heart: New insights from Ca2+ sparks. Cell Calcium. 1996;20:129–40. doi: 10.1016/s0143-4160(96)90102-5. [DOI] [PubMed] [Google Scholar]
- 44.Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol. 1996;270:C148–59. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- 45.Jiang D, Chen W, Wang R, Zhang L, Chen SRW. Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. PNAS. 2007;104:18309–18314. doi: 10.1073/pnas.0706573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao B, Tian X, Xie W, Jones PP, Cai S, Wang X, Jiang D, Kong H, Zhang L, Chen K, Walsh MP, Cheng H, Chen SR. Functional consequence of protein kinase A-dependent phosphorylation of the cardiac ryanodine receptor: Sensitization of store overload-induced Ca2+ release. J Biol Chem. 2007;282:30256–30264. doi: 10.1074/jbc.M703510200. [DOI] [PubMed] [Google Scholar]
- 47.Gyorke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008;77:245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- 48.Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac purkinje cell. J Gen Physiol. 1985;85:247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fabiato A. Two kinds of calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cardiac cells. Adv Exp Med Biol. 1992;311:245–62. doi: 10.1007/978-1-4615-3362-7_18. [DOI] [PubMed] [Google Scholar]
- 50.Diaz M, Trafford A, O’Neill S, Eisner D. Measurement of sarcoplasmic reticulum Ca2+ content and sarcolemmal Ca2+ fluxes in isolated rat ventricular myocytes during spontaneous Ca2+ release. J Physiol (Lond) 1997;501:3–16. doi: 10.1111/j.1469-7793.1997.003bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tripathy A, Meissner G. Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys J. 1996;70:2600–215. doi: 10.1016/S0006-3495(96)79831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gyorke I, Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ching LL, Williams AJ, Sitsapesan R. Evidence for ca(2+) activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ Res. 2000;87:201–26. doi: 10.1161/01.res.87.3.201. [DOI] [PubMed] [Google Scholar]
- 54.Laver DR. Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Biophys J. 2007;92:3541–3555. doi: 10.1529/biophysj.106.099028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qin J, Valle G, Nani A, Nori A, Rizzi N, Priori SG, Volpe P, Fill M. Luminal Ca2+ regulation of single cardiac ryanodine receptors: Insights provided by calsequestrin and its mutants. J Gen Physiol. 2008;131:325–334. doi: 10.1085/jgp.200709907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sitsapesan R, Williams AJ. Regulation of current flow through ryanodine receptors by luminal Ca2+ J Membr Biol. 1997;159:179–185. doi: 10.1007/s002329900281. [DOI] [PubMed] [Google Scholar]
- 58.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]