Abstract
Vascular endothelial growth factor (VEGF) is a well-established stimulator of vascular permeability and angiogenesis, whereas thrombospondin-1 (TSP-1) is a potent angiogenic inhibitor. In this study, we have found that the TSP-1 receptors CD36 and β1 integrin associate with the VEGF receptor 2 (VEGFR2). The coclustering of receptors that regulate angiogenesis may provide the endothelial cell with a platform for integration of positive and negative signals in the plane of the membrane. Thus, this complex may represent a molecular switch that regulates angiogenesis and determines endothelial cell behavior. In this context, physiological levels of TSP-1 appear to support VEGFR2 function on both the cellular and tissue level, because phosphorylation of VEGFR2 and vascular permeability in response to VEGF are decreased in TSP-1-null mice and isolated endothelial cells. A therapeutic agent based on the antiangiogenic domain of TSP-1, designated 3TSR (for three TSP-1 type 1 repeats), has significant antiangiogenic and antitumor efficacy. Systemic treatment of wild-type mice with 3TSR significantly decreased VEGF-induced permeability. Consistent with this result, VEGF-stimulated phosphorylation of VEGFR2 was also significantly decreased in lung extracts from 3TSR-treated mice. Moreover, 3TSR significantly decreased VEGF-stimulated VEGFR2 phosphorylation in human dermal microvascular endothelial cells in culture. Taken together, the results indicate that TSP-1 and 3TSR modulate the function of VEGFR2.—Zhang, X., Kazerounian, S., Duquette, M., Perruzzi, C., Nagy, J. A., Dvorak, H. J., Parangi, S., and Lawler, J. Thrombospondin-1 modulates vascular endothelial growth factor activity at the receptor level.
Keywords: KDR, angiogenesis, integrin, CD36, VEGFR2
Physiological angiogenesis is regulated by the opposing activities of stimulators and inhibitors, such as vascular endothelial growth factor (VEGF) and thrombospondin-1 (TSP-1), respectively (1). VEGF is a potent stimulator of angiogenesis during vascular development and wound healing and is also a key driver of pathological angiogenesis in the tumor microenvironment (2). TSP-1 has been shown to limit angiogenesis during physiological and pathological processes, such as hair growth and tumor progression (3, 4). Control of angiogenesis by temporal regulation of the expression of VEGF and TSP-1 is exemplified by the hair follicle in mouse skin (5, 6). VEGF and angiogenesis are up-regulated during the anagen phase, whereas the catagen phase is associated with decreased VEGF mRNA, increased TSP-1 production, and vessel regression. Consistent with this pattern of expression, vessel regression is significantly delayed in the hair follicle of TSP-1-null mice (6). TSP-1 specifically antagonizes VEGF function by inhibiting the activation of matrix metalloproteinase 9 (MMP9), which, in turn, inhibits the mobilization of VEGF from the extracellular matrix (7). In addition, TSP-1 mediates the uptake and clearance of VEGF (8). These data suggest that TSP-1 has evolved to have multiple mechanisms to antagonize VEGF activity.
The antiangiogenic activity of TSP-1 has been mapped to the type 1 repeats (TSRs) (4, 9, 10). There are three TSRs in TSP-1, and recombinant proteins that contain all three, designated 3TSR, or just the second TSR are potent inhibitors of angiogenesis in vivo (10,11,12). The antiangiogenic therapeutic agent ABT-510 is based on the sequence of a portion of the second TSR (13, 14). A recent report indicates that the type 3 repeats may also contribute to the inhibition of basic fibroblast growth factor-induced angiogenesis (15).
Inhibition of angiogenesis by the TSRs involves induction of endothelial cell apoptosis and inhibition of endothelial cell migration (9, 16, 17). The TSRs induce apoptosis of human dermal microvascular endothelial cells (HDMECs) through the membrane protein CD36, Fyn, p38 mitogen-activated protein kinase, and caspases (16). The inhibition of migration of HDMECs is mediated by CD36, whereas the inhibition of migration of large vessel endothelial cells, which lack CD36, is mediated by β1 integrins (9, 17). CD36 associates with β1 integrins in platelets, melanoma cells, and endothelial cells (11, 18, 19). This association is dependent on the extracellular portion of CD36 and a cysteine residue (C464) in the C-terminal cytoplasmic tail of CD36 (18, 19). Treatment of human umbilical vein endothelial cells (HUVECs) that have been engineered to express CD36 with TSP-1 results in decreased VEGF-induced phosphorylation of VEGFR2 (18). Mutation of C464 in CD36 abrogates the ability of TSP-1 to inhibit VEGFR2 phosphorylation, suggesting that the formation of the CD36/β1 integrin complex is required.
Inhibition of nitric oxide (NO) by TSP-1 has also been shown to inhibit angiogenesis (20). Treatment of endothelial cells with TSP-1 or 3TSR inhibits angiogenic responses to NO through a CD36- and cGMP-dependent mechanism. In addition, NO signaling is enhanced in TSP-1-null mice (20). This effect has been proposed to be partially due to the ability of TSP-1 to bind to CD36 and inhibit uptake of myristate, which results in decreased endothelial nitric oxide synthase (eNOS) activity (21). In addition, a pathway that also includes CD47, which is reportedly a receptor for the C-terminal domain of TSP-1, and cGMP is involved in suppression of NO signaling by TSP-1 (21, 22).
In this study, we have used several different experimental models to explore the effect of TSP-1 on VEGF activity and signal transduction in endothelial cells. We have identified an association of VEGFR2 with two TSP-1 receptors, CD36 and β1 integrin. VEGF-induced vascular permeability and signal transduction are suppressed in the absence of TSP-1. A similar decrease is also observed after 3TSR treatment. The results indicate that the level of activation of VEGFR2 by VEGF is modulated by the presence of TSP-1. They also reveal a novel mechanism for the inhibition of angiogenesis by TSP-1 and 3TSR. TSP-1 and 3TSR inhibit the activation of proangiogenic signal transduction by VEGF, in addition to activating antiangiogenic pathways through CD36 and β1 integrins. Together with published results, the data indicate that a receptor complex in the plane of the membrane serves as a molecular switch that integrates pro- and antiangiogenic signals and determines endothelial cell behavior.
MATERIALS AND METHODS
Mice
TSP-1-null mice on the FVB background were produced as described previously (23). All animal studies were approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center.
Isolation of human and murine endothelial cells
HDMECs were isolated from human foreskins by the method of Richard et al. (24). The cells were cultured in Vitrogen-coated dishes with EBM-2 medium (Lonza, Allendale, NJ, USA) containing 20% FBS, 1 μg/ml hydrocortisone acetate, 50 μM dibutyryl-cAMP, 200 U/ml penicillin, 100 U/ml streptomycin, and 250 μg/ml amphotericin. In some experiments, the medium was supplemented with 1 μM rosiglitazone 16 h before solubilization to increase CD36 expression levels (25).
