Abstract
Staphylococcus aureus infections are associated with abscess formation and bacterial persistence; however, the genes that enable this lifestyle are not known. We show here that following intravenous infection of mice, S. aureus disseminates rapidly into organ tissues and elicits abscess lesions that develop over weeks but cannot be cleared by the host. Staphylococci grow as communities at the center of abscess lesions and are enclosed by pseudocapsules, separating the pathogen from immune cells. By testing insertional variants in genes for cell wall-anchored surface proteins, we are able to infer the stage at which these molecules function. Fibrinogen-binding proteins ClfA and ClfB are required during the early phase of staphylococcal dissemination. The heme scavenging factors IsdA and IsdB, as well as SdrD and protein A, are necessary for abscess formation. Envelope-associated proteins, Emp and Eap, are either required for abscess formation or contribute to persistence. Fluorescence microscopy revealed Eap deposition within the pseudocapsule, whereas Emp was localized within staphylococcal abscess communities. Antibodies directed against envelope-associated proteins generated vaccine protection against staphylococcal abscess formation. Thus, staphylococci employ envelope proteins at discrete stages of a developmental program that enables abscess formation and bacterial persistence in host tissues.—Cheng, A. G., Kim, H. K., Burts, M. L., Krausz, T., Schneewind, O., Missiakas, D. M. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues.
Keywords: surface proteins, vaccine, protective immunity, sortase, envelope-associated proteins
Abscess lesions in human or animal tissues have been thought of as default host responses to biological, chemical, or physical injury, where localized release of proinflammatory cytokines initiates vascular leakage, followed by attraction of polymorphonuclear leukocytes and macrophages (1). Phagocytes then degrade defective tissues, generating liquefaction necroses that are eventually drained to organ surfaces (2). Another host response to injury, the deposition of fibrin at the periphery of necrotic lesions, aims to delineate areas of inflammation from healthy tissues and limit the extent of tissue replacement with fibrotic scars (3). Localized drainage of pus, the byproduct of liquefaction necrosis, can therefore be appreciated as a clinical sign of healing following injury (4).
Staphylococcus aureus is a commensal of the human skin and nares, and the leading cause of bloodstream, skin, and soft tissue infections (5). The pathogenesis of staphylococcal infections is initiated as bacteria invade tissues or bloodstream via trauma, surgical wounds, or medical devices (6). Although the invading pathogen may be phagocytosed and killed, staphylococci can also escape innate immune defenses and seed infections in organ tissues, inducing inflammatory responses that attract macrophages, neutrophils, and other phagocytes (6). The responsive invasion of immune cells to the site of infection is accompanied by liquefaction necrosis as the host seeks to prevent staphylococcal spread and allow for removal of necrotic tissue debris (7). Such lesions can be observed by microscopy as hypercellular areas containing necrotic tissue, leukocytes, and a central nidus of bacteria (7). Unless staphylococcal abscesses are surgically drained and treated with antibiotics, disseminated infection and septicemia produce a lethal outcome (8).
We tested here the hypothesis that abscess formation in response to S. aureus infection may be a developmental process, primarily driven by the invading pathogen rather than by default host processes that accompany the clearance of microbes. If so, one would presume that some pathogen genes may be uniquely required at discrete stages during the establishment of abscess lesions or staphylococcal persistence. By perturbing a mouse model of infection with genetic and immunological variables, we examined bacterial mutants for phenotypic defects and compared these with the impact of humoral immune responses to staphylococcal envelope components.
MATERIALS AND METHODS
Bacterial strains and growth
Staphylococci were cultured on tryptic soy agar or broth at 37°C. Escherichia coli strains DH5α and BL21 (DE3) were cultured on Luria agar or broth at 37°C. Ampicillin (100 μg/ml) and erythromycin (10 μg/ml) were used for plasmid and transposon mutant selection, respectively.
Transposon mutagenesis
Insertional mutations from the Phoenix library were transduced into the human clinical isolate S. aureus Newman (9). Each mutant carries the transposon bursa aurealis containing an erythromycin resistance cassette in the gene of interest, and mutations were verified as described previously (9).
Cloning, purification, and antibody generation
Coding sequences for Eap and Emp were PCR-amplified using S. aureus Newman template DNA (10). PCR products were cloned into pET15b to express recombinant proteins with an N-terminal His6 tag fusion. Bacteria were disrupted in a French press; membrane and insoluble components were sedimented by ultracentrifugation. His-tagged Emp was purified by affinity chromatography in its native state. Extract containing Eap was solubilized at room temperature in 8 M urea, 50 mM Tris-HCl, pH 8.0 for 4–5 h, then centrifuged at 10,000 g. The supernatant containing the denatured protein was subjected to nickel-nitrilotriacetic acid (Ni-NTA) affinity chromatography (Promega, Madison, WI, USA). Protein was eluted in PBS-8 M urea containing successively higher concentrations of imidazole (100–500 mM). Eluate fractions positive for Eap were pooled, diluted into PBS-1 M urea and passed over a second Ni-NTA column. Refolded Eap was eluted with PBS buffer containing imidazole. Protein concentration was determined by absorbance at 280 nm. Rabbits (6-mo-old New Zealand white, females, Charles River Laboratories, Germantown, MD, USA) were immunized with 500 μg protein emulsified in CFA (Difco, Franklin Lakes, NJ, USA) for initial immunization or IFA for booster immunizations on d 24 and 48. On d 60, rabbits were bled, and serum was recovered for immunoblotting, immune-fluorescence microscopy, or passive transfer experiments.
Scanning electron microscopy
Infected kidneys were fixed for 24–48 h in 8% glutaraldehyde at 4°C and sectioned into 2- to 5-mm pieces to expose internal tissues or abscesses. Samples were dehydrated by successive incubations in 25, 50, 75, 90, and 100% ethanol, followed by 100% hexamethyldisilazane. Following dehydration, samples were mounted and sputter-coated with 80% Pt/20% Pd to 8 nm prior to viewing with a Fei Nova NanoSEM 200 scanning electron microscope (FEI Co., Hillsboro, OR, USA).
Renal abscess
Overnight cultures of S. aureus Newman were inoculated 1:100 into fresh tryptic soy broth and grown for 2 h at 37°C. Staphylococci were sedimented, washed with 1× PBS, and suspended in a volume of PBS to yield an A600 of 0.6 [1×108 colony-forming units (CFU)/ml]. The inoculum was verified by plating and colony enumeration. Mice were anesthetized by intraperitoneal injection of 100 mg/ml of ketamine and 2 mg/ml of xylazine per kilogram of body weight. Six- to 8-wk-old female BALB/c mice (Charles River Laboratories) were infected with 100 μl of bacterial suspension (1×107 CFU) by retro-orbital injection. Cohorts of 20 mice were infected per staphylococcal strain. On d 5 following infection, mice were killed by CO2 inhalation; then they were dissected, and their kidneys were excised and homogenized in 0.01% Triton X-100 using a sonicator. Aliquots (20 μl) were serially diluted, plated, and incubated for determination of CFU. At least 8 right kidneys from each cohort of mice were fixed in 10% formalin for 24 h at room temperature. Tissues were embedded in paraffin, thin-sectioned, stained with hematoxylin and eosin (H&E), and examined by microscopy. Three- to 4-wk-old female BALB/c mice were used for persistence studies.
