Abstract
Background
Isoflurane causes long-term hippocampal-dependent learning deficits in rats despite limited isoflurane-induced hippocampal cell death, raising questions about the causality between isoflurane-induced cell death and isoflurane-induced cognitive function. Neurogenesis in the dentate gyrus is required for hippocampal-dependent learning and thus constitutes a potential alternative mechanism by which cognition can be altered after neonatal anesthesia. We tested the hypothesis that isoflurane alters proliferation and differentiation of hippocampal neural progenitor cells.
Methods
Multipotent neural progenitor cells were isolated from pooled rat hippocampi (postnatal day 2) and grown in culture. These cells were exposed to isoflurane and evaluated for cell death using lactate dehydrogenase release, caspase activity and immunocytochemistry for nuclear localization of cleaved caspase 3. Growth was assessed by cell counting and BrdU incorporation. Expression of markers of stemness (Sox2) and cell division (Ki67) were determined by quantitative polymerase chain reaction. Cell fate selection was assessed using immunocytochemistry to stain for neuronal and glial markers.
Results
Isoflurane did not change lactate dehydrogenase release, activity of caspase 3/7, or the amount of nuclear cleaved caspase 3. Isoflurane decreased caspase 9 activity, inhibited proliferation and decreased the proportion of cells in s-phase. mRNA expression of Sox2 (stem cells) and Ki67 (proliferation) were decreased. Differentiating neural progenitor cells more often select a neuronal fate after isoflurane exposure.
Conclusions
We conclude that isoflurane does not cause cell death, but does act directly on neural progenitor cells, independent of effects on the surrounding brain, to decrease proliferation and increase neuronal fate selection. These changes could adversely affect cognition after isoflurane anesthesia.
INTRODUCTION
Over the past 5 years a wide variety of anesthetic agents used alone or in combination in neonatal rodents have been reported to cause cell death and cognitive dysfunction (reviewed in1). Isoflurane alone has been shown to produce both cell death and long term cognitive deficits when given to neonatal rodents2–5. The cognitive deficits that have been reported after anesthetic exposure are largely hippocampal in origin despite modest or no cell death in the hippocampus compared to other brain structures3,4,6,7.
Neurogenesis in the hippocampal dentate gyrus (DG) is required for certain types of learning and memory and inhibiting it by genetic manipulation or radiation leads to specific hippocampal cognitive deficits8–12 similar to those seen in postnatal day 7 rats following exposure to Isoflurane4. We have recently reported a decrease in proliferation of precursors in the DG of both adult and postnatal day 7 rats after exposure to isoflurane4, however it is unknown if this effect is mediated by isoflurane acting directly on precursor cells or by its action on the surrounding brain where it inhibits neural signaling in neonates and adults and causes cell death in neonates.
Gamma-aminobutyric acid type A (GABAA) and N-methyl-D-aspartate receptors provide important cues for proliferation and differentiation of neural progenitor or stem cells in the developing and adult brain13–16 and isoflurane acts at both of these receptors. Besides acting to decrease signaling of nearby established neurons, isoflurane could act directly on precursor cells to alter their growth and differentiation. We propose that a direct effect of isoflurane on precursor cells could be an alternative or additional explanation for the cognitive deficits observed in rodents.
In this study, we hypothesize that isoflurane acts on neural progenitor cells (NPCs) to decrease proliferation independent of its effects (cell death and decreased neural transmission) on the surrounding brain. To test this hypothesis NPCs isolated from rat postnatal day 2 hippocampus were grown in culture and exposed to isoflurane. In the following experiments we demonstrate that isoflurane is not toxic to NPCs and acts independently of surrounding brain to induce cell cycle exit and increase neuronal fate selection.