In some experiments, HDMECs were treated with a recombinant version of the antiangiogenic domain of TSP-1, designated 3TSR, and VEGF before solubilization (26). A recombinant version of the procollagen homology domain of TSP-1, designated ProCol, was used as a control. The preparation of 3TSR was described previously (26). ProCol was prepared using the forward primer 652hTSP-1f (GAT GAT CCC GGG GAT GAG CTG TCC AGC ATG) and the reverse primer 760TSP-1r (GAT ACC GGT GTC GCT GGG CCA ACA GCG). The PCR product was sequenced and cloned between the XmaI and AgeI sites of the vector pMT/BiP/V5-HisA (Invitrogen, Carlsbad, CA, USA). The recombinant protein includes the vector-derived sequences RSPWPG at the N terminus and TGHHHHHH at the C terminus. Vector transfection, cell selection, and protein expression and purification were performed as described previously (26). The HDMECs were grown overnight in the above medium with reduced FBS (2%) and were washed 3 times in PBS. The cells were then incubated at 37°C for 35 min in PBS containing 0.5% FBS with and without 3TSR or ProCol. The cells were washed once in PBS containing 0.5% FBS before addition of 50 ng/ml of VEGF. The cells were incubated at 37°C for 10 min before solubilization in 250 mM NaCl, 25 mM Tris-HCl (pH 7.5), 5 mM EDTA, 1% Brij99, 1 mM EGTA, 1 mM sodium vanadate, 20 mM NaF, and 1× protease inhibitor cocktail (Halt; Pierce Biotechnology, Rockford, IL, USA).
To isolate murine endothelial cells, the hearts and lungs of 3 to 4 12- to 15-d-old mice were removed and collected in a tube containing cold Hanks’ balanced salt solution (HBSS) with antibiotics. Tissues were trimmed of extraneous tissue (thymus, trachea, etc.), washed with HBSS, finely minced, and mixed in a 50-ml tube with 20–30 ml of 2% Worthington type 1 collagenase in Dulbecco’s PBS with calcium/magnesium that had been predigested at 37°C for 1 h. The tissue was digested for 30 min at 37°C with gentle agitation. Digested tissues were triturated through a 14-gauge cannula 10–15 times and then were filtered through a 70-μm cell strainer (BD Discovery Labware, San Jose, CA, USA). The digested filtrate was centrifuged, and the pellet was washed once and resuspended in PBS with 0.1% BSA added (PBS/BSA). The filtrate was then incubated for 15 min at room temperature with 25 μl of magnetic beads (Invitrogen) that had been conjugated with anti-mouse CD31 antibody (BD Biosciences, San Jose, CA, USA). Cells with beads attached were collected using an MPC magnet (Invitrogen) and washed 6 to 8 times with PBS/BSA. Washed cells were collected and plated in endothelial medium in a 100-mm tissue culture plate that had been precoated with gelatin (0.1% in PBS). After 24–48 h, nonattached cells and excess beads were removed, and fresh medium was added. Cells were further purified by repeating the beading using beads that had been conjugated to intercellular adhesion molecule-2 (BD Biosciences). Purity was assessed by DiI-acetylated low-density-lipoprotein staining (Biomedical Technologies, Inc., Stoughton, MA, USA). The cells were grown in DMEM basal mediim with 4.5 g/L glucose, 1 mM sodium pyruvate, 2 mM glutamine, 1 mM nonessential amino acids, 20% FBS, 50 mg/500 ml ECGS (Biomedical Technologies, Inc.), 0.1 mg/ml heparin, and antibiotic/antimycotic.
Immunoprecipitation and Western blotting
Cells were trypsinized, washed twice with ice-cold PBS, and then used for protein extraction. The whole-cell or lung tissue extracts were prepared with either T-PER Tissue Protein Extraction Reagent (Pierce Biotechnology) with added proteinase and phosphatase inhibitors or 1% Brij99, 1% Brij96, or 1% Brij96 containing 5 mM MgCl2 lysis buffer [150 or 250 mM NaCl, 25 mM Tris-HCl or HEPES (pH 7.4), 5 mM EDTA, and protease inhibitors]. The cells were treated with lysis buffer for 20 min at 4°C, and the samples were centrifuged at 45,000 rpm for 30 min at 4°C in an SW55 rotor. The cell lysate was either used immediately for immunoprecipitation experiments or stored at −80°C. To preclear the samples, 1 ml of cell lysate, 5 μg of nonimmune IgG, and 25 μl (pellet volume) of protein G agarose beads were mixed for 1 h at 4°C. After removal of the protein G beads by centrifugation, 5 μg of anti-CD36 antibodies [2 μg of FA6–152 (Immunotech, Marseille, France), and 3 μg of CLB-IV C7 (Accurate Chemical & Scientific, Westbury, NY, USA)], anti-VEGFR2 (Cell Signaling Technology Inc., Danvers, MA, USA), or anti-β1 integrin antibody (kindly provided by Dr. Richard O. Hynes, Massachusetts Institute of Technology, Cambridge, MA, USA) was added, and the samples were incubated for 2–3 h at 4°C. Then 25 μl (pellet volume) of protein G beads were added, and the samples were incubated for an additional 2–3 h at 4°C. The beads were washed 3 times with lysis buffer, and the precipitated immunocomplex was eluted in 50 μl of 2× SDS-PAGE loading buffer by boiling for 4 min. The eluted samples were separated by SDS-PAGE in the presence of 1% dithiothreitol as described previously (27). After SDS-PAGE, the proteins were transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA, USA) as described previously (27). For immunological detection, the transfer membranes were incubated in 5% nonfat dry milk in Tris-buffered saline-Tween 20 (TBS-T) for 1 h. The primary antibodies (diluted in the blocking solution) were added, and the membranes were incubated overnight at 4°C with mixing. After 5 washes for 5 min each in TBS-T, the horseradish peroxidase-conjugated secondary antibodies were added, and the blots were incubated for 2 h at room temperature. The membranes were washed 5 times for 5 min each in TBS-T, and the bands were visualized using ECL detection (Pierce Biotechnology).
Vascular permeability
Vascular permeability was quantified using the Miles assay in wild-type and TSP-1-null FVB mice (28). In some experiments, mice were pretreated with 3TSR (3 mg/kg/d) for 5 d before vascular permeability was quantified. The backs of the mice were shaved 2 or 3 d before intraperitoneal injection with pyrilamine (0.4 mg/kg). Thirty minutes after pyrilamine injection, the mice were anesthetized with 2.5% Avertin (10 μl/g), and 100 μl of 0.5% Evans blue was injected into the tail vein, followed immediately by intradermal VEGF (or HBSS as control) injections at the back skin (50 ng in 50 μl) and ear (25 ng in 10 μl). Mice were euthanized 30 min later, and tissue was collected with an 8-mm skin punch. The tissue was incubated in 300 μl of formamide at 56°C for 48 h. The quantity of Evans blue in tissues and standards was determined by reading optical density at 605 nm.
