Abstract
Although a battery of neuropsychological tests are often used in making a clinical diagnosis of Alzheimer’s disease (AD), definitive diagnosis still relies on pathological evaluation at autopsy. The identification of AD biomarkers may allow for a less invasive and more accurate diagnosis as well as serve as a predictor of future disease progression and treatment response. Importantly, biomarkers may also allow for the identification of individuals who are already developing the underlying pathology of AD such as plaques and tangles yet who are not yet demented, i.e. “preclinical” AD. Attempts to identify biomarkers have included fluid and imaging studies, with a number of candidate markers showing significant potential. More recently, better reagent availability and novel methods of assessment have further spurred the search for biomarkers of AD. This review will discuss promising fluid and imaging markers to date.
Keywords: Alzheimer’s disease, amyloid-β, biomarker, cerebrospinal fluid, neuroimaging, proteomics, tau
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder estimated to affect 5.1 million individuals in 2007 (Alzheimer’s Association, 2007). The diagnosis of AD is largely based upon clinical assessment, with definitive diagnosis still requiring pathological evaluation at autopsy. The identification of biomarkers for AD would allow for a less invasive and more accurate diagnosis in the antemortem period. Additionally, biomarkers may facilitate early diagnosis, which is particularly difficult given that there are no signs or symptoms unique to AD. More importantly, they may allow for the identification of individuals with preclinical AD (those with AD neuropathology that do not yet display clinical symptoms) (Gomez-Isla et al., 1996; Hulette et al., 1998; Markesbery et al., 2006; Morris and Price, 2001; Price et al., 2001). Biomarkers may be instrumental not only in the diagnosis of disease cases, but may aid in following disease progression and response to treatment as well. Finally, biomarkers are key in advancing our understanding of the pathophysiology of AD, which in turn has important implications for patient diagnosis and treatment.
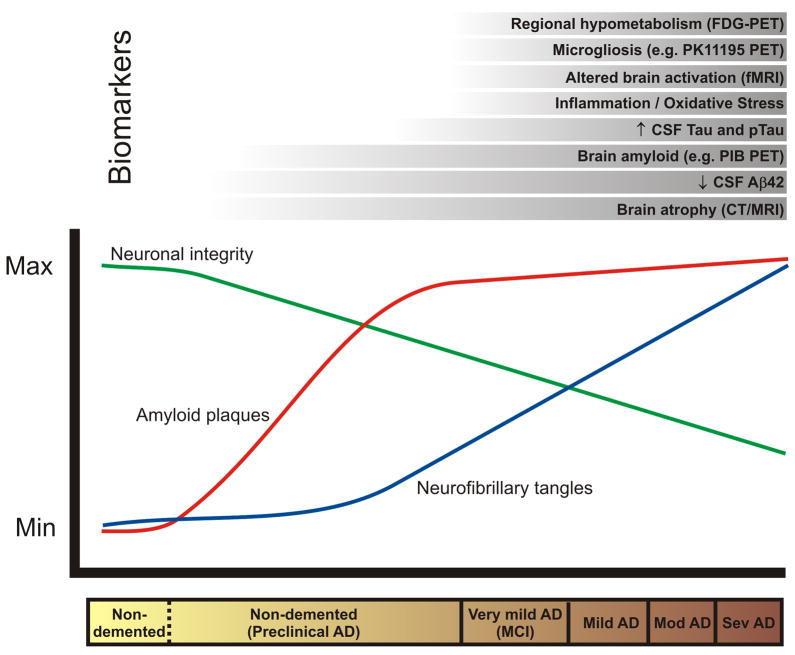
The identification of reliable biomarkers has been hindered by the fact that patient classification relies on clinical diagnosis which is not always accurate, especially at early stages of the disease. Requiring postmortem confirmation of disease diagnosis has been impractical for biomarker studies. Moreover, control groups are likely to contain individuals with preclinical AD. Limited patient sample size and lack of adjustment for covariates such as age, gender, ethnicity, and APOE genotype have restricted the application of results from some studies to the general population. In addition, protocols for sample collection, preparation, and analysis often vary widely between labs, thus contributing additional methodological variability. Adopting standardized protocols for clinical assessment, sample analysis, and statistical evaluation would help overcome many of these shortcomings. Given the multifactorial nature of the disease, it is unlikely that a single biomarker will meet the needs for clinical diagnosis, while a panel of biomarkers may offer the appropriate sensitivity, specificity, and positive and negative predictive values. These limitations not withstanding, many potential biomarkers have been identified, the most promising of which are discussed below and in the accompanying Table. Where in the disease course these various candidate markers may be useful is shown in the Figure.
Table.
Select candidate fluid and imaging biomarkers of AD
AD, indiates clinical diagnosis of dementia believed to be Alzheimer’s disease, not necessarily autopsy confirmed AD cases.
FAD, Familial Alzheimer’s disease
MCI, mild cognitive impairment
Figure 1.
Hypothesized relationship between the timecourse of changes in various biomarkers in relation to the neuropathology and clinical changes of Alzheimer’s disease.
Fluid Biomarkers
APP
The postmortem pathological diagnosis of an AD brain relies on the presence of senile plaques and neurofibrillary tangles. These senile plaques are composed of β amyloid (Aβ), a proteolytic fragment of the Amyloid Precursor Protein (APP). If altered proteolytic processing of APP underlies AD, then measures of APP or its derivatives may serve as diagnostic markers. Indeed, early studies (Ghiso et al., 1989; Kitaguchi et al., 1990; Weidemann et al., 1989) observed increased levels of APP and/or its secreted forms in the cerebrospinal fluid (CSF) of AD individuals. However, later studies have reported decreased (Henriksson et al., 1991; Prior et al., 1991; Van Nostrand et al., 1992) or unchanged (Chong et al., 1990) levels. Several studies of AD patients have shown reduced CSF levels of sAPPα, the soluble product released following α-secretase cleavage of APP (Palmert et al., 1990; Sennvik et al., 2000; Van Nostrand et al., 1992). These inconsistent findings between studies do not currently support a consensus of CSF APP being a useful biomarker for AD.
Aβ
APP is expressed in all tissues and undergoes cleavage by β-secretase to release the ectodomain (sAPP-β) and subsequent cleavage by γ-secretase to release Aβ peptides of 38–43 amino acids (Evin and Weidemann, 2002). Because Aβ42 is the dominant component of the plaques seen in AD (Roher et al., 1993a), many groups have investigated the use of Aβ42, as well as the other Aβ species, as a diagnostic tool. The amount of total Aβ in CSF is not well correlated with the diagnosis of AD (Lannfelt et al., 1995; Southwick et al., 1996; van Gool et al., 1995). The majority of studies have demonstrated a decrease in CSF Aβ42 in AD patients (Andreasen et al., 1999a; Andreasen et al., 2001; Clark et al., 2003; Engelborghs et al., 2008; Fagan et al., 2007; Galasko et al., 1998; Hampel et al., 2004a; Hulstaert et al., 1999; Ida et al., 1996; Kanai et al., 1998; Kanemaru et al., 2000; Kapaki et al., 2001; Kapaki et al., 2003; Lewczuk et al., 2004; Mehta et al., 2000; Motter et al., 1995; Mulder et al., 2002; Otto et al., 2000; Riemenschneider et al., 2000; Rosler et al., 2001a; Sjogren et al., 2002; Sjogren et al., 2000; Skoog et al., 2003; Tamaoka et al., 1997; Vanderstichele et al., 2000); however, there have been a few reports of increased (Jensen et al., 1999) or unchanged (Csernansky et al., 2002; Fukuyama et al., 2000) CSF Aβ42. These discrepancies are likely due to differing methods for assaying samples and varying sizes and selection criteria of patient groups, including the usage of subjects at different points along the disease spectrum.
A number of studies have investigated CSF Aβ42 levels in conjunction with those of tau, the primary protein component of neurofibrillary tangles. In perhaps the most comprehensive analysis of Aβ42 and tau levels to date, Sunderland et al. (2003) assayed 131 AD patients and 72 controls, and performed a meta-analysis of 17 studies of CSF Aβ42 levels and 34 studies of CSF tau levels. In their own patient cohort, they observed significantly lower mean levels of CSF Aβ42 and higher CSF tau in AD compared to controls, but significant overlap between the groups. The results of the meta-analysis mimicked their findings, with an effect size, or difference in levels between AD and controls, of 1.53 for Aβ42 and 1.31 for tau. Several interesting correlations were observed, with tau correlating with the age of the controls but not of the AD individuals, with gender for the AD group only, and with Clinical Dementia Rating (CDR) and Mini Mental State Examination (MMSE) scores, but not duration of illness. While the meta-analysis did not reveal correlations between CSF Aβ42 and any score of dementia severity, age, or duration of illness, there have been studies reporting a negative correlation between Aβ42 and dementia severity (Galasko et al., 1998; Jensen et al., 1999; Samuels et al., 1999) and APOE ε4 dosage (Galasko et al., 1998).
In addition to distinguishing AD from non-demented subjects, decreased levels of CSF Aβ42 have been shown to be predictive of future dementia in MCI patients (Andreasen et al., 2003; Blennow and Hampel, 2003; Hampel et al., 2004a; Hansson et al., 2007; Hansson et al., 2006; Hansson et al., 2008; Herukka et al., 2005; Herukka et al., 2007; Riemenschneider et al., 2002a). Interestingly, significantly decreased CSF Aβ42 has been observed in patients with very mild dementia (MMSE score of 25–28 or CDR 0.5) (Fagan et al., 2007; Riemenschneider et al., 2000), and levels have been reported to decrease from mild to more severe dementia (Jensen et al., 1999; Riemenschneider et al., 2000), suggesting that Aβ42 may be useful in tracking the clinical course of patients.
It is important to consider whether a given biomarker makes sense in the context of the disease pathophysiology. Mouse models of AD have shown that CSF Aβ levels are related to the amount of plaque in the brain (DeMattos et al., 2002), and human studies have shown that increased neocortical and hippocampal plaque burden and cerebral amyloid angiopathy is highly associated with decreased Aβ42 in postmortem CSF (Strozyk et al., 2003). These finding were furthered by Fagan and colleagues (Fagan et al., 2006; Fagan et al., 2007) who reported an inverse relationship between CSF Aβ42 and in vivo plaque load using the amyloid imaging agent Pittsburgh Compound B (PIB) in living humans, supporting the authors’ claim that plaques can function as “sinks” or “traps” of Aβ42, thus decreasing the amount of Aβ42 clearing the brain to the CSF. Other groups have likewise proposed this hypothesis (Motter et al., 1995; Samuels et al., 1999). Recent studies have shown that CSF Aβ42 levels can identify PIB-positive individuals with near 100% sensitivity and greater than 80% specificity (Fagan and Holtzman, unpublished data).
One possible limitation of Aβ42 for AD diagnosis is that decreased CSF levels have also been reported in Frontotemporal Dementia (FTD) (Hulstaert et al., 1999; Riemenschneider et al., 2002b; Sjogren et al., 2000), Creutzfeldt-Jakob disease (CJD) (Clark et al., 2003; Kapaki et al., 2001; Otto et al., 2000), Gerstmann-Straussler-Scheinker syndrome (Clark et al., 2003), 6 amyotrophic lateral sclerosis (Sjogren et al., 2002), multiple system atrophy (Holmberg et al., 2003), and dementia with Lewy bodies (DLB) (Clark et al., 2003; Kanemaru et al., 2000; Vanderstichele et al., 2000). While a number of studies have shown that CSF Aβ40 is unchanged in AD (Fagan et al., 2007; Fukuyama et al., 2000; Lewczuk et al., 2004; Mehta et al., 2000; Shoji et al., 1998), the ratio of Aβ42 to Aβ40, rather than either marker alone, has been demonstrated to better distinguish AD subjects from controls or other dementias and to identify incipient AD in subjects with mild cognitive impairment (MCI) (Hansson et al., 2007; Kanai et al., 1998; Lewczuk et al., 2004; Shoji et al., 1998). The ratios of other markers such as tau / Aβ (Csernansky et al., 2002) tau / Aβ42 (Csernansky et al., 2002; Fagan et al., 2007; Kapaki et al., 2003; Li et al., 2007) and p-tau181 / Aβ42 (Fagan et al., 2007; Maddalena et al., 2003) have similarly been used, and the CSF tau / Aβ42 ratio has been shown to strongly predict future dementia in non-demented cohorts (Li et al., 2007).
While CSF is thought to more closely reflect what is happening in the brain, CSF is not as routinely obtained as blood. However, there has been little consensus among studies as to the relationship between plasma/serum Aβ and AD. Although Mehta et al., (2000) reported increased plasma Aβ40 and Pesaresi et al., (2006) found decreased plasma Aβ42 in AD, most groups have reported no difference in plasma/serum Aβ levels between sporadic AD and controls (Aβ40 and Aβ42 (Fukumoto et al., 2003; Kosaka et al., 1997; Tamaoka et al., 1996), Aβ42 (Mehta et al., 2000; Vanderstichele et al., 2000)). In contrast, plasma Aβ42 has been found to be increased (Kosaka et al., 1997; Scheuner et al., 1996) and Aβ40 decreased (Kosaka et al., 1997) in individuals with autosomal dominant, disease-causing mutations (familial AD, FAD). Based on the findings of an early study showing that plasma Aβ42 is elevated in presymptomatic FAD mutation carriers (Scheuner et al., 1996), a recent study investigated the levels of Aβ42 in asymptomatic first-degree relatives of individuals with sporadic AD (Ertekin-Taner et al., 2008). As compared to controls, plasma Aβ42 was found to be elevated in these subjects, irrespective of APOE ε4 or FAD mutations. The difference between the Aβ42 levels of the sporadic AD relatives and the controls was small, however (14.2±0.6 and 12.3±0.7 pM, respectively). It will be interesting to see in longitudinal studies whether these relatives with increased plasma Aβ42 will go on to develop AD dementia.
