Abstract
Activation of poly(ADP-ribose) polymerase-1 (PARP1) has been shown to mediate cell death induced by genotoxic stimuli. The role of poly(ADP-ribose) glycohydrolase (PARG), the enzyme responsible for polymer degradation, has been largely unexplored in the regulation of cell death. Using lentiviral gene silencing we generated A549 lung adenocarcinoma cell lines with stably suppressed PARG and PARP1 expression (shPARG and shPARP1 cell lines, respectively) and determined parameters of apoptotic and necrotic cell death following hydrogen peroxide exposure. shPARG cells accumulated large amounts of poly(ADP-ribosyl)ated proteins and exhibited reduced PARP activation. Hydrogen peroxide-induced cell death is regulated by PARG in a dual fashion. Whereas the shPARG cell line (similarly to shPARP1 cells) was resistant to the necrotic effect of high concentrations of hydrogen peroxide, these cells exhibited stronger apoptotic response. Both shPARP1 and especially shPARG cells displayed a delayed repair of DNA breaks and exhibited reduced clonogenic survival following hydrogen peroxide treatment. Translocation of apoptosis-inducing factor could not be observed, but cells could be saved by methyl pyruvate and α-ketoglutarate, indicating that energy failure may mediate cytotoxicity in our model. These data indicate that PARG is a survival factor at mild oxidative damage but contributes to the apoptosis-necrosis switch in severely damaged cells.—Erdélyi, K., Bai, P., Kovács, I., Szabó, E., Mocsár, G., Kakuk, A., Szabó, C., Gergely, P., Virág, L. Dual role of poly(ADP-ribose) glycohydrolase in the regulation of cell death in oxidatively stressed A549 cells.
Keywords: DNA damage, gene silencing, apoptosis, necrosis, hydrogen peroxide, apoptosis-inducing factor
Poly(ADP-ribosyl)ation (parylation) is a post-translational protein modification catalyzed by poly(ADP-ribose) polymerase (PARP) enzymes (1). PARylation regulates various cellular processes (1), such as cell division, transcription, and DNA repair, and different forms of cell death ranging from necrosis to apoptosis and autophagy. The PARP enzyme family consists of 17 homologues (2), with most enzymes being vaguely characterized. On the contrary, PARP1, the founding member of the PARP family has been extensively studied in recent decades. PARP1 is a nuclear enzyme activated mainly by DNA single- and double-strand breaks. On binding to DNA nicks, the enzyme cleaves NAD+ to nicotinamide and ADP-ribose and attaches the latter to glutamate or aspartate residues of suitable acceptor proteins. In turn, several ADP-ribose units are added to the first monomer, resulting in the formation of a branched poly(ADP-ribose) (PAR) polymer. In addition to histones and PARP1, many other PAR acceptor proteins have been identified, with most of them being involved in DNA repair, replication, and transcription, RNA processing, and translation initiation (3).
In severely DNA-damaged cells, intense PARylation mediates consumption of NAD+ and consequently ATP, leading to necrotic cell death (4). Alternatively, PAR can trigger the release of apoptosis-inducing factor (AIF) from the mitochondria and lead to AIF-mediated cell death (5, 6). Which of these two mechanisms predominates in different models may depend on cell type and the nature and intensity of the genotoxic stimuli used. Pharmacological inhibition of PARP enzymes or genetic ablation of the PARP1 gene has been shown to provide protection from necrosis (as measured by loss of plasma membrane integrity and ATP depletion) in oxidatively stressed cells (4). Moreover, PARP inhibitors or the PARP1-deficient (knockout) phenotype reduces tissue injury or dysfunction in oxidative stress-related pathologies, as demonstrated in animal models of stroke, myocardial or intestinal ischemia-reperfusion, shock, Parkinson’s disease, diabetes, diabetes-associated vasculopathy, and neuropathy (4). These preclinical studies provided the basis for clinical trials aimed at investigating the possible beneficial effects of PARP inhibitors in oxidative stress-related pathologies.
In contrast to the high number of papers focusing on the biological roles of PAR-synthesizing enzymes (PARPs), until recently little attention has been paid to PAR decomposition. PAR degradation is carried out mainly by poly(ADP-ribose) glycohydrolase (PARG) enzymes, although ARH3 (ADP-ribose hydrolase-like) (7) and ADP-ribosyl protein lyase have also been assigned accessory roles in PAR decomposition (8, 9). PARGs, the principal PAR-degrading enzymes, are products of a single PARG gene. In addition to the full-length (110 kDa) PARG, alternative splice variants (102 and 99 kDa) have also been shown to be produced and to possess catalytic activity (10). Moreover, catalytically active fragments produced on caspase-3 cleavage (85 and 74 kDa), as well as recently identified smaller splice variants of PARG (PARG60 and PARG55) (11), may also contribute to cellular PAR degrading activity. Interestingly, cytoplasmic PARG activity predominates in healthy cells with only the less abundant full-length isoform localizing to nuclei. However, the highest amount of PAR is produced in the nuclei following genotoxic stimuli. The predominantly cytoplasmic occurrence of PARG enzymes does not limit cellular PAR decomposition, because PARG isoforms have been shown to shuttle between cytoplasm and nuclei following genotoxic noxa (12). Moreover, PAR degrading as well as synthesizing activity has also been detected in mitochondria (13,14,15), although its biological function has not yet been determined. As PARGs possess both exoglycosidase and endoglycosidase activities, they are responsible for the hydrolysis of ribose-ribosyl glycosidic bonds between ADP-ribose units located at the extremity and within the polymer.
Investigating the biological roles of PARG enzymes has been challenging. Specific cell-permeable inhibitors are not available, and the PARG-knockout mice lacking all 3 splice variants are embryonically lethal (16). Trophoblast stem-cell lines established before embryonic failure required the presence of a PARP inhibitor for growth and were more sensitive to the toxic action of menadione and the DNA alkylating agent MNNG (16). PARG-mutant Drosophila exhibited lethality in the larval stages, and mutants grown at a permissive temperature exhibited progressive neurodegeneration (17). These data indicate that PARylation is required for embryonic development and accumulation of the polymer may cause toxicity, especially to neuronal cells. Cortes et al. (18) established PARG110-deficient mice by targeting exons 2 and 3. These mice lacking the full-length enzyme but expressing PARG60 were viable and fertile. However, similarly to the PARP1-knockout phenotype, these mice were hypersensitive to alkylating agents and ionizing radiation, and, in contrast to PARP1-knockout animals, they were susceptible to streptozotocin-induced diabetes and endotoxic shock.
The role of PARG in cell death is controversial. Using tannins such as gallotannin or nobotanin B for PARG inhibition, it has been reported that PARG inhibition provides protection to oxidatively stressed neuronal cells (19). However, tannins are potent antioxidants and have pleiotropic biological effects. Therefore, because of the lack of potent, specific, and cell-permeable pharmacological inhibitors, it is difficult to establish the role of PARG in cell death. Studies based on PARG-specific siRNA yielded conflicting results ranging from protection from hydrogen peroxide-induced cell death to lack of effect in N-methyl-N′-nitro-N-nitrosoguanidine (MNNG)-induced cytotoxicity (20, 21). Moreover, the role of PARG in regulating the form of cell death has not yet been investigated. In view of the scarcity of information presently available on the role of PARG in cell-death regulation, we conducted investigations to elucidate the role of PARG in oxidative-stress-induced cytotoxicity. We report the generation of A549 cell lines with stable silencing of PARG and PARP1 genes using lentiviral vectors. We demonstrate that, similarly to PARP1, PARG also serves as an apoptosis to necrosis switch in severe oxidative stress.
