Abstract
Here, we describe the zebrafish (Danio rerio) as a vertebrate model system to study liver regeneration with the added benefit of its powerful genetics and screening possibilities to uncover the molecular pathways underlying liver regeneration. We developed a partial hepatectomy (PH) protocol in zebrafish and investigated in detail the cellular and morphological changes during the process of liver regeneration. We show that the type of regenerative response is dependent on the size of the injury sustained by the zebrafish liver. Furthermore, we demonstrate for the first time that the mechanisms of liver regeneration in zebrafish after PH are strikingly similar to those of rodents and humans, with 100% recovery of the liver mass after 6–7 d postsurgery. This occurs via compensatory growth mediated by proliferation of hepatocytes throughout the entire liver remnant. By analyzing transgenic fish expressing dominant-negative forms of either bone morphogenetic protein (BMP) receptor or fibroblast growth factor (FGF) receptor 1, we demonstrate that the BMP and FGF signaling pathways are crucial regulators of the early events during liver regeneration after PH. Our study demonstrates that the mechanisms of liver regeneration in zebrafish are highly similar to the processes ongoing during mammalian liver regeneration and make the adult zebrafish a suitable model system to study the mechanisms of liver regeneration.—Kan, N. G., Junghans, D., Belmonte, J. C. I. Compensatory growth mechanisms regulated by BMP and FGF signaling mediate liver regeneration in zebrafish after partial hepatectomy.
Keywords: hepatocyte proliferation, liver-to-body weight ratio, liver mass restoration, Danio rerio
The liver of higher vertebrates, including humans, can precisely regulate its growth and mass and possesses the remarkable feature to regenerate even after massive tissue loss. Nevertheless, an inability to regenerate damaged liver tissue is observed in diseases such as cirrhosis or hepatitis, which are often accompanied by the development of hepatocellular carcinomas, the fifth most common cancer in the world. In mammals, the liver regenerates in a compensatory fashion, which means that the original liver weight is regained by a compensatory growth of the remaining liver tissue (1, 2). This process is mainly mediated by proliferation of hepatocytes that reenter the cell cycle. This is in contrast to epimorphic regeneration, where damaged or lost tissue is directly restored. Extensive studies (2, 3) in rodents have uncovered a role for cytokines, growth factors, and cellular events as major players in regulating the complex process of liver regeneration. However, the initial signal that triggers the molecular machinery of regeneration in the liver and the mechanisms that regulate the restoration of the precise liver size remain largely unknown.
The zebrafish model represents a well-established genetic model to study molecular mechanisms of vertebrate development (4, 5). To take advantage of this excellent genetic model system and extend it into the area of regenerative hepatology and hepatopathology, we aimed to establish more powerful methods to study liver regeneration in zebrafish. In mice and rats, a 2/3 partial hepatectomy (PH) is commonly used to study hepatocyte-mediated liver regeneration. After surgical removal of 60–70% of the liver mass, hepatocytes, which under normal conditions are quiescent, synchronously reenter the cell cycle and begin to proliferate, with a peak of proliferation between 12–48 h postsurgery, depending on the species studied (6). Within the following 7 d, the original liver mass is completely reconstituted by this compensatory growth. Zebrafish, in contrast, are known for their ability for epimorphic regeneration, which means amputated fins, heart, spinal cord, and retina can regrow via an intermediate step, the formation of a blastema, a mass of undifferentiated cells that finally differentiate again and reconstitute the lost tissue (reviewed in ref. 7). To use the zebrafish as a model organism for liver regeneration studies and to be able to extrapolate this knowledge to higher vertebrates, including humans, we examined whether the zebrafish liver regenerates in a manner similar to that described for mammals. Previous studies (8, 9) addressed this question only marginally, and the chosen experimental setup did not allow a full description of the regenerative capacity of the zebrafish liver.
Here, we developed an extended PH technique and a liver weight analysis protocol in adult zebrafish and describe for the first time in detail the cellular and morphological events in the regenerating zebrafish liver in response to a 1/3 PH and discuss our findings in light of the previously published protocols. We show that, in contrast to previous studies and other organs that are known to regenerate epimorphically, the zebrafish liver regains its original organ mass via compensatory growth mechanisms similar to those of the mammalian liver. We demonstrate that after our PH protocol, hepatocytes throughout the entire zebrafish liver reenter the cell cycle and reconstitute the presurgical liver mass. The dynamics of liver mass restoration were found to be strikingly similar to those of mammals. In contrast to the effect seen after PH, which leads to a compensatory growth of the remaining liver, we found in addition that local injuries result only in local proliferative responses, again mediated by hepatocytes.
To demonstrate that the molecular events during zebrafish regeneration after PH are also comparable to those in mammals, we analyzed the bone morphogenetic protein (BMP), fibroblast growth factor (FGF), and Wnt/β-catenin signaling pathways, which have recently been demonstrated to be involved in the regenerative response of the liver in mice and humans and are important regulators of liver development in zebrafish (10,11,12,13,14). We also show that zebrafish liver regeneration after PH is heavily impaired in the absence of either BMP or FGF signaling and demonstrate that BMP signaling is able to directly regulate proliferation of hepatocytes in vitro, supporting the hypothesis that growth factors are crucial key players in regulating liver regeneration. Finally, we also provide evidence that the Wnt/β-catenin pathway is activated during regeneration after PH in zebrafish.
This study demonstrates that the mechanisms of liver regeneration in zebrafish are strikingly similar to the processes taking place during liver regeneration in mammals.
MATERIALS AND METHODS
Animal strains
All zebrafish used in this study were between 4 and 12 mo of age. The following lines were used: AB, Wlk, and Ekkwill wild-type strains and transgenic fish lines Tg(hsp70l:dnbmpr-GFP) (15) and Tg(hsp70l:dnfgfr1-EGFP) (16). All animals were maintained and crossed according to standard methods (17).
PH, surgeries, and liver weight analysis
Adult zebrafish were deprived of food 24 h before surgery. Fish were anesthetized in 0.015% Tricaine solution for 1 min and placed on a wet sponge with the ventral side up, and the scales were removed from the ventral body wall using forceps. Next, the ventral body wall was opened by a 3–4 mm incision. The ventral liver lobe was carefully pulled out of the peritoneal cavity and resected at the very base of the lobe. Special care was taken to resect the whole ventral lobe without damaging other areas of the remaining liver tissue. Removal of the ventral lobe led to a 30% PH. For local, incomplete PH and surface scratch experiments, the ventral lobe was exposed, and a 0.5-mm piece of liver tissue from the tip of the lobe was resected, or a small scratch (0.5–1 mm) was introduced to the liver surface using sharp forceps. The remaining liver was placed back carefully into the peritoneal cavity. Finally, the body wall was closed with Nexaband liquid topical tissue adhesive (5295-04-01; Abbott Laboratories, Abbott Park, IL, USA). The animals were placed into fresh fish water and monitored for full recovery for 2–4 h at room temperature before transfer to 28°C water tanks. Sham-treated animals were subjected to the same procedure excluding liver resection. The postoperative survival rate was ∼90%, with most deaths occurring on the day of surgery.
