Abstract
Heat shock protein 25/27 (Hsp25/27) is a cytoprotective protein that is ubiquitously expressed in most cells, and is up-regulated in response to cellular stress. Previous work, in nonmuscle cells, has shown that Hsp27 inhibits TNF-α-induced NF-κB activation. During skeletal muscle disuse, Hsp25/27 levels are decreased and NF-κB activity increased, and this increase in NF-κB activity is required for disuse muscle atrophy. Therefore, the purpose of the current study was to determine whether electrotransfer of Hsp27 into the soleus muscle of rats, prior to skeletal muscle disuse, is sufficient to inhibit skeletal muscle disuse atrophy and NF-κB activation. The 35% disuse muscle-fiber atrophy observed in nontransfected fibers was attenuated by 50% in fibers transfected with Hsp27. Hsp27 also inhibited the disuse-induced increase in MuRF1 and atrogin-1 transcription by 82 and 40%, respectively. Furthermore, disuse- and IKKβ-induced NF-κB transactivation were abolished by Hsp27. In contrast, Hsp27 had no effect on Foxo transactivation. In conclusion, Hsp27 is a negative regulator of NF-κB in skeletal muscle, in vivo, and is sufficient to inhibit MuRF1 and atrogin-1 and attenuate skeletal muscle disuse atrophy.—Dodd, S. L., Hain, B., Senf, S. M., Judge, A. R. Hsp27 inhibits IKKβ-induced NF-κB activity and skeletal muscle atrophy.
Keywords: MuRF1, atrogin-1/MAFbx, immobilization
Heat shock proteins (Hsps) are a family of proteins constitutively expressed in cells whose expression is further induced by a variety of stimuli, including heat stress, oxidative stress, and hypoxia (1, 2). Given the diverse roles of Hsps, which includes chaperoning, protein folding and transport, assisting in the removal of damaged proteins, and regulating cell signaling pathways (3,4,5), this induction is important in providing protection against tissue and cellular stress.
In skeletal muscle, two members of the Hsp family, Hsp25/27 (murine Hsp25 is the homologue of human Hsp27) and Hsp70, are significantly up-regulated during increased physical activity (6,7,8) and decreased during reduced activity, or disuse (9,10,11). Hsp25 and Hsp27 share >80% homology at the amino acid level (12) and are functionally similar (13). Because of this homology, several investigators refer to murine Hsp25 as Hsp27. However, from here on, we will refer to the protein as Hsp25/27 unless the specific isoform is known.
Given the down-regulation of both Hsp25/27 and Hsp70 during skeletal muscle disuse, and their role in providing cellular protection, we and others previously used whole-body hyperthermia prior to and during disuse to increase the expression of these Hsps in rat skeletal muscle (14, 15). Although hyperthermia increased the expression of Hsp25 and Hsp70 and significantly attenuated disuse muscle atrophy, whole-body hyperthermia may differentially regulate the expression of a wide variety of proteins. Therefore, these studies fail to provide direct evidence for Hsp25 or Hsp70 in the attenuation of disuse muscle atrophy.
In order to determine directly whether Hsp70 is sufficient to attenuate muscle atrophy, we recently injected and electrotransferred an Hsp70 expression plasmid into the soleus muscle of rats prior to disuse (16). When we used this technique, Hsp70 was exclusively up-regulated, and it prevented the muscle-fiber atrophy induced by 7 d of disuse. Furthermore, the increased transcriptional activities of nuclear factor of κB (NF-κB) and forkhead box O (Foxo) during disuse were abolished by Hsp70 overexpression. The inhibition of these transcriptional pathways may explain the prevention of muscle-fiber atrophy by Hsp70, since NF-κB and Foxo are independently sufficient to cause skeletal muscle atrophy (17,18,19), and NF-κB is required for disuse muscle atrophy (17, 20, 21).
Although we have identified a specific role of Hsp70 in the regulation of skeletal muscle mass during disuse, a similar understanding for Hsp25/27 is currently lacking. Therefore, in the current study, we injected and electrotransferred an Hsp27 expression plasmid into the soleus muscle of rats prior to hind-limb immobilization. Our results show that Hsp27 significantly attenuated skeletal muscle disuse atrophy. Furthermore, Hsp27 abolished the activation of NF-κB and MuRF1 transcription, induced by both disuse and IKKβ overexpression. These findings demonstrate that an increase in Hsp27 expression is sufficient to attenuate skeletal muscle disuse atrophy and suggest that this is mediated, in part, through the inhibition of NF-κB transactivation, and the inhibition of MuRF1 transcription.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (200 g), purchased from Charles River Laboratories (Wilmington, MA, USA), were used for all animal experiments. All animal procedures were approved by the University of Florida Institutional Animal Care and Use Committee.
Expression and reporter plasmids
The pCMV5-Hsp27 plasmid was obtained from Dr. Bianca Brundel (University of Groningen, Groningen, The Netherlands). To create the Hsp27-EGFP plasmid, we used PCR to amplify the Hsp27 insert out of pCMV5 and subcloned it, in frame, into the HindIII and SalI sites on the COOH terminus of an EGFP plasmid (EGFP-c1; Clontech, Palo Alto, CA, USA). Verification that Hsp27 was in frame was confirmed by DNA sequencing (DNA Sequencing Core, University of Florida, Gainesville, FL, USA). The pcDNA3-WT IKKβ-FLAG expression plasmid was obtained from Denis Guttridge (Ohio State University, Columbus, OH, USA). The NF-κB-GL3 reporter plasmid was obtained from Dr. Steffan Ho and the Foxo (DAF-16)-GL3 reporter from Dr. Alex Toker (Beth Israel Deaconess Medical Center, Boston, MA, USA); both have been previously used and described (20, 22, 23). The MuRF1-GL3 reporter plasmid, which has been previously described (17), was a kind gift of Dr. Steve Shoelson (Joslin Diabetes Center and Harvard Medical School, Boston, MA, USA). The plasmid DNA was prepared and isolated using an Endotoxin-Free Maxi or Mega Prep Kit (Qiagen, Valencia, CA, USA).