MATERIALS AND METHODS
Unless specified otherwise, all reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Cell culture and treatments
The human lung adenocarcinoma cell line A549 was grown in RPMI 1640 medium containing 10% heated fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C, 5% CO2. The transformed human kidney cell line 293T was maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated FBS, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C, 5% CO2. Mesenchymal stem cells were isolated from umbilical cords according to ref. 22 after obtaining informed consent from donors. The protocol was approved by the Ethical Board of the Medical and Heath Science Center of the University of Debrecen under permission no. 2754-2008.
Lentiviral infection of A549 cells
For stable gene silencing, 293T cells were transfected with the pLKO.1-puro vector carrying the U6 promoter-human PARP1 and PARG (sense-linker-antisense) insert [constructs TRCN0000007929 and TRCN0000051307; Sigma Mission shRNA (small hairpin RNA)] or the pLKO.1-puro empty vector as a control using polyethylenimine (PEI) as a transfection reagent. Infectious lentiviruses were harvested at 48 h post-transfection and filtered through 0.45-μm nitrocellulose filters. The infection of A549 cells with shPARP1, shPARG, and control lentiviruses was carried out by the addition of 10 MOI lentivirus. Virus-containing supernatant was removed after 12 h. Following transduction, cells were selected with 5 μg/ml puromycine.
Reverse transcription and PCR
Total RNA was isolated using TRIzol (Applied Biosystems/Ambion, Austin, TX, USA) according to the manufacturer’s instruction. Concentration and purity of the isolated RNA was measured spectrophotometrically at 260 nm and 280 nm.
Reverse transcription was performed using MMLV Reverse Transcriptase (Promega, Madison, WI, USA). A mix of 2 μg total RNA and 2 μl of random primers (Promega) was incubated in a total volume of 15 μl for 5 min at 70°C and cooled on ice. After adding 5 μl of MMLV 5× Reaction Buffer (Promega), 10 mM dNTPs,1 μl ribonuclease inhibitor (Promega), and 2 μl of MMLV Reverse Transcriptase to reach a total volume of 25 μl, the reaction mix was incubated again for 1 h at 37°C.
PCR reactions were performed using GoTaqPolymerase (Promega) in reaction mixes containing 5 U polymerase, 10 nmol of each primer, 1–4 μl cDNA, and PCR buffers as supplied by the manufacturer, in a total volume of 25 μl.
PCR primers used for the analysis were designed based on sequences deposited in the UniGene database: hPARG forward GCCCAAAGCAGAGGACAGAAGA, reverse CGGTTTCCTTGATGAACGTCCC; hPARP1 forward CGTCACTGCCTGGACCAAGTG, reverse TCCACCAGGCCAAGGGTGGC; hACTIN forward CGGGAAATCGTGCGTGACAT, reverse GAACTTTGGGGGATGCTCGC. Products were run on 1% agarose gel and visualized by ethidium bromide staining.
Real-time quantitative PCR
Quantitative real-time PCR analysis was performed using an ABI Prism 7500 sequence detector system (Applied Biosystems, Foster City, CA, USA) with Power SYBR Green PCR Master Mix (Applied Biosystems) according to the manufacturer’s protocol. The relative abundance of mRNAs was calculated by the comparative cycle of threshold (CT) method with β-actin mRNA as the invariant control. The following primers were used for PCR: hACTIN forward GACAGGATGCAGAAGGAGATTACT, reverse TGATCCACATCTGCTGGAAGGT; hPARP1 forward CAGCTTCATAACCGAAGATTGCT, reverse CGAAATAGATCCCTTTACCAAACATG; hPARG forward TGTTGGAGATGTGTATAAGCTGTTG, reverse GGACTCGACAGCATGGTATATGAA.
PARP activity
PARP activity of cell lysates has been determined by measuring the incorporation of isotope from 3H-NAD+ into trichloroacetic acid (TCA)-precipitable proteins, as described previously (23).
Immunocytochemical detection of PAR
PAR was detected by immunocytochemistry as described earlier (24), with slight modifications as follows. Cells were fixed in ice-cold methanol for 10 min and hydrated by successive 5-min washes in PBS. Coverslips were blocked in 1% BSA diluted in PBS-Triton X-100 for 1 h and were then incubated for 2 h at room temperature with 10H monoclonal anti-PAR antibody diluted 1:100. After 4 × 5-min washes in PBS, coverslips were incubated with biotinylated horse anti-mouse IgG, diluted 1:300, for 1 h at room temperature. Excess antibody was removed by 4 × 5-min washes in PBS, and the bound antibody was visualized with the ABC detection system (Vector Laboratories, Burlingame, CA, USA) and 3,3′-diaminobenzidene substrate. Coverslips were viewed with a Zeiss Axiolab microscope (Carl Zeiss, Oberkochen, Germany). Pictures were taken with a Zeiss Axiocam digital camera.
Immunofluorescent staining for AIF
For immunodetection of AIF, cells were stained with a rabbit polyclonal anti-AIF antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) using standard protocol. Biotinylated secondary antibody (Vector Laboratories) was used at a dilution of 1:300 (45 min at room temperature) followed by incubation with streptavidin-Alexa 488 conjugate (1:100; Invitrogen, Carlsbad, CA, USA) for 30 min at room temperature. Nuclei were counterstained with propidium iodide (1 μg/ml). Images were analyzed on the Olympus FluoView 1000 confocal microscope (Olympus, Tokyo, Japan).
Subcellular fractionation
Nuclear and postnuclear (designated as cytosolic) fractions were prepared as described previously (25).
Western blot analysis
Cells were washed once in PBS and collected by scraping into 200 μl ice-cold lysis buffer containing 62.5 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 50 mM dithiothreitol (DTT), 1 mM PMSF, 1 mM NaF, 1 mM Na3VO4, and protease inhibitors. The extracts were sonicated, and supernatants were collected after centrifugation. Protein concentrations were determined using the Coomassie assay. Protein (20 μg/lane) was loaded onto 8% polyacrylamide gels. Proteins were separated by electrophoresis and then transferred to nitrocellulose membranes. For immunoblotting, membranes were blocked with 5% nonfat dried milk in Tris-buffered saline (TBS) for 90 min. Primary antibodies against PAR (clone 10H), hATP5B, and rabbit polyclonal hPARP1 were applied at 1 μg/ml concentrations overnight at 4°C. After washing 3 times in TBS containing 0.05% Tween-20 (TBST), secondary antibodies (peroxidase-conjugated goat anti-mouse and goat anti-rabbit) were applied at 1:5000 dilution for 1 h. Blots were washed 3 times in TBST, once in TBS, and incubated in enhanced chemiluminescence reagents (Supersignal WestPico Chemiluminescent substrate; Pierce Biotechnology, Rockford, IL, USA) and exposed to photographic film.