Immunofluorescence microscopy
Kidneys of infected animals were dissected, placed in 1× PBS on ice, and then flash frozen in Tissue Tek OCT Compound (Sakura Finetek, Torrance, CA, USA) within cryomolds. Samples were thin sliced (4-μm thick), mounted on slides, and stored at −80°C. Before staining, slides were warmed to room temperature for 30 min, fixed in ice-cold acetone for 10 min, and washed twice with ice-cold PBS. The slides were blocked in 3% BSA, 1:20 human IgG (Sigma, St. Louis, MO, USA), 1× PBS, and 0.1% Tween-80 for 1 h at room temperature with shaking. Specific rabbit antibody (1:2000) was added to the mixture, and slides were allowed to incubate for another hour. The solution was decanted, and glass slides were washed 3× with PBS and 10-min incubations each. Slides were placed in 3% BSA, 1× PBS, 0.1% Tween-80, and 1:200 AlexaFluor-647 mouse anti-rabbit secondary antibody (Molecular Probes, Eugene, OR, USA) and allowed to incubate at room temperature in the dark, with shaking. The solution was decanted; slides were washed 3× with PBS, placed in PBS containing 1:1000 Hoechst dye (Invitrogen, Carlsbad, CA, USA) as well as 1 μg/ml boron-dipyrromethene (BODIPY)-vancomycin, and allowed to incubate in the dark for 5 min with shaking. The slides were washed once more with PBS, mounted in N-propylgallate, and viewed under a Leica SP5 AOBS spectral 2-photon confocal microscope (Leica Microsystems, Wetzlar, Germany).
Active and passive immunization
BALB/c mice (n=15) were immunized with purified Eap or Emp or PBS on d 0 and 11. On d 20 following immunization, 5 mice were bled to obtain sera to determine antibody titers; on d 21, all mice were challenged with 1 × 107 CFU S. aureus Newman. Five days following infection, kidneys were removed during necropsy, and renal tissue was analyzed for staphylococcal load or histopathology. Rabbit Eap or Emp antibodies were purified by affinity chromatography (purified Eap or Emp covalently linked to Sepharose) and transferred by intraperitoneal injection into mice. Passively immunized animals were challenged 24 h later by retro-orbital injection with 1 × 107 CFU S. aureus Newman. Serum IgG titers of actively or passively immunized animals were analyzed by ELISA. Four days following infection, kidneys were removed during necropsy, and renal tissue was analyzed for staphylococcal load or histopathology.
RESULTS
Animal model for staphylococcal abscess formation and persistent infection
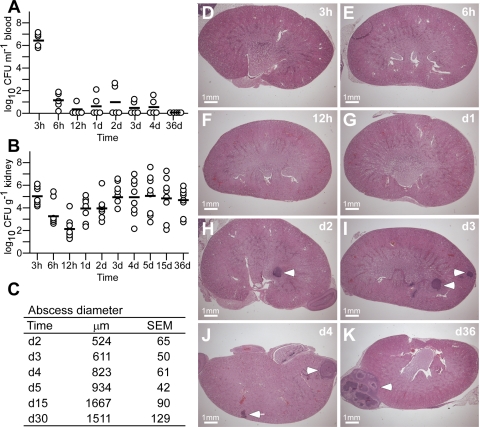
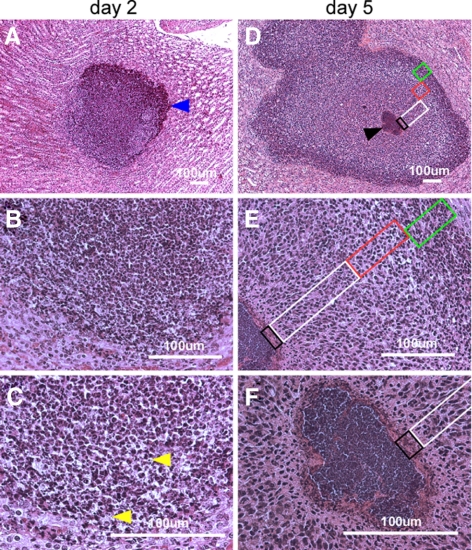
To characterize the pathogenesis of S. aureus abscess formation, we modified the renal abscess model (11), wherein BALB/c mice were infected by intravenous injection with 1 × 107 CFU of the human clinical isolate S. aureus Newman (10). Within 6 h after infection, 99.999% of staphylococci disappeared from the bloodstream and were distributed via the vasculature (Fig. 1A). Staphylococcal dissemination to peripheral tissues occurred rapidly, as the bacterial load in kidney and other peripheral organ tissues reached 1 × 105 CFU/g within the first 3 h (Fig. 1B). The staphylococcal load in kidney tissues increased by 1.5 log CFU within 24 h (Fig. 1B). Forty-eight hours following infection, mice developed disseminated abscesses in multiple organs, detectable by light microscopy of H&E-stained, thin-sectioned kidney tissue (Fig. 1D–K). The initial average abscess diameter was 524 ± 65 μM; lesions were initially marked by an influx of polymorphonuclear leukocytes (PMNs) and harbored no discernable organization of staphylococci, most of which appeared to reside within PMNs (Fig. 2A–C). On d 5 of infection, abscesses had increased in size and enclosed a central population of staphylococci, surrounded by a layer of eosinophilic, amorphous material, and a large cuff of PMNs (Fig. 2D–F). Histopathology revealed massive necrosis of PMNs in proximity to the staphylococcal nidus at the center of abscess lesions, as well as a mantle of healthy phagocytes (Fig. 2D–F). We also observed a rim of necrotic PMNs at the periphery of abscess lesions, bordering eosinophilic, amorphous material that separated healthy renal tissue from the infected lesion (Fig. 2D–F). Abscesses eventually reached a diameter of ≥1500 μM on d 15 or 36 (Fig. 1K). At later time intervals, staphylococcal load was increased to 104–106 CFU/g, and growing abscess lesions migrated toward the organ’s capsule (Fig. 1J, K). Peripheral lesions were prone to rupture, thereby releasing necrotic material and staphylococci into the peritoneal cavity or the retroperitoneal space. These events resulted in bacteremia, as well as a secondary wave of abscesses, eventually precipitating a lethal outcome of these infections (data not shown).
Figure 1.
Staphylococcal abscess formation following intravenous infection of mice. A) BALB/c mice were infected with 1 × 107 CFU of S. aureus Newman by retro-orbital injection. Cohorts of 5 mice were examined by cardiac puncture at timed intervals for bacterial load in blood; sample aliquots were plated on agar medium, and colony formation was enumerated (CFU/ml blood). Black bars indicate means of observations. B) Dissemination of S. aureus Newman into peripheral organ tissues and replication of the pathogen was measured at timed intervals in the kidneys of mice (cohorts of 10 animals), which were homogenized and plated on agar medium for colony formation and enumeration. C) Diameter of abscess lesions was measured in thin-sectioned H&E-stained tissues of infected kidneys at timed intervals. D–K) Images of infected kidneys at timed intervals analyzed in thin-sectioned H&E-stained tissues. Arrowheads indicate abscess lesions.
