Abstract
Impairments in language and communication are core features of autism spectrum disorder (ASD). The anatomy of critical language areas has been studied in ASD with inconsistent findings. We used MRI to measure gray matter volume and asymmetry of Heschl's gyrus, planum temporale, pars triangularis, and pars opercularis in 40 children and adolescents with ASD and 40 typically developing individuals, each divided into younger (7-11 years) and older (12-19 years) cohorts. The older group had larger left planum temporale volume and stronger leftward asymmetry than the younger group, regardless of diagnosis. The pars triangularis and opercularis together were larger in ASD than controls. Correlations between frontal language areas with language and symptom severity scores were significant in younger ASD children. Results suggest similar developmental changes in planum temporale anatomy in both groups, but group differences in pars triangularis and opercularis that may be related to language abilities and autism symptom severity.
Keywords: autism, language, MRI, asymmetry, development
Introduction
Cortical Language Regions
Perisylvian regions critical for language functions include posterior regions, which consist of Heschl's gyrus and the posterior superior temporal gyrus, including the planum temporale, and frontal areas, which are comprised of the pars triangularis and pars opercularis. The anatomy of these regions has been the focus of many studies in normal healthy adults. Consistent asymmetry patterns of Heschl's gyrus, however, have not been established in normal subjects. Leftward planum temporale asymmetry has been found repeatedly and is one of the most consistent asymmetries found in the human cortex (for review, Shapleske et al., 1999). Less consistent asymmetry has been found for the pars triangularis and pars opercularis, with leftward asymmetry of these areas being found in some studies (Foundas et al., 1995; Foundas et al., 1996; Foundas et al., 1998; Foundas et al., 2001; Gauger et al., 1997), but not in others (Knaus et al., 2006; Knaus et al., 2007; Tomaiuolo et al., 1999). The pars triangularis and pars opercularis together make up Broca's area and when measured together, again some studies have demonstrated leftward asymmetry (Albanese et al., 1989; Falzi et al., 1982), while others have not (Robichon et al., 2000; Wada et al., 1975).
Although anatomical asymmetries of some of these language areas have been found in infant and fetal brains (Chi et al., 1977; Wada et al., 1975; Witelson & Pallie, 1973), studies have indicated that the anatomy and asymmetry of these critical language regions continue to develop throughout childhood. Wada et al. (1975) found greater degree of planum temporale asymmetry in adult compared to infant brains and suggested that left planum size increases with age. Cross-sectional and longitudinal studies have indicated increases in gray matter density of left perisylvian regions in children and adolescents up to age 30 years (Sowell et al., 2001; Sowell et al., 2003; Sowell et al., 2004). Blanton et al. (2001) demonstrated increases with age in complexity of inferior frontal region, length of the left inferior frontal sulcus, and asymmetry of inferior frontal and temporal regions in children. Similarly, Sowell et al. (2002) found increased asymmetry of the Sylvian fissure in adolescents and adults relative to children. Another study showed that the temporal lobe was the last to mature, with the posterior superior temporal gyrus maturing last within the temporal lobe (Gogtay et al., 2004). There is some evidence that these structural asymmetries may be related to language functions (Foundas et al., 1994; Foundas et al., 1996; Tzourio et al., 1998), providing support for a close relationship between brain and behavioral changes related to language development.
Cortical Language Regions in ASD
Pervasive developmental disorders are characterized by severe and pervasive impairments in reciprocal social interaction, communication and the presences of stereotyped behavior, interests and activities (American Psychiatric Association, 1994). Nowadays, the term autism spectrum disorder (ASD) is used as an umbrella term to cover three of these disorders, which appear to share a common etiology and developmental profile: autism, pervasive developmental disorder-not otherwise specified (PDD-NOS), and Asperger syndrome (Volkmar & Lord, 2007). We use this term ASD throughout the paper to acknowledge the range of participants used in prior studies as well as in our sample. Individuals with ASD demonstrate a wide range of language abilities, with some individuals never developing functional language and others having vocabulary, articulation, and grammar skills in the normal range or above (Kjelgaard & Tager-Flusberg, 2001). By definition, Asperger syndrome is characterized by normal range language skills, however both autism and PDD-NOS include individuals with normal, impaired, or absent language. Across the spectrum, children and adults with ASD have deficits in pragmatic and discourse aspects of language (for review, Tager-Flusberg et al., 2005).
Several studies have investigated the neurobiological substrates of language in ASD. Posterior language regions have been studied in ASD, but results have been inconsistent. Rojas et al. (2002) used manual tracings to measure gray matter volume and asymmetry. They found no differences in Heschl's gyrus and decreased left planum temporale volume in adults with ASD compared to normal controls, resulting in stronger leftward planum asymmetry in controls. They later replicated this finding in a sample of children (Rojas et al., 2005). McAlonan et al. (2005), using voxel-based morphometry, had similar results, with decreased gray matter volume of left superior temporal gyrus in children with ASD compared to normal controls. However, other studies using semi-automated methods and focusing on asymmetry, have found the opposite, with stronger leftward planum temporale asymmetry in boys with ASD than in typically developing boys (de Fossé et al., 2004; Herbert et al., 2002; Herbert et al., 2005). de Fossé et al. (2004) compared ASD children with and without language impairment to non-ASD children with and without language impairment. They found leftward planum asymmetry in both language impaired groups, but not in the language normal groups. Still other results have been reported; Boddaert et al. (2004) found decreased gray matter bilaterally in the ASD group compared to controls in the superior temporal gyrus, with voxel-based morphometry methods, while Bigler et al. (2003) utilizing semi-automated volume measurements found no group differences in superior temporal gyrus volume or asymmetry.
Anterior language areas have also been measured in individuals with ASD, again with inconsistent findings across studies. One voxel-based morphometry study found decreased left gray matter volume of the pars triangularis in adults with ASD compared to normal controls (Abell et al., 1999). Utilizing semi-automated measurements, Herbert et al. (2005), reported similar results, with decreased leftward pars triangularis asymmetry and increased rightward pars opercularis asymmetry in boys with ASD compared to typically developing boys. Other studies examining asymmetry, however, have found rightward asymmetry of the pars triangularis in boys on the autism spectrum and leftward pars triangularis asymmetry in controls (de Fossé et al., 2004; Herbert et al., 2002). de Fossé et al. (2004) found that boys with specific language impairment and boys with ASD with language impairment had rightward pars triangularis and opercularis asymmetry, while typically developing boys and boys with ASD without language impairment had leftward pars triangularis and opercularis asymmetry. In contrast, McAlonan et al. (2005) found smaller right volume of the pars triangularis in ASD children compared to normal controls, using voxel-based morphometry. For review of these studies, see Table 1.
Table 1.
