Abstract
Water sorption (WS), mechanical strength, and ion release of polymeric composites formulated with 40 % as-made or milled amorphous calcium phosphate (ACP) are compared after 1, 2 and 3 months of aqueous exposure. Ethoxylated bisphenol A dimethacrylate, triethylene glycol dimethacrylate, 2-hydroxyethyl methacrylate and methacryloxyethyl phthalate comprised the resin. The WS (mass %) peaked at 3 months. WS of as-made ACP composites was significantly higher than WS of milled ACP composites and copolymers. Both composite groups experienced decreases in biaxial flexural strength (BFS) with water aging, with milled ACP composites retaining a significantly higher BFS throughout immersion. Ion release was moderately reduced in milled ACP composites, though they remained superior to as-made ACP composites due to significantly lower WS and higher BFS after prolonged aqueous exposure.
Keywords: amorphous calcium phosphate, biaxial flexure strength, ion release kinetics, particle size distribution, water sorption
INTRODUCTION
Bioactive polymeric dental composites containing calcium phosphates have tremendous appeal due to their potential to arrest demineralization and even remineralize defective tooth structures.1 Such materials are expected to be osteoconductive and, depending on the polymeric phase, also can be biocompatible.2–4 Amorphous calcium phosphate (ACP)-based materials, by providing an extended supply of Ca and PO4 ions needed to reform damaged mineral structures, may counteract recurrent decay, known to develop near the surfaces of teeth in contact with conventional fillings (almost 50 % of all dental fillings require replacement because of recurrent caries). Such anti-demineralizing/remineralizing composites may be particularly useful for patients that are susceptible to cavities as a result of radiation therapy or medications that cause dry mouth. When embedded in polymerized methacrylate matrices and exposed to an aqueous environment, ACP releases sufficient levels of remineralizing Ca and PO4 ions in a sustained manner to promote redeposition of thermodynamically stable, apatitic tooth mineral.5 A problem with dental composites of all types is their inability to resist cracking under masticatory stress due to their relatively low strength and toughness. In the case of ACP composites, the uncontrolled agglomeration of ACP particles was identified as one of the main reasons for a poor interfacial interaction with dental resins that can lead to mechanical instability for these materials, especially when compared to silanized glass-reinforced composites.6 To overcome this shortcoming we have focused on systematically investigating structure-composition-property relationships of ACP fillers, developing strategies that better control the dispersion of ACP particles in the polymer matrix, thereby learning more about the complex interaction(s) occurring at the critical interface that connects the inorganic filler/organic matrix regions.
In this work we compare the water sorption (WS), mechanical strength, and ion release profiles of photo-curable polymeric composites formulated with as-made and milled ACP after prolonged exposure to the same aqueous environments. The hypothesis tested was that the milling of ACP leads to an improved interfacial interaction of the filler phase with the resin, resulting in composites with lower water sorption and improved mechanical strength while still maintaining adequate ion release properties.
MATERIALS AND METHODS
Synthesis and Evaluation of the Fillers
Zirconia (Zr)-ACP precipitated instantaneously at 23 °C upon rapidly mixing equal volumes of a 800 mmol/L Ca(NO3)2 solution, a 536 mmol/L Na2HPO4 solution that contained a molar fraction of 2 % Na4P2O7 and an appropriate volume of a 250 mmol/L ZrOCl2 solution (mole fraction of 10 % ZrOCl2 based on the Ca reactant).7 The reaction pH varied between 8.6 and 9.0. The suspension was filtered, the solid phase subsequently washed with ice-cold ammoniated water, then acetone, and finally lyophilized.
For milling (planetary ball mill PM100; Retch, Inc., Newtown, PA, USA), approx. 23 g of as-made Zr-ACP solid were mixed with 550 g of very high density ZrO2 balls (d = 3 mm; Glen Mills Inc., Clifton, NJ, USA) and 150 mL of analytical grade isopropanol (J.T. Baker, Phillipsburg, NJ, USA) and then sealed in a grinding jar (total mass = 7.4 kg). The milling was performed at 42 rad/s for 3.5 h with rotation reversed every 15 min. Milled Zr-ACP solid was removed from the ZrO2 balls by sieving. Isopropanol was then evaporated in a vacuum oven (Squaroid Labline vacuum oven, Melrose Park, IL, USA) at 70 °C for 24 h.
The amorphous state of both as-made and milled ACP powders was verified by powder X-ray diffraction (XRD: Rigaku Dmax diffractometer, Rigaku/USA Inc., Danvers, MA, USA) and Fourier-transform spectroscopy (FTIR: Nicolet Magna-IR FTIR System 550 spectrophotometer, Nicolet Instrument Corporation, Madison, WI, USA). Morphological/topological features of the fillers were examined by scanning electron microscopy after the specimens were sputter-coated with gold (SEM: JEOL 35C, JEOL Inc., Peabody, MA, USA).
The particle size distribution (PSD) of the fillers was determined using laser obscuration concurrently with a computerized inspection system (CIS-100 Particle Size Analyzer: Ankersmid LTD., Yokneam, Israel). With this technique, a He-Ne laser focused to a known spot size is passed through a rotating wedge prism, imparting a constant angular velocity. The laser scans the particles, and a photodiode detector measures the time the laser takes to traverse the particle diameter (i.e., the amount of time the laser is obscured). This time of obscuration is then directly related to the particle diameter:
| (1) |
where D = particle diameter, v = velocity of the laser, and t = time of obscuration. Approximately 3 mg of filler were dispersed in 3 g of resin (see Table 1), and the suspension briefly ground with a mortar and pestle to simulate the effect of hand spatulation. Five repetitive measurements were performed for each filler type.
Table 1.
Monomers and components of photo-initiator system employed in the study.
| Chemical name | Acronym | Type | Mass fraction, % |
|---|---|---|---|
| ethoxylated bisphenol A dimethacrylate | EBPADMA | Base monomer | 62.8 |
| triethylene glycol dimethacrylate | TEGDMA | Diluent monomer | 23.2 |
| 2-hydroxyethyl methacrylate | HEMA | Surface active monomer |
10.4 |
| methacryloxyethyl phthalate | MEP | Surface active monomer |
2.6 |
| camphorquinone | CQ | Photo-activator | 0.2 |
| ethyl-4-N,N-dimethylaminobenzoate | 4EDMAB | Photo-reductant | 0.8 |
Resin Formulation
The experimental resin was formulated from the following commercially available dental monomers (Esstech, Essington, PA). The chemical names and the acronyms for the monomers used throughout this manuscript and the composition of the resin are provided in Table 1. The chemical structure of the utilized components is shown in Figure 1.
Figure 1.
Chemical structure of the monomers and photo-curing agents utilized in the study.
Fabrication of Copolymer and ACP Composite Specimens
The photoactivated resin ETHM was made from mixing EBPADMA, TEGDMA, HEMA and MEP with the photoinitiator system comprising CQ and 4EDMAB. Composite pastes were made by mixing the ETHM resin (mass fraction 60 %) and either as-made or milled Zr-ACP filler (mass fraction 40 %). The homogenized pastes were kept under a moderate vacuum (2.7 kPa) overnight to eliminate the air entrained during mixing. The pastes were placed into Teflon molds 15.8 mm to 16.8 mm in diameter and 1.5 mm to 2.1 mm in thickness to prepare specimens for biaxial flexure strength (BFS) testing. Each opening of the mold was covered with a thin Mylar film and a glass slide, which were clamped in place by spring clips. The clamped specimens were then photo-polymerized by irradiating sequentially each face of the mold assembly for 120 s with visible light (Triad 2000, Dentsply International, York, PA, USA). The BFS specimens were randomly selected and stored for 24 h at 22 °C in air before being tested for dry strength. The remaining specimens were immersed in distilled water at 22 °C for 1 month, 2 months, and 3 months prior to testing for wet strength. The identical procedure was utilized in the fabrication and testing of unfilled copolymer specimens derived from the ETHM resin.