Figure 2.
Histopathology of SACs. BALB/c mice were infected with S. aureus Newman via retro-orbital injection. Thin-sectioned, H&E-stained tissues of infected kidneys on d 2 (A–C) and d 5 following infection (D–F) were analyzed by light microscopy, and images were captured. On d 2, a massive infiltrate (A, blue arrowhead) of PMNs with occasional intracellular staphylococci (C, yellow arrowheads) are characteristic of early infectious lesions. By d 5, staphylococcal abscess communities developed as a central nidus (D, black arrowhead). Staphylococci were enclosed by an amorphous, eosinophilic pseudocapsule (black box) and surrounded by a zone of dead PMNs (white box), a zone of apparently healthy PMNs (red box), and a rim of necrotic PMNs (green box), separated through an eosinophilic layer from healthy kidney tissue.
Staphylococcal abscess communities (SACs) are enclosed by a pseudocapsule
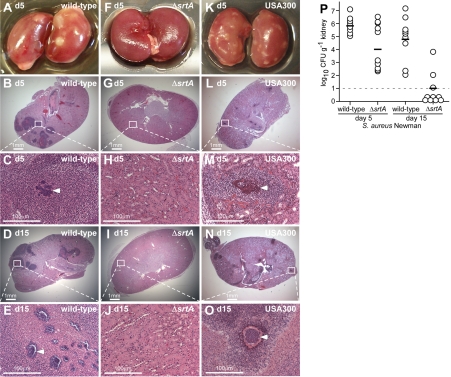
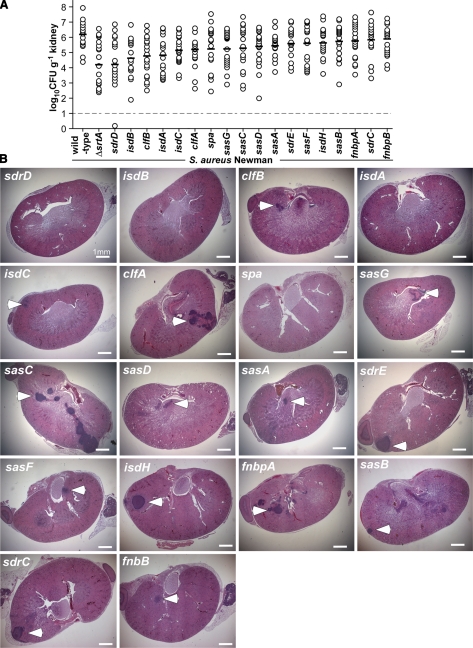
To enumerate staphylococcal load in renal tissue, animals were killed, their kidneys were excised, and tissue homogenate was spread on agar medium for colony formation. On d 5 of infection, we observed a mean of 1 × 106 CFU/g renal tissue for S. aureus Newman (Fig. 3). To quantify abscess formation, kidneys were visually inspected, and each individual organ was given a score of 1 (Fig. 3A) or 0 (Fig. 3F). The final sum was divided by the total number of kidneys to calculate percentage surface abscesses (Table 1). In addition, randomly chosen kidneys were fixed in formalin, embedded, thin sectioned, and stained with H&E. For each kidney, 4 sagittal sections at 200-μM intervals were viewed by microscopy (Fig. 3). The numbers of lesions were counted for each section and averaged to quantify the number of abscesses within kidneys. S. aureus Newman caused 4.4 ± 0.9 abscesses/kidney, and surface abscesses were observed on 14 of 20 kidneys (70%) (Table 1).
Figure 3.
Sortase A is required for abscess formation and staphylococcal persistence in host tissues. Kidneys of BALB/c mice (cohorts of 10 animals) infected with S. aureus Newman, its isogenic sortase A mutant (ΔsrtA), or methicillin-resistant S. aureus USA300 were removed during necropsy of animals at d 5 and 15 following inoculation. A–O) Kidneys were inspected for surface abscesses (A, F, K) or fixed in formalin, embedded, thin sectioned, and stained with H&E. Histopathology images were acquired with light microscopy at ×12.5 (B, G, L, D, I, N) and ×200 (C, H, M, E, J, O). P) Staphylococcal replication and persistence in kidney tissue was measured at d 5 and 15 following infection. Kidneys were removed from infected mice during necropsy; tissue was homogenized and plated on agar medium for colony formation and enumeration.
TABLE 1.
Genetic requirements for S. aureus Newman abscess formation in mice
| Genotype
|
Staphylococcal load in kidney tissue
|
Abscess formation in kidney tissue
|
||||
|---|---|---|---|---|---|---|
| Load (log10 CFU/g)a | Significance (P)b | Reduction (log10 CFU/g)c | Surface abscesses (%)d | Abscesses per kidneye | Significance (P)b | |
| Wild type | 6.141 ± 0.192 | — | — | 70 | 4.4 ± 0.9 | — |
| ΔsrtA
|
4.095 ± 0.347
|
6.7 × 10−6
|
2.046
|
0
|
0.0 ± 0.0
|
0.0216
|
| Surface protein genes
| ||||||
| sdrD | 4.092 ± 0.454 | 0.0001 | 2.049 | 15 | 0.6 ± 0.3 | 0.0265 |
| isdB | 4.535 ± 0.298 | 5.7 × 10−5 | 1.606 | 5 | 0.5 ± 0.2 | 0.0227 |
| clfB | 4.672 ± 0.302 | 0.0001 | 1.469 | 30 | 1.9 ± 0.7 | 0.1298 |
| isdA | 4.723 ± 0.299 | 0.0002 | 1.418 | 15 | 0.4 ± 0.2 | 0.0350 |
| isdC | 5.050 ± 0.208 | 0.0004 | 1.091 | 27 | 1.0 ± 0.3 | 0.0737 |
| clfA | 5.103 ± 0.260 | 0.0025 | 1.038 | 40 | 1.1 ± 0.4 | 0.0848 |
| spa | 5.137 ± 0.374 | 0.0144 | 1.004 | 13 | 0.4 ± 0.4 | 0.0356 |
| sasG | 5.139 ± 0.287 | 0.0054 | 1.002 | 45 | 1.2 ± 0.4 | 0.0770 |
| sasC | 5.193 ± 0.337 | 0.0167 | 0.948 | 56 | 1.4 ± 0.6 | 0.1335 |
| sasD | 5.312 ± 0.291 | 0.0212 | 0.829 | 48 | 1.5 ± 0.5 | 0.1272 |
| sasA | 5.355 ± 0.217 | 0.0102 | 0.786 | 39 | 2.3 ± 0.5 | 0.2568 |
| sdrE | 5.498 ± 0.255 | 0.0475 | 0.643 | 65 | 2.3 ± 0.7 | 0.5023 |
| sasF | 5.518 ± 0.318 | 0.0884 | 0.623 | 47 | 1.3 ± 0.4 | 0.3187 |
| isdH | 5.555 ± 0.251 | 0.0676 | 0.586 | 44 | 1.1 ± 0.5 | 0.0859 |
| sasB | 5.650 ± 0.255 | 0.1641 | 0.491 | 59 | 1.7 ± 0.6 | 0.1651 |
| fnbA | 5.678 ± 0.270 | 0.1294 | 0.463 | 51 | 2.1 ± 0.7 | 0.2338 |
| sdrC | 5.693 ± 0.287 | 0.1908 | 0.448 | 33 | 1.0 ± 0.4 | 0.0741 |
| fnbB | 5.823 ± 0.246 | 0.3124 | 0.318 | 54 | 2.0 ± 0.6 | 0.2074 |
| PNAG (PIA) genes | ||||||
| icaA | 5.326 ± 0.452 | 0.1122 | 0.815 | 40 | 2.7 ± 1.5 | 0.5768 |
