Abstract
BNIP3 (Bcl-2/adenovirus E1B 19-kDa interacting protein 3) is a BH3-only proapoptotic member of the Bcl-2 family. Because the interaction of Bcl-2 proteins with intracellular Ca2+ stores has been linked to apoptosis, the role of Ca2+ transfer between endoplasmic reticulum (ER) and mitochondria in BNIP3-mediated cell death was determined in a rat dopaminergic neuronal cell line, Mes 23.5. BNIP3 mutants were constructed to target either ER or mitochondria. Localization of BNIP3 to the ER membrane facilitated release of Ca2+ and subsequently increased uptake of Ca2+ into mitochondria. Excessive accumulation of mitochondrial Ca2+ decreased mitochondrial membrane potential (ΔΨm), resulting in execution of a caspase-independent cell death. Reduction of ER Ca2+ induced by ER-targeted BNIP3 and the subsequent cell death was blocked by the antiapoptotic protein, Bcl-2. On the other hand, mitochondria-targeted BNIP3 initiated apoptosis by a Ca2+-independent mechanism by inducing mitochondrial pore transition and dissipation of ΔΨm. The disruption of ΔΨm and cell death was not blocked by Bcl-2 overexpression. These findings show that BNIP3 undergoes a dual subcellular localization and initiates different cell death signaling events in the ER and mitochondria. Bcl-2 counters the BNIP3-initiated mobilization of ER Ca2+ depletion to reduce the level of apoptosis.—Zhang, L., Li, L., Liu, H., Borowitz, J. L., Isom, G. E. BNIP3 mediates cell death by different pathways following localization to endoplasmic reticulum and mitochondrion.
Keywords: apoptosis, Bcl-2, calcium, thapsagargin
Bnip3 (Bcl-2/adenovirus E1B 19-kDa interacting protein 3) is a proapoptotic protein that contains a single Bcl-2 homology 3 (BH3) domain and is classified in the BH3-only subfamily of Bcl-2 proteins. Unlike other BH3 proteins, the C-terminal transmembrane (TM) domain of BNIP3 is required for mitochondrial targeting and for the prodeath function (1). In the initial phase of apoptosis, BNIP3 inserts into the outer mitochondrial membrane, with the N terminus oriented into the cytoplasm and the C terminus inside the mitochondria. BNIP3 may initiate mitochondria-mediated apoptosis by inducing mitochondrial permeability transition (MPT) pore opening to disrupt the proton electrochemical gradient across the inner mitochondrial membrane (2). The result is reduced mitochondrial respiration, excess generation of reactive oxygen species, and reduced ATP synthesis that can lead to cell death (3). Interestingly, targeting BNIP3 to the endoplasmic reticulum (ER) by replacing the TM domain with ER anchor residues in rat hepatic cytochrome b5 (Cb5) does not affect the prodeath properties (1). It appears that BNIP3-mediated cell death involves not only mitochondria, but other target organelles.
Mitochondria play a critical role in amplifying cell-death signals. Upstream of the execution events in cell death is MPT, which is accompanied by mitochondrial swelling, rupture of the outer mitochondrial membrane, and release of small proapoptotic molecules to initiate either caspase-dependent or -independent cell death (4). MPT can be triggered by mitochondrial Ca2+ overload and is regulated by Bcl-2 proteins. For instance, the proapoptotic proteins Bax/Bak or antiapoptotic Bcl-2 can induce or block MPT by affecting the mitochondrial voltage-dependent anion channel (VDAC) and adenine nucleotide translocator (ANT) (5).
The ER participates in apoptosis by regulating cellular Ca2+ stores. Under normal conditions, ER is a primary Ca2+ intracellular storage site. A high concentration of Ca2+ is maintained in ER through action of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) pump located in the ER lumen. On the other hand, inositol 1,4,5-triphosphate (IP3) can activate IP3 receptors (IP3Rs) to trigger Ca2+ release from the ER. The Ca2+ is then rapidly taken up and accumulated by juxtaposed mitochondria. Bax and the BH3-only protein Bik can localize in ER to facilitate mobilization of ER Ca2+, thus increasing propagation of the calcium signal to mitochondria and eventually producing Ca2+-dependent MPT (6, 7). In contrast, Bcl-2 exerts an antiapoptotic effect by binding with IP3Rs to reduce Ca2+ leakage from ER (8, 9, 10).
Since BNIP3 participates in cell death by localizing in mitochondria and ER, the objective of this study was to determine whether Ca2+ signaling between ER mitochondria is involved in BNIP3-mediated apoptosis. Mes 23.5 cells, an immortalized dopaminergic cell line, were transfected with BNIP3 wild-type and site-directed BNIP3 constructs, and subsequent changes in Ca2+ and mitochondrial function were correlated with cell death.
MATERIALS AND METHODS
Cell culture
The Mes 23.5 cell line, obtained from Dr. C. Rochet (Purdue University), was derived from somatic cell fusion of rat embryonic mesencephalic cells and the murine neuroblastoma-glioma cell line N18TG2 (11). Mes 23.5 cells have been used to study Ca2+-mediated neurotoxicity (11, 12). To establish a stable cell line overexpressing Bcl-2, the Bcl-2 construct in the LTR-SV neo vector (a kind gift from Dr. David M. Hockenbery, University of Washington, Seattle, WA, USA) was introduced into Mes 23.5 cells by transfection and selected with Geneticin (Life Technologies, Grand Island, NY, USA). Cells were seeded on poly-l-lysine-precoated plates and maintained in DMEM supplemented with 5% FBS, 2% newborn calf serum (NBS), 15 mM HEPES, and SATO components (5 μg/ml insulin, 5 μg/ml transferrin, 48.6 μg/ml pyruvic acid, 4 μg/ml putrescine, 5 ng/ml sodium selenite, and 6.3 ng/ml progesterone) at 37°C in an atmosphere of 5% CO2 and 95% air.
Cell-death assay
Apoptosis was detected by using a nuclear staining kit from Molecular Probes (Eugene, OR, USA), as described previously (13). Cells were washed twice with PBS, and fixed in 4% paraformaldehyde in PBS, then treated with 2 μM Hoechst 33258 dye, and examined under fluorescence microscopy. Cells with condensed and fragmented DNA were considered apoptotic. Apoptosis was quantified by averaging cell counts in four random ×400 fields.
The in situ terminal deoxynucleotidyl transferase-mediated DNA nick-end labeling (TUNEL) assay was used to confirm apoptosis. The TUNEL assay was performed on paraformaldehyde (4% in PBS)-fixed cells using the ApopTag™ in situ apoptosis detection kit (Oncor, Gaithersburg, MD, USA).
During apoptosis, phosphatidylserine (PS) is translocated from the cytoplasmic face of the plasma membrane to the cell surface. Annexin V has a high affinity for PS and can be used to further confirm apoptosis. Cells were incubated with annexin V-FITC in annexin V-binding buffer (BD Bioscience, San Jose, CA, USA) for 15 min; then fluorescence intensity was detected with a microtiter plate reader at an excitation wavelength of 488 nm and an emission wavelength of 520 nm.