In vivo phosphorylation of VEGFR2
Wild-type and TSP-1-null mice received tail vein injection of 2 μg of VEGF, and lung tissue was harvested 5 min later (29). In some experiments, the mice were treated with 3TSR as described above. The lung tissue was solubilized in either T-PER Tissue Protein Extraction Reagent (Pierce Biotechnology) or extraction buffer containing 250 mM NaCl, 25 mM Tris-HCl (pH 7.5), 5 mM EDTA, 1% Brij99, and 1 mM EGTA. Both solutions contained 1 mM sodium vanadate, 20 mM NaF, and 1× protease inhibitor cocktail (Halt). The extracts were Western-blotted with antibodies to total VEGFR2 or p-1175 VEGFR2 (Cell Signaling Technology Inc.) diluted 1:500. The intensity of the bands was quantified with a Bio-Rad densitometer. The quantity of signal in each lane was divided by the signal in the corresponding β-actin blot.
RESULTS
TSP-1 receptors associate with VEGFR2
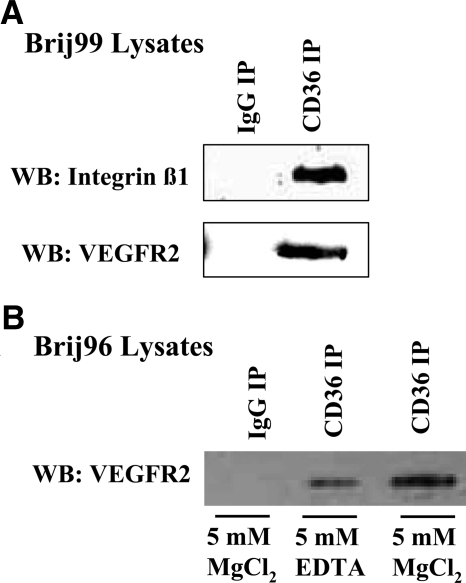
Several groups, including our own, have previously found that receptors for TSP-1, CD36, and β1 integrins associate with each other in the plane of the membrane in multiple cell types (11, 18, 19). We have used an antibody array strategy to identify other components of these protein complexes on unstimulated HDMECs in culture and have identified a potential interaction of VEGFR2 with CD36 (unpublished results). To validate the array data, we performed immunoprecipitations for CD36 and Western blotting for VEGFR2 or β1 integrin. VEGFR2 and β1 integrin were identified by Western blotting in anti-CD36 immunoprecipitates of Brij99-solubilized HDMECs but not in nonimmune IgG control immunoprecipitates (Fig. 1). In contrast, the associations of CD36 with VEGFR2 and β1 integrin were disrupted when the cells were solubilized in Triton X-100 (11) (data not shown). A weak VEGFR2 band was detected in cellular extracts that were prepared with Brij96, a detergent with intermediate stringency (Fig. 1). However, when the cells were solubilized in Brij96 containing 5 mM magnesium, a strong VEGFR2 band was detected (Fig. 1). Magnesium has been reported to increase the specificity of the interactions that are detected in Brij96 (30, 31).
Figure 1.
CD36, β1 integrins, and VEGFR2 are associated in the endothelial cell membrane. HDMECs were solubilized in extraction buffers containing either Brij99 (A) or Brij96 (B), and immunoprecipitations (IPs) were performed with anti-CD36 antibodies. Integrin β1 or VEGFR2 was detected by Western blotting, as indicated.
TSP-1-null mice exhibit decreased VEGF-induced phosphorylation of VEGFR2 in vivo and in vitro
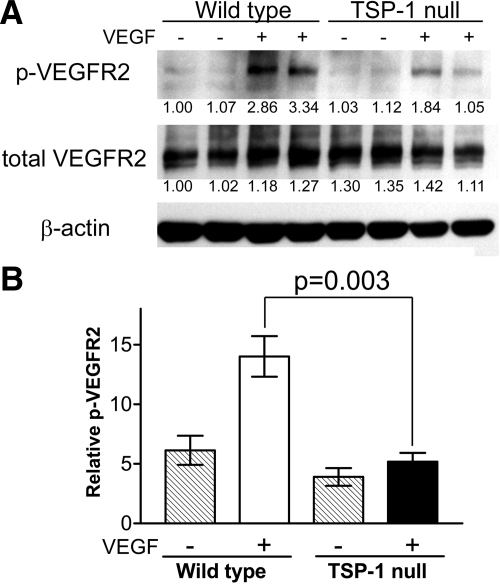
The observation that VEGFR2 associates with CD36 and β1 integrin raised the possibility that TSP-1 may affect signal transduction by VEGFR2. To explore this possibility, we performed an in vivo assay for VEGFR2 phosphorylation in lung tissue, which is ∼50% endothelial cells. Wild-type and TSP-1-null mice received a tail vein injection of 2 μg of VEGF, lung tissue was harvested and solubilized 5 min later, and the tissue extracts were blotted with an antibody that detects phosphorylation at tyrosine 1173 of mouse VEGFR2 (equivalent to tyrosine 1175 in human VEGFR2). As shown in Fig. 2, the level of phosphorylation of VEGFR2 subsequent to VEGF injection was significantly decreased in the TSP-1-null mice compared with wild-type mice, whereas the levels of total VEGFR2 were comparable.
Figure 2.
VEGF-induced phosphorylation of VEGFR2 in wild-type and TSP-1-null mice. A) Lung tissue was harvested 5 min after mice received an injection of 2 μg of VEGF via the tail vein. Tissue was solubilized using T-PER Tissue Protein Extraction Reagent with added proteinase and phosphatase inhibitors and subjected to SDS-PAGE followed by Western blotting using an antibody that recognizes VEGFR2 phosphorylated at tyrosine 1173. A parallel sample was blotted with an antibody that detects total VEGFR2. Intensity of the bands was quantified with a Bio-Rad densitometer. The quantity of signal in each lane was divided by the signal in the corresponding β-actin blot. Number under each lane represents fold change compared with lane 1. B) Percentage of phosphorylated VEGFR2 was determined by dividing the intensity of the bands detected with the antiphosphorylated VEGFR2 antibody by that detected with the total anti-VEGFR2 antibody. ▒, untreated mice; □, VEGF-treated wild-type mice; ▪, VEGF-treated TSP-1-null mice. Values for 3 mice in each group were averaged.