MATERIALS AND METHODS
Hippocampal precursor cell isolation and culture
All animals were cared for following procedures approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco. NPCs were isolated following methods previously described with slight modification17–20. Un-anesthetized postnatal day 2 Sprague Dawley rats were separated from the dam and decapitated using a guillotine. Hippocampi were immediately dissected out and placed in 10mL ice cold Hanks Basic Salt Solution without calcium. Whole hippocampi pooled from 10 animals were collected by centrifugation for 2 minutes at 300rcf (relative centrifugal force). Supernatant was removed and hippocampi were minced with a razor blade then triturated 5 times using a 200 microliter pipette. Cells were then re-suspended in 10mL Hanks Basic Salt Solution for 2 minutes. After settling for 2 minutes the supernatant was transferred to a new tube. Cells were then collected by centrifugation at 300rcf for 5 minutes. Supernatant was discarded and cells were re-suspended in proliferation medium consisting of 3:1 Dubelco’s Modified Eagles Medium: Ham’s F12 (UCSF cell culture facility), 1% penicillin and streptomycin, 1× B-27 supplement (Invitrogen, Grand Isle, NY), 20ng/mL basic fibroblast growth factor (Chemicon, Temecula, CA), 0.75units heparin/mL (Abraxis, Schaumburg, Il). Hippocampal precursor cells were then grown in 5% carbon dioxide in air, at 37°C with media changed 3× per weak and cells transferred to new flasks every 5 days so that adherent differentiated cells were left behind and non-adherent proliferating NPCs were moved to the new flask. NPCs had been grown in culture for 2 to 12 weeks at the time of use. To differentiate NPCs, basic fibroblast growth factor containing medium was replaced with differentiation medium consisting of Neurobasal-A (Invitrogen, Grand Isle NY), B27 supplement, 1% penicillin-streptomycin (UCSF cell culture facility, San Francisco, CA), L-glutamine (Invitrogen, Carlsbad CA), and 5% fetal bovine serum (UCSF cell culture facility, San Francisco, CA) on day one. The next day medium was replaced with differentiation medium lacking serum.
Immunocytochemistry
Cells were plated at a density of 20,000 per well on 8-chamber microscope slides that were pre-coated with poly-L-ornithine (Sigma-Aldrich, St. Louis, MO) in water overnight and then with laminin (Sigma-Aldrich, St. Louis, MO) in phosphate-buffered saline, pH7.4 (PBS) overnight. At the end of the experiment the slides were fixed with 4% para-formaldehyde (Sigma-Aldrich, St. Louis, MO in PBS for 15 minutes at room temperature and blocked with 10% goat serum and 0.03% triton X-100 in PBS for 2 hours at room temperature. Primary antibodies were diluted in PBS and added for overnight incubation at 4°C. Secondary antibodies were diluted 1:1000 in PBS and added for 2 hours at room temperature. Nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI) for 10 minutes at room temperature. Slides were washed with PBS and coverslips applied with Aquapolymount (Polysciences, Warring, PA). Table 1 gives a summary of the antibodies used.
Table 1.
List of antibody sources and dilutions.
| Antibody | Name | Company | Dilution |
|---|---|---|---|
| BrdU | SC56258 | Santa Cruz (Santa Cruz, CA) | 1/75 |
| Tuj-1 | MMS-435P | Covance (Billerica, MA) | 1/200 |
| GFAP | AB5804 | Chemicon (Emeryville, CA) | 1/1500 |
| Map2 | MAB3418 | Chemicon | 1/200 |
| Sox2 | 2003600 | Chemicon | 1/500 |
| Nestin | MAB353 | Chemicon | 1/200 |
| Goat anti mouse IgG | Alexa 488 | Invitrogen (Grand Isle, NY) | 1/1000 |
| Goat anti rabbit IgG | Alexa 594 | Invitrogen | 1/1000 |
Isoflurane exposure
NPCs in slides or flasks were placed in a Billups-Rothenburg chamber (Billups-Rothenburg, Del Mar, CA). The chamber was flushed with isoflurane in humidified air with 5% carbon dioxied at 37°C for 15 minutes while monitoring the temperature and concentration of gases inside the chamber. The chamber was then sealed and placed inside a 37°C incubator. The concentrations of oxygen, isoflurane and carbon dioxide in the chamber were monitored using a Datex Capnomac Ultima gas analyzer (Datex Ohmeda, Helsinki Finland) at 2-hour intervals and the chamber re-flushed, if there was any change. Control experiments were performed in the same manner except no isoflurane was added when flushing the chamber. 3.4% isoflurane was chosen for these experiments based on our experience anesthetizing postnatal day 7 animals. Using the method of Merkel & Eger21 we found the inspired minimum alveolar concentration of isoflurane to be 3.6–4% initially, with a slow decline thereafter4.