Interestingly, several longitudinal studies have found that baseline plasma Aβ42 levels were significantly higher in those cognitively normal individuals who later progressed to AD as compared to those who did not (Mayeux et al., 2003; Mayeux et al., 1999). Additionally, Aβ42 levels were observed to decrease over time in these individuals, suggesting that while plasma Aβ42 does not appear to be a suitable diagnostic marker for AD, it may be a marker for progression (Mayeux et al., 2003). Similarly, a case-cohort study originating from the prospective Rotterdam study found that increased plasma Aβ40 at baseline was associated with an increased risk of AD as well as vascular dementia (VD) (van Oijen et al., 2006).
In a recent study however, any association between plasma Aβ40 or Aβ42 levels and progression from a normal to demented state was lost after adjusting for covariates such as age, cognitive status, cerebrovascular disease, APOE genotype, and kidney function (Lopez et al., 2008). A longitudinal study of MCI patients similarly found no correlation between plasma Aβ species and progression to AD (Hansson et al., 2008). This lack of association between plasma Aβ and AD is further supported by studies demonstrating that plasma Aβ40 and Aβ42 levels do not reflect brain Aβ or plaque levels (Fagan et al., 2006; Freeman et al., 2007) and that there is no correlation between plasma and CSF Aβ42or Aβ40 (Mehta et al., 2001; Vanderstichele et al., 2000).
A number of anti-amyloid clinical trials have aimed at slowing or stopping the progression of AD by decreasing the production of Aβ42, increasing its clearance, or reducing its aggregation. Based on animal findings that immunization with Aβ42 resulted in a reduction of brain amyloid plaques (Janus et al., 2000; Morgan et al., 2000; Schenk et al., 1999), a phase II clinical trial (AN1792, Elan Pharmaceuticals) was undertaken to study its effects in humans. While this trial was cut short because of an increased incidence of meningoencephalitis (6%), a six-year follow up of a subset of the patients from the earlier phase I trial revealed a positive effect on Aβ load and plaque removal, but no effect on cognitive function, clinical outcomes, or long-term survival (Holmes et al., 2008). These findings would appear to cast doubt on the role of Aβ as a culprit in the cognitive decline characteristic of AD. The lack of correlation between amyloid load and dementia severity in clinicopathologic studies would also support this assertion (Arriagada et al., 1992; Bierer et al., 1995). It may be, however, that the immunizations were given too late in the disease course, as the subjects already had mild to moderate dementia at the time of treatment. Studies have shown that brain accumulation of Aβ probably begins 10–20 years before clinical manifestations of the disease (Price and Morris, 1999) and can be imaged with a variety of compounds that can be visualized by PET (see below), and that this accumulation may drive the further accumulation of tau aggregates within vulnerable neurons (Lewis et al., 2001). If these demented patients already have substantial tau aggregation, it may be that the reduction of Aβ cannot reverse the tau-associated pathology and consequent cognitive impairment once the disease has progressed too far. However, this does not mean that Aβ is not promising as a candidate biomarker of AD. A repertoire of biomarkers that can serve as surrogates of underlying disease pathology would be crucial to our diagnosing of AD and following its progression and response to treatment. While it has been shown that CSF Aβ42 reflects the presence of brain amyloid, the results from the Aβ42 immunization trial suggests that tau is likely a better marker to follow for clinical disease progression and clinical outcomes. However, since Aβ load in the brain does not correlate with dementia severity (Arriagada et al., 1992; Bierer et al., 1995), and some degree of tangle pathology can exist in older individuals in the absence of dementia (Bouras et al., 1993; Haroutunian et al., 1999; Price et al., 1991), accurate diagnosis and prognosis of AD will most likely require a combination of these pathology-related biomarkers.
Tau and p-tau
The other pathognomic feature of AD brains, neurofibrillary tangles, is composed primarily of tau, a microtubule-associated protein which has similarly been extensively investigated as a biomarker. Many studies have demonstrated that CSF tau is increased in AD patients (Andreasen et al., 1999b; Andreasen et al., 2001; Andreasen et al., 1998; Arai et al., 1998; Arai et al., 1995; Arai et al., 1997; Blennow et al., 1995; Burger nee Buch et al., 1999; Csernansky et al., 2002; Fagan et al., 2007; Galasko et al., 1998; Golombowski et al., 1997; Green et al., 1999; Hampel et al., 2001; Hampel et al., 1999; Hock et al., 1995; Itoh et al., 2001; Jensen et al., 1995; Kahle et al., 2000; Kanai et al., 1998; Kanemaru et al., 2000; Kurz et al., 1998; Mecocci et al., 1998; Molina et al., 1999; Mori et al., 1995; Motter et al., 1995; Munroe et al., 1995; Nishimura et al., 1998; Rosler et al., 1996; Rosler et al., 2001a; Shoji et al., 1998; Shoji et al., 2002; Sjogren et al., 2002; Sjogren et al., 2000; Skoog et al., 1995; Tato et al., 1995; Vandermeeren et al., 1993; Vigo-Pelfrey et al., 1995).
In AD, tau undergoes abnormal hyperphosphorylation at many sites, and enzyme linked immunosorbent assays (ELISAs) have been developed to recognize various phosphorylated epitopes such as threonine 181 and 231 and serine 199, 235, 396, and 404 (Blennow and Hampel, 2003). As a result of this aberrant phosphorylation, tau is likely unable to bind and stabilize microtubules, possibly leading to axon degeneration (Mandelkow and Mandelkow, 1998). Thus, one possibility is that the increase in tau seen in AD CSF is due to the release of tau from degenerating neurons and its subsequent diffusion into the CSF (Mandelkow and Mandelkow, 1998). With the disturbance of the tau-microtubule binding equilibrium, there is a resulting increase in the cytosolic unbound levels of tau as well, and consequently an increased likelihood of protein misfolding and subsequent aggregation as neuropil threads in dystrophic neurites and as neurofibrillary tangles (Ballatore et al., 2007). While these observations suggest possible reasons for the increases in CSF tau level in AD, it is still unclear what is really happening in the human disease process.
Given that increased levels of CSF tau can be seen in other neurodegenerative disorders, in particular FTD, stroke, corticobasal degeneration, and CJD (Itoh et al., 2001), studies have begun looking specifically at phosphorylated forms of tau as diagnostic markers for AD. Hampel et al., (2004b) compared the accuracy of CSF p-tau231, p-tau181, and p-tau199 in discriminating AD from FTD, LBD, VD, and normal controls. They found that all three proteins were significantly increased in AD as compared to the other groups; however, the discriminative power of each differed, with p-tau231 providing for the greatest discrimination between AD and non-AD, AD and controls, and AD and FTD. The combined use of the three p-tau markers did not provide further discrimination. Several studies have similarly shown that p-tau231 and p-tau199 can discriminate AD from other neurological disorders with sensitivies and specificities in the 80%–90% range (Buerger et al., 2002; Itoh et al., 2001; Kohnken et al., 2000).
While Aβ42 and tau are specific markers of AD pathogenesis, a recent study has investigated the utility of a marker of neuronal death in the diagnosis of AD (Lee et al., 2008). Visinin-like protein 1 (VLP-1), a cytoplasmic calcium sensor protein that is thought to leak from damaged or dying neurons, was found to be significantly increased in the CSF of AD subjects compared to controls (Lee et al., 2008). Although VLP-1 is not specific to AD and indeed was originally studied in ischemic stroke subjects (Laterza et al., 2006), the combined use of Aβ42, tau, p-tau, and VLP-1 resulted in increased diagnostic accuracy over any marker individually. Several studies have shown little correlation between amyloid plaque load and dementia severity (Arriagada et al., 1992; Bierer et al., 1995), thus VLP-1, in representing the end-result of the disease process, may provide a better reflection of the degree of dementia. Indeed, in this preliminary study, only VLP-1 and none of the other markers were found to correlate with MMSE (Lee et al., 2008). Clearly additional study of this molecule as a potential biomarker of cell death in AD is warranted.
Isoprostanes
Growing evidence suggests that oxidative damage may be important in the pathogenesis of AD. Isoprostanes, the end-products of lipid peroxidation, and in particular F2-isoprostanes, have been investigated in relation to AD. They have been found to be increased in the frontal and temporal cortex of AD compared to control and FTD brains, suggesting a specificity for AD (Pratico et al., 1998; Yao et al., 2003). Studies have shown F2-isoprostanes to be increased in postmortem ventricular CSF obtained from autopsy-verified AD cases (Montine et al., 1998; Montine et al., 1999b; Pratico et al., 1998), as well as in antemortem CSF from individuals diagnosed with AD dementia (Grossman et al., 2005; Montine et al., 1999a; Montine et al., 2001; Pratico et al., 2000; Pratico et al., 2002). CSF F2-isoprostanes have been shown to correlate with brain weight, degree of cortical atrophy, and Braak stage (Montine et al., 1999b), as well as dementia severity (Pratico et al., 2000). Several longitudinal studies have shown that over one and two year periods, CSF F2-isoprostanes increase in MCI and AD patients (de Leon et al., 2006; Quinn et al., 2004), and that baseline measurements could distinguish individuals that progress to MCI or AD from stable patients with 100% accuracy (de Leon et al., 2007). Moreover, the addition of isoprostane measurements to conventional memory testing or to quantitative MRI measurements resulted in increased diagnostic and prognostic power (de Leon et al., 2007), although confirmation awaits investigation in a larger number of subjects. Preclinical FAD mutation carriers have been shown to have increased CSF F2-isoprostanes as well, indicating that this marker may be suitable for both sporadic and familial AD (Ringman et al., 2008). Using a combined analysis of CSF Aβ42, tau, and F2-isoprostanes, Montine et al., (2001) were able to diagnose AD with a sensitivity of 84% and specificity of 89%, while Grossman et al., (2005) were able to classify 88.5% of patients in accordance with their clinical or autopsy diagnosis using this same panel of markers.
Results have been less consistent in regards to peripheral F2-isoprostanes, with several studies reporting increased plasma levels (Pratico et al., 2000; Pratico et al., 2002), and others reporting no significant difference (Feillet-Coudray et al., 1999; Irizarry et al., 2007; Montine et al., 2000) in AD compared to controls. Similarly, urinary F2-isoprostanes have been reported to be increased (Pratico et al., 2000; Pratico et al., 2002; Tuppo et al., 2001) or unchanged (Bohnstedt et al., 2003; Montine et al., 2000). The discrepancies concerning peripheral F2-isoprostanes may be due to differences in patient selection criteria between studies, as smoking and other conditions associated with oxidative stress, such as cardiovascular disease and diabetes, can significantly alter isoprostane levels (Flirski and Sobow, 2005).
Inflammatory markers
In addition to the classical pathological features of amyloid plaques and neurofibrillary tangles, AD brains display characteristics of inflammatory processes (Akiyama et al., 2000). One well investigated potential inflammatory marker of AD is α1-antichymotrypsin (ACT), a serine protease inhibitor that is a colocalized with Aβ in senile/neuritic plaques (Abraham et al., 1988; Roher et al., 1993b; Shoji et al., 1991). Early studies of ACT yielded inconsistent results, however, with reports of increased ACT in AD serum (Brugge et al., 1992; Hinds et al., 1994; Lieberman et al., 1995; Matsubara et al., 1990) or CSF (Harigaya et al., 1995; Matsubara et al., 1990), along with reports of unchanged ACT in AD serum (Furby et al., 1991; Lanzrein et al., 1998; Pirttila et al., 1994) or CSF (Furby et al., 1991; Lanzrein et al., 1998; Pirttila et al., 1994). Four recent studies, however, have attempted to put this controversy to rest by measuring ACT levels in large groups of subjects or by including additional controls. In a study of 196 subjects, Licastro et al., (2000) observed increased plasma ACT in AD, and found that levels inversely correlated with cognitive performance. DeKosky et al., (2003) carried out a large study of 516 individuals, with AD subjects stratified by dementia severity, and similarly found that plasma and CSF ACT were increased, and that levels were negatively correlated with dementia severity. This study excluded those with systemic inflammatory diseases or those taking anti-inflammatory medications in an attempt to achieve as homogeneous a study population as possible. Additionally, plasma ACT was significantly increased in women compared to men, perhaps further explaining why previous studies which did not control for gender yielded inconsistent results. A proteomics approach, using gel electrophoresis and mass spectrometry, also identified ACT as being differentially expressed in AD versus controls, and findings were confirmed by ELISA validation in an independent sample set (Hu et al., 2007). In a 700+ subject case-cohort study within the Rotterdam Study, Engelhart et al., (2004) found that increased plasma ACT was associated with increased risk of dementia, AD, and VD. CSF ACT has also been found to be elevated in DLB, suggesting that it may be ineffective in distinguishing between these types of dementia (Nielsen et al., 2007).