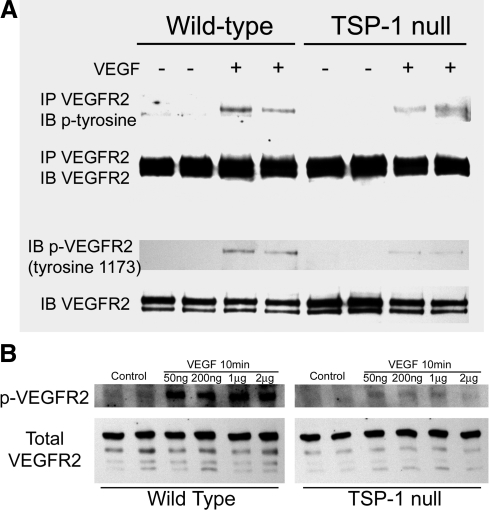
To establish that the decreased response of VEGFR2 was specifically due to decreased responsiveness of endothelial cells, we isolated endothelial cells from the hearts and lungs of wild-type and TSP-1-null mice and treated them with 50 ng/ml of VEGF. Maximum phosphorylation of VEGFR2 was observed ∼5–10 min after VEGF treatment in both wild-type and TSP-1-null serum-starved endothelial cells (data not shown). Western blotting of cell extracts with the anti-p1173 antibody revealed decreased phosphorylation of VEGFR2 in the TSP-1-null endothelial cells (Fig. 3). Decreased phosphorylation of VEGFR2 was also detected by immunoprecipitating VEGFR2 and Western blotting with an antibody to phosphotyrosine (Fig. 3). The level of total VEGFR2 was comparable in TSP-1-null and wild-type endothelial cells. To determine whether the difference in response to VEGF was due to a decreased sensitivity to VEGF, we treated endothelial cells with higher concentrations of VEGF. Decreased phosphorylation of VEGFR2 in TSP-1-null endothelial cells was also observed after treatment with 200 ng/ml, 1 μg/ml, or 2 μg/ml of VEGF (Fig. 3).
Figure 3.
VEGF-induced phosphorylation of VEGFR2 in wild-type and TSP-1-null endothelial cells. A) Duplicate samples of VEGF-treated and untreated heart endothelial cells from wild-type and TSP-1-null mice were immunoblotted with the antibody to VEGFR2 that is phosphorylated at tyrosine 1173 (bottom) or were immunoprecipitated with anti-VEGFR2 antibody and immunoblotted with an antibody to phosphotyrosine (top). Samples were also immunoblotted with an antibody to total VEFGR2 to ensure consistent protein loading. B) Lung endothelial cells from wild-type and TSP-1-null mice were treated with varying doses of VEGF as indicated for 10 min, and samples were immunoblotted for total or phosphorylated VEGFR2.
TSP-1-null mice exhibit diminished vascular permeability in response to VEGF
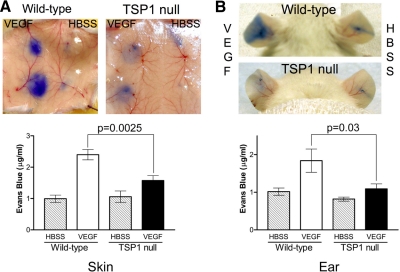
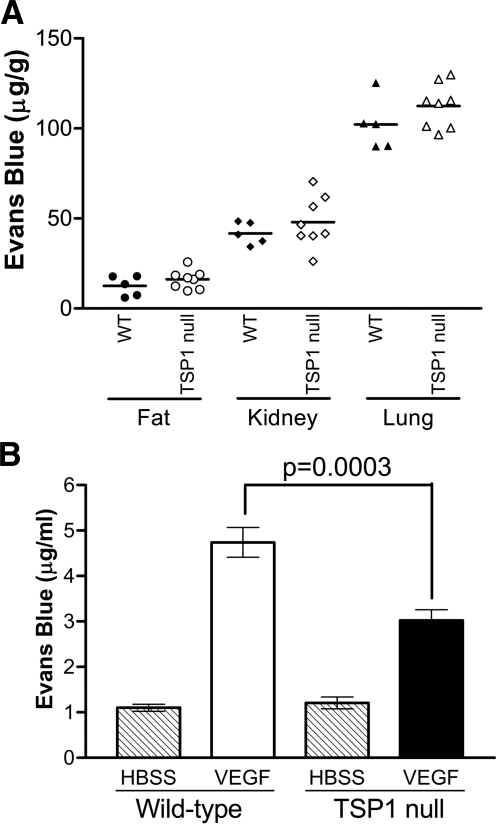
We performed the Miles permeability assay to establish that the decreases in VEGFR2 phosphorylation observed in TSP-1-null mice are sufficient to affect physiological responses to VEGF in an acute setting. Whereas wild-type and TSP-1-null mice displayed similar levels of basal vascular permeability in skin, fat, kidney, and lung tissue, vascular permeability induced by 50 ng of VEGF was significantly decreased in TSP-1-null mice as measured with the Miles assay in the flank skin and in the ear (Figs. 4 and 5A). Permeability in the flank skin was decreased by 34.5% (P=0.0025), and permeability in the ear was decreased by 40.8% (P=0.035) in the TSP-1-null mice compared with the wild-type controls. A comparable decrease in vascular permeability in the TSP-1-null mice was also seen when the dose of VEGF was increased to 200 ng (Fig. 5B).
Figure 4.
VEGF-induced vascular permeability in wild-type and TSP-1-null mice. Miles assay was performed with 50 ng of VEGF in flank skin (A) and ear (B) of wild-type and TSP-1-null mice. Evan’s blue dye extravasation was quantified in 3 independent experiments and averaged (mean±sd). ▒, untreated mice; □, VEGF-treated wild-type mice; ▪, VEGF-treated TSP-1-null mice.
Figure 5.
Basal and VEGF-induced permeability in wild-type (WT) and TSP-1-null mice. A) Basal permeability in fat, kidney, and lung tissue in wild-type and TSP-1-null mice. B) Miles assay was performed with 200 ng of VEGF in skin of wild-type and TSP-1-null mice. Evan’s blue dye extravasation into tissue was quantified for 3 independent experiments (mean±sd). ▒, untreated mice; □, VEGF-treated wild-type mice; ▪, VEGF-treated TSP-1-null mice.
3TSR suppresses VEGF-induced vascular permeability
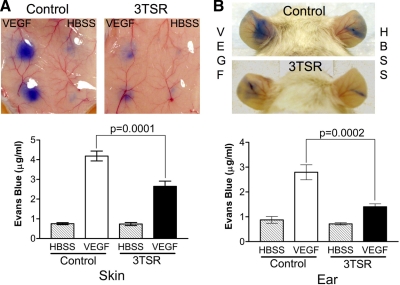
The effect of TSP-1 on VEGF signal transduction raised the possibility that an antiangiogenic domain of TSP-1, designated 3TSR, might influence VEGFR2 function. To explore this possibility, the Miles assay was performed in wild-type mice that were treated with 3TSR (3 mg/kg/d) or an equivalent volume of PBS by intraperitoneal injections for 5 d (n=7 for each group). Whereas treatment with 3TSR did not have an effect on the basal permeability on either ear or skin, VEGF-induced vascular permeability was significantly reduced in both ear (59.3% reduction, P=0.0002) and flank skin (36.8% reduction, P=0.0001) of the 3TSR-treated mice compared with that in the untreated control mice (Fig. 6). In contrast, a single 3TSR intraperitoneal injection did not affect vascular permeability in the Miles assay (data not shown). However, localized 3TSR injection did inhibit VEGF-induced permeability in the same site. Mice were treated with a single 3TSR intradermal injection (30 μg in 100 μl) on one flank, and the other flank was injected with PBS. VEGF was injected intradermally into the same spot 20 min later (n=12). VEGF-induced permeability was significantly decreased in the sites that were pretreated with 3TSR compared with that in buffer-pretreated sites in the same mouse (28.0% reduction, P=0.0003, data not shown).