Cell Death/Necrosis
NPCs were grown as neurospheres in suspension as described above and plated in 96-well plates in proliferation medium. The next day, the cells were exposed to 3.4% isoflurane for 4 hours as described above (isoflurane exposure) and returned to standard incubator growth conditions for 18 hours. NPCs were then analyzed for release of lactate dehydrogenase (LDH) using the Cytox-96 assay kit (Promega, Madison, WI) and following the manufacturers protocol. Treatment with triton X-100 (0.9% volume/volume, provided by manufacturer) for 45 minutes at 37°C was used as a positive control.
Caspase activation
1×104 NPCs were plated in proliferation medium and grown as neurospheres in suspension in opaque 96-well plates. The following day plates were exposed to 3.4% isoflurane, 500ng/mL fentanyl citrate (0.95μM), or control for 4 hours, followed by standard incubator conditions for 2 hours for caspase 3/7 or as indicated in the figure for caspase 9. Fentanyl has previously been shown to activate opiod receptors on cultured differentiated neurons at concentrations of 0.1 to 2.0 μM22–24 and did not induce caspase activation in the brain when used as a sole anesthetic25. Fentanyl was used as a control treatment because it does not interact with GABAA or N-methyl-D-aspartate receptors and did not cause cell death in vivo but is commonly used for clinical anesthesia. Cells were then lysed and analyzed for caspase 3/7 or caspase 9 activity (Promega, Caspase-Glo 3/7 or Glo 9 kit, Promega, Madison, WI). Nuclear cleaved caspase 3 was quantified using immunocytochemistry methods described above and performed on NPCs plated in 8-chamber glass slides coated with poly-L-ornithine and laminin. The total (DAPI labeled) and cleaved caspase 3 (immunofluorescence labeled) nuclei were counted in multiple fields of view to determine the fraction of nuclei positive for cleaved caspase 3.
Growth
After triturating to a single cell suspension equal numbers of NPCs were plated into 6- or 12-well plates in proliferation medium and grown floating in culture. The next day cultures were exposed to 3.4% isoflurane, or control for 6 hours before returning to the incubator for growth under standard conditions. Forty-eight (48) hours later the contents of each well were collected by centrifugation, triturated to a single cell suspension and then re-suspended in 500 μL medium before counting the cells by trypan blue exclusion using a hemocytometer (VWR, Batavia, IL).
Quantitative polymerase chain reaction
0.5–1×105 NPCs grown as neurospheres in suspension in T-25 flasks(Corning supplied by Fisher, Pittsburg, PA) were exposed to isoflurane for 4 hours. At specified times cells were collected by centrifugation and RNA was isolated using Trizol (Invitrogen, Carlsbad, CA), following the manufacturer’s directions 6, 12 and 24 hours after treatment. 1 microgram of RNA was reverse transcribed to make cDNA using the Quigen Omniscript kit (Quiagen, Valencia, CA) following manufactures instructions and using a mix of poly d-thymidine and random hexamers. Quantitative polymerase chain reaction was performed using Quiagen Quantitect SYBR Green kit (Quiagen, Valencia, CA) on an Stratagene MX 3000P thermocycler (Agilent Technologies, La Jolla, CA). Changes in gene expression were determined using the delta-delta cT method26. ®-actin was used as a reference gene for loading control and expression of the gene of interest was defined relative to untreated cultures, which were arbitrarily defined as 100%. Primer sequences for target genes are listed in table 2.