The results of cytokine studies in AD have been highly inconsistent between groups. For example, in AD patients, CSF interleukin-6 (IL-6) has been found to be increased (Blum-Degen et al., 1995; Jia et al., 2005; Martinez et al., 2000; Rosler et al., 2001b), decreased (Yamada et al., 1995), or unchanged (Engelborghs et al., 1999; Hampel et al., 1997; Hasegawa et al., 2000; Lanzrein et al., 1998; Marz et al., 1997; Tarkowski et al., 1999). Plasma/serum IL-6 results have similarly been mixed (Angelis et al., 1998; Kalman et al., 1997; Lanzrein et al., 1998; Licastro et al., 2000; Maes et al., 1999; Tarkowski et al., 1999). Additionally, whereas Wada-Isoe et al., (2004) were able to discriminate VD from AD by CSF IL-6 levels, Jia et al., (2005) found no difference in levels between VD and AD. These inconsistencies have been mimicked in studies of IL-6 receptor, Gp130, IL-1β, TNF-α, and Hp 2-1 (Teunissen et al., 2002). Moreover, most studies have either found no concentration differences or have yielded inconsistent results for additional cytokines such as IL-4, IL-8, IL-10, interferon-gamma, complement C1q, and TGF-β (Flirski and Sobow, 2005). These discrepancies between studies are likely due to several significant obstacles to the evaluation of cytokines in AD. Cytokine concentration can vary considerably over time, and can be influenced by an individual’s genetic background, comorbid systemic inflammatory processes, usage of anti-inflammatory drugs, and exposure to environmental factors (Flirski and Sobow, 2005). Moreover, many studies use subjects with neurological diseases other than AD, such as Parkinson’s disease (PD) or amyotrophic lateral sclerosis, as “controls.”
Proteomics
A newer field of biomarker studies is moving away from the traditional approach of investigating levels of a single, or several, candidate biomarkers that have been implicated in the pathogenesis of AD, and is instead focusing on nonbiased profiling of human fluids in an attempt to discover novel biomarkers. As a result of improved mass spectrometry (MS) techniques, proteomics has emerged as a powerful tool for biomarker discovery. General methodologies in proteomic studies typically include protein preparation by two-dimensional gel electrophoresis (2-DE), liquid chromatography (LC), or protein-chip arrays, followed by MS or tandem MS and database searches to determine protein identity. Recent efforts to characterize the human CSF proteome have identified 2,594 proteins (Pan et al., 2007) and 563 peptide forms and 798 proteins (Zougman et al., 2008) using a combination of approaches. By comparing the differences in protein expression levels between AD and control CSF samples, a number of studies have identified potential diagnostic markers (Abdi et al., 2006; Carrette et al., 2003; Davidsson et al., 2002; Hu et al., 2007; Puchades et al., 2003; Selle et al., 2005; Zhang et al., 2005a). Additional studies have carried out similar analyses in samples with postmortem neuropathological confirmation of AD (Castano et al., 2006; Choe et al., 2002), with one study analyzing both antemortem and postmortem CSF from the same individuals (Finehout et al., 2006a).
An important concern of proteomics-based discovery, however, is that often candidate markers identified by a study are not confirmed in independent studies or by other more quantitative methods, indicating the present need for large validation studies and corroboration by alternative techniques. While some candidates have been identified in multiple studies, and furthermore have been implicated in AD pathogenesis, they unfortunately have not been consistently reported as increased or decreased in AD CSF. For example, β2-microglobulin, the constant component of the class I major histocompatibility complex, has been identified as increased (Carrette et al., 2003; Davidsson et al., 2002; Hu et al., 2005; Simonsen et al., 2007; Zhang et al., 2005b) and decreased (Puchades et al., 2003) in AD CSF. Although the function of this protein is still unclear, it has been shown to accumulate as amyloid fibrils in dialysis patients (Gejyo et al., 1985; Gorevic et al., 1985). Similarly, transthyretin has been reported to be increased (Davidsson et al., 2002; Zhang et al., 2005b) and decreased (Castano et al., 2006; Puchades et al., 2003) in AD CSF. Transthyretin is thought to play a role in AD pathogenesis, as it can form complexes with Aβ40 and Aβ42, thus preventing Aβ aggregation (Schwarzman and Goldgaber, 1996; Schwarzman et al., 1994; Tsuzuki et al., 2000) and has been shown to negatively correlate with senile plaque abundance (Merched et al., 1998).
Many studies have formulated panels of proteins for the discrimination of AD from normal cohorts. For example, using 2-DE and tandem MS, Finehout et al., (2006b) formulated a panel of 23 protein spots that differentiated AD from non-AD with a sensitivity and specificity of 94% and a predictive error rate of 5.9%; the application of this same panel to a validation cohort yielded only slightly lower values. Moreover, panels derived from proteomic studies have been shown to differentiate AD, PD, and DBL with high accuracy (Abdi et al., 2006) and to distinguish MCI individuals who progress to AD from those who do not (Simonsen et al., 2007). Interestingly, the fragment signature of four CSF Aβ species (Aβ1–16, Aβ1–33, Aβ1–39, and Aβ1–42) as analyzed by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight MS (MALDI-TOF-MS) has been shown to discriminate AD with a sensitivity of 89%, specificity of 83%, and accuracy of 86% (Portelius et al., 2006). Finally, the few proteomic studies of serum (German et al., 2007; Lopez et al., 2005) and of plasma (Hye et al., 2006; Ray et al., 2007) have similarly yielded markers to distinguish AD from controls, as well as to predict progression from MCI to AD (Ray et al., 2007), although verification in independent cohorts is needed.
Imaging Biomarkers
Neuroimaging techniques have increasingly been used to detect brain changes associated with AD, and thus have potential as markers of disease progression, monitors of therapeutic effects, and predictors of future dementia prior to symptoms.
Structural
Computed tomography (CT) and Magnetic Resonance Imaging (MRI)
Neuropathological studies have documented an abundance of neurofibrillary tangles (Braak and Braak, 1991; Schmitt et al., 2000) and significant neuronal loss in the hippocampus and entorhinal cortex of AD patients (Gomez-Isla et al., 1996; Kordower et al., 2001; Price et al., 2001; West, 1993; West et al., 1994); therefore, these areas have frequently been targeted by imaging techniques. Atrophy of medial temporal regions, the area where AD pathology is seen early in the disease, has been observed by CT (De Leon et al., 1997; Jobst et al., 1992), but MRI has more recently surpassed CT in AD studies due to its greater accuracy, manipulability, and precision (Frisoni, 2001; Scheltens et al., 2002). Meta-analysis confirms the ability of MRI to distinguish AD subjects from normal controls, with volumetric studies of the medial temporal lobe and hippocampus having a sensitivity of 78–94% and specificity of 60–100% (Bosscher and Scheltens, 2001). In addition to discriminating AD from control cases in cross-sectional studies, measurements of the banks of the superior temporal sulcus, the anterior cingulate, and the entorhinal cortex have been shown to discriminate with high (93%) accuracy MCI patients who later convert to AD from those who do not (Killiany et al., 2000). Similar predictive power has been reported for entorhinal cortex and hippocampal volumes (deToledo-Morrell et al., 2004; Tapiola et al., 2008). Longitudinal studies have demonstrated that the rate of whole brain atrophy increases in early AD, from two (Fotenos et al., 2005) to five times (Thompson et al., 2003) that observed in age-matched non-demented controls. In addition, a recent study reported that the rate of ventricular volume expansion could predict future MCI in non-demented cohorts followed for up to 15 years, and that this rate further accelerated years prior to the diagnosis of MCI, suggesting this measurement may also be useful in preclinical diagnosis (Carlson et al., 2008). MRI has also revealed patterns of atrophy that differ between AD, FTLD, and DLB subjects, suggesting its use in the discrimination of these dementias (Barnes et al., 2007; Barnes et al., 2006; Whitwell and Jack, 2005; Whitwell et al., 2007).
Blood Flow and Metabolism
Functional magnetic resonance imaging (fMRI)
Functional MRI studies have revealed abnormalities in brain activation in AD, such as decreased activation of entorhinal cortex (Dickerson et al., 2005; Kato et al., 2001), supramarginal gyrus, prefrontal regions, anterior inferior temporal lobe (Kato et al., 2001), medial temporal lobe (Machulda et al., 2003; Rombouts et al., 2000), and hippocampal regions (Celone et al., 2006; Dickerson et al., 2005; Sperling et al., 2003), and increased activation of medial parietal cortex and posterior cingulate (Sperling et al., 2003). Results in MCI patients have been less consistent, with reports of decreased (Machulda et al., 2003) and increased (Dickerson et al., 2004; Kircher et al., 2007) medial temporal lobe activation. Additional studies have reported decreased activation in posterior cingulate, frontal cortex, hippocampus, and cerebellum (Johnson et al., 2006; Petrella et al., 2006). However, other studies have reported increased hippocampal activation in MCI cohorts (Celone et al., 2006; Dickerson et al., 2005; Hamalainen et al., 2007). Such disparities are likely due to differences in the clinical group assessment, the tasks patients are asked to perform (e.g. modality, underlying mechanism, etc.) and how the data are analyzed. It has been proposed that an increased or altered area of activation seen early in MCI may represent a compensatory recruitment for the neuronal loss and degeneration that is occurring, while in later stages of MCI and in AD this activation is not possible (Dickerson and Sperling, 2008). For a recent review on fMRI studies of the medial temporal lobe memory system in AD and MCI, see (Dickerson and Sperling, 2008)
Positron emission tomography (PET)
PET has been employed in many AD studies to examine regional cerebral metabolism using 18F-2-deoxy-2-fluoro-D-glucose as a marker (CMRglc using FDG-PET). Observed changes in AD brains include decreased metabolism in temporoparietal (Hoffman et al., 2000; Sakamoto et al., 2002), posterior cingulate (Minoshima et al., 1997; Nestor et al., 2003), hippocampal complex, medial thalamic regions, and mamillary bodies (Nestor et al., 2003). A number of studies have shown that decreased CMRglc in similar areas—temporoparietal, entorhinal, and posterior cingulate— is indicative of who will progress from MCI to AD (Arnaiz et al., 2001; Chetelat et al., 2005; de Leon et al., 2001; Drzezga et al., 2005; Mosconi et al., 2004). Additionally, PET studies using H215O to measure regional cerebral blood perfusion, and hence cerebral activation, have shown that AD and MCI brains have patterns of activation that differ significantly from normal controls when performing the same task (Becker et al., 1996; Grady et al., 2001; Moulin et al., 2007; Woodard et al., 1998).
Single photon emission computed tomography (SPECT)
Many SPECT studies have demonstrated decreased regional cerebral perfusion in the temporoparietal cortex in AD compared to normal controls (Burns et al., 1989; Harris et al., 1998; Hunter et al., 1989; Jobst et al., 1992; Leys et al., 1989; Montaldi et al., 1990; Perani et al., 1988). SPECT has also been used to accurately discriminate MCI patients who convert to AD from nonconverters and normal controls by measuring perfusion increases in cerebellum and frontal lobe, decreases in parietal lobe (Huang et al., 2007; Huang et al., 2003), posterior cingulate (Borroni et al., 2006; Hirao et al., 2005; Huang et al., 2002; Kogure et al., 2000), and precuneus (Borroni et al., 2006; Hirao et al., 2005; Kogure et al., 2000). Importantly, in a study of 70 autopsy-verified dementia patients and 14 control patients, Jagust et al., (2001) found the accuracy of premortem clinical AD diagnosis was enhanced using SPECT. Furthermore, antemortem SPECT changes have been shown to correlate well with postmortem Braak staging within the same individuals, and the areas displaying perfusion defects appeared to evolve with disease progression (Bradley et al., 2002).
Cerebral perfusion defects in AD have also been assessed by PET and MR, yielding hypoperfusion patterns similar to those obtained with SPECT. Contrast-based MR imaging methods have shown decreased temporoparietal perfusion in AD (Bozzao et al., 2001; Gonzalez et al., 1995; Harris et al., 1998), while arterial spin-labeling perfusion MR imaging (ASL-MRI) has shown decreases in areas including temporoparietal, frontal, and posterior cingulate cortices (Alsop et al., 2000; Johnson et al., 2005). ASL-MRI has also revealed decreased perfusion in MCI (Johnson et al., 2005) and distinct patterns of hypoperfusion that differ between FTD and AD (Du et al., 2006). Since ASL-MRI does not use ionizing radiation, radioactive isotopes, or contrast injection, and allows for a structural MRI to be performed during the same imaging session, this technique may be particularly attractive for clinical applications.
Molecular
Definitive diagnosis of AD relies on the presence of amyloid plaques and neurofibrillary tangles assessed at biopsy or, more commonly, at autopsy. Recent studies, however, have aimed at developing compounds for the in vivo imaging of brain amyloid, neurofibrillary tangles, and activated microglia. These imaging markers would allow for earlier diagnosis, as AD pathology precedes the onset of dementia symptoms by many years, and for the monitoring of disease progression and treatment efficacy.
Amyloid
Five amyloid PET ligands have been tested in AD patients and have yielded promising results.
FDDNP
The first probe for imaging amyloid plaques that was used in living patients was 2-(1-{6-[(2-[F-18]fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene)malononitrile, or [18F]FDDNP (Barrio et al., 1999). This label has been shown to bind to neurofibrillary tangles in vivo (Shoghi-Jadid et al., 2002) and prion plaques ex vivo as well (Bresjanac et al., 2003). An early AD case study using [18F]FDDNP (Agdeppa et al., 2001) was followed by a study of 16 subjects showing that the relative residence time (RRT) of [18F]FDDNP was highest in hippocampus-amygdala-entorhinal regions-- areas most dense in plaques and tangles (Shoghi-Jadid et al., 2002). Additionally, [18F]FDDNP RRT was strongly correlated with performance on memory tests and MMSE scores (Shoghi-Jadid et al., 2002). A later study of 83 subjects found that retention in MCI individuals was intermediate between that of normal controls and AD, and that FDDNP-PET was better able to discriminate the three groups than FDG-PET or volumetric MRI (Small et al., 2006).