Figure 6.
Effect of 3TSR on VEGF-induced vascular permeability. Miles assay was performed with injection of 50 ng of VEGF into flank skin (A) and ear (B) of untreated and 3TSR-treated mice. Mice received intraperitoneal injections of 3TSR (3 mg/kg/d) for 5 d. Evan’s blue dye extravasation was quantified for 3 independent experiments (mean±sd). ▒, untreated mice; □, VEGF-treated wild-type mice; ▪, VEGF-treated TSP-1-null mice.
3TSR suppresses VEGF-induced phosphorylation of VEGFR2 in vivo and in vitro
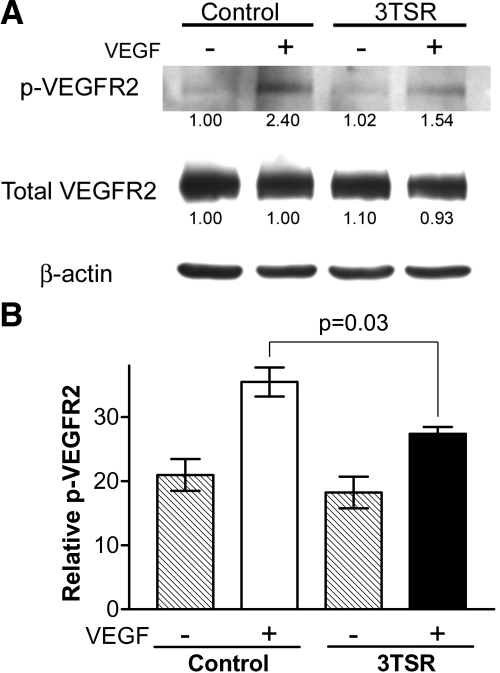
To determine whether the decreased permeability that was observed in the 3TSR-treated mice corresponded to decreased phosphorylation of VEGFR2, we treated mice for 5 d with 3TSR (3 mg/kg/d) or an equivalent volume of PBS, and the in vivo VEGFR2 phosphorylation assay was performed as described above. As shown in Fig. 7, the level of phosphorylation of VEGFR2 in response to VEGF was significantly decreased in the 3TSR-treated mice compared with that in their wild-type counterparts (Fig. 7).
Figure 7.
Effect of 3TSR on VEGF-induced phosphorylation of VEGFR2. Mice received intraperitoneal injections of 3TSR (3 mg/kg/d) for 5 d. A) Lung tissue was harvested 5 min after mice received an injection of 2 μg of VEGF via the tail vein. Tissue was solubilized using T-PER Tissue Protein Extraction Reagent with added proteinase and phosphatase inhibitors and subjected to SDS-PAGE followed by Western blotting using an antibody to VEGFR2 that is phosphorylated at tyrosine 1173. A parallel sample was blotted with an antibody that detects total VEGFR2. The quantity of signal in each lane was divided by the signal in the corresponding β-actin blot. Number under each lane represents fold change compared with lane 1. B) Percentage of phosphorylated VEGFR2 was determined by dividing intensity of bands detected with antiphosphorylated VEGFR2 antibody by that detected with total anti-VEGFR2 antibody. Values for 3 mice in each group were averaged. ▒, untreated mice; □, VEGF-treated wild-type mice; ▪, VEGF-treated TSP-1-null mice.
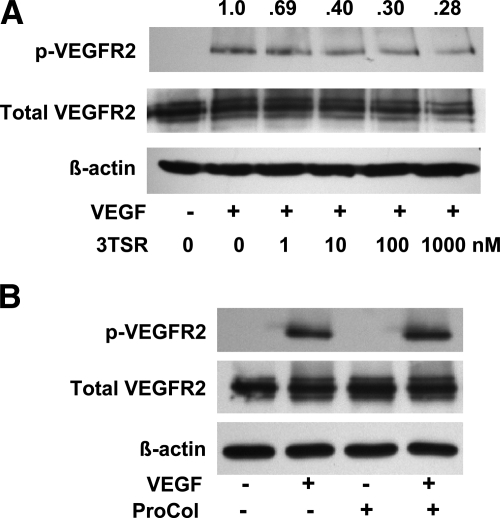
Treatment of serum-starved HDMECs in culture with 50 ng/ml of VEGF resulted in a significant increase in phosphorylation of VEGFR2 at tyrosine 1175 (Fig. 8). Pretreatment of the cells for 35 min with 3TSR (1 μM) resulted in a decrease in VEGFR2 phosphorylation (40.0±24.3% of control, P=0.0103, n=4). This decrease in VEGFR2 phosphorylation exhibited a dose-dependent response to 3TSR (Fig. 8A). Comparable levels of total VEGFR2 were observed in untreated and 3TSR-treated HDMECs. The procollagen homology domain of TSP-1, designated ProCol, is adjacent to 3TSR and is comparably sized (32). In three experiments, pretreatment of HDMECs with ProCol did not decrease VEGF-induced phosphorylation of VEGFR2 (Fig. 8B).
Figure 8.
Effect of 3TSR on VEGF-induced phosphorylation of VEGFR2 in HDMECs. HDMECs were cultured in medium containing 2% FBS overnight. A) Cells were incubated in PBS containing 5% FBS, with or without 3TSR (1–1000 nM, as indicated below lanes), for 35 min. Cells were washed once before the addition of fresh mediun and VEGF (50 ng/ml, as indicated below each lane). Cells were solubilized in extraction buffer containing 1% Brij99 after a 10-min incubation, and samples were immunoblotted for VEGFR2 phosphorylated at tyrosine 1175. Quantity of phospho-VEGFR2 relative to the sample that was not treated with 3TSR is indicated above each lane. B) An experiment equivalent to that described in A was performed using the procollagen homology domain of TSP-1 (ProCol) at 1 μM, as indicated below each lane.
To determine whether 3TSR affects the association of CD36 with VEGFR2, we treated HDMECs with 1 μM 3TSR for 35 min and then solubilized the cells in buffer containing 1% Brij99. CD36 immunoprecipitates of these extracts were Western-blotted for VEGFR2, and the bands were quantified by densitometry. A small increase (10.2±37.3%, n=5) in the amount of VEGFR2, which was not statistically significant, was detected in the CD36 immunoprecipitates from cells treated with 3TSR, indicating that 3TSR does not disrupt the association of CD36 with VEGFR2.