Plasmid injection and electroporation
Plasmid injection and sequential transfection of skeletal muscle have been detailed previously (16, 24). The plasmid amounts injected were 10 μg of the expression plasmids (Hsp27, Hsp27-EGFP, WT IKKβ) and/or 40 μg of the reporter plasmids (NF-κB-GL3, Foxo-GL3, or MuRF1-GL3) in a total volume of 50 μl 1XPBS. When skeletal muscle is injected with a mixture containing two vectors, cotransduction of a given fiber occurs 75–95% of the time (25, 26). Thus, a fiber that takes up one vector will most likely also take up the other.
Immobilization
Four days following plasmid injection, animals were cast-immobilized bilaterally with the ankle joint in the plantar-flexed position to induce maximal atrophy of the soleus muscle, as described previously (14, 16, 27). The soleus muscle was selected for study since this postural muscle shows the greatest degree of atrophy during periods of disuse (28).
Muscle preparation and analysis
Muscles of immobilized and weight-bearing animals were removed following either 3 d of immobilization (7 d after plasmid injection) or following 7 d of immobilization (11 d following plasmid injection). Following removal, muscles were either rapidly frozen in liquid nitrogen and stored at −80°C for subsequent biochemical analyses, processed immediately for RNA isolation, or placed on a tongue depressor at a consistent muscle length in tissue-freezing medium and frozen in dry ice-cooled isopentane for fiber sectioning and subsequent immunohistochemical analysis.
NF-κB, Foxo, and MuRF1 reporter activity
Muscles were homogenized in a passive lysis buffer (Promega, Madison, WI, USA), and centrifuged for 20 min at 5000 g. Twenty microliters of the supernatant was added to 100 μl of luciferase reagent (Promega) for determination of total muscle luciferase activity, using an LMax II microplate luminometer (Molecular Devices Corp., Sunnyvale, CA, USA).
Immunohistochemistry
Cross sections (10 μm) were cut with a cryostat microtome (Microm HM 550; Microm International, Walldorf, Germany) from the midbelly of the soleus muscle and fixed in 4% paraformaldehyde. The muscle sections were incubated with wheat germ agglutinin Texas Red-X conjugate (Invitrogen, Carlsbad, CA, USA) for visualization of muscle fibers under fluorescence microscopy, and images were captured with an Olympus IX50 camera (Olympus, Tokyo, Japan). The muscle-fiber area of ∼200 fibers from each muscle was traced and measured using Image Pro Discovery software (Media Cybernetics, Bethesda, MD, USA).
Western blot analysis
Whole-cell lysates used for measurement of reporter activity were subsequently used for Western blot analysis. Protein concentrations were determined using a detergent-compatible assay (Bio-Rad, Hercules, CA, USA). Samples were diluted in loading buffer (Bio-Rad) containing 5% β-mercaptoethanol, heat denatured, and equal amounts of protein were loaded onto linear gradient gels and separated using SDS-PAGE. Proteins were transferred onto an Immobilon-FL polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA), blocked for 1 h, and incubated overnight with primary antibody diluted in blocking buffer. The following primary antibodies were used: anti-Hsp27 (SPA-800; Stressgen, Ann Arbor, MI, USA); anti-Hsp25 (SPA-801; Stressgen); anti-GFP (sc-8334; Santa Cruz Biotechnology, Santa Cruz, CA, USA); and anti-IκBα (sc-371; Santa Cruz Biotechnology). Following a series of washes, the membranes were incubated with Alexa Fluor 680 or IRDye800 (LI-COR Biosciences, Lincoln, NE, USA) fluorescent-dye-conjugated secondary antibodies and visualized using the Odyssey infrared imaging system (LI-COR Biosciences). Relative quantification of proteins was determined by measuring the fluorescence of each lane at the appropriate molecular weight.
Coimmunoprecipitation
Immunoprecipitation of Hsp27 was performed using a Catch and Release kit (Upstate, Charlottesville, VA, USA) according to the manufacturer’s instructions. Briefly, 500 μg of total protein and 2 μg of an anti-Hsp27 antibody (SPA-800; Stressgen, Ann Arbor, MI, USA) or anti-normal mouse IgG antibody (12–371; Millipore, Bedford, MA, USA), as a negative control, were used for immunoprecipitation. Hsp27-bound proteins were eluted, heat denatured, separated by electrophoresis, and blotted with an anti-FLAG antibody (F3165; Sigma-Aldrich, St. Louis, MO, USA) or anti-IκBα (sc-371; Santa Cruz Biotechnology), as described above.
RNA isolation, cDNA synthesis, and RT-PCR
Following 3 d of immobilization or weight-bearing activity, the soleus muscles were removed, and total RNA was isolated using the TRIzol-based method previously described (29). The quality of the RNA was subsequently improved by using RNeasy columns (Qiagen). Total RNA (1 μg) and oligo dT primers were used to synthesize cDNA using the RETROscript First Strand Synthesis Kit (Ambion, Austin, TX, USA), according to the manufacturers instructions. cDNA (5 μl) was then used as a template for real-time qRT-PCR using primer sets (Hspb1, GenBank accession no. NM_031970; atrogin-1, GenBank NM_133521; MuRF1, GenBank NM_080903; and 18S, GenBank X03205.1). TaqMan probe-based chemistry was used to allow detection of PCR products, and quantitation of gene expression was performed using the relative standard curve method.
Statistical analysis
Hsp25 protein expression was analyzed using a 2-tailed Student’s t test; WT IKKβ-induced NF-κB and MuRF1 promoter reporter activity were analyzed using a 1-way ANOVA followed by Bonferroni corrections for multiple comparisons when appropriate; all other data were analyzed using a 2-way ANOVA (GraphPad Software, San Diego, CA, USA). All data are expressed as means ± se; significance was established at the P < 0.05 level.
