Abstract
Merkel cell polyomavirus (MCV) is a newly-discovered human tumor virus found in ∼80% of Merkel cell carcinoma (MCC). The rate of MCV infection among persons without MCC is unknown. We developed a MCV virus-like particle (VLP) enzyme-linked immunoassay (EIA) that does not cross-react with human BK or murine polyomaviruses. Peptide mapping of the MCV VP1 gene and immunoblotting with denatured MCV VLP are less sensitive than the MCV EIA in detecting MCV antibodies suggesting antibody reactivity in this assay primarily targets conformational but not linear epitopes. Among MCC patients, all 21 (100%) patients tested with MCV-positive tumors had high serum MCV IgG but not high MCV IgM levels. Only 3 of 6 (50%) MCC patients with MCV-negative tumors were positive for MCV antibodies. Sera from most adults, including 107 of 166 (64%) blood donors, 63 of 100 (63%) commercial donors and 37 of 50 (74%) systemic lupus erythematosus patients, show evidence for prior MCV exposure. Age-specific MCV prevalence was determined by examining a cross-sectional distribution of 150 Langerhans cell histiocytosis (an unrelated neoplasm) patient sera. MCV prevalence increases from 50% among children age 15 years or younger to 80% among persons older than 50 years. We did not find evidence for vertical transmission among infants. Although past exposure to MCV is common among all adult groups, MCC patients have a markedly elevated MCV IgG response compared with control patients. Our study demonstrates that MCV is a widespread but previously unrecognized human infection.
Keywords: Merkel cell polyomavirus, Merkel cell carcinoma, virus-like particles, enzyme-linked immunosorbent assay, serologic assay
Merkel cell carcinoma (MCC) is an uncommon skin cancer frequently having a poor prognosis.1 It most often arises in chronically sun-exposed skin and occurs more commonly than expected among immunosuppressed persons, including AIDS patients, transplant recipients and elderly persons.2 Feng et al.3 used digital transcriptome subtraction, a high-throughput cDNA sequencing technique to search for viral sequences in MCC. Transcripts encoding a unique polyomavirus large tumor (T) antigen were recovered from one MCC tumor.4 This led to full-length sequencing of a 5.4 kbp Merkel cell polyomavirus (MCV) genome encoding viral protein (VP)1 and VP2 capsid genes and a multiply-spliced T antigen oncogene locus. Subsequent studies showed that MCV DNA is present in 70–80% of MCC tumors in persons from different geographic locations.5-8
Substantial biological evidence supports MCV having an etiopathologic role for the majority of human MCC tumors.9 Within MCC tumors, MCV is monoclonally-integrated into the host genome4 and acquires T antigen mutations that prevent autonomous viral DNA replication but still allow the virus to target the retinoblastoma tumor suppressor protein.10 These tumor-specific mutations eliminate the possibility that MCV is a secondary infection of MCC tumors. Tumor cells in MCV-positive tumors express abundant MCV T antigen protein and tissue surveys of hematologic malignancies show that MCV DNA and T antigen protein expression are specific to MCC tumors.11 Nevertheless, ∼20–30% of MCC tumors are not MCV infected indicating that this cancer has at least 2 distinct etiologies.
The rate of human exposure to MCV infection is currently not known. Other polyomaviruses, including BK (BKV) and JC (JCV) viruses, are near-ubiquitous infections among adults. These viruses are closely related to each other and to the primate virus simian virus 40 (SV40), leading to frequent serologic cross-reactivity.12,13 MCV is distantly related to these polyomaviruses; however, and the divergence of its protein sequences from those of known human polyomaviruses suggests that antibodies generated during natural MCV infection antigens might be specifically distinguished on blood tests. For example, a panel of 23 antibodies raised against different SV40 T antigen epitopes was tested and found to be completely nonreactive to the MCV T antigen.11 Several of these antibodies are highly cross-reactive to BKV and JCV T antigen proteins and are used in human clinical diagnosis. Conversely, a monoclonal antibody (CM2B4) raised against MCV T antigen does not react with T antigens from the SV40, BKV or JCV.11
We show here that artificially-expressed MCV VP1 and VP2 proteins self-assemble into virus-like particles (VLP) that have unique conformational epitopes recognized by sera from MCV-infected MCC patients. Antibodies to MCV VLP are not cross-reactive to either murine or BK polyomavirus VLP. MCC patients with MCV-infected tumors have uniformly high anti-MCV IgG antibody levels, whereas MCC patients with uninfected tumors have antibody patterns similar to those of control populations. Using this assay, we find that exposure to this virus increases with age and is common among children and adults from various US populations.