Mechanical Testing
Biaxial flexure strength (BFS) of the specimens was determined using a computer-controlled Universal Testing Machine (Instron 5500R, Instron Corp., Canton, MA, USA) operated by Instron Merlin Software Series 9. For BFS measurements (number of specimens per group, 4 ≤ n ≤ 15), the strength of a disk specimen supported along a lower support circle was determined under a static load utilizing a piston-on-three-ball loading arrangement with a crosshead speed of 0.5 mm/min. The failure stress was calculated according to the following equation8:
| (2) |
where
| (3) |
and
| (4) |
| (5) |
where ν = Poisson’s ratio, rl = radius of the piston applying the load at the surface of contact, rsc = radius of the support circle, rs = radius of disk specimen, L = applied load at failure, and t = thickness of the disk specimen.
Water Sorption
To determine the water sorption (WS) of copolymers and composite specimens two types of experiments were performed. For each type of WS experiment, pieces of specimens fractured during mechanical testing were dried over anhydrous CaSO4 until a constant mass was achieved (± 0.1 mg). The specimens were then either exposed to an air atmosphere of 75 % relative humidity (rH) at room temperature (22 °C) by keeping them suspended over saturated aqueous NaCl slurry in closed systems (number of specimens n ≤ 16/group) or immersed into buffered saline solution (saline immersion, SI: 30 mL per specimen; number of specimens n ≤ 21/group). Gravimetric changes were recorded at predetermined time intervals of exposure to rH and SI for up to 3 months. Immersed specimens were blotted dry before recording their mass. The WS or mass gain (in percent mass fraction) of any individual specimen at any given time interval (t) was calculated according to the expression:
| (6) |
where Wt represents specimen mass at the time t, and Wo is the initial mass of the dry specimen. The WS values of the composites in each experiment were normalized for the same amount of resin (copolymer disks = 100 % resin, composites disks = 60 % resin by mass).
Ion Release Profiles
Ion release from the two types of composite specimens (as-made vs. milled ACP) was screened in the following manner. Individual disk specimens with the geometry of BFS specimens (three specimens per group) were immersed into a buffered saline solution (pH = 7.40, ionic strength = 0.13 mol NaCl/L, 23 °C, continuous magnetic stirring) and aliquots for Ca and PO4 analysis9–10 were taken at 1 month time intervals for 3 months.
STATISTICAL ANALYSIS
One standard deviation (SD) is given in this paper for comparative purposes as the estimated standard uncertainty of the measurements. These values should not be compared with data obtained in other laboratories under different conditions. Experimental data were analyzed by ANOVA (α = 0.05). Significant differences between specific groups were determined by pair-wise multiple comparisons (two-tail t-test; unequal variances).
RESULTS
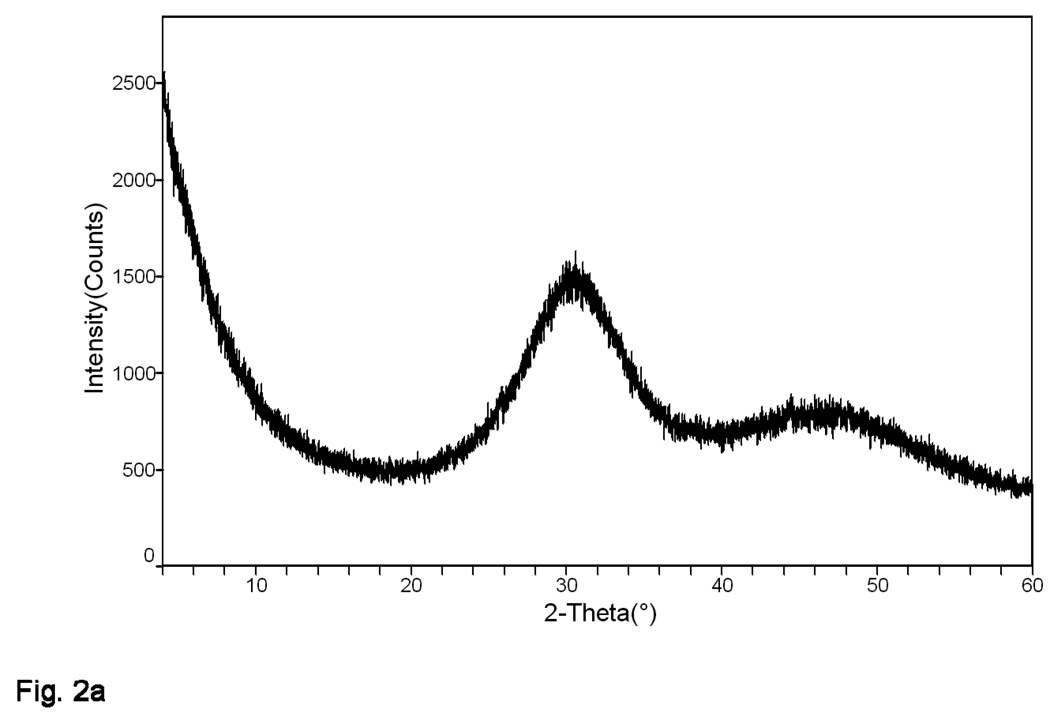
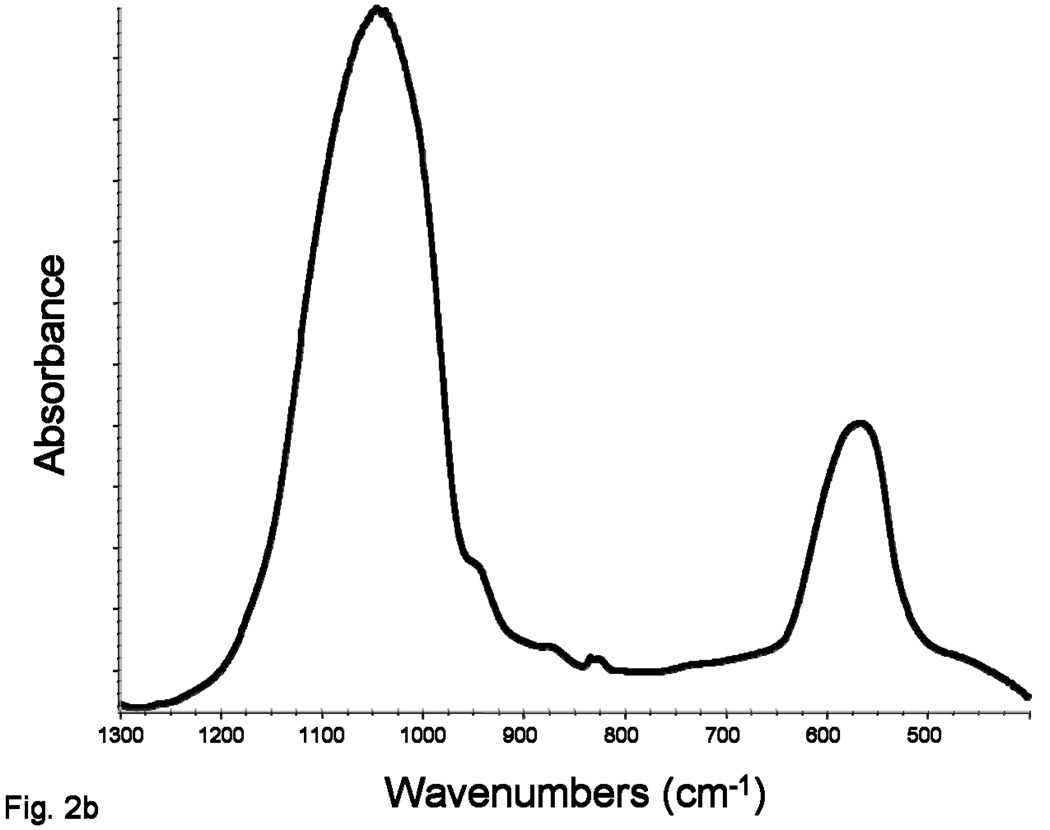
Both the as-made and milled Zr-ACP filler employed in this study showed no discrete XRD peaks; their XRD patterns consisted of two diffuse, broad bands resembling XRD spectra of noncrystalline substances such as glasses and certain polymers (Figure 2a). Corresponding FTIR spectra (Figure 2b) showed only two wide bands typical for phosphate stretching and phosphate bending of noncrystalline calcium phosphate in the region of (1200 to 900) cm−1 and (630 to 500) cm−1, respectively. Results from both XRD and FTIR confirm the amorphous nature of the Zr-ACP used in this study.