| icaB | 5.894 ± 0.306 | 0.4917 | 0.247 | 35 | 1.0 ± 0.3 | 0.2690 |
| icaC | 5.651 ± 0.441 | 0.3004 | 0.491 | 35 | 2.0 ± 1.5 | 0.4384 |
| icaD | 5.886 ± 0.278 | 0.4394 | 0.255 | 45 | 1.7 ± 0.7 | 0.3741 |
| icaR | 6.201 ± 0.309 | 0.8837 | +0.06 | 60 | 2.3 ± 0.3 | 0.5018 |
| ica:tet | 5.692 ± 0.280 | 0.1909 | 0.449 | 55 | 2.3 ± 0.7 | 0.5023 |
| Envelope-associated protein genes | ||||||
| eap | 6.530 ± 0.385 | 0.1217 | +0.49 | 55 | 1.3 ± 0.4 | 0.0971 |
| emp | 5.540 ± 0.040 | 0.0576 | 0.601 | 20 | 0.8 ± 0.4 | 0.0361 |
| Capsular polysaccharide genes | ||||||
| capO | 6.028 ± 0.579 | 0.9825 | 0.113 | 50 | 3.0 ± 1.0 | 0.6035 |
Means ± se of staphylococcal load were calculated as log10 CFU/g in homogenized renal tissues 5 d following infection in cohorts of 15 BALB/c mice per challenge strain.
Statistical significance was calculated with the Students t test; values of P < 0.05 were deemed significant.
Reduction in bacterial load calculated as log10 CFU/g.
Abscess formation in kidney tissues 5 d following infection was measured by macroscopic inspection (% positive).
Histopathology of H&E-stained, thin-sectioned kidneys from 8–10 animals; average number of abscesses per kidney was recorded and averaged again for the final mean ± se.
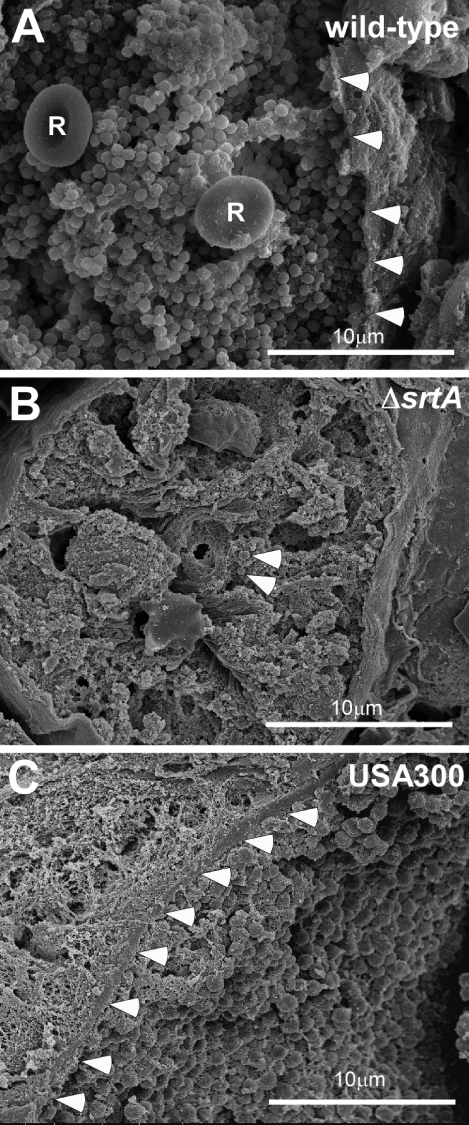
Kidneys were sectioned, fixed, dehydrated, and sputter-coated with platinum/palladium for scanning electron microscopy. Figure 4A shows S. aureus Newman in tightly associated lawns at the center of abscesses. Staphylococci were contained by an amorphous pseudocapsule (Fig. 4A, arrowheads) that separated bacteria from the cuff of abscess leukocytes. No immune cells were observed in these central nests of staphylococci; however, occasional red blood cells were located among the bacteria (Fig. 4A, R). Bacterial populations at the abscess center, designated SACs, appeared homogenous and were coated by an electron-dense, granular material. The kinetics of the appearance of infectious lesions and the morphological attributes of abscesses formed by S. aureus Newman were similar to those observed following mouse infection with S. aureus USA300 (LAC), the current epidemic community-acquired methicillin-resistant S. aureus (CA-MRSA) clone in the United States (12) (Figs. 3K–O and 4C).
Figure 4.
Staphylococcal communities at the center of abscess lesions. Kidney tissue from mice infected with S. aureus Newman (wild type), its isogenic sortase A mutant (ΔsrtA), or MRSA strain USA300 was sectioned, fixed, dehydrated, and sputter-coated with 80% Pt/20% Pd for scanning electron microscopy. A) Wild-type pathogen is organized as a tightly associated lawn, the SAC, at the abscess center that is contained within an amorphous pseudocapsule (arrowheads), separating SACs from the cuff of leukocytes. Red blood cells (R) are located among staphylococci. B) The sortase mutant, ΔsrtA (arrowheads) did not form SACs, and isolated staphylococci were found in healthy kidney tissue. C) Similar to S. aureus Newman, MRSA strain USA300 also formed SACs contained within a pseudocapsule (arrowheads).
Sortase mutants cannot establish abscess lesions and fail to persist
Sortase A is a transpeptidase that immobilizes 19 surface proteins in the envelope of S. aureus strain Newman (13, 14). Earlier work identified sortase A as a virulence factor in multiple animal model systems; however, the contributions of this enzyme and its anchored surface proteins to abscess formation or persistence have not yet been revealed (15, 16). Compared to the wild-type parent (10), an isogenic srtA variant (ΔsrtA) failed to form abscess lesions on either macroscopic or histopathology examination on d 2, 5, or 15 (Fig. 3F–J and Table 1). In mice infected with the strA mutant, only 1 × 104 CFU/g was recovered from kidney tissue on d 5 of infection, which is a 2.046 log10 CFU/g reduction compared to the wild-type parent strain (P=6.73×10−6) (Fig. 2P). A similar defect was observed for the srtA mutant of MRSA strain USA300 (data not shown). Scanning electron microscopy showed that srtA mutants (Fig. 4B, arrowheads) were highly dispersed and often associated with leukocytes in otherwise healthy renal tissue. On d 15 following infection, srtA mutants were cleared from renal tissues, a ≥3.5 log10 CFU/g reduction compared to the wild-type (Fig. 2P). Thus, sortase A-anchored surface proteins enable the formation of abscess lesions and the persistence of bacteria in host tissues, wherein staphylococci replicate as communities embedded in an extracellular matrix and shielded from surrounding leukocytes by an amorphous pseudocapsule.