RESULTS
Hsp25 expression
In agreement with previous findings from our lab and others (9, 10, 30), Hsp25 mRNA and protein expression were significantly decreased during skeletal muscle disuse. Hsp25 mRNA was decreased by 40% following 3 d of immobilization (Fig. 1A), and Hsp25 protein levels were decreased by 25 and 20% following 3 and 7 d of immobilization, respectively (Fig. 1B).
Figure 1.
Hind-limb immobilization decreases Hsp25 mRNA and protein levels. A) Hsp25 mRNA expression from weight-bearing and immobilized solei after 3 d. B) Hsp25 protein expression from weight-bearing and immobilized solei after 3 and 7 d. Bars represent means ± se; 6 muscles/group. *P < 0.05 vs. weight-bearing control. C) Representative Western blots of whole-cell lysates from solei injected with control plasmid (lanes 1 and 3), Hsp27 expression plasmid (lane 2), or Hsp27-EGFP expression plasmid (lane 4). Control-injected samples show endogenous Hsp25 (25 kDa) only; Hsp27- and Hsp27-EGFP-injected muscles show both endogenous Hsp25 and overexpressed Hsp27 (27 kDa) and Hsp27-EGFP (54 kDa), respectively.
To overexpress Hsp27 in the soleus muscle of rats, we injected and electrotransferred an empty vector (pCMV5) or Hsp27 expression plasmid (pCMV5-Hsp27 or Hsp27-EGFP) (Fig. 1C).
Hsp27 attenuates disuse muscle-fiber atrophy
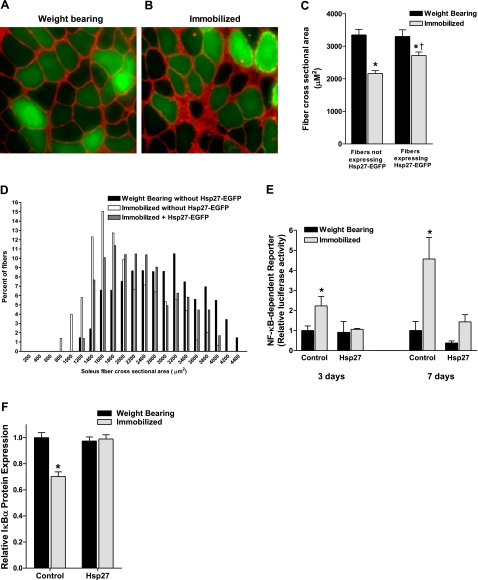
To determine whether overexpression of Hsp27 could attenuate skeletal muscle disuse atrophy, we injected and electrotransferred the Hsp27-EGFP plasmid into the soleus muscles of rats selected to be immobilized for 7 d or to be weight-bearing controls. The 32% decrease in soleus muscle weight/body weight ratio following 7 d of immobilization was attenuated by 29% in muscles expressing Hsp27 (data not shown). Although this attenuation did not reach statistical significance, these findings at the whole-muscle level are diluted given that the transfection efficiency in our hands is ∼60–70%. Therefore, measurement of muscle-fiber cross-sectional area is necessary to more accurately determine the degree of atrophy attenuation. Given the 60–70% transfection efficiency, visualization of soleus cross sections using fluorescent microscopy reveals both green (Hsp27-EGFP transfected) and black (nontransfected) fibers (Fig. 2A, B). Hind-limb immobilization caused a 35% decrease in the mean cross-sectional area of nontransfected fibers (weight bearing= 3348±168 μm2; immobilized=2163±86 μm2), which was attenuated by 50% in transfected fibers, i.e., those fibers overexpressing Hsp27 (weight bearing=3302±201 μm2; immobilized=2714±109 μm2) (Fig. 2C). We have previously verified that EGFP has no effect on muscle-fiber cross-sectional area (20). The fiber cross-sectional area frequency distribution curve (Fig. 2D) shows a left shift following immobilization in fibers not overexpressing Hsp27, illustrating an increase in the percentage of smaller fibers. However, this shift is attenuated following immobilization in fibers expressing Hsp27. The range of fiber sizes expressing Hsp27 demonstrates the nonspecificity of fibers sizes that are transfected by the Hsp27 plasmid. These findings show, for the first time, that an increase in Hsp27 expression is sufficient to attenuate skeletal muscle disuse atrophy.
Figure 2.
Hsp27 overexpression attenuates disuse muscle atrophy and disuse-induced NF-κB activity. A, B) Representative cross-sections of Hsp27-EGFP-injected soleus muscle from weight-bearing (A) and immobilized rats after 7 d (B). Mean cross-sectional area of fibers expressing Hsp27-EGFP (green fluorescing fibers) was compared to the mean cross-sectional area of fibers not expressing Hsp27-EGFP (nonfluorescing fibers) within the same muscle. C) Muscle-fiber cross-sectional area of ∼250 fibers/muscle from 6 muscles/group. *P < 0.05 vs. weight-bearing fibers not expressing Hsp27-EGFP. †P < 0.05 vs. immobilized fibers not expressing Hsp27-EGFP. D) Frequency distribution of fiber cross-sectional area from weight-bearing muscles, immobilized muscles, and immobilized muscles expressing Hsp27. E) NF-κB transactivation is increased 2- and 4-fold following 3 and 7 d of immobilization in control-injected muscles, respectively, but is completely abolished in muscles overexpressing Hsp27 at both time points. Bars represent means ± se; 8 muscles/group. *P < 0.05 vs. weight-bearing control. F) IκBα protein expression is significantly decreased following 7 d of immobilization, and overexpression of Hsp27 prevents this decrease. Bars represent means ± se; 8 muscles/group. *P < 0.05 vs. weight-bearing control.