Figure 2.
X-ray diffraction pattern (a) and Fourier-transform infrared spectrum (b) typical for both the as-made and milled ACP.
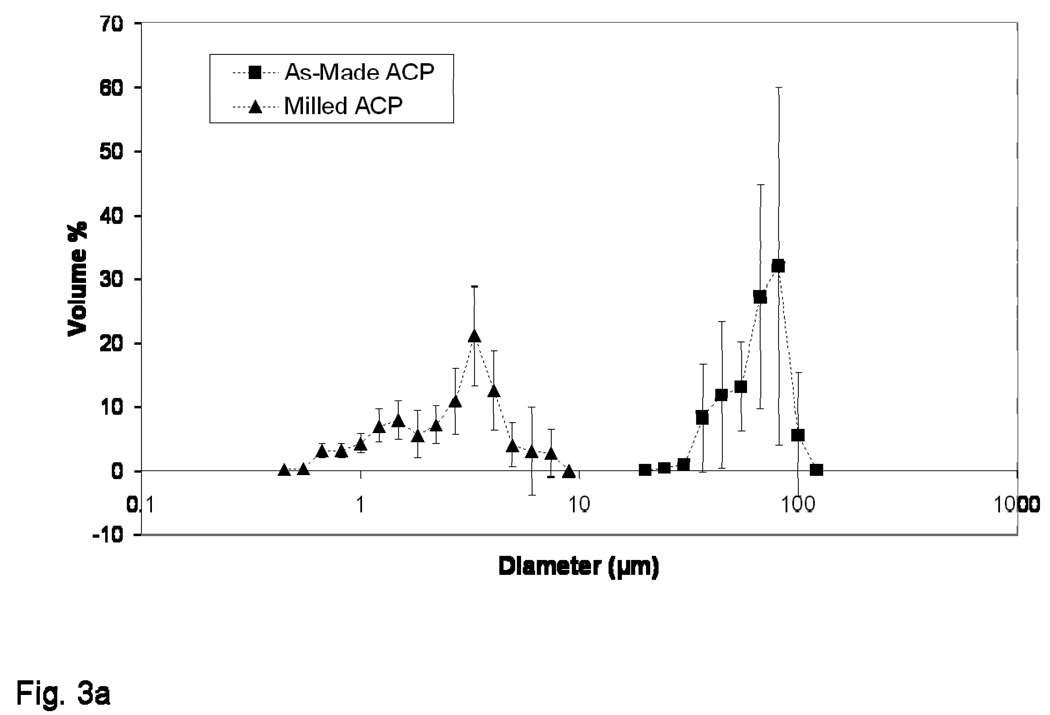
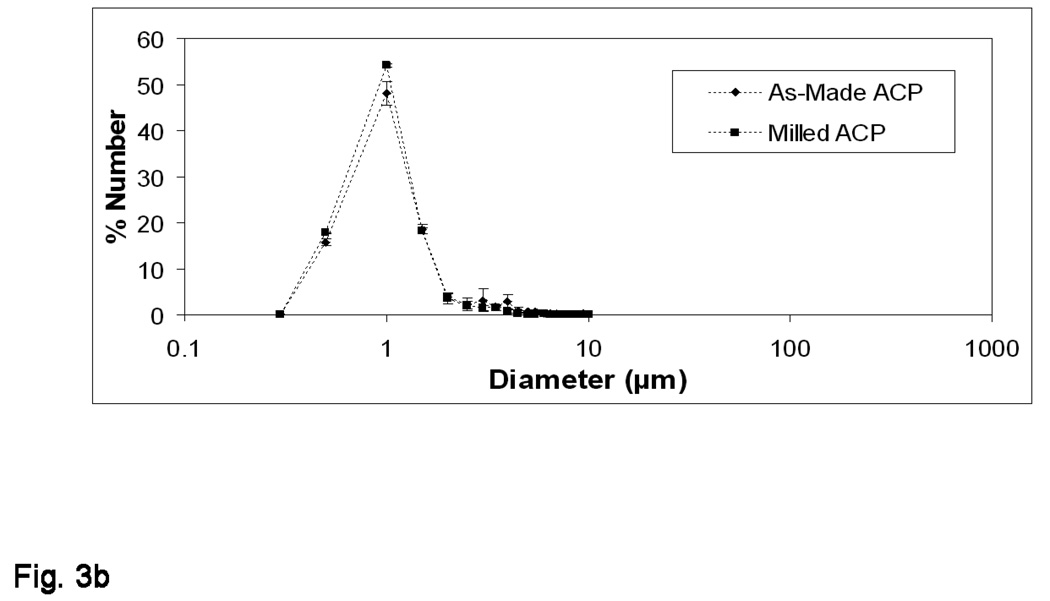
The results of PSD analysis are shown in Figures 3a and 3b. When distributed by percentage of sample volume (% Volume; Figure 3a), a reduction in median diameter, dm, from (70.88 ± 13.27) µm to (3.10 ± 0.88) µm for as-made ACP vs. milled ACP was observed. In terms of percentage of particles scanned (% Number; Figure 3b) a reduction in dm from (0.85 ± 0.03) µm to (0.78 ± 0.01) µm for as-made ACP vs. milled ACP was observed.
Figure 3.
Volume density (a) and number density (b) particle size distributions of as-made ACP and milled ACP used in the study. The standard deviation (SD; indicated by a bar) is taken as a measure of the standard uncertainty.