Cell viability
Cell viability was assessed by the colorimetric MTT assay, as described previously (23). Briefly, cells were treated with hydrogen peroxide in a 96-well plate. Then 24 h later, MTT was added to the cells (0.5 mg/ml) and incubated for an additional hour. The medium was then aspirated, and the formazan crystals were dissolved by the addition of 100 μl dimethyl sulfoxide. Optical density was determined in a Multiskan MS plate reader (Labsystem, Vantaa, Finland) at 550-nm test wavelength with 690 nm as a reference wavelength.
Clonogenic survival
For the determination of clonogenic activity, a single-cell suspension was prepared at a density of 5 × 105 cells/ml, and cells were treated with hydrogen peroxide for 1 h at 37°C. Cells were washed to remove the hydrogen peroxide, diluted further in culture medium to densities of 102–104 cells/ml, and plated onto 6-well plates, followed by culture at 37°C for 10 d. Cells were fixed with 4% formaldehyde, then stained with hematoxylin for 10 min. After intensive washing with tap water, plates were air dried, and colonies were counted. Survival was calculated as a percentage, using the equation T/C × 100, where T and C are numbers of colonies in treated and control (untreated) plates, respectively.
Plasma membrane integrity
Plasma membrane integrity was measured by propidium iodide uptake as described previously (26). Briefly, cells were stained with 5 μg/ml propidium iodide for 15 min. Detached and trypsinized cells were then collected to tubes, washed once with PBS, and analyzed by flow cytometry.
Caspase activity
Caspase-3-like activity was measured by the cleavage of the fluorogenic tetrapeptide-amino-4-methylcoumarine conjugate (DEVD-AMC) as described previously (23), with modifications as follows. Twenty-four hours after hydrogen peroxide exposure, floating and adherent cells were pooled and resuspended in lysis buffer (10 mM HEPES, 0.1% w/v CHAPS, 5 mM DTT, 2 mM EDTA, 10 μg/ml aprotinin, 20 μg/ml leupeptin, 10 μg/ml pepstatin A, and 1 mM PMSF, pH 7.25). Cell lysates and substrates (50 μM) were combined in triplicates in caspase reaction buffer (100 mM HEPES, 10% sucrose, 5 mM DTT, 0.1% CHAPS, pH 7.25) at 37°C. Fluorescence of released AMC has been measured by a microplate fluorimeter (Labsystems) at excitation wavelength of 380 nm and emission wavelength of 460 nm.
DNA fragmentation
Cells were grown in 6-well plates and exposed to hydrogen peroxide. After 24 h, floating and trypsinized cells were pooled and resuspended in 200 μl of lysis buffer supplied by the manufacturer and incubated for 30 min at room temperature. Internucleosomal DNA fragmentation was determined with the Cell Death Detection ELISA Plus kit (Roche Molecular Biochemicals, Indianapolis, IN, USA). The assay is based on the preparation of cytoplasmic fractions and detection of cytoplasmic oligonucleosome-associated histone-DNA complexes in ELISA (23).
NAD+ content
The recycling assay was used with minor modifications, as described previously (19). Cells were extracted in 0.5 N HClO4, neutralized with 3 M KOH/125 mM Gly-Gly buffer (pH 7.4), and centrifuged at 10,000 g for 5 min. Supernatants were mixed with a reaction medium containing 0.1 mM 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide, 0.9 mM phenazine methosulfate, 13 U/ml alcohol dehydrogenase, 100 mM nicotinamide, and 5.7% ethanol in 61 mM Gly-Gly buffer (pH 7.4). Absorbance was determined at 560 nm immediately, and after 10 min. NAD+ levels were calculated from standard curves generated with known concentrations of NAD+.
Intracellular ATP level
To measure intracellular ATP levels, we used a luminometric assay with ApoSensor Cell Viability Assay Kit (BioVision, Mountain View, CA, USA) according to the manufacturer’s protocol. ATP was measured at 24 h after hydrogen peroxide treatment, and ATP level was calculated as a percentage of untreated control. Assays were performed in 3 independent experiments.
Single-cell gel electrophoresis (comet assay)
Broken DNA unwinds under alkaline conditions and forms comet-like structures after cell lysis and electrophoresis. Single-stranded DNA strand breaks were assayed by single-cell gel electrophoresis (comet assay) as described previously (27).
Mitochondrial membrane potential
Depolarization of mitochondria was determined with JC-1 staining. JC-1 is a cationic dye that is used as an indicator of mitochondrial potential in cells (28). In cells with intact mitochondria, JC-1 accumulates in the mitochondria as red fluorescent aggregates. In depolarized mitochondria, red fluoresence fades and green fluorescence of JC-1 monomers predominates. Cells attached to coverslips were treated with different concentrations of hydrogen peroxide for 24 h. Coverslips were then rinsed in PBS, and cells were loaded with 1 μM JC-1 for 30 min. The same microscopic fields were imaged first with red and then with green filters.
Statistical analysis
All experiments were preformed at least 3 times on different days. Student’s t test was applied for statistical analysis and for the determination of significance, with P < 0.05 considered as significant. For the statistical analysis of the comet assay experiments, Mann and Whitney’s U test was applied.
RESULTS
Establishment and characterization of A549 cell lines with stably suppressed hPARG and hPARP1
To investigate the contribution of PARG to oxidative-stress-induced cell death in A549 cells, we have generated cell lines with suppressed expression of hPARG. As reference, a PARP1-knockdown cell line has also been established. Silencing hPARG and hPARP1 was achieved through the use of lentiviral vector-mediated short hairpin RNA (shRNA) interference. Five individual clones from Mission shRNA target sets NM 003631and NM 001618 (for PARG and PAPR1, respectively) were cotransfected with a lentivirus packaging plasmid into HEK 293T cells. The resulting lentiviral particles were used to infect A549 cells, followed by selection in the presence of puromycin. Data obtained with the most effective clones are presented.
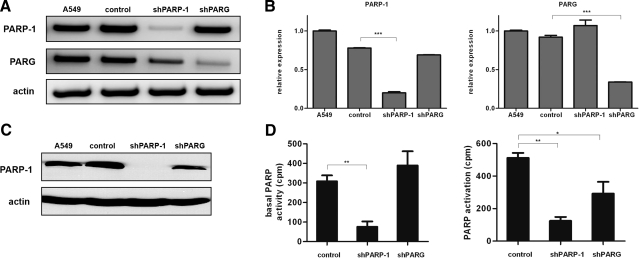
PARG and PARP1 mRNA levels were detected by reverse transcription-coupled PCR (Fig. 1A) and real-time quantitative PCR (Fig. 1B). Actin mRNA was used as an internal control. Knockdown of PARP1 has also been confirmed at the protein level by Western blotting (Fig. 1C). Expression of PARG protein was not detected because of lack of commercially available antibody that would be able to detect endogenous PARG. However, functional characterization of the shPARG cell line, as shown in Fig. 2, proves the efficient suppression of PARG protein levels. Our data demonstrate that the shPARG and shPARP1 cell lines display efficient reduction of targeted mRNAs and (in the case of PARP1) the corresponding protein. Of note, slightly decreased PARP1 mRNA and protein levels could be observed in the shPARG cell lines. This is in line with previous data reporting the regulation of PARP1 expression by PARylation and may indicate that PARG also plays a role in the regulation of PARP1 expression.