Review of previous studies of anatomical language regions in ASD. TD = typically developing, LI = language impaired, LN = language normal, SLI = specific language impaired, DLD = developmental language delayed, RH = right-handed, L = left, R = right, PTR = pars triangularis, HG = Heschl's gyrus, PT = planum temporale, POP = pars opercularis
| Study | Participants | Method | Measurement | Findings |
|---|---|---|---|---|
| Abell et al., 1999 |
|
Voxel-Based Morphometry | Volume |
|
| Herbert et al., 2002 |
|
Semi-Automated | Asymmetry |
|
| Rojas et al., 2002 |
|
Manual | HG and PT Volume and Asymmetry |
|
| Bigler et al., 2003 |
|
Semi-Automated | Volume and Asymmetry |
|
| Boddaert et al., 2004 |
|
Voxel-Based Morphometry | Volume |
|
| De Fosse et al., 2004 |
|
Semi-Automated | Volume and Asymmetry |
|
| Herbert et al., 2005 |
|
Semi-Automated | Asymmetry |
|
| McAlonan et al., 2005 |
|
Voxel-Based Morphometry | Volume |
|
| Rojas et al., 2005 |
|
Manual | HG and PT Volume and Asymmetry |
|
Brain Development in ASD
Developmental changes in cortical language areas in ASD have not been extensively studied, but several studies examining total brain size or lobar size have demonstrated a different developmental trajectory of brain growth in ASD relative to typically developing individuals (for reviews see, Courchesne, 2004; Courchesne & Pierce, 2005; McCaffery & Deutsh, 2005; Redcay & Courchesne, 2005). Some cross-sectional studies have found larger brain size in young children with ASD (2-4 year olds) relative to typically developing children, with no differences in older children (Carper et al., 2002; Courchesne et al., 2001). This has led to the hypothesis that in ASD, rapid brain growth occurs during the first 1-2 years of life, which is then followed by slow or discontinued growth. Providing further support for this idea are studies examining brain volume in toddlers, which have demonstrated larger volume in ASD compared to typically developing toddlers (Hazlett et al., 2005; Sparks et al., 2002) and studies of adolescents and adults which have not found differences in brain size (Herbert et al., 2003; Rojas et al., 2002). Aylward et al. (2002), however, found enlarged brain size in ASD children relative to typically developing children, 8-12 years old, with no differences in individuals over 12 years old. In addition, some studies of adolescents and adults have shown larger brain volume in ASD compared to normal controls (Hardan et al., 2001; Piven et al., 1995; Piven et al., 1996). These studies have demonstrated different developmental trajectories in brain growth in individuals with ASD, however the exact developmental trajectory in ASD remains unresolved. One cross-sectional study has investigated developmental patterns in superior temporal gyrus, however they did not find any differences between ASD and controls, regardless of age (Bigler et al., 2003).
Current Study
The purpose of this study was to examine age-related changes in gray matter volume and asymmetry of critical language areas, including Heschl's gyrus, the planum temporale, the pars triangularis, and the pars opercularis, in a large sample of children and adolescents with ASD and typically developing children and adolescents. In typically developing individuals, studies have demonstrated developmental changes in gray matter and asymmetry of some of these language regions and studies of ASD have indicated differences in the developmental trajectory of brain growth. No previous studies, however, have examined developmental changes in the anatomy of these language areas in individuals with ASD. Manual measurements were used because of their increased sensitivity to subtle group differences. There is great individual variability in the cortical anatomy of language regions and manual measurements are better able to take into account these individual differences, by maintaining scans in native space, instead of warping them into a standard space, as is usually necessary for automated or semi-automated methods. Based on previous studies of developmental changes in normal controls (Blanton et al., 2001; Sowell et al., 2001; Sowell et al., 2002; Sowell et al., 2004), we predicted that in the typically developing group, older children would have larger gray matter volume of left perisylvian language regions and increased leftward asymmetry relative to younger children. Anatomical differences of cortical language areas in individuals with ASD compared to normal controls have been demonstrated, but there are not prior studies that have examined age-related changes in volume or asymmetry of these regions in ASD. However, since ASD is a developmental disorder with studies of brain growth indicating a different developmental pattern, we expected that the ASD group would not show the same differences in volume and asymmetry between older and younger children as the typically developing individuals.
Materials and Methods
Participants
Participants were 40 children and adolescents with ASD (autism or PDD-NOS), ages 7-19 years (mean = 12.22, SD = 3.30) and 40 typically developing children and adolescents, ages 7-19 years (mean = 12.24, SD = 3.25). Participants in each group were divided into a younger group of 20, ages 7-11 years and an older group (n = 20), ages 12-19 years. Twelve years was used as the division between the older and younger groups as this roughly corresponds to the division between pre-puberty and puberty and is similar to cutoffs to classify children and adolescents used in previous studies (Blanton et al., 2001; Sowell et al., 2002; Aylward et al., 2002). All participants spoke English as their first language. There were 4 females in the ASD group (1 younger and 3 older) and 4 in the control group (2 younger and 2 older). Based on writing hand, there were 8 left-handers with ASD (4 younger and 4 older) and 3 left-handed controls (2 younger and 1 older). A modified version of the Dean handedness inventory (Piro, 1998) was also administered to all but 4 participants. The modified Dean handedness consists of 12 unimanual tasks and scores range from −24, indicating complete left-handedness, to +24, indicating complete right-handedness. Table 2 presents the descriptive statistics for all participants. An analysis of variance (ANOVA) with the factors diagnosis (ASD, typically developing) and age (young, old) revealed no main or interaction effects on handedness. However, since there are some indications that language laterality may be different in left- and right-handers, with structural studies of these language regions indicating differences between handedness groups (Foundas et al., 1995; Habib, 1989; Jäncke et al., 1994; Steinmetz et al., 1991), we also ran all of our analyses with the left-handers removed.
Table 2.
Means and standard deviations of demographic information for each group.
| ASD | Controls | |||
|---|---|---|---|---|
| Younger | Older | Younger | Older | |
| Age | 9.50 (1.32) | 14.95 (2.23) | 9.54 (1.38) | 14.93 (2.13) |
| Handedness | 10.58 (15.08) | 11.22 (13.75) | 15.15 (9.01) | 17.42 (9.08) |
| K-BIT VIQ | 100.45 (20.35) | 102.74 (21.83) | 117.50 (13.03) | 119.53 (14.53) |
| K-BIT NVIQ | 109.25 (13.88) | 105.05 (13.33) | 113.90 (8.81) | 109.95 (11.06) |
| K-BIT FSIQ | 105.85 (17.18) | 104.79 (17.27) | 118.40 (9.93) | 117.21 (12.12) |
| CELF Receptive | 100.16 (16.55) | 96.80 (21.12) | 118.11 (13.83) | 113.15 (10.66) |
| CELF Expressive | 97.42 (16.19) | 90.35 (19.35) | 115.16 (12.65) | 107.60 (11.21) |
| CELF Total | 98.79 (16.69) | 92.95 (21.17) | 118.37 (11.42) | 111.45 (10.05) |
| ADOS Communication | 3.55 (1.79) | 3.10 (1.68) | - | - |
| ADOS Social | 9.80 (2.33) | 8.65 (3.08) | - | - |
| ADOS Total | 13.35 (3.92) | 11.75 (4.39) | - | - |
The Kaufman Brief Intelligence Test (KBIT-II; Kaufman & Kaufman, 2004) was administered to assess IQ, except for two participants (1 older ASD, 1 older control) who were administered the Differential Ability Scales (DAS; Elliot, 1990) and the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999), respectively. The Clinical Evaluation of Language Fundamentals (CELF, 3rd ed; Semel et al., 1995) was administered to all but two participants (1 younger ASD, 1 younger control) to assess language abilities. When left-handers were removed there was only 1 younger control who did not have a CELF. Four subtests were administered, which varied based on the age of the subject. For participants 7- 8 years old, Concepts and Directions, Sentence Structure (receptive language), Recalling Sentences, and Word Structure (expressive language) subtests were given. For participants 9 years and older, Concepts and Directions, Word Classes (receptive language), Formulated Sentences, and Recalling Sentences (expressive language) subtests were administered. Together, the receptive and expressive subtest scores yielded a total language score.