Hsp27 inhibits disuse-induced NF-κB activation
Because an increase in NF-κB activity is required for disuse muscle atrophy (17, 20, 21), and Hsp27 inhibits NF-κB activity in HeLa cells (31) and keratinocytes (32), we determined whether an increase in Hsp27 expression was sufficient to inhibit disuse-induced NF-κB activation in skeletal muscle. The ∼2-fold and ∼4.5-fold increases in NF-κB activity following 3 and 7 d of immobilization, respectively, in control-injected muscles were abolished in muscles overexpressing Hsp27 (Fig. 2E). To ensure that the Hsp27-EGFP fusion protein used in the atrophy experiment functioned in a similar manner to the Hsp27 expressed from the pCMV5-Hsp27, we injected either pCMV5, EGFP, pCMV5-Hsp27, or Hsp27-EGFP into the soleus muscle of rats and immobilized all hind limbs for 7 d. NF-κB activation was inhibited by the same magnitude in the Hsp27-EGFP-injected muscles as the pCMV5-Hsp27 muscles (data not shown). This confirms that the Hsp27-EGFP fusion protein functions the same as Hsp27.
Given that IκBα is the endogenous inhibitor of NF-κB and its degradation is required for NF-κB activation and muscle-fiber atrophy during disuse (17, 20), we determined the effect of Hsp27 overexpression on IκBα protein expression during immobilization. IκBα protein levels were decreased by 30% following 7 d of hind-limb immobilization in control-injected muscles. However, this decrease was completely prevented in muscles overexpressing Hsp27, suggesting that Hsp27 inhibits NF-κB activation by preventing IκBα degradation (Fig. 2F). Because degradation of IκBα is initiated by its phosphorylation, we also measured the phosphorylation status of IκBα after both 3 and 7 d of immobilization; however, we were unable to detect any differences with immobilization or with Hsp27 overexpression (data not shown). This lack of a detectable difference in phospho-IκBα levels following sustained disuse has been reported previously (20, 22) and may be due to the short half-life of serine phosphorylated IκBα, which has been shown in Jurkat cells to be 25 min (33).
Hsp27 inhibits IKKβ-induced NF-κB activation
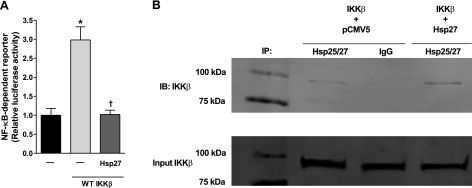
Because IκBα levels are regulated by IKKβ and Hsp27 inhibits NF-κB activation in HeLa cells through its interaction with IKKβ (31), we next sought to determine whether Hsp27 was capable of inhibiting IKKβ-induced NF-κB activity in skeletal muscle. Injection and electrotransfer of WT IKKβ caused a ∼3-fold increase in NF-κB activity, which was abolished in muscles coinjected with WT IKKβ plus Hsp27 (Fig. 3A). These findings show that Hsp27 is sufficient to inhibit IKKβ-induced NF-κB activity in skeletal muscle, in vivo.
Figure 3.
Hsp27 associates with IKKβ and inhibits IKKβ-induced NF-κB activity. A) WT IKKβ causes a 3-fold increase in NF-κB activation, which is abolished in muscles coinjected with WT IKKβ and Hsp27. Bars represent means ± se; 6 muscles/group. *P < 0.05 vs. control-injected muscles. †P < 0.05 vs. WT IKKβ-injected muscles. B) Immunoprecipitation of Hsp25/27, using an anti-Hsp25/27 antibody, from muscles injected with IKKβ or IKKβ plus Hsp27. Immunoprecipitated samples were separated on a denaturing gel and blotted with anti-FLAG antibody for IKKβ. Endogenous Hsp25 (lane 2) and ectopic Hsp27 (lane 4) both bound in complex with IKKβ-FLAG. A normal IgG antibody was used as negative control for immunoprecipitation. Corresponding samples used for coimmunoprecipitation experiments were subjected to SDS-PAGE and immunoblotted with an anti-FLAG antibody to show that the level of overexpressed IKKβ is similar in each sample.
We subsequently used coimmunoprecipitation experiments to determine whether Hsp25/27 binds to IKKβ and found that both endogenous Hsp25 and ectopic Hsp27 associated with IKKβ (Fig. 3B). If this association affects the function of IKKβ, it could explain the inhibition of IKKβ-induced NF-κB activation by Hsp27, since IKKβ is the kinase responsible for phosphorylating IκBα, leading to IκBα degradation and NF-κB activation. The immunoprecipitated samples were also immunoblotted with an anti-IκBα antibody, but no association was found (data not shown).
Hsp27 inhibits MuRF1 transcription
The regulation of muscle mass by NF-κB is believed to be through the regulation of atrophy genes, particularly MuRF1, since its mRNA expression is increased by IKKβ-induced NF-κB activation (17). Because Hsp27 inhibits both IKKβ- and disuse-induced NF-κB activation, we sought to determine whether Hsp27 expression can inhibit disuse-induced MuRF1 transcription. To do this, we coinjected a MuRF1 promoter reporter plasmid with either a control plasmid or Hsp27 expression plasmid into the soleus muscles of rats that were subsequently assigned to weight-bearing activity or 3 d of hind-limb immobilization. Immobilization caused a ∼2-fold increase in MuRF1 promoter activity in control-injected muscles, which was abolished in muscles injected with the Hsp27 plasmid (Fig. 4A). To substantiate this finding and determine whether the inhibition of MuRF1 transcription by Hsp27 could be due to its inhibition of NF-κB, we subsequently measured MuRF1 mRNA expression in solei injected with a control, Hsp27, or IκBα super repressor (IκBα SR) plasmid and assigned to weight-bearing activity or 3 d of hind-limb immobilization. The ∼3-fold increase in MuRF1 mRNA expression in control-injected immobilized muscles was completely abolished in immobilized muscles overexpressing Hsp27 but unaffected in immobilized muscles injected with the IκBα SR (Fig. 4B). 18S rRNA was unchanged across treatments and groups (data not shown). These combined findings demonstrate that Hsp27 overexpression is sufficient to inhibit MuRF1 transcription during physiological muscle wasting but that this inhibition is not mediated through Hsp27’s inhibition of NF-κB.
Figure 4.