Transgenic zebrafish lines were subjected to heat shock (37°C for 12 h) directly before and every following night after surgery until the fish were analyzed. Transgenic fish were identified by ultraviolet light scanning, and only GFP-positive animals were subjected to surgery. Control wild-type animals were subjected to the same heat-shock protocol. For analysis, animals were anesthetized in tricaine solution, and livers were dissected out and processed. All fish were weighed directly before and after surgery. All animals were placed on dry KimWipe paper (Kimberley-Clark, Irving, TX, USA) before weighing to remove excess water. Dissected livers were also carefully dried on paper (KimWipe) and weighed on an analytical balance.
For bromodeoxyuridine (BrdU) incorporation experiments, 25 μl of BrdU solution (5 mg/ml PBS) was injected intraperitoneally 8 h before liver dissection.
Immunohistochemistry
Zebrafish livers were fixed in 4% paraformaldehyde (PFA) overnight at 4°C, washed in PBS, dehydrated, and embedded in paraffin. Histological staining of 7-μm-thick paraffin sections was performed according to standard protocols. Immunohistochemical analysis on paraffin sections was performed as described elsewhere (18). Antibodies were as follows: anti-proliferating cell nuclear antigen (PCNA) (P8825; Sigma, St. Louis, MO, USA), anti-Prox1 (AB5475; Chemicon, Temecula, CA, USA), anti-BrdU (OBT0030; Accurate Chemicals, Westbury, NY, USA), anti-β-catenin (610153; BD Biosciences, San Jose, CA, USA), and Alexa 488 and 596 coupled anti-rabbit and anti-mouse (Molecular Probes, Eugene, OR, USA).
Zebrafish liver cell culture
Liver cell cultures were established as described elsewhere, with minor modifications (19). In brief, adult male zebrafish were deeply anesthetized in Tricaine solution and rinsed with 70% ethanol and sterile PBS. Livers were dissected, rinsed with PBS, and incubated in 0.25% trypsin-EDTA solution (Life Technologies, Inc., Carlsbad, CA, USA) for 5 min at room temperature. The tissue was triturated to a single-cell suspension by gentle pipetting and collected by centrifugation (500 g, 5 min). Liver cells were resuspended and plated in LDF (50% Leibovitz’s L-15, 35% Dulbecco’s modified Eagle’s, and 15% Ham’s F12) medium (19) supplemented with 5% heat inactivated fetal bovine serum (Life Technologies, Inc.), 50 ng/ml epidermal growth factor (R&D Systems, Minneapolis, MN, USA), 0.01 mg/ml insulin (Sigma), 15 mM HEPES, and 0.15 g/L sodium bicarbonate. Liver cells from wild-type and dominant-negative (dn)BMPR transgenic fish were cultured in 100% atmospheric air, either at 28°C (normal culture conditions) or heat shocked at 37°C (for 8 h every day) to induce expression of dnBMPR. Twenty-four hours after plating, cells were treated with the following recombinant proteins: 1) BMP2; 2) Noggin; or 3) BMP2 + Noggin (provided by Dr. Senyon Choe, Salk Institute) at different concentrations for 2–4 d. On the day of final analysis, BrdU was added to the medium for 4 h (final concentration 0.5 mg/ml). Finally, cells were washed with PBS, fixed with 4% PFA, and subjected to immunofluorescence analysis. All experiments were performed in independent triplicates.
RESULTS
Morphology of the zebrafish liver
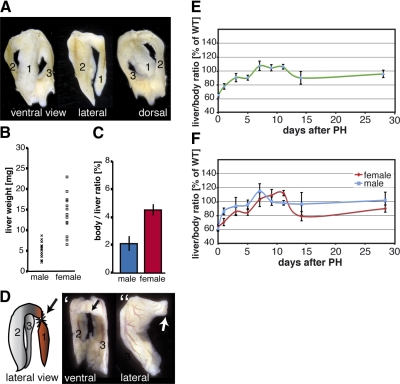
The adult zebrafish liver consists of 3 lobes. Besides the ventral lobe (Fig. 1A, lobe 1), there are two more dorsal lobes (Fig. 1A, lobes 2 and 3). Sometimes fusion of the two dorsal lobes at their posterior end can be observed (Fig. 1A). Because of its soft and gel-like consistency, dissection of nonfixed zebrafish liver tissue often leads to a loss of one of the dorsal lobes. This might explain the differences to previous studies in which the zebrafish liver has been described as a bilobed organ (9). However, all zebrafish analyzed in this study (∼200) exhibited the morphology of a trilobed organ. Furthermore, to exclude strain-specific differences, 3 wild-type strains were analyzed, and no differences in the number of liver lobes were found.
Figure 1.
Zebrafish liver regeneration after PH. A) Morphology of an intact trilobed zebrafish liver is shown from 3 different views. Right dorsal lobe (2) and left dorsal lobe (3) are located behind the ventral lobe (1). Dorsal liver lobes are often fused at their posterior ends. B) Weight of individual zebrafish livers ranged from 2.2 to 8.7 mg (n=15) for males and from 6.5 to 22.9 mg (n=13) for females. C) Average LBR in zebrafish is 2.10 ± 0.65% for males and 4.51 ± 0.40 for females. Significant difference in LBR between males and females (P<0.001, Student’s t test) indicates a sexual dimorphism in zebrafish. D) Cartoon demonstrating the site of surgery for 1/3 PH in zebrafish. Ventral lobe (1) is resected at the anterior base of the lobe. Dorsal lobes (2, 3) remain intact. D′, D″) Ventral (D′) and lateral (D″) views of the surgically treated liver. Arrows indicate site of resection. E) Dynamics of zebrafish liver mass restoration after PH (male and female fish). LBR of ≥5 animals was determined at d 1, 3, 5, 7, 9, 11, 14, and 28 postsurgery. All values were normalized to the LBR of sham-treated fish. F) Analysis of liver mass recovery after PH with male and female animals separated. Error bars = sd.