Genetic requirements for staphylococcal surface proteins
Sortase A anchors a large spectrum of proteins with LPXTG motif sorting signals to the cell-wall envelope, thereby providing for the surface display of many virulence factors (17). To identify surface proteins required for staphylococcal abscess formation, we introduced bursa aurealis insertions in 5′ coding sequences of genes that encode polypeptides with LPXTG motif proteins (9) and transduced these mutations into S. aureus Newman. Following intravenous infection of mice and analysis through the renal abscess model, the severity of observed virulence defects was rank ordered as the log10 reduction of the means of staphylococcal CFU/g (Fig. 5 and Table 1). Mutations in sdrD, isdB, clfB, isdA, clfA, and isdC caused reduced bacterial load (Table 1). We considered mutants with <30% surface abscesses and histology abscess average P < 0.05 as significant for defects in abscess formation, which included variants with mutations in sdrD, isdB, and isdA (Table 1). Interestingly, mutations in clfA and clfB exhibited defects in staphylococcal load but not in abscess formation (Fig. 5). These virulence findings are in agreement with previous studies suggesting that clumping factor proteins mediate fibrinogen binding, as well as resistance to phagocytic clearance, attributes required for pathogen survival and dissemination in blood (18, 19). Protein A impedes phagocytosis by binding the Fc component of immunoglobulin (20, 21), activates platelet aggregation via the von Willebrand factor (22), functions as a B-cell superantigen by capturing the Fab region of VH3 bearing IgM (23), and, through its activation of TNFR1, can initiate staphylococcal pneumonia (24). Protein A mutants (spa) exhibited a modest reduction in staphylococcal load (d 5); however, in contrast to wild-type, clfA, and clfB strains, the ability of spa variants to form abscesses was diminished (Fig. 5 and Table 1).
Figure 5.
Formation of SACs requires specific surface proteins. A) S. aureus Newman variants with bursa aurealis insertions in surface-protein genes were examined 5 d following infection of BALB/c mice (cohorts of 20 animals) for bacterial load in homogenized kidney tissues. B) H&E-stained thin sections of infected kidneys were examined by light microscopy at ×12.5 for abscess lesions (arrowheads).
Staphylococcal carbohydrates and envelope-associated proteins
S. aureus elaborates two carbohydrate structures, capsular polysaccharide (CPS) (25) and poly-N-acetylglucosamine (PNAG) (26). S. aureus Newman and USA300 synthesize type 5 CPS, which is composed of a repeating trisaccharide subunit [→4)-β-D-ManAcA-(1→4)-α-L-FucNAc(3OAc)-(1→3)-β-D-FucNAc-(1→] (10). Nucleotide sequences of the cap5 gene cluster comprise a 16-gene operon (capA-P) and two of its products, CapP and CapO, function as epimerase and dehydrogenase in the synthesis UDP-N-acetylmannosaminuronic acid (UDP-ManNAcA) (27, 28). As expected, bursa aurealis insertion into capO abrogated CPS5 synthesis (data not shown). PNAG (or PIA), a linear β (1–6)-linked glucosaminoglycan, is composed of 2-deoxy-2-amino-d-glucopyranosyl residues, of which 80–85% are N-acetylated (29); the remaining glucosamine residues are positively charged and promote association of the polysaccharide with the bacterial envelope (30). PNAG is synthesized by products of the intercellular adhesin locus (icaADBC) (31, 32). Both S. aureus carbohydrate structures were dispensable for the pathogenesis of animal infections, as mutations in capO, as well as icaADBC or the regulator icaR did not affect bacterial load on d 5, the establishment of staphylococcal communities or renal abscess formation (Table 1).
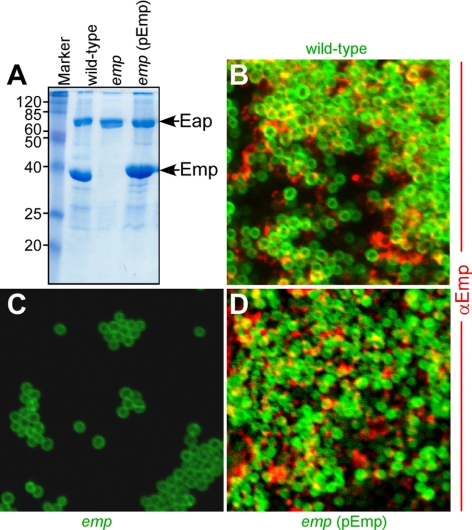
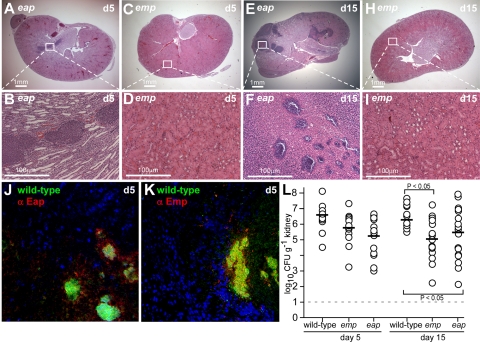
We also examined the contribution of envelope-associated proteins to staphylococcal abscess formation. The hallmark of envelope-associated proteins is that they can be extracted by boiling in hot SDS. We used this method to detect the deposition of two such proteins, Eap and Emp, in the envelope of S. aureus Newman (Fig. 6). A mutant with bursa aurealis insertion in emp displayed reduced bacterial load in kidney tissue on d 5 of infection, in addition to significant defects in the formation of abscesses and in bacterial persistence within host tissues (Fig. 7, Table 1). No reduction in abscess formation was observed for the eap mutant; however, the reduced staphylococcal load on d 5 and 15 indicates a defect in bacterial persistence within host tissues (Fig. 7 and Table 1). Expression of Emp and Eap during infection was detected with immunofluorescence experiments (Fig. 7J, K). Eap was found deposited within the pseudocapsule, whereas Emp was detected in SACs. These observations support a model, whereby Emp contributes to the formation of staphylococcal communities that elicit abscess lesions, whereas Eap deposition in the pseudocapsule promotes bacterial persistence in host tissues.
Figure 6.