PIB
Arguably the most successful of the imaging amyloid agents has been 11C-labelled Pittsburgh compound B, or PIB, (2-[4’-(methylamino)phenyl]-6-hydrobenzothiazole) (Mathis et al., 2003). In AD, PIB retention is increased in the frontal, parietal, temporal, and occipital cortices and striatum, and studies have consistently shown that nearly all patients diagnosed with Alzheimer’s dementia test PIB-positive (Klunk et al., 2004; Pike et al., 2007; Rabinovici et al., 2007; Rowe et al., 2007). Additionally, the difference in ligand binding in AD versus controls is significantly more robust with PIB than with FDDNP (Kemppainen et al., 2006; Klunk et al., 2004; Price et al., 2005; Small et al., 2006). PIB binding correlates well with rates of cerebral atrophy in AD (Archer et al., 2006) and with reductions in CSF Aβ42 (Fagan et al., 2006; Fagan et al., 2007; Forsberg et al., 2007). PIB retention is also inversely correlated with cerebral glucose metabolism as determined by FDG-PET (Klunk et al., 2004), and is strongly related to the degree of memory impairment in MCI and AD (Engler et al., 2006; Forsberg et al., 2007; Pike et al., 2007). Interestingly, a longitudinal study of early AD patients taking cholinesterase inhibitors and/or the NMDA antagonist memantine, found that PIB retention did not change over a two-year follow-up, although cortical rCMRGlc decreased (Engler et al., 2006). This suggests that amyloid burden reaches a maximum early in the course of the disease, and indeed, several studies have found that certain MCI individuals have PIB uptake in the AD range (Jack et al., 2008; Kemppainen et al., 2007; Pike et al., 2007; Rowe et al., 2007). Importantly, studies have also shown PIB uptake in a proportion of non-demented elderly controls (Fagan et al., 2006; Jack et al., 2008; Klunk et al., 2007; Lopresti et al., 2005; Mintun et al., 2006; Pike et al., 2007; Rowe et al., 2007), consistent with the known presence of AD pathology in a subset of cognitively normal elders as reported in clinicopathological studies (Hulette et al., 1998; Price and Morris, 1999). These subjects presumably have preclinical AD, but longitudinal studies are needed to test this hypothesis before we can conclude that brain amyloid has adequate sensitivity and specificity to be considered a viable biomarker of AD. PIB retention is not observed in frontotemporal lobar degeneration (FTLD) (Rabinovici et al., 2007), or more specifically in its two syndromes FTD (Rowe et al., 2007) and semantic dementia (Drzezga et al., 2008). PIB imaging has also been shown to detect cerebral amyloid angiopathy (Bacskai et al., 2007; Johnson et al., 2007). Together these studies suggest that PIB imaging may be suitable for confirming the diagnosis of AD in symptomatic cases as well as for identifying individuals in the pre-symptomatic (preclinical) stages of the disease.
18F-BAY94-9172, 11C-SB-13, 11C-BF-227
18F-BAY94-9172, a stilbene derivative with structural similarity to PIB, has been shown to bind in a nearly identical pattern to that of 11C-PIB, and to provide a similar effect size and accuracy in discriminating AD from controls and FTLD (Rowe et al., 2008). The roughly six-fold longer half-life of 18F over 11C may allow for its easier integration into clinical settings (Rowe et al., 2008). Another stilbene derivative, 11C-SB-13, has shown a binding performance similar to that of 11C-PIB in a preliminary study of a small group of patients (Verhoeff et al., 2004). Finally, 11C-BF-227 is a benzoxazole derivative that has been shown to label cerebral amyloid in a pattern distinct from that of 11C-PIB (Kudo et al., 2007). The study’s authors suggest that the difference in cortical distribution of the two amyloid labeling agents can be explained by the preferential binding of BF-227 to more dense amyloid, thus increasing retention in temporoparieto-occipital cortex where neuritic (dense core) plaques are most abundant (Kudo et al., 2007).
Microglia
Inflammatory mechanisms may play an important role in the neurodegeneration of AD, as various inflammatory mediators have been reported in AD brains, and epidemiological studies have reported altered AD risk with the use of anti-inflammatory drugs. Indeed, activated microglia closely associate with neuritic plaques (Sheng et al., 1997), and Aβ and APP species can act as microglial activators (Barger and Harmon, 1997; Combs et al., 1999; Rogers and Lue, 2001). Upon microglial activation, expression of the peripheral benzodiazepine receptor (PBR) is up-regulated, providing a marker for inflammatory processes. A PET study measuring microglial activation with [11C](R)-PK11195, a ligand for the PBR binding site, found increased binding in the entorhinal, temporoparietal, and cingulate cortices in AD, and a similar pattern in an MCI individual (Cagnin et al., 2001) . A 1-2 year follow-up with MRI revealed that the areas of highest [11C](R)-PK11195 binding had the greatest rates of atrophy (Cagnin et al., 2001). The authors point to differences in the acquisition and analysis of PET data and their use of the higher affinity R-enantiomer of the ligand as reasons why their results differed from those of an initial study that did not detect any differences between AD and non-demented patients (Groom et al., 1995). Using [123I]iodo-PK11195 as a SPECT ligand, Versijpt et al., (2003) observed significantly increased binding in the frontal and right mesotemporal regions of AD patients, and a correlation between ligand binding and performance on tasks of cognition. An additional PET ligand for PBR, [18F] FE-DAA1106 (Zhang et al., 2004), has also been shown to be useful in assessing glial activation in in vivo AD mouse models (Maeda et al., 2007). To our knowledge, this compound has not yet been tested in humans. While microglial activation is not specific to AD, indeed PK1195 binding has been studied in glial neoplasms, ischemic stroke, and multiple sclerosis among other diseases, the unique pattern of ligand binding within AD brains along with the concomitant use of other more specific markers of AD may prove useful to diagnosis.
Conclusions
The field of AD biomarkers has experienced a renewed level of enthusiasm due to better availability of reagents and novel methods for the assessment of a variety of fluid and imaging measures. CSF Aβ42 and tau have stood the test of time, proving particularly promising as potential predictors of cognitive decline in individuals with very mild cognitive impairment as well as future dementia in non-demented cohorts. Further, low levels of CSF Aβ42 are an excellent marker for the presence of neocortical amyloid deposition, in the presence or absence of dementia. Whether brain amyloid invariably leads to subsequent dementia in AD is not known at present but is currently being studied. More rigorous investigation of fluid markers of inflammation, oxidative damage, and neuronal death are clearly warranted. Plasma and serum analytes have been notoriously difficult to interrogate but results from recent array-based panels are promising. Development of “molecular” imaging agents for the detection of AD pathologies (amyloid, tangles, activated microglia) has propelled the imaging field forward. It is likely that panels of markers and multiple biomarker modalities, especially combinations of fluid and imaging measures, will be required. Not only may these biomarkers eventually be useful as diagnostic tools in the clinic but also, in the more immediate future, for the design and evaluation of clinical trials of disease-modifying therapies by helping to reduce sample size, reduce trial duration, and evaluate treatment efficacy.
Acknowledgments
The authors thank Dr. John Cirrito for help with graphic design. This work was supported by NIH grants AG03991, AG026276, AG05681
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures 2007. 2007. [Google Scholar]
- Abdi F, et al. Detection of biomarkers with a multiplex quantitative proteomic platform in cerebrospinal fluid of patients with neurodegenerative disorders. J Alzheimers Dis. 2006;9:293–348. doi: 10.3233/jad-2006-9309. [DOI] [PubMed] [Google Scholar]
- Abraham C, et al. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer’s disease. Cell. 1988;52:487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Agdeppa ED, et al. In vivo and in vitro labeling of plaques and tangles in the brain of an Alzheimer’s disease patient: a case study. J Nucl Med. 2001;42:65P. [Google Scholar]
- Akiyama H, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop DC, et al. Assessment of cerebral blood flow in Alzheimer’s disease by spin-labeled magnetic resonance imaging. Ann Neurol. 2000;47:93–100. [PubMed] [Google Scholar]
- Andreasen N, et al. Cerebrospinal fluid β-amyloid(1-42) in Alzheimer’s disease: Differences between early- and late-onset Alzheimer’s disease and stability during the course of disease. Arch Neurol. 1999a;56:673–680. doi: 10.1001/archneur.56.6.673. [DOI] [PubMed] [Google Scholar]
- Andreasen N, et al. Sensitivity, specificity, and stability of CSF-tau in AD in a community-based patient sample. Neurology. 1999b;53:1488–94. doi: 10.1212/wnl.53.7.1488. [DOI] [PubMed] [Google Scholar]
- Andreasen N, et al. Evaluation of CSF-tau and CSF-Aβ42 as diagnostic markers for Alzheimer’s disease in clinical practice. Arch Neurol. 2001;58:373–379. doi: 10.1001/archneur.58.3.373. [DOI] [PubMed] [Google Scholar]
- Andreasen N, et al. Cerebrospinal fluid tau protein as a biochemical marker for Alzheimer’s disease: A community based follow up study. J Neurol Neurosurg Psych. 1998;64:298–305. doi: 10.1136/jnnp.64.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N, et al. Cerebrospinal fluid levels of total-tau, phospho-tau and A beta 42 predicts development of Alzheimer’s disease in patients with mild cognitive impairment. Acta Neurol Scand Suppl. 2003;179:47–51. doi: 10.1034/j.1600-0404.107.s179.9.x. [DOI] [PubMed] [Google Scholar]
- Angelis P, et al. Serum interleukin-6 and interleukin-6 soluble receptor in Alzheimer’s disease. Neurosci Lett. 1998;244:106–8. doi: 10.1016/s0304-3940(98)00136-0. [DOI] [PubMed] [Google Scholar]
- Arai H, et al. No increase in cerebrospinal fluid tau protein levels in patients with vascular dementia. Neurosci Lett. 1998;256:174–6. doi: 10.1016/s0304-3940(98)00781-2. [DOI] [PubMed] [Google Scholar]
- Arai H, et al. Tau in cerebrospinal fluid: A potential diagnostic marker in Alzheimer’s disease. Ann Neurol. 1995;38:649–652. doi: 10.1002/ana.410380414. [DOI] [PubMed] [Google Scholar]
- Arai H, et al. Effect of genetic risk factors and disease progression on the cerebrospinal fluid tau levels in Alzheimer’s disease. J Am Geriatr Soc. 1997;45:1228–31. doi: 10.1111/j.1532-5415.1997.tb03775.x. [DOI] [PubMed] [Google Scholar]
- Archer H, et al. Amyloid load and cerebral atrophy in Alzheimer’s disease: An 11C-PIB positron emission tomography study. Ann Neurol. 2006;60:145–7. doi: 10.1002/ana.20889. [DOI] [PubMed] [Google Scholar]
- Arnaiz E, et al. Impaired cerebral glucose metabolism and cognitive functioning predict deterioration in mild cognitive impairment. Neuroreport. 2001;12:851–5. doi: 10.1097/00001756-200103260-00045. [DOI] [PubMed] [Google Scholar]
- Arriagada PV, et al. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–9. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Bacskai BJ, et al. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: a case report. Arch Neurol. 2007;64:431–4. doi: 10.1001/archneur.64.3.431. [DOI] [PubMed] [Google Scholar]
- Ballatore C, et al. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–72. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Barger SW, Harmon AD. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature. 1997;388:878–881. doi: 10.1038/42257. [DOI] [PubMed] [Google Scholar]
- Barnes J, et al. Atrophy rates of the cingulate gyrus and hippocampus in AD and FTLD. Neurobiol Aging. 2007;28:20–8. doi: 10.1016/j.neurobiolaging.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Barnes J, et al. Measurements of the amygdala and hippocampus in pathologically confirmed Alzheimer disease and frontotemporal lobar degeneration. Arch Neurol. 2006;63:1434–9. doi: 10.1001/archneur.63.10.1434. [DOI] [PubMed] [Google Scholar]
- Barrio JR, et al. PET imaging of tangles and plaques in Alzheimer disease with a highly hydrophobic probe. J Labelled Compd Radiopharm. 1999;42:S194–195. [Google Scholar]
- Becker JT, et al. Compensatory reallocation of brain resources supporting verbal episodic memory in Alzheimer’s disease. Neurology. 1996;46:692–700. doi: 10.1212/wnl.46.3.692. [DOI] [PubMed] [Google Scholar]
- Bierer LM, et al. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch Neurol. 1995;52:81–8. doi: 10.1001/archneur.1995.00540250089017. [DOI] [PubMed] [Google Scholar]
- Blennow K, Hampel H. CSF Markers for Incipient Alzheimer’s Disease. Lancet Neurol. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- Blennow K, et al. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995;26:231–45. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- Blum-Degen D, et al. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett. 1995;202:17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- Bohnstedt KC, et al. Determination of isoprostanes in urine samples from Alzheimer patients using porous graphitic carbon liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;796:11–9. doi: 10.1016/s1570-0232(03)00600-7. [DOI] [PubMed] [Google Scholar]