Western blot analysis
Following treatments, whole-cell lysates were prepared using a lysis buffer containing 220 mM mannitol, 68 mM sucrose, 20 mM HEPES (pH 7.4), 50 mM KCl, 5 mM EGTA, 1 mM EDTA, 2 mM MgCl2, 1 mM dithiothreitol, 0.1% Triton X-100, and protease inhibitors. Western blot analysis was carried out with the ECF Western blot kit (Amersham Biosciences, Piscataway, NJ, USA), as described by the manufacturer. The primary antibodies were mouse anti-BNIP3 and mouse anti-β-actin (Sigma Chemical Co, St. Louis, MO, USA) and goat anti-BNIP3, mouse anti-COX IV, and rabbit anti-calnexin antibodies (Abcam Inc., Cambridge, MA, USA).
Subcellular fractionation
Isolation of mitochondria and ER fractions were performed as described by Wang and Chan (14), with modifications. Cells were collected; suspended in hypotonic buffer containing 250 mM sucrose, 20 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, and protease inhibitor cocktail; and homogenized by passing through a syringe needle (26-gauge). Homogenates were first centrifuged at 800 g for 10 min at 4°C, and the supernatant was centrifuged at 10,000 g for 20 min at 4°C to pellet the mitochondrial fraction (heavy membrane, HM). The supernatant was centrifuged at 100,000 g for 1.5 h at 4°C, and the final pellet was the ER (light membrane, LM)-enriched fraction. Fractionation purity was confirmed by assessing the presence of COX IV for mitochondria and calnexin for ER.
Confocal microscopy
Cells grown on poly-l-lysine-precoated coverslips were labeled with 100 nM MitoTracker Red (Invitrogen, Eugene, OR, USA) for 10 min at 37°C. After washing with PBS, cells were fixed in 4% paraformaldehyde and permeabilized with 0.2% Triton X-100 for 30 min at room temperature. Cells were then exposed to blocking solution (20% goat serum in PBS), followed by incubation with a mouse monoclonal anti-BNIP3 primary antibody at room temperature for 3 h. Cells were then rinsed with PBS 3 times, followed by incubation with Alexa Fluor 488-conjugated goat anti-mouse secondary antibody (Molecular Probes) for 1 h at room temperature.
Double-labeling immunofluorescence was used to characterize cellular distribution of BNIP3-immunoreactive proteins in cells. Rabbit polyclonal anti-calnexin antibodies were added with the BNIP3 antibody. After 3 h of incubation, an Alexa Fluor 568-conjugated goat anti-rabbit secondary antibody was applied with the Alexa Fluor 488-conjuated goat anti-mouse secondary antibody for 1 h at room temperature.
Coverslips were then mounted onto glass slides, and images were acquired and analyzed using a Zeiss LSM 510 laser-scanning confocal microscope (Zeiss, Oberkochen, Germany) equipped with an ×60 oil-immersion objective.
Plasmid constructs and transient transfection
BNIP3 mediates cell death following insertion into the mitochondrial outer membrane; however, it is possible that nonmitochondrial sites may participate in the cell death (15, 16). To selectively target BNIP3, mutants were constructed to include either mitochondrial- or ER-targeting sequences. The listerial protein Acta contains a 27-aa insertion sequence that targets heterologous proteins to the mitochondrial outer membrane with the proteins oriented toward the cytosol (17). Cb5 is an integral membrane protein located on the outer surface of the ER. The 35-aa residue at the C terminal of Cb5 selectively targets a heterologous protein to the cytoplasmic face of the ER membrane (18). Wild-type BNIP3 and BNIP3ΔTM (TM domain-deleted form) were constructed as described previously (13). The nucleotide sequences encoding Acta (Listeria) and Cb5 (rat) (18) were added to the C terminus of the nucleotide sequence encoding BNIP3ΔTM by PCR reaction. The whole cDNA sequence encoding the chimeric proteins BNIP3-Acta and BNIP3-Cb5 was then subcloned into the pHA-CMV vector between the EcoRI/KpnI restriction sites. Positive clones were confirmed by DNA sequencing. Transient transfections were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s recommendations, and the transfection efficiency was ∼90% when assessed using enhanced yellow fluorescent protein (eYFP; data not shown).
Cytosolic free Ca2+
Cells were loaded with 2.5 μM of the fluorescence Ca2+ indicator dye fluo-4AM (Invitrogen, Eugene, OR, USA) in Krebs-Ringer (K-R) buffer consisting of (in mM) 125 NaCl, 5 KCl, 1 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 5 NaHCO3, and 25 HEPES (pH 7.4) for 30 min at 37°C. Then, cells were washed with K-R buffer and incubated for 30 min at room temperature for deesterification of the Fluo-4AM dye. Fluorescence was recorded with a FluoMax stirring cell spectroflurometer (Horiba; Jobin Yvon Inc., Edison, NJ, USA). A 2.0-ml sample (2×106 cell/ml) was thermostatically maintained at 37°C in a quartz cuvette, Fluo-4 was excited with a 485-nm laser, and emission was detected at 517 nm. After obtaining the basal signal (F0), fluorescence intensity was acquired at 3-s intervals for 5–10 min with continuous stirring of the cell suspension. The relative fluorescence intensity F/F0 was used as an indicator of [Ca2+]i, where F represents fluorescence intensity at a given time, and F0 represents initial fluorescence. In some experiments, fluorescence intensity was converted to [Ca2+]i by using the calcium calibration buffer kit 2 (Invitrogen, Carlsbad, CA, USA) and the formula [Ca2+]i = Kd[(F − Fmin)/(Fmax − F)], where Kd = 410 nM. Fmin was the minimal fluorescent intensity following removal of free Ca2+ deletion by ionomycin (10 μM) and EGTA (2 mM). Fmax was obtained from cells incubated with excess Ca2+ (10 mM CaCl2).
For indirect measurement of the ER calcium pool, Fluo-4AM-loaded cells were bathed in K-R buffer in the absence of extracellular free Ca2+ by chelating with 2 mM EGTA. After the basal [Ca2+]i was established, ER Ca2+ was mobilized by the addition of thapsigargin (TG; 2 μM). The measured Fluo-4AM fluorescence ratio between basal and peak fluorescence after TG stimulation was an index of modifiable [Ca2+]ER.
Mitochondrial-to-cytosolic Ca2+ ratio
Cells were loaded with the low-affinity Ca2+ dye Rhod-2AM (4 μM) and Fluo-4AM (2.5 μM) for 30 min at 37°C (19). Specificity of mitochondria staining with Rhod-2AM was confirmed by colocalization with the mitochondria-specific probe MitoTracker Green FM (Invitrogen, Eugene, OR, USA)and by producing >95% loss of rhod-2 fluorescence within 5 min after dissipation of the mitochondrial membrane potential (ΔΨm) by the addition of 1 μM carbonyl cyanide p-trifluoromethoxy phenylhydrazone (FCCP). Mitochondrial and cytosolic Ca2+ levels were monitored by dual emission fluorometry at 485- and 550-nm excitation and 517- and 580-nm emission wavelengths.
