Abstract
Objective
Mesenchymal stem cells (MSCs) have been shown to possess immunomodulatory properties on a diverse array of immune cell lineages. However, their effect on B-lymphocytes has remained unclear. We investigated the effect of MSCs on B cell modulation with a special emphasis on gene regulation mediated by MSC humoral factors.
Methods
MSCs were isolated from C57BL/6 bone marrow and expanded in culture. Splenic B cells were purified using anti-CD43 antibody and immunomagnetic beads. B cells and MSCs were co-cultured in separate compartments in a transwell system. For B cell stimulation, lipopolysaccharide (LPS) was used in vitro and T-dependent and T-independent antigens were used in vivo.
Results
In MSC co-cultures, LPS-stimulated B cell proliferation was suppressed, CD138+ cell percentage decreased, and the number of apoptotic CD138+ cells decreased. In the B/MSC co-culture, the IgM+ cell percentage was higher and the IgM amount released in the medium was lower than in the control. The Blimp-1 mRNA expression in the co-culture was suppressed throughout the 3 day culture period. Conditioned media derived from MSC cultures prevented the terminal differentiation of B cells in vitro and significantly suppressed the antigen specific IgM and IgG1 secretion in mice immunized with T cell-independent as well as T cell-dependent antigens in vivo.
Conclusion
Results indicate that humoral factor(s) released by MSCs exert a suppressive effect on the B cell terminal differentiation. The suppression may be mediated through inhibition of Blimp-1 expression, but the nature of the factor(s) is yet to be determined.
Keywords: Mesenchymal stem cells, B-Cell Differentiation, BLIMP-1 protein
Introduction
Bone Marrow (BM) is a complex tissue containing diverse lineages of hematopoietic and stromal cells that support hematopoiesis [1]. Marrow stroma contains a small subpopulation of undifferentiated cells referred to mesenchymal stem cells (MSCs). MSCs are capable of rapidly proliferating ex vivo and differentiating into various mesenchymal lineages [2, 3], offering a tool for clinical applications [4, 5]. MSCs have also shown immune regulatory properties [6-8]. We have shown in rats that MSCs facilitate the induction of mixed hematopoietic chimerism and islet allograft tolerance [9]. MSCs exert suppressive effects on T cells [10-12], NK cells [13], as well as dendritic cells [14]. With respect to mature B cells, human MSCs have been shown to inhibit B cell proliferation, differentiation, and chemotaxis in vitro [15], though the mechanism involving in B cell modulation is largely unknown.
Exposure of mature B cells to lipopolysaccharides (LPS) induces expression of B lymphocyte-induced maturation protein-1 (Blimp-1), leading to the terminal differentiation of B cells into plasma cells [16]. Blimp-1 is postulated to be the master transcriptional regulator required for B cell terminal differentiation by directly repressing transcription factors that, in turn, regulate several important gene programs [17]. The ectopic expression of Blimp-1 has been shown to be sufficient for inducing B cell terminal differentiation in BCL-1 lymphoma, a model used for testing differentiation of mature B cells into plasma cells [18, 19]. In the Blimp-1-conditional knock out mouse, Blimp-1 is required for the differentiation of plasma cells, pre-plasma memory B cells [20], and maintenance of plasma cell longevity in the BM [21].
We investigated the potential of MSCs to modulate mature B cells using mice, focusing on gene regulation and MSC-released humoral factors. Our results show that MSCs reduce the plasma cell generation in co-cultured B cells in vitro. Humoral factor(s) from MSCs released in culture suppress antigen (Ag)-specific IgM and IgG1 secretion in vivo in animals immunized with T cell-independent (T-ID) as well as T cell-dependent (T-D) antigens. B-cell suppression is mediated by MSC-released humoral factor(s), does not require cell-cell contact, and is associated with reduced Blimp-1 mRNA expression. MCP-1, IL-10, TGF-β, and IDO are not involved in the B cell suppression, and the nature of humoral factor(s) remains to be elucidated.
Materials and Methods
Animals and immunization
Female C57BL/6 and BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and maintained in the City of Hope Animal Resources Center. BALB/c mice were immunized with intraperitoneal (i.p.) injections of either a T-D Ag (50 μg of NP12-Ficoll) or a T-ID Ag (50 μg of alum-precipitated NP19-KLH, both from Biosearch Technologies, Novato, CA, USA) in 250 mL phosphate buffered saline (PBS). The animal protocol used in this study was approved by the City of Hope Research Animal Care Committee.
Isolation and expansion of MSCs and preparation of splenic B cells
BM cells from C57BL/6 mice were cultured in 25 cm2 tissue culture flasks (8×105 cells/cm2) using Murine MesenCult Basal Medium containing 20% MSC Stimulatory Supplements (StemCell Technologies, Vancouver, BC, Canada) at 37°C in air plus 5% CO2. After 72 hours, non-adherent cells were decanted and thereafter the medium was changed every 3 to 4 days. When adherent cells reached 70-80% confluence, they were trypsinized and passaged. MSCs were differentiated to adipocytes or osteocytes using the culture method described by Peister et al [22]. Adipocytes were detected by Oil Red O (Sigma-Aldrich, St. Louis, MO, USA) staining and osteocytes by alkaline phosphatase staining. B cells were prepared from the spleen by depleting non-B cells using a PE-anti-CD43 antibody (Ab) (BD Biosciences, San Jose, CA, USA) and magnetic beads coated with anti-PE Ab (Miltenyi Biotec, Gladbach, Germany). The Ab-labeled cells were then separated by a MACS system (Miltenyi Biotec). The resulting B cell fraction contained >95% CD19+ B cells.
Monoclonal antibodies and FACS analysis
Fc receptors were blocked by incubating cells with 5 μg/mL of anti-CD16/32 Ab (BD Biosciences). Antibodies used for labeling included: monoclonal Abs conjugated to APC-anti-CD19; Biotin: -anti-H-2Kb, -anti-I-Ab, -anti-FAS-L, -anti-CD40L, -anti-IgMb, -anti-IgDb, -anti-IgG3; FITC: -anti-Sca-1, -anti-CD34, -anti-CD40; -anti IgG3; and PE: -anti-c-kit, -anti-CD11b, -anti-CD45, -anti-CD80, -anti-CD86, -anti-CD138 (all from BD Biosciences). Cells labeled with biotinylated Abs were visualized by incubating with allophycocyanin (APC)-conjugated streptavidin. For cell proliferation assays, B cells were labeled with carboxyfluorescein diacetate, succinimidyl ester (CFSE; Molecular Probes/Invitrogen, Carlsbad, CA, USA) as described elsewhere [21] and analyzed using FACSCalibur (Becton Dickinson, San Jose, CA, USA).