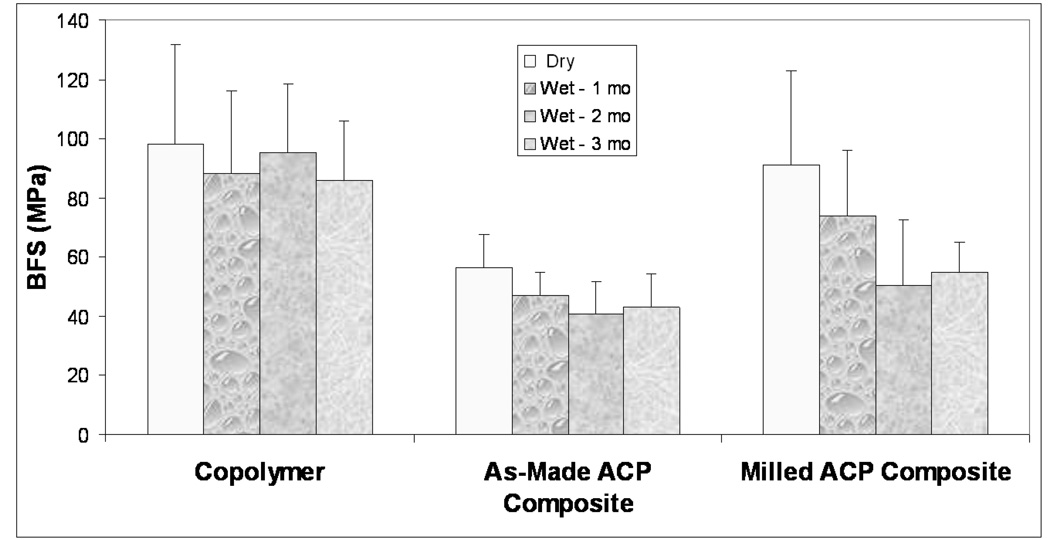
The results of the BFS testing of dry (before immersion) and wet (after 1 month, 2 months, and 3 months of SI) copolymer, as-made and milled Zr-ACP composite specimens are summarized in Figure 4. The average BFS values of dry copolymer and milled Zr-ACP composites ((98 ± 33) MPa and (91 ± 32) MPa, respectively) were significantly higher than the average BFS value of the as-made ACP composites ((56 ± 11) MPa). The same order of descending BFS existed after 1 month of aqueous exposure: {copolymer ((88 ± 28)MPa) and milled ACP composites ((74 ± 22) MPa)} > as-made ACP composites ((47 ± 8) MPa). After 2 months, no significant difference exists between the composite groups: copolymer ((95 ± 23) MPa) > {milled ACP composites ((50 ± 9) MPa) and as-made ACP composites ((41 ± 11) MPa)}. Finally, after 3 months of aqueous immersion the following order of significantly decreasing BFS values existed: copolymer ((86 ± 20) MPa) > milled ACP composites ((55 ± 10) MPa) > as-made ACP composites ((43 ± 11) MPa). Despite significant deterioration compared to the dry BFS values (up to 40 %), the mechanical strength of milled ACP composites remained 28 % higher than the BFS of the composites fabricated with the as-made ACP after 3 months of SI.
Figure 4.
Biaxial flexure strength (BFS; mean + SD) of dry and wet (immersion times as indicated) copolymer, as-made ACP composite and milled ACP composite specimens (number of samples per group, n ≤ 15).
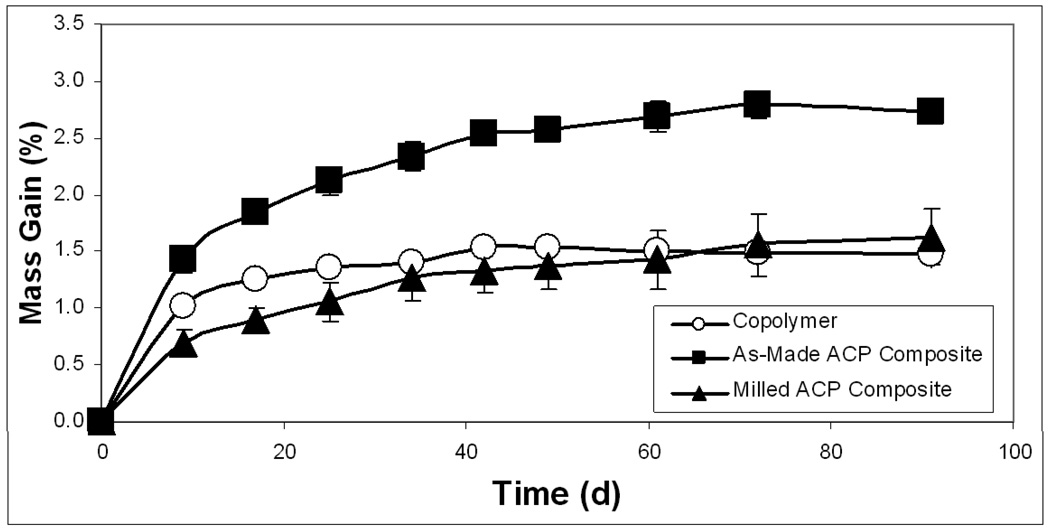
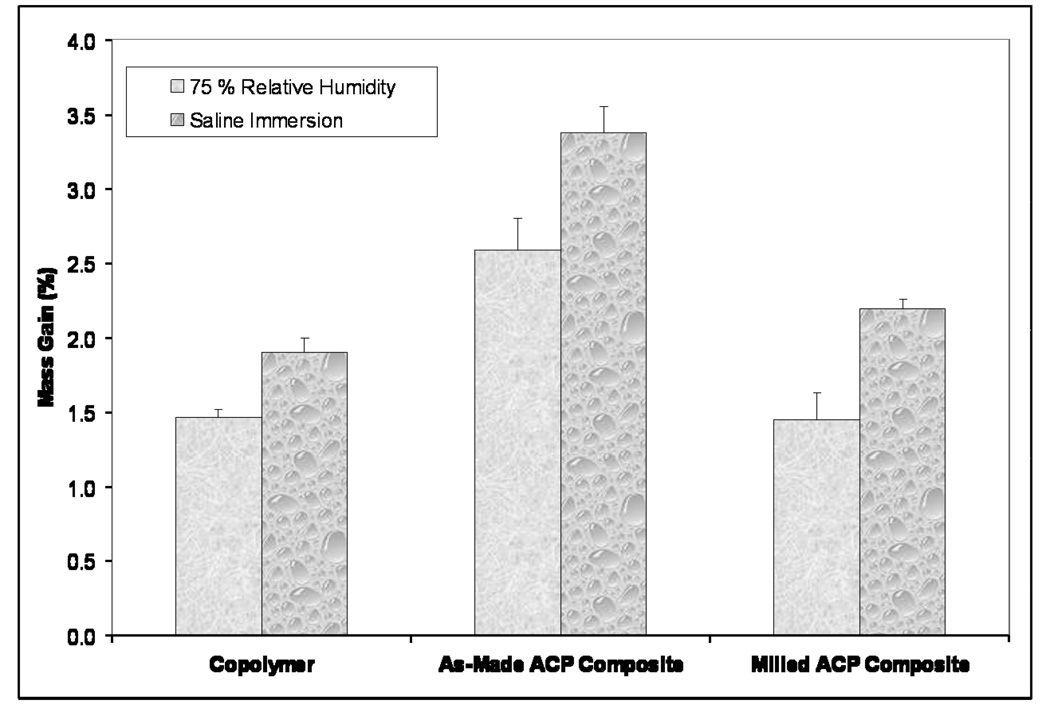
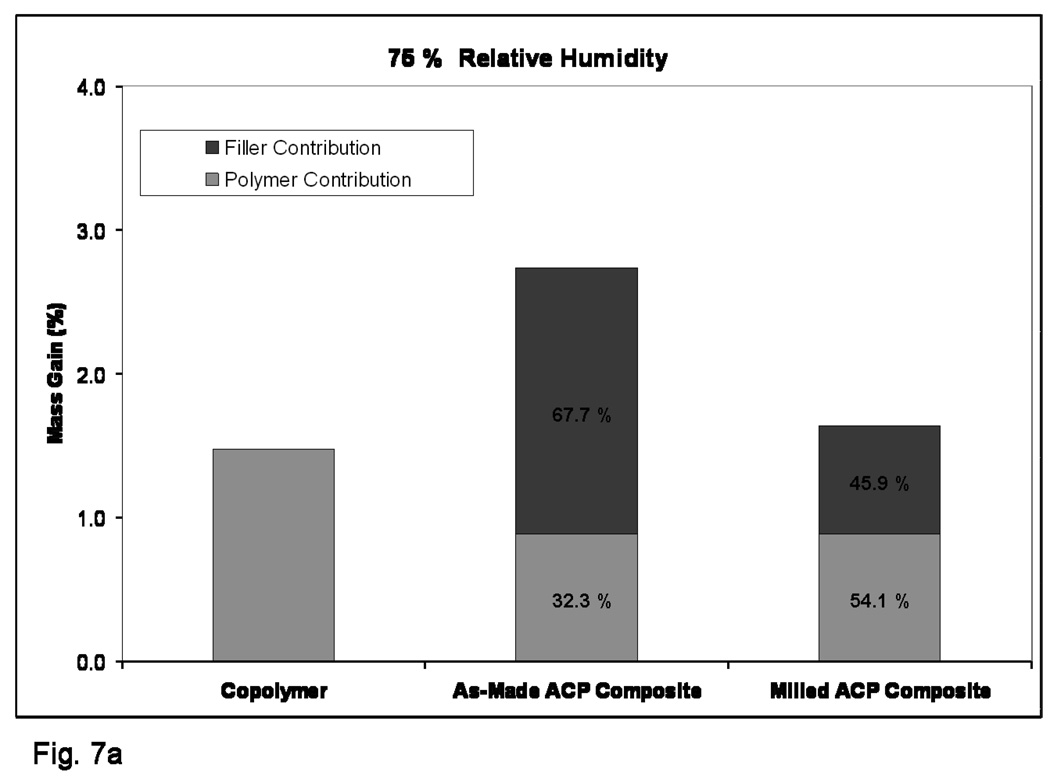
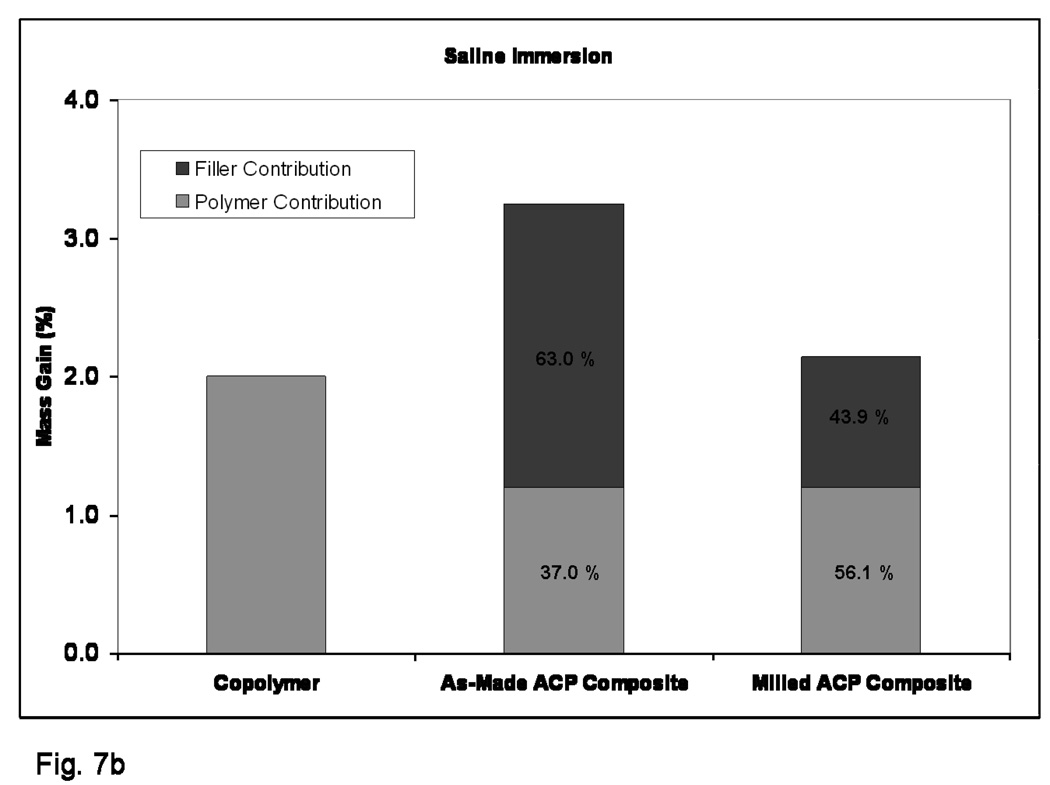
Kinetic WS data shown in Figure 5 indicates that plateau values were reached within 40 d for copolymer specimens and within 70 d for the as-made ACP composites exposed to 75 % RH. The composite specimens based on the milled ACP may not yet have reached their maximum WS at 90 d. The maximum WS values reached within 3 months of RH exposure decreased in the following order: as-made ACP composites > (milled ACP composites and copolymer