DISCUSSION
TSP-1 is well established as an endogenous inhibitor of angiogenesis (4). In this study, we have made several novel observations regarding the function of TSP-1. First, we have found that TSP-1 receptors (CD36 and β1 integrins) associate with VEGFR2 and that this association has physiological significance. Paradoxically, however, we have shown that TSP-1-null mice exhibit decreased VEGF-induced phosphorylation of VEGFR in response to VEGF, both in vivo and in vitro. Consistent with this observation, TSP-1-null mice exhibit decreased vascular permeability response to VEGF. Finally, we have shown that 3TSR, a therapeutic agent based on the antiangiogenic domain of TSP-1, suppresses VEGF-induced vascular permeability and phosphorylation of VEGFR2 in vivo and in vitro. Together, these findings indicate a heretofore unrecognized complexity in VEGF signaling that is mediated, at least in part, by interactions between VEGFR2 and TSP-1 receptors.
The binding of TSP-1 to CD36 results in the suppression of angiogenesis by inhibiting endothelial cell migration and inducing endothelial cell apoptosis (9, 16). In addition, β1 integrins mediate the inhibition of migration of HUVECs, which are known to express low to undetectable levels of CD36 (17). When CD36 is expressed in HUVECs, it forms a complex with β1 integrin that mediates a reduction in VEGF-induced phosphorylation of VEGFR2 when TSP-1 is present (18). Primo et al. (18) did not detect an association of CD36 with VEGFR2 in cell extracts prepared with Brij97. This result is consistent with our observation that only a weak association of CD36 with VEGFR2 is seen in HDMEC extracts that are prepared in Brij96. Claas et al. (33) have classified detergents in terms of their ability to disrupt protein-protein interactions in the membrane. Brij96 and Brij97 are considered to be intermediate in stringency, whereas Triton X-100 is more stringent, and Brij99 and 3-[3-cholamidopropyl) diethylammonio]-1 propane sulfonate (CHAPS) are less so. The inclusion of 5 mM magnesium chloride with Brij96 improves the specificity of interactions by a mechanism that is yet to be determined (30, 31). The fact that the association of CD36 with VEGFR2 is preserved in Brij96 when 5 mM magnesium chloride is present suggests that the interaction is specific; however, it is not necessarily direct. The biochemical properties of the molecular complex described in this study, together with our prior data showing that the tetraspanin CD9 complexes with CD36 and integrins in platelets, indicate that a portion of VEGFR2 localizes to tetraspanin-enriched microdomains with CD36 and β1 integrins (11).
Our data identify a novel mechanism by which 3TSR can inhibit angiogenesis, that is, by antagonizing VEGF activity at the receptor level. TSP-1 has been reported to suppress the activity of VEGF by antagonizing VEGF mobilization from the extracellular matrix through inhibition of MMP9 and by promoting VEGF clearance through receptor-mediated uptake (7, 8). In this study, we have found that the antiangiogenic domain of TSP-1 (3TSR) reduces the level of VEGFR2 phosphorylation in response to VEGF treatment. In separate studies, we have found that 3TSR also decreases VEGF-induced phosphorylation of Akt, which is downstream of VEGFR2 (34, 35). Because these effects can be observed in vitro after short-term exposure to VEGF, they do not appear to involve effects on mobilization or clearance of VEGF. We can detect decreased phosphorylation of VEGFR2 in response to VEGF after the 3TSR recombinant protein is washed out of the sample; therefore, it does not seem that our results can be explained by a direct interaction of 3TSR with VEGF (36). However, we cannot exclude the possibility that 3TSR remains associated with the cell and acts as a sink for VEGF. Two other inhibitors of angiogenesis, endostatin and tissue inhibitor of metalloproteinase 2, have also been shown to decrease VEGF-induced phosphorylation of VEGFR2 by distinct mechanisms (37, 38).
Mutations in CD36 that disrupt its association with β1 integrin abrogate the ability of TSP-1 to inhibit VEGF-induced phosphorylation of VEGFR2 (18). In a parallel study, we have detected a decreased association of CD36 with VEGFR2 in the absence of TSP-1 (unpublished results). These data indicate that TSP-1 promotes the assembly of a molecular complex that is necessary for its inhibitory activity. However, we have found that 3TSR does not affect the association of CD36 with VEGFR2. These data imply that TSP-1 and 3TSR are probably acting through distinct mechanisms.
We made use of the acute vascular permeability that is induced in response to VEGFR2 stimulation by its cognate ligand VEGF-A to ask whether the inhibitory effects of TSP-1 and 3TSR on VEGFR2 phosphorylation that we observed in vitro are sufficient to hinder the biological activity of this receptor in vivo. Notably, the reduction in VEGFR2 phosphorylation that we detected in tissues from mice treated systemically with 3TSR correlated well with the concomitant attenuation in plasma extravasation observed in the Miles assay. These data suggest that overexpression of TSP-1 can significantly down-regulate VEGFR2 function in vivo.
Vascular permeability is a complex process that involves numerous cellular events including initiation of signal transduction cascades, cytoskeletal reorganization, subcellular organelle activation, interaction with extracellular matrix, and cell-to-cell junctional communication (39, 40). It can also be affected by local blood flow and systemic blood pressure. Therefore, because TSP-1 has been shown to suppress NO-mediated signaling (21, 41), the suppressive effect of 3TSR on VEGF-A-induced acute vascular permeability may not be solely restricted to the regulation of VEGFR2 phosphorylation. However, our data clearly document an additional point of control mediated by TSP-1 that occurs very early in the VEGF-A/VEGFR2/eNOS/NO signaling cascade. In contrast, the reasons for the reduction in VEGF-A-induced acute tracer leakage that we observed in the TSP-1-null mice remain unclear. The antagonistic relationship between TSP-1 and NO signaling would predict that acute vascular permeability mediated by VEGF-A should be increased in the absence of TSP-1. However, this supposition is in direct opposition to our experimental data. Our finding is specific in that similar studies performed in TSP-2-null mice yielded no such inhibition (data not shown). Hence, further studies will be needed to unravel this complexity and discern the molecular mechanism by which the absence of TSP-1 reduces acute VEGF-A-driven vascular permeability.