Transwell cultures
Transwell cultures were set up in 6-well culture plates. Each well contained an insert with a 0.4 μm pore size membrane (Corning, Corning, NY, USA) to separately culture B cells from MSCs. MSCs (105 cells/well) were seeded in wells 6 to 8 hours before placing 106 B cells in the insert. Complete culture medium (CCM) was added in a volume of 4 mL/well. This medium was RPMI1640 supplemented with 3 μg/mL LPS, 10% FBS, 50 μM 2-mercaptoethanol, and antibiotics. The cells were cultured for 3 days in a tissue culture incubator.
ELISA for antibody titration
The titer of IgM and IgG3 in B-cell cultures, or NP-specific IgM, IgG1, IgG2a, IgG2b, or IgG3 in serum samples from the immunized mice was measured by ELISA. The ELISA plates were prepared by coating 96-well plates with either goat anti-mouse IgG plus IgM (Caltag Laboratories, Burlingame, CA, USA) or NP20-BSA (Biosearch Technology, Novato, CA, USA). After incubation, the wells were washed then incubated with biotinylated goat anti-mouse IgM, IgG1, IgG2a, IgG2b, or IgG3 Ab (Caltag Laboratories), followed by incubation with avidin-peroxidase (Sigma-Aldrich) using o-phenylenediamine dihydrochloride (Sigma-Aldrich) in citrate buffer as substrate. The reaction was read at 450 nm on a multiscan 96-well plate reader (GENios, TECAN US Inc., NC, USA).
Cell proliferation assay by 3H-thymidine uptake
B cells (106 cells/well) were cultured 48 hours in a 96-well plate in the presence of LPS either with or without MSCs (1 or 2 × 105/well). Cells were pulsed with 1 μCi 3H-thymidine for the last 8 hours and 3H-thymidine uptake was measured by a liquid scintillation counter (Microbeta Trilux1450, Wallac, Waltham, Mass, USA).
Apoptosis analysis
An Annexin V kit (BD Biosciences) was used to detect apoptotic cells in the CD138+ and CFSE- cell fractions by FACS analysis.
qRT-PCR analysis
Total RNA was extracted from the cultured B cells using TRI-REAGENT RNA isolation reagent (Molecular Research Center, Cincinnati, OH, USA). RNA was reverse-transcribed using Superscript III and Oligo (dT) (Invitrogen, Carlsbad, CA, USA) in a final volume of 50 mL. Semi-quantitative PCR using 1 mL cDNA was performed as follows. An initial 2 minute incubation at 92-95°C for denaturation was followed by annealing at 30 or 35 cycles of PCR at 50°C for μS (secretion form of IgM heavy chain mRNA) and at 52°C for μM (membrane form of IgM heavy chain mRNA), and for 45 cycles at 55°C for hypoxanthine phosphoribosyltransferase (HPRT). Polymerization was done at 72°C for 1 min. The following PCR primers were used for the cDNA amplification: the μM primers, 5′-GGCTTTGAGAACCTGTGGA-3′ and 5′-TTACAGCTCAGCTGTCTGT-3′; the μS primers, 5′-TCTGCCTTCACCACAGAAG-3′ and 5′-TAGCATGGTCAATAGCAGG-3′; and the HPRT primers, 5′-GCTGGTGAAAAGGACCTCT-3′ and 5′-CACAGGACTAGAACACCTGC-3′. The following primers and probes were purchased from Applied Biosystems (Foster City, CA, USA) for cDNA amplification to perform quantitative real-time PCR: Blimp-1 (Mm01187285ml); XBP-1 (Mm01187751ml); IRF-4 (Mm00516431ml); Bcl-6 (Mm01342169ml); PAX-5 (Mm01345231ml); β-actin (401846). TaqMan Universal PCR master mix (Applied Biosystems) was used for quantitative real time (RT) PCR with 2 mL of cDNA in five replicates. The average threshold cycles of the replicates were used to calculate the fold change between endogenous gene expression in the day 0 sample and the specific gene expression in the day 1, 2, and 3samples. Cycle for β-actin was used to normalize the results. Relative quantification was calculated using the comparative Ct method.
Determination of cytokines and chemokines released in the culture medium
Cytokines and chemokines released into the culture medium were detected using RayBio Mouse Cytokine Array I (RayBiotech, Norcross, GA, USA). Membrane-bound cytokines/chemokines were revealed by horseradish peroxidase (HRP)-conjugated streptavidin.
Preparation and tests of conditioned media
Five types of conditioned medium (Table 1) were prepared by culturing cells with CCM (4 mL/well) in the transwell system. CM1 was CCM containing LPS (3 μg/mL, CCM-LPS) with no cells; CM2 was produced by culturing MSCs (105/well) in CCM without LPS; CM3, 4 and 5 used CCM-LPS to culture MSCs, B cells (106/well), and MSCs and B cells, respectively. At the end of 3 day-culture, the supernatant was collected, aliquoted, and stored at -80°C until use. For in vitro experiments, purified B cells (106/well) were cultured in a 24-well plate with 1 mL/well of a desired CM and the generation of CD138+ cells was analyzed on day 3 by FACS. For in vivo experiments, BALB/c mice immunized with NP12-Ficoll were injected i.p. with 300 mL of a desired CM or PBS (control) on days 0 (day of first immunization), 2, and 4. Mice immunized with alum-precipitated NP19-KLH were treated on days 2, 4, and 6. Serum samples were collected for Ab assays on weeks 1, 2, and 3. Control serum was from BALB/c mice immunized with alum-precipitated NP19-KLH (50 mg) without CM-treatment.
Table 1.
MSC-conditioned media
| CCM | CM1 | CM2 | CM3 | CM4 | CM5 | |
|---|---|---|---|---|---|---|
| LPS | - | + | - | + | + | + |
| MSCs | - | - | + | + | - | + |
| B cells | - | - | - | - | + | + |
CCM, complete culture medium; CM, conditioned medium
Statistical analysis
Statistical analysis was performed using unpaired Student's t-test. P values less than 0.05 were considered to be significant.
Results
Characteristics of mouse MSCs
Cells isolated from C57BL/6 BM and passaged more than 5 times in culture exhibited a spindle-shaped morphology (Fig. 1A–a) and differentiated into adipocytes (Fig. 1A–b) and osteocytes (Fig. 1A–c). The cells were negative for hematopoietic markers (c-kit, CD34, CD45 and CD11b) and immunophenotypic makers (H-2Kb, I-Ab, CD86, CD40, CD40L and FAS-L), but expressed Sca-1 and CD80 Ags (Fig. 1B). Thus, the characteristics of our cells were comparable to those of murine MSCs reported by others [3, 10, 22- 24]. However, we did not test if these cells met specified stem cell criteria including long-term self-renewing, our MSCs would have been more appropriate to be expressed as multipotent mesenchymal stromal cells as suggested by the International Society for Cellular Therapy [25].
FIGURE 1.