For ASD participants, diagnoses were based on DSM-IV criteria (American Psychiatric Association, 1994) using the Autism Diagnostic Interview-Revised (ADI-R; Rutter et al., 2003) and the Autism Diagnostic Observation Schedule, Module 3 or 4 (ADOS; Lord et al., 1999). In addition, an expert clinician (RMJ) confirmed that all individuals met criteria for ASD. Based on ADI-R scores, the majority of participants met criteria for a diagnosis of autism. Among the younger group, 18 met criteria for autism and 2 for PDD-NOS (one child missed by 1 point on the communication algorithm score and one missed by 1 point on the repetitive behavior algorithm score). Among the older group 17 met criteria for autism and 3 for PDD-NOS (each missed by one point on the repetitive behavior algorithm score). Individuals with frank neurological damage, with a known genetic disorder, who were born prematurely (less than 35 weeks), or who had had seizures within the last three years were excluded from the study. Typically developing individuals also had no history or current diagnosis of developmental, learning, psychiatric, or neurologic disorders.
Participants 18 years and older were informed of the procedures and gave written consent prior to participation in the study. For participants under 18 years old, parents and participants were informed of the procedures and parents gave written consent prior to the child's participation in the study. Children 12 years and older also provided written assent prior to participation. All data in this manuscript were collected in compliance with the Boston University School of Medicine Institutional Review Board.
MRI Acquisition
All participants were trained in a mock scanner prior to the actual MR scanning. Volumetric MR images were acquired on a Philips 3 Tesla Intera scanner. T1-weighted images were obtained as a series of 160, 1 mm gapless sagittal images. TFE was used, with technical factors of: TR = 9.9 seconds, TE = 4.6, 256×256 pixel matrix, 24 cm field of view, and 8 degree flip angle. Data sets were rotated into alignment in the sagittal, axial, and coronal planes in order to eliminate any head rotation and MRI scans were maintained in real space. Each MRI scan series was assigned a blind number to assure subject confidentiality and to ensure that all volume measurements were performed blind to group and subject.
MRI Measurements
Total Hemisphere Volume
Total hemisphere volume was measured with the MEASURE program (Barta et al., 1997). The BET tool in FSL (Smith, 2002) was first used to remove as much skull as possible, without removing any brain. The contrast was then set and the MEASURE program automatically outlined the brain. The midline slice was identified and all outlining on this image was removed. This slice was not included in the measure of left or right hemisphere volume. The outlining was then manually edited in each slice to remove any remaining non-brain regions from the outline. This measurement included gray and white matter and the thalamus, but excluded the cerebellum and brainstem. For inter-rater reliability, a subset of 5 brains (10 hemispheres) was measured by 4 investigators. An intra-class correlation (ICC) was calculated between the investigators' volume measurements across both hemispheres of the 5 brains. The ICC for total brain volume was .97.
Regional Volumes
For each region of interest, the MEASURE program (Barta et al., 1997) was used to trace the gray matter, using a mouse-driven cursor, while viewing sagittal, coronal, and axial planes simultaneously. Boundaries were marked and saved in all three planes, ensuring the accurate identification of the boundaries of each region, and enabling the investigator to measure the full gray matter volume using a three-dimensional approach. A region was highlighted first in one of the three planes and then viewed and corrected in the other planes. The MEASURE program calculated the volume of each region in cm3, based on the slice thickness and the area highlighted on each image.
Inter-rater reliability has previously been established for this methodology for measuring each of these regions. For this, ICCs were calculated between the first author's, who completed all the measurements for this study, and a second rater's measurements of each region, across both hemispheres of 5 brains (Knaus et al., 2006). Intra-rater reliability was re-established. For this, a subset of 5 brains (10 hemispheres) was re-measured and ICCs were calculated between the first measurements and the second measurements of each region, across both hemispheres of the 5 brains. ICC data are provided for each region in the following sections.
Heschl's Gyrus
Heschl's is located within the Sylvian fossa, just anterior to the planum temporale, and has a characteristic configuration in the sagittal plane, which appears most commonly as a “mushroom” shape with variation including a “heart-shape” or a double gyrus. The sagittal and coronal planes were primarily used for measuring this region. The coronal plane was used to define the anterior and posterior boundaries. The anterior boundary was defined as the most anterior image in which Heschl's gyrus was clearly visible, with a small amount of white matter lateral to the gyrus. In the coronal slice immediately anterior to this image, the ledge of white matter lateral to Heschl's was not present because Heschl's gyrus had merged with it. The posterior boundary was defined by Heschl's sulcus, as the most posterior coronal slice, which had a protrusion of gray matter, at the junction of Heschl's gyrus with the insula. Heschl's is often bifurcated, at least partially, by an intermediate sulcus. If Heschl's fully split into two separate gyri in the sagittal plane, only the most anterior gyrus (first Heschl's gyrus) was included in the measurement. When Heschl's completely split in the coronal plane, only the most medial gyrus, which corresponds to the most anterior (first) Heschl's gyrus, was included in the measure. However, when Heschl's was only partially bifurcated, in the sagittal or coronal plane, creating a heart-shaped gyrus, the entire gyrus was included in the Heschl's gyrus measurement. The gray matter of Heschl's gyrus was measured in the coronal plane and then checked and corrected in the sagittal plane. For inter-rater reliability, the ICC was .84 for intra-rater, the ICC was .97.