Hsp27 inhibits IKKβ-induced MuRF1 promoter activation and disuse-induced MuRF1 and atrogin-1 mRNA expression. A) MuRF1 promoter reporter activity is increased following 3 d of immobilization, and overexpression of Hsp27 completely inhibits this activation. *P < 0.05 vs. weight-bearing control. B) Hsp27, but not IκBα SR, abolishes the increase in MuRF1 mRNA expression following 3 d of immobilization. *P < 0.05 vs. weight-bearing control. †P < 0.05 vs. immobilized control. C) WT IKKβ increases MuRF1 promoter activity, which is abolished in muscles coinjected with IKKβ and Hsp27. *P < 0.05 vs. control-injected muscles. †P < 0.05 vs. WT IKKβ-injected muscles. D) Hsp27, but not IκBα SR, attenuates the increase in atrogin-1 mRNA expression observed following 3 d of immobilization. *P < 0.05 vs. weight-bearing control. †P < 0.05 vs. immobilized control. Bars represent means ± se; ≥6 muscles/group.
Although we demonstrate that NF-κB is not required for MuRF1 activation during skeletal muscle disuse, IKKβ-induced NF-κB is sufficient to activate MuRF1 transcription (17). Therefore, activation of NF-κB through IKKβ may play a more substantial role in regulating MuRF1 in other forms of muscle wasting, such as those involving systemic inflammation. In fact, we have previously observed that the IκBα SR abolishes the increase in MURF1 mRNA expression following ischemia-reperfusion (I-R) in skeletal muscle (unpublished results). Given that I-R initiates an inflammatory response, this substantiates our speculation that NF-κB may be required for MuRF1 transcription during some conditions, and not others. We therefore sought to determine whether Hsp27 could inhibit IKKβ-induced MuRF1 transcription. To do this, we coinjected a MuRF1 promoter reporter plasmid with a control plasmid, WT IKKβ, or WT IKKβ plus Hsp27 expression plasmid into the soleus muscles of rats. WT IKKβ caused an ∼1.8-fold increase in MuRF1 promoter activity, which was abolished in muscles coinjected with WT IKKβ plus Hsp27 (Fig. 4C).
These findings are in agreement with the report of Cai et al. (17) that IKKβ-induced NF-κB activation is sufficient to increase MuRF1 transcription. Our findings further show that Hsp27 can inhibit IKKβ (NF-κB)-induced MuRF1 transcription. However, since specific inhibition of NF-κB does not prevent MuRF1 transcription during skeletal muscle disuse, other pathways must also regulate MuRF1 transcription during physiological muscle wasting, which may be regulated by Hsp27.
We next determined whether overexpression of Hsp27 is sufficient to inhibit another important atrophy gene required for muscle wasting, atrogin-1. Three days of hind-limb immobilization caused an ∼2.7-fold increase in atrogin-1 mRNA expression in control-injected muscles, which was significantly attenuated in immobilized muscles injected with the Hsp27 plasmid. Although it is largely believed that NF-κB does not regulate atrogin-1 transcription during muscle wasting, we next sought to confirm that Hsp27 inhibits atrogin-1 transcription, independent of its inhibition of NF-κB. Indeed, specific inhibition of NF-κB, through expression of IκBα SR, during 3 d of immobilization did not inhibit disuse-induced atrogin-1 mRNA expression (Fig. 4D). Again, 18S rRNA was unchanged across treatments and groups (data not shown).
Hsp27 does not inhibit Foxo activation during disuse
Our finding that Hsp27 attenuates atrogin-1 and MuRF1 expression during disuse, independently of NF-κB, suggests that Hsp27 may also inhibit Foxo. This suggestion is based on the significant up-regulation of Foxo during skeletal muscle disuse (16) and its ability to regulate atrogin-1 and MuRF1 transcription (16, 19, 34). We therefore determined whether Hsp27 overexpression also inhibits the increase in Foxo transactivation during disuse. The ∼2.5-fold increase in Foxo transactivation following 3 d of immobilization in control-injected muscles was unchanged by Hsp27 overexpression. These findings demonstrate that, unlike Hsp70 (16), Hsp27 overexpression does not inhibit Foxo activation during skeletal muscle disuse (Fig. 5).
Figure 5.
Foxo transcriptional activity from the soleus muscles of rats injected with a Foxo (DAF-16) reporter plasmid plus either a control or Hsp27 expression plasmid and immobilized for 7 d. Bars represent means ± se; 6 muscles/group. *P < 0.05 vs. weight-bearing control.
DISCUSSION
Hsp25/Hsp27 is decreased in skeletal muscle during various models of disuse (9, 10, 35), including cast immobilization (9), hind-limb suspension (10), sciatic nerve denervation (36), and spinal cord transection (35), as well as other models of muscle atrophy (37), and this decrease is speculated to contribute to the atrophy process. However, despite the speculation, there is no direct evidence to confirm this. In the current study, we specifically overexpressed Hsp27 in the soleus muscle of rats prior to 7 d of hind-limb immobilization and found a 50% attenuation of disuse muscle-fiber atrophy in those fibers overexpressing Hsp27. This demonstrates that an increase in Hsp27 is sufficient to attenuate disuse muscle-fiber atrophy. These findings, in conjunction with our recent study showing that specific overexpression of Hsp70 abolished disuse muscle atrophy (16), clearly identify a key role for specific Hsps in the regulation of skeletal muscle mass.
The potential mechanisms to explain how Hsp25/27 might regulate skeletal muscle mass are as broad as its cellular functions, which include stabilizing the cytoskeleton during periods of stress (38), acting as a molecular chaperone (3), and inhibiting apoptosis and oxidative stress (39, 40). Although we cannot rule out these possibilities, we show here that overexpression of Hsp27 completely abolishes NF-κB transactivation. Since specific inhibition of NF-κB activity during disuse consistently attenuates skeletal muscle disuse fiber atrophy (17, 20, 21), the attenuation of muscle-fiber atrophy by Hsp27 is likely due, at least in part, to its inhibition of NF-κB activation.