- Borroni B, et al. Combined 99mTc-ECD SPECT and neuropsychological studies in MCI for the assessment of conversion to AD. Neurobiol Aging. 2006;27:24–31. doi: 10.1016/j.neurobiolaging.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Bosscher L, Scheltens PH. Evidence based dementia. Oxford: Blackwell; 2001. MRI of the temporal lobe. [Google Scholar]
- Bouras C, et al. Neurofibrillary tangle densities in the hippocampal formation in a non-demented population define subgroups of patients with differential early pathologic changes. Neurosci Lett. 1993;153:131–5. doi: 10.1016/0304-3940(93)90305-5. [DOI] [PubMed] [Google Scholar]
- Bozzao A, et al. Diffusion and perfusion MR imaging in cases of Alzheimer’s disease: correlations with cortical atrophy and lesion load. AJNR Am J Neuroradiol. 2001;22:1030–6. [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Bradley KM, et al. Cerebral perfusion SPET correlated with Braak pathological stage in Alzheimer’s disease. Brain. 2002;125:1772–81. doi: 10.1093/brain/awf185. [DOI] [PubMed] [Google Scholar]
- Bresjanac M, et al. Molecular-imaging probe 2-(1-[6-[(2-fluoroethyl)(methyl) amino]-2-naphthyl]ethylidene) malononitrile labels prion plaques in vitro. J Neurosci. 2003;23:8029–33. doi: 10.1523/JNEUROSCI.23-22-08029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge K, et al. Serological alpha 1-antichymotrypsin in Down’s syndrome and Alzheimer’s disease. Ann Neurol. 1992;32:193–7. doi: 10.1002/ana.410320211. [DOI] [PubMed] [Google Scholar]
- Buerger K, et al. Differential diagnosis of Alzheimer’s disease with cerebrospinal fluid levels of tau protein phosphorylated at threonine 231. Arch Neurol. 2002;59:1267–1272. doi: 10.1001/archneur.59.8.1267. [DOI] [PubMed] [Google Scholar]
- Burger nee Buch K, et al. Cerebrospinal fluid tau protein shows a better discrimination in young old (<70 years) than in old old patients with Alzheimer’s disease compared with controls. Neurosci Lett. 1999;277:21–4. doi: 10.1016/s0304-3940(99)00845-9. [DOI] [PubMed] [Google Scholar]
- Burns A, et al. The investigation of Alzheimer’s disease with single photon emission tomography. J Neurol Neurosurg Psychiatry. 1989;52:248–53. doi: 10.1136/jnnp.52.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnin A, et al. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358:461–7. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- Carlson NE, et al. Trajectories of brain loss in aging and the development of cognitive impairment. Neurology. 2008;70:828–33. doi: 10.1212/01.wnl.0000280577.43413.d9. [DOI] [PubMed] [Google Scholar]
- Carrette O, et al. A panel of cerebrospinal fluid potential biomarkers for the diagnosis of Alzheimer’s disease. Proteomics. 2003;3:1486–94. doi: 10.1002/pmic.200300470. [DOI] [PubMed] [Google Scholar]
- Castano E, et al. Comparative proteomics of cerebrospinal fluid in neuropathologically-confirmed Alzheimer’s disease and non-demented elderly subjects. Neurol Res. 2006;28:155–163. doi: 10.1179/016164106X98035. [DOI] [PubMed] [Google Scholar]
- Celone KA, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. J Neurosci. 2006;26:10222–31. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat G, et al. Using Voxel-Based Morphometry to Map the Structural Changes Associated with Rapid Conversion in MCI: A Longitudinal MRI Study. NeuroImage. 2005:1–13. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Choe L, et al. Studies of potential cerebrospinal fluid molecular markers for Alzheimer’s disease. Electrophoresis. 2002;23:2247–2251. doi: 10.1002/1522-2683(200207)23:14<2247::AID-ELPS2247>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Chong JK, et al. Detection of amyloid beta protein precursor immunoreactivity in normal and Alzheimer’s disease cerebrospinal fluid. Life Sci. 1990;47:1163–71. doi: 10.1016/0024-3205(90)90177-s. [DOI] [PubMed] [Google Scholar]
- Clark C, et al. Cerebrospinal fluid tau and beta-amyloid: How well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch. Neurol. 2003;60:1696–702. doi: 10.1001/archneur.60.12.1696. [DOI] [PubMed] [Google Scholar]
- Combs CK, et al. Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of beta-amyloid and prion proteins. J Neurosci. 1999;19:928–39. doi: 10.1523/JNEUROSCI.19-03-00928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, et al. Relationships among cerebrospinal fluid biomarkers in dementia of the Alzheimer type. Alzheimer Dis Assoc Disord. 2002;16:144–9. doi: 10.1097/00002093-200207000-00003. [DOI] [PubMed] [Google Scholar]
- Davidsson P, et al. Proteome analysis of cerebrospinal fluid proteins in Alzheimer patients. NeuroReport. 2002;13:611–615. doi: 10.1097/00001756-200204160-00015. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, et al. Plasma and cerebrospinal fluid alpha-1-antichymotrypsin levels in Alzheimer’s disease: correlation with cognitive impairment. Ann Neurol. 2003;53:81–90. doi: 10.1002/ana.10414. [DOI] [PubMed] [Google Scholar]
- de Leon M, et al. Longitudinal CSF isoprostane and MRI atrophy in the progression to AD. J Neurol Sci. 2007 Nov 14; doi: 10.1007/s00415-007-0610-z. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET) Proc Natl Acad Sci U S A. 2001;98:10966–71. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon MJ, et al. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol Aging. 2006;27:394–401. doi: 10.1016/j.neurobiolaging.2005.07.003. [DOI] [PubMed] [Google Scholar]
- De Leon MJ, et al. Frequency of hippocampal formation atrophy in normal aging and Alzheimer’s disease. Neurobiol Aging. 1997;18:1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, et al. Plaque-associated disruption of CSF and plasma amyloid-β (Aβ) equilibrium in a mouse model of Alzheimer’s disease. J Neurochem. 2002;81:229–236. doi: 10.1046/j.1471-4159.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, et al. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol Aging. 2004;25:1197–203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Dickerson B, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–11. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA. Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: Insights from functional MRI studies. Neuropsychologia. 2008;46:1624–1635. doi: 10.1016/j.neuropsychologia.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzezga A, et al. Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer’s disease. Neuroimage. 2008;39:619–33. doi: 10.1016/j.neuroimage.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Drzezga A, et al. Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)F-FDG PET. J Nucl Med. 2005;46:1625–32. [PubMed] [Google Scholar]
- Du AT, et al. Hypoperfusion in frontotemporal dementia and Alzheimer disease by arterial spin labeling MRI. Neurology. 2006;67:1215–20. doi: 10.1212/01.wnl.0000238163.71349.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelborghs S, et al. Unchanged levels of interleukins, neopterin, interferon-gamma and tumor necrosis factor-alpha in cerebrospinal fluid of patients with dementia of the Alzheimer type. Neurochem Int. 1999;34:523–30. doi: 10.1016/s0197-0186(99)00031-5. [DOI] [PubMed] [Google Scholar]
- Engelborghs S, et al. Diagnostic performance of a CSF-biomarker panel in autopsy-confirmed dementia. Neurobiol Aging. 2008;29:1143–59. doi: 10.1016/j.neurobiolaging.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Engelhart MJ, et al. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- Engler H, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129:2856–66. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- Ertekin-Taner N, et al. Plasma amyloid beta protein is elevated in late-onset Alzheimer disease families. Neurology. 2008;70:596–606. doi: 10.1212/01.WNL.0000278386.00035.21. [DOI] [PubMed] [Google Scholar]
- Evin G, Weidemann A. Biogenesis and metabolism of Alzheimer’s disease Abeta amyloid peptides. Peptides. 2002;23:1285–97. doi: 10.1016/s0196-9781(02)00063-3. [DOI] [PubMed] [Google Scholar]
- Fagan A, et al. Inverse relation between in vivo amyloid imaging load and CSF Aβ42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Fagan A, et al. Cerebrospinal fluid tau/Aβ42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–49. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- Feillet-Coudray C, et al. Plasma levels of 8-epiPGF2alpha, an in vivo marker of oxidative stress, are not affected by aging or Alzheimer’s disease. Free Radic Biol Med. 1999;27:463–9. doi: 10.1016/s0891-5849(99)00096-9. [DOI] [PubMed] [Google Scholar]
- Finehout EJ, et al. Proteomic analysis of cerebrospinal fluid changes related to postmortem interval. Clin Chem. 2006a;52:1906–13. doi: 10.1373/clinchem.2006.070508. [DOI] [PubMed] [Google Scholar]
- Finehout EJ, et al. Cerebrospinal fluid proteomic biomarkers for Alzheimer’s disease. Ann Neurol. 2006b;61:120–129. doi: 10.1002/ana.21038. [DOI] [PubMed] [Google Scholar]
- Flirski M, Sobow T. Biochemical markers and risk factors of Alzheimer’s disease. Curr Alzheimer Res. 2005;2:47–64. doi: 10.2174/1567205052772704. [DOI] [PubMed] [Google Scholar]
- Forsberg A, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, et al. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64:1032–9. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Freeman SH, et al. Plasma Abeta levels do not reflect brain Abeta levels. J Neuropathol Exp Neurol. 2007;66:264–71. doi: 10.1097/NEN.0b013e31803d3ae4. [DOI] [PubMed] [Google Scholar]
- Frisoni GB. Structural imaging in the clinical diagnosis of Alzheimer’s disease: problems and tools. J Neurol Neurosurg Psychiatry. 2001;70:711–8. doi: 10.1136/jnnp.70.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H, et al. Age but not diagnosis is the main predictor of plasma amyloid beta-protein levels. Arch Neurol. 2003;60:958–64. doi: 10.1001/archneur.60.7.958. [DOI] [PubMed] [Google Scholar]
- Fukuyama R, et al. Age-dependent changes in the levels of Aβ40 and Aβ42 in cerebrospinal fluid from control subjects, and a decrease in the ratio of Aβ42 to Aβ40 level in cerebrospinal fluid from Alzheimer’s disease patients. Eur Neurol. 2000;43:155–160. doi: 10.1159/000008156. [DOI] [PubMed] [Google Scholar]
- Furby A, et al. Are alpha-1-antichymotrypsin and inter-alpha-trypsin inhibitor peripheral markers of Alzheimer’s disease? J Neurol Neurosurg Psychiatry. 1991;54:469. doi: 10.1136/jnnp.54.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasko D, et al. High cerebrospinal fluid tau and low amyloid β42 levels in the clinical diagnosis of Alzheimer’s disease and relation to apolipoprotein E genotype. Arch Neurol. 1998;55:937–945. doi: 10.1001/archneur.55.7.937. [DOI] [PubMed] [Google Scholar]
- Gejyo F, et al. A new form of amyloid protein associated with chronic hemodialysis was identified as beta 2-microglobulin. Biochem Biophys Res Commun. 1985;129:701–6. doi: 10.1016/0006-291x(85)91948-5. [DOI] [PubMed] [Google Scholar]
- German DC, et al. Serum biomarkers for Alzheimer’s disease: proteomic discovery. Biomed Pharmacother. 2007;61:383–9. doi: 10.1016/j.biopha.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Ghiso J, et al. Alzheimer’s disease amyloid precursor protein is present in senile plaques and cerebrospinal fluid: immunohistochemical and biochemical characterization. Biochem Biophys Res Commun. 1989;163:430–7. doi: 10.1016/0006-291x(89)92154-2. [DOI] [PubMed] [Google Scholar]
- Golombowski S, et al. Dependence of cerebrospinal fluid Tau protein levels on apolipoprotein E4 allele frequency in patients with Alzheimer’s disease. Neurosci Lett. 1997;225:213–5. doi: 10.1016/s0304-3940(97)00228-0. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, et al. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez RG, et al. Functional MR in the evaluation of dementia: correlation of abnormal dynamic cerebral blood volume measurements with changes in cerebral metabolism on positron emission tomography with fludeoxyglucose F 18. AJNR Am J Neuroradiol. 1995;16:1763–70. [PMC free article] [PubMed] [Google Scholar]
- Gorevic PD, et al. Beta-2 microglobulin is an amyloidogenic protein in man. J Clin Invest. 1985;76:2425–9. doi: 10.1172/JCI112257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, et al. Altered brain functional connectivity and impaired short-term memory in Alzheimer’s disease. Brain. 2001;124:739–56. doi: 10.1093/brain/124.4.739. [DOI] [PubMed] [Google Scholar]
- Green AJ, et al. Increased tau in the cerebrospinal fluid of patients with frontotemporal dementia and Alzheimer’s disease. Neurosci Lett. 1999;259:133–5. doi: 10.1016/s0304-3940(98)00904-5. [DOI] [PubMed] [Google Scholar]
- Groom GN, et al. PET of peripheral benzodiazepine binding sites in the microgliosis of Alzheimer’s disease. J Nucl Med. 1995;36:2207–10. [PubMed] [Google Scholar]
- Grossman M, et al. Cerebrospinal Fluid Profile in Frontotemporal Dementia and Alzheimer’s Disease. Ann Neurol. 2005;57:721–729. doi: 10.1002/ana.20477. [DOI] [PubMed] [Google Scholar]
- Hamalainen A, et al. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging. 2007;28:1889–903. doi: 10.1016/j.neurobiolaging.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Hampel H, et al. Tracking of Alzheimer’s disease progression with cerebrospinal fluid tau protein phosphorylated at threonine 231. Ann Neurol. 2001;49:545–6. [PubMed] [Google Scholar]