Figure 1.
Knockdown of PARG and PARP1 in A549 cells. A, B) Following transduction with control (CTL), PARP1-specific and PARG-specific shRNA-knockdown efficiency on gene expression were determined by PCR and real-time quantitative PCR; β-actin was used as the invariant control. Significant drops in PARP1 and PARG mRNA were observed (B). C) Western blot analysis of whole-protein extracts from nontransduced A549 cells, cells infected with control viruses, shPARP1 cells, and shPARG cells. Immunoblotting was performed with the anti-PARP1 antibody; the anti-actin antibody as a loading control. D) PARP activity was determined by 3H-NAD incorporation in unstimulated and 400 μM hydrogen peroxide-treated cells. shPARP-1 cells exhibited significantly lower basal PARP activity. Hydrogen peroxide induced PARP activation. PARP activation was significantly lower in shPARP1 and, to a lesser extent, in shPARG cells. *P < 0.05; **P < 0.01; ***P < 0.001.
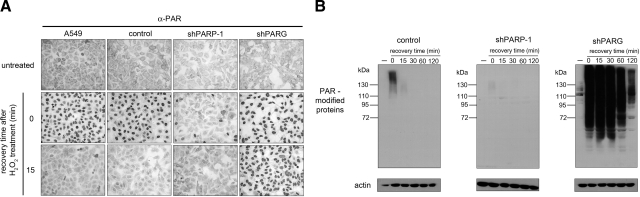
Figure 2.
Functional characterization of shPARG and shPARP1 cells. Oxidative stress-induced PAR accumulation in A549, control, shPARP1-, and shPARG cells was detected by immunocytochemistry (A) or immunoblotting (B). Following treatment with 400 μM hydrogen peroxide, PAR polymers were detected immediately (0 time) or after increasing recovery times. β-Actin was used as the loading control (B). Lack of PAR polymer synthesis was observed in shPARP1 cells, while delayed PAR polymer degradation was detected in shPARG cells.
Both basal and hydrogen peroxide-induced PARP activity was reduced in the shPARP1 cell line (Fig. 1D). Knockdown of PARG had no effect on basal PARP activity, whereas hydrogen peroxide-induced PARP activity was significantly reduced. These data indicate that by removing inhibitory PAR polymers from PARP1, PARG permits constantly high PARP1 activity in oxidatively stressed cells.
Functional characterization of the shPARG and shPARP1cell lines
To further characterize our cell lines and to learn how knockdown of PARG and PARP1 affects PAR metabolism, the cell lines were subjected to functional characterization. In A549 cells, hydrogen peroxide treatment caused transient nuclear synthesis of PAR, as assessed by immunocytochemical analysis using a polymer-specific antibody. The polymer level was restored in 15 min (Fig. 2). Cells infected with control lentiviruses showed the same response. In the shPARP1 cell line, however, PAR synthesis was strongly suppressed, with only a few scattered cells displaying immunopositivity for PAR. PAR synthesis was apparently unchanged in shPARG cells; however, degradation of the polymer was significantly delayed, with no obvious sign of PAR degradation at 15 min (Fig. 2) and strong immunopositivity detected even 120 min after hydrogen peroxide treatment (not shown). Lack of PAR synthesis in shPARP1 cells and blocked PAR degradation in shPARG cells has also been confirmed in Western blot experiments (Fig. 2B). In the untreated control cells, no PAR-modified proteins could be detected. After hydrogen peroxide treatment, a smear between 115 and 160 kDa, indicating automodified PARP-1, became visible, but the signal disappeared during 15 min of recovery (Fig. 2B). In the shPARP1 cells, only a very faint signal indicated a low degree of PAR synthesis. Unstimulated shPARG cells displayed 3 distinct bands of PAR-modified proteins in the 110+-kDa region. Hydrogen peroxide treatment triggered a robust PARP activation response, with polymers showing a markedly prolonged half-life (Fig. 2B).
These data clearly demonstrate that efficient suppression of the main PAR synthesizing and degrading enzyme has been achieved in A549 cells.
Role of PARG in oxidative stress-induced cell death
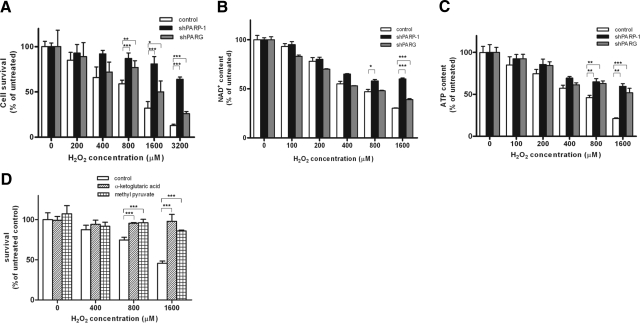
Similarly to most cell types, oxidative stress-induced cell death of A549 cells is mediated, at least in part, by PARylation (29). The role of PARG in the regulation of cell death is not known. Hydrogen peroxide caused a concentration-dependent decrease in the viability of control A549 cells. shPARG and shPARP1 cells were significantly protected from toxicity of the oxidant, with PARG knockdown providing weaker protection (Fig. 3A). Similar patterns could be observed in the drop of cellular NAD+ and ATP levels (Fig. 3B, C), indicating that compromised cellular energetics could explain the PARP/PARG-dependent component of hydrogen peroxide-induced cytotoxicity. Indeed, TCA-cycle substrates methyl pyruvate and α-ketoglutarate provided significant protection from hydrogen peroxide-induced cell death (Fig. 3D and Supplemental Fig. S1), indicating that slowdown of glycolysis contributes to loss of viability in this model. Inhibition of glycolysis may result in mitochondrial depolarization, an event mediated by PARP activation in cells exposed to DNA damage. In our model, hydrogen peroxide triggered a concentration-dependent mitochondrial depolarization that was blocked in shPARP1 and shPARG cells (Supplemental Fig. S2).
Figure 3.
Hydrogen peroxide-induced cell death: apoptotic and energetic parameters. A–C) Concentration-dependent cell death (A), NAD+ consumption (B), and ATP consumption (C) induced by hydrogen peroxide in control, shPARP1 and shPARG cells. Hydrogen peroxide caused significant cell death (as determined by MTT reduction) and a significant drop in cellular levels of NAD+ and ATP at 24 h in control cells. Cell death and loss of cellular NAD+ and ATP was significantly prevented by gene silencing of PARP1 and PARG as compared to control. D) Hydrogen peroxide-induced cytotoxicity was prevented by TCA-cycle substrates methyl pyruvate and α-ketoglutarate. Data represent means ± se of 4 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Concerted action of PARG and PARP1 mediate apoptosis to necrosis switch in severe oxidative stress
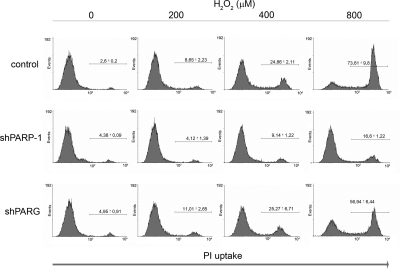
As we have shown here (Fig. 3C), high concentrations of hydrogen peroxide caused a drop in cellular ATP level, a common feature of necrotic cell death and an important regulator of apoptosis to necrosis switch (30). To further characterize the mode of cell death in our model, we have determined plasma membrane permeability. Exposure of cells to high concentrations of hydrogen peroxide lead to increased plasma membrane permeability, as indicated by propidium iodide uptake (Fig. 4). Loss of plasma membrane integrity is mediated by the activation of PARP1, as shPARP1 cells were resistant to hydrogen peroxide-induced plasma membrane permeabilization. PARG may also contribute to the permeabilization of the plasma membrane, as shPARG cells were, although to a lesser extent, also protected from the loss of plasma membrane integrity.