disks). The WS (mass gain) of the specimens directly immersed into saline solution showed practically no change after 1 month of immersion. WS of fully immersed specimens followed the trend observed with the specimens exposed to RH. The WS values obtained under immersing conditions were, however, consistently higher [(30 to 52) %] compared to the WS values attained upon exposure to RH (Figure 6). When normalized for the amount of copolymer present, the contributions from filler and polymer to each composite’s mass gain compares much more favorably between the relative humidity and saline immersion WS experiments (Figures 7a and 7b, respectively). The contribution of as-made ACP filler to the mass gain in saline immersed composites was 63.0 %, compared to 67.7 % for as-made ACP composites exposed to RH. Milled ACP, on the other hand, accounted for 43.9 % of the mass gain when immersed in saline and 45.9 % in a relative humidity environment.
Figure 5.
Kinetic water sorption (WS) profiles for ETHM copolymers and the corresponding as-made and milled ACP composites. Total time of exposure to 75 % rH: 3 months. Number of specimens per group n ≤ 21. The SDs of the measurements indicated by bars.
Figure 6.
Comparison of the mass gain due to the water sorption of copolymer and composite specimens exposed to 75 % rH and immersed in saline. Indicated values represent the WS (mean + SD) determined at 1 month, 2 months and 3 months for each type of experiment. (number of specimens per group was ≤ 16 and ≤ 21 for rH and SI, respectively).
Figure 7.
Comparison of normalized composite mass gains for 75 % rH (a) and SI (b).
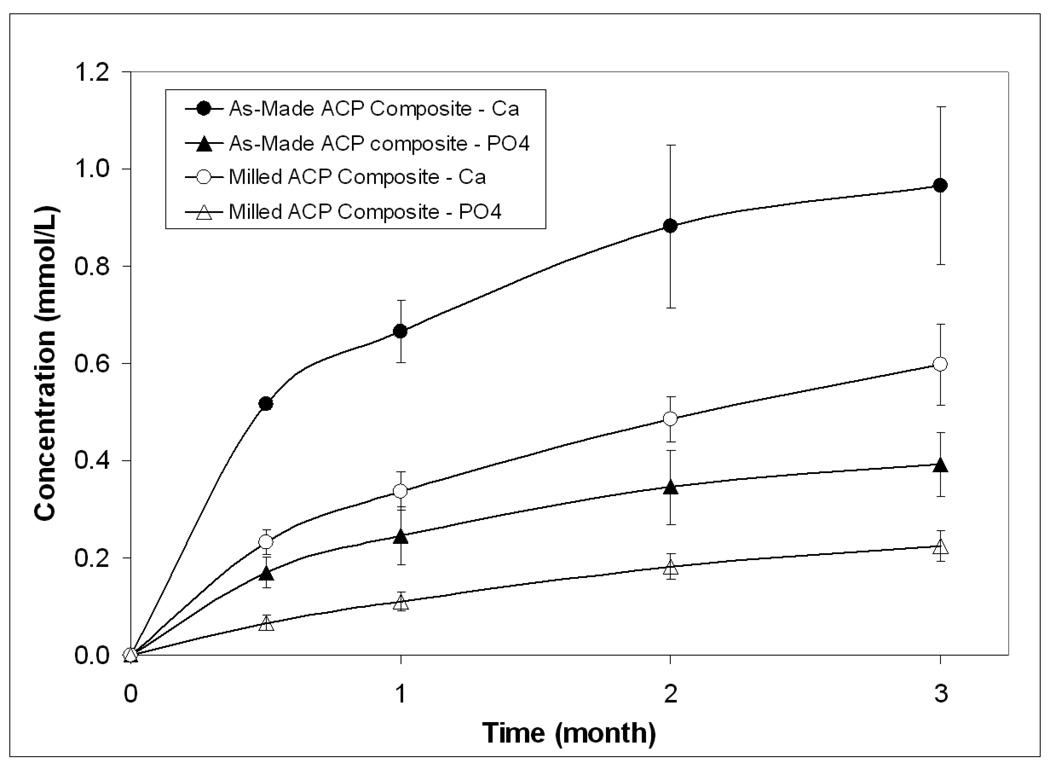
Kinetic profiles of Ca and PO4 release from composites are presented in Figure 8. The levels of the ions released from the as-made ACP composites into aqueous milieu were systematically higher than the levels of these ions released from the milled ACP specimens. However, the immersion solutions containing the maximum concentrations of Ca and PO4 were all supersaturated with respect to apatite in both groups of composites.