Table 2.
Primers used for quantitative polymerase chain reaction.
| Gene | Primer Sequence |
|---|---|
| Sox2 | CTCTGCACATGAAGGAGCAC |
| CTCCGGGAAGCGTGTACTTA | |
| Ki-67 | CGATCGCAGAAACCTAGGAG |
| GGCTCTGTGCAGGAGAAGAC | |
| Actin | ACAGCTGAGAGGGAAATCGT |
| TTCTCCAGGGAGGAAAGAGG |
5-bromodeoxyuridine (BrdU) incorporation
NPCs were plated in coated 8-chamber microscope slides in proliferation medium. The next morning NPCs were exposed to isoflurane as described above (isoflurane exposure). BrdU, a thymidine analog, was added to the medium for 2 hours at the end of the isoflurane exposure or 12 hours later. Slides were fixed and stained as described above then photographed with 40× or 100× objectives on a E400 (Nikon, Melville, NY) fluorescence microscope equipped with filters at 385, 490, and 570 nM. Six to twelve sets of images were acquired at different locations and were subsequently merged using NIH Image J software (NIH, Bethesda, MD), and the total number of cells (DAPI) as well as the number of BrdU-positive cells was determined.
Differentiation
NPCs growing in suspension in proliferation medium were collected, switched to differentiation medium and plated on coated 8-chamber microscope slides. The slides were immediately exposed to isoflurane as described above under isoflurane exposure, then returned to standard incubator conditions for 4 days. Media was replaced on day 2 with differentiation medium lacking serum. Slides were fixed and stained as described above (immunocytochemistry) then photographed with 40× or 100× objectives on a Nikon E400 fluorescence microscope with filters at 385, 490, and 570 nM. Six to twelve sets of images were acquired at random locations and were subsequently merged using NIH Image J software. The total number of cells (DAPI) and the ratio of cells positive for neuron specific beta 3 tubulin (Tuj1) to mark neurons or glial fibrillary acidic protein (GFAP) to mark astrocytes was determined.
Statistical Analysis
All statistical analyses were performed and all graphs produced using Prism 4 (GraphPad Software, San Diego, California). Data were expressed as mean ± standard error, and analyzed using parametric test except in case where assumptions required for parametric analyses (homogeneity of variance, normality) were violated, when data were expressed as medians ± interquartile ranges and analyzed using nonparametric tests. Caspase 3/7 and 9 enzyme activity was evaluated by analysis of variance (ANOVA), and Bonferroni post test to correct for multiple comparisons. BrdU incorporation immediately after isoflurane exposure was analyzed by Kruskal-Wallis with Dunn’s post test. Nuclear localization of cleaved caspase 3, BrdU incorporation at 12 hours, and expression of Tuj1 or GFAP by differentiated NPCs were analyzed by Mann Whitney U test. Growth experiments were analyzed by Wilcoxon signed rank test with fold increases from control values being compared to the theoretical value of 1, assigned to the control group. Subsequent comparison of fold increase in untreated vs isoflurane treated cultures was done with Wilcoxon matched pairs test. Changes in gene expression in quantitative polymerase chain reaction experiments were analyzed by ANOVA with Dunnett’s post test to compare all time points to control untreated cultures.