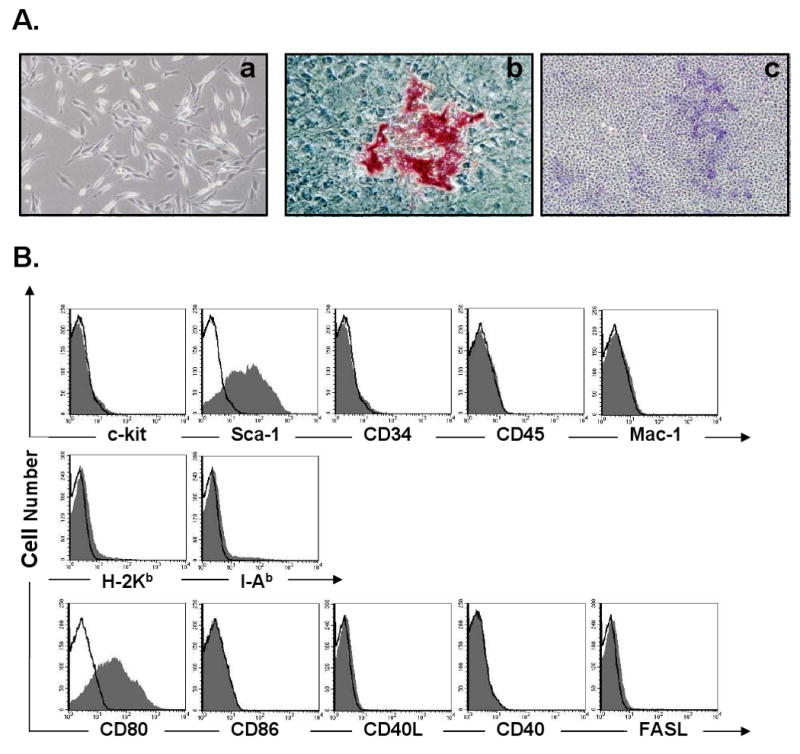
Characteristics of MSCs isolated from mouse bone marrow and expanded in culture: (A) MSCs were spindle-shape in monolayer culture (A-a, 100×), differentiated into adipocytes (A-b, Oil Red O staining, 200×) and osteocytes (A-c, alkaline phosphatase staining, 40×); (B) FACS analysis of MSCs using hematopoietic and immunophenotypic markers. Shaded histograms represent cells stained with a specific Ab and open histograms represent un-stained control cells.
MSCs prevent the terminal differentiation of LPS-stimulated B cells into plasma cells
In control transwell cultures without MSCs, the CD138+ cell percentage increased by LPS stimulation from 0.5% pre-culture to 7.8% on day 3 and remained at the similar level on day 4. In contrast, in the B/MSC (at a 10: 1 ratio) co-cultures, the CD138+ cell percentage increased to 2.0% on day 3 with no further increase on day 4 (Fig. 2A). Thus, the presence of MSCs reduced the plasma cell number to approximately 1 /4 of the control level. To determine if MSCs prevent B cell terminal differentiation, CFSE-labeled B cells were stimulated with LPS and cultured with or without MSCs for 3 days to measure the expression of CFSE and several differentiation markers by FACS. B cells in both groups divided up to seven cell divisions during this period (Fig. 2B–a), indicating that MSCs does not accelerate cell cycle progression. The expression of IgM and IgD decreased gradually as cell division progressed. The reduction of IgM on the B cells co-cultured with MSCs was a slightly slower than the control B cells (Fig. 2B–b). In contrast, the IgD expression was still high on divisions 3 and 4 of B cells co-cultured with MSCs and then slowly decreased. CD138 expression was detected on some of the control B cells after five cell divisions, but the less detectable with B cells co-cultured with MSCs. The slow reduction of surface IgD and the slow induction of CD138 expression on the B cells co-cultured with MSCs might indicate that MSCs decelerate the terminal differentiation of B cells stimulated with LPS.
FIGURE 2.
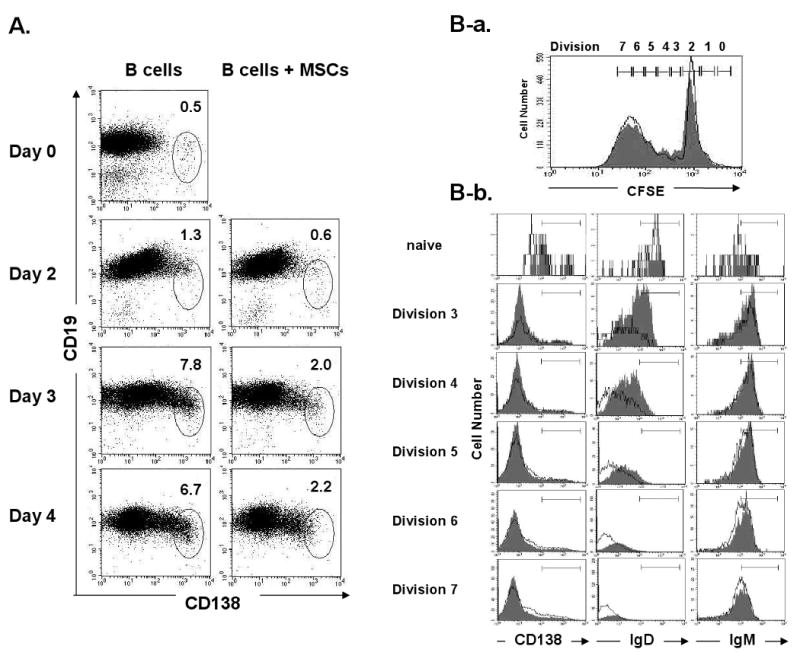
MSCs inhibit B cell terminal differentiation: (A) CD138 expression was analyzed daily for 4 days by FACS on BALB/c B cells co-cultured with C57BL/6 MSCs (1: 10 ratio) in separate compartments in the transwell system; (B-a) Cell division of the CFSE-labeled B cells cultured with or without MSCs was analyzed by FACS on day 3; (B-b) Expression of IgM, IgD, and CD138 on the co-cultured (filled histograms) and control (open histograms) B cells was analyzed by FACS (Representative of three independent experiments).
MSCs selectively suppress LPS-stimulated B cell differentiation into IgM-forming cells
To determine whether MSCs suppress immunoglobulin production, the B cell expression of μM and μS mRNA on day 3 was examined using RT-PCR but no clear difference was observed (Fig. 3A). IgM antibody production was significantly lower in the medium taken from the B/MSC co-cultures than in the control medium (29.0 ± 1.6 ng/mL vs. 69.9 ± 23.2 ng/mL, n = 3, p<0.05) and the IgG3 titer was 2-fold higher in the co-culture medium (5.1 ± 0.7 ng/mL vs.2.9 ± 0.2 ng/mL, n = 3, p<0.01) (Figure 3B). Furthermore, the IgG3+ B cell percentage was significantly higher in the co-cultures than in the controls (2.1 ± 1.6 % vs. 0.9 ± 0.2%, n = 5, p<0.01) (Fig. 3C–a, C-b). CFSE labeling revealed the presence of a higher number of IgG3+CFSE- B cells in the co-cultures than the control cultures (Fig. 3D). These results indicate that MSCs augment IgG3 expression of B cells and influence the Ig class switch recombination from IgM to IgG3.