ΔΨm
ΔΨm was monitored with rhodamine 123 (R123), as described previously (20). Uptake of R123 into mitochondria directly reflects the membrane potential; increased R123 fluorescence reflects a lowering of ΔΨm. After treatment, cells were loaded with 10 μM R123 and incubated at 37°C for 30 min in the dark. Cells were then washed twice with PBS, and changes in R123 fluorescence were monitored using a fluorescence plate reader at 485-nm excitation and 525-nm emission.
Caspase-3 protease activity
Cleavage of the substrate Ac-DEVD-pNA was used to determine caspase-3 protease activity, as previously described (13). After treatment, cells were harvested in PBS and centrifuged at 500 g for 5 min. The cell pellet was then incubated on ice with lysis buffer for 10 min and then centrifuged at 13,000 rpm for 1 min at 4°C. The supernatant was harvested, and 80 μg of total protein was incubated with Ac-DEVD-pNA at 37°C. Generation of the chromophore P-nitroanilide (pNA) was determined spectrophotometically at 405 nm with a microtiter plate reader.
Statistics
Data are expressed as means ± se. One-way ANOVA followed by the Tukey-Kramer procedure for multiple comparisons was used to determine statistical differences between treatments. Differences were considered significant at P < 0.05.
RESULTS
Wild-type BNIP3 and constructs localize to ER and mitochondria
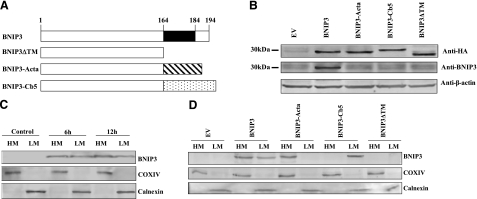
The BNIP3 TM domain was substituted with the targeting sequences of Acta or Cb5 to enable delivery of BNIP3 to specific organelles (Fig. 1A). Expression of each construct was determined at 24 h after transfection by Western blot analysis (Fig. 1B). Ectopic expression of BNIP3 and the constructs were at similar levels, as determined by an antibody specific for N-terminal HA tag expressed by each construct. In addition, a goat anti-rat BNIP3 antibody recognizing the C terminus of BNIP3 was used to determine wild-type BNIP3 expression. In transfected cells, endogenous BNIP3 was expressed at low levels, with no significant difference from empty vector (EV)-transfected cells. It was concluded that Mes 23.5 cells express low levels of constitutive BNIP3 that were not altered by transfection with mutant cDNA.
Figure 1.
BNIP3 expression in Mes 23.5 cells. A) Schematic representation of BNIP3 wild-type and constructs. C-terminal TM domain of BNIP3 was deleted (ΔTM) or replaced with the mitochondrial-targeting sequence of Acta or the ER-targeting sequence of Cb5. B) Cells were transiently transfected with wild-type BNIP3 or constructs for 24 h, and whole-cell lysates were subjected to Western blot analysis. Expression of proteins was examined by an antibody specific for N-terminal HA tag of each construct. A goat anti-rat BNIP3 antibody was used to determine wild-type BNIP3 levels. C) Cells were transfected with BNIP3 for 6 h or 12 h, followed by subcellular fractionation. BNIP3 expression was determined by Western blot analysis of the mitochondrial (heavy membrane, HM) and endoplasmic reticulum (light membrane, LM) fractions. Quality of the fractionation was confirmed by assessing distribution of COX IV in mitochondria and calnexin in ER. D) Cells were transfected with BNIP3 wild-type and constructs for 6 h, and subcellular fractionation and Western blot analysis were conducted.
Subcellular fractionation was performed to confirm intracellular localization of BNIP3 and the constructs. In cells transfected with wild-type BNIP3, the protein was observed in both mitochondria (HM) and ER (LM) within 6 h post-transfection, and expression continued to increased over 12 h (Fig. 1C). In contrast, BNIP3-Acta was detected only in mitochondria, and BNIP3-Cb5 was expressed exclusively in ER (Fig. 1D). On the other hand, the BNIP3ΔTM protein was not detected in either mitochondria or ER, indicating that the TM domain was essential for organelle binding.
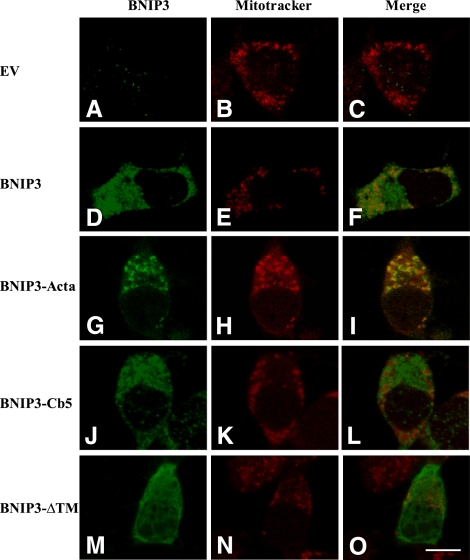
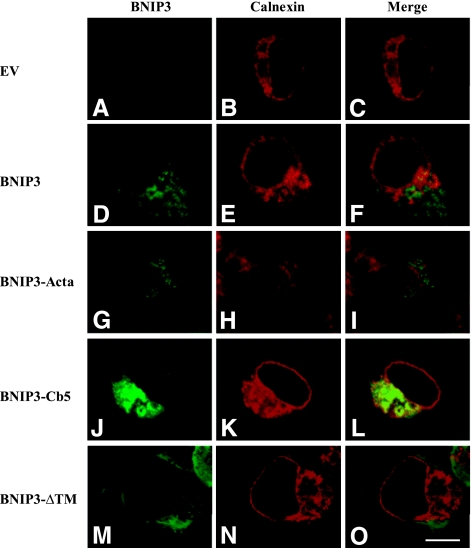
Subcellular localization of BNIP3 proteins was further examined by immunofluorescence. As shown in Figs. 2 and 3, cells were stained with a mouse monoclonal antibody that recognizes aa 112–124 of the BNIP3 molecule (immunofluorescence green indicates presence of BNIP3). For mitochondria and ER imaging, cells were costained with mitochondria-specific fluorescent dye, MitoTracker Red (21), and antibodies to calnexin, an ER marker protein (22). Consistent with the Western blots, in cells expressing wild-type BNIP3, the imaging pattern showed subcellular distribution to mitochondria (Fig. 2D–F, yellow fluorescence) and in perinuclear membranes resembling ER (Fig. 3D–F, yellow fluorescence). Therefore, it appears that expressed wild-type BNIP3 proteins are located in both mitochondria and ER. On the other hand, BNIP3-Cb5 did not colocalize with MitoTracker Red (Fig. 2J–L) but was observed in ER (Fig. 3J–L). Immunofluorescence micrographs of cells expressing BNIP3-Acta show that the protein localized to mitochondria (Figs. 2G–I and 3G–I). In BNIP3ΔTM-transfected cells, the protein distribution displayed a different pattern in which BNIP3ΔTM was widely distributed throughout cell cytoplasm (Figs. 2M–O and 3M–O). It was concluded that BNIP3 constructs localized to the targeted subcellular compartments.
Figure 2.