- Hampel H, et al. Interleukin-6 is not altered in cerebrospinal fluid of first-degree relatives and patients with Alzheimer’s disease. Neurosci Lett. 1997;228:143–6. doi: 10.1016/s0304-3940(97)00379-0. [DOI] [PubMed] [Google Scholar]
- Hampel H, et al. Value of CSF beta-amyloid1-42 and tau as predictors of Alzheimer’s disease in patients with mild cognitive impairment. Mol Psychiatry. 2004a;9:705–10. doi: 10.1038/sj.mp.4001473. [DOI] [PubMed] [Google Scholar]
- Hampel H, et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch Gen Psychiatry. 2004b;61:95–102. doi: 10.1001/archpsyc.61.1.95. [DOI] [PubMed] [Google Scholar]
- Hampel H, et al. Discriminant power of combined cerebrospinal fluid tau protein and of the soluble interleukin-6 receptor complex in the diagnosis of Alzheimer’s disease. Brain Res. 1999;823:104–12. doi: 10.1016/s0006-8993(99)01146-4. [DOI] [PubMed] [Google Scholar]
- Hansson O, et al. Prediction of Alzheimer’s disease using the CSF Abeta42/Abeta40 ratio in patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23:316–20. doi: 10.1159/000100926. [DOI] [PubMed] [Google Scholar]
- Hansson O, et al. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol. 2006;5:228–34. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- Hansson O, et al. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Harigaya Y, et al. Alpha 1-antichymotrypsin level in cerebrospinal fluid is closely associated with late onset Alzheimer’s disease. Intern Med. 1995;34:481–4. doi: 10.2169/internalmedicine.34.481. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, et al. Neurofibrillary tangles in nondemented elderly subjects and mild Alzheimer disease. Arch Neurol. 1999;56:713–8. doi: 10.1001/archneur.56.6.713. [DOI] [PubMed] [Google Scholar]
- Harris GJ, et al. Dynamic susceptibility contrast MR imaging of regional cerebral blood volume in Alzheimer disease: a promising alternative to nuclear medicine. AJNR Am J Neuroradiol. 1998;19:1727–32. [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, et al. Increased soluble tumor necrosis factor receptor levels in the serum of elderly people. Gerontology. 2000;46:185–8. doi: 10.1159/000022157. [DOI] [PubMed] [Google Scholar]
- Henriksson T, et al. Analysis and quantitation of the beta-amyloid precursor protein in the cerebrospinal fluid of Alzheimer’s disease patients with a monoclonal antibody-based immunoassay. J Neurochem. 1991;56:1037–42. doi: 10.1111/j.1471-4159.1991.tb02026.x. [DOI] [PubMed] [Google Scholar]
- Herukka S, et al. CSF Aβ42 and tau or phosphorylated tau and prediction of progressive mild cognitive impairment. Neurology. 2005;64:1294–7. doi: 10.1212/01.WNL.0000156914.16988.56. [DOI] [PubMed] [Google Scholar]
- Herukka SK, et al. CSF Abeta42, Tau and phosphorylated Tau, APOE epsilon4 allele and MCI type in progressive MCI. Neurobiol Aging. 2007;28:507–14. doi: 10.1016/j.neurobiolaging.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hinds TR, et al. Relationship between serum alpha 1-antichymotrypsin and Alzheimer’s disease. Neurobiol Aging. 1994;15:21–7. doi: 10.1016/0197-4580(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Hirao K, et al. The prediction of rapid conversion to Alzheimer’s disease in mild cognitive impairment using regional cerebral blood flow SPECT. Neuroimage. 2005;28:1014–21. doi: 10.1016/j.neuroimage.2005.06.066. [DOI] [PubMed] [Google Scholar]
- Hock C, et al. Increased levels of tau protein in cerebrospinal fluid of patients with Alzheimer’s disease--correlation with degree of cognitive impairment. Ann Neurol. 1995;37:414–5. doi: 10.1002/ana.410370325. [DOI] [PubMed] [Google Scholar]
- Hoffman JM, et al. FDG PET imaging in patients with pathologically verified dementia. J Nucl Med. 2000;41:1920–8. [PubMed] [Google Scholar]
- Holmberg B, et al. Cerebrospinal fluid Abeta42 is reduced in multiple system atrophy but normal in Parkinson’s disease and progressive supranuclear palsy. Mov Disord. 2003;18:186–90. doi: 10.1002/mds.10321. [DOI] [PubMed] [Google Scholar]
- Holmes C, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–23. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Hu Y, et al. Identification and validation of novel CSF biomarkers for early stages of Alzheimer’s disease. Proteomics - Clin Appl. 2007;1:1373–1384. doi: 10.1002/prca.200600999. [DOI] [PubMed] [Google Scholar]
- Hu Y, et al. Comparative proteomic analysis of intra- and interindividual variation in human cerebrospinal fluid& Cell. Mol Proteom. 2005;4:2000–2009. doi: 10.1074/mcp.M500207-MCP200. [DOI] [PubMed] [Google Scholar]
- Huang C, et al. Imaging markers of mild cognitive impairment: multivariate analysis of CBF SPECT. Neurobiol Aging. 2007;28:1062–9. doi: 10.1016/j.neurobiolaging.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Huang C, et al. Voxel- and VOI-based analysis of SPECT CBF in relation to clinical and psychological heterogeneity of mild cognitive impairment. Neuroimage. 2003;19:1137–44. doi: 10.1016/s1053-8119(03)00168-x. [DOI] [PubMed] [Google Scholar]
- Huang C, et al. Cingulate cortex hypoperfusion predicts Alzheimer’s disease in mild cognitive impairment. BMC Neurol. 2002;2:9. doi: 10.1186/1471-2377-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulette CM, et al. Neuropathological and neuropsychological changes in “normal” aging: Evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- Hulstaert F, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52:1555–62. doi: 10.1212/wnl.52.8.1555. [DOI] [PubMed] [Google Scholar]
- Hunter R, et al. The pattern of function-related regional cerebral blood flow investigated by single photon emission tomography with 99mTc-HMPAO in patients with presenile Alzheimer’s disease and Korsakoff’s psychosis. Psychol Med. 1989;19:847–55. doi: 10.1017/s0033291700005560. [DOI] [PubMed] [Google Scholar]
- Hye A, et al. Proteome-based plasma biomarkers for Alzheimer’s disease. Brain. 2006;129:3042–50. doi: 10.1093/brain/awl279. [DOI] [PubMed] [Google Scholar]
- Ida N, et al. Analysis of heterogeneous βA4 peptides in human cerebrospinal fluid and blood by a newly developed sensitive Western blot assay. J Biol Chem. 1996;271:22908–22914. doi: 10.1074/jbc.271.37.22908. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, et al. Plasma F2A isoprostane levels in Alzheimer’s and Parkinson’s disease. Neurodegener Dis. 2007;4:403–5. doi: 10.1159/000107699. [DOI] [PubMed] [Google Scholar]
- Itoh N, et al. Large-scale, multicenter study of cerebrospinal fluid tau protein phosphorylated at serine 199 for the antemortem diagnosis of Alzheimer’s disease. Ann Neurol. 2001;50:150–6. doi: 10.1002/ana.1054. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W, et al. SPECT perfusion imaging in the diagnosis of Alzheimer’s disease: a clinical-pathologic study. Neurology. 2001;56:950–956. doi: 10.1212/wnl.56.7.950. [DOI] [PubMed] [Google Scholar]
- Janus C, et al. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–82. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Jensen M, et al. Increased cerebrospinal fluid tau in patients with Alzheimer’s disease. Neurosci Lett. 1995;186:189–91. doi: 10.1016/0304-3940(95)11297-a. [DOI] [PubMed] [Google Scholar]
- Jensen M, et al. Cerebrospinal fluid Aβ42 is increased early in sporadic Alzheimer’s disease and declines with disease progression. Annals of Neurology. 1999;45:504–511. doi: 10.1002/1531-8249(199904)45:4<504::aid-ana12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Jia JP, et al. Cerebrospinal fluid tau, Abeta1-42 and inflammatory cytokines in patients with Alzheimer’s disease and vascular dementia. Neurosci Lett. 2005;383:12–6. doi: 10.1016/j.neulet.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Jobst KA, et al. Association of atrophy of the medial temporal lobe with reduced blood flow in the posterior parietotemporal cortex in patients with a clinical and pathological diagnosis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1992;55:190–4. doi: 10.1136/jnnp.55.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–34. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- Johnson NA, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–9. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, et al. Activation of brain regions vulnerable to Alzheimer’s disease: the effect of mild cognitive impairment. Neurobiol Aging. 2006;27:1604–12. doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle PJ, et al. Combined assessment of tau and neuronal thread protein in Alzheimer’s disease CSF. Neurology. 2000;54:1498–504. doi: 10.1212/wnl.54.7.1498. [DOI] [PubMed] [Google Scholar]
- Kalman J, et al. Serum interleukin-6 levels correlate with the severity of dementia in Down syndrome and in Alzheimer’s disease. Acta Neurol Scand. 1997;96:236–40. doi: 10.1111/j.1600-0404.1997.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Kanai M, et al. Longitudinal study of cerebrospinal fluid levels of tau, Aβ1-40, and Aβ1-42(43) in Alzheimer’s disease: A study in Japan. Ann Neurol. 1998;44 doi: 10.1002/ana.410440108. [DOI] [PubMed] [Google Scholar]
- Kanemaru K, et al. Decreased CSF amyloid beta42 and normal tau levels in dementia with Lewy bodies. Neurology. 2000;54:1875–6. doi: 10.1212/wnl.54.9.1875. [DOI] [PubMed] [Google Scholar]
- Kapaki E, et al. Highly increased CSF tau protein and decreased beta-amyloid (1-42) in sporadic CJD: a discrimination from Alzheimer’s disease? J Neurol Neurosurg Psychiatry. 2001;71:401–3. doi: 10.1136/jnnp.71.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapaki E, et al. CSF tau protein and beta-amyloid (1-42) in Alzheimer’s disease diagnosis: discrimination from normal ageing and other dementias in the Greek population. Eur J Neurol. 2003;10:119–28. doi: 10.1046/j.1468-1331.2003.00562.x. [DOI] [PubMed] [Google Scholar]
- Kato T, et al. Dissociation of regional activation in mild AD during visual encoding: a functional MRI study. Neurology. 2001;57:812–6. doi: 10.1212/wnl.57.5.812. [DOI] [PubMed] [Google Scholar]
- Kemppainen NM, et al. Voxel-based analysis of PET amyloid ligand [11C]PIB uptake in Alzheimer disease. Neurology. 2006;67:1575–80. doi: 10.1212/01.wnl.0000240117.55680.0a. [DOI] [PubMed] [Google Scholar]
- Kemppainen NM, et al. PET amyloid ligand [11C]PIB uptake is increased in mild cognitive impairment. Neurology. 2007;68:1603–6. doi: 10.1212/01.wnl.0000260969.94695.56. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann Neurol. 2000;47:430–9. [PubMed] [Google Scholar]
- Kircher TT, et al. Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. J Neurol Neurosurg Psychiatry. 2007;78:812–8. doi: 10.1136/jnnp.2006.104877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaguchi N, et al. Determination of amyloid beta protein precursors harboring active form of proteinase inhibitor domains in cerebrospinal fluid of Alzheimer’s disease patients by trypsin-antibody sandwich ELISA. Biochem Biophys Res Commun. 1990;166:1453–9. doi: 10.1016/0006-291x(90)91030-v. [DOI] [PubMed] [Google Scholar]
- Klunk W, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Klunk WE, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–84. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure D, et al. Longitudinal evaluation of early Alzheimer’s disease using brain perfusion SPECT. J Nucl Med. 2000;41:1155–62. [PubMed] [Google Scholar]
- Kohnken R, et al. Detection of tau phosphorylated at threonine 231 in cerebrospinal fluid of Alzheimer’s disease patients. Neurosci Lett. 2000;287:187–90. doi: 10.1016/s0304-3940(00)01178-2. [DOI] [PubMed] [Google Scholar]
- Kordower JH, et al. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol. 2001;49:202–13. [PubMed] [Google Scholar]
- Kosaka T, et al. The beta APP717 Alzheimer mutation increases the percentage of plasma amyloid-beta protein ending at Abeta42(43) Neurology. 1997;48:741–5. doi: 10.1212/wnl.48.3.741. [DOI] [PubMed] [Google Scholar]
- Kudo Y, et al. 2-(2-[2-Dimethylaminothiazol-5-yl]ethenyl)-6- (2- [fluoro]ethoxy)benzoxazole: a novel PET agent for in vivo detection of dense amyloid plaques in Alzheimer’s disease patients. J Nucl Med. 2007;48:553–61. doi: 10.2967/jnumed.106.037556. [DOI] [PubMed] [Google Scholar]
- Kurz A, et al. Tau protein in cerebrospinal fluid is significantly increased at the earliest clinical stage of Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12:372–7. doi: 10.1097/00002093-199812000-00020. [DOI] [PubMed] [Google Scholar]
- Lannfelt L, et al. Amyloid beta-peptide in cerebrospinal fluid in individuals with the Swedish Alzheimer amyloid precursor protein mutation. Neurosci Lett. 1995;199:203–206. doi: 10.1016/0304-3940(95)12059-d. [DOI] [PubMed] [Google Scholar]
- Lanzrein AS, et al. Longitudinal study of inflammatory factors in serum, cerebrospinal fluid, and brain tissue in Alzheimer disease: interleukin-1beta, interleukin-6, interleukin-1 receptor antagonist, tumor necrosis factor-alpha, the soluble tumor necrosis factor receptors I and II, and alpha1-antichymotrypsin. Alzheimer Dis Assoc Disord. 1998;12:215–27. doi: 10.1097/00002093-199809000-00016. [DOI] [PubMed] [Google Scholar]
- Laterza OF, et al. Identification of novel brain biomarkers. Clin Chem. 2006;52:1713–21. doi: 10.1373/clinchem.2006.070912. [DOI] [PubMed] [Google Scholar]