Zebrafish liver-to-body weight ratio (LBR) is a constant parameter
In mammals, PH leads to a compensatory growth of the liver until the original organ weight is regained (1, 3). Since the liver weight varies between different animals, the standard parameter used to study liver regrowth is the LBR, which has been shown to be constant within species, including mice and humans (6). To be able to use this parameter in zebrafish liver regeneration studies, we evaluated two parameters: absolute liver mass and the LBR of individual zebrafish (Fig. 1B, C). Absolute liver mass of 13 females and 15 males was determined, and we observed dramatic variations among individual specimens (Fig. 1B). This variance makes it difficult to use this parameter to monitor liver regeneration reliably. However, we found that, as in other species, the zebrafish LBR is constant. (Fig. 1C). We did not find age-dependant variations of the LBR when comparing animals of different ages (12 and 4 mo, data not shown). Interestingly, and similar to absolute liver mass values, we observed a difference in LBR between males and females. In males, the liver constitutes ∼2.10% of total body weight, whereas in females, the liver is ∼4.51% of the total body weight, indicating that the LBR displays sexual dimorphism in zebrafish. Due to these results, we decided to use the LBR as a parameter to study liver regeneration in zebrafish, with males and females separated in all further studies (see Materials and Methods for the precise procedure).
PH
PH in mammals involves the resection of ∼2/3 of the liver. In our study, we removed the whole zebrafish ventral lobe, which results in a loss of ∼1/3 of the liver weight as monitored by calculating the LBR. The cartoon in Fig. 1D shows a zebrafish liver from a lateral view and the cut site used to remove the ventral lobe. Figure 1D′, D″ shows ventral and lateral views of a zebrafish liver after resection of the ventral lobe (see Fig. 1A for intact livers with different views). After surgery, the animals moved slower and became less active for the first 1–2 h. After this period, no behavioral differences were observed between nontreated and treated zebrafish (sham surgery and hepatectomy). The survival rate was >90% after overnight recovery for both sham-treated (45/49) and hepatectomized animals (76/81). The removal of 2 liver lobes (ventral and either the left or right dorsal lobe, resulting in a 2/3 hepatectomy) led to strong bleeding and, most likely, dramatic perturbations in blood circulation accompanied by a dramatic reduction in overnight survival rates (1/14), with no survivors after 2 d of recovery. Therefore, we used the 1/3 PH protocol for all further studies.
Zebrafish liver mass restoration after PH
To follow liver regeneration and anatomical changes after PH, animals were sacrificed at different time points after surgery. For control experiments, sham surgeries were performed by opening and closing the body wall without perturbing the liver. Due to its gel-like consistency, it is difficult to dissect the entire liver without damaging or losing parts of the tissue. Therefore, we applied in situ fixation with 4% PFA before organ resection. This technique allowed organ preservation for anatomical and histological analysis and allowed us to dissect out the livers without causing organ damage. Representative images of intact and hepatectomized livers are shown in Fig. 1A, D, respectively.
To study liver mass recovery after PH, adult animals were sacrificed at d 0, 1, 2, 3, 4, 5, 7, 11, 14, and 28 after surgery and at least 5 animals were analyzed for each time point of each group. As shown in Fig. 1E, immediately after surgery LBRs were reduced to ∼65% compared with the LBR of control animals, indicating a 1/3 PH. However, within 7 d postsurgery, the liver mass returned to normal levels, as calculated by monitoring the LBR. In the following 2–3 d, a further increase in liver mass was observed, resulting in 10% higher LBRs than controls. This increase was followed by a LBR decrease to 90% in regenerating animals by d 14 postsurgery. Finally, 28 d after surgery, the LBR of hepatectomized animals exhibited values comparable to control animals. This dynamic of liver mass recovery in zebrafish after PH is remarkably similar to liver regeneration processes seen in mammals, where regenerating livers also exhibit an overshooting of the liver mass, followed by a weight fine-tuning before reaching the original liver mass (20). We next evaluated whether the dynamic of liver mass recovery is different for male and female zebrafish (Fig. 1F). As show in Fig. 1F, both males and females follow similar dynamics of liver mass recovery. However, the regeneration process appears to be slightly delayed in female zebrafish. Nonetheless, both males and females reach 100% recovery of liver weight between d 6 and 7 after the hepatectomy.
PH results in a compensatory growth of hepatocytes in zebrafish liver
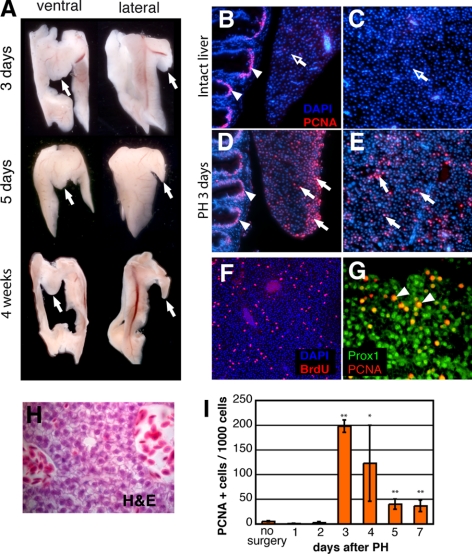
Liver regeneration in mammals occurs in a compensatory fashion, and ablated liver lobes never grow back. The original liver mass and function are reached by compensatory growth of the remaining liver lobes, a process mainly mediated by hepatocytes that reenter the cell cycle (20, 21). Therefore, we addressed the question of how the zebrafish liver recovers after 30% PH and analyzed the liver morphology at different time points after resection. We observed that after PH, the resected ventral lobe was absent at all stages analyzed. Figure 2A shows representative pictures of zebrafish livers at 3, 5, and 28 d postsurgery. Because we never observed a reappearance of the resected ventral lobe in any of the analyzed animals (we analyzed fish up to 6 mo post-PH, not shown), we concluded that the zebrafish liver, similar to that of mammals, also regenerates in a compensatory fashion.
Figure 2.
One-third PH results in compensatory regeneration of zebrafish liver. A) Gross morphology of a zebrafish liver at d 3, 5, and 28 after PH, with ventral and lateral views. Arrows indicate site of resection at base of the ventral lobe. Note that the resected lobe does not show signs of regrowth, even 1 mo after 1/3 PH. B–E) Anti-PCNA immunostaining of intact (sham surgery; B, C) and regenerating (D, E) livers 3 d after 1/3 PH. In the intact livers, the tip of the ventral lobe (B), the outer area of the base of the ventral liver lobe (not shown), and the inner dorsal liver lobe parenchyma (C) only rarely showed anti-PCNA-positive cells (open arrows). Transient amplifying cells in the gut crypts serve as an internal positive control (B, D; arrowheads). One-third PH induces proliferation throughout the whole liver (D, E). Anti-PCNA-positive cells are seen at the site of resection (D) and in the liver parenchyma of the dorsal lobes (E, white arrows). F) Strong BrdU labeling of liver parenchyma of the dorsal lobe is seen 3 d after 1/3 PH. G) Coimmunostaining reveals that almost all PCNA-positive cells also costained for Prox1 (arrowheads). H) H&E staining after PH reveals the typical polygonal cell shape and large basophilic nuclei of hepatocytes. I) Dynamics of cell proliferation after PH were determined by analyzing the number of proliferating cells at various time points post-PH. At least 3 animals for each individual time point were hepatectomized, and 30–40 sections covering all areas of each hepatectomized liver were analyzed by anti-PCNA/DAPI staining. Proliferation index: number of PCNA positive nuclei/1000 cells; 100-fold increase in PCNA-positive cells from d 2–3 after PH is statistically highly significant. *P < 0.05, **P < 0.01; Student’s t test. Error bars = sd.