Figure 8.
Ca and PO4 release from the as-made and milled ACP composite disk specimens immersed in saline for three months. The number of repetitive runs in each experimental group n = 3. The SDs of the measurements indicated by bars.
DISCUSSION
Small ceramic particles are known to enhance the mechanical and tribological properties of polymers.11 When introduced into the resin phase of composites, filler morphology, size, shape and amount, as well as the state of dispersion of filler particles in polymeric matrices, significantly determine the performance of composites. Both the type and amount of inorganic filler are particularly important in dental material applications; in most cases spherical and/or irregularly shaped fillers are dispersed randomly into polymeric matrices.12 Water sorption and solubility are primarily influenced by the type of components (both organic and inorganic) that comprise the microstructure of the composite materials. Variations occurring between composites with the same type of filler phase usually result from differences in matrix composition.13 For conventional dental composites, WS typically increases until equilibrium is reached and is largely a function of resin composition. The solubility of composite resin materials also is largely influenced by the resin composition. TEGDMA appears to be the main monomer released from various dental composites, while the leaching of base monomers such as Bis-GMA is considerably less.14 Although both the inorganic (glass, ceramic) filler phase and silane derived interface can influence WS and solubility, their contributions are significantly less than the resin phase of composites.
In this study we explored the effects that ball milling ACP has on the properties of this bioactive filler and also some key properties of resin composites utilizing milled ACP as the filler phase. In the milling process, the rotation of the milling jar imparts kinetic energy to the grinding media, which in this case are very high density ZrO2 balls. The balls, in turn, impact each other and the jar walls, imparting mechanical energy from the balls to the wall and any powder caught in between. In addition to breaking up agglomerates, this also raises the temperature of the jar significantly, so care must be taken not to mill for too long. This is especially true when using alcohols to disperse the powder (common practice in ultra-fine grinding), as the combination of hydrophilic solvents and extreme heat greatly increases the likelihood of apatitic conversion.
Compared to other methods that calculate particle size from a secondary property such as settling speed (sedimentation technique) or refractive index (laser diffraction technique), particle size analysis by laser obscuration uses the time-of-transition principle to directly measure a particle’s size. As such, there is a much greater freedom in choosing the dispersing medium for the sample. In fact, one may choose to use no dispersing medium and measure only the dry sample. In this case, however, both as-made and milled ACP were dispersed in the ETHM resin used in this study. Though it was not possible to perform this test using the same ratio of filler/resin present in the composite pastes (the opacity and high viscosity would preclude measurement), this should provide a closer approximation of the filler particle size distribution within the resin matrix.
The different ways of expressing particle size distributions shown in Figures 3a and 3b seem to be contradictory. A glance at the number distribution (Figure 3b) would seem to indicate almost no difference in particle size between as-made and milled ACP, while the volume distribution (Figure 3a) demonstrates quite the opposite. Together, however, the plots prove complementary, and allow us to make several significant observations: 1) the shift and narrowing of the majority sample volume from (20 to 100) µm to (0.45 to 7.4) µm as well as the reduction in scatter between milled ACP measurements point to the effectiveness of the milling process and the consequential increase in sample homogeneity; 2) large agglomerates (large and fine defined here quite arbitrarily as diameters greater than 10 µm and less than 1 µm, respectively) accounted for approximately 1 % of the as-made particles scanned but 99.5 % of the overall sample volume, while the milled ACP sample contained no large agglomerates; and 3) as-made and milled ACP contained similar amounts of fine particles (64 % vs. 72 %, respectively), but they constituted a much higher volume of the milled sample (11 % vs. 0.02 % for as-made).
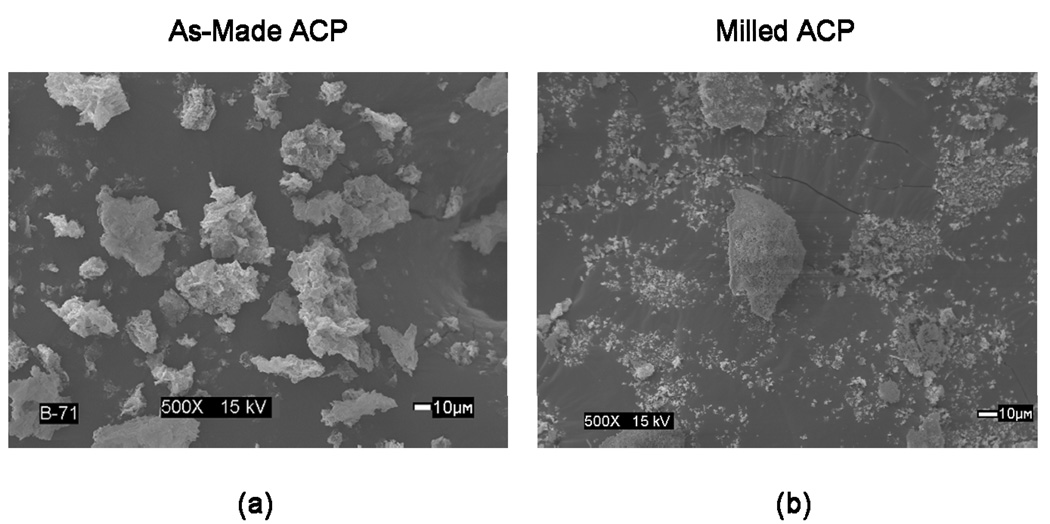
One way to characterize a powder sample is by measuring its bulk density. This value is obtained by dividing the mass of the sample by the volume it occupies, which includes the interstices between particles as well as the space inside their pores. Breaking up large agglomerates in a powder allows it to pack more efficiently, thereby increasing the bulk density of the sample. Though not quantitatively measured here, several qualitative observations have been made which confirm this. SEM microphotographs of as-made and milled ACP (Figures 9a and 9b, respectively) clearly show milled ACP to have significantly more fine particles than as-made ACP. One may note the presence of several large agglomerates in the milled ACP microphotograph that are not represented in its PSD. This is to be expected, as microscopy requires the powders to be dried, causing the reformation of agglomerates broken up by the milling process. The handling properties of composite pastes also provide an indirect characterization of a filler’s bulk density. In this case, utilizing milled ACP as the filler phase resulted in a composite paste with handling properties appreciably different from as-made ACP composite paste. As-made ACP composite pastes were highly viscous and resistant to flow, while pastes with milled ACP were fluid and very flowable.