Figure 4.
Permeabilization of the plasma membrane in oxidatively stressed cells requires the concerted action of PARP1 and PARG. Cells were exposed to indicated concentrations of hydrogen peroxide. Plasma membrane injury was assessed after 12 h by propidium iodide staining. Significant protection from oxidative stress was observed in shPARP1 and shPARG cells as compared to control. Data represent means ± sd of triplicate samples.
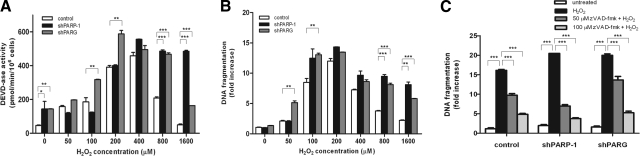
Hydrogen peroxide also induces caspase activation and DNA fragmentation, biochemical markers of apoptosis (Fig. 5). In parallel to the appearance of necrotic parameters (loss of plasma membrane integrity, drop in ATP level), apoptotic parameters such as caspase-3-like activity and internucleosomal DNA fragmentation declined at higher concentrations of the oxidant. In shPARP1 cells and, to a lesser extent, in shPARG cells, these apoptotic parameters were significantly more preserved at higher concentrations of hydrogen peroxide (Fig. 5A, B), indicating that a concerted action of PARP1 and PARG is required for the apoptosis-to-necrosis switch in oxidatively stressed A549 cells. We also show that DNA fragmentation is caspase-dependent in our model because DNA fragmentation could be abolished by the pancaspase inhibitor zVAD-fmk (Fig. 5C).
Figure 5.
Effect of silencing PARP1 and PARG on oxidative stress-induced caspase-3-like activity and DNA fragmentation in A549 cells. A, B) Cells were exposed to indicated concentrations of hydrogen peroxide. After 24 h, cells were collected and lysed. Caspase-3-like activity (A) and DNA fragmentation (B) were quantified. C) Cells pretreated with the cell-permeable pancaspase inhibitor z-VAD-fmk (50 or 100 μM) for 1 h followed by exposure to 200 μM of hydrogen peroxide exhibited decreased caspase activity. Data represent means ± sd of quadruplicate samples. Similar data were obtained in 3 independent experiments. *P<0.05, **P<0.01, ***P<0.001 vs. untreated control cells.
Hydrogen peroxide-induced alterations in cell morphology as observed in May-Grünwald-Giemsa-stained cells also support apoptotic death (condensed chromatin) at low hydrogen peroxide concentrations (400 μM) and necrotic morphology at higher concentrations (800–1600 μM). In the shPARP-1 line and, to a lesser extent, in shPARG cells, necrosis was inhibited at 800–1600 μM of hydrogen peroxide, and most cells displayed apoptotic morphology (Supplemental Fig. S3). Moreover, at low concentrations (200 μM) of hydrogen peroxide, when no morphological alterations could be observed in control cells, shPARG cells have shown signs of apoptosis (Supplemental Fig. S3).
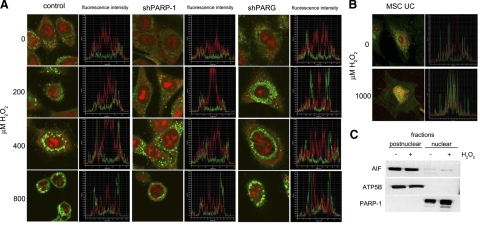
As AIF release has been proposed to propagate PAR-mediated cell death, we sought to determine AIF relocation following hydrogen peroxide treatment (Fig. 6). In untreated cells, AIF exhibited a punctuate distribution in the cytoplasm, corresponding to the mitochondrial localization of the protein. Hydrogen peroxide failed to induce AIF translocation from the mitochondria to the nucleus, indicating that AIF is not likely to mediate cell death in our model (Fig. 6A). Human umbilical cord-derived mesenchymal cells (MSC UCs) were used as a positive control. Hydrogen peroxide induced nuclear translocation of AIF in MSC UCs (Fig. 6B). Lack of nuclear translocation of AIF in A549 cells could also be confirmed in subcellular fractionation experiments (Fig. 6C). Cells were fractionated into nuclear and postnuclear fractions, and AIF was detected with Western blotting. ATP5B was used as mitochondrial marker, whereas PARP1 served as a nuclear marker.
Figure 6.
Lack of AIF translocation in A549 cells. A) Cells exposed to indicated concentrations of hydrogen peroxide for 24 h were stained for AIF (green) as described in Materials and Methods. Nuclei were counterstained with propidium iodide (red). AIF translocation was not observed in any of the 3 cell lines. B) Z-stack fluorescent intensity plots prove lack of nuclear translocation of AIF. Umbilical cord-derived human mesenchymal stem cells (UC MSCs) were used as positive control. UC MSCs were treated with 1000 μM hydrogen peroxide for 16 h and were then stained similarly to A549 cells. UD MSCs showed clear signs of AIF translocation. C) Lack of AIF translocation in A549 cells was also confirmed by subcellular fractionation into nuclear and postnuclear (including cytoplasmic and mitochondrial) fractions. Proteins were separated by electrophoresis and stained for AIF, ATP5B (mitochondrial marker), and PARP1 (nuclear marker).
Concerted action of PARG and PARP1 is required for the repair of oxidative stress-induced DNA breaks
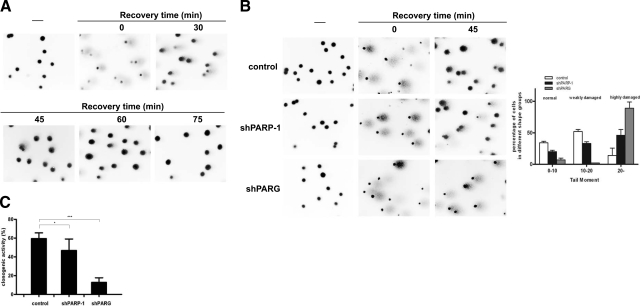
One of the best-described biological functions of PARylation is to assist DNA repair. Hydrogen peroxide (100, 200, and 400 μM) induced a concentration-dependent DNA breakage (data not shown) as assessed in single-cell gel electrophoresis (comet assay). At 400 μM of hydrogen peroxide, massive DNA breakage could be observed. Considering the severity of DNA damage, cells coped remarkably well with the repair of DNA breaks, as shown by images obtained during the recovery phase (Fig. 7A). As an almost complete repair of damage could be observed at 45 min following hydrogen peroxide treatment, we compared the repair capacity of our cell lines at this time point. We observed an impaired DNA strand break repair in shPARP1 cells and an almost complete blockage of repair in shPARG cells (Fig. 7B). Compromised DNA single-strand break repair may result in limited long-term clonogenic survival of shPARG and shPARP1 cells. Indeed, we determined clonogenic survival following treatment with 200 μΜ hydrogen peroxide, and both knockdown cells exhibited impaired clonogenicity (Fig. 7C).