FIGURE 3.
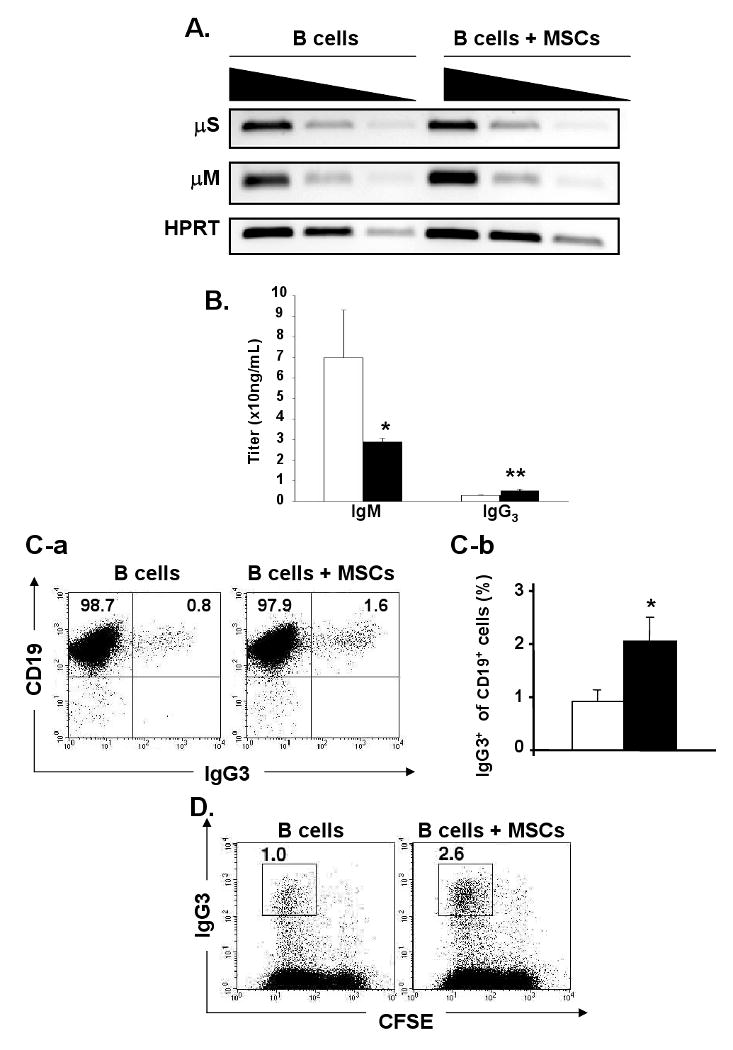
MSCs selectively suppress LPS-stimulated B cell differentiation into IgM-forming cells and augment IgG3 expression: (A) mRNA was extracted on day 3 from the B cells cultured with or without MSCs. The expression of μM and μS mRNA was analyzed by semi-quantitative RT-PCR. Four-fold dilution series of the cDNA were used as input material for the PCR with primers specific for μM, μS, or HPRT as a reference and HPRT mRNA as an internal control for the amount of mRNAs (representative of n=2); (B) Titers of IgM and IgG3 were measured by ELISA in the supernatant of B cells cultured alone (open bar) or co-cultured with MSCs (solid bar) for 3 days. (Titers are shown by mean ± SD, n=3; *, p < 0.05; **, p < 0.01); (C) MSCs and CFSE-labeled B cells (1: 10 ratio) were co-cultured in the presence of LPS. (C-a) The numbers indicate IgG3-CD19+ and IgG3+CD19+ fractions on day 3; (C-b) The percentage of surface IgG3+ cells in CD19+ cells in B cells cultured alone (open bar) or with MSCs (solid bar) (n=5; **, p < 0.01); (D) The percentages of CFSE-CD138+ cells were determined on day 3 by FACS and shown in each square (representative of n=5).
MSCs also suppress B cell proliferation, but do not induce plasma cell apoptosis
3H-thymidine incorporation performed on day 2 demonstrated the suppression of LPS-stimulated B cell proliferation by MSCs (Fig 4A). At a 5: 1 of B and MSC ratio, MSCs suppressed the 3H-thymidine uptake of B cells to approximately half of the control levels (1.6 ± 0.8 ×104 cpm vs. 2.9 ± 0.1 ×104 cpm, n = 3, p<0.05). In contrast, no suppression was observed at a 10: 1 ratio. MSCs also suppressed B cell division as tested on day 3 using CFSE-labeled B cells (Fig. 4B). The number of CFSElow dividing B cells was significantly lower in the B/MSC co-cultures at all ratios tested (10: 1, 5: 1, 2: 1) as compared to that of B cell alone cultures (Fig. 4B). These results show that the suppression of LPS-mediated B cell proliferation requires higher number of MSCs than that required for the suppression of B cell differentiation. The number of dividing (CFSE-) cells expressing CD138 was significantly lower in the B/MSC co-cultures than in the control cultures (2.6 vs. 5.4 %) (Fig. 4C–a). To determine whether the low CD138+ cell number in the co-cultures was a result of cell apoptosis, B cells were stained on day 3 for CD138 and Annexin V. The percentage of Annexin V+PI-CD138+ cells was significantly lower in the B/MSCs than that in the B cells alone (10.8± 0.5% vs. 17.2 ± 0.8%, n=4, p<0.01) (Fig. 4C–b and C-c), suggesting that the decreased plasma cell numbers was not due to apoptosis caused by the presence of MSCs.
FIGURE 4.