The envelope-associated protein Emp is a component of the extracellular matrix in staphylococcal biofilms. A) Coomassie-stained SDS-PAGE identified envelope-associated proteins Eap and Emp and characterized a bursa aurealis mutant (emp) with (pEmp) and without complementing plasmid. B–D) Expression of Emp in the extracellular matrix of in vitro-formed biofilms was detected by microscopy, using BODIPY-vancomycin-stained staphylococci (green) and AlexaFluor-647-labeled secondary antibody to recognize the deposition of rabbit anti-Emp (red).
Figure 7.
Emp and Eap in staphylococcal abscess lesions. Kidneys of BALB/c mice infected with S. aureus Newman variants carrying bursa aurealis insertions in emp or eap were removed at d 5 and 15 following inoculation. A–K) Kidneys were stained with H&E, and histopathology images were acquired with light microscopy at ×12.5 (A, C, E, H) and ×200 (B, D, F, I). Expression of Eap (J) and Emp (K) in abscess lesions of wild-type S. aureus Newman was detected with rabbit anti-Emp or anti-Eap and secondary AlexaFluor-647 labeled antibodies (red) in renal tissue stained with Hoechst dye (blue) to detect nuclei of polymorphonuclear leukocytes, and with BODIPY-vancomycin (green) to reveal staphylococcal abscess communities. L) Staphylococcal replication and persistence in kidney tissue was measured at d 5, 15, and 30 following intravenous inoculation. Kidneys were removed from infected mice (cohorts of 10 animals), and tissue was homogenized and plated on agar medium for colony formation and enumeration.
Envelope-associated proteins as vaccine antigens
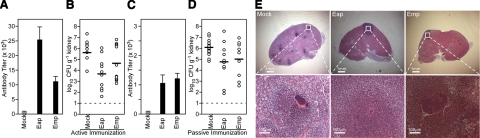
Previous work sought to characterize S. aureus vaccine antigens by interrogating purified sortase A substrates for their ability to elicit protective immunity toward staphylococcal disease (33). When used as individual subunit vaccine antigens, surface proteins generated variable degrees of protection; immunization with SdrD, IsdA, IsdB, SdrE, SpA, ClfA, and ClfB achieved a significant reduction in bacterial load; however, none of these vaccines afforded complete protection. In contrast, a combination of four antigens generated much more robust vaccine protection against abscess formation or lethal challenge with several different S. aureus strains (33). We purified recombinant Eap and Emp from E. coli and used these proteins to immunize BALB/c mice for subsequent challenge with S. aureus Newman (Fig. 8). Following immunization, mice developed humoral immune responses against both envelope-associated proteins (Fig. 8A). Immunization with Emp caused a modest 0.959 log10 CFU/g reduction in staphylococcal load within kidney tissues (P=0.5114), whereas a significant level of protection was achieved with Eap (1.939 log10 CFU/g reduction in bacterial load, P=0.0079) (Fig. 8B). To test whether Emp- or Eap-specific antibodies can provide protection against staphylococcal challenge, we immunized rabbits and purified Emp- as well as Eap-specific antibodies by affinity chromatography. Passive immunization with 5 mg/kg (85 μg/animal) purified antibodies into the peritoneal cavity of naive BALB/c mice resulted in low, but detectable, levels of serum IgG 24 h following transfer (antibody titers of 1000±110 for Eap and 1124±236 for Emp, Fig. 8C). In parallel, passively immunized animals were challenged by intravenous inoculation with S. aureus Newman, which, when compared to mock controls, resulted in a 1.36 log10 CFU/g reduction in staphylococcal load for Eap-immunized animals (n=10) on d 4 (P=0.0085) and a reduction in the number of abscesses formed (4.6±1.1 vs. 1.4±0.5 abscesses/kidney for mock-treated and Eap-immunized mice; P=0.028, n=14 and 10; Fig. 8D, E). Animals (n=9) that received Emp-specific antibodies displayed a 1.20 log10 CFU/g reduction in staphylococcal load on d 4 (P=0.0132), but only a slightly reduced number of abscesses formed (2.0±1, P=0.1362; Fig. 8D, E). In summary, similar to sortase-anchored surface proteins, antibodies against envelope-associated factors can generate protection against staphylococcal infection in mice.
Figure 8.
Active and passive immunization with Eap generates protection from staphylococcal challenge. A) BALB/c mice were immunized with purified Eap or Emp or mock treated with adjuvant alone, and serum IgG titers were analyzed by ELISA. B) Three weeks following immunization, animals were challenged via intravenous inoculation of staphylococci. Five days following infection, kidneys were removed during necropsy, and renal tissue was analyzed for staphylococcal load or histopathology. C) Rabbit antibodies directed against Eap or Emp were purified by affinity chromatography and passively transferred by intraperitoneal injection into mice. Twenty-four hours later, serum IgG titers of passively immunized animals were analyzed by ELISA. D) Animals passively immunized with purified antibodies against Eap or Emp, as well as mock-immunized animals were subsequently challenged with S. aureus Newman, and bacterial load was enumerated on d 4. E) Abscess formation in kidneys was detected in thin-sectioned, H&E-stained tissues.
DISCUSSION
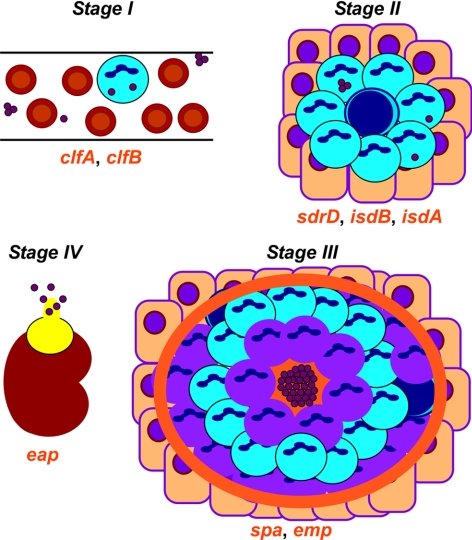
We propose that S. aureus employs its virulence factors to establish abscess lesions as a prerequisite for its persistence in host tissues. A working model for this proposed developmental process is illustrated in Fig. 9. Following intravenous inoculation of mice, staphylococci survive in the bloodstream and rapidly disseminate to peripheral organ tissues (stage 1). In a recent study, we observed that mutations in two surface-protein genes, clfA and clfB, display defects of survival in murine blood (unpublished results). This defect is associated with a reduction in staphylococcal load in kidney tissue on d 5; however, it does not affect the ability of clfA and clfB variants to form abscess lesions, in agreement with their proposed role for escape from opsonophagocytosis (Fig. 5). We propose that stage 2 commences ∼48 h after infection with localized polymorphonuclear leukocytic infiltrates and few associated staphylococci (Fig. 2). Within 4–5 d, these lesions have converted to a complex structure with staphylococcal communities at their center, enclosed by an amorphous pseudocapsule (stage 3). The eosinophilic pseudocapsule is reminiscent of the Splendore-Hoeppli phenomenon, a histopathology finding of immunoglobulin deposits surrounding nocardia (34). It seems plausible that the pseudocapsule functions as a barrier, separating staphylococcal communities from surrounding immune cells. The cuff of polymorphonuclear leukocytes can be further characterized as a broad inner layer of mostly necrotic cells, followed by a second layer of apparently healthy cells, and finally a third and outer layer with intense cell death and a rim of eosinophilic material, separating healthy tissue from the lesion. Mutations in heme-scavenging genes (isdA and isdB), as well as sdrD, cause large reductions in bacterial load on d 5 in addition to defects in abscess formation, consistent with the possibility that these genes act during stages 2 and 3. Most certainly, staphylococci must require heme-iron scavenging via IsdA and IsdB for expansive growth during these stages. Protein A (spa) and emp mutations do not greatly affect the bacterial load on d 5 and instead reduce the number of abscesses, suggesting that these genes function at a later time (stage 3). Staphylococcal abscesses mature over weeks and, following rupture and release into the peritoneal cavity, lead to new infectious lesions (stage 4). Mutations in eap have no effect on staphylococcal load or early abscess formation (d 5); however, these variants are defective in persistence and are, therefore, assigned to stage 4 (35). Notably, C57/BL6 mice, as well as BALB/c mice, are unable to clear S. aureus from their tissues and eventually succumb to infection. At the time of death, a large number of abscesses at all stages of development can be found in the kidneys, liver, spleen, brain, heart, and skeletal system, as well as in the skin and soft tissues (data not shown).