- Lee JM, et al. The brain injury biomarker VLP-1 is increased in the cerebrospinal fluid of Alzheimer disease patients. Clin Chem. 2008;54:1617–23. doi: 10.1373/clinchem.2008.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewczuk P, et al. Neurochemical diagnosis of Alzheimer’s dementia by CSF Abeta42, Abeta42/Abeta40 ratio and total tau. Neurobiol Aging. 2004;25:273–81. doi: 10.1016/S0197-4580(03)00086-1. [DOI] [PubMed] [Google Scholar]
- Lewis J, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–91. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Leys D, et al. [Alzheimer’s disease: study by single photon emission tomography (Hm PAO Tc99m)] Rev Neurol (Paris) 1989;145:443–50. [PubMed] [Google Scholar]
- Li G, et al. CSF tau/Aβ42 ratio for increased risk of mild cognitive impairment: A follow- up study. Neurology. 2007;69:631–39. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- Licastro F, et al. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer’s disease: peripheral inflammation or signals from the brain? J Neuroimmunol. 2000;103:97–102. doi: 10.1016/s0165-5728(99)00226-x. [DOI] [PubMed] [Google Scholar]
- Lieberman J, et al. Serum alpha 1-antichymotrypsin level as a marker for Alzheimer-type dementia. Neurobiol Aging. 1995;16:747–53. doi: 10.1016/0197-4580(95)00056-k. [DOI] [PubMed] [Google Scholar]
- Lopez MF, et al. High-resolution serum proteomic profiling of Alzheimer disease samples reveals disease-specific, carrier-protein-bound mass signatures. Clin Chem. 2005;51:1946–54. doi: 10.1373/clinchem.2005.053090. [DOI] [PubMed] [Google Scholar]
- Lopez OL, et al. Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology. 2008;70:1664–71. doi: 10.1212/01.wnl.0000306696.82017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopresti BJ, et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46:1959–72. [PubMed] [Google Scholar]
- Machulda MM, et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology. 2003;61:500–6. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddalena A, et al. Biochemical diagnosis of Alzheimer disease by measuring the cerebrospinal fluid ratio of phosphorylated tau protein to beta-amyloid peptide42. Arch Neurol. 2003;60:1202–6. doi: 10.1001/archneur.60.9.1202. [DOI] [PubMed] [Google Scholar]
- Maeda J, et al. Longitudinal, quantitative assessment of amyloid, neuroinflammation, and anti-amyloid treatment in a living mouse model of Alzheimer’s disease enabled by positron emission tomography. J Neurosci. 2007;27:10957–68. doi: 10.1523/JNEUROSCI.0673-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, et al. Inflammatory markers in younger vs elderly normal volunteers and in patients with Alzheimer’s disease. J Psychiatr Res. 1999;33:397–405. doi: 10.1016/s0022-3956(99)00016-3. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Mandelkow E. Tau in Alzheimer’s disease. Trends Cell Biol. 1998;8:425–7. doi: 10.1016/s0962-8924(98)01368-3. [DOI] [PubMed] [Google Scholar]
- Markesbery W, et al. Neuropathologic substrate of Mild Cognitive Impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- Martinez M, et al. Increased cerebrospinal fluid fas (Apo-1) levels in Alzheimer’s disease. Relationship with IL-6 concentrations. Brain Res. 2000;869:216–9. doi: 10.1016/s0006-8993(00)02363-5. [DOI] [PubMed] [Google Scholar]
- Marz P, et al. Interleukin-6 (IL-6) and soluble forms of IL-6 receptors are not altered in cerebrospinal fluid of Alzheimer’s disease patients. Neurosci Lett. 1997;239:29–32. doi: 10.1016/s0304-3940(97)00886-0. [DOI] [PubMed] [Google Scholar]
- Mathis C, et al. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- Matsubara E, et al. Alpha 1-antichymotrypsin as a possible biochemical marker for Alzheimer-type dementia. Ann Neurol. 1990;28:561–7. doi: 10.1002/ana.410280414. [DOI] [PubMed] [Google Scholar]
- Mayeux R, et al. Plasma A[beta]40 and A[beta]42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology. 2003;61:1185–90. doi: 10.1212/01.wnl.0000091890.32140.8f. [DOI] [PubMed] [Google Scholar]
- Mayeux R, et al. Plasma amyloid beta-peptide 1-42 and incipient Alzheimer’s disease. Ann Neurol. 1999;46:412–6. doi: 10.1002/1531-8249(199909)46:3<412::aid-ana19>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Mecocci P, et al. Tau protein in cerebrospinal fluid: a new diagnostic and prognostic marker in Alzheimer disease? Alzheimer Dis Assoc Disord. 1998;12:211–4. doi: 10.1097/00002093-199809000-00015. [DOI] [PubMed] [Google Scholar]
- Mehta P, et al. Amyloid β protein 1-40 and 1-42 levels in matched cerebrospinal fluid and plasma from patients with Alzheimer disease. Neurosci Lett. 2001;304:102–6. doi: 10.1016/s0304-3940(01)01754-2. [DOI] [PubMed] [Google Scholar]
- Mehta PD, et al. Plasma and cerebrospinal fluid levels of amyloid β proteins 1-40 and 1-42 in Alzheimer’s disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- Merched A, et al. Apolipoprotein E, transthyretin and actin in the CSF of Alzheimer’s patients: Relation with the senile plaques and cytoskeleton biochemistry. FEBS Lett. 1998;425:225–228. doi: 10.1016/s0014-5793(98)00234-8. [DOI] [PubMed] [Google Scholar]
- Minoshima S, et al. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Mintun M, et al. [11C]PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–52. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Molina L, et al. Tau and apo E in CSF: potential aid for discriminating Alzheimer’s disease from other dementias. Neuroreport. 1999;10:3491–5. doi: 10.1097/00001756-199911260-00005. [DOI] [PubMed] [Google Scholar]
- Montaldi D, et al. Measurements of regional cerebral blood flow and cognitive performance in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1990;53:33–8. doi: 10.1136/jnnp.53.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine T, et al. Increased CSF F2-isoprostane concentration in probable AD. Neurology. 1999a;52:562–5. doi: 10.1212/wnl.52.3.562. [DOI] [PubMed] [Google Scholar]
- Montine T, et al. Cerebrospinal fluid F2-isoprostane levels are increased in Alzheimer’s disease. Ann Neurol. 1998;44:410–413. doi: 10.1002/ana.410440322. [DOI] [PubMed] [Google Scholar]
- Montine TJ, et al. Cerebrospinal fluid abeta42, tau, and f2-isoprostane concentrations in patients with Alzheimer disease, other dementias, and in age-matched controls. Arch Pathol Lab Med. 2001;125:510–2. doi: 10.5858/2001-125-0510-CFATAF. [DOI] [PubMed] [Google Scholar]
- Montine TJ, et al. The magnitude of brain lipid peroxidation correlates with the extent of degeneration but not with density of neuritic plaques or neurofibrillary tangles or with APOE genotype in Alzheimer’s disease patients. Am J Pathol. 1999b;155:863–8. doi: 10.1016/S0002-9440(10)65185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, et al. No difference in plasma or urinary F2-isoprostanes among patients with Huntington’s disease or Alzheimer’s disease and controls. Ann Neurol. 2000;48:950. [PubMed] [Google Scholar]
- Morgan D, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–5. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Mori H, et al. Tau in cerebrospinal fluids: establishment of the sandwich ELISA with antibody specific to the repeat sequence in tau. Neurosci Lett. 1995;186:181–3. doi: 10.1016/0304-3940(95)11291-4. [DOI] [PubMed] [Google Scholar]
- Morris J, Price J. Pathologic correlates of nondemented aging, mild cognitive impairment, and early stage Alzheimer’s disease. J Mol Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- Mosconi L, et al. MCI conversion to dementia and the APOE genotype: A prediction study with FDG-PET. Neurology. 2004;63:2332–40. doi: 10.1212/01.wnl.0000147469.18313.3b. [DOI] [PubMed] [Google Scholar]
- Motter R, et al. Reduction of β-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer’s disease. Ann Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- Moulin CJ, et al. Brain function during multi-trial learning in mild cognitive impairment: a PET activation study. Brain Res. 2007;1136:132–41. doi: 10.1016/j.brainres.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Mulder C, et al. CSF markers related to pathogenetic mechanisms in Alzheimer’s disease. J Neural Transm. 2002;109:1491–8. doi: 10.1007/s00702-002-0763-y. [DOI] [PubMed] [Google Scholar]
- Munroe WA, et al. Tau protein in cerebrospinal fluid as an aid in the diagnosis of Alzheimer’s disease. Ann Clin Lab Sci. 1995;25:207–17. [PubMed] [Google Scholar]
- Nestor P, et al. Limbic hypometabolism in Alzheimer’s disease and mild cognitive impairment. Ann Neurol. 2003;54:343–351. doi: 10.1002/ana.10669. [DOI] [PubMed] [Google Scholar]
- Nielsen HM, et al. Plasma and CSF serpins in Alzheimer disease and dementia with Lewy bodies. Neurology. 2007;69:1569–79. doi: 10.1212/01.wnl.0000271077.82508.a0. [DOI] [PubMed] [Google Scholar]
- Nishimura T, et al. Basic and clinical studies on the measurement of tau protein in cerebrospinal fluid as a biological marker for Alzheimer’s disease and related disorders: multicenter study in Japan. Methods Find Exp Clin Pharmacol. 1998;20:227–35. [PubMed] [Google Scholar]
- Otto M, et al. Decreased beta-amyloid1-42 in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Neurology. 2000;54:1099–102. doi: 10.1212/wnl.54.5.1099. [DOI] [PubMed] [Google Scholar]
- Palmert MR, et al. Soluble derivatives of the beta amyloid protein precursor in cerebrospinal fluid: alterations in normal aging and in Alzheimer’s disease. Neurology. 1990;40:1028–34. doi: 10.1212/wnl.40.7.1028. [DOI] [PubMed] [Google Scholar]
- Pan S, et al. A combined dataset of human cerebrospinal fluid proteins identified by multi-dimensional chromatography and tandem mass spectrometry. Proteomics. 2007;7:469–73. doi: 10.1002/pmic.200600756. [DOI] [PubMed] [Google Scholar]
- Perani D, et al. Technetium-99m HM-PAO-SPECT study of regional cerebral perfusion in early Alzheimer’s disease. J Nucl Med. 1988;29:1507–14. [PubMed] [Google Scholar]
- Pesaresi M, et al. Plasma levels of beta-amyloid(1-42) in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27:904–905. doi: 10.1016/j.neurobiolaging.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Petrella JR, et al. Mild cognitive impairment: evaluation with 4-T functional MR imaging. Radiology. 2006;240:177–86. doi: 10.1148/radiol.2401050739. [DOI] [PubMed] [Google Scholar]
- Pike KE, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130:2837–44. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- Pirttila T, et al. Alpha 1-antichymotrypsin and IL-1 beta are not increased in CSF or serum in Alzheimer’s disease. Neurobiol Aging. 1994;15:313–7. doi: 10.1016/0197-4580(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Portelius E, et al. An Alzheimer’s Disease-Specific B-Amyloid Fragment Signature in Cerebrospinal Fluid. Neurosci Lett. 2006;409:215–219. doi: 10.1016/j.neulet.2006.09.044. [DOI] [PubMed] [Google Scholar]
- Pratico D, et al. Increased 8,12-iso-iPF2alpha-VI in Alzheimer’s disease: Correlation of a noninvasive index of lipid peroxidation with disease severity. Ann Neurol. 2000;48:809–12. [PubMed] [Google Scholar]
- Pratico D, et al. Increased brain oxidative stress in mild cognitive impairment: A possible predictor of Alzheimer’s disease. Arch Neurol. 2002;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- Pratico D, et al. Increased F2-isoprostanes in Alzheimer’s disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998;12:1777–83. doi: 10.1096/fasebj.12.15.1777. [DOI] [PubMed] [Google Scholar]
- Price J, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow & Metab. 2005 doi: 10.1038/sj.jcbfm.9600146. in press. [DOI] [PubMed] [Google Scholar]
- Price J, et al. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer’s disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- Price JL, et al. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Prior R, et al. Quantitative changes in the amyloid beta A4 precursor protein in Alzheimer cerebrospinal fluid. Neurosci Lett. 1991;124:69–73. doi: 10.1016/0304-3940(91)90824-d. [DOI] [PubMed] [Google Scholar]
- Puchades M, et al. Proteomic studies of potential cerebrospinal fluid protein markers for Alzheimer’s disease. Mol Brain Res. 2003;118:140–146. doi: 10.1016/j.molbrainres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Quinn JF, et al. Suppression of longitudinal increase in CSF F2-isoprostanes in Alzheimer’s disease. J Alzheimers Dis. 2004;6:93–7. doi: 10.3233/jad-2004-6110. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007;68:1205–12. doi: 10.1212/01.wnl.0000259035.98480.ed. [DOI] [PubMed] [Google Scholar]
- Ray S, et al. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–62. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- Riemenschneider M, et al. Cerebrospinal Fluid Tau and B-Amyloid 42 Proteins Identify Alzheimer Disease in Subjects With Mild Cognitive Impairment. Arch Neurol. 2002a;59:1729–1734. doi: 10.1001/archneur.59.11.1729. [DOI] [PubMed] [Google Scholar]
- Riemenschneider M, et al. Cerebrospinal beta-amyloid ((1-42)) in early Alzheimer’s disease: association with apolipoprotein E genotype and cognitive decline. Neurosci Lett. 2000;284:85–8. doi: 10.1016/s0304-3940(00)00976-9. [DOI] [PubMed] [Google Scholar]
- Riemenschneider M, et al. Tau and Abeta42 protein in CSF of patients with frontotemporal degeneration. Neurology. 2002b;58:1622–8. doi: 10.1212/wnl.58.11.1622. [DOI] [PubMed] [Google Scholar]