Next, to substantiate these findings, we analyzed cell proliferation after PH. Zebrafish were subjected to PH and sacrificed at different time points after PH, and their livers were analyzed. The complete livers were sectioned, and proliferating cells were detected by PCNA immunohistochemistry. Representative pictures are shown in Fig. 2B–E. In control livers, we rarely observed PCNA-positive cells. Both areas close to the surface of the lobes (Fig. 2B) as well as the parenchyma in deeper areas contained only a very few proliferating cells (Figs. 2C and 3A). In contrast, widely distributed and strong anti-PCNA staining was observed in all areas of hepatectomized livers (Figs. 2D, E and 3B, C). The strong PCNA labeling of proliferating cells in the gut served as an internal control. Comparable results were obtained by BrdU incorporation studies, which also showed strong proliferation in the dorsal lobes distant from the cut site at the ventral lobe (Fig. 2F). These findings are in agreement with findings in mammals, where the liver has been described as a quiescent organ that begins to proliferate strongly after PH (1, 3). Furthermore, we observed that again, similar to mammals, hepatocytes are the main cell type that begin to proliferate in response to PH as shown by colocalization of PCNA-positive cells with Prox1, a hepatocyte-specific transcription factor, 3 d after PH (Fig. 2G; ref. 22). In response to PH, hepatocytes in mammals reenter the cell cycle, with a peak of cell proliferation 24–48 h after PH (6). To determine the proliferation profile of the zebrafish liver, we analyzed the number of proliferating cells at various time points after PH. For each liver, ≥30 sections covering all areas of the liver were analyzed by anti-PCNA/DAPI staining and imaged, and a proliferation index was calculated (number of PCNA-positive nuclei per 1000 cells; Fig. 2I). We observed no significant increase in the number of PCNA-positive cells during the first 2 d after removal of the ventral lobe. However, by d 3 after PH, the number of PCNA-positive cells increased dramatically (∼100-fold) and declined again slowly during the following 4 d. At d 7 after PH, we still observed on average 40 PCNA-positive cells/1000 cells, compared with 1–3 cells under control conditions. Again, these results are in strong agreement with the mammalian model, where PH induces a synchronous and robust proliferative response of hepatocytes, with a species-specific timing for the peak of proliferation (6).
Figure 3.
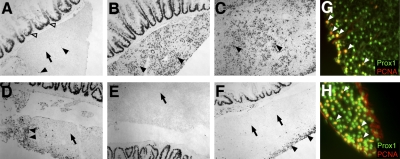
PH in zebrafish may induce two different regenerative responses. A–F) Anti-PCNA immunostaining on liver sections. A) Intact liver after sham surgery. Some proliferating cells are seen in intact livers (arrowheads). However, most areas of the liver show no signs of proliferation (arrow). Proliferating cells in the gut serve as internal control (open arrowheads). B) Strong increase in cell proliferation (arrowheads) is seen at base of ventral lobe 3 d after 1/3 PH. C) Dorsal lobe 3 d after 1/3 PH of ventral lobe. Strong cell proliferation (arrowheads) is seen in areas far away from the site of surgery. D) Site of resection at ventral lobe after removal of posterior tip of ventral lobe (6–8% PH). Strong proliferation is only observed directly at the cut site (arrowheads). Areas of ventral lobe distal from the cut site show only very little proliferation (arrow). E) Dorsal lobe after removal of posterior tip of ventral lobe (6–8% PH) shows no signs of increased proliferation (arrow). F) Ventral lobe 3 d after surface of lobe was scratched. An increase in cell proliferation is only seen in the vicinity of the injured liver surface (arrowheads). Distal areas show no increase in proliferation (arrows). G) Double immunohistochemistry after scratching the ventral liver lobe surface. Most proliferating cells are found close to the scratch side and appear to be Prox1-positive hepatocytes (arrowheads). H) Double immunohistochemistry using anti-Prox1 and anti-PCNA after removal of posterior tip of ventral lobe (6–8% PH). Most proliferating cells are Prox1-positive hepatocytes (arrowheads).
Local injury in the zebrafish liver induces a local regenerative response
In previous studies (9), it has been postulated that PH in zebrafish leads to an increase in PCNA-positive cells exclusively at the site of resection. However, we observed in our studies an increase in PCNA-positive proliferating cells throughout the remaining liver lobes after resection of the complete ventral lobe resulting in a 30% hepatectomized liver. In contrast to controls, which very rarely showed proliferating cells in intact liver tissues (Fig. 3A), a strong increase in the number of proliferating cells in the parenchyma of both uncut dorsal lobes far away from the site of resection was observed at d 3 post-PH (Fig. 3B, C). BrdU incorporation studies after PH showed similar results (Fig. 2F and data not shown). Since the resection of 30% of the liver mass led to a proliferative response throughout the remaining lobes, we asked whether smaller, local injuries would induce a different regenerative response. Therefore, we removed only the tip of the ventral lobe, which represents ∼10–15% of the ventral lobe (∼3–4% of the total liver mass). Anti-PCNA analysis of the liver 3 d after removal of the ventral liver tip showed a strong increase in the number of proliferating cells at the cut site of the ventral lobe (Fig. 3D). However, little anti-PCNA staining was found in deeper areas of the ventral lobe (Fig. 3D). Furthermore, we did not find any increase in cell proliferation in the dorsal lobe (Fig. 3E). Similar results, with no increase in cell proliferation in areas far away from the cut site and a strong increase in the vicinity of the cut site, were obtained at d 5 and 7 after removal of the ventral liver tip (data not shown). Most cells that reentered the cell cycle were identified as hepatocytes, as demonstrated by anti-Prox1 immunohistochemistry (Fig. 2H). Next, we introduced local injuries at the ventral liver lobe surface by scratching the tissue with forceps, analyzed the livers 3 d later, and obtained similar results as with the removal of the ventral lobe tip. Again, cells reentered the cell cycle in close vicinity to the scratched surface (Fig. 3F) and were found to be mostly hepatocytes (Fig. 3G). Also comparable to the previous experiment, very little anti-PCNA staining was found in deeper areas of the ventral lobe and, as in controls, almost no staining was observed in dorsal lobes (Fig. 3F and data not shown). These findings demonstrate that the type of regenerative response of the zebrafish liver is dependent on the size of the injury sustained by the liver.