Figure 9.
A working model for staphylococcal abscess formation and persistence in host tissues. Stage I: following intravenous inoculation, S. aureus survives in the bloodstream and disseminates via the vasculature to peripheral organ tissues. Stage II: in renal tissues, staphylococci attract a massive infiltrate of polymorphonuclear leukocytes and other immune cells. Stage III: abscesses mature with a central accumulation of the pathogen (SAC), enclosed by an eosinophilic pseudocapsule. The SAC is surrounded by a zone of dead PMNs, apparently healthy PMNs, and finally an outer zone of dead PMNs with a rim of eosinophilic material. Stage 4: abscesses mature and rupture on the organ surface, thereby releasing staphylococci into circulation and initiating new rounds of abscess development. Genes for bacterial envelope components that are required for specific stages of staphylococcal abscess development are in red below the corresponding stage during which these genes function. See text for details.
By comparing the virulence attributes of surface proteins toward establishment of SACs and persistence in host tissues with their ability to raise protective immunity, one can draw several conclusions. Three surface proteins of S. aureus are required for abscess formation and represent leading protective antigens for a combination vaccine—IsdA, IsdB, and SdrD (33). Two surface proteins ensure staphylococcal escape from phagocytic clearance but are not required for abscess formation—ClfA and ClfB (18, 19). Antibodies to ClfA or ClfB reduce staphylococcal load similar to insertion mutations in clfA and clfB, suggesting that these antibodies may remove staphylococci from circulation, causing secondary effects on the number of abscesses or the speed with which they can be formed (33). Antibodies against protein A also generate significant protection, although it is still unclear whether these antibodies cause phagocytic clearance prior to abscess formation or, alternatively, impact the formation of the eosinophilic barrier that separates staphylococcal communities from immune cells (33). Antibodies against Eap, which is deposited outside the pseudocapsule, afford a significant level of disease protection, whereas antibodies against Emp, which is located within staphylococcal communities, generate limited protective immunity. Thus, with the exception of emp, whose product may not be accessible for adaptive immune responses, all other surface proteins involved in disease pathogenesis can also be thought of as leading candidates for vaccine development.
The microscopic images of SACs resemble biofilm growth, microcolonies on glass or other surfaces that are embedded within an extracellular matrix (36, 37). Many bacterial species switch between planktonic growth and the formation of biofilms, thereby displaying increased antibiotic resistance (38), evasion from host immune defenses (39), or the ability to establish chronic infections in humans (40). Previous research associated biofilms of staphylococcal species with several diseases, including endocarditis (41), osteomyelitis (42), and various implant-mediated infections, including urinary catheters, prosthetic heart valves, and artificial joints (43). This applies in particular to Staphylococcus epidermidis, an opportunistic pathogen that avidly forms biofilms in vitro and in vivo (29). However, several genes that are known to be involved in biofilm growth, e.g., icaADBC and sasG, were here found to be dispensable for the ability of S. aureus Newman to form abscesses or to persist in host tissues. We propose that the ability of staphylococcal communities to elicit the complex architecture of abscess lesions is best appreciated as a series of complex interactions between the pathogen’s virulence factors and the molecules, cells, and tissues of its host; it should not be mistaken for biofilm growth. Further, we think it is likely that many findings on the genetic requirements for disease pathogenesis in S. aureus Newman may be broadly applicable to other staphylococcal strains; however, future work must generate experimental tests for this conjecture.
Acknowledgments
We thank Qiti Guo for electron microscopy, Shirley Bond for light microscopy, Vytas Bindokas for fluorescence microscopy, and members of our laboratories, as well as Guido Grandi and Fabio Bagnoli (Novartis) for discussion. This work was supported, in part, by grants from the National Institute of Allergy and Infectious Diseases, Infectious Diseases Branch (AI42797 to O.S.) and by Novartis Vaccines and Diagnostics (Siena, Italy). A.G.C. was a trainee of the National Institutes of Health Medical Scientist Training Program at The University of Chicago (GM07281). O.S. and D.M.M. acknowledge membership within and support from the Region V (Great Lakes) Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (GLRCE, National Institute of Allergy and Infectious Diseases award 1-U54-AI-057153).