RESULTS
Hippocampal neural progenitor cells grown in culture
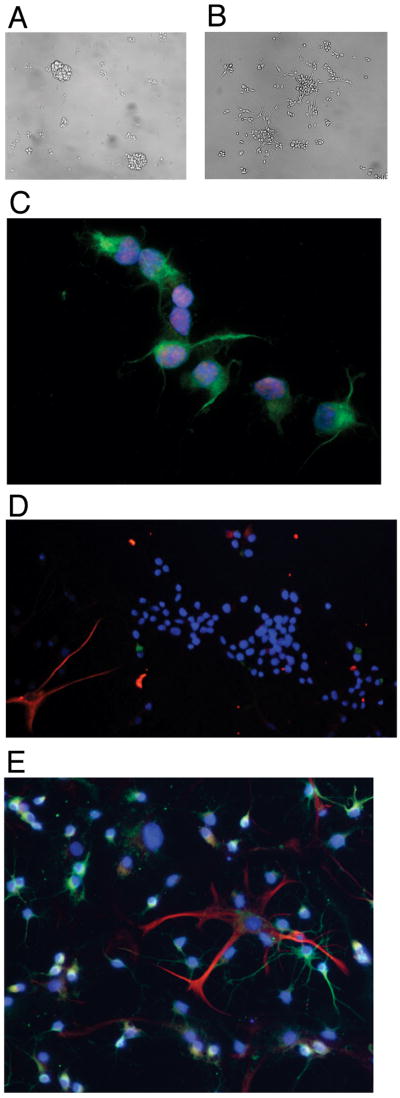
Two to four days after isolation, NPCs in proliferation medium begin to grown in characteristic neurospheres as has been described previously17–20,27,28 (fig. 1A). The same NPCs plated on slides with poly-L-ornithine and laminin become adherent and put out processes (fig. 1B), however they remain largely undifferentiated as evidenced by expression of stem cell markers nestin and Sox2 (fig. 1C) but little expression of neuronal marker Tuj1 or the astrocyte marker GFAP (fig. 1D). After 4 days in differentiation medium cells positive for GFAP (fig. 1E, red) and Tuj1 (fig. 1E, green) were observed confirming that the precursor cells had been multi-potent prior to differentiation.
Figure 1.
Hippocampal neural progenitor cells (NPCs) grown in culture are multi-potent. A) NPCs grown in culture flasks in proliferation form floating neurospheres, but become adherent when grown in poly-L-ornithine and laminin coated glass slides (B). C) NPCs grown in 8-chamber slides in proliferation media express Sox2 (red) and nestin (green). 4′,6-diamidino-2-phenylindole (DAPI) marks the DNA, or nuclei (blue). D) NPCs grown on coated slides in proliferation media for 24 hours express very little glial fibrillary acidic protein (GFAP, red) or neuron-specific class III beta tubulin (Tuj1, green). E) NPCs grown on coated slides in differentiation media for 4 days after basic fibroblast growth factor withdrawal and addition of serum show Tuj1 (green) staining neurons, and GFAP (red) positive astrocytes. DAPI stains all nuclei (blue).
Isoflurane does not cause caspase activation or LDH release
The activity of the apoptotic proteins caspase 3/7 and caspase 9 was determined (fig 2). While 1μM staurosporine increased caspase 3/7 activity, neither 4 hours of 3.4% isoflurane nor 4 hours of 500ng/mL fentanyl citrate changed the activity of caspase 3/7 2 hours after exposure (n=3, ANOVA P=0.0005, Bonferroni’s post test P>0.05 for control vs isoflurane and control vs fentany, fig. 2A). Similarly, when cells were grown adherently on glass slides, no increase was seen in nuclear activated caspase 3 staining 2 hours after a 4-hour isoflurane exposure (control n=3, isoflurane n=4 staurosporine n=2, Mann Whitney U P>0.05, fig. 2C). 1μM staurosporine did induce nuclear localization of cleaved caspase 3 (fig 2C and D). Caspase 9 activity was increased in the presence of 1μM staurosporine but was unchanged by fentanyl and decreased with isoflurane after 2 or 4 hours of exposure and remained decreased 2 hours after removal of cells from isoflurane and return of cultures to standard incubator conditions. Caspase 9 activity was increased in the presence of 1μM staurosporine but was unchanged by fentanyl and decreased after 2 or 4 hours isoflurane or 4 hours isoflurane + 2 hours standard incubator conditions (n=3, except staurosporine n=5, ANOVA P<0.0001, Bonferroni post test P<0.001 control vs any isoflurane group, P<0.05 control vs staurosporine, and P>0.05 control vs fentanyl, fig. 2B).