Figure 7.
Knockdown of PARG and PARP1 delays single-strand break repair. DNA single-strand breakage was measured with the comet assay. A) A549 cells were treated with 400 μM hydrogen peroxide for 15 min and then allowed to recover in culture medium for indicated time periods. Single cells were then exposed to an electric field in agarose gel and stained with ethidium bromide, as described in Materials and Methods. Labeled DNA was visualized under fluorescent microscope. B) Control, shPARP1, and shPARG cells were exposed to 400 μM hydrogen peroxide for 15 min and then allowed to recover in culture medium for 45 min. Single cells then underwent electrophoresis and were processed as described above. Distribution of olive tail moments is shown at right. Representative images are presented (A, B). C) Clonogenic activity of 200 μM hydrogen peroxide-treated control, shPARP1, and shPARG cells.
DISCUSSION
Once regarded as a DNA repair-assisting process, poly(ADP-ribosyl)ation has emerged in recent years as a pleiotropic mediator of a wide range of biological phenomena. Moreover, it has also been suggested that in cases of severe DNA damage, such as the ones observed in oxidative stress-related pathologies, accelerated PAR turnover may also mediate cellular suicide (4). A high number of studies based on the use of pharmacological inhibitors or knockout cells proved the role of PARP1 in mediating genotoxicity-induced cell death (4). Moreover, we and others also demonstrated that PARP1 acts as a molecular switch between apoptosis and necrosis (4, 23, 31,32,33,34). Recently the role of PARylation has also been suggested in the regulation of a third form of cell death, autophagy (35, 36). Of note, in line with the dual role of PARP1 in cell death regulation, as proposed by us (4), activation of PARP1 may also act as a protective mechanism in mild oxidative stress (37), likely via assisting the repair of DNA breaks.
In contrast to the plethora of papers dealing with the role of PARP1 in cell death, the role of PARG in the PARP1-mediated suicidal pathway is largely unexplored. Studies based on the use of tannins as PARG inhibitors suggested that PARG is a mediator of cell death, whereas other studies reported opposite results (38). However, the cellular effects of gallotannin in models of oxidative stress are difficult to interpret due to the potent antioxidant properties of tannins (38, 39). Moreover, embryonic lethality of PARG KO mice and neurotoxicity observed in PARG mutant Drosophila indicated that PARG activity may promote cell survival in genotoxic stress. More recently, PAR has been identified as a death-signaling molecule that, on release from the nucleus, may trigger AIF-mediated cell death. The role of PARG in the regulation of the mode of cell death has not yet been investigated. To investigate the role of PARG in cell-death regulation, we established an A549 cell line with stably suppressed PARG. Knocking down PARG did not cause toxicity in untreated cells. Hydrogen peroxide-induced PAR accumulation clearly demonstrated efficient knockdown of the protein.
Our current work underscores the dual role of PARG in cell-death regulation. Knockdown of PARG provided protection from hydrogen peroxide-induced loss of viability; however, the degree of protection was less than that observed in shPARP1 cells. [Of note, similar but less pronounced protection could be observed in shPARP1 and shPARG cells if other genotoxic stimuli (doxorubicine or MNNG) were used as cytotoxic agents (Supplemental Fig. S4).] Increased short-term viability of hydrogen peroxide-treated shPARG cells, however, did not translate to increased long-term clonogenic survival. This is likely because of deficient repair of oxidative DNA breaks both in shPARP1 and especially in shPARG cells. Concerted action of PARG and PARP1 in DNA repair has been previously demonstrated (40, 41). Disruption of the PAR cycle on the side of either polymer synthesis or degradation leads to delayed DNA repair. Fisher et al. (40) also reported decreased clonogenic survival in PARP1- and PARG-knockdown cells; however, the effect of PARP1 knockdown appeared to be more pronounced. Our data are in line with those of Fisher et al. (40), with the exception that PARG knockdown compromised DNA repair and clonogenic survival more than PARP1 knockdown did, which might be due to different knockdown efficiency. Contribution of PARG to DNA repair may explain the increased sensitivity of shPARG cells to mild genotoxic stimuli.
What is the mechanism of oxidative stress-induced cytotoxicity in the absence of PARG? Suppression of PARG expression decreased necrosis (as assessed by membrane permeability and ATP measurements) and led to increased apoptotic parameters, such as caspase-3 like activity and oligonucleosomal DNA fragmentation. These data indicate that PARG, similarly to PARP1, mediates apoptosis-to-necrosis switch in severe oxidative stress. According to this scenario, PARG suppression leads to increased automodification of PARP1, which is known to lead to PARP1 autoinhibition. In support of this, the major band of PAR-modified proteins (Fig. 2B, right panel, first lane) in untreated shPARG cells corresponds to the size of PARP1. Furthermore, PARG knockdown resulted in reduced PARP activation in hydrogen peroxide-treated cells, suggesting that auto-PARylation indeed down-regulates PARP1 activity.
Previously, it has been suggested that in neurons the lethal effects of PARP activation are mediated by AIF. If released from the nuclei, PAR may trigger AIF release from the mitochondria, which may propagate cell death. In our model, however, nuclear translocation of AIF could not be detected, and therefore AIF is not likely to mediate the cell-death response in oxidatively stressed A549 cells. Lack of AIF translocation has also been observed in a model of mild endogenous oxidative stress in neuronal cells (37). Furthermore, heat shock protein 70 (Hsp70) has been shown to prevent nuclear relocation of AIF (42). As lung tumors have been shown to express Hsp70 (43), and stressed A549 cells also up-regulate Hsp70 (44), therefore induction of Hsp70 may explain the absence of AIF translocation in our model. However, Hsp70 does not interfere with the release of AIF from the mitochondria; instead, it sequesters released AIF in the cytoplasm, thus blocking nuclear translocation. In our model, however, AIF did not appear to be released from the mitochondria, because a punctuate staining pattern was maintained. (AIF release would be indicated by a diffuse staining pattern.) Whether Hsp70 interferes with AIF signaling in oxidatively stressed A549 cells requires further investigation. Nonetheless, our data underscore the importance of cell type- and death-model-dependent differences in the dependence of cell death on AIF or other mediators. In light of the potential clinical applications of PARG inhibitors, these differences should be especially carefully evaluated by comparing responses of primary untransformed cells (e.g., neurons) with those of tumor cell lines.
As the AIF pathway does not play a role in mediating hydrogen peroxide-induced cell death of A549 cells, compromised cellular energetics may underlie the PARP1/PARG-mediated cell death. This “classic” scenario of PARylation-mediated cell death is based on PARP1-mediated depletion of NAD+, which slows glycolysis at the level of the NAD+-dependent glyceraldehyde-3-phosphate dehydrogenase, leading to mitochondrial depolarization. NAD+ depletion, mitochondrial depolarization, and the protective effect of TCA-cycle substrates support this scenario in our model.