References
- Gorbach S L. Good and laudable pus. J Clin Invest. 1995;96:2545. doi: 10.1172/JCI118316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzianabos A O, Kasper D L, Cisneros R L, Smith R S, Onderdonk A B. Polysaccharide-mediated protection against abscess formation in experimental intra-abdominal sepsis. J Clin Invest. 1995;96:2727–2731. doi: 10.1172/JCI118340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenfang T, Bohl J, Kretzschmar K. Evolution of brain abscess in cats formation of capsule and resolution of brain edema. Neurosurg Rev. 1980;3:101–111. doi: 10.1007/BF01644062. [DOI] [PubMed] [Google Scholar]
- Russell D G. Staphylococcus and the healing power of pus. Cell Host Microbe. 2008;3:115–116. doi: 10.1016/j.chom.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Klevens R M, Morrison M A, Nadle J, Petit S, Gershman K, Ray S, Harrison L H, Lynfield R, Dumyati G, Townes J M, Craig A S, Zell E R, Fosheim G E, McDougal L K, Carey R B, Fridkin S K, Investigators for the Active Bacterial Core surveillance (ABCs) MRSA Investigators Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- Lowy F D. Staphylococcus aureus infections. New Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- Lam G T, Sweeney F J J, Witmer C M, Wise R I. Abscess forming factors produced by Staphylococcus aureus. II. Abscess formation and immunity by Staphylococcus and its mutants. J Bacteriol. 1963;86:87–91. doi: 10.1128/jb.86.1.87-91.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheagren J N. Staphylococcus aureus. The persistent pathogen. N Engl J Med. 1984;310:1368–1373. doi: 10.1056/NEJM198405243102107. [DOI] [PubMed] [Google Scholar]
- Bae T, Banger A K, Wallace A, Glass E M, Aslund F, Schneewind O, Missiakas D M. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc Natl Acad Sci U S A. 2004;101:12312–12317. doi: 10.1073/pnas.0404728101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Bae T, Schneewind O, Takeuchi F, Hiramatsu K. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes. J Bacteriol. 2007;190:300–310. doi: 10.1128/JB.01000-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albus A, Arbeit R D, Lee J C. Virulence studies of Staphylococcus aureus mutants altered in type 5 capsule production. Infect Immun. 1991;59:1008–1014. doi: 10.1128/iai.59.3.1008-1014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep B A, Gill S R, Chang R F, Phan T H, Chen J H, Davidson M G, Lin F, Lin J, Carleton H A, Mongodin E F, Sensabaugh G F, Perdreau-Remington F. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- Mazmanian S K, Liu G, Ton-That H, Schneewind O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science. 1999;285:760–763. doi: 10.1126/science.285.5428.760. [DOI] [PubMed] [Google Scholar]
- Mazmanian S K, Liu G, Jensen E R, Lenoy E, Schneewind O. Staphylococcus aureus mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc Natl Acad Sci U S A. 2000;97:5510–5515. doi: 10.1073/pnas.080520697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson I M, Mazamanian S K, Schneewind O, Vendrengh M, Bremell T, Tarkowski A. On the role of Staphylococcus aureus sortase and sortase-catalyzed surface protein anchoring in murine septic arthritis. J Infect Dis. 2002;185:1417–1424. doi: 10.1086/340503. [DOI] [PubMed] [Google Scholar]
- Weiss W J, Lenoy E, Murphy T, Tardio L, Burgio P, Projan S J, Schneewind O, Alksne L. Effect of srtA and srtB gene expression on the virulence of Staphylococcus aureus in animal infection. J Antimicrob Chemother. 2004;53:480–486. doi: 10.1093/jac/dkh078. [DOI] [PubMed] [Google Scholar]
- Mazmanian S K, Ton-That H, Su K, Schneewind O. An iron-regulated sortase enzyme anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc Natl Acad Sci U S A. 2002;99:2293–2298. doi: 10.1073/pnas.032523999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt D, Francois P, Vaudaux P, Foster T J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- Ní Eidhin D, Perkins S, Francois P, Vaudaux P, Höök M, Foster T J. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol Microbiol. 1998;30:245–257. doi: 10.1046/j.1365-2958.1998.01050.x. [DOI] [PubMed] [Google Scholar]
- Uhlén M, Guss B, Nilsson B, Gatenbeck S, Philipson L, Lindberg M. Complete sequence of the staphylococcal gene encoding protein A. J Biol Chem. 1984;259:1695–1702. and 13628 (Corr.) [PubMed] [Google Scholar]
- Jensen K. A normally occuring staphylococcus antibody in human serum. Acta Path Microbiol Scandin. 1958;44:421–428. doi: 10.1111/j.1600-0463.2007.apm_731a.x. [DOI] [PubMed] [Google Scholar]
- Hartleib J, Kohler N, Dickinson R, Chhatwal G, Sixma J, Hartford O, Foster T J, Peters G, Kehrl B, Herrmann M. Protein A is the von Willebrand factor binding protein of Staphylococcus aureus. Blood. 2000;96:2149–2156. [PubMed] [Google Scholar]
- Roben P W, Salem A N, Silverman G J. Human IgM antibodies bind domain D of staphylococcal protein A. J Immunol. 1995;154:6437–6445. [PubMed] [Google Scholar]
- Gomez M I, Lee A, Reddy B, Muir A, Soong G, Pitt A, Cheung A, Prince A. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med. 2004;10:842–848. doi: 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- Jones C. Revised structures for the capsular polysaccharides from Staphylococcus aureus types 5 and 8, components of novel glycoconjugate vaccines. Carbohydr Res. 2005;340:1097–1106. doi: 10.1016/j.carres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Götz F. Staphylococcus and biofilms. Mol Microbiol. 2002;43:1367–1378. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- O'Riordan K, Lee J C. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev. 2004;17:218–234. doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sau S, Bhasin N, Wann E R, Lee J C, Foster T J, Lee C Y. The Staphylococcus aureus allelic genetic loci for serotype 5 and 8 capsule expression contain the type-specific genes flanked by common genes. Microbiology. 1997;143:2395–2405. doi: 10.1099/00221287-143-7-2395. [DOI] [PubMed] [Google Scholar]
- Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996;178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong C, Kocianova S, Voyich J M, Yao Y, Fischer E R, DeLeo F R, Otto M. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J Biol Chem. 2004;279:54881–54886. doi: 10.1074/jbc.M411374200. [DOI] [PubMed] [Google Scholar]
- Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Götz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol. 1996;20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- Cramton S E, Gerke C, Schnell N F, Nichols W W, Götz F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun. 1999;67:5427–5433. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranger-Jones Y K, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A. 2006;103:16942–16947. doi: 10.1073/pnas.0606863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodig S J, Dorfman D M. Splendore-Hoeppli phenomenon. Arch Pathol Lab Med. 2001;125:1515–1516. doi: 10.5858/2001-125-1515-SHP. [DOI] [PubMed] [Google Scholar]
- Lee L Y, Miyamoto Y J, McIntyre B W, Höök M, McCrea K W, McDevitt D, Brown E L. The Staphylococcus aureus Map protein is an immunomodulator that interferes with T cell-mediated responses. J Clin Invest. 2002;110:1461–1471. doi: 10.1172/JCI16318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton J W, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Kolter R, Greenberg E P. The superficial life of microbes. Nature. 2006;441:300–302. doi: 10.1038/441300a. [DOI] [PubMed] [Google Scholar]
- Drenkard E, Ausubel F M. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Nature. 2002;416:740–743. doi: 10.1038/416740a. [DOI] [PubMed] [Google Scholar]
- Singh P K, Parsek M R, Greenberg E P, Welsh M J. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417:552–555. doi: 10.1038/417552a. [DOI] [PubMed] [Google Scholar]
- Brady R A, Leid J G, Calhoun J H, Costerton J W, Shirtliff M E. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol. 2008;52:13–22. doi: 10.1111/j.1574-695X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- Xiong Y Q, Willard J, Kadurugamuwa J L, Yu J, Francis K P, Bayer A S. Real-time in vivo bioluminescent imaging for evaluating the efficacy of antibiotics in a rat Staphylococcus aureus endocarditis model. Antimicrob Agents Chemother. 2005;49:380–387. doi: 10.1128/AAC.49.1.380-387.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady R A, Leid J G, Camper A K, Costerton J W, Shirtliff M E. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect Immun. 2006;74:3415–3426. doi: 10.1128/IAI.00392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassat J E, Lee C Y, Smeltzer M S. Investigation of biofilm formation in clinical isolates of Staphylococcus aureus. Methods Mol Biol. 2007;391:127–144. doi: 10.1007/978-1-59745-468-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]