Figure 2. No caspase activation or lactate dehydrogenase release following isoflurane treatment.
NPCs grown in proliferative conditions were exposed to isoflurane (Iso) and then lysed to determine the activity of caspase enzymes, fixed to identify nuclear translocation of cleaved caspase 3, or media tested for lactate dehydrogenase release. A) 1μM staurosporine (positive control) increased activity of caspase 3/7 more than 3 fold, but 4 hours of 3.4% isoflurane or 6 hours of 500 ng/mL fentanyl citrate had no effect (Bonferroni post test ** P<0.01). B) Immediately after 2 or 4 hours exposure to 3.4% isoflurane (Iso 2hr, Iso 4hr) the baseline activity of caspase 9 decreased. Caspase 9 activity remained decreased 2 hours after removal from isoflurane following a 4 hour exposure (Iso 4hr + 2). Fentanyl citrate (500ng/mL) had no effect on activity of caspase 9 (Bonferroni post test * P<0.05, *** P<0.001). C) 1μM staurosporine dramatically increased the number of cells that were positive for nuclear cleaved caspase 3 while isoflurane did not. D) Following addition of 1μM staurosporine all nuclei stained with DAPI are blue and cleaved caspase 3 positive nuclei are red. E) To determine if non-apoptotic cell death occurred NPCs were exposed to isoflurane for 4 hours and the media assayed for lactate dehydrogenase activity. No difference was seen between control and isoflurane exposed groups (t-test P=0.80). Triton X-100 (0.9% v/v) for 45 minutes at 37°C was used as a positive control.
To determine if non-apoptotic cell death occurred NPCs were exposed to isoflurane for 4 hours and the amount of LDH released into the media was determined. No difference was seen between control and isoflurane exposed groups but addition of detergent (0.9% Triton X-100) to the medium did increase LDH release (n=8, isoflurane vs control t-test P=0.80, fig. 2E).
Isoflurane inhibits proliferation
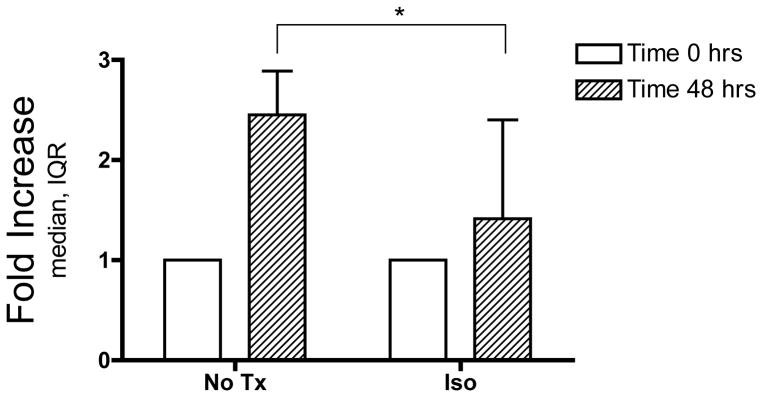
NPCs were plated in 6 or 12 well plates, grown in non-adherent proliferative conditions, and exposed to 3.4% isoflurane for 6 hours. Both untreated and isoflurane-treated cultures proliferated relative to time 0 (Wilcoxon signed rank test relative to theoretical value of 1 at time 0, control P<0.01, isoflurane P<0.05,). However, the fold increase in the number of cells 48 hours later was smaller in isoflurane-treated than in untreated cultures (n=9, Wilcoxon matched pairs t-test: P<0.05, fig. 3).
Figure 3. Isoflurane inhibits proliferation.
Neural progenitor cells were plated in equal numbers and exposed to 3.4% isoflurane for 6 hours. Fold increase relative to control cultures was determined 48 hours later. All cultures showed growth over 48 hours, but isoflurane exposure inhibited proliferation by 42% relative to untreated cultures (Wilcoxon matched pairs t-test * P<0.05, IQR=interquartile range).