Material and methods
Patient populations and recruitment
MCC patients were recruited at the University Clinic of Würzburg, department of dermatology, venerology and allergy (Germany) and the University of Pittsburgh Cancer Institute, US. All patient tumors were histologically confirmed to be MCC by pathologic diagnosis with cytokeratin 20 immunostaining. Tumor infection with MCV was determined by PCR assay4 or MCV T antigen immunostaining11 for all but 2 patients whose tumors were not available, but whose peripheral blood mononuclear cells were positive by MCV PCR. Deidentified blood donor samples were obtained from individuals over 18 years old in Arizona, Pennsylvania and New York in 1994–1996. Samples from paid donors (commercial donors) over age 47 years old, chosen to more closely match the older age-range of MCC patients, were obtained from 2 commercial sources (Equitech-Bio, Kerrville, TX) and Innovative Research, Novi, MI). Systemic lupus erythematosus (SLE) patient sera were obtained from an ambulatory clinic population as previously described.14 Langerhans cell histiocytosis (LCH) patient sera were obtained from participants recruited by their physicians as a part of a study on the epidemiology of LCH through mailings to members of the Histiocytosis Association of America (Pitman, NJ). These patients had a prior history of pathology-confirmed LCH diagnosis at the time of sample collection. All serum samples were stored at -80°C until tested. All samples and data were collected after written consent under study protocols approved by the institutional review boards of the University of Pittsburgh and the University Clinic of Würzberg.
Plasmids
VP1 and VP2 genes were designed according to a silent codon modification scheme (GenBank accession FJ548568-FJ54871)15 and synthesized by Blue Heron Biotechnology (Bothell, WA) based on MCV339 (accession EU375804),4 and murine polyomavirus (MPyV) strain LID (accession PSU27813).16 BKV VLP produced in a baculovirus system17 were a kind gift of Dr. John T. Schiller.
Virus-like particle production
VLPs were produced in human embryonic kidney 293TT cells18 as previously described19 (detailed protocol available at http://home.ccr.cancer.gov/LCO/ and in Supporting Materials). Briefly, cells were cotransfected with expression constructs encoding VP1 and VP2 at a 3:1 mass ratio, harvested after 48 hr following transfection, and incubated at 37°C overnight for virion maturation.19 VLP were isolated on a 27–33–39% Optiprep (Sigma, St. Louis, MO) step gradient after ultracentrifugation for 3.5 hr at 234,000g. Gradient fractions were tested for encapsidated DNA concentration using Quant-iT Picogreen dsDNA Reagent (Invitrogen). Fractions were also screened for protein content using NuPage polyacrylamide gels (Invitrogen, Carlsbad, CA) with SYPRO Ruby protein staining (Invitrogen). VLP yields were ∼1 mg purified protein particles per transfected 225 cm2 flask of cells. Electron microscopy was performed by Kunio Nagashima of the National Cancer Institute’s Image Analysis Laboratory (SAIC-Frederick, MD).
Enzyme-linked immunosorbent assays
Each serum was initially tested in a blinded and randomized fashion for MCV VLP reactivity and confirmed with a second unblinded test. Each determination was performed in duplicate and optical density (OD) values were adjusted by background subtraction using wells without antigen as previously described.14 Enzyme-linked immunoassay (EIAs) (Supporting Materials for detailed protocol) were performed on Immulon (Thermo Scientific, Waltham, MA) HB2 plates coated overnight with fraction purified MCV, BKV or murine polyomavirus VLPs at 100 ng protein per well in phosphate-buffered saline blocked with 0.5% nonfat dry milk. Unless stated otherwise, all IgG results are based on duplicate 1:500 dilutions of patient sera.
VLP competition assays
Serum samples were tested using the MCV EIA at 1:500, 1:1,000, 1:2,000, 1:4,000, 1:8,000, 1:16,000, and 1:32,000 dilution to estimate the 50% effective concentration (EC50) for MCV antibody reactivity. Sera diluted to their respective EC50 were incubated with increasing amounts of MCV or BKV VLP (0.01–1 μg per 100 μl diluted sera) for one hour at room temperature. After VLP incubation, sera were directly added to wells and used for the MCV EIA as described above (Supporting Materials).