- Ringman JM, et al. Biochemical markers in persons with preclinical familial Alzheimer disease. Neurology. 2008;71:85–92. doi: 10.1212/01.wnl.0000303973.71803.81. [DOI] [PubMed] [Google Scholar]
- Rogers J, Lue LF. Microglial chemotaxis, activation, and phagocytosis of amyloid beta-peptide as linked phenomena in Alzheimer’s disease. Neurochem Int. 2001;39:333–40. doi: 10.1016/s0197-0186(01)00040-7. [DOI] [PubMed] [Google Scholar]
- Roher AE, et al. beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci U S A. 1993a;90:10836–40. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roher AE, et al. Morphological and biochemical analyses of amyloid plaque core proteins purified from Alzheimer disease brain tissue. J Neurochem. 1993b;61:1916–26. doi: 10.1111/j.1471-4159.1993.tb09834.x. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, et al. Functional MR imaging in Alzheimer’s disease during memory encoding. AJNR Am J Neuroradiol. 2000;21:1869–75. [PMC free article] [PubMed] [Google Scholar]
- Rosler N, et al. Total tau protein immunoreactivity in lumbar cerebrospinal fluid of patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1996;60:237–8. doi: 10.1136/jnnp.60.2.237-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosler N, et al. Clinical significance of neurobiochemical profiles in the lumbar cerebrospinal fluid of Alzheimer’s disease patients. J Neural Transm. 2001a;108:231–46. doi: 10.1007/s007020170091. [DOI] [PubMed] [Google Scholar]
- Rosler N, et al. Intra vitam lumbar and post mortem ventricular cerebrospinal fluid immunoreactive interleukin-6 in Alzheimer’s disease patients. Acta Neurol Scand. 2001b;103:126–30. doi: 10.1034/j.1600-0404.2001.103002126.x. [DOI] [PubMed] [Google Scholar]
- Rowe CC, et al. Imaging of amyloid beta in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008;7:129–35. doi: 10.1016/S1474-4422(08)70001-2. [DOI] [PubMed] [Google Scholar]
- Rowe CC, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–25. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- Sakamoto S, et al. Differences in cerebral metabolic impairment between early and late onset types of Alzheimer’s disease. J Neurol Sci. 2002;200:27–32. doi: 10.1016/s0022-510x(02)00114-4. [DOI] [PubMed] [Google Scholar]
- Samuels SC, et al. CSF beta-amyloid, cognition, and APOE genotype in Alzheimer’s disease. Neurology. 1999;52:547–51. doi: 10.1212/wnl.52.3.547. [DOI] [PubMed] [Google Scholar]
- Scheltens P, et al. Structural magnetic resonance imaging in the practical assessment of dementia: beyond exclusion. Lancet Neurol. 2002;1:13–21. doi: 10.1016/s1474-4422(02)00002-9. [DOI] [PubMed] [Google Scholar]
- Schenk D, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–7. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Scheuner D, et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 APP mutations linked to familial Alzheimer’s disease. Nature Med. 1996;2:864–852. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Schmitt F, et al. “Preclinical” AD revisited: Neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- Schwarzman AL, Goldgaber D. Interaction of transthyretin with amyloid beta-protein: binding and inhibition of amyloid formation. Ciba Found Symp. 1996;199:146–60. doi: 10.1002/9780470514924.ch10. discussion 160-4. [DOI] [PubMed] [Google Scholar]
- Schwarzman AL, et al. Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc Natl Acad Sci U S A. 1994;91:8368–72. doi: 10.1073/pnas.91.18.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selle H, et al. Identification of novel biomarker candidates by differential peptidomics analysis of cerebrospinal fluid in Alzheimer’s disease. Comb Chem High Throughput Screen. 2005;8:801–6. doi: 10.2174/138620705774962391. [DOI] [PubMed] [Google Scholar]
- Sennvik K, et al. Levels of alpha- and beta-secretase cleaved amyloid precursor protein in the cerebrospinal fluid of Alzheimer’s disease patients. Neurosci Lett. 2000;278:169–72. doi: 10.1016/s0304-3940(99)00929-5. [DOI] [PubMed] [Google Scholar]
- Sheng JG, et al. Neuritic plaque evolution in Alzheimer’s disease is accompanied by transition of activated microglia from primed to enlarged to phagocytic forms. Acta Neuropathol. 1997;94:1–5. doi: 10.1007/s004010050664. [DOI] [PubMed] [Google Scholar]
- Shoghi-Jadid K, et al. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry. 2002;10:24–35. [PubMed] [Google Scholar]
- Shoji M, et al. Alpha 1-antichymotrypsin is present in diffuse senile plaques. A comparative study of beta-protein and alpha 1-antichymotrypsin immunostaining in the Alzheimer brain. Am J Pathol. 1991;138:247–57. [PMC free article] [PubMed] [Google Scholar]
- Shoji M, et al. Combination assay of CSF tau, A beta 1-40 and A beta 1-42(43) as a biochemical marker of Alzheimer’s disease. J Neurol Sci. 1998;158:134–40. doi: 10.1016/s0022-510x(98)00122-1. [DOI] [PubMed] [Google Scholar]
- Shoji M, et al. Cerebrospinal fluid tau in dementia disorders: a large scale multicenter study by a Japanese study group. Neurobiol Aging. 2002;23:363–70. doi: 10.1016/s0197-4580(01)00309-8. [DOI] [PubMed] [Google Scholar]
- Simonsen A, et al. Novel panel of cerebrospinal fluid biomarkers for the prediction of progression to Alzheimer dementia in patients with mild cognitive impairment. Arch Neurol. 2007;64:366–70. doi: 10.1001/archneur.64.3.366. [DOI] [PubMed] [Google Scholar]
- Sjogren M, et al. Decreased CSF-beta-amyloid 42 in Alzheimer’s disease and amyotrophic lateral sclerosis may reflect mismetabolism of beta-amyloid induced by disparate mechanisms. Dement Geriatr Cogn Disord. 2002;13:112–8. doi: 10.1159/000048642. [DOI] [PubMed] [Google Scholar]
- Sjogren M, et al. CSF levels of tau, beta-amyloid(1-42) and GAP-43 in frontotemporal dementia, other types of dementia and normal aging. J Neural Transm. 2000;107:563–79. doi: 10.1007/s007020070079. [DOI] [PubMed] [Google Scholar]
- Skoog I, et al. Cerebrospinal fluid beta-amyloid 42 is reduced before the onset of sporadic dementia: A population-based study in 85-year-olds. Dement Geriatr Cogn Disord. 2003;15:169–176. doi: 10.1159/000068478. [DOI] [PubMed] [Google Scholar]
- Skoog I, et al. A population-based study of tau protein and ubiquitin in cerebrospinal fluid in 85-year-olds: relation to severity of dementia and cerebral atrophy, but not to the apolipoprotein E4 allele. Neurodegeneration. 1995;4:433–42. doi: 10.1006/neur.1995.0052. [DOI] [PubMed] [Google Scholar]
- Small GW, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006;355:2652–63. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- Southwick PC, et al. Assessment of amyloid beta protein in cerebrospinal fluid as an aid in the diagnosis of Alzheimer’s disease. J Neurochem. 1996;66:259–65. doi: 10.1046/j.1471-4159.1996.66010259.x. [DOI] [PubMed] [Google Scholar]
- Sperling RA, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strozyk D, et al. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60:652–6. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- Sunderland T, et al. Decreased beta-amyloid(1-42) and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003;289:2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- Tamaoka A, et al. Amyloid beta protein in plasma from patients with sporadic Alzheimer’s disease. J Neurol Sci. 1996;141:65–8. doi: 10.1016/0022-510x(96)00143-8. [DOI] [PubMed] [Google Scholar]
- Tamaoka A, et al. Amyloid β protein 42(43) in cerebrospinal fluid of patients with Alzheimer’s disease. Neurol J Sci. 1997;1997:41–45. doi: 10.1016/s0022-510x(96)00314-0. [DOI] [PubMed] [Google Scholar]
- Tapiola T, et al. MRI of hippocampus and entorhinal cortex in mild cognitive impairment: a follow-up study. Neurobiol Aging. 2008;29:31–8. doi: 10.1016/j.neurobiolaging.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, et al. Intracerebral production of tumor necrosis factor-alpha, a local neuroprotective agent, in Alzheimer disease and vascular dementia. J Clin Immunol. 1999;19:223–30. doi: 10.1023/a:1020568013953. [DOI] [PubMed] [Google Scholar]
- Tato RE, et al. Tau protein concentrations in cerebrospinal fluid of patients with dementia of the Alzheimer type. J Neurol Neurosurg Psychiatry. 1995;59:280–3. doi: 10.1136/jnnp.59.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen CE, et al. Biochemical markers related to Alzheimer’s dementia in serum and cerebrospinal fluid. Neurobiol Aging. 2002;23:485–508. doi: 10.1016/s0197-4580(01)00328-1. [DOI] [PubMed] [Google Scholar]
- Thompson PM, et al. Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki K, et al. Transthyretin binds amyloid beta peptides, Abeta1-42 and Abeta1-40 to form complex in the autopsied human kidney - possible role of transthyretin for abeta sequestration. Neurosci Lett. 2000;281:171–4. doi: 10.1016/s0304-3940(00)00834-x. [DOI] [PubMed] [Google Scholar]
- Tuppo EE, et al. Sign of lipid peroxidation as measured in the urine of patients with probable Alzheimer’s disease. Brain Res Bull. 2001;54:565–8. doi: 10.1016/s0361-9230(01)00450-6. [DOI] [PubMed] [Google Scholar]
- van Gool WA, et al. Concentrations of amyloid beta protein in cerebrospinal fluid of patients with Alzheimer’s disease. Ann Neurol. 1995;37:277–9. doi: 10.1002/ana.410370221. [DOI] [PubMed] [Google Scholar]
- Van Nostrand WE, et al. Decreased levels of soluble amyloid beta-protein precursor in cerebrospinal fluid of live Alzheimer disease patients. Proc Natl Acad Sci U S A. 1992;89:2551–5. doi: 10.1073/pnas.89.7.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oijen M, et al. Plasma Aβ1-40 and Aβ1-42 and the risk of dementia: A prospective case-cohort study. Lancet Neurol. 2006;5:655–60. doi: 10.1016/S1474-4422(06)70501-4. [DOI] [PubMed] [Google Scholar]
- Vandermeeren M, et al. Detection of tau proteins in normal and Alzheimer’s disease cerebrospinal fluid with a sensitive sandwich enzyme-linked immunosorbant assay. J Neurochem. 1993;61:1828–1834. doi: 10.1111/j.1471-4159.1993.tb09823.x. [DOI] [PubMed] [Google Scholar]
- Vanderstichele H, et al. Standardization of measurement of β-amyloid(1-42) in cerebrospinal fluid and plasma. Int J Exp Clin Invest. 2000;7:245–258. doi: 10.3109/13506120009146438. [DOI] [PubMed] [Google Scholar]
- Verhoeff NP, et al. In-vivo imaging of Alzheimer disease beta-amyloid with [11C]SB-13 PET. Am J Geriatr Psychiatry. 2004;12:584–95. doi: 10.1176/appi.ajgp.12.6.584. [DOI] [PubMed] [Google Scholar]
- Versijpt JJ, et al. Assessment of neuroinflammation and microglial activation in Alzheimer’s disease with radiolabelled PK11195 and single photon emission computed tomography. A pilot study. Eur Neurol. 2003;50:39–47. doi: 10.1159/000070857. [DOI] [PubMed] [Google Scholar]
- Vigo-Pelfrey C, et al. Elevation of microtubule-associated protein tau in the cerebrospinal fluid of patients with Alzheimer’s disease. Neurology. 1995;45:788–93. doi: 10.1212/wnl.45.4.788. [DOI] [PubMed] [Google Scholar]
- Wada-Isoe K, et al. Elevated interleukin-6 levels in cerebrospinal fluid of vascular dementia patients. Acta Neurol Scand. 2004;110:124–127. doi: 10.1111/j.1600-0404.2004.00286.x. [DOI] [PubMed] [Google Scholar]
- Weidemann A, et al. Identification, biogenesis, and localization of precursors of Alzheimer’s disease A4 amyloid protein. Cell. 1989;57:115–26. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging. 1993;14:287–93. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- West MJ, et al. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–72. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR., Jr Comparisons between Alzheimer disease, frontotemporal lobar degeneration, and normal aging with brain mapping. Top Magn Reson Imaging. 2005;16:409–25. doi: 10.1097/01.rmr.0000245457.98029.e1. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain. 2007;130:708–19. doi: 10.1093/brain/awl388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard JL, et al. Compensatory recruitment of neural resources during overt rehearsal of word lists in Alzheimer’s disease. Neuropsychology. 1998;12:491–504. doi: 10.1037//0894-4105.12.4.491. [DOI] [PubMed] [Google Scholar]
- Yamada K, et al. Decreased interleukin-6 level in the cerebrospinal fluid of patients with Alzheimer-type dementia. Neurosci Lett. 1995;186:219–21. doi: 10.1016/0304-3940(95)11318-q. [DOI] [PubMed] [Google Scholar]
- Yao Y, et al. Enhanced brain levels of 8,12-iso-iPF2alpha-VI differentiate AD from frontotemporal dementia. Neurology. 2003;61:475–8. doi: 10.1212/01.wnl.0000070185.02546.5d. [DOI] [PubMed] [Google Scholar]
- Zhang J, et al. Quantitative proteomics of cerebrospinal fluid from patients with Alzheimer’s disease. J Alz Dis. 2005a;7:125–133. doi: 10.3233/jad-2005-7205. [DOI] [PubMed] [Google Scholar]
- Zhang J, et al. Quantitative proteomics of cerebrospinal fluid from patients with Alzheimer disease. J Alzheimers Dis. 2005b;7:125–33. doi: 10.3233/jad-2005-7205. discussion 173-80. [DOI] [PubMed] [Google Scholar]
- Zhang MR, et al. Development of a new radioligand, N-(5-fluoro-2-phenoxyphenyl)-N-(2-[18F]fluoroethyl-5-methoxybenzyl)acetami de, for pet imaging of peripheral benzodiazepine receptor in primate brain. J Med Chem. 2004;47:2228–35. doi: 10.1021/jm0304919. [DOI] [PubMed] [Google Scholar]
- Zougman A, et al. Integrated analysis of the cerebrospinal fluid peptidome and proteome. J Proteome Res. 2008;7:386–99. doi: 10.1021/pr070501k. [DOI] [PubMed] [Google Scholar]