Isoflurane decreases expression of Ki67 and Sox2 mRNA
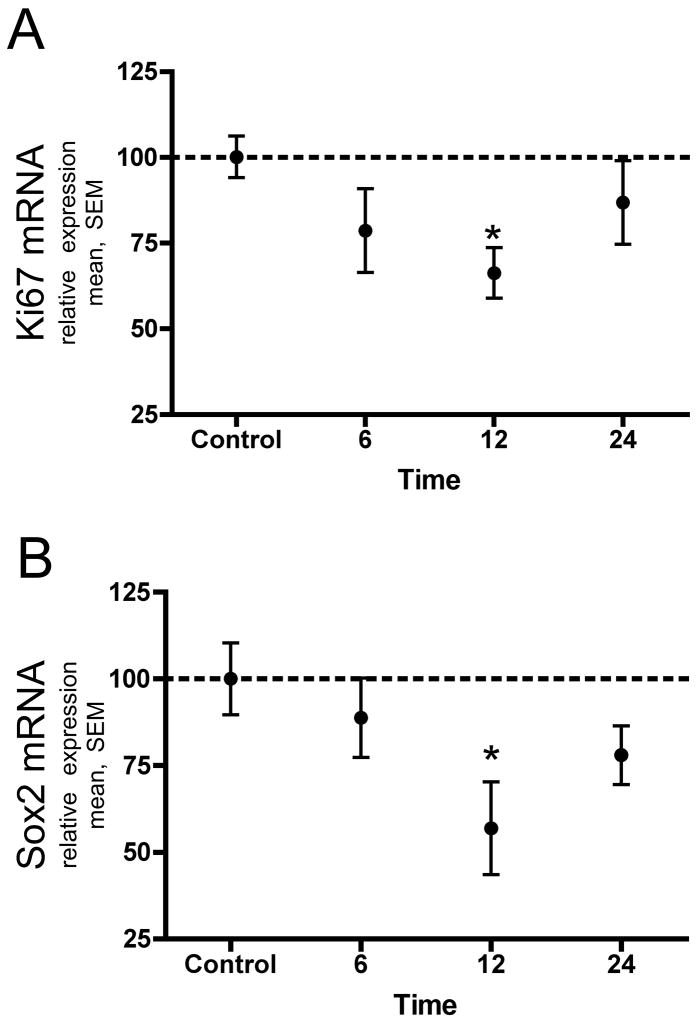
NPCs grown in proliferative conditions were exposed to 3.4% isoflurane for 4 hours and expression of the cell cycle regulator Ki-67 and the stem cell marker Sox2 was evaluated 6, 12 and 24 hours later using quantitative polymerase chain reaction. Both Ki-67 and Sox2 messenger RNA were decreased maximally at 12 hours (34 and 45% lower) and began to return to baseline by 24 hours (Ki-67: n=6, ANOVA P>0.05 Dunnett’s post test 12 hours P<0.05, fig. 4A; Sox2: n=5, ANOVA P<0.05, Dunnett’s post test 12 hours P<0.05, fig. 4B).
Figure 4. Isoflurane decreases expression of Ki67 and Sox2 mRNA.
Quantitative polymerase chain reaction 6, 12 and 24 hours after exposure to 3.4% isoflurane for 4 hours shows a decrease in the amount of the cell cycle gene Ki-67 and the stem cell gene Sox2 that is maximal at 12 hours and begins to return to baseline by 24 hours. The amount of mRNA for each time-point is expressed relative to control cultures which were arbitrarily assigned a value of 100%. A) Ki67 decreased by 34% from baseline at 12 hours (Dunnett’s post test * P<0.05). B) Sox2 decreased by 43% from baseline at 12 hours (Dunnett’s post test * P<0.05).