Figure 9.
Microphotographs showing a typical morphology of the as-made ACP (a) and milled ACP filler (b) (SEM data).
The highly agglomerated nature of as-made ACP makes it nearly impossible to achieve fill levels higher than 40 weight % with this particular resin system. Data currently being prepared for publication show no significant differences in BFS between 30, 35 and 40 weight % as-made ACP. Given that higher fill levels yield improvements in shrinkage stress (decreases) and Ca/PO4 release (increases), using the upper limit of 40 weight % as-made ACP seems most sensible. Though higher fill levels can be achieved with milled ACP, 40 weight % was in this case used for comparative purposes.
Since equal filler mass fractions were used in both composites, the filler with the higher bulk density (milled ACP) constituted an overall smaller volume of its composite. This, of course, entails an increased volume of the matrix phase and a corresponding increase in the polymerization shrinkage stress of the cured composite. This is potentially problematic when considering milled ACP composites for clinical use in composite restorations, as high volumetric shrinkage and stress leaves the tooth subject to marginal leakage, recurrent decay, or even mechanical failure. In that case, it is recommended the filler mass fraction be increased to achieve lower polymerization shrinkage and a more acceptable shrinkage stress value.
The primary aim of this paper was to investigate the effect large agglomerates have on specific physical and chemical properties of ACP composites. As a non-reinforcing filler, ACP itself does not contribute to the development of shrinkage stress during polymerization; this is largely governed by the type and relative amount of matrix phase present. At most, the elimination of highly agglomerated ACP allows for an increase in filler load level, reducing matrix volume and, in turn, shrinkage stress. Previous publications on ACP composites have reported findings to this effect.15–17
For several reasons, BFS testing was felt to be the most appropriate means of characterization the composites used in this study. While three- and four-point bending tests are both long-established techniques in characterizing the mechanical properties of brittle materials, the measured strength depends upon both the condition of the surface in tension and the condition of the edges in tension.18 This sensitivity to edge flaws can result in large strength variations among specimens with very similar surfaces. Given the difficulty in separating these effects from one another, the biaxial flexure test, which is indifferent to edge conditions, is often used. Based on the work of Kirstein and Woolley,19 this test method involves supporting a thin circular disk using three balls positioned near its periphery (equidistant from the center) and loading its center using a piston. Since the area of maximum tensile stress falls at the center of the disk’s lower face, the strength is independent of the edge conditions of the disk.18,20 Both the diametral tensile strength15,21 (DTS) and compressive strength (unpublished data) of ACP composites have previously been evaluated, and show trends similar to those seen in BFS testing. Additionally, it is important to demonstrate the effect of filler by comparing the WS and BFS of composites to those of copolymer disks, and previous work has shown that these materials are not amenable to DTS testing.21 The ductility of unfilled copolymer DTS disks causes significant deviations from the idealized pure tension across specimen diameters. As the material plastically deforms, small flat areas develop at the contact points, redistributing stresses and causing failure in shear and compression.
Examining the water-related behavior of ETHM copolymer disks in both a relative humidity and immersed saline environment has proven useful. In these cases, the closed system contains only two interacting components (water and disk), so any observed effects on the copolymer disks (i.e., water uptake, dissolution) can be seen to have a direct causal link to the environment. Since copolymer disks can technically be viewed as unfilled composites, their WS values can be normalized to match the resin matrix mass % of the filler reinforced composites and overlaid with their total mass gain. In this way, the effects of polymer matrix and filler on water uptake can be separated, allowing for a comparison of the otherwise differing WS values from relative humidity and saline immersion environments. Immersing the composites yielded WS values that were 30 % and 52 % higher for as-made and milled ACP, respectively, than for the same composites exposed to rH. Once the values are normalized, however, we see only a 4.7 % difference in filler contribution between SI and rH for as-made ACP composites, and a 2.0 % difference for milled ACP composites.
The overall hydrophilicity of the resin system drives the uptake of water into the composite disks, which (to varying degrees) facilitates the diffusion of unreacted monomer and Ca and PO4 ions. In both cases (rH and SI), milled ACP shows that most of the mass gain is due to the polymer matrix, whereas the water uptake in as-made ACP composites is dominated by the filler. This suggests that as-made ACP is more hydrophilic than milled ACP. This also compares well with the earlier observation regarding the bulk density and PSD of both fillers. The increased filler volume in as-made ACP composites provides more paths of diffusion, and their highly agglomerated and porous nature increases their capacity to absorb water. Milling results in the diminution of particle sizes and porosity, increasing both the filler’s bulk density and volume of polymer required in the composites. Thus, although the chemical and fundamental morphological character of ACP itself was not altered by milling, the breaking up of agglomerates has, in effect, reduced the hydrophilicity of the filler phase and, in turn, that of the milled ACP composites.
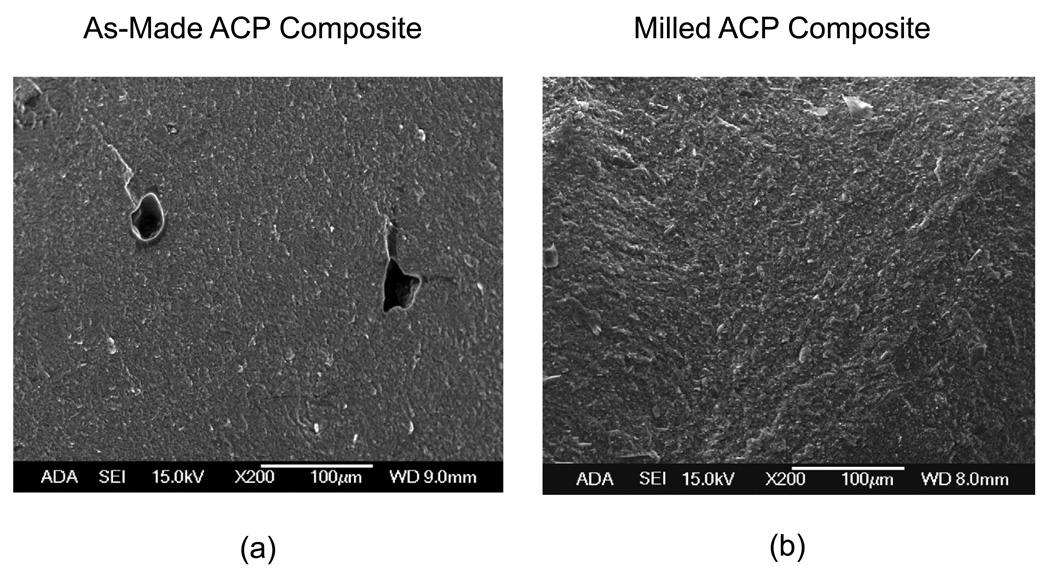
The results, as a whole, show that a definite interplay exists between the physical and chemical properties of ACP composites. As-made ACP is by nature highly agglomerated and heterogeneous, which makes it difficult to incorporate it into resin, yielding an extremely viscous composite paste with little or no cohesiveness. This, in turn, leads to the inclusion of more voids (Figures 10a and 10b), which act both as stress concentrations (reducing the mechanical strength of the cured composite) and as paths of diffusion (increasing the water uptake and ion release of the composite). The loss of ACP mineral may also act to structurally weaken the composites, further contributing to the observed differences in mechanical strength for similarly immersed as-made and milled ACP composites. By contrast, milled ACP has a more homogeneous PSD and finer particle sizes, allowing it to easily mix with the resin to yield a flowable cohesive paste. With a higher volume of matrix phase, these composites proved stronger in biaxial flexure and experienced a decrease in water uptake. As the main mechanism for ion release involves water-assisted diffusion, this reduction in water uptake also reduced the concentrations of Ca and PO4 released, though they were still above the minimum necessary for re-deposition of apatite to occur.