BMP, FGF, and Wnt/β-catenin signaling pathways are involved in zebrafish liver regeneration
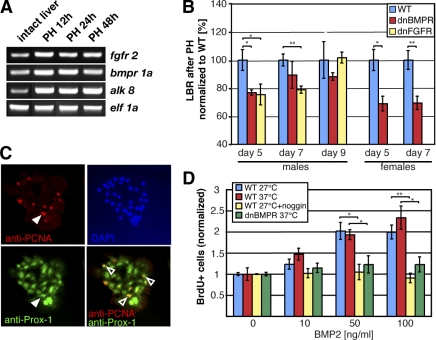
In mammals, several signaling pathways have been shown to be involved in regulating liver regeneration after PH, including BMP, FGF, and Wnt signaling, which are capable of modulating hepatocyte proliferation (10,11,12,13, 23, 24). Moreover, all 3 pathways were also recently identified as important regulators of liver development in the zebrafish embryo (14, 25). Therefore, we first analyzed the presence of FGF receptor (FGFR)and BMP receptor (BMPR) in the adult zebrafish before and after PH. RT-PCR analysis revealed that mRNAs of the corresponding receptors could be found in the zebrafish liver (Fig. 4A). To investigate whether BMP and FGF signaling pathways are also involved in zebrafish liver regeneration and to prove that our zebrafish PH model system can be used to address these kinds of questions, we analyzed the liver regeneration potential of two transgenic zebrafish lines. Both transgenic lines express, in an inducible manner, dominant-negative forms of either BMPR or FGFR. Both dnBMPR and dnFGFR1 proteins have been demonstrated to block most and possibly all BMP and FGF signaling, respectively (15, 16, 26, 27).
Figure 4.
BMP and FGF signaling pathways are involved in liver regeneration in zebrafish. A) RT-PCR demonstrating the presence of FGFR2, BMPR1a, and ALK 8 mRNA in zebrafish liver before and after PH. B) Liver mass reconstitution after PH in two transgenic fish lines, hs70::dnBMPR-GFP and hs70::dnFGFR1-eGFP. LBR of ≥5 animals was determined at d 5, 7, and 9 after PH. Males and females were analyzed separately; heat-shock-treated wild-type was used as control. All values were normalized to PH heat-shock-treated wild-type fish. Male transgenic fish expressing dnFGFR1 show a significant decrease of LBR compared with control animals at d 5 (75.4±7.4%) and d 7 after PH (78.8±2.5%). In the case of the dnBMPR mutant fish, liver recovery is significantly decreased at d 5 (76.8±1.9%) and appeared reduced at d 7 and 9 (88.2 and 89.4%), however, without statistical significance. Female transgenic dnBMPR fish analyzed at postoperative d 5 and 7 also show decreased LBR (69±5.3% at d 5; 69.5±4.7% at d 7). C) Zebrafish liver cells cultured for 5 d and stained with anti-PCNA and anti-Prox1 antibodies. White arrowheads show proliferating cells; open arrowheads show colocalization of PCNA with Prox1 in hepatocytes. D) BMP signaling enhances proliferation of zebrafish hepatocytes in vitro. Zebrafish liver cells were treated with 0, 10, 50, or 100 ng/ml BMP2 for 4 d. Cell proliferation index: ratio of BrdU-positive cells to total number of cells in 10 field images. Each experiment was repeated at least 3 times. Fold differences in cell proliferation indices between nontreated and BMP2-treated cells are shown. Noggin was given in double concentrations (20, 100, or 200 ng/ml). *P < 0.05, **P < 0.01; Student’s t test.
Both transgenic fish and controls were subjected to 37°C overnight heat shock to induce the expression of dnBMPR or dnFGFR1, respectively. The onset of expression was monitored by the expression of a GFP reporter, and only GFP-positive fish were used for PH experiments. PHs were performed on both heat-shocked transgenic and heat-shocked control wild-type fish. Animals subjected to overnight heat shock followed by PH showed slightly lower recovery than animals surgically treated without prior heat shock. All fish were subjected daily after PH to overnight heat shock before final analysis, and expression driven from the heat-shock-sensitive promoter was monitored under ultraviolet light. We analyzed liver mass regeneration by measuring the LBR at different time points after 1/3 PH (Fig. 4B). As shown in Fig. 4B, 5 d after PH, LBRs in both male dnBMPR and dnFGFR1 transgenic fish were remarkably lower compared with wild-type heat-shock-treated animals. At d 7 after PH, fish expressing dnFGFR1 still had reduced LBRs of ∼20% compared with controls. dnBMPR transgenic fish also showed reduced liver mass at d 7, although the difference was not statistically significant, due to the large variation seen within these fish. By d 9 both transgenic and control fish had comparable LBRs. To clarify that the effect seen at d 5 and 7 in the transgenic lines is not sex specific, we also analyzed female fish with inhibited BMP signaling (Fig. 4B). As expected, female dnBMPR transgenic fish also showed a remarkably reduced liver mass 5 and 7 d after 1/3 PH. In summary, these data indicate that in zebrafish, FGF and BMP signaling also contribute to the correct orchestration of liver mass recovery after PH.
To determine whether BMP signaling is capable of regulating hepatocyte proliferation, we analyzed liver cell proliferation in vitro. Under standard culture conditions, adult zebrafish liver cells begin to reenter the cell cycle 3 d after plating, with a peak of proliferation at d 4–5, as analyzed by BrdU incorporation studies (data not shown). To assess a direct role of BMP signaling on cell proliferation, we treated zebrafish liver cells 24 h after plating for 4 d with different concentrations of recombinant human BMP2 and analyzed cell proliferation by BrdU incorporation (4 h BrdU pulse; Fig. 4D). In the presence of BMP2, cultured adult liver cells revealed increased proliferation. Both non-heat-shock- and heat-shock-treated wild-type cultures showed a 1.8- to 2.2-fold increase in the number of BrdU-positive liver cells with 50 and 100 ng/ml of BMP2. However, the effect on cell proliferation was completely abolished when BMP2 was given together with the BMP antagonist Noggin or when liver cells expressing a dnBMPR were used (Fig. 4D). We again identified hepatocytes as the major cell type that started to proliferate after addition of BMP2 (Fig. 4C), indicating that BMP signaling is able to regulate proliferation of hepatocytes in vitro and also during regeneration in the zebrafish liver.