Although, to our knowledge, this is the first study to identify Hsp27 as a negative regulator of NF-κB in skeletal muscle, it is in agreement with data collected in HeLa cells (31) and keratinocytes (32). However, in U937 human leukemic cells, MEF cells, and rat colon carcinoma REG cells, Hsp27 appears to enhance NF-κB activation in response to either etoposide or TNF-α treatment (41), suggesting the outcome of NF-κB regulation by Hsp27 may vary with cell types or stimuli. Our finding that Hsp27 associates with IKKβ and prevents the typical decrease in IκBα levels during disuse (20, 22) is also in agreement with others (31, 32). IKKβ is the upstream kinase responsible for phosphorylating IκBα at serines 32 and 36, which subsequently leads to its degradation. This degradation of IκBα is required for disuse-induced NF-κB activation (17, 20). Therefore the prevention of IκBα degradation by Hsp27, shown here, could be due to the association between Hsp27 and IKKβ. In this scenario, the interaction between Hsp27 and IKKβ could prevent IKKβ from phosphorylating IκBα, which would decrease IκBα degradation and explain the inhibition of NF-κB activity.
Although NF-κB activation is required for disuse muscle atrophy (17, 20, 21), the atrophy genes that require NF-κB activation during disuse remain to be determined. The candidate gene that has received the most attention as an NF-κB target gene is MuRF1. This is largely based on the findings of Cai et al. (17), who demonstrated that transgenic mice overexpressing a muscle-specific constitutively active IKKβ show a 15-fold increase in NF-κB activity and a 3.3-fold increase in MuRF1 mRNA expression. Furthermore, treatment of C2C12 myotubes with either TNF-α or IL-1β, both of which are potent activators of NF-κB, increased the activation of a MuRF1 promoter reporter, and transfection of the IκBα SR prior to these treatments abolished this increase. These findings demonstrate that an increase in NF-κB activity is sufficient to increase MuRF1 and that NF-κB is required for TNF-α- and IL-1β-induced MuRF1 promoter activation in myotubes. In agreement with this, we show here that overexpression of WT IKKβ, in vivo, increases NF-κB activity ∼3-fold, and MuRF1 promoter activation ∼80%. These findings, in combination with those of Cai et al. (17), also suggest that the magnitude of MuRF1 activation is dependent on the magnitude of NF-κB activation. We further show that the increase in both variables is abolished by Hsp27.
Although an increase in NF-κB activity is sufficient to increase MuRF1, we recently showed that expression of the IκBα SR during disuse abolishes the increase in NF-κB activity, but not the increase in MuRF1 promoter activity. In agreement with this, we show here that expression of the IκBα SR does not prevent the immobilization-induced increase in MuRF1 mRNA expression. The fact that NF-κB is sufficient to increase MuRF1 transcription and required for IKKβ-induced MuRF1 transcription (17), but that NF-κB is not required for MuRF1 transcription during disuse, is likely due to the complexities of MuRF1 transcription (34). Likewise, atrogin-1 is known to be regulated by Foxo factors, yet we show here that Hsp27 attenuates the disuse-induced increase in atrogin-1 mRNA expression, independently of Foxo. These findings suggest that Hsp27 may regulate the transcription of MuRF1 and atrogin-1 independently of NF-κB and Foxo. The possibility that additional transcription factors regulate MuRF1 and atrogin-1 is substantiated by analysis of the 5′-flanking region of these genes, using transcription element search software (TESS). This analysis reveals a number of potential Foxo and NF-κB binding sites, as previously reported (19, 34), but also potential binding sites of numerous other transcription factors. Alternatively, Hsp27 may regulate Foxo cofactors that are necessary for coactivation by Foxo of the single Foxo binding sites dispersed throughout the promoter region of MuRF1 and atrogin-1, but not necessary for Foxo activation of the Foxo (DAF-16) reporter, which contains 6 copies of the DAF-16 family protein-binding element (DBE). In this instance, inhibition of the necessary Foxo coactivators by Hsp27 could have a similar inhibitory effect on the mRNA expression of MuRF1 and atrogin-1, as occurs through the direct inhibition of Foxo. Clearly, much more work is needed to understand the complete transcriptional regulation of these important genes during physiological muscle wasting.
It is not currently known whether the inhibition of NF-κB transactivation by Hsp27 overexpression seen in the current study is redundant to the inhibition of NF-κB transactivation by Hsp70 overexpression previously reported (16). Certainly, there seems to be a general involvement of Hsps in NF-κB signaling. Hsp90 regulates IKK activation in lymphoma cells (42); 293 cells, HeLa cells, B cells, and T cells (43); and rat alveolar epithelial type II (ATII) cells (44); and NF-κB DNA binding is enhanced in astrocytes null for αB crystallin (45). However, in addition to regulating NF-κB signaling, Hsp70 overexpression is sufficient to inhibit Foxo transactivation, whereas overexpression of Hsp27 had no effect on Foxo activity. Therefore, even if Hsp25/27 and Hsp70 have redundant roles in the regulation of NF-κB, they clearly have distinct differences in the regulation of other atrophy-related pathways. Therefore, subsequent work is clearly needed to determine whether cooverexpression of Hsp27 and Hsp70 provides greater atrophy protection than overexpression of each Hsp independently.
Since a down-regulation of Hsp25/27 is associated with other atrophy-inducing conditions, such as denervation (36), spinal cord transaction (35), and unweighting (10), overexpression of Hsp27 could potentially attenuate muscle atrophy during these conditions. Furthermore, given the finding here that Hsp27 is a negative regulator of NF-κB, overexpression of Hsp27 could potentially attenuate muscle-fiber atrophy during any atrophy condition that is associated with an increase in NF-κB activity. However, additional work is needed to address these possibilities.