Figure 10.
Microphotographs showing voids incorporated into the microstructure of as-made ACP composite disks (a), compared to a milled ACP composite disk (b) (SEM data).
CONCLUSIONS
Particle size analysis of as-made ACP revealed that an extremely small amount of highly agglomerated particles constituted an overwhelming majority of the sample volume, while the PSD of milled ACP showed a relative dearth of such particles. The fact that as-made and milled ACP composites show significant differences in nearly every physico-chemical category measured suggests that this relatively small number of large agglomerates plays a disproportionately large role in determining the physico-chemical profile of ACP composites. This study has shown the mechanical milling of as-made ACP to be an effective method of eliminating these particles and creating a filler particle size distribution more favorable to more homogeneous dispersion in a resin matrix. Utilizing milled ACP as a filler yields composites with reduced water sorption and aids in the retention of mechanical strength when exposed to aqueous milieu, while maintaining a Ca and PO4 release profile that is still conductive to remineralization via apatite deposition.
Acknowledgements
Support for this research was provided from the grant R01 DE13169-08 from the National Institute of Dental and Craniofacial Research to Dr. Skrtic. We gratefully acknowledge generous contribution of monomers from Esstech, Essington, PA, USA.
Disclaimer: Certain commercial materials and equipment are identified in this work for adequate definition of the experimental procedures. In no instance does such identification imply recommendation or endorsement by the American Dental Association or the National Institute of Standards and Technology, or that the material and the equipment identified is necessarily the best available for the purpose.
Biographies
Justin O’Donnell is a Research Assistant at the Paffenbarger Research Center (PRC), American Dental Association Foundation (ADAF) in Gaithersburg, MD. His main interests include the physicochemical properties of bioactive polymeric composites, and the development of a dynamic, fatigue-based approach to the study of dentin bonding.
Dr. Antonucci is a Research Chemist in the Biomaterials Group of the Polymers Division, Materials Science and Engineering Laboratory of the National Institute of Standards and Technology, Gaithersburg, MD. His main interests include synthetic and polymer chemistry, dental composites, cements and adhesion, initiator systems, interfacial coupling agents, and remineralizing polymer systems.
Dr. Skrtic is a Senior Project Leader at PRC, ADAF. His main interests include physicochemical studies of the formation and stability of slightly soluble salts, relevant to biomedicine, dentistry and/or industry.
REFERENCES
- 1.Skrtic D, Hailer AW, Takagi S, Antonucci JM, Eanes ED. Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. J Dent Res. 1996;75(9):1679–1686. doi: 10.1177/00220345960750091001. [DOI] [PubMed] [Google Scholar]
- 2.Hollinger JO, Brekke J, Gruskin E, Lee D. Role of bone substitutes. Clin Orthop. 1996;324:55–65. doi: 10.1097/00003086-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 3.LeGeros RZ, LeGeros JP. Calcium phosphate biomaterials in medical application. Bioceramics. 1996;9:7–10. [Google Scholar]
- 4.Yuan H, Li Y, de Bruijn J, de Groot K, Zhang X. Tissue responses of calcium phosphate cement. A study in dogs. Biomaterials. 2000;21(12):1283–1290. doi: 10.1016/s0142-9612(00)00016-8. [DOI] [PubMed] [Google Scholar]
- 5.Skrtic D, Antonucci JM, Eanes ED. Amorphous calcium phosphate-based bioactive polymeric composites for mineralized tissue regeneration. J Res Natl Inst Stands Technol. 2003;108(3):167–182. doi: 10.6028/jres.108.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skrtic D, Antonucci JM, Eanes ED, Eidelman N. Dental composites based on hybrid and surface-modified amorphous calcium phosphates - A FTIR microspectroscopic study. Biomaterials. 2004;25(7–8):1141–1150. doi: 10.1016/j.biomaterials.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Eanes ED, Gillessen IH, Posner AS. Intermediate states in the precipitation of hydroxyapatite. Nature. 1965;208:365–367. doi: 10.1038/208365a0. [DOI] [PubMed] [Google Scholar]
- 8.ASTM F394-78. Standard test method for biaxial strength of ceramic substrates. 1991. [Google Scholar]
- 9.Vogel GL, Chow LC, Brown WE. A microanalytical procedure for the determination of calcium, phosphate and fluoride in enamel biopsy samples. Caries Res. 1983;17:23–31. doi: 10.1159/000260645. [DOI] [PubMed] [Google Scholar]
- 10.Murphy J, Riley JP. Single solution method for the determination of phosphate in natural waters. Anal Chim Acta. 1962;27:31–36. [Google Scholar]
- 11.Wetzel B, Haupert F, Zhang MQ. Epoxy nanocomposites with high mechnaical and tribological performance. Comp Sci Technol. 2003;63(14):2055–2067. [Google Scholar]
- 12.Ramalho A, Antunes PV, de Carvalho MDB, Gil H, Rocha J. Mechanical properties of particle reinforced resin composites. Advanced Materials Forum. 2006:514–516. 619–623. III, PTS 1 and 2; Material Science Forum. [Google Scholar]
- 13.Toledano M, Osorio R, Osorio E, Fuentes V, Prati C, Garcia-Godoy F. Sorption and solubility of resin-based restorative dental materials. J Dent. 2003;31(1):43–50. doi: 10.1016/s0300-5712(02)00083-0. [DOI] [PubMed] [Google Scholar]
- 14.Ortengren U, Wellendorf H, Karlsson S, Ruyter IE. Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. J Oral Rehab. 2001;28(12):1106–1115. doi: 10.1046/j.1365-2842.2001.00802.x. [DOI] [PubMed] [Google Scholar]
- 15.Antonucci JM, Skrtic D. Polymers for Dental and Orthopedic Applications. Boca Raton, FL: Taylor and Francis Group LLC; 2007. Physicochemical Properties of Bioactive Polymeric Composites: Effects of Resin Matrix and the Type of Amorphous Calcium Phosphate Filler; pp. 217–242. [Google Scholar]
- 16.O'Antonucci JM, O'Donnell JNR, Skrtic D. Polymerization shrinkage stress development and mechanical strength of ACP acrylic resin composites. Polym Mater: Sci and Eng. 2007;96:229–231. [Google Scholar]
- 17.Antonucci JM, Icenogle TB, Regnault WF, Liu DW, O’Donnell JNR, Skrtic D. Polymerization shrinkage and stress development in bioactive urethane acrylic resin composites. Polymer Preprints. 2006;47(1):498–499. doi: 10.1016/j.polymer.2005.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wachtman JB, Capps W, Mandel J. Biaxial flexure tests of ceramic substrates. J Matls. 1972;7:188–194. [Google Scholar]
- 19.Kirstein AF, Woolley RM. Symmetrical bending of thin circular elastic plates on equally spaced point supports. J Res Natl Bur Stands. 1967;71C:1–10. [Google Scholar]
- 20.Ban S, Anusavice KJ. Influence of test method on failure stress of brittle dental material. J Dent Res. 1990;69:1791–1799. doi: 10.1177/00220345900690120201. [DOI] [PubMed] [Google Scholar]
- 21.O'Donnell JNR, Antonucci JM, Skrtic D. Amorphous calcium phosphate composites with improved mechanical properties. J Bioact Compat Polym. 2006;21(3):169–184. doi: 10.1177/0883911506064476. [DOI] [PMC free article] [PubMed] [Google Scholar]