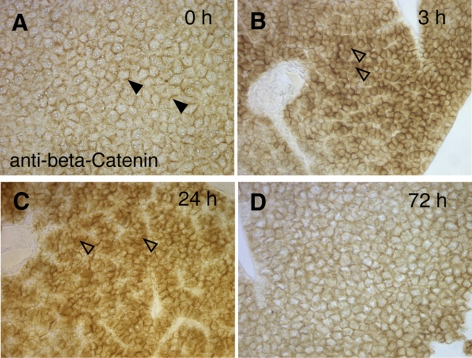
A third signaling pathway playing crucial roles during liver development and liver regeneration is the Wnt/β-catenin pathway (25, 28). In rats and mice, it has been shown that after 70% liver hepatectomy, β-catenin is translocated to the cytosol/nucleus, a hallmark of active canonical Wnt signaling (23, 24). In intact livers, β-catenin is found almost exclusively at the cell membrane in a complex with cadherins to form adhesion junctions. To test whether Wnt signaling is also activated in the zebrafish liver after PH, we performed immunohistochemistry to monitor β-catenin localization before and after PH (Fig. 5). Similar to rodents, we found a transient activation of canonical Wnt signaling in the zebrafish after PH, as shown by the cytosolic/nuclear immunostaining for β-catenin in hepatocytes (Fig. 5B, C). Seventy-two hours after PH, only little cytosolic/nuclear β-catenin was found in hepatocytes (Fig. 5D). In control animals, β-catenin was found almost exclusively localized to the cell membrane.
Figure 5.
PH in zebrafish induces cytoplasmic accumulation of β-catenin. A) Nonhepatectomized liver demonstrates membrane localization of β-catenin. Almost no cytoplasmic β-catenin is observed. B) At 3 h after PH, increased cytoplasmic β-catenin is observed throughout liver lobes (open arrowheads). C) Majority of hepatocytes display high cytoplasmic staining for β-catenin 24 h postsurgery (open arrowheads). D) Decreased cytoplasmic staining for β-catenin is seen 72 h postsurgery. All sections were stained in parallel under the same conditions.
DISCUSSION
The 2/3 PH in mice represents the best established mammalian liver regeneration model. We aimed to establish a liver regeneration model in zebrafish to take advantage of the powerful zebrafish genetics and its excellent eligibility for both genetic and compound screenings to study the molecular mechanisms of liver regeneration. The size of the zebrafish liver varies between different animals, as does the animal size itself. However, liver regeneration after PH is often studied by taking advantage of the fact that the LBR is constant in many species. Our finding that the LBR is also constant in zebrafish allowed us to combine this parameter with PH protocols to study liver regeneration in zebrafish.
The zebrafish liver is composed of 3 lobes, and subjecting it to a 2/3 hepatectomy surgery, similar to what is done in mammals, led to a massive loss of blood shortly after resection, leading to 100% lethality within 48 h postsurgery. Therefore, we decided to remove only the ventral lobe, which results in a 1/3 hepatectomized liver. Afterward, the surgical procedure led to a strong increase in survival rates and allowed us to analyze regeneration after PH. We could demonstrate that the zebrafish liver responds to a loss of 1/3 of its liver mass by compensatory growth mediated by hepatocytes, which leads to a complete restoration of the liver mass within 7 d after surgery. In addition, we observed that the dynamics of liver mass restoration are also similar to those of mammals, with an increase above the preoperative liver mass, followed by fine-tuning in the following days (20). Our observation that a 1/3 liver hepatectomy leads to an onset of proliferation throughout the entire liver is in contrast to an earlier study (9) that showed a regrowth of the amputated tissue within 7 d. The authors observed massive proliferation localized to the site of resection. However, the authors also documented some compensatory proliferation of hepatocytes distant to the cut site. This discrepancy led us to investigate whether the zebrafish liver is able to respond with different regenerative programs dependent on the injury size. It is documented that in mice and rats, the type of liver proliferative response is dependent on the degree of liver mass loss, with small local liver injuries leading to local response and a liver mass loss of >30% resulting in a strong regenerative response throughout the entire liver remnant and regenerative factors circulating in the bloodstream (20). We believe that similar mechanisms are also present in the zebrafish liver, based on 3 experiments: 1/3 PH led to global liver proliferation response and local surface injuries, and removal of the tip of a liver lobe resulted in local hepatic proliferative response at the site of injury. These results are in agreement with the results of Sadler et al. (9) that described regrowth of the resected tissue. However, the authors describe in their study a PH protocol based on the assumptions that the adult zebrafish is a bilobed organ (1 ventral and 1 dorsal lobe). We have shown that the zebrafish liver is a trilobed organ composed of 1 ventral and 2 dorsal lobes. This discrepancy might result from different dissection methods, since liver dissection before fixation often leads to a loss of one of the dorsal lobes due to its gel-like structure. We presume that in this previous study only local injuries were introduced by resecting a piece of liver tissue, based on the assumption of a bilobed zebrafish liver, that was too small to induce a strong global liver response (9). Local injuries sustained by the zebrafish liver, such as removal of the tip of a lobe or scratching the liver surface, result in a localized increase of the liver mass. Many organs in zebrafish, such as fins and heart, are able to regenerate in an epimorphic fashion, which includes the formation of a blastema. Our study shows that induction of local injuries induces proliferation of hepatocytes. We observed only very rarely proliferating and Prox1-negative cells close to the site of injury, very similar to the findings in mammals. Therefore, we conclude that liver regeneration after local injury is also similar to that of mammals, which is hepatocyte mediated and not of an epimorphic/blastema-mediated nature. Nevertheless, the precise mechanisms of this process in zebrafish need to be analyzed in more detail. However, removal of >30–40% of the total zebrafish liver mass, as described in this study, results in a regenerative response of the entire remaining liver without regrowth of the resected tissue. Our observations indicate that the ability of the liver to regenerate in a compensatory growth fashion is not an evolutionary atavism inherited by mammals from lower vertebrates but rather a unique organ-specific feature of the liver that is already present in lower vertebrates, such as teleost fish.
Although the precise molecular mechanisms that orchestrate the regenerative response as well as regeneration itself are still not known, several key signaling pathways have been described in mammals to be involved in or to be able to regulate liver regeneration after larger tissue damage, such as applied in the 2/3 PH protocol (2). In our approach in establishing a liver regeneration model, we also checked for the appearance of 3 signaling pathways, namely BMP, FGF, and Wnt/β-catenin signaling, which have been previously shown to be activated during liver regeneration in mammals (10,11,12,13, 23, 24) and which, in addition, play crucial roles during liver development (14, 25, 26, 29,30,31,32). One of the strengths of the zebrafish model is the availability of an enormous mutant animal pool and its easy and straightforward handling in mutagenesis screens and the generation of transgenic lines. Therefore, to study the involvement of FGF or BMP signaling during zebrafish liver regeneration, we used transgenic animals that express dnFGFR or dnBMPR, resulting in a blockage of the respective pathways (15, 16). We found in our study, that FGF and BMP signaling indeed play important roles in zebrafish during liver regeneration after PH. The blockage of either of these pathways led to a strong impairment in liver recovery, during the first week after PH. Despite the multiple roles of BMP proteins during liver development, surprisingly little has been studied in regards to their roles during liver regeneration. Our findings that liver regeneration is perturbed during the first 5 d after PH, when BMP signaling is blocked, are in agreement with recent reports (10) that administration of neutralizing Bmp-7 antibodies or Alk3-Fc chimeric proteins in mice result in impaired liver regeneration after PH. Administration of rhBMP-7, in contrast, significantly enhances liver regeneration and function during the first 4 d after PH, suggesting that it primarily functions in enhancing the proliferation of hepatocytes (10). Our in vitro findings, which demonstrate a positive effect of BMP-2 on proliferation of hepatocytes in culture, support the findings that BMPs are playing a crucial role in liver regeneration in zebrafish, most likely via regulating hepatocyte proliferation during the first days after PH.