The decreased response to VEGF in TSP-1-null mice may at first seem counterintuitive because increased angiogenesis and tumor growth have been reported in TSP-1-null mice (42, 43). Angiogenesis is a multistep process that occurs over a period of days to weeks. During this time, a wide range of proteins and cells contribute to the formation of new blood vessels. We have preliminary data (not shown) indicating that VEGF-induced angiogenesis is, in fact, increased in TSP-1-null mice. There are many possible reasons that the angiogenic response in TSP-1-null mice is enhanced even though VEGFR2 phosphorylation is decreased. TSP-1-null mice have been shown to have significantly higher levels of circulating endothelial progenitor cells (44). TSP-1-null endothelial cells have increased proliferation and migration in serum-containing media (45). The study of TSP-1-null endothelial cells also reveals that the absence of TSP-1 affects the expression or activation of platelet-endothelial cell adhesion molecule, fibronectin, Src, Shp-2, phosphatidylinositol 3-kinase, Akt, Rac1, and p38 (45, 46). We have found that there is decreased association of Fyn with CD36 in TSP-1-null endothelial cells (35). Because Fyn has been reported to mediate TSP-1-induced apoptosis of endothelial cells, the decreased association of CD36 and Fyn may reduce the antiangiogenic activity of TSP-1. Thus, concomitant decreases in antiangiogenic responses and increases in prosurvival signals or circulating endothelial progenitor cells in TSP-1-null mice may compensate for the decrease in VEGFR2 activity. In the TSP-1-null tumor environment, the net effect appears to support enhanced angiogenesis and tumor growth.
Localization of VEGFR2 to specific regions of the membrane may facilitate signal transduction in that it may promote dimer formation or colocalization with downstream signaling proteins or coreceptors such as neuropilins (47). The coclustering of receptors that regulate angiogenesis may provide the endothelial cell with a platform for integration of positive and negative signals in the plane of the membrane (48). Thus, this complex may represent a molecular switch that regulates angiogenesis and determines endothelial cell behavior. In this context, physiological levels of TSP-1 appear to support VEGFR2 function on both the cellular and tissue levels because VEGF-induced phosphorylation of VEGFR2 and vascular permeability are decreased in TSP-1-null mice. This is, at least in part, a cell autonomous effect in that decreased VEGF-induced phosphorylation of VEGFR2 is observed in cultured heart and lung endothelial cells from TSP-1-null mice compared with that in their wild-type counterparts. We propose that physiological levels of TSP-1 function on the endothelial cell membrane to guide the assembly of multiprotein complexes that act as a molecular switch that regulates angiogenesis.
Acknowledgments
We thank Dr. Richard O. Hynes (Massachusetts Institute of Technology, Cambridge, MA, USA) for antibodies of β1 integrin and Sami Lawler and Raji Bhat for help in preparing the manuscript. This work was supported by NIH grants CA92644 and CA130895.
References
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12:5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- Kazerounian S, Yee K O, Lawler J. Thrombospondins in cancer. Cell Mol Life Sci. 2008;65:700–712. doi: 10.1007/s00018-007-7486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lawler J. Thrombospondin-based antiangiogenic therapy. Microvasc Res. 2007;74:90–99. doi: 10.1016/j.mvr.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Brown L F, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107:409–417. doi: 10.1172/JCI11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Brown L F, Lawler J, Miyakawa T, Detmar M. Thrombospondin-1 plays a critical role in the induction of hair follicle involution and vascular regression during the catagen phase. J Invest Dermatol. 2003;120:14–19. doi: 10.1046/j.1523-1747.2003.12045.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque J C, Lane T F, Ortega M A, Hynes R O, Lawler J, Iruela-Arispe M L. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenaway J, Lawler J, Moorehead R, Bornstein P, Lamarre J, Petrik J. Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1) J Cell Physiol. 2007;210:807–818. doi: 10.1002/jcp.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D W, Volpert O V, Pearce S F, Schneider A J, Silverstein R L, Henkin J, Bouck N P. Three distinct d-amino acid substitutions confer potent antiangiogenic activity on an inactive peptide derived from a thrombospondin-1 type 1 repeat. Mol Pharmacol. 1999;55:332–338. doi: 10.1124/mol.55.2.332. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe M L, Lombardo M, Krutzsch H C, Lawler J, Roberts D D. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation. 1999;100:1423–1431. doi: 10.1161/01.cir.100.13.1423. [DOI] [PubMed] [Google Scholar]
- Miao W M, Vasile E, Lane W S, Lawler J. CD36 associates with CD9 and integrins on human blood platelets. Blood. 2001;97:1689–1696. doi: 10.1182/blood.v97.6.1689. [DOI] [PubMed] [Google Scholar]
- Reiher F K, Volpert O V, Jimenez B, Crawford S E, Dinney C P, Henkin J, Haviv F, Bouck N P, Campbell S C. Inhibition of tumor growth by systemic treatment with thrombospondin-1 peptide mimetics. Int J Cancer. 2002;98:682–689. doi: 10.1002/ijc.10247. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus S, Hussain M, Tannir N, Gordon M, Desai A A, Knight R A, Humerickhouse R A, Qian J, Gordon G B, Figlin R. Phase 2 study of ABT-510 in patients with previously untreated advanced renal cell carcinoma. Clin Cancer Res. 2007;13:6689–6695. doi: 10.1158/1078-0432.CCR-07-1477. [DOI] [PubMed] [Google Scholar]
- Markovic S N, Suman V J, Rao R A, Ingle J N, Kaur J S, Erickson L A, Pitot H C, Croghan G A, McWilliams R R, Merchan J, Kottschade L A, Nevala W K, Uhl C B, Allred J, Creagan E T. A phase II study of ABT-510 (thrombospondin-1 analog) for the treatment of metastatic melanoma. Am J Clin Oncol. 2007;30:303–309. doi: 10.1097/01.coc.0000256104.80089.35. [DOI] [PubMed] [Google Scholar]
- Margosio B, Rusnati M, Bonezzi K, Cordes B L, Annis D S, Urbinati C, Giavazzi R, Presta M, Ribatti D, Mosher D F, Taraboletti G. Fibroblast growth factor-2 binding to the thrombospondin-1 type III repeats, a novel antiangiogenic domain. Int J Biochem Cell Biol. 2008;40:700–709. doi: 10.1016/j.biocel.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez B, Volpert O V, Crawford S E, Febbraio M, Silverstein R L, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- Short S M, Derrien A, Narsimhan R P, Lawler J, Ingber D E, Zetter B R. Inhibition of endothelial cell migration by thrombospondin-1 type-1 repeats is mediated by beta1 integrins. J Cell Biol. 2005;168:643–653. doi: 10.1083/jcb.200407060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primo L, Ferrandi C, Roca C, Marchio S, di Blasio L, Alessio M, Bussolino F. Identification of CD36 molecular features required for its in vitro angiostatic activity. FASEB J. 2005;19:1713–1715. doi: 10.1096/fj.05-3697fje. [DOI] [PubMed] [Google Scholar]