Statistical methods
MCC patients having known MCV infection (MCV-positive MCC patients) determined by tumor PCR or T antigen immunostaining were defined as gold standards for a positive test result. There are no obvious negative gold standards to measure lack of exposure to this virus since asymptomatic individuals may have had previous MCV exposure; therefore, a negative MCV EIA result was determined by comparison to murine polyomavirus (a nonhuman infection) VLP EIA reactivity and by MCV VLP competition. The average reactivity for MCV-positive MCC sera tested at 1:500 dilution against murine polyomavirus VLP was determined to be 0.048 O.D. units (95% confidence interval 0.029–0.067 OD units), an EIA optical density level associated with seronegativity in our laboratory for other viral EIA assays.14 This value was used as a negative cut-off value on the MCV EIA test. All sera reactive on the MCV EIA between 0.05 and 0.2 OD units were retested for specific MCV competition. Any of these sera having greater than 50% reduction in MCV EIA absorbance after competition with 200 ng MCV VLP per 100 μl, were then considered to be MCV antibody positive. All sera having >0.2 OD unit optical density on the MCV EIA were considered positive for MCV antibodies. Analyses were conducted using GraphPad Prism (La Jolla, CA) software and a VasserStats online statistical calculator (http://faculty.vassar.edu/lowry/odds2×2.html). Continuous data were analyzed by a nonparametric Mann-Whitney test and categorical data were analyzed using a two-sided, Fisher’s exact test and by chi-squared tests for trend.
Results
VLP production
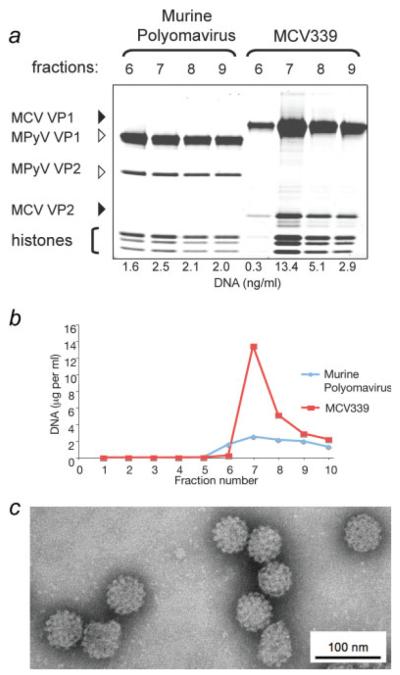
MCV virus-like particles (VLP) were produced by expression from synthetic MCV339 VP1 and VP2 protein genes in 293TT cells. Control VLP based on MPyV, a near relative of MCV that is not known to infect humans, were also identically produced. Abundant MCV VLP resembling typical icosahedral polyomavirus capsids were isolated having a uniform 55–58 nm diameter with phosphotungstic acid staining and possessing capsomer features characteristic for polyomavirus virions (Fig. 1 and Supporting Fig. 1).
FIGURE 1.
(a) MCV VP1 and VP2 proteins expressed in 293TT cells self-assemble into virus-like particles (VLP). This panel shows protein staining of gradient fractions from cell lysates expressing MCV strain 339 VP1 and VP2 proteins compared with murine polyomavirus VP1 and VP2, which are known to coassemble into VLP (Supporting Fig. 1). Each lane was loaded with 2.5 μl of fraction material, electrophoresed on a polyacrylamide gel and stained with SYPRO Ruby protein stain. Murine polyomavirus and MCV VP1 and VP2 proteins coassemble into high molecular weight complexes (fractions 7–9) with histone-associated DNA, which is characteristic for VLP production. (b) DNA concentrations, representing VLP encapsidation of cellular and plasmid DNA fragments, peak in high molecular weight gradient fractions 7–9 for both MCV and murine polyomavirus VLP. (c) Transmission electron microscopy of MCV VLP at ×50,000 magnification shows characteristic polyomaviral icosahedral capsid structures. Bar represents 100 nm.
MCC seroreactivity to MCV VLP
Thirty-four MCC patients from the United States and Germany were initially enrolled, of whom 27 had tumor and blood specimens available and were included in the analysis. These patients (Table I) included 21 (78%) persons with confirmed MCV tumor infection (median age: 74 years, range 14–95 years) and 6 MCC patients (22%) with tumors negative for MCV (median age: 75, range 44–88 years), a proportion similar to those found in other settings.8
TABLE I.