Mitochondrial distribution of BNIP3 and constructs. Cells were transfected with EV (A–C), wild-type BNIP3 (D–F), BNIP3-Acta (G–I), BNIP3-Cb5 (J–L) and BNIP3-ΔTM (M–O) for 6 h, and then expression was determined by immunostaining. Mitochondria were labeled with the fluorescent dye MitoTracker Red. Fluorescence microscopy shows localization of mitochondria (red; B, E, H, K, N) and BNIP3 and constructs (green; A, D, G, J, M). Images were merged to visualize colocalization of BNIP3 and mitochondria (C, F, I, L, O). Scale bar = 10 μm.
Figure 3.
ER distribution of BNIP3 and targeted constructs. Cells were transfected with EV (A–C), wild-type BNIP3 (D–F), BNIP3-Acta (G–I), BNIP3-Cb5 (J–L) and BNIP3-ΔTM (M–O) for 6 h, and then BNIP3 expression was determined by immunostaining. ER was specifically labeled with rabbit anti-calnexin antibodies. Confocal microscopy shows localization of calnexin (red; B, E, H, K, N) and BNIP3 and constructs (green; A, D, G, J, M). Images were merged to visualize colocalization of BNIP3 in ER (C, F, I, L, O). Scale bar = 10 μm.
BNIP3 and mutants induced cell death via different mechanisms
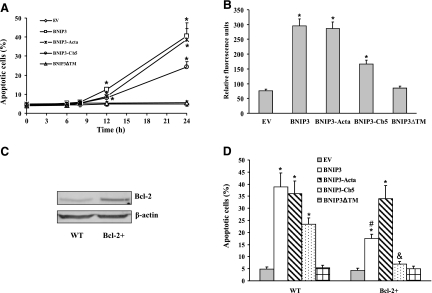
The prodeath action of the constructs was accessed by TUNEL assay and Hoechst 33258 staining. Cells displayed apoptosis within 12 h after transfection with wild-type BNIP3, BNIP3-Acta, or BNIP3-Cb5, as determined by the presence of chromatin condensation (Fig. 4A). The level of cell death progressively increased over the next 12 h, although BNIP3-Cb5 showed a lower level of apoptosis. BNIP3ΔTM did not exert a prodeath effect, consistent with previous reports (23). Apoptotic cell death was further confirmed by measuring Annexin V-FITC fluorescence intensity (Fig. 4B). It appeared that the TM domain of BNIP3 was responsible for membrane targeting and prodeath action, and the BNIP3-Acta and BNIP3-Cb5 molecules were appropriately localized by the recombinant targeting domains.
Figure 4.
BNIP3-induced apoptotic cell death. Cells were transfected with EV control, wild-type BNIP3, BNIP3-Acta, BNIP3-Cb5, or BNIP3ΔTM. A) At varying times post-transfection, apoptosis was determined by Hoechest 33258 staining. B) Twenty-four hours post-transfection, cells were incubated with Annexin V, and fluorescence intensity was recorded. C) Bcl-2 expression was determined by Western blot analysis in wild-type (control) Mes 23.5 or Bcl-2+-expressing cells. D) Mes 23.5 or Bcl-2+ cells were transfected for 24 h, and then apoptosis was determined. Data represent means ± se of 6 separate determinations. *P < 0.05 vs. EV group. #P < 0.05 vs. BNIP3 group. &P < 0.05 vs. BNIP3-Cb5 group.
It has been established that Bcl-2 localized to ER can regulate ER Ca2+ homeostasis (24). To assess the effect of Bcl-2 on BNIP3 function, Mes 23.5 cells were stably transfected with Bcl-2 cDNA, and the subsequent response to BNIP3 overexpression was characterized. The Bcl-2+ cells expressed high levels of Bcl-2 (Fig. 4C). In cell-death studies, it was observed that Bcl-2 overexpression prevented the apoptosis induced by transfection with BNIP3 or BNIP3-Cb5, whereas BNIP3-Acta-induced apoptosis was not affected (Fig. 4D). Thus, it appeared that different mechanisms are involved in BNIP3- and BNIP3-Acta-induced cell death.
BNIP3 and BNIP3-Cb5 deplete ER calcium
To determine whether the BNIP3-induced alteration of intracellular Ca2+ homeostasis was involved in cell death, cytosolic free Ca2+ levels were assessed in cells transfected with EV, Bcl-2, BNIP3, or BNIP3 mutants. Under resting conditions, cytosolic free Ca2+ concentration was ∼125 nM, with no difference between control (no transfection) cells and cells at 3, 6, and 12 h post-transfection (data not shown); thus, the dysregulation of cytosolic Ca2+ did not play a significant role in cell death produced by wild-type BNIP3 or other BNIP3 proteins.
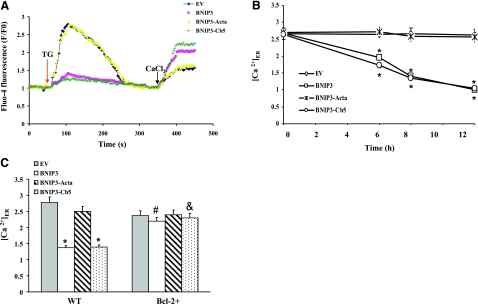
The level of Ca2+ stored in ER was determined by measuring TG-induced release of Ca2+. The TG-releasable Ca2+ pool was reduced within 6 h of transfection with wild-type BNIP3 and BNIP3-Cb5, whereas transfection with BNIP3-Acta did not affect the magnitude of the TG-releasable Ca2+ pool (Fig. 5A, B). To confirm that reduction of the [Ca2+]i spike was due to depletion of the ER Ca2+, cells were exposed to excess extracellular Ca2+. Wild-type BNIP3- or BNIP3-Cb5-expressing cells displayed a higher capacitative Ca2+ entry. Because depletion of intracellular Ca2+ stores stimulates activation of capacitative Ca2+ entry through store-operated Ca2+ channels (25), it is concluded that BNIP3- and BNIP3-Cb5-overexpressing cells had a lower [Ca2+]ER, and thus a higher store-operated Ca2+ entry. Interestingly, depletion of ER Ca2+ in wild-type BNIP3- or BNIP3-Cb5-expressing cells was not blocked by pretreatment with Z-VAD-fmk, a pan-caspase inhibitor (data not shown), indicating that caspase activation was not involved in mobilization of ER Ca2+. The level of TG-releasable [Ca2+]ER was also examined in Bcl-2+ cells stably expressing Bcl-2 (Fig. 5C). Expression of wild-type BNIP3 or BNIP3-Cb5 failed to deplete ER Ca2+. Thus, expression of Bcl-2 blocked BNIP3- and BNIP3-Cb5-induced ER Ca2+ depletion and subsequent induction of apoptosis.
Figure 5.