Recent findings indicate that DNA-damaging signals, including doxorubicin and hydrogen peroxide, may also trigger autophagy (35, 36). As autophagy may inhibit other types of cell death, e.g., necrosis (35), it is plausable to hypothesize that autophagic process may be important in our model. Hydrogen peroxide-induced and PARP1-mediated autophagy may down-regulate cell death seen at low concentrations of the oxidant. This effect may contribute to increased sensitivity of shPARP1 and shPARG cells, as observed in clonogenic assays. Similarly to our model, PARP1-mediated autophagy could also be inhibited by the TCA-cycle substrate methyl pyruvate (36), indicating that suppressed cellular energetics may be a central regulator of the autophagic process. The exact role of autophagy in hydrogen peroxide-induced cytotoxicity in A549 cells and the interrelationship of PARP1/PARG and the autophagic process require further investigation.
Use of PARP inhibitors as adjuvant anticancer agents and to reduce tissue injury in oxidative stress-related pathologies has previously been proposed and is being tested in clinical trials. Our data indicate that the effects of PARG inhibition are similar to the inhibition of PARP1. Therefore, it appears to be plausible to test the effects of PARG inhibition in the same pathological conditions in which PARP inhibitors proved beneficial. Potent, selective, and cell-permeable PARG inhibitors are clearly needed to test whether PARG inhibition represents a valid approach in these disease models.
Supplementary Material
Acknowledgments
This work was supported by grants from the Hungarian Ministry of Health (12/2006), the Hungarian National Science Research Fund (OTKA K60780, K75864, K60620, NNF 78498, K77600), the University of Debrecen Medical and Health Science Center (Mec-1/2008), and the U.S. National Institutes of Health (NIH R01 GM060915). É.S. and P.B. were supported by a Bolyai Fellowship from the Hungarian Academy of Sciences. L.V. was supported by an István Széchenyi Fellowship. We thank Krisztián Csomós for his help with the lentiviral system. The excellent technical assistance of Ms. Erzsébet Herbály is greatly appreciated.
References
- Diefenbach J, Burkle A. Introduction to poly(ADP-ribose) metabolism. Cell Mol Life Sci. 2005;62:721–730. doi: 10.1007/s00018-004-4503-3. [DOI] [PubMed] [Google Scholar]
- Ame J C, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–893. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- Gagne J P, Isabelle M, Lo K S, Bourassa S, Hendzel M J, Dawson V L, Dawson T M, Poirier G G. Proteome-wide identification of poly(ADP-ribose) binding proteins and poly(ADP-ribose)-associated protein complexes. Nucleic Acids Res. 2008;36:6959–6976. doi: 10.1093/nar/gkn771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- Andrabi S A, Kim N S, Yu S W, Wang H, Koh D W, Sasaki M, Klaus J A, Otsuka T, Zhang Z, Koehler R C, Hurn P D, Poirier G G, Dawson V L, Dawson T M. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci U S A. 2006;103:18308–18313. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S W, Wang H, Poitras M F, Coombs C, Bowers W J, Federoff H J, Poirier G G, Dawson T M, Dawson V L. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- Oka S, Kato J, Moss J. Identification and characterization of a mammalian 39-kDa poly(ADP-ribose) glycohydrolase. J Biol Chem. 2006;281:705–713. doi: 10.1074/jbc.M510290200. [DOI] [PubMed] [Google Scholar]
- Bonicalzi M E, Haince J F, Droit A, Poirier G G. Regulation of poly(ADP-ribose) metabolism by poly(ADP-ribose) glycohydrolase: where and when? Cell Mol Life Sci. 2005;62:739–750. doi: 10.1007/s00018-004-4505-1. [DOI] [PubMed] [Google Scholar]
- Gagne J P, Hendzel M J, Droit A, Poirier G G. The expanding role of poly(ADP-ribose) metabolism: current challenges and new perspectives. Curr Opin Cell Biol. 2006;18:145–151. doi: 10.1016/j.ceb.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Meyer-Ficca M L, Meyer R G, Coyle D L, Jacobson E L, Jacobson M K. Human poly(ADP-ribose) glycohydrolase is expressed in alternative splice variants yielding isoforms that localize to different cell compartments. Exp Cell Res. 2004;297:521–532. doi: 10.1016/j.yexcr.2004.03.050. [DOI] [PubMed] [Google Scholar]
- Meyer R G, Meyer-Ficca M L, Whatcott C J, Jacobson E L, Jacobson M K. Two small enzyme isoforms mediate mammalian mitochondrial poly(ADP-ribose) glycohydrolase (PARG) activity. Exp Cell Res. 2007;313:2920–2936. doi: 10.1016/j.yexcr.2007.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haince J F, Ouellet M E, McDonald D, Hendzel M J, Poirier G G. Dynamic relocation of poly(ADP-ribose) glycohydrolase isoforms during radiation-induced DNA damage. Biochim Biophys Acta. 2006;1763:226–237. doi: 10.1016/j.bbamcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Du L, Zhang X, Han Y Y, Burke N A, Kochanek P M, Watkins S C, Graham S H, Carcillo J A, Szabo C, Clark R S. Intra-mitochondrial poly(ADP-ribosylation) contributes to NAD+ depletion and cell death induced by oxidative stress. J Biol Chem. 2003;278:18426–18433. doi: 10.1074/jbc.M301295200. [DOI] [PubMed] [Google Scholar]
- Scovassi A I. Mitochondrial poly(ADP-ribosylation): from old data to new perspectives. FASEB J. 2004;18:1487–1488. doi: 10.1096/fj.04-1841rev. [DOI] [PubMed] [Google Scholar]
- Niere M, Kernstock S, Koch-Nolte F, Ziegler M. Functional localization of two poly(ADP-ribose)-degrading enzymes to the mitochondrial matrix. Mol Cell Biol. 2008;28:814–824. doi: 10.1128/MCB.01766-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D W, Lawler A M, Poitras M F, Sasaki M, Wattler S, Nehls M C, Stoger T, Poirier G G, Dawson V L, Dawson T M. Failure to degrade poly(ADP-ribose) causes increased sensitivity to cytotoxicity and early embryonic lethality. Proc Natl Acad Sci U S A. 2004;101:17699–17704. doi: 10.1073/pnas.0406182101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanai S, Kanai M, Ohashi S, Okamoto K, Yamada M, Takahashi H, Miwa M. Loss of poly(ADP-ribose) glycohydrolase causes progressive neurodegeneration in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:82–86. doi: 10.1073/pnas.2237114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes U, Tong W M, Coyle D L, Meyer-Ficca M L, Meyer R G, Petrilli V, Herceg Z, Jacobson E L, Jacobson M K, Wang Z Q. Depletion of the 110-kilodalton isoform of poly(ADP-ribose) glycohydrolase increases sensitivity to