CHARACTERISTICS OF MCC PATIENTS
| Patient no. |
Age at diagnosis (yr) |
MCV-tumor status |
Tumor stage1 |
Alive/dead1 | MCV IgG2 (OD units) |
|---|---|---|---|---|---|
| 1 | 81 | Pos | 1 | Alive | 2.670 |
| 2 | 95 | Pos | 3 | Dead | 2.473 |
| 3 | 84 | Pos | 2 | Alive | 2.646 |
| 4 | 64 | Pos | 3 | Alive | 2.526 |
| 5 | 74 | Pos | 3 | Alive | 2.614 |
| 6 | 68 | Pos | 1 | Alive | 2.508 |
| 7 | 55 | Pos | 3 | Alive | 2.512 |
| 8 | 55 | Pos | 1 | Alive | 2.195 |
| 9 | 87 | Pos | 1/2 | Alive | 2.348 |
| 10 | 90 | Pos | 2 | Dead | 0.914 |
| 11 | 78 | Pos | 1 | Alive | 1.802 |
| 12 | 72 | Pos | 1 | Alive | 1.911 |
| 13 | 73 | Pos | 1 | Alive | 2.216 |
| 14 | 77 | Pos | 2 | Alive | 2.477 |
| 15 | 73 | Pos | 1 | Alive | 2.219 |
| 16 | 83 | Pos | 1 | Alive | 2.513 |
| 17 | 59 | Pos | 3 | Alive | 2.567 |
| 18 | 83 | Pos | 1/2 | Alive | 2.476 |
| 19 | 14 | Pos | 3 | Alive | 2.114 |
| 20 | 57 | Pos | 3 | Alive | 2.478 |
| 21 | 81 | Pos | 2 | Alive | 2.530 |
| 22 | 47 | Neg | 3 | Alive | 2.413 |
| 23 | 44 | Neg | 3 | Alive | 2.746 |
| 24 | 83 | Neg | 2 | Alive | 2.347 |
| 25 | 88 | Neg | 2 | Alive | 0.008 |
| 26 | 67 | Neg | 3 | Alive | 0.147 |
| 27 | 81 | Neg | 3 | Alive | 0.108 |
At entry into study.
MCV VLP EIA.
IgG antibody levels against MCV VLP were markedly elevated overall for the MCC patients, with most sera having absorbance values >2.0 optical density (OD) units (Table I). All 21 MCV-positive MCC patient sera exceeded 0.9 OD units (median 2.477, range 0.914–2.670 OD units). In comparison, the median MCV EIA result for sera from 6 MCV-negative MCC patients was 1.247 OD units (range 0.008–2.746 OD units). Three of these patients had MCV EIA values below 0.15 OD units and showed no competition with MCV VLP (see Methods). None of the MCC patient sera were reactive to highly homologous murine polyomavirus VLP at greater than 0.2 OD units, with most sera having reactivities less than 0.05 OD units, demonstrating that these antibodies are not likely to result from cross-reactivity to other nonMCV polyomavirus infections.
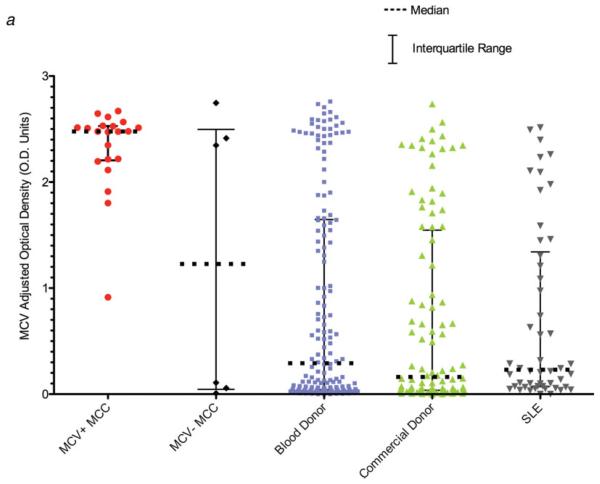
MCV IgG antibody levels were significantly lower among persons without MCC compared with MCV-positive MCC patients (Mann-Whitney two-tailed tests, p < 0.001 for all comparisons). Median MCV EIA values (Fig. 2a) for 166 blood donors, 100 commercial donors and 50 SLE patients were 0.292 (range 0.0–2.760), 0.162 (range 0.0–2.737) and 0.231 (range 0.0–2.513) OD units, respectively, and did not significantly differ between each other (Mann-Whitney two-tailed tests, p = 0.25–0.91). Although the SLE patient sera had previously been found to have high rates of nonspecific reactivity to other infectious agents on EIA testing,14 we found no evidence for nonspecific reactivity using the MCV VLP-based EIA.
FIGURE 2.