BNIP3-mediated depletion of the ER Ca2+. A) Representative fluorometric tracing shows effect of BNIP3 constructs on the ER Ca2+ pool. After transfection (8 h), cells were loaded with Fluo-4AM, and then ER Ca2+ release was measured in a Ca2+-free K-R buffer. Arrow indicates addition of TG. After reaching baseline, CaCl2 (10 mM; arrow) was added to the extracellular medium, and cytosolic Ca2+ was determined. B) Level of TG-releasable Ca2+ at different times after transfection. C) Mes 23.5 or Bcl-2+ cells were transfected with BNIP3 constructs for 8 h, and then TG-releasable ER Ca2+ was measured. Data represent means ± se of 3–6 separate determinations. *P < 0.05 vs. EV group. #P < 0.05 vs. BNIP3 group. &P < 0.05 vs. BNIP3-Cb5 group.
BNIP3 and BINP3-Cd5 induce mitochondrial Ca2+ overload and dysfunction
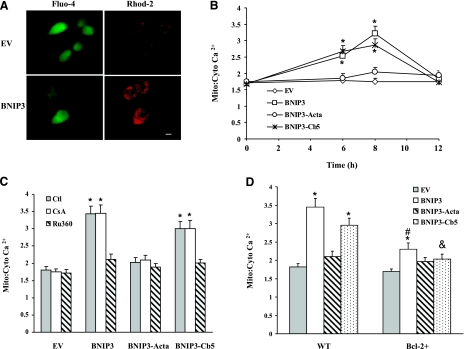
Because Ca2+ uptake by mitochondria can occur following Ca2+ efflux from ER, mitochondrial Ca2+ levels were determined by the calcium-sensitive dye, Rhod-2AM. Specific mitochondrial staining of Rhod-2AM was confirmed by colocalization of Rhod-2 with the mitochondrion-specific probe MitoTracker Green and by loss of >95% of Rhod-2 fluorescence within 5 min after addition of 1 μM FCCP (data not shown). Wild-type BNIP3 or BNIP3-Cb5 overexpression transiently increased the mitochondrial-to-cytosolic Ca2+ ratio in 6 to 8 h compared to EV cells (Fig. 6A, B). These results corresponded to depletion of ER Ca2+. In contrast, mitochondrial Ca2+ levels in the BNIP3-Acta group were not significantly different from EV cells. Cells were also pretreated with a mitochondrial uniporter inhibitor, Ru360, before transfection (Fig. 6C). Ru360 prevented the BNIP3- or BNIP3-Cb5-induced increase in mitochondrial Ca2+. It was concluded that wild-type BNIP3 or BNIP3-Cb5 stimulated mitochondrial Ca2+ uptake, resulting in Ca2+ accumulation. To determine the role of MPT in mitochondrial Ca2+ uptake, cells were pretreated with CsA, an inhibitor of MPT pore opening. CsA did not change mitochondrial Ca2+ levels in either transfection group, indicating that MPT was not involved (Fig. 6D). In contrast, overexpression of Bcl-2 significantly suppressed the BNIP3- or BNIP3-Cb5-induced mitochondrial Ca2+ overload. These results indicated that Bcl-2 protected cells from BNIP3-induced cell damage by reducing mitochondrial Ca2+ accumulation.
Figure 6.
BNIP3-induced mitochondrial Ca2+ overload. A) Representative images of BNIP3-expressing cells. Cells were loaded with Fluo-4AM and Rhod-2AM for cytosolic (green) and mitochondrial Ca2+ (red), respectively, and images were captured by fluorescence microscopy. Scale bar = 10 μm. B) Cells were transfected with various BNIP3 constructs for 0–12 h, and then the ratio of mitochondrial-to-cytosolic Ca2+ levels was determined. C) Cells were transfected with BNIP3 constructs in the presence or absence of CsA (1 μM) or Ru360 (10 μM) for 8 h; then the ratio of mitochondrial-to-cytosolic Ca2+ was examined. D) Control or Bcl-2+ cells were transfected for 8 h, and the ratio of mitochondrial-to-cytosolic Ca2+ was determined. Data represent means ± se of 3–6 separate determinations. *P < 0.05 vs. control (no treatment) group. #P < 0.05 vs. BNIP3 group. &P < 0.05 vs. BNIP3-Cb5 group.
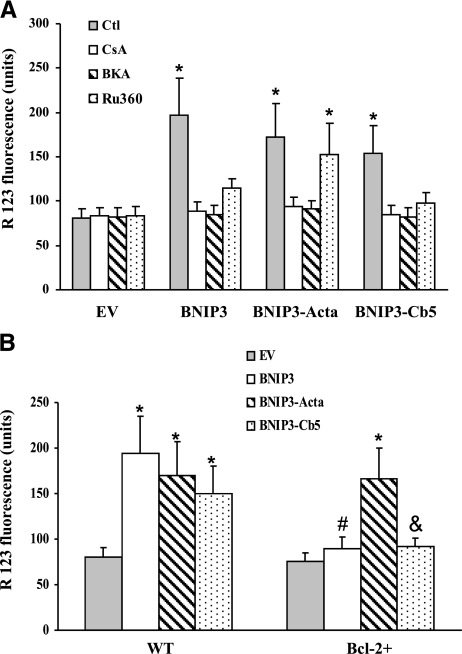
The effect of the BNIP3 constructs on mitochondrial function was determined by monitoring ΔΨm (Fig. 7A). Compared to EV-expressing cells, BNIP3, BNIP3-Acta, or BNIP3-Cb5 overexpression reduced ΔΨm. Because collapse of ΔΨm is associated with MPT pore opening, CsA was used to preserve ΔΨm, and bongkrekic acid (BKA) was used to block the pore by binding to ANT. Both compounds prevented ΔΨm collapse, showing involvement of MPT in the mitochondrial dysfunction produced by the BNIP3 constructs. On the other hand, pretreatment with Ru360 reduced the lowering of ΔΨm in wild-type BNIP3- and BNIP3-Cb5 expressing cells, consistent with triggering MPT pore opening by overloading mitochondria with Ca2+ (26). In Bcl-2+ cells, BNIP3 or BNIP3-Cb5 failed to dissipate the ΔΨm (Fig. 7B). Thus, Bcl-2 protected cells from ER-targeted BNIP3.
Figure 7.
BNIP3-mediated mitochondrial dysfunction. A) Cells were pretreated with CsA (1 μM), BKA (20 μM), or Ru360 (10 μM) for 1 h before transfection with BNIP3 constructs for 12 h, and then the relative ΔΨm was monitored. B) ΔΨm of Mes 23.5 or Bcl-2+ cells at 12 h after transfection with constructs. Data represent means ± se of 3–6 separate determinations. *P < 0.05 vs. EV group. #P < 0.05 vs. BNIP3 group. &P < 0.05 vs. BNIP3-Cb5 group.
BNIP3 induces mitochondria-mediated cell death
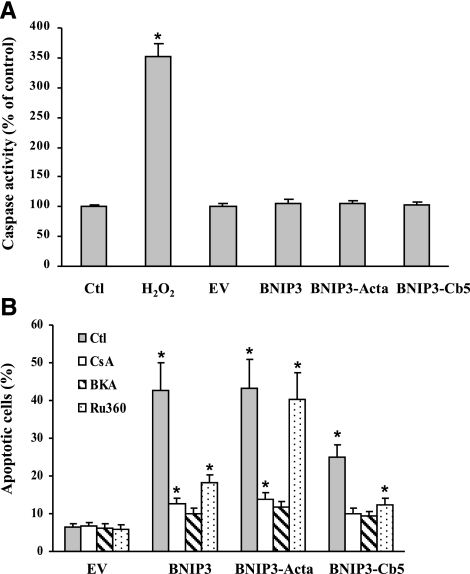
The mode of cell death was further characterized in cells expressing wild-type BNIP3 and the mutants by measuring caspase activity 24 h after transfection. As shown in Fig. 8A, H2O2 (positive control) induced a 3.5-fold increase in caspase substrate cleavage; however, the lysates from cells transfected with the BNIP3 constructs showed no difference compared to untreated control cells. Thus, cell death produced under these conditions is a caspase-independent apoptosis.