In mice, FGFs have been described as mitogens for adult hepatocytes during regeneration (33, 34). Injection of FGF-2 in hepatectomized rats was found to induce hepatocyte proliferation. Furthermore, FGF-2 protein is elevated during the first 4 d after PH in mammals (12). However, FGF-2-knockout mice display no difference in liver regeneration dynamics after PH, most likely due to functional substitution by VEGF or functional redundancy by other FGF family members (12, 35). We found that in zebrafish the blockage of FGF signaling after PH led to a strong reduction in liver mass recovery, especially during the first 5–7 d after PH, comparable to the effects seen in fish that express dnBMPR. Interestingly, many studies in zebrafish revealed a crucial role for FGF as a key regulator of epimorphic or facultative regeneration by controlling the formation and function of the blastema (16, 36,37,38). Heat-induced expression of dnFGFR1 in transgenic fish results in a block of blastema formation and a block of fin regeneration after amputation (16, 39). Indirectly, these results support our findings that the zebrafish liver does not regenerate via formation of a blastema after PH and, therefore, most likely does not regenerate in an epimorphic manner because the expression of dnFGFR1 led to a delayed regenerative response but not a complete block. Both dnFGFR and dnBMPR mutant lines showed only a temporary impairment of regeneration after PH, predominantly during the first 4–5 d. The mutant and control fish were subjected every night to heat shock and controlled for GFP expression, a protocol previously used by others successfully to block FGF/BMP signaling in adult zebrafish (16, 40). Therefore, we can exclude down-regulation of the expression of the dominant-negative receptors driven by the heat-shock-sensitive promoter. The most likely explanation for the recovery of the regeneration potential after d 5 and, similar to the findings in mice, is a redundancy in molecular signaling pathways that might have compensated for the loss of FGF and BMP signaling during regeneration. Both pathways seem to play crucial roles at the early steps of regeneration, and it is known that BMP and FGF signaling can also compensate for each other, e.g., in hepatoblast specification, where BMP2b can partially compensate for FGF (14). However, in order to define the precise function and epistatic relationship between these pathways, it will be necessary to identify the ligands that activate the FGF and BMP signaling cascade after PH. In addition, we have shown that in zebrafish the Wnt/β-catenin pathway is also rapidly activated after 1/3 PH and almost completely diminished by d 3 post-PH, as seen by nuclear β-catenin staining. These findings are in strong agreement with the findings observed in rodents and support our hypothesis of a compensatory regeneration process. A previous study(8) that demonstrated the importance of the Wnt/β-catenin pathway in liver regeneration after PH following the published protocols in zebrafish mutants is also in agreement with our findings, as these authors also found, only very rarely, nuclear β-catenin 3 d after PH in wild-type animals.
By establishing a detailed PH protocol, we have shown that the zebrafish liver regenerates in a fashion remarkably similar to that seen in mammalian liver. The similar kinetics and proliferative response mediated by hepatocytes as well as the presence of key signaling cascades known to play crucial roles during mammalian liver regeneration make the zebrafish, with its possibilities for fast chemical and genetic screens, an extremely attractive model system to study the process of regeneration. Recent comparisons of gene expression signatures between zebrafish and human liver tumors revealed high molecular conservation at various levels between fish and human liver tumors (41). Therefore, we believe that our PH protocol combined with the advantages of the zebrafish system will help to identify the molecular mechanisms underlying liver regeneration under normal as well as pathological conditions.
Acknowledgments
The authors greatly appreciate the generous gift of BMP2 and Noggin recombinant proteins from Dr. Senyon Choe (Salk Institute, La Jolla, CA, USA). We thank Verdon Taylor, Gerald Pao and Karl-Dimiter Bissig for helpful discussions and constructive comments on the manuscript, Michael Schorpp (Max-Planck Institute of Immunobiology, Freiburg, Germany) for providing zebrafish; and May Schwarz for proofreading the manuscript. This work was supported by grants from the G. Harold and Leila Y. Mathers Charitable Foundation, the U.S. National Institutes of Health, the Marato, and Fundacion Cellex. N.G.K., D.J., and J.C.I.B. designed the research; N.G.K. and D.J. performed the research; N.G.K., D.J., and J.C.I.B analyzed the data; and N.G.K., D.J., and J.C.I.B. wrote the paper.