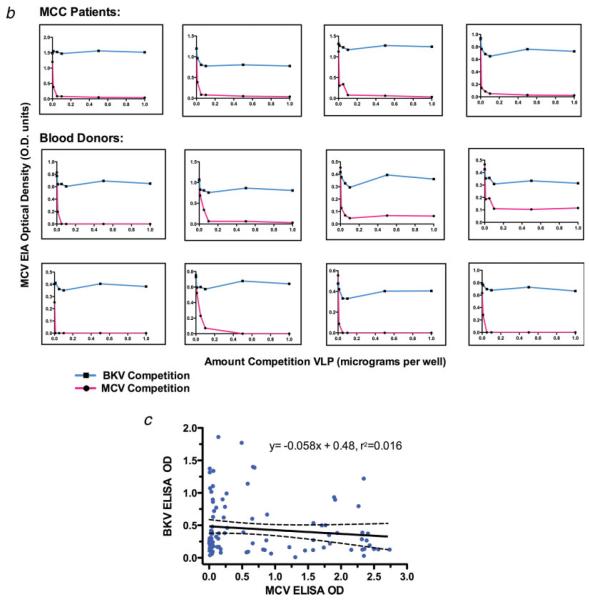
(a) Anti-MCV VLP antibodies levels are significantly elevated in sera from MCV-positive MCC patients compared with other patient populations. IgG antibody levels against MCV were measured with an MCV VLP EIA for MCC patients, blood donors, commercial donors and SLE patients. Median MCV optical density values are indicated by solid horizontal lines, with bars representing interquartile values. Each sample was tested in duplicate, in a randomized and blinded fashion. Background subtraction in the absence of antigen was performed to obtain the adjusted optical density value for each serum. (b) Immune competition experiments for 4 MCC patient sera and 8 blood donor sera reactive on the MCV EIA. For the competitions, each serum was diluted to its EC50 concentration, preincubated with increasing amounts of either MCV (red) or BKV VLP, and then tested on the MCV EIA. MCV VLP but not BKV VLP compete for MCV reactivity among all of the sera. (c) No correlation is present between BKV and MCV IgG antibody responses in human sera measured by EIA (100 ng VLP protein per well). One hundred commercial donor samples were tested by BKV and MCV EIA at 1:500 dilution. The regression line between BKV and MCV antibody levels shows no correlation, with a slope that includes zero (95% CI, -0.15 to 0.03). Dotted lines represent 95% confidence bounds for the regression line.
In contrast to MCV IgG, MCV VLP IgM titers at 1:100 dilution were not significantly elevated for the 21 MCV-positive MCC patients compared with 166 blood donors (median 0.348 vs. 0.284 O.D. units, respectively, Mann-Whitney 2-tailed test p = 0.30). For sera from MCV-positive MCC patients, MCV and BKV VLP IgM levels also were not significantly different from each other (p = 0.32). These results indicate that MCV IgM levels, unlike MCV IgG levels, are not significantly correlated to MCC.
To determine if the MCV EIA measures specific MCV antibodies, competition studies using BKV and MCV VLP were performed on 4 MCV-positive MCC sera and 8 reactive blood donor sera (Fig. 2b). Preincubation with as little as 50 ng soluble MCV VLP was sufficient to block MCV EIA reactivity. Preincubation with up to 1.0 μg BKV VLP, however, failed to compete the MCV EIA reactivity. Further, antibody reactivity using a BKV VLP EIA showed no correlation to patterns of MCV EIA reactivity (Fig. 2c). Finally, 48 commercial donor sera were tested in parallel EIA against MCV VLP and murine polyomavirus VLP-a virus that has higher protein sequence similarity to MCV than any of the known human polyomaviruses or SV40. Reactivity against murine polyomavirus VLP was low for all human sera and showed no correlation with MCV reactivity (Supporting Fig. 2). These results demonstrate that the MCV VLP EIA is not cross-reactive for antibodies against polyomaviruses belonging to the SV40 subgroup or to murine polyomavirus.
Peptide library screening and denaturing gel immunoblotting (not shown) against MCV antigens failed to identify a similar pattern of reactivity, indicating that immunodominant antibodies detected with the MCV VLP EIA are mainly reactive to conformational epitopes (Supporting Fig. 3).
Prevalence and age-dependence of MCV infection
Sera having an OD value less than 0.05 OD units, or between 0.05 and 0.2 that did not diminish after competition with soluble MCV VLP, were classified as negative for MCV antibodies (see Methods). Using this standard, all MCV-positive MCC patients were positive for MCV antibodies (100% sensitivity, 95% CI 82–100%) compared with only 3 of 6 (50%, p = 0.007) MCV-negative MCC patients. Exposure to MCV infection was prevalent but significantly less common among asymptomatic blood donors (107 of 166, 64%, p < 0.001), commercial donors (63 of 100, 63%, p = 0.001) and SLE patients (37 of 50, 74%, p = 0.015).