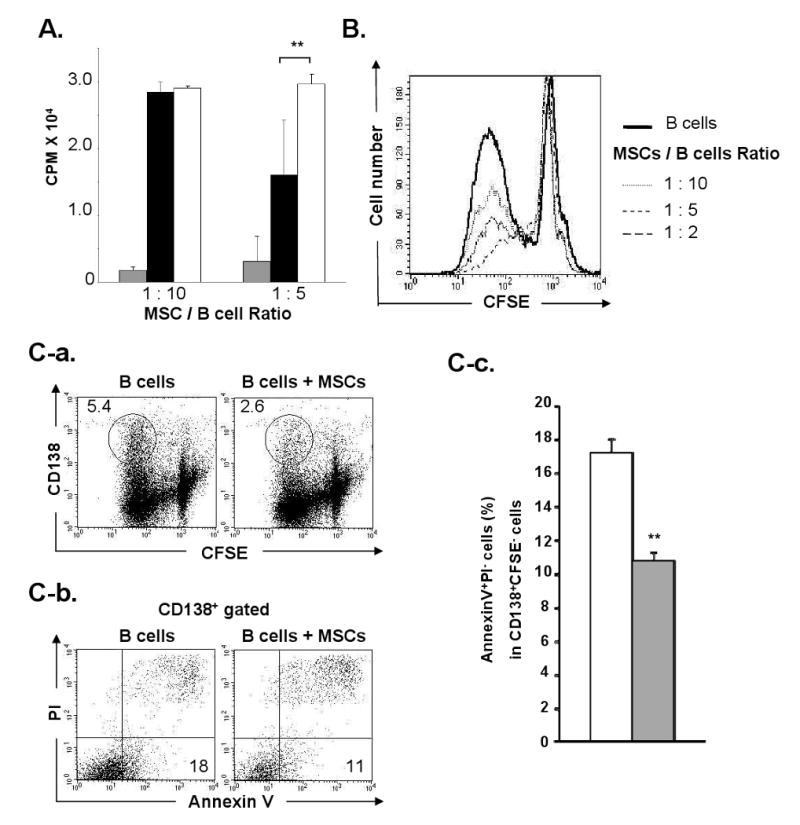
MSCs suppress B cell proliferation, but do not induce plasma cell apoptosis: (A) 3H-thymidine uptake on day 2. B cells with MSCs at 1: 10 or 1: 5 ratio (solid bar), B cells cultured alone (open bar), and MSC alone (shaded bar) were cultured in the presence of LPS (cpm; mean ± SD, n=3); (B) MSCs and CFSE-labeled B cells were cultured at three different ratios (1: 10, 1: 5, and 1: 2) in transwells in the presence of LPS using B cells alone as controls. Histograms show the B cell division on day 3; (C) MSCs and CFSE-labeled B cells were co-cultured (1: 10) in the presence of LPS. (C-a) The number in each oval indicates the percentage of CFSE-CD138+ cells on day 3; (C-b) The numbers at low-right corners indicate the percentage of Annexin V+PI- cells in the CD138+ population in B/MSC and B alone cultures; (C-c) The shaded bar shows the percentage of Annexin V+PI- cells in CD138+CFSE- cells in B/MSC cultures (10: 1) and the open bar shows that in the B cell alone cultures (mean ± SD, triplicate tests of n=4; **, p< 0.01).
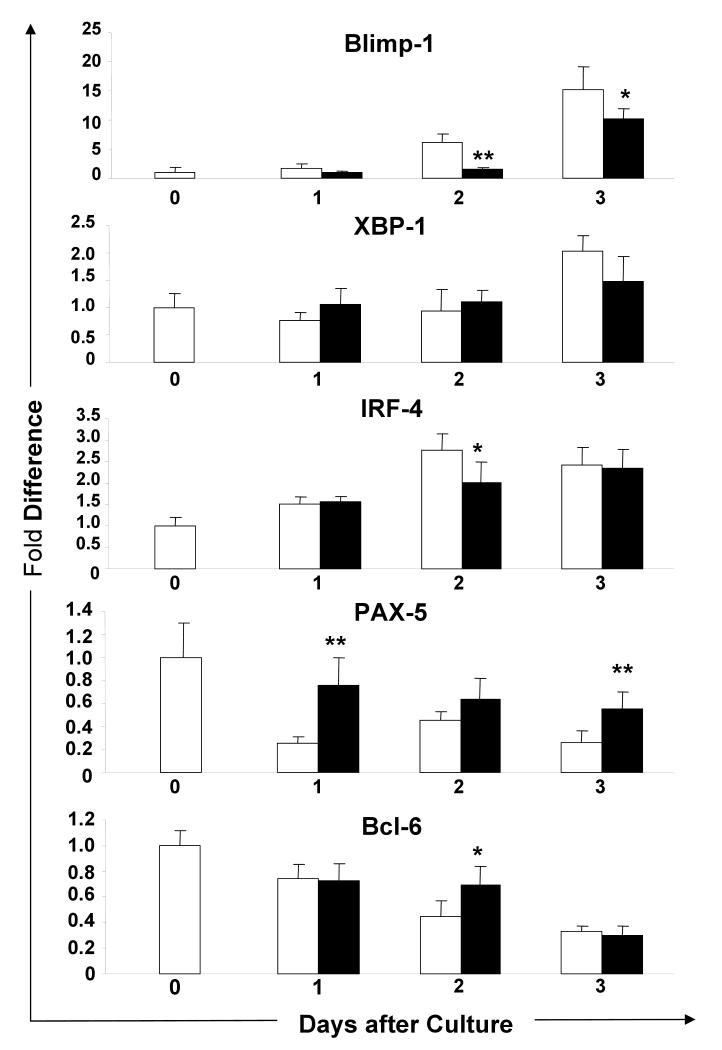
MSCs down-regulate the expression of Blimp-1 mRNA during the B cell terminal differentiation
To examine genes involved in the suppression of B cell terminal differentiation by MSCs, the expression of Blimp-1, XBP-1, IRF-4, PAX-5, and Bcl-6 mRNA by B cells was examined using RT-PCR. To compare expression levels, the endogenous mRNA level on day 0 was defined as 1.0. Blimp-1 expression continuously increased in control B cells during the 3-day culture period (Fig. 5). Blimp-1 expression was significantly lower in B cells co-cultured with MSCs and the difference was highest on day 2 (co-cultured vs. control B cells: 1.5 ± 0.3 vs. 6.1 ± 1.5, n = 5, p<0.01). Blimp-1 expression in the co-cultured B cells was also lower on day 3 (10.2 ± 1.7 vs. 15.2 ± 4.0, n = 5, p<0.05). There was no significant difference in the XBP-1 expression in B cells in both groups. The IRF-4 expression in the co-cultured B cells was significantly suppressed only on day 2 (2.0 ± 0.5, vs. 2.8 ± 0.4, n = 5, p<0.05) and the PAX-5 expression was significantly increased as compared to the control B cells (co-culture vs. control: 0.8 ± 0.2 vs. 0.3 ± 0.1 on day 1 and 0.6 ± 0.1 vs. 0.3 ± 0.1 on day 3, n = 5, p<0.01). During culture, the Bcl-6 expression gradually decreased as the Blimp-1 expression increased in control B cells, while it was expressed significantly higher in co-cultured B cells but only on day 2 (0.7 ± 0.1 vs. 0.4 ± 0.1, n = 5, p<0.05). In summary, the expression of Blimp-1 mRNA was suppressed throughout the culture period in the B cells co-cultured with MSCs. Conversely, PAX-5 expression increased. These results demonstrate that MSCs prevent the terminal differentiation of B cells by down-regulation of Blimp-1.