Figure 8.
BNIP3-induced caspase-independent apoptosis. A) Cells were transfected with EV control or BNIP3 constructs for 24 h; then caspase-3 activity was determined. H2O2 (100 μM) treatment was used as positive control. B) Cells were pretreated with CsA (1 μM), BKA (20 μM), or Ru360 (10 μM) for 1 h before transfection with the BNIP3 constructs for 24 h, and then apoptosis was determined. Data represent means ± se of 3–6 separate determinations. *P < 0.05 vs. control (no treatment) group.
It is well established that mitochondria play a critical role in BNIP3-induced cell death (27). CsA protected cells in the BNIP3, BNIP3-Acta, and BNIP3-Cb5 groups from apoptosis (Fig. 8B), indicating mitochondrial dysfunction was involved in the cell damage. On the other hand, Ru360 protected only wild-type BNIP3 and BNIP3-Cb5 cells, but not BNIP3-Acta-expressing cells, showing that different mechanisms mediated cell death when BNIP3’s action was restricted to either mitochondria or ER.
DISCUSSION
BNIP3 initiated different modes of cell death depending on the subcellular site of localization. Wild-type BNIP3 localized to both ER and mitochondria, and both sites participated in initiation of cell death. By selectively expressing BNIP3 in ER or mitochondria, different pathways to cell death were activated. Targeting BNIP3 to ER released Ca2+, followed by increased Ca2+ influx into mitochondria. Ca2+ accumulation in mitochondria induced ΔΨm dissipation, resulting in execution of a caspase-independent cell-death mechanism. Bcl-2 blocked ER Ca2+ release and the subsequent mitochondrial Ca2+ overload and cell death. On the other hand, mitochondria-targeted BNIP3 initiated Ca2+-independent apoptosis that was not blocked by Bcl-2 overexpression, indicating that different mechanisms are involved in cell death induced by the site-targeted BNIP3 mutants.
BNIP3 normally resides in cytosol or is loosely attached to the mitochondrial outer membrane (23, 28). When apoptosis is activated, BNIP3 undergoes translocation from cytosol to mitochondria, where it is inserted into the outer membrane to initiate mitochondrial dysfunction. In Mes 23.5 cells, wild-type BNIP3 was expressed in both ER and mitochondria. To target BNIP3 to either mitochondrial outer membrane or ER, the TM domain was substituted with Acta or Cb5 insertion sequences (29). As designed, BNIP3-Acta and BNIP3-Cb5 localized to mitochondria and ER, respectively, whereas wild-type BNIP3 was detected at both subcellular sites. Note that targeting of BNIP3 to either mitochondria or ER was as effective as wild-type BNIP3 in inducing cell death. Substitution of the TM domain with site-specific sequences did not diminish the prodeath potential. Thus, the first 163 aa of BNIP3 are sufficient for proapoptotic function, provided they are fused to either a mitochondrial- or ER-targeting sequence. On the other hand, expression of the mutant lacking the TM insertion domain (BNIP3ΔTM) produced a diffuse cellular distribution pattern and a low level of cell death. As noted previously (23, 30), it was apparent that removal of the TM domain abolished the ability of BNIP3 to undergo site-specific localization and reduced cell death.
ER and mitochondria are intracellular Ca2+ storage sites from which Ca2+ can be mobilized to activate numerous signaling pathways, including control of cell fate (31). It was observed that rapid changes in intracellular Ca2+ homeostasis occurred following BNIP3 overexpression. At 6 h post-transfection, apoptotic cells were not detected, and it was concluded that the Ca2+ dyshomeostasis was not secondary to apoptotic alterations. At 6–12 h post-transfection, wild-type BNIP3- and BNIP3-Cb5-expressing cells had reduced TG-releasable ER Ca2+ and increased mitochondrial Ca2+ loading. The proximity of mitochondria and ER facilitates local Ca2+ communication between the organelles. Ca2+ redistribution can have important consequences for cell fate, since overloading of mitochondria with Ca2+ can initiate apoptosis (22, 33). Consistent with this relationship, Ru360, a specific mitochondrial Ca2+ uptake inhibitor, blocked the increase of mitochondrial Ca2+ produced by overexpressing BNIP3-Cb5, indicating that mitochondrial Ca2+ overload was directly linked to a BNIP3-mediated action in ER. Mitochondrial Ca2+ was reduced within 12 h post-transfection, most likely due to progression into the final stages of apoptosis, as shown by dissipation of ΔΨm and chromatin condensation.
It has previously been shown that BNIP3 localizes in the ER (1), but this study shows for the first time that localization of BNIP3 in the ER mobilizes Ca2+. This action may involve an interaction of BNIP3 with Bcl-2 in the ER. Bcl-2 sustains ER Ca2+ and/or inhibits Ca2+ release from the ER by interacting with the IP3R to attenuate IP3-evoked Ca2+ fluxes (9, 34). It was observed that ER-targeted BNIP3 depleted ER Ca2+, and this was suppressed by overexpressing Bcl-2. It is possible that BNIP3 interacts with Bcl-2 in the ER to induce a conformational change to counter Bcl-2’s actions on ER Ca2+ efflux. Other Bcl-2 proteins may also localize in the ER membrane to alter Ca2+ homeostasis (35). Since BNIP3 initiates a prodeath action through activation of Bax or Bak during ischemia/reperfusion (2), it is possible that BNIP3 interacts with Bax/Bak in the ER membrane to initiate Ca2+ mobilization.
Because BNIP3 induces cell death by promoting the onset of MPT (3), MPT pore inhibitors were tested and found to block dissipation of ΔΨm in Mes 23.5 cells transfected with wild-type BNIP3 and the constructs, confirming involvement of MPT in the apoptosis. The mechanism underlying the MPT is related to increased mitochondrial Ca2+ levels observed in both wild-type BNIP3 and BNIP3-Cb5-expressing cells. Excessive mitochondrial Ca2+ overload is a major trigger for MPT pore opening, followed by generation of excessive mitochondrial ROS and onset of MPT (36). BNIP3-stimulated mitochondrial Ca2+ overload appears to be upstream of MPT, since mitochondrial Ca2+ levels were not influenced by the MPT inhibitor CsA.