Patients with symptomatic MCC have highly elevated MCV antibody levels compared with MCV seropositive control patients without MCC. Among persons seropositive for MCV antibodies, 9 of 21 (43%) MCV-positive MCC patients had high MCV EIA absorbances (>2.5 OD units) compared with only 16 of 107 (15%, p = 0.006) blood donor, 2 of 63 (3%, p < 0.001) commercial donor and 1 of 37 (3%, p < 0.001) SLE patient sera. These results indicate that while low levels of MCV antibodies, indicative of past MCV infection, are common among adults, very high levels of MCV antibodies are most likely to be found among sera from MCV-positive MCC patients, possibly representing ongoing immune stimulation from infected tumors.
To assess changes in MCV antibodies with age, we examined a convenience cross-sectional cohort sample of 150 sera from LCH patients ranging in age from 1 month to 72 years old (Figure 3). None of 6 sera from children 1 year or younger were positive for MCV antibodies. Prevalence of MCV antibody positivity increased to 43% among children aged 2–5 years old (12 of 28 patients; 95% CI, 26–61%) and 49% of children and young adults aged 6–15 years old (26 of 53 patients; 95% CI, 34–59%). This correlation between MCV antibody prevalence and age continued among older LCH patients reaching 80% (12 of 15 patients; 95% CI 47–90%) among LCH patients older than 50 years (chi-squared test for trend with age, p < 0.001).
FIGURE 3.
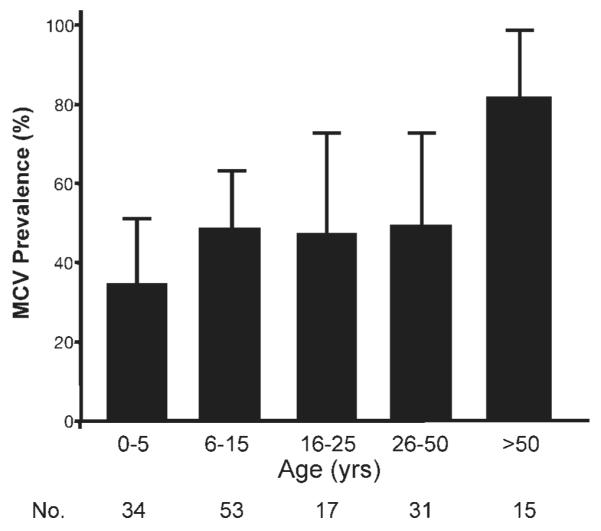
Age-dependent prevalence of MCV antibodies among Langerhans cell histiocytosis (LCH) patients. Sera from a cross-sectional cohort of persons with a history of LCH were tested for IgG antibodies using the MCV EIA. Antibodies were absent from children 1 year and younger but increased in an age-dependent manner, reaching 80% prevalence among adults over age 50. Some children as young as 2 years old were strongly reactive on this assay.
Discussion
Our study is consistent with previous PCR-based tissue surveys. Using a serologic test, we are able to examine comparable tissues (i.e., sera) from cases and controls as well as to assay for past and current MCV exposure. Our results are all consistent with MCV having a causal role in most but not all MCC tumors. Antibodies specific to MCV are found at high levels in sera from most MCC patients and do not cross-react with BK virus or other polyomaviruses. The robust antibody response against MCV VLP among these patients frequently distinguishes them from MCC patients whose tumors are not infected with MCV. The reasons why MCV-positive MCC patients have elevated MCC IgG antibody levels compared with persons who have been asymptomatically-exposed to MCV are not known but may represent either a continued viral antigen production during tumor development or a possible higher virus burden being a risk factor for MCC tumorigenesis. It is unlikely that MCC patients newly acquire MCV infection since MCV IgM is not significantly elevated among these patients. Presence of anti-VLP antibodies among MCC patients suggests that humoral immunity is not protective once a tumor is formed, but it remains possible that neutralizing immunity induced by MCV VLP vaccination might prevent primary infection.
Average MCV antibody levels are highest among MCV-positive MCC patients, but a substantial proportion of asymptomatic adults in each control group that we examined also have elevated MCV antibodies. Several studies have identified MCV genome in tissues from persons without MCC tumor consistent with MCV carriage without symptoms.4,6,7,20,21 Detection of MCV in respiratory aspirates suggests that the virus might be readily transmitted to and among children by a respiratory route.22,23 This is similar to studies for other human polyomaviruses. Stolt et al.24 report near universal prevalence for BKV antibodies among Swedish children under age 10 years and 72% prevalence for JCV antibodies among women age greater than 25 years old. If MCV behaves like other human polyomaviruses, persistent infection after initial exposure may be prolonged or even life-long. Given the rarity of MCC compared with the prevalence of MCV exposure, the large majority of persons who become infected with MCV do not develop MCC and other factors (e.g., sun exposure, immune deficiency) probably determine the risk for cancer development.