- Thorne R F, Marshall J F, Shafren D R, Gibson P G, Hart I R, Burns G F. The integrins α3β1 and α6β1 physically and functionally associate with CD36 in human melanoma cells: requirement for the extracellular domain of CD36. J Biol Chem. 2000;275:35264–35275. doi: 10.1074/jbc.M003969200. [DOI] [PubMed] [Google Scholar]
- Isenberg J S, Ridnour L A, Perruccio E M, Espey M G, Wink D A, Roberts D D. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci U S A. 2005;102:13141–13146. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg J S, Jia Y, Fukuyama J, Switzer C H, Wink D A, Roberts D D. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem. 2007;282:15404–15415. doi: 10.1074/jbc.M701638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg J S, Frazier W A, Roberts D D. Thrombospondin-1: a physiological regulator of nitric oxide signaling. Cell Mol Life Sci. 2008;65:728–742. doi: 10.1007/s00018-007-7488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agah A, Kyriakides T R, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161:831–839. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard L, Velasco P, Detmar M. A simple immunomagnetic protocol for the selective isolation and long-term culture of human dermal microvascular endothelial cells. Exp Cell Res. 1998;240:1–6. doi: 10.1006/excr.1998.3936. [DOI] [PubMed] [Google Scholar]
- Huang H, Campbell S C, Bedford D F, Nelius T, Veliceasa D, Shroff E H, Henkin J, Schneider A, Bouck N, Volpert O V. Peroxisome proliferator-activated receptor γ ligands improve the antitumor efficacy of thrombospondin peptide ABT510. Mol Cancer Res. 2004;2:541–550. [PubMed] [Google Scholar]
- Miao W M, Seng W L, Duquette M, Lawler P, Laus C, Lawler J. Thrombospondin-1 type 1 repeat recombinant proteins inhibit tumor growth through transforming growth factor-β-dependent and -independent mechanisms. Cancer Res. 2001;61:7830–7839. [PubMed] [Google Scholar]
- Lawler J, Derick L H, Connolly J E, Chen J-H, Chao F C. The structure of human platelet thrombospondin. J Biol Chem. 1985;260:3762–3772. [PubMed] [Google Scholar]
- Miles A A, Miles E M. Vascular reactions to histamine, histamine-liberator and leukotaxine in the skin of guinea-pigs. J Physiol. 1952;118:228–257. doi: 10.1113/jphysiol.1952.sp004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P M, Yu J, Chen Y, Hickey R, Bernatchez P N, Looft-Wilson R, Huang Y, Giordano F, Stan R V, Sessa W C. Endothelial-specific expression of caveolin-1 impairs microvascular permeability and angiogenesis. Proc Natl Acad Sci U S A. 2005;102:204–209. doi: 10.1073/pnas.0406092102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M E. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 2003;19:397–422. doi: 10.1146/annurev.cellbio.19.111301.153609. [DOI] [PubMed] [Google Scholar]
- Stipp C S, Orlicky D, Hemler M E. FPRP, a major, highly stoichiometric, highly specific CD81- and CD9-associated protein. J Biol Chem. 2001;276:4853–4862. doi: 10.1074/jbc.M009859200. [DOI] [PubMed] [Google Scholar]
- Lawler J, Hynes R O. The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. J Cell Biol. 1986;103:1635–1648. doi: 10.1083/jcb.103.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claas C, Stipp C S, Hemler M E. Evaluation of prototype transmembrane 4 superfamily protein complexes and their relation to lipid rafts. J Biol Chem. 2001;276:7974–7984. doi: 10.1074/jbc.M008650200. [DOI] [PubMed] [Google Scholar]
- Ren B, Song K, Parangi S, Jin T, Ye M, Humphreys R, Duquette M, Zhang X, Benhaga N, Lawler J, Khosravi-Far R. A double hit to kill tumor and endothelial cells by TRAIL and antiangiogenic 3TSR. Cancer Res. 2009;69:3856–3865. doi: 10.1158/0008-5472.CAN-08-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Hopkins B D, Tsujikawa K, Perruzzi C, Adini I, Swerlick R, Bornstein P, Lawler J, Benjamin L E. Thrombospondin-1 modulates VEGF-A-mediated Akt signaling and capillary survival in the developing retina. Am J Physiol Heart Circ Physiol. 2009;296:H1344–H1351. doi: 10.1152/ajpheart.01246.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K, Gupta P, Wild R, Ramakrishnan S, Hebbel R P. Binding and displacement of vascular endothelial growth factor (VEGF) by thrombospondin: effect on human microvascular endothelial cell proliferation and angiogenesis. Angiogenesis. 1999;3:147–158. doi: 10.1023/a:1009018702832. [DOI] [PubMed] [Google Scholar]
- Ling Y, Yang Y, Lu N, You Q D, Wang S, Gao Y, Chen Y, Guo Q L. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys Res Commun. 2007;361:79–84. doi: 10.1016/j.bbrc.2007.06.155. [DOI] [PubMed] [Google Scholar]
- Seo D W, Li H, Guedez L, Wingfield P T, Diaz T, Salloum R, Wei B Y, Stetler-Stevenson W G. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik A B. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Nagy P D, Pogany J. Multiple roles of viral replication proteins in plant RNA virus replication. Methods Mol Biol. 2008;451:55–68. doi: 10.1007/978-1-59745-102-4_4. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Martin-Manso G, Maxhimer J B, Roberts D D. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat Rev Cancer. 2009;9:182–194. doi: 10.1038/nrc2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J, Miao W M, Duquette M, Bouck N, Bronson R T, Hynes R O. Thrombospondin-1 gene expression affects survival and tumor spectrum of p53-deficient mice. Am J Pathol. 2001;159:1949–1956. doi: 10.1016/S0002-9440(10)63042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee KO, Connolly CM, Duquette M, Kazerounian S, Washington R, Lawler J. The effect of thrombospondin-1 on breast cancer metastasis. Breast Cancer Res Treat. 2009;114:85–96. doi: 10.1007/s10549-008-9992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaked Y, Bertolini F, Man S, Rogers M S, Cervi D, Foutz T, Rawn K, Voskas D, Dumont D J, Ben-David Y, Lawler J, Henkin J, Huber J, Hicklin D J, D'Amato R J, Kerbel R S. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; Implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7:101–111. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Scheef E A, Huang Q, Wang S, Sorenson C M, Sheibani N. Isolation and characterization of corneal endothelial cells from wild type and thrombospondin-1 deficient mice. Mol Vis. 2007;13:1483–1495. [PubMed] [Google Scholar]
- Wang Y, Su X, Wu Z, Sheibani N. Thrombospondin-1 deficient mice exhibit an altered expression pattern of alternatively spliced PECAM-1 isoforms in retinal vasculature and endothelial cells. J Cell Physiol. 2005;204:352–361. doi: 10.1002/jcp.20290. [DOI] [PubMed] [Google Scholar]
- Geretti E, Shimizu A, Klagsbrun M. Neuropilin structure governs VEGF and semaphorin binding and regulates angiogenesis. Angiogenesis. 2008;11:31–39. doi: 10.1007/s10456-008-9097-1. [DOI] [PubMed] [Google Scholar]
- Hoffman R. Do the signalling proteins for angiogenesis exist as a modular complex? The case for the angosome. Med Hypotheses. 2004;63:675–680. doi: 10.1016/j.mehy.2004.01.038. [DOI] [PubMed] [Google Scholar]