Planum Temporale
The planum temporale is located within the Sylvian fossa, posterior to Heschl's gyrus and lies on the superior surface of the posterior superior temporal gyrus. The anterior boundary was defined as Heschl's sulcus, and when present the second Heschl's gyrus was included in the planum measure. In the coronal plane, this image was the same one used to define the anterior boundary for Heschl's gyrus, where Heschl's was fully visible and there was a small amount of white matter lateral to it. If Heschl's was completely bifurcated by an intermediate sulcus, the posterior portion of the gyrus was included in the planum measure. The posterior boundary was defined in the sagittal plane, as the point where the horizontal ramus of the Sylvian fissure turns upward into the posterior ascending ramus, so neither the posterior ascending ramus nor posterior descending ramus was included in the planum temporale measurement. In cases where the Sylvian fissure gently sloped upward, the knife-cut method (Witelson & Kigar, 1992) was utilized. The horizontal ramus of the Sylvian fissure was followed to the point where it intersected the vertical wall. When this point changed from medial to lateral, more lateral images were used for determining this boundary. If there was no posterior ascending ramus, however, the end of the Sylvian fissure was used as the posterior boundary. This boundary was defined in the coronal plane as the most posterior slice where the Sylvian fissure was clearly visible, before it became intermixed with white matter. However, if the Sylvian fissure extended into parietal regions, the posterior boundary was defined as the image just anterior to the one where the intraparietal sulcus appeared to encircle the Sylvian fissure. This boundary appeared as small amounts of gray matter visible through the white matter around the Sylvian fissure. The planum temporale was measured in the coronal plane, with Heschl's sulcus, when present, as the medial boundary. Laterally, the boundary for the planum was the edge of the Sylvian fissure, not including the lateral wall of the posterior superior temporal gyrus. The planum temporale measurement was then checked for accuracy in the sagittal plane. For inter-rater reliability, the ICC for this structure was .91 and for intra-rater reliability, it was .95.
Pars Triangularis
The pars triangularis is located in the anterior inferior frontal gyrus and is a triangular shaped region, which extends from the Sylvian fissure. The sagittal plane was predominantly used to measure this region because it provides the clearest view of the pars triangularis. Defined in the sagittal plane, the anterior boundary was the anterior horizontal ramus and the posterior boundary was the anterior ascending ramus. Thus, the posterior/superior bank of the anterior horizontal ramus and the anterior/superior bank of the anterior ascending ramus were included in the pars triangularis measurement. When extra sulci, internal notches, occurred between these two rami, the banks of these sulci were included in the measurement. The lateral boundary was defined as the most lateral sagittal image, prior to the rami being cut off, so that surface gray matter was not included. At times, only one of the sulci, either the anterior horizontal ramus or the anterior ascending ramus, was cut off, while the full extent of the other sulcus remained, in which case the bank of the remaining sulcus was still measured on images as far lateral as possible, until the sulcus was cut off. The medial boundary was defined as the most medial sagittal image in which the insula was clearly defined, prior to the white matter intruding. At this boundary, the insula appeared with clearly defined strips of white matter. The superior boundary was the inferior frontal sulcus. The inter-rater reliability ICC for this structure was .83. For intra-rater reliability, the ICC was .99.
Pars Opercularis
The pars opercularis is also located in the inferior frontal gyrus, posterior to the pars triangularis. It is generally a U-shaped area, which extends from the Sylvian fissure. The sagittal plane provides the best view of the pars opercularis, so this plane of section was primarily used to measure this region. In the sagittal plane, the anterior boundary of the pars opercularis was defined as the anterior ascending ramus and the posterior boundary was defined as the precentral sulcus. Thus, the posterior/inferior bank of the anterior ascending ramus and the anterior bank of the precentral sulcus, up to the inferior frontal sulcus, were included in the measurement. The inferior frontal sulcus, also defined in the sagittal plane, was used as the superior boundary. While on some images the precentral sulcus connects with the Sylvian fissure, it was not continuous with the Sylvian fissure throughout the full extent of the pars opercularis. In addition, in some brains, the precentral sulcus never fully connected with the Sylvian fissure. When the precentral sulcus did not connect with the Sylvian fissure, the pars opercularis was measured to the point where the Sylvian fissure would have connected with the precentral sulcus and the anterior bank of the precentral sulcus was measured from the inferior frontal sulcus, down until it disappeared. The lateral boundary was defined on sagittal images as the most lateral image prior to the rami being cut off, so that surface gray matter was not included. At times, only one of the sulci, the anterior ascending ramus or the precentral sulcus was cut off, while the other one remained, in which case the bank of the remaining sulcus was still measured on images as far lateral as possible, until the sulcus was cut off. The medial boundary was defined as one sagittal image lateral to the medial boundary of the pars triangularis. The diagonal sulcus was not included in the pars opercularis measurement, however, other extra sulci that appeared between the anterior ascending ramus and the precentral sulcus, were included. The ICC for inter-rater reliability for this structure was .89 and for intra-rater reliability was .97.
Analyses
Behavioral Measures
A multivariate analysis of variance (MANOVA) was used to examine differences in IQ between the ASD and control groups and between the younger and older groups. Diagnosis and age group were the independent variables and K-BIT verbal and non-verbal standard scores were the dependent variables. Similarly, differences in language scores were examined via MANOVA, with diagnosis and age group as independent variables and CELF receptive and expressive standard scores as dependent variables.
Volume
To control for differences in total brain size, each region was calculated as a proportion of total hemisphere volume for all analyses. To examine differences in volume between the ASD and control groups and between the younger and older groups, MANOVA was used with hemisphere as the within-subject independent variable, diagnosis and age group as the between-subjects independent variables, and Heschl's gyrus, planum temporale, pars triangularis, and pars opercularis gray matter volumes as the dependent variables. Since the pars triangularis and pars opercularis together comprise Broca's area and some studies have examined these regions together, the pars triangularis and pars opercularis measures were also combined, creating a total frontal language region. To examine differences in this total frontal area, an ANOVA was performed with hemisphere as the within-subject independent variable, diagnosis and age group as the between-subjects independent variables, and pars triangularis + pars opercularis gray matter volume as the dependent variable.
Differences in Heschl's gyrus configurations were also examined. Chi-squares were performed for left and right Heschl's to examine the frequency of a partial split, full split, or no split in each group. ANOVAs were also performed to see if there were differences in size related to the different configurations. Left or right Heschl's gyrus volume was the dependent variable and left or right Heschl's gyrus configuration (partial, full, or no split) was the independent variable.
Asymmetry Quotients
For each region, asymmetry quotients were calculated as (L-R)/[(L+R)/2], so that a positive asymmetry quotient indicated leftward asymmetry and a negative quotient indicated rightward asymmetry. To examine group and age group differences in asymmetry, MANOVA was utilized, with diagnosis and age group as the independent variables and asymmetry quotients of Heschl's, planum, pars triangularis and pars opercularis as the dependent variables. An ANOVA was also performed, with diagnosis and age group as the independent variables and pars triangularis + pars opercularis asymmetry quotient as the dependent variable.
Correlations
Pearson correlations were computed to examine the relationship between behavioral and anatomical measures. For all correlations, regions proportional to total hemisphere volume were used. Correlations between left and right volume of each region with age were calculated for the ASD and control groups. Correlations were also computed for each group, between left and right volumes of each region with CELF total standard score and with autism symptom severity in the ASD groups. Symptom severity was measured using the communication and social algorithm scores from the ADOS Module 3 or 4. Higher ADOS scores reflect increased symptom severity.