By testing sera at 1:500 dilution, we used a stringent protocol that reduces the possibility of cross-reactivity to other circulating human polyomaviruses. Even under these conditions, our test achieved 100% sensitivity in correctly identifying MCV-positive MCC patients. Antibodies reactive to the MCV VLP are not cross-reactive to BKV, a member of the SV40 family, or to the more closely-related murine polyomavirus, indicating that this test is highly specific for MCV. None of the children in our study under age 1 were positive for MCV antibodies and so vertical MCV transmission, if it occurs, is not likely to be common. MCV infection rates, however, are high even among very young children consistent with a casual transmission mechanism. Two other recently-discovered human polyomaviruses belonging to the SV40 subgroup, KI and WU viruses, have been found in respiratory secretions from symptomatic children and adults25,26 suggesting respiratory transmission as one possible route for polyomavirus infection. We do not know the transmission mechanisms for MCV but use of the MCV VLP EIA in longitudinal studies will help to reveal the transmission dynamics for this virus. Additional studies are also needed to determine if this first generation assay is sufficiently sensitive to detect all exposures to MCV among asymptomatically infected adults.
Our data support MCV being the seventh known human cancer virus and that it is directly involved in the pathogenesis of most MCC, a tumor for which prevention options are limited. Given the efficacy of human papillomavirus vaccines based on VLP antigens,27 our assay may be useful for measuring immune responses to MCV VLP-based vaccine candidates. This assay will also be useful for surveys of other neoplastic or nonneoplastic disorders to determine if MCV contributes to diseases beyond MCC. Added in proof: Kean et al. have recently reported a 25–42% MCV prevalence rate among adults using a VP1-GST recombinant antigen assay (Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog 2009;5(3):epub).
Supplementary Material
Acknowledgements
The authors thank Dr. John Kirkwood and Ms. Cindy Sander for providing patient sera from patients enrolled in the University of Pittsburgh Cancer Institute Melanoma Serum, Lymphocyte and Tissue Bank. We thank Ms. Chrissie Usher, Ms. Susan Scudiere, Dr. Masahiro Shuda and Ms. Reety Arora for data management, sample preparation and patient specimen testing and Dr. Susan Manzi and faculty of the University of Pittsburgh Lupus Center of Excellence for providing SLE patient sera. Dr. Raphael P. Viscidi, Johns Hopkins University, kindly provided expert advice on protocols for BKV VLP IgM and IgG EIA testing and we thank Dr. Michael Pawlita for the gift of LPV antibodies and antisera used in preliminary analyses. We thank Gary Peacock for help in preparing the manuscript. YT was supported in part through a fellowship from the University of Pittsburgh Catalyst Program, and YC and PSM are funded as American Cancer Society Professors.
Grant sponsor: NIH; Grant numbers: CA136363, CA120726; Grant sponsors: The Al Copeland Foundation, University of Pittsburgh EXPLORER Award, University of Pittsburgh Clinical and Translational Science Institute Catalyst Training Program.
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- EIA
enzyme-linked immunosorbent assay
- LCH
Langerhans cell histiocytosis
- MCC
Merkel cell carcinoma
- MCV
Merkel cell polyomavirus
- OD
optical density
- PCR
polymerase chain reaction
- SLE
systemic lupus erythematosus
- VLP
virus-like particles
- VP
viral protein.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Dinh V, Feun L, Elgart G, Savaraj N. Merkel cell carcinomas. Hematol Oncol Clin North Am. 2007;21:527–44. doi: 10.1016/j.hoc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet. 2002;359:497–8. doi: 10.1016/S0140-6736(02)07668-7. [DOI] [PubMed] [Google Scholar]
- 3.Feng H, Taylor JL, Benos PV, Newton R, Waddell K, Lucas SB, Chang Y, Moore PS. Human transcriptome subtraction by using short sequence tags to search for tumor viruses in conjunctival carcinoma. J Virol. 2007;81:11332–40. doi: 10.1128/JVI.00875-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foulongne V, Kluger N, Dereure O, Brieu N, Guillot B, Segondy M. Merkel cell polyomavirus and Merkel cell carcinoma. Fr Emerg Infect Dis. 2008;14:1491–3. doi: 10.3201/eid1409.080651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridd K, Yu S, Bastian BC. The presence of polyomavirus in non-melanoma skin cancer in organ transplant recipients is rare. J Invest Dermatol. 2009;129:250–2. doi: 10.1038/jid.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker JC, Houben R, Ugurel S, Trefzer U, Pfohler C, Schrama D. MC polyomavirus is frequently present in Merkel cell carcinoma of European patients. J Invest Dermatol. 2009;129:248–50. doi: 10.1038/jid.2008.198. [DOI] [PubMed] [Google Scholar]