As a result of insertion into the outer mitochondrial membrane, BNIP3 appears to activate a Ca2+-independent pathway for MPT. Structural analysis suggests that the TM domain of BNIP3 contains a right-handed parallel helix-helix structure with a hydrogen bond-rich His-Ser node (26). This structure may form a proton-conducting pathway through the membrane (26). Thus, insertion of BNIP3 into the mitochondria outer membrane may lead to proton influx from the cytosol, resulting in MPT pore opening. It is also possible that insertion of BNIP3 into the outer membrane may lead to an interaction with either MPT pore proteins or other Bcl-2 proteins (Bax, Bak), thereby facilitating pore opening. This would explain why BNIP3-Acta-expressing cells exhibit MPT, followed by Ca2+-independent apoptosis. Interestingly, current study shows that gene silencing of Bax prevented the mitochondrial dysfunction and the subsequent cell death in BNIP3-overexpressing cells, further suggesting the requirement of Bax in BNIP3-mediated cell damage.
Apoptosis is regulated by relative expression levels and competing interaction between pro- and antiapoptotic Bcl-2 family members (37). BNIP3 heterodimerizes with Bcl-2 and Bcl-XL, as determined in in vitro and in vivo coimmunoprecipitation experiments and a yeast two-hybrid system (1, 38). To study the interaction between BNIP3 and Bcl-2, a stably expressed Bcl-2+ cell line was employed, since Bcl-2 transient transfection has been reported to produce cell toxicity (39). Deletion mapping has shown that the BH3 domain of BNIP3 is not involved in a heterodimeric interaction with Bcl-2 (1). However, the TM domain is essential for heterodimerization with Bcl-2 following association with membranes. Substitution of the TM domain with membrane-targeting sequences disrupts the interaction of BNIP3 and Bcl-2. Therefore, it appears that reduction of cell death observed in Bcl-2+ cells transfected with BNIP3-Cb5 is not due to heterodimerization of the two proteins, but to a functional interaction in the ER.
It is important to note that Bcl-2 overexpression only prevented MPT induced by ER-targeted BNIP3 but did not block MPT in cells expressing mitochondria-targeted BNIP3. Similarly, Bcl-2 protects against Bax-induced cell death by an interaction in the ER membrane (40). It is concluded that localization of Bcl-2 in mitochondria is not necessary to inhibit BNIP3-mediated apoptosis, since Bcl-2 in the ER antagonized BNIP3-induced apoptosis, but Bcl-2 did not block cell death when BNIP3 was localized to the mitochondrial membrane.
In summary, selective targeting of BNIP3 can produce cell death through different signaling processes, depending on the subcellular site of localization. Wild-type BNIP3 produces a mixed mode of cell death due to localization in both ER and mitochondria. In addition, it is shown for the first time that BNIP3 can modulate Ca2+ mobilization between ER and mitochondria to initiate a proapoptotic action. BNIP3-induced Ca2+ mobilization is reversed by overexpression of Bcl-2 suggests that BNIP3 interacts with Bcl-2 proteins in the ER to modulate Ca2+ release.
Acknowledgments
This work was supported by a grant from the U.S. National Institutes of Health (ES04140). The authors thank Andrew D. Monnot (School of Health Science, Purdue University) for technical assistance in conducting the confocal microscopy and image analysis.
References
- Ray R, Chen G, Vande Velde C, Cizeau J, Park J H, Reed J C, Gietz R D, Greenberg A H. BNIP3 heterodimerizes with Bcl-2/Bcl-X(L) and induces cell death independent of a Bcl-2 homology 3 (BH3) domain at both mitochondrial and nonmitochondrial sites. J Biol Chem. 2000;275:1439–1448. doi: 10.1074/jbc.275.2.1439. [DOI] [PubMed] [Google Scholar]
- Kubli D A, Ycaza J E, Gustafsson A B. Bnip3 mediates mitochondrial dysfunction and cell death through Bax and Bak. Biochem J. 2007;405:407–415. doi: 10.1042/BJ20070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Velde C, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, Hakem R, Greenberg A H. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol. 2000;20:5454–5468. doi: 10.1128/mcb.20.15.5454-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R, Emi M, Tanabe K. Caspase-dependent and -independent cell death pathways after DNA damage. Oncol Rep. 2005;14:595–599. [PubMed] [Google Scholar]
- Juhaszova M, Wang S, Zorov D B, Nuss H B, Gleichmann M, Mattson M P, Sollott S J. The identity and regulation of the mitochondrial permeability transition pore: where the known meets the unknown. Ann N Y Acad Sci. 2008;1123:197–212. doi: 10.1196/annals.1420.023. [DOI] [PubMed] [Google Scholar]
- Mathai J P, Germain M, Shore G C. BH3-only BIK regulates BAX, BAK-dependent release of Ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J Biol Chem. 2005;280:23829–23836. doi: 10.1074/jbc.M500800200. [DOI] [PubMed] [Google Scholar]
- Nutt L K, Chandra J, Pataer A, Fang B, Roth J A, Swisher S G, O'Neil R G, McConkey D J. Bax-mediated Ca2+ mobilization promotes cytochrome c release during apoptosis. J Biol Chem. 2002;277:20301–20308. doi: 10.1074/jbc.M201604200. [DOI] [PubMed] [Google Scholar]
- White C, Li C, Yang J, Petrenko N B, Madesh M, Thompson C B, Foskett J K. The endoplasmic reticulum gateway to apoptosis by BclL modulation of the InsP3R. Nat Cell Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamvoki M, Roizman B. Bcl-2 blocks accretion or depletion of stored calcium but has no effect on the redistribution of IP3 receptor I mediated by glycoprotein E of herpes simplex virus 1. J Virol. 2007;81:6316–6325. doi: 10.1128/JVI.00311-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y P, Aromolaran A S, Bultynck G, Zhong F, Li X, McColl K, Matsuyama S, Herlitze S, Roderick H L, Bootman M D, Mignery G A, Parys J B, De Smedt H, Distelhorst C W. Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2’s inhibition of apoptotic calcium signals. Mol Cell. 2008;31:255–265. doi: 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le W D, Colom L V, Xie W J, Smith R G, Alexianu M, Appel S H. Cell death induced by beta-amyloid 1–40 in MES 23.5 hybrid clone: the role of nitric oxide and NMDA-gated channel activation leading to apoptosis. Brain Res. 1995;686:49–60. doi: 10.1016/0006-8993(95)00450-5. [DOI] [PubMed] [Google Scholar]