Results
Behavioral Measures
The group data on IQ and language scores are presented in Table 2. For IQ, at the multivariate level, there was a significant main effect of diagnosis (F2,73 = 8.66, p < .001). The univariate level indicated significantly higher verbal IQ (F1,74 = 17.56, p < .001) scores in controls than in the ASD group, but no significant group differences in non-verbal IQ scores. There was no significant difference between age groups or interaction between diagnosis and age group. For the CELF, there was a significant main effect of diagnosis (F2,73 = 14.76, p < .001), with no significant differences between age groups or interaction between diagnosis and age group. For receptive (F1,74 = 22.31, p < .001) and expressive CELF (F1,74 = 25.79, p < .001) the control group had significantly higher scores than the ASD group. Results were the same when left-handers were removed.
Brain Volume
The means and standard deviations of the volumes for total and regional brain volumes are presented in Table 3. At the multivariate level, there was a significant hemisphere effect (F4,73 = 14.84, p < .001, partial η2 = .449). This was significant for Heschl's gyrus (F1,76 = 31.71, p < .001, partial η2 = .294) and planum temporale (F1,76 = 27.74, p < .001, partial η2 = .267), indicating significantly larger volume in the left hemisphere than in the right. There was also a significant hemisphere by age group interaction (F4,73 = 2.51, p = .049, partial η2 = .121). However, this was significant only for the planum temporale (F1,76 = 4.66, p = .034, partial η2 = .058) and indicated that leftward asymmetry of the planum temporale was greater in the older group than in the younger group, across both diagnostic groups. At the multivariate level, there was no significant effect of diagnosis. When left-handers were removed the hemisphere effect was also significant for the pars opercularis, indicating significantly larger right volume than left. All other results were the same with left-handers removed.
Table 3.
Means and standard deviations of total hemisphere volume and gray matter volume of each region, proportional to total hemisphere volume for each group. HG = Heschl's gyrus, PT = planum temporale, PTR = pars triangularis, POP = pars opercularis
| ROI | Hemi | ASD | Controls | ||
|---|---|---|---|---|---|
| Younger (n = 20) | Older (n = 20) | Younger (n = 20) | Older (n = 20) | ||
| Total | Left | 670.94 (72.10) | 682.93 (79.66) | 670.70 (43.96) | 691.87 (54.91) |
| Right | 669.13 (65.61) | 677.41 (75.26) | 663.48 (45.68) | 689.76 (60.97) | |
| HG | Left | .0025 (.00056) | .0024 (.00072) | .0025 (.00053) | .0024 (.00059) |
| Right | .0019 (.00053) | .0021 (.00049) | 0021 (.00065) | .0021 (.00048) | |
| PT | Left | .0036 (.00130) | .0034 (.00091) | .0033 (.00091) | .0038 (.00112) |
| Right | .0030 (.00084) | .0024 (.00096) | .0030 (.00103) | .0027 (.00089) | |
| PTR | Left | .0017 (.00068) | .0015 (.00056) | .0013 (.00054) | .0013 (.00068) |
| Right | .0016 (.00066) | .0015 (.00073) | .0013 (.00062) | .0014 (.00063) | |
| POP | Left | .0028 (.00068) | .0027 (.00079) | .0024 (.00073) | .0026 (.00060) |
| Right | .0029 (.00087) | .0028 (.00081) | .0027 (.00075) | .0026 (.00063) | |
| PTR+POP | Left | .0045 (.00124) | .0042 (.00108) | .0037 (.00113) | .0040 (.00117) |
| Right | .0045 (.00127) | .0043 (.00129) | .0040 (.00115) | .0041 (.00097) | |
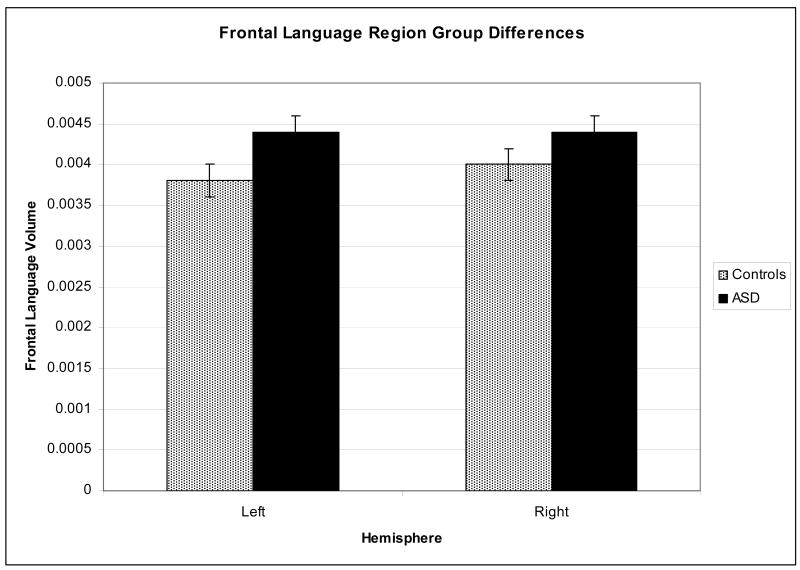
For the ANOVA examining total frontal language volume, there was a significant main effect of diagnosis (F1,76 = 4.51, p = .037, partial η2 = .056), indicating that the ASD group had larger pars triangularis + pars opercularis volume than the control group (Figure 1). There were no significant hemisphere or age effects or interactions. This result was the same without left-handers in the sample.
Figure 1.
Differences between ASD and controls in pars triangularis+pars opercularis volume.
The chi-squares examining differences in frequencies of Heschl's gyrus configurations were not significant. In all 4 groups, for both right and left Heschl's, most individuals had no split. The next most common configuration in all groups in both hemispheres was a fully split Heschl's, with very few individuals having a partially split gyrus. The ANOVAs examining configuration differences in size were also not significant for either the left or the right Heschl's gyrus. Results were the same when left-handers were removed.