In summary, the data presented here further our understanding of the role that specific Hsps play in the regulation of muscle mass. Specifically, we show that Hsp27 is sufficient to abolish NF-κB activation, inhibit the increase in MuRF1 and atrogin-1, and attenuate skeletal muscle disuse atrophy. Given the pleiotropic functions of Hsp27, we are unable to conclude that Hsp27 attenuates disuse muscle atrophy specifically through NF-κB, MuRF1, or atrogin-1. Certainly, we acknowledge that continued work is needed to determine the involvement of other potential pathways and proteins. However, given the known requirements of NF-κB (17, 20, 21), MuRF1, and atrogin-1 (46) in disuse muscle atrophy, it seems likely that the attenuation of muscle atrophy by Hsp27 is mediated, at least in part, through these mechanisms.
Acknowledgments
This work was supported by U.S. National Institutes of Health grant R03AR056418 (to A.R.J.).
References
- Lindquist S, Craig E A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Welch W J. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Parsell D A, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gampert L, Nething K, Steinacker J M. Response and function of skeletal muscle heat shock protein 70. Front Biosci. 2006;11:2802–2827. doi: 10.2741/2011. [DOI] [PubMed] [Google Scholar]
- Murlasits Z, Cutlip R G, Geronilla K B, Rao K M, Wonderlin W F, Alway S E. Resistance training increases heat shock protein levels in skeletal muscle of young and old rats. Exp Gerontol. 2006;41:398–406. doi: 10.1016/j.exger.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Locke M, Noble E G, Atkinson B G. Exercising mammals synthesize stress proteins. Am J Physiol Cell Physiol. 1990;258:C723–C729. doi: 10.1152/ajpcell.1990.258.4.C723. [DOI] [PubMed] [Google Scholar]
- Salo D C, Donovan C M, Davies K J. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic Biol Med. 1991;11:239–246. doi: 10.1016/0891-5849(91)90119-n. [DOI] [PubMed] [Google Scholar]
- Selsby J T, Rother S, Tsuda S, Pracash O, Quindry J, Dodd S L. Intermittent hyperthermia enhances skeletal muscle regrowth and attenuates oxidative damage following reloading. J Appl Physiol. 2007;102:1702–1707. doi: 10.1152/japplphysiol.00722.2006. [DOI] [PubMed] [Google Scholar]
- Lawler J M, Song W, Kwak H B. Differential response of heat shock proteins to hindlimb unloading and reloading in the soleus. Muscle Nerve. 2006;33:200–207. doi: 10.1002/mus.20454. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden E E, Houle J D, Dennis R A, Zhang J, Knox M, Wagoner G, Peterson C A. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle Nerve. 2004;29:73–81. doi: 10.1002/mus.10511. [DOI] [PubMed] [Google Scholar]
- Gaestel M, Gross B, Benndorf R, Strauss M, Schunk W H, Kraft R, Otto A, Bohm H, Stahl J, Drabsch H, Bielka H. Molecular cloning, sequencing and expression in Escherichia coli of the 25-kDa growth-related protein of Ehrlich ascites tumor and its homology to mammalian stress proteins. FEBS J. 1989;179:209–213. doi: 10.1111/j.1432-1033.1989.tb14542.x. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan C D, Dunsmore K, Wong H, Roy S, Sen C K, Wani A, Zweier J L, Ilangovan G. HSP27 regulates p53 transcriptional activity in doxorubicin-treated fibroblasts and cardiac H9c2 cells: p21 upregulation and G2/M phase cell cycle arrest. Am J Physiol Heart Circ Physiol. 2008;294:H1736–H1744. doi: 10.1152/ajpheart.91507.2007. [DOI] [PubMed] [Google Scholar]
- Selsby J T, Dodd S L. Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am J Physiol Regul Integr Comp Physiol. 2005;289:R134–R139. doi: 10.1152/ajpregu.00497.2004. [DOI] [PubMed] [Google Scholar]
- Naito H, Powers S K, Demirel H A, Sugiura T, Dodd S L, Aoki J. Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J Appl Physiol. 2000;88:359–363. doi: 10.1152/jappl.2000.88.1.359. [DOI] [PubMed] [Google Scholar]
- Senf S M, Dodd S L, McClung J M, Judge A R. Hsp70 overexpression inhibits NF-κB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J. 2008;22:3836–3845. doi: 10.1096/fj.08-110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Frantz J D, Tawa N E, Jr, Melendez P A, Oh B C, Lidov H G, Hasselgren P O, Frontera W R, Lee J, Glass D J, Shoelson S E. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Mizukami J, Miura S, Suzuki M, Takahashi N, Kawada T, Taniguchi T, Ezaki O. A forkhead transcription factor FKHR up-regulates lipoprotein lipase expression in skeletal muscle. FEBS Lett. 2003;536:232–236. doi: 10.1016/s0014-5793(03)00062-0. [DOI] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker S H, Goldberg A L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge A R, Koncarevic A, Hunter R B, Liou H C, Jackman R W, Kandarian S C. Role for IκBα, but not c-Rel, in skeletal muscle atrophy. Am J Physiol Cell Physiol. 2007;292:C372–C382. doi: 10.1152/ajpcell.00293.2006. [DOI] [PubMed] [Google Scholar]
- Hunter R B, Kandarian S C. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest. 2004;114:1504–1511. doi: 10.1172/JCI21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter R B, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig D A, Kandarian S C. Activation of an alternative NF-κB pathway in skeletal muscle during disuse atrophy. FASEB J. 2002;16:529–538. doi: 10.1096/fj.01-0866com. [DOI] [PubMed] [Google Scholar]
- Storz P, Doppler H, Toker A. Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol Cell Biol. 2005;25:8520–8530. doi: 10.1128/MCB.25.19.8520-8530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell-Felton H, Kandarian S C. Normalization of muscle plasmid uptake by Southern blot: application to SERCA1 promoter analysis. Am J Physiol. 1999;277:C1269–C1276. doi: 10.1152/ajpcell.1999.277.6.C1269. [DOI] [PubMed] [Google Scholar]