- 8.Kassem A, Schopflin A, Diaz C, Weyers W, Stickeler E, Werner M, Zur Hausen A. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res. 2008;68:5009–13. doi: 10.1158/0008-5472.CAN-08-0949. [DOI] [PubMed] [Google Scholar]
- 9.Buck CB, Lowy DR. Getting stronger: the relationship between a newly identified virus and Merkel cell carcinoma. J Invest Dermatol. 2009;129:9–11. doi: 10.1038/jid.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shuda M, Feng H, Kwun HJ, Rosen ST, Gjoerup O, Moore PS, Chang Y. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci USA. 2008;105:16272–7. doi: 10.1073/pnas.0806526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shuda M, Arora R, Kwun HJ, Feng HC, Sarid R, Fernández-Figueras MT, Tolstov Y, Gjoerup O, Mansukhani M, Swerdlow SH, Chaudhary PM, Kirkwood JM, et al. Human Merkel cell polyomavirus infection. I. MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors. Int J Cancer. 2009 doi: 10.1002/ijc.24510. DOI: 10.1002/ijc.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viscidi RP, Clayman B. Serological cross reactivity between polyomavirus capsids. Adv Exp Med Biol. 2006;577:73–84. doi: 10.1007/0-387-32957-9_5. [DOI] [PubMed] [Google Scholar]
- 13.Poulin DL, DeCaprio JA. Is there a role for SV40 in human cancer? J Clin Oncol. 2006;24:4356–65. doi: 10.1200/JCO.2005.03.7101. [DOI] [PubMed] [Google Scholar]
- 14.Laney AS, Peters JS, Manzi SM, Kingsley LA, Chang Y, Moore PS. Use of a multiantigen detection algorithm for diagnosis of Kaposi’s sarcoma-associated herpesvirus infection. J Clin Microbiol. 2006;44:3734–41. doi: 10.1128/JCM.00191-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, Kjaer S Kruger, Lowy DR, Schiller JT. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–16. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Bauer PH, Bronson RT, Fung SC, Freund R, Stehle T, Harrison SC, Benjamin TL. Genetic and structural analysis of a virulence determinant in polyomavirus VP1. J Virol. 1995;69:7925–31. doi: 10.1128/jvi.69.12.7925-7931.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenz P, Day PM, Pang YY, Frye SA, Jensen PN, Lowy DR, Schiller JT. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol. 2001;166:5346–55. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- 18.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004;78:751–7. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med. 2005;119:445–62. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- 20.Giraud G, Ramqvist T, Ragnarsson-Olding B, Dalianis T. DNA from BK virus and JC virus and from KI, WU and MC polyomaviruses as well as from simian virus 40 is not detected in non-UV-light-associated primary malignant melanomas of mucous membranes. J Clin Microbiol. 2008;46:3595–8. doi: 10.1128/JCM.01635-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garneski KM, DeCaprio JA, Nghiem P. Does a new polyomavirus contribute to Merkel cell carcinoma? Genome Biol. 2008;9:228. doi: 10.1186/gb-2008-9-6-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bialasiewicz S, Lambert SB, Whiley DM, Nissen MD, Sloots TP. Merkel cell polyomavirus DNA in respiratory specimens from children and adults. Emerg Infect Dis. 2009;15:492–4. doi: 10.3201/eid1503.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goh S, Lindau C, Tiveljung-Lindell A, Allander T. Merkel cell polyomavirus in respiratory tract secretions. Emerg Infect Dis. 2009;15:489–91. doi: 10.3201/eid1503.081206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stolt A, Sasnauskas K, Koskela P, Lehtinen M, Dillner J. Seroepidemiology of the human polyomaviruses. J Gen Virol. 2003;84(Pt 6):1499–504. doi: 10.1099/vir.0.18842-0. [DOI] [PubMed] [Google Scholar]
- 25.Allander T, Andreasson K, Gupta S, Bjerkner A, Bogdanovic G, Persson MA, Dalianis T, Ramqvist T, Andersson B. Identification of a third human polyomavirus. J Virol. 2007;81:4130–6. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaynor AM, Nissen MD, Whiley DM, Mackay IM, Lambert SB, Wu G, Brennan DC, Storch GA, Sloots TP, Wang D. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3:e64. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, Chiacchierini LM, Jansen KU. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–51. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.