FIGURE 5.
Blimp-1 expression is suppressed in B cells co-cultured with MSCs: mRNA was extracted from the B cells cultured alone or with MSCs in the presence of LPS in transwells for 3 days. The expression levels of Blimp-1, XBP-1, IRF-4, PAX-5, and Bcl-6 mRNA were analyzed by RT-PCR. The results were calculated by the comparative threshold cycle (Ct) method, with the Ct for the β-actin used to normalize the results. Expression of each gene was calculated with the endogenous level of the corresponding gene in untreated B cells defined as 1. Each of X-axis indicates the days of culture. Solid and open bars show results of B/MSC cell co-cultures and B cell alone, respectively (mean ± SD of 5 mRNA samples from two independent experiments; *, p < 0.05; **, p < 0.01).
Inhibition of B cell differentiation is mediated by MSC-released humoral factor(s)
The culture system used in this study did not allow direct cell-cell contact between MSCs and B cells, and therefore the suppression of B cell differentiation did not require cell-cell contact and must have been mediated through humoral factor(s) secreted by MSCs. To further confirm this, purified BALB/c B cells were cultured for 3 days in 24-well plates using three different concentrations (100%, 50%, and 25%) of three different conditioned media, CM3, CM4, and CM5 (Table 1). Control conditioned media (CCM) containing 3 μg/mL LPS was used for control cultures, as well as to dilute the test CMs. The CM3 and CM5 cultures containing MSC humoral factor(s) suppressed the generation of CD138+ cells, while the CCM and CM4 cultures did not (Fig. 6A).
FIGURE 6.
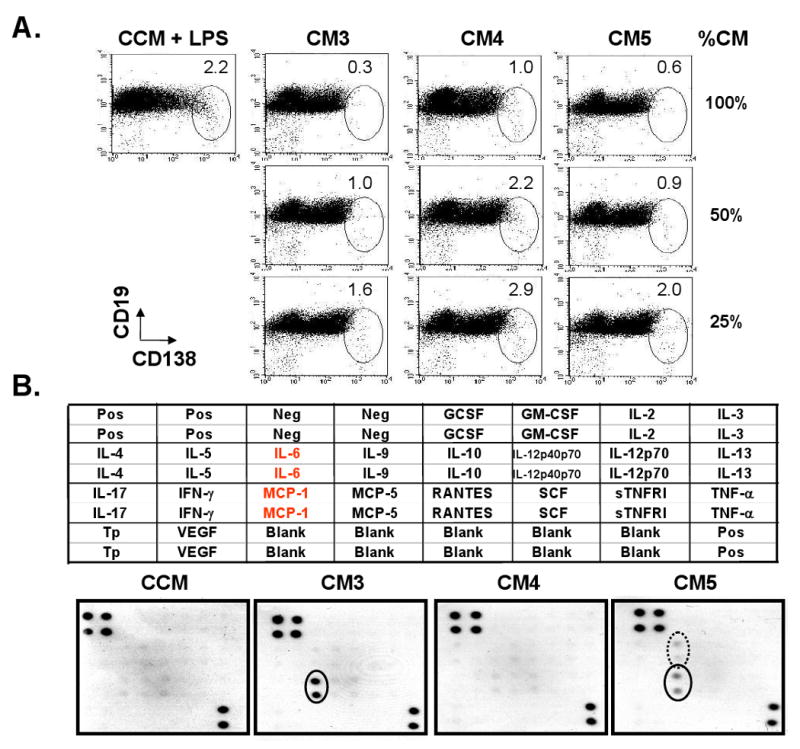
MSCs prevent B cell differentiation into plasma cells by releasing humoral factor(s): (A) 106 B cells were cultured with 3 different concentrations (100, 50 or 25%) of CM3, CM4 or CM5 in 24-well plates for 3 days. CMs containing LPS were diluted with LPS-containing CCM, and those without LPS were diluted with CCM. B cells cultured with CCM containing LPS were used as controls. The numbers in each oval indicate the percentages of CD138+ CD19+/- cell fractions (n=3); (B) The presence of cytokines/chemokines in CM3, CM4, and CM5 was screened using the RayBio Mouse Cytokine Array I. The layout of the array is shown on the top. The protein array was incubated separately with test CM and CCM as a control. The results are shown at the bottom. Solid dots indicate the signal for MCP-1 and the circled dots indicates the signal for IL-6. (Pos: positive control; Neg: negative control; Tp: thrombopoietin). Spot intensity relative to the positive and negative control offers an indication of the relative amount of chemokines/cytokines present in the CM.
Possible humoral factors involved in B cell suppression could be cytokines. Therefore, cytokines and chemokines present in CMs were assayed using the Cytokine Antibody Array I. As shown in figure 6B, only MCP-1 was detected in CM3, MCP-1 and IL-6 were positive in CM5, and no cytokine or chemokine was detected in CM4 derived from the B cell alone culture. We then tested a possible involvement of MCP-1 in the CD138+ cell suppression by adding anti-MCP-1 monoclonal Ab at various concentrations to B cells cultured in CM3 or CM5. Three days later, only a few CD138+ cells were recovered from all of these cultures, suggesting that MCP-1 was not responsible for the suppression of B cell differentiation (data not shown).
Administration of MSC-derived CMs reduces antigen-specific IgM and IgG1 production in mice immunized with T-independent or T -dependent antigen
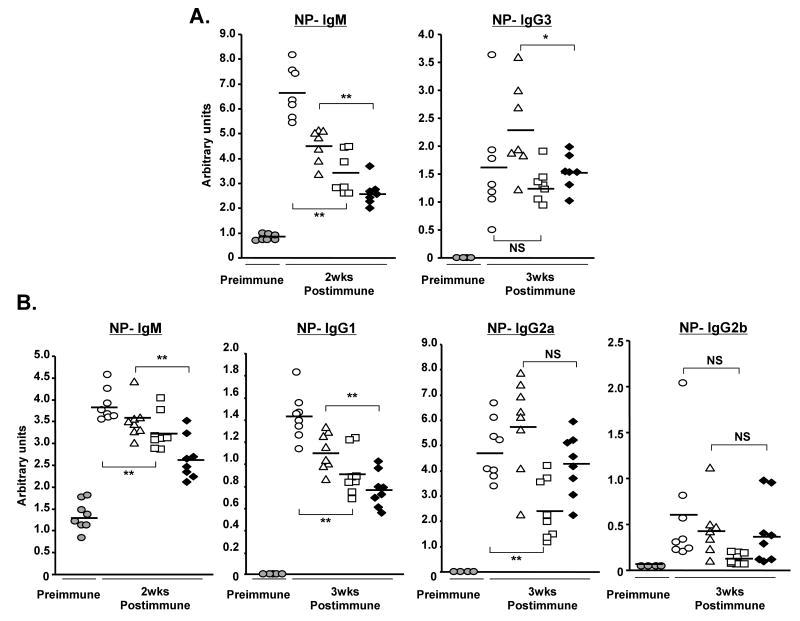
To test the suppression of B cell function by MSC-humoral factor(s) in vivo, test CMs were injected i.p. to mice immunized with NP12-Ficoll from day 0, or alum-precipitated NP19-KLH from day 2. Serum samples were collected after 2 and 3 weeks for the measurement of NP-specific Ab titers. Since some of the injected CMs contained LPS which might influence the recipient Ab response, the NP-specific Ig titers were compared between the groups injected with CM not containing LPS (PBS vs. CM2) and between the groups injected with LPS-containing CM (CM1 vs. CM3). In addition, CM2 and CM3 contained MSC products. In mice treated with CM2 or CM3, the titers of all isotypes of NP-specific Igs were lower than those measured in mice administered LPS-free PBS or CM1 (Fig. 7A and 7B). The NP-specific IgM-titer was significantly lower with ranges of 51 - 84% in mice immunized with T-D as well as T-ID antigens (Table 2). Similarly, the NP-specific IgG1 titer in mice immunized with T-D Ag was reduced to 64 – 69% of the controls. These results clearly show that humoral factor(s) from MSCs released in culture medium are capable of suppressing B cell function in vivo.