- Parikh H N, Chiodo L A, Koss J, Crawford G D, Freeman A S. Cholecystokinin mobilizes intracellular Ca2+ in hybrid N18TG2 X mesencephalon (MES-23.5) cells. Synapse. 1995;21:278–280. doi: 10.1002/syn.890210304. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li L, Liu H, Prabhakaran K, Zhang X, Borowitz J L, Isom G E. HIF-1α activation by a redox-sensitive pathway mediates cyanide-induced BNIP3 upregulation and mitochondrial-dependent cell death. Free Radic Biol Med. 2007;43:117–127. doi: 10.1016/j.freeradbiomed.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Chan J Y. Nrf1 is targeted to the endoplasmic reticulum membrane by an N-terminal transmembrane domain. Inhibition of nuclear translocation and transacting function. J Biol Chem. 2006;281:19676–19687. doi: 10.1074/jbc.M602802200. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Aguirre-Chen C, Kietzmann T, Saul I, Busto R, Ginsberg M D. Nuclear localization of the hypoxia-regulated pro-apoptotic protein BNIP3 after global brain ischemia in the rat hippocampus. Brain Res. 2004;1001:133–142. doi: 10.1016/j.brainres.2003.11.065. [DOI] [PubMed] [Google Scholar]
- Tan E Y, Campo L, Han C, Turley H, Pezzella F, Gatter K C, Harris A L, Fox S B. BNIP3 as a progression marker in primary human breast cancer; opposing functions in in situ versus invasive cancer. Clin Cancer Res. 2007;13:467–474. doi: 10.1158/1078-0432.CCR-06-1466. [DOI] [PubMed] [Google Scholar]
- Soucie E L, Annis M G, Sedivy J, Filmus J, Leber B, Andrews D W, Penn L Z. Myc potentiates apoptosis by stimulating Bax activity at the mitochondria. Mol Cell Biol. 2001;21:4725–4736. doi: 10.1128/MCB.21.14.4725-4736.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Cowie A, Wasfy G W, Penn L Z, Leber B, Andrews D W. Bcl-2 mutants with restricted subcellular location reveal spatially distinct pathways for apoptosis in different cell types. EMBO J. 1996;15:4130–4141. [PMC free article] [PubMed] [Google Scholar]
- Kolar S S, Barhoumi R, Lupton J R, Chapkin R S. Docosahexaenoic acid and butyrate synergistically induce colonocyte apoptosis by enhancing mitochondrial Ca2+ accumulation. Cancer Res. 2007;67:5561–5568. doi: 10.1158/0008-5472.CAN-06-4716. [DOI] [PubMed] [Google Scholar]
- Prabhakaran K, Li L, Mills E M, Borowitz J L, Isom G E. Up-regulation of uncoupling protein-2 by cyanide is linked with cytotoxicity in mesencephalic cells. J Pharmacol Exp Ther. 2005;314:1338–1345. doi: 10.1124/jpet.105.088625. [DOI] [PubMed] [Google Scholar]
- Gustavsson N, Abedi G, Larsson-Nyren G, Lindstrom P. Timing of Ca2+ response in pancreatic beta-cells is related to mitochondrial mass. Biochem Biophys Res Commun. 2006;340:1119–1124. doi: 10.1016/j.bbrc.2005.12.119. [DOI] [PubMed] [Google Scholar]
- Orlandi F, Venanzi F M, Concetti A, Yamauchi H, Tiwari S, Norton L, Wolchok J D, Houghton A N, Gregor P D. Antibody and CD8+ T cell responses against HER2/neu required for tumor eradication after DNA immunization with a Flt-3 ligand fusion vaccine. Clin Cancer Res. 2007;13:6195–6203. doi: 10.1158/1078-0432.CCR-07-0258. [DOI] [PubMed] [Google Scholar]
- Prabhakaran K, Li L, Zhang L, Borowitz J L, Isom G E. Upregulation of BNIP3 and translocation to mitochondria mediates cyanide-induced apoptosis in cortical cells. Neuroscience. 2007;150:159–167. doi: 10.1016/j.neuroscience.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Rizzuto R. (2006) Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ. 2006;13:1409–1418. doi: 10.1038/sj.cdd.4401960. [DOI] [PubMed] [Google Scholar]
- Putney J W., Jr Capacitative calcium entry: sensing the calcium stores. J Cell Biol. 2005;169:381–382. doi: 10.1083/jcb.200503161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocharov E V, Pustovalova Y E, Pavlov K V, Volynsky P E, Goncharuk M V, Ermolyuk Y S, Karpunin D V, Schulga A A, Kirpichnikov M P, Efremov R G, Maslennikov I V, Arseniev A S. Unique dimeric structure of BNip3 transmembrane domain suggests membrane permeabilization as a cell death trigger. J Biol Chem. 2007;282:16256–16266. doi: 10.1074/jbc.M701745200. [DOI] [PubMed] [Google Scholar]
- Webster K A, Graham R M, Bishopric N H. BNip3 and signal-specific programmed death in the heart. J Mol Cell Cardiol. 2005;38:35–45. doi: 10.1016/j.yjmcc.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Frazier D P, Wilson A, Graham R M, Thompson J W, Bishopric N H, Webster K A. Acidosis regulates the stability, hydrophobicity, and activity of the BH3-only protein Bnip3. Antioxid Redox Signal. 2006;8:1625–1634. doi: 10.1089/ars.2006.8.1625. [DOI] [PubMed] [Google Scholar]
- Handrick R, Rudner J, Muller I, Eibl H, Belka C, Jendrossek V. Bcl-2-mediated inhibition of erucylphosphocholine-induced apoptosis depends on its subcellular localisation. Biochem Pharmacol. 2005;70:837–850. doi: 10.1016/j.bcp.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Kanzawa T, Zhang L, Xiao L, Germano I M, Kondo Y, Kondo S. Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3. Oncogene. 2005;24:980–991. doi: 10.1038/sj.onc.1208095. [DOI] [PubMed] [Google Scholar]
- Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol. 2007;17:511–517. doi: 10.1016/j.tcb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su T P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay F S, Fogarty K E, Lifshitz L M, Tuft R A, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Chen R, Valencia I, Zhong F, McColl K S, Roderick H L, Bootman M D, Berridge M J, Conway S J, Holmes A B, Mignery G A, Velez P, Distelhorst C W. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol. 2004;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong W X, Li C, Hatzivassiliou G, Lindsten T, Yu Q C, Yuan J, Thompson C B. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Kadono T, Hoshiai M, Eguchi Y, Nakazawa S, Nakazawa H, Higashijima N, Ishida H. Na+/H+ exchanger inhibitor cariporide attenuates the mitochondrial Ca2+ overload and PTP opening. Am J Physiol Heart Circ Physiol. 2007;293:H3517–H3523. doi: 10.1152/ajpheart.00483.2006. [DOI] [PubMed] [Google Scholar]
- Degterev A, Yuan J. Expansion and evolution of cell death programs. Nat Rev Mol Cell Biol. 2008;9:378–390. doi: 10.1038/nrm2393. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Theodorakis P, Subramanian T, Chinnadurai G. Adenovirus E1B–19K/BCL-2 interacting protein BNIP3 contains a BH3 domain and a mitochondrial targeting sequence. J Biol Chem. 1998;273:12415–12421. doi: 10.1074/jbc.273.20.12415. [DOI] [PubMed] [Google Scholar]
- Hanson C J, Bootman M D, Distelhorst C W, Maraldi T, Roderick H L. The cellular concentration of Bcl-2 determines its pro- or anti-apoptotic effect. Cell Calcium. 2008;44:243–258. doi: 10.1016/j.ceca.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Wang N S, Unkila M T, Reineks E Z, Distelhorst C W. Transient expression of wild-type or mitochondrially targeted Bcl-2 induces apoptosis, whereas transient expression of endoplasmic reticulum-targeted Bcl-2 is protective against Bax-induced cell death. J Biol Chem. 2001;276:44117–44128. doi: 10.1074/jbc.M101958200. [DOI] [PubMed] [Google Scholar]