References
- Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G K. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N, Campbell J S, Riehle K J. Liver regeneration. Hepatology. 2006;43:S45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- Driever W, Solnica-Krezel L, Schier A F, Neuhauss S C, Malicki J, Stemple D L, Stainier D Y, Zwartkruis F, Abdelilah S, Rangini Z, Belak J, Boggs C. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- Haffter P, Granato M, Brand M, Mullins M C, Hammerschmidt M, Kane D A, Odenthal J, van Eeden F J, Jiang Y J, Heisenberg C P, Kelsh R N, Furutani-Seiki M, Vogelsang E, Beuchle D, Schach U, Fabian C, Nusslein-Volhard C. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- Weglarz T C, Sandgren E P. Timing of hepatocyte entry into DNA synthesis after partial hepatectomy is cell autonomous. Proc Natl Acad Sci U S A. 2000;97:12595–12600. doi: 10.1073/pnas.220430497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss K D, Keating M T, Nechiporuk A. Tales of regeneration in zebrafish. Dev Dyn. 2003;226:202–210. doi: 10.1002/dvdy.10220. [DOI] [PubMed] [Google Scholar]
- Goessling W, North T E, Lord A M, Ceol C, Lee S, Weidinger G, Bourque C, Strijbosch R, Haramis A P, Puder M, Clevers H, Moon R T, Zon L I. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev Biol. 2008;320:161–174. doi: 10.1016/j.ydbio.2008.05.526. [DOI] [PubMed] [Google Scholar]
- Sadler K C, Krahn K N, Gaur N A, Ukomadu C. Liver growth in the embryo and during liver regeneration in zebrafish requires the cell cycle regulator, uhrf1. Proc Natl Acad Sci U S A. 2007;104:1570–1575. doi: 10.1073/pnas.0610774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, Yang C, LeBleu V S, Soubasakos M A, Giraldo M, Zeisberg M, Kalluri R. BMP-7 functions as a novel hormone to facilitate liver regeneration. FASEB J. 2007;21:256–264. doi: 10.1096/fj.06-6837com. [DOI] [PubMed] [Google Scholar]
- Nakatsuka R, Taniguchi M, Hirata M, Shiota G, Sato K. Transient expression of bone morphogenic protein-2 in acute liver injury by carbon tetrachloride. J Biochem. 2007;141:113–119. doi: 10.1093/jb/mvm012. [DOI] [PubMed] [Google Scholar]
- Sturm J, Keese M, Zhang H, Bonninghoff R, Magdeburg R, Vajkoczy P, Dono R, Zeller R, Gretz N. Liver regeneration in FGF-2-deficient mice: VEGF acts as potential functional substitute for FGF-2. Liver Int. 2004;24:161–168. doi: 10.1111/j.1478-3231.2004.0896.x. [DOI] [PubMed] [Google Scholar]
- Steiling H, Wustefeld T, Bugnon P, Brauchle M, Fassler R, Teupser D, Thiery J, Gordon J I, Trautwein C, Werner S. Fibroblast growth factor receptor signalling is crucial for liver homeostasis and regeneration. Oncogene. 2003;22:4380–4388. doi: 10.1038/sj.onc.1206499. [DOI] [PubMed] [Google Scholar]
- Shin D, Shin C H, Tucker J, Ober E A, Rentzsch F, Poss K D, Hammerschmidt M, Mullins M C, Stainier D Y. Bmp and Fgf signaling are essential for liver specification in zebrafish. Development. 2007;134:2041–2050. doi: 10.1242/dev.000281. [DOI] [PubMed] [Google Scholar]
- Pyati U J, Webb A E, Kimelman D. Transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development. Development. 2005;132:2333–2343. doi: 10.1242/dev.01806. [DOI] [PubMed] [Google Scholar]
- Lee Y, Grill S, Sanchez A, Murphy-Ryan M, Poss K D. Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development. 2005;132:5173–5183. doi: 10.1242/dev.02101. [DOI] [PubMed] [Google Scholar]
- Westerfield M. Eugene, OR, USA: University of Oregon Press; The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) (4th ed.) 2000 [Google Scholar]
- Junghans D, Hack I, Frotscher M, Taylor V, Kemler R. Beta-catenin-mediated cell-adhesion is vital for embryonic forebrain development. Dev Dyn. 2005;233:528–539. doi: 10.1002/dvdy.20365. [DOI] [PubMed] [Google Scholar]
- Ghosh C, Zhou Y L, Collodi P. Derivation and characterization of a zebrafish liver cell line. Cell Biol Toxicol. 1994;10:167–176. doi: 10.1007/BF00757560. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G K, DeFrances M C. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Grisham J W. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-H3. Cancer Res. 1962;22:842–849. [PubMed] [Google Scholar]
- Sosa-Pineda B, Wigle J T, Oliver G. Hepatocyte migration during liver development requires Prox1. Nat Genet. 2000;25:254–255. doi: 10.1038/76996. [DOI] [PubMed] [Google Scholar]
- Monga S P, Pediaditakis P, Mule K, Stolz D B, Michalopoulos G K. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 2001;33:1098–1109. doi: 10.1053/jhep.2001.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Behari J, Cieply B, Michalopoulos G K, Monga S P. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Ober E A, Verkade H, Field H A, Stainier D Y. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 2006;442:688–691. doi: 10.1038/nature04888. [DOI] [PubMed] [Google Scholar]
- Graff J M, Thies R S, Song J J, Celeste A J, Melton D A. Studies with a Xenopus BMP receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell. 1994;79:169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- Amaya E, Musci T J, Kirschner M W. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Burke Z D, Thowfeequ S, Tosh D. Liver specification: a new role for Wnts in liver development. Curr Biol. 2006;16:R688–690. doi: 10.1016/j.cub.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Calmont A, Wandzioch E, Tremblay K D, Minowada G, Kaestner K H, Martin G R, Zaret K S. An FGF response pathway that mediates hepatic gene induction in embryonic endoderm cells. Dev Cell. 2006;11:339–348. doi: 10.1016/j.devcel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Jung J, Zheng M, Goldfarb M, Zaret K S. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- Rossi J M, Dunn N R, Hogan B L, Zaret K S. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret K S. Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev Genet. 2002;3:499–512. doi: 10.1038/nrg837. [DOI] [PubMed] [Google Scholar]
- Baruch Y, Shoshany G, Neufeld G, Enat R. Basic fibroblast growth factor is hepatotropic for rat liver in regeneration. J Hepatol. 1995;23:328–332. [PubMed] [Google Scholar]
- Hioki O, Minemura M, Shimizu Y, Kasii Y, Nishimori H, Takahara T, Higuchi K, Yoshitake Y, Nishikawa K, Watanabe A. Expression and localization of basic fibroblast growth factor (bFGF) in the repair process of rat liver injury. J Hepatol. 1996;24:217–224. doi: 10.1016/s0168-8278(96)80032-8. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz D M. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Poss K D, Shen J, Nechiporuk A, McMahon G, Thisse B, Thisse C, Keating M T. Roles for Fgf signaling during zebrafish fin regeneration. Dev Biol. 2000;222:347–358. doi: 10.1006/dbio.2000.9722. [DOI] [PubMed] [Google Scholar]
- Whitehead G G, Makino S, Lien C L, Keating M T. fgf20 is essential for initiating zebrafish fin regeneration. Science. 2005;310:1957–1960. doi: 10.1126/science.1117637. [DOI] [PubMed] [Google Scholar]
- Thummel R, Bai S, Sarras M P, Jr, Song P, McDermott J, Brewer J, Perry M, Zhang X, Hyde D R, Godwin A R. Inhibition of zebrafish fin regeneration using in vivo electroporation of morpholinos against fgfr1 and msxb. Dev Dyn. 2006;235:336–346. doi: 10.1002/dvdy.20630. [DOI] [PubMed] [Google Scholar]
- Yin V P, Thomson J M, Thummel R, Hyde D R, Hammond S M, Poss K D. Fgf-dependent depletion of microRNA-133 promotes appendage regeneration in zebrafish. Genes Dev. 2008;22:728–733. doi: 10.1101/gad.1641808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepilina A, Coon A N, Kikuchi K, Holdway J E, Roberts R W, Burns C G, Poss K D. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Lam S H, Wu Y L, Vega V B, Miller L D, Spitsbergen J, Tong Y, Zhan H, Govindarajan K R, Lee S, Mathavan S, Murthy K R, Buhler D R, Liu E T, Gong Z. Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat Biotechnol. 2006;24:73–75. doi: 10.1038/nbt1169. [DOI] [PubMed] [Google Scholar]