FIGURE 7.
Antigen-specific Ig secretion is suppressed in the immunized mice treated with CM, a culture supernatant obtained from C57BL/6 MSCs cultures: (A) CM or control PBS was injected i.p. on days 0, 2, and 4 into BALB/c mice immunized on day 0 with 50 μg NP12-Ficoll (T-dependent Ag). Serum samples were analyzed for NP-specific IgM on days 0 and 14, and IgG3 on days 0 and 21; (B) CM or PBS was injected i.p. on days 2, 4, and 6 into BALB/c mice immunized with 50 μg alum-precipitated NP19-KLH (T-independent Ag). Serum samples were analyzed for NP-specific IgM on days 0 and 14, and IgG1, IgG2a, and IgG2b Abs on days 0 and 21. Ab titers were shown as arbitrary units by ELISA. Shaded circles, open circles, triangles, squares, or black diamonds represent pre-immune, PBS, CM1, CM2, or CM3, respectively (n = 7 for fig. 7A; n = 8 for fig. 7B; NS, not significant; *, p < 0.05; **, p < 0.01).
Table 2. Reduction of NP-specific Ig titers by injections of MSC-culture supernatants.
| Antigen | T -independent | T-dependent | ||||
|---|---|---|---|---|---|---|
| Antibody isotype | IgM | IgG3 | IgM | IgG1 | IgG2a | IgG2b |
| CM2/PBS (no LPS) | 51** | 81NS | 84** | 64** | 51** | 21NS |
| CM3/CM1 (LPS) | 58** | 66* | 76** | 69** | 73NS | 91NS |
The percentage of reductive antigen NP-specific Ig titers was calculated from the average of each group injected with CM or PBS (n=8 for T-ID antigen and n=7 for T-D). CM: conditioned medium;
not significant;
p < 0.05;
p < 0.01
Discussion
The culture-expanded MSCs consistently suppressed the terminal differentiation of B cells into plasma cells. Previous investigations measured MSC effects by 3H-thymidine uptake of B cells stimulated by various other antigens [11, 15, 26] and all of these studies with one exception [15], showed the MSC's inhibitory effect on B cell proliferation as we did. However, the recent studies have reported two opposite effects on B cell differentiation. Two studies showed “suppression of B cell differentiation” [15, 27] and the other two showed “augmentation” [28, 29]. However, even in the latter studies, Rasmusson I et al. found the suppression of LPS-stimulated B cell differentiation by MSC-secreted humoral factors [28]. These discrepancies may be due to various factors and conditions, including different signaling pathways initiated by the stimuli through the BCR, TLR, or CD40 molecules, via the cell-cell contact, or humoral factors, the strength of the stimuli, the species of the MSC origin, the purity of B cells, and/or MSCs. We have shown that the suppression of LPS-stimulated B cell proliferation in vitro requires a higher MSC: B cell ratio than that required for suppressing B cell differentiation. Moreover, our study has suggested that the decreased numbers of differentiated plasma cells in the B/MSC co-cultures is not mediated by apoptosis.
Transcription factors Blimp-1 [19], XBP-1 [30] and IRF-4 [31] have been postulated to be the master regulators of B cell terminal differentiation [16]. Blimp-1 represses the expression of both PAX-5 [32, 33] and Bcl-6 [34-36] that are required for preservation of B cell phenotypes and germinal center reactions. The generation of plasma cells also requires the repression of PAX-5 and Bcl-6 expression [16]. Among these genes, only the expression of Blimp-1 mRNA was continuously suppressed during the 3 day-culture period in the B/MSC co-cultures, although the decreased Blimp-1 expression may possibly be due to decreased plasma cell numbers. The expression of PAX-5 mRNA increased relative to Blimp-1 suppression. TLR-4, bound to LPS on B cells, sends signals to initiate the transcription factors NF-κB and AP-1 [37] which subsequently induce Blimp-1 expression [38, 39]. The Blimp-1 promoter is directly regulated by Bcl-6 [40] or AP-1 [38, 39]. Thus, the humoral factor(s) released by MSCs may influence this signaling pathway, leading to the suppression of Blimp-1. The simultaneous suppression of the Blimp-1and IRF-4 mRNAs on day 2 may further enhance the inhibition of B cell terminal differentiation.
Using the transwell culture system, we have shown that cell-cell contact is not necessary for MSCs to suppress B cell function. The involvement of the humoral factor(s) was further demonstrated by culturing B cells in MSC culture supernatants (CM3 and CM5). The presence of MCP-1 in both CM3 and CM5 detected by the Cytokine Antibody Array I is consistent with the previous finding that MSCs are capable of secreting MCP-1 [41]. However, the addition of anti-MCP-1 mAb to the CM3 and CM5 did not inhibit the suppression of B cell differentiation, indicating no direct involvement of this cytokine. MSCs are also shown to secret IL-10, TGF-β [42], and IDO [43] in response to IFN-γ stimulation. IL-10 and TGF-β are representative of suppressive cytokines [44], and IDO is shown to be involved in the suppression of T cell activation by catalyzing tryptophan conversion to kynurenine [45]. However, the Cytokine Antibody Array analysis did not detect IL-10 in MSC culture supernatants. Moreover, neither TGF-β nor IDO appeared to be involved in B cell suppression as indicated by our neutralizing experiments using anti-TGF-βmAb or 1-methyl-D-tryptophan (data not shown).