- Rana Z A, Ekmark M, Gundersen K. Coexpression after electroporation of plasmid mixtures into muscle in vivo. Acta Physiol Scand. 2004;181:233–238. doi: 10.1111/j.1365-201X.2004.01282.x. [DOI] [PubMed] [Google Scholar]
- Alzghoul M B, Gerrard D, Watkins B A, Hannon K. Ectopic expression of IGF-I and Shh by skeletal muscle inhibits disuse-mediated skeletal muscle atrophy and bone osteopenia in vivo. FASEB J. 2004;18:221–223. doi: 10.1096/fj.03-0293fje. [DOI] [PubMed] [Google Scholar]
- Booth F W, Kelso J R. Production of rat muscle atrophy by cast fixation. J Appl Physiol. 1973;34:404–406. doi: 10.1152/jappl.1973.34.3.404. [DOI] [PubMed] [Google Scholar]
- Vazeille E, Codran A, Claustre A, Averous J, Listrat A, Bechet D, Taillandier D, Dardevet D, Attaix D, Combaret L. The ubiquitin-proteasome and the mitochondria-associated apoptotic pathways are sequentially downregulated during recovery after immobilization-induced muscle atrophy. Am J Physiol. 2008;295:E1181–E1190. doi: 10.1152/ajpendo.90532.2008. [DOI] [PubMed] [Google Scholar]
- Ahtikoski A M, Koskinen S O, Virtanen P, Kovanen V, Risteli J, Takala T E. Synthesis and degradation of type IV collagen in rat skeletal muscle during immobilization in shortened and lengthened positions. Acta Physiol Scand. 2003;177:473–481. doi: 10.1046/j.1365-201X.2003.01061.x. [DOI] [PubMed] [Google Scholar]
- Stevenson E J, Giresi P G, Koncarevic A, Kandarian S C. Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J Physiol. 2003;551:33–48. doi: 10.1113/jphysiol.2003.044701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K J, Gaynor R B, Kwak Y T. Heat shock protein 27 association with the I κB kinase complex regulates tumor necrosis factor α-induced NF-κB activation. J Biol Chem. 2003;278:35272–35278. doi: 10.1074/jbc.M305095200. [DOI] [PubMed] [Google Scholar]
- Sur R, Lyte P A, Southall M D. Hsp27 regulates pro-inflammatory mediator release in keratinocytes by modulating NF-κB signaling. J Invest Dermatol. 2008;128:1116–1122. doi: 10.1038/sj.jid.5701157. [DOI] [PubMed] [Google Scholar]
- Imbert V, Rupec R A, Livolsi A, Pahl H L, Traenckner E B, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle P A, Peyron J F. Tyrosine phosphorylation of IκB-α activates NF-κB without proteolytic degradation of IκB-α. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- Waddell D S, Baehr L M, van den Brandt J, Johnsen S A, Reichardt H M, Furlow J D, Bodine S C. The glucocorticoid receptor and Foxo1 synergistically activate the skeletal muscle atrophy associated Murf1 gene. Am J Physiol Endocrinol Metab. 2008;295:E785–E797. doi: 10.1152/ajpendo.00646.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey K A, Roy R R, Zhong H, Lullo C. Time-dependent changes in caspase-3 activity and heat shock protein 25 after spinal cord transection in adult rats. Exp Physiol. 2008;93:415–425. doi: 10.1113/expphysiol.2007.041228. [DOI] [PubMed] [Google Scholar]
- Inaguma Y, Goto S, Shinohara H, Hasegawa K, Ohshima K, Kato K. Physiological and pathological changes in levels of the two small stress proteins, HSP27 and αB crystallin, in rat hindlimb muscles. J Biochem. 1993;114:378–384. doi: 10.1093/oxfordjournals.jbchem.a124184. [DOI] [PubMed] [Google Scholar]
- Lecker S H, Jagoe R T, Gilbert A, Gomes M, Baracos V, Bailey J, Price S R, Mitch W E, Goldberg A L. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- Mounier N, Arrigo A P. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones. 2002;7:167–176. doi: 10.1379/1466-1268(2002)007<0167:acashs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C, Schmitt E, Cande C, Vahsen N, Parcellier A, Kroemer G. HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell Cycle. 2003;2:579–584. [PubMed] [Google Scholar]
- Arrigo A P. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 1998;379:19–26. [PubMed] [Google Scholar]
- Parcellier A, Schmitt E, Gurbuxani S, Seigneurin-Berny D, Pance A, Chantome A, Plenchette S, Khochbin S, Solary E, Garrido C. HSP27 is a ubiquitin-binding protein involved in I-κBα proteasomal degradation. Mol Cell Biol. 2003;23:5790–5802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broemer M, Krappmann D, Scheidereit C. Requirement of Hsp90 activity for IκB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-κB activation. Oncogene. 2004;23:5378–5386. doi: 10.1038/sj.onc.1207705. [DOI] [PubMed] [Google Scholar]
- Qing G, Yan P, Xiao G. Hsp90 inhibition results in autophagy-mediated proteasome-independent degradation of IκB kinase (IKK) Cell Res. 2006;16:895–901. doi: 10.1038/sj.cr.7310109. [DOI] [PubMed] [Google Scholar]
- Pittet J F, Lee H, Pespeni M, O'Mahony A, Roux J, Welch W J. Stress-induced inhibition of the NF-κB signaling pathway results from the insolubilization of the IκB kinase complex following its dissociation from heat shock protein 90. J Immunol. 2005;174:384–394. doi: 10.4049/jimmunol.174.1.384. [DOI] [PubMed] [Google Scholar]
- Ousman S S, Tomooka B H, van Noort J M, Wawrousek E F, O'Connor K C, Hafler D A, Sobel R A, Robinson W H, Steinman L. Protective and therapeutic role for αB-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- Bodine S C, Latres E, Baumhueter S, Lai V K, Nunez L, Clarke B A, Poueymirou W T, Panaro F J, Na E, Dharmarajan K, Pan Z Q, Valenzuela D M, DeChiara T M, Stitt T N, Yancopoulos G D, Glass D J. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]