Asymmetry Quotients
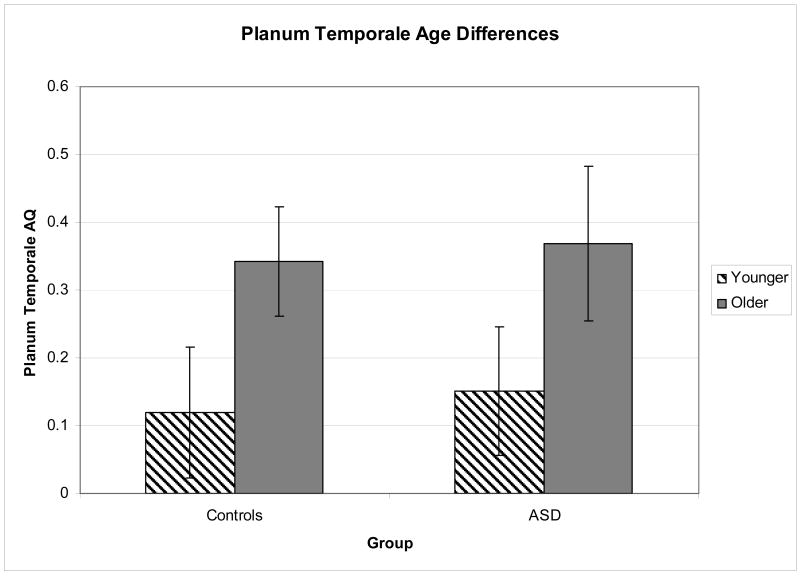
Table 4 presents the data on asymmetry quotients for each group. At the multivariate level, there was a significant main effect of age group (F4,73 = 2.88, p = .029, partial η2 = .136). At the univariate level, this was significant for the planum temporale (F1,76 = 5.13, p = .026, partial η2 = .063), indicating that the older group had significantly larger planum asymmetry quotient (more leftward) than the younger group (Figure 2). There was no significant effect of diagnosis.
Table 4.
Means and standard deviations of asymmetry quotients for each region for each group. A positive asymmetry quotient indicates leftward asymmetry and a negative asymmetry quotient indicates rightward asymmetry. AQ = asymmetry quotient
| ROI AQ | ASD | Controls | ||
|---|---|---|---|---|
| Younger (n = 20) | Older (n = 20) | Younger (n = 20) | Older (n = 20) | |
| HG | .267 (.25) | .156 (.24) | .231 (.35) | .116 (.28) |
| PT | .151 (.42) | .368 (.51) | .119 (.43) | .342 (.36) |
| PTR | .088 (.36) | .023 (.51) | .050 (.64) | -.064 (.59) |
| POP | -.011 (.40) | -.016 (.33) | -.120 (.29) | -.009 (.26) |
| PTR+POP | .016 (.35) | -.006 (.33) | -.068 (.35) | -.036 (.29) |
Figure 2.
Age differences in planum temporale asymmetry quotients for both the control and ASD groups. AQ = asymmetry quotient
For the total frontal language region, there were no significant age differences for pars triangularis + pars opercularis asymmetry quotient and no differences in asymmetry quotients were found comparing ASD and controls. All asymmetry quotient results were the same when left-handers were removed.
Correlations
There was a significant positive correlation between age and left planum temporale size in the control group (r = .364, p = .021). Other significant brain-behavior correlations were found only in the younger ASD group. The right pars triangularis volume was negatively correlated with the CELF total language score (r = -.516, p = .024), and left pars triangularis volume was positively correlated with ADOS communication scores (r = .595, p = .006) and ADOS social scores (r = .552, p = .012). The left pars triangularis + pars opercularis volume was also correlated with ADOS communication (r = .497, p = .026) and ADOS social (r = .467, p = .038) scores. When left-handers were removed from the sample, there were a few additional correlations. In the ASD group, age was negatively correlated with the right planum temporale volume (r = -.369. p = .038). In addition, in the younger ASD group, the right pars triangularis was correlated with ADOS communication (r = .526, p = .036) and social (r = .497, p = .050) scores. There was no significant correlation between left pars triangularis + pars opercularis and ADOS social scores.
Discussion
There were several interesting findings in this study. In controls, there was significantly stronger leftward planum temporale asymmetry in the older group than the younger group. This was also found in the ASD group. There was also a significant correlation between age and left planum temporale size, found only in controls. We also found larger frontal language region volume in the ASD group compared to controls and this was found across the age groups. There were significant correlations in the younger ASD group between frontal language regions and measures of language and autism severity.
The finding of increased leftward asymmetry of the planum temporale in controls partly supported our hypothesis of increased left volume and leftward asymmetry of language areas in older compared to younger individuals. This finding is consistent with previous studies of typically developing individuals, demonstrating increased asymmetry of temporal language regions in adolescents compared to adults (Sowell et al., 2001) and differences in asymmetry of the Sylvian fissure in adolescents and adults compared to children (Sowell et al., 2002). These developmental changes in anatomy may also be related to age-related changes in language functions. It was surprising that we did not find a significant interaction between diagnosis and age group, rather increases in leftward asymmetry with age were equivalent between the typically developing and ASD groups. This did not support our hypothesis, but is consistent with one previous study that found no developmental differences in the superior temporal gyrus between ASD and controls. One interesting difference, however, was in the correlations with age. In the control group, the left planar volume increased with age. In ASD, we did not find this correlation and when left-handers were removed, the right planum temporale decreased with age in this group. So, although both groups had increased leftward asymmetry, these correlations suggest that there may be different mechanisms. More studies, however, need to examine developmental changes in these critical language areas.
An unexpected finding in our study was increased frontal language region volume in the ASD group compared to controls. Broca's area has been shown to be involved in the organization of articulatory sequences and inner speech (Stuss & Benson, 1986, cited in Démonet et al., 1992), in subvocal rehearsal (Paulesu et al., 1993), and in the initiation of speech (Alexander et al., 1990). The pars triangularis and pars opercularis are also involved in semantic encoding and retrieval and in on-line manipulation of semantic representations (Blumenfeld et al., 2006). Functional neuroimaging studies have demonstrated that this region may also be important for phonological (Démonet et al., 1992; Zatorre et al., 1996) and syntactic processing (Dapretto & Bookheimer, 1999; Embick et al., 2000). Both anatomical (Abell et al., 1999; de Fossé et al., 2004; Herbert et al., 2002; Herbert et al., 2005; McAlonan et al., 2005) and functional studies utilizing language tasks (Gaffrey et al., 2007; Harris et al., 2006; Just et al., 2004; Kana et al., 2006) have indicated differences in these frontal areas in individuals on the autism spectrum, which may be related to differences in language development. Findings, however, of anatomical studies have been inconsistent and increased pars triangularis and opercularis volume in ASD has not previously been reported. This, however, is the only study that has performed manual measurements of these critical frontal language regions in ASD, which may better take into account differences in individual anatomy than more automated methods. The sample size was also larger than many previous anatomical studies of ASD. More studies need to be conducted that focus on both anatomical and functional differences of these frontal areas.
Differences in pars triangularis and pars opercularis volume could be due to differences in language abilities between the groups, as controls had significantly higher CELF scores than the ASD group, however, significant correlations of these regions with behavioral measures were only found in the younger ASD group. Correlations in the control groups were probably not found due to the smaller range of CELF scores found in those groups. In the younger ASD group, larger right pars triangularis volume was associated with lower language scores on the CELF. This finding may be related to suggestions that a lack of pruning of tissue, especially in the right hemisphere, in individuals with neurodevelopmental language disorders may contribute to problems with language (Beaton, 1997; Morgan & Hynd, 1998). It is unclear, however, why this correlation would not be found in the older group of ASD individuals. In the younger group, larger left pars triangularis and left frontal language volumes were associated with more severe communication and social autism symptoms, indicating that these regions may be involved in language and social functions.