Immunomodulatory properties of MSCs on mature B cells have never been investigated in vivo. To determine whether the humoral factors from MSCs can also effectively suppress B cell function in vivo, Ig titers were measured in serum samples taken from mice immunized with T-ID or T-D Ag and treated with a specific CM. The class switch recombination from IgM to IgG-subtypes in B cells is influenced by various cytokines secreted by CD4+ T cells and antigen presenting cells (APC). In order to exclude the effects of LPS on T cells and APCs, the results of CMs with or without LPS were compared to those obtained with appropriate controls. These in vivo results have clearly shown a significant reduction of IgM and IgG1 titers specific to NP by mice treated with MSC culture supernatant. We speculate that MSCs and MSC-derived conditioned medium would also suppress memory B cell differentiation into plasma cells, leading to the suppression of antibody production, although the timing of conditioned medium administration may be critical.
In summary, we have demonstrated that MSCs exert a suppressive effect on the terminal differentiation of B cells both in vitro and in vivo by releasing humoral factor(s). The suppression of B cell differentiation may be mediated by the down-regulation of Blimp-1 expression by MSC-humoral factor(s).
Acknowledgments
This study was supported by grants from the Nora Eccles Treadwell Foundation and NIH. The authors gratefully acknowledge Dr. Taihei Ito for stimulating discussion, Jonathan Shintaku for editing the manuscript, and Jeffrey Rawson for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu Rev Immunol. 1990;8:111–137. doi: 10.1146/annurev.iy.08.040190.000551. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 4.Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–121. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Kalka C, Isner JM. Stem cell therapy and gene transfer for regeneration. Gene ther. 2000;7:451–457. doi: 10.1038/sj.gt.3301142. [DOI] [PubMed] [Google Scholar]
- 6.Krampera M, Pasini A, Pizzolo G, Cosmi L, Romagnani S, Annunziato F. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr Opin Pharmacol. 2006;6:435–441. doi: 10.1016/j.coph.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 8.Uccelli A, Pistoia V, Lorenzo Moretta. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol. 2007;28:219–226. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Itakura S, Asari S, Rawson J, et al. Mesenchymal stem cells facilitate the induction of mixed hematopoietic chimerism and islet allograft tolerance without GVHD in the rat. Am J Transplant. 2007;7:336–346. doi: 10.1111/j.1600-6143.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 10.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 11.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 12.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 13.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 14.Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 15.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 17.Lin KI, Tunyaplin C, Calame K. Transcriptional regulatory cascades controlling plasma cell differentiation. Immunol Rev. 2003;194:19–28. doi: 10.1034/j.1600-065x.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 18.Turner CA, Jr, Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y, Wong K, Calame K. Repression of c-myc transcription by Blimp-1, an inducer of terminal B cell differentiation. Science. 1997;276:596–599. doi: 10.1126/science.276.5312.596. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–620. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro-Shelef M, Lin KI, Savitsky D, Liao J, Calame K. Blimp-1 is required for maintenance of long-lived plasma cells in the bone marrow. J Exp Med. 2005;202:1471–1476. doi: 10.1084/jem.20051611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 23.Meirelles Lda S, Nardi NB. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol. 2003;123:702–711. doi: 10.1046/j.1365-2141.2003.04669.x. [DOI] [PubMed] [Google Scholar]
- 24.Sun S, Guo Z, Xiao X, et al. Isolation of mouse marrow mesenchymal progenitors by a novel and reliable method. Stem cells. 2003;21:527–535. doi: 10.1634/stemcells.21-5-527. [DOI] [PubMed] [Google Scholar]
- 25.Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 26.Augello A, Tasso R, Negrini SM, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 27.Comoli P, Ginevri F, Maccario R, et al. Human mesenchymal stem cells inhibit antibody production induced in vitro by allostimulation. Nephrol Dial Transplant. 2008;23:1196–1202. doi: 10.1093/ndt/gfm740. [DOI] [PubMed] [Google Scholar]
- 28.Rasmusson I, Le Blanc K, Sundberg B, Rinden O. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol. 2007;65:336–343. doi: 10.1111/j.1365-3083.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 29.Traggiai E, Volpi S, Schena F, et al. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008;26:562–569. doi: 10.1634/stemcells.2007-0528. [DOI] [PubMed] [Google Scholar]
- 30.Reimold AM, Iwakoshi NN, Manis J, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 31.Klein U, Casola S, Cattoretti G, et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat immunol. 2006;7:773–782. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- 32.Nera KP, Kohonen P, Narvi E, et al. Loss of Pax5 promotes plasma cell differentiation. Immunity. 2006;24:283–293. doi: 10.1016/j.immuni.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Delogu A, Schebesta A, Sun Q, Aschenbrenner K, Perlot T, Busslinger M. Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity. 2006;24:269–281. doi: 10.1016/j.immuni.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Ye BH, Cattoretti G, Shen Q, et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet. 1997;16:161–170. doi: 10.1038/ng0697-161. [DOI] [PubMed] [Google Scholar]
- 35.Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997;276:589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- 36.Fukuda T, Yoshida T, Okada S, et al. Disruption of the Bcl6 gene results in an impaired germinal center formation. J Exp Med. 1997;186:439–448. doi: 10.1084/jem.186.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzgerald KA, Chen ZJ. Sorting out Toll signals. Cell. 2006;125:834–836. doi: 10.1016/j.cell.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Vasanwala FH, Kusam S, Toney LM, Dent AL. Repression of AP-1 function: a mechanism for the regulation of Blimp-1 expression and B lymphocyte differentiation by the B cell lymphoma-6 protooncogene. J Immunol. 2002;169:1922–1929. doi: 10.4049/jimmunol.169.4.1922. [DOI] [PubMed] [Google Scholar]
- 39.Ohkubo Y, Arima M, Arguni E, et al. A role for c-fos/activator protein 1 in B lymphocyte terminal differentiation. J Immunol. 2005;174:7703–7710. doi: 10.4049/jimmunol.174.12.7703. [DOI] [PubMed] [Google Scholar]
- 40.Tunyaplin C, Shaffer AL, Angelin-Duclos CD, Yu X, Staudt LM, Calame KL. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J Immunol. 2004;173:1158–1165. doi: 10.4049/jimmunol.173.2.1158. [DOI] [PubMed] [Google Scholar]
- 41.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 42.Liu H, Kemeny DM, Heng BC, Ouyang HW, Melendez AJ, Cao T. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. J Immunol. 2006;176:2864–2871. doi: 10.4049/jimmunol.176.5.2864. [DOI] [PubMed] [Google Scholar]
- 43.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 44.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. 2006;117:433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mellor A. Indoleamine 2,3 dioxygenase and regulation of T cell immunity. Biochem Biophys Res Commun. 2005;338:20–24. doi: 10.1016/j.bbrc.2005.08.232. [DOI] [PubMed] [Google Scholar]