genotoxic and endotoxic stress in mice. Mol Cell Biol. 2004;24:7163–7178. doi: 10.1128/MCB.24.16.7163-7178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W, Sevigny M B, Chen Y, Swanson R A. Poly(ADP-ribose) glycohydrolase mediates oxidative and excitotoxic neuronal death. Proc Natl Acad Sci U S A. 2001;98:12227–12232. doi: 10.1073/pnas.211202598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenn C, Althaus F R, Malanga M. Poly(ADP-ribose) glycohydrolase silencing protects against H2O2-induced cell death. Biochem J. 2006;396:419–429. doi: 10.1042/BJ20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohausz O, Blenn C, Malanga M, Althaus F R. The roles of poly(ADP-ribose)-metabolizing enzymes in alkylation-induced cell death. Cell Mol Life Sci. 2008;65:644–655. doi: 10.1007/s00018-008-7516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo C H, Kim O S, Park E Y, Kim B J, Lee J H, Kang S B, Han H S, Rhee S H, Yoon K S. Fetal mesenchymal stem cells derived from human umbilical cord sustain primitive characteristics during extensive expansion. Cell Tissue Res. 2008;334:423–433. doi: 10.1007/s00441-008-0696-3. [DOI] [PubMed] [Google Scholar]
- Virag L, Scott G S, Cuzzocrea S, Marmer D, Salzman A L, Szabo C. Peroxynitrite-induced thymocyte apoptosis: the role of caspases and poly (ADP-ribose) synthetase (PARS) activation. Immunology. 1998;94:345–355. doi: 10.1046/j.1365-2567.1998.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkle A, Chen G, Kupper J H, Grube K, Zeller W J. Increased poly(ADP-ribosyl)ation in intact cells by cisplatin treatment. Carcinogenesis. 1993;14:559–561. doi: 10.1093/carcin/14.4.559. [DOI] [PubMed] [Google Scholar]
- Lontay B, Kiss A, Gergely P, Hartshorne D J, Erdodi F. Okadaic acid induces phosphorylation and translocation of myosin phosphatase target subunit 1 influencing myosin phosphorylation, stress fiber assembly and cell migration in HepG2 cells. Cell Signal. 2005;17:1265–1275. doi: 10.1016/j.cellsig.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Bai P, Bakondi E, Szabo E, Gergely P, Szabo C, Virag L. Partial protection by poly(ADP-ribose) polymerase inhibitors from nitroxyl-induced cytotoxity in thymocytes. Free Radic Biol Med. 2001;31:1616–1623. doi: 10.1016/s0891-5849(01)00756-0. [DOI] [PubMed] [Google Scholar]
- Hegedus C, Lakatos P, Olah G, Toth B I, Gergely S, Szabo E, Biro T, Szabo C, Virag L. Protein kinase C protects from DNA damage-induced necrotic cell death by inhibiting poly(ADP-ribose) polymerase-1. FEBS Lett. 2008;582:1672–1678. doi: 10.1016/j.febslet.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- Nanavaty U B, Pawliczak R, Doniger J, Gladwin M T, Cowan M J, Logun C, Shelhamer J H. Oxidant-induced cell death in respiratory epithelial cells is due to DNA damage and loss of ATP. Exp Lung Res. 2002;28:591–607. doi: 10.1080/01902140260426715. [DOI] [PubMed] [Google Scholar]
- Leist M, Single B, Castoldi A F, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virag L, Salzman A L, Szabo C. Poly(ADP-ribose) synthetase activation mediates mitochondrial injury during oxidant-induced cell death. J Immunol. 1998;161:3753–3759. [PubMed] [Google Scholar]
- Ha H C, Snyder S H. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci U S A. 1999;96:13978–13982. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni F, Meli E, Peruginelli F, Chiarugi A, Cozzi A, Picca R, Romagnoli P, Pellicciari R, Pellegrini-Giampietro D E. Poly(ADP-ribose) polymerase inhibitors attenuate necrotic but not apoptotic neuronal death in experimental models of cerebral ischemia. Cell Death Differ. 2001;8:921–932. doi: 10.1038/sj.cdd.4400884. [DOI] [PubMed] [Google Scholar]
- Tentori L, Balduzzi A, Portarena I, Levati L, Vernole P, Gold B, Bonmassar E, Graziani G. Poly (ADP-ribose) polymerase inhibitor increases apoptosis and reduces necrosis induced by a DNA minor groove binding methyl sulfonate ester. Cell Death Differ. 2001;8:817–828. doi: 10.1038/sj.cdd.4400863. [DOI] [PubMed] [Google Scholar]
- Huang Q, Wu Y T, Tan H L, Ong C N, Shen H M. A novel function of poly(ADP-ribose) polymerase-1 in modulation of autophagy and necrosis under oxidative stress. Cell Death Differ. 2009;16:264–277. doi: 10.1038/cdd.2008.151. [DOI] [PubMed] [Google Scholar]
- Muñoz-Gámez J A, Rodríguez-Vargas J M, Quiles-Pérez R, Aguilar-Quesada R, Martín-Oliva D, de Murcia G, Menissier de Murcia J, Almendros A, Ruiz de Almodóvar M, Oliver F J. PARP-1 is involved in autophagy induced by DNA damage. Autophagy. 2009;5:61–74. doi: 10.4161/auto.5.1.7272. [DOI] [PubMed] [Google Scholar]
- Diaz-Hernandez J I, Moncada S, Bolanos J P, Almeida A. Poly(ADP-ribose) polymerase-1 protects neurons against apoptosis induced by oxidative stress. Cell Death Differ. 2007;14:1211–1221. doi: 10.1038/sj.cdd.4402117. [DOI] [PubMed] [Google Scholar]
- Falsig J, Christiansen S H, Feuerhahn S, Burkle A, Oei S L, Keil C, Leist M. Poly(ADP-ribose) glycohydrolase as a target for neuroprotective intervention: assessment of currently available pharmacological tools. Eur J Pharmacol. 2004;497:7–16. doi: 10.1016/j.ejphar.2004.06.042. [DOI] [PubMed] [Google Scholar]
- Erdelyi K, Kiss A, Bakondi E, Bai P, Szabo C, Gergely P, Erdodi F, Virag L. Gallotannin inhibits the expression of chemokines and inflammatory cytokines in a549 cells. Mol Pharmacol. 2005;68:895–904. doi: 10.1124/mol.105.012518. [DOI] [PubMed] [Google Scholar]
- Fisher A E, Hochegger H, Takeda S, Caldecott K W. Poly(ADP-ribose) polymerase 1 accelerates single-strand break repair in concert with poly(ADP-ribose) glycohydrolase. Mol Cell Biol. 2007;27:5597–5605. doi: 10.1128/MCB.02248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil C, Grobe T, Oei S L. MNNG-induced cell death is controlled by interactions between PARP-1, poly(ADP-ribose) glycohydrolase, and XRCC1. J Biol Chem. 2006;281:34394–34405. doi: 10.1074/jbc.M606470200. [DOI] [PubMed] [Google Scholar]
- Ravagnan L, Gurbuxani S, Susin S A, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger J M, Garrido C, Kroemer G. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- Ciocca D R, Calderwood S K. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H R, Menendez I Y, Ryan M A, Denenberg A G, Wispe J R. Increased expression of heat shock protein-70 protects A549 cells against hyperoxia. Am J Physiol. 1998;275:L836–L841. doi: 10.1152/ajplung.1998.275.4.L836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.