In summary, there were age-related changes in the planum temporale in both typically developing individuals and individuals on the autism spectrum, with both groups having increased leftward asymmetry in older than younger individuals. Group differences were found in frontal language regions, with the ASD group having larger volume than controls, with no age effects. There are several limitations to this study. One limitation is that this was a cross-sectional study, rather than a longitudinal study, which would be able to directly assess developmental changes in behavior and brain anatomy. Another limitation is that the ASD individuals in this study had a wide range of functioning levels and language abilities. Larger sample sizes would be necessary, so that these groups could be further broken down based on functioning level or language abilities or so that variability related to these could be examined within the group. Another limitation is that because the study involved manual tracings, only specific regions, with well-defined anatomical boundaries were examined and other potentially important language regions were not examined in this study. Future studies should use longitudinal designs to further explore the developmental trajectory of cortical language regions in ASD.
Acknowledgments
This study was supported by a program project grant from the National Institute on Deafness and Other Communication Disorders (U19 DC 03610), which is part of the NICHD/NIDCD funded Collaborative Programs on Excellence in Autism, as well as funding for the GCRC at Boston University School of Medicine (M01-RR0533). This study was also supported by NINDS F30 NS055511. We thank Lin Themelis for help with screening and scheduling participants and Danielle Delosh for help with measurements of total hemisphere volume. We also extend our sincere gratitude to the children and families who participated in this study.
References
- Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, Happé F, Frith C, Frith U. The neuroanatomy of autism: A voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–1651. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- Albanese E, Merlo A, Albanese A, Gomez E. Anterior speech region. Asymmetry and weight-surface correlation. Neurology. 1989;40:353–362. doi: 10.1001/archneur.1989.00520390073019. [DOI] [PubMed] [Google Scholar]
- Alexander MP, Naeser MA, Palumbo C. Broca's area aphasias: Aphasia after lesions including the frontal operculum. Neurology. 1990;40:353–362. doi: 10.1212/wnl.40.2.353. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association Press; 1994. [Google Scholar]
- Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59:175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Barta PE, Dhingra L, Royall R, Schwartz E. Improving stereological estimates for the volume of structures identified in three-dimensional arrays of spatial data. Journal of Neuroscience Methods. 1997;75:111–118. doi: 10.1016/s0165-0270(97)00049-6. [DOI] [PubMed] [Google Scholar]
- Beaton AA. The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender, and dyslexia: A review of the evidence. Brain and Language. 1997;60:255–322. doi: 10.1006/brln.1997.1825. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Tate DF, Neeley ES, Wolfson LJ, Miller MJ, Rice SA, Cleavinger H, Anderson C, Coon H, Ozonoff S, Johnson M, Dinh E, Lu J, McMahon WM, Lainhart JE. Temporal lobe, autism, and macrocephaly. AJNR: American Journal of Neuroradiology. 2003;24:2066–2076. [PMC free article] [PubMed] [Google Scholar]
- Blanton RE, Levitt JG, Thompson PM, Narr KL, Capetillo-Cunliffe L, Nobel A, Singerman JD, McCracken JT, Toga AW. Mapping cortical asymmetry and complexity patterns in normal children. Psychiatry Research: Neuroimaging Section. 2001;107:29–43. doi: 10.1016/s0925-4927(01)00091-9. [DOI] [PubMed] [Google Scholar]
- Blumenfeld HK, Booth JR, Burman DD. Differential prefrontal-temporal neural correlates of semantic processing in children. Brain and Language. 2006;99:226–235. doi: 10.1016/j.bandl.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, Barthélemy C, Mouren MC, Artiges E, Samson Y, Brunelle F, Frackowiak RSJ, Zilbovicius M. Superior temporal sulcus anatomical abnormalities in childhood autism: A voxel-based morphometry MRI study. Neuroimage. 2004;23:364–369. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: Early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Chi JG, Dooling EC, Gilles FH. Left-right asymmetries of the temporal speech areas of the human fetus. Archives of Neurology. 1977;34:346–348. doi: 10.1001/archneur.1977.00500180040008. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Brain development in autism: early overgrowth followed by premature arrest of growth. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:106–111. doi: 10.1002/mrdd.20020. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: Implications for frontal pyramidal neuron and interneuron development and connectivity. International Journal of Developmental Neuroscience. 2005;23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Bookheimer SY. Form and content: dissociating syntax and semantics in sentence comprehension. Neuron. 1999;24:427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- de Fossé L, Hodge SM, Makris N, Kennedy DN, Caviness VS, Jr, McGrath L, Steele S, Ziegler DA, Herbert MR, Frazier JA, Tager-Flusberg H, Harris GJ. Language-association cortex asymmetry in autism and specific language impairment. Annals of Neurology. 2004;56:757–766. doi: 10.1002/ana.20275. [DOI] [PubMed] [Google Scholar]
- Démonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- Elliot CD. Differential Ability Scales: Introductory and Technical Handbook. New York: The Psychological Corporation; 1990. [Google Scholar]
- Embick D, Marantz A, Miyashita Y, O'Neil W, Sakai KL. A syntactic specialization for Broca's area. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6150–6154. doi: 10.1073/pnas.100098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzi G, Perrone P, Vignolo LA. Right-left asymmetry in anterior speech region. Archives of Neurology. 1982;39:239–240. doi: 10.1001/archneur.1982.00510160045009. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Eure KF, Luevano LF, Weinberger DR. MRI asymmetries of Broca's area: The pars triangularis and pars opercularis. Brain and Language. 1998;64:282–296. doi: 10.1006/brln.1998.1974. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Gilmore RL, Fennell EB, Heilman KM. Planum temporale asymmetry and language dominance. Neuropsychologia. 1994;32:1225–1231. doi: 10.1016/0028-3932(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Gilmore RL, Fennell EB, Heilman KM. Pars triangularis asymmetry and language dominance. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:719–722. doi: 10.1073/pnas.93.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Heilman KM. Morphologic cerebral asymmetries and handedness the pars triangularis and planum temporale. Archives of Neurology. 1995;52:501–508. doi: 10.1001/archneur.1995.00540290091023. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Weisberg A, Browning CA, Weinberger DR. Morphology of the frontal operculum: A volumetric magnetic resonance imaging study of the pars triangularis. Journal of Neuroimaging. 2001;11:153–159. doi: 10.1111/j.1552-6569.2001.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Gaffrey MS, Kleinhaus NM, Haist F, Akshoomoff N, Campbell A, Courchesne E, Müller RA. A typical participation of visual cortex during word processing in autism: An fMRI study of semantic decision. Neuropsychologia. 2007;45:1672–1684. doi: 10.1016/j.neuropsychologia.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauger LM, Lombardino LJ, Leonard CM. Brain morphology in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 1997;40:1272–1284. doi: 10.1044/jslhr.4006.1272. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TFI, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib M. Anatomical asymmetries of the human cerebral cortex. International Journal of Neuroscience. 1989;47:67–80. doi: 10.3109/00207458908987419. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Mallikarjuhn M, Keshavan MS. Brain volume in autism. Journal of Child Neurology. 2001;16:421–424. doi: 10.1177/088307380101600607. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Chabris CF, Clark J, Urban T, Aharon I, Steele S, McGrath L, Condouris K, Tager-Flusberg H. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain and Cognition. 2006;61:54–68. doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Harris GJ, Adrien KT, Ziegler DA, Makris N, Kennedy DN, Lange NT, Chabris CF, Bakardjiev A, Hodgson J, Takeoka M, Tager-Flusberg H, Caviness VS., Jr Abnormal asymmetry in language association cortex in autism. Annals of Neurology. 2002;52:588–596. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Kennedy DN, Filipek PA, Bakardjiev AI, Hodgson J, Takeoka M, Makris N, Caviness VS., Jr Brain asymmetries in autism and developmental language disorder: A nested whole-brain analysis. Brain. 2005;128:213–226. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Lange N, Bakardjiev A, Hodgson J, Adrien KT, Steele S, Makris N, Kennedy D, Harris GJ, Caviness VS., Jr Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–1192. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Schlaug G, Huang Y, Steinmetz H. Asymmetry of the planum parietale. Neuroreport. 1994;5:1161–1163. doi: 10.1097/00001756-199405000-00035. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: Thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. 2nd. Circle Pines, MN: AGS Publishing; 2004. [Google Scholar]
- Kjelgaard M, Tager-Flusberg H. An investigation of language impairment in autism: Implications for genetic subgroups. Language and Cognitive Processes. 2001;16:287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus TA, Bollich AM, Corey DM, Lemen LC, Foundas AL. Variability in perisylvian brain anatomy in healthy adults. Brain and Language. 2006;97:219–232. doi: 10.1016/j.bandl.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Knaus TA, Corey DM, Bollich AM, Lemen LC, Foundas AL. Anatomical asymmetries of anterior perisylvian speech-language regions. Cortex. 2007;43:499–510. doi: 10.1016/s0010-9452(08)70244-2. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule - WPS (ADOS-WPS) Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, Yip L, Murphy DGM, Chua SE. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain. 2005;128:268–276. doi: 10.1093/brain/awh332. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Deutsh CK. Macrocephaly and the control of brain growth in autistic disorders. Progress in Neurobiology. 2005;77:38–56. doi: 10.1016/j.pneurobio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Morgan AE, Hynd GW. Dyslexia, neurolinguistic ability, and anatomical variation of the planum temporale. Neuropsychology Review. 1998;8:79–93. doi: 10.1023/a:1025609216841. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Piro JM. Handedness and intelligence: Patterns of hand preference in gifted and nongifted children. Developmental Neuropsychology. 1998;14:619–630. [Google Scholar]
- Piven J, Arndt S, Bailey J, Andreasen N. Regional brain enlargement in autism: a magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:530–536. doi: 10.1097/00004583-199604000-00020. [DOI] [PubMed] [Google Scholar]
- Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P. An MRI study of brain size in autism. American Journal of Psychiatry. 1995;152:1145–1149. doi: 10.1176/ajp.152.8.1145. [DOI] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biological Psychiatry. 2005;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Robichon F, Levrier O, Farnarier P, Habib M. Developmental dyslexia: Atypical cortical asymmetries and functional significance. European Journal of Neurology. 2000;7:35–46. doi: 10.1046/j.1468-1331.2000.00020.x. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Bawn SD, Benkers TL, Reite ML, Rogers SJ. Smaller left hemisphere planum temporale in adults with autistic disorder. Neuroscience Letters. 2002;328:237–240. doi: 10.1016/s0304-3940(02)00521-9. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Camou SL, Reite ML, Rogers SJ. Planum temporale volume in children and adolescents with autism. Journal of Autism and Developmental Disorders. 2005;35:479–486. doi: 10.1007/s10803-005-5038-7. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview - Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals. 3rd. San Antonio, TX: The Psychological Corporation, Harcourt Brace and Co.; 1995. [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PWR, David AS. The planum temporale: A systematic, quantitative review of its structural, functional, and clinical significance. Brain Research Brain Research Review. 1999;29:26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- Smith S. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. The Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Rex D, Kornsand D, Tessner KD, Jernigan TL, Toga AW. Mapping sulcal pattern asymmetry and local cortical surface gray matter distribution in vivo: Maturation in perisylvian cortices. Cerebral Cortex. 2002;12:17–26. doi: 10.1093/cercor/12.1.17. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. The Journal of Neuroscience. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Volkmann J, Jancke L, Freund HJ. Anatomical left-right asymmetry of language-related temporal cortex is different in left- and right-handers. Annals of Neurology. 1991;29:315–319. doi: 10.1002/ana.410290314. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Paul R, Lord CE. Language and communication in autism. In: Volkmar F, Paul R, Klin A, Cohen DJ, editors. Handbook of autism and pervasive developmental disorder. 3rd. New York: Wiley; 2005. pp. 335–364. [Google Scholar]
- Tomaiuolo F, MacDonald JD, Caramanos Z, Posner G, Chiavaras M, Evans AC, Petrides M. Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: An in vivo MRI analysis. European Journal of Neuroscience. 1999;11:3033–3046. doi: 10.1046/j.1460-9568.1999.00718.x. [DOI] [PubMed] [Google Scholar]
- Tzourio N, Nkanga-Ngila B, Mazoyer B. Left planum temporale surface correlates with functional dominance during story listening. Neuroreport. 1998;9:829–833. doi: 10.1097/00001756-199803300-00012. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Lord C. Diagnosis and definition of autism and other pervasive developmental disorders. In: Volkmar FR, editor. Autism and pervasive developmental disorders. 2nd. New York: Cambridge University Press; 2007. pp. 1–31. [Google Scholar]
- Wada JJ, Clarke R, Hamm A. Cerebral hemispheric asymmetry in humans. Archives of Neurology. 1975;32:239–246. doi: 10.1001/archneur.1975.00490460055007. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York: Harcourt Association; 1999. [Google Scholar]
- Witelson SF, Kigar DL. Sylvian fissure morphology and asymmetry in men and women: Bilateral differences in relation to handedness in men. Journal of Comparative Neurology. 1992;323:236–340. doi: 10.1002/cne.903230303. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Pallie W. Left hemisphere specialization for language in the newborn. Neuroanatomical evidence of asymmetry. Brain. 1973;96:641–646. doi: 10.1093/brain/96.3.641. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Meyer E, Gjedde A, Evans AC. PET studies of phonetic processing of speech: Review, replication, and reanalysis. Cerebral Cortex. 1996;6:21–30. doi: 10.1093/cercor/6.1.21. [DOI] [PubMed] [Google Scholar]