Isoflurane decreased the number of cells in S-phase
To determine if the isoflurane-mediated decrease in proliferation was due to a change in cell cycle, progenitors were exposed to 4 hours of 3.4% isoflurane followed immediately by 2 hours of BrdU. Control cultures showed a median of 21.5% of cells incorporated BrdU compared to only 13.1% of isoflurane-exposed cells (Kruskal-Wallis P=0.029, Dunn’s post test P<0.05, control vs isoflurane, fig. 5A). 500ng/mL fentanyl citrate given for 6 hours did not change BrdU incorporation (fig. 5A). To assess the duration of the effect BrdU was added 12 hours after completion of the isoflurane treatment leading to a median 18.6% BrdU positive cells in the isoflurane group compared to 23.3% in control (Mann-Whitney P<0.05, fig. 5B).
Figure 5. Decreased number of cells in S-Phase.
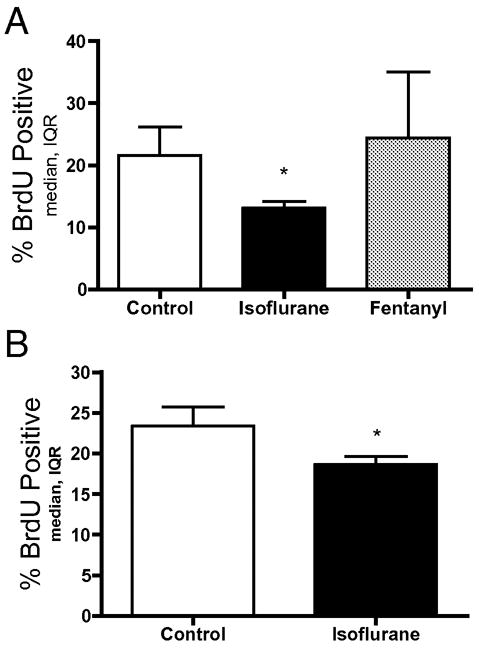
To determine whether isoflurane exposure changes the cell cycle, neural precursor cells were exposed to isoflurane for 4 hours then removed and BrdU was added to the media for 2 hours either immediately or 12 hours later. A) 3.4% isoflurane for 4 hours, but not 500 ng/mL fentanyl citrate decreased the number of cells that took up BrdU during the subsequent 2 hours (Dunn’s post test * P<0.05). B) The inhibitory effect of isoflurane on cells taking up BrdU persists until at least 12 hours after isoflurane exposure (Mann-Whitney * P<0.05)(IQR=interquartile range).
Isoflurane increased neuronal fate selection
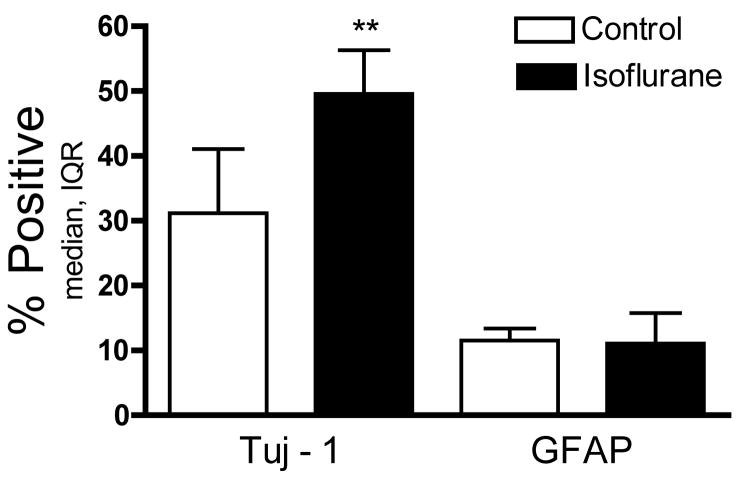
We next evaluated the effect of isoflurane on fate selection and differentiation by exposing NPCs to isoflurane for 4 hours at the time of plating in differentiation medium. Cells were fixed 4 days later and stained for Tuj1 (neurons), GFAP (astrocytes), and DAPI (all nuclei). NPCs exposed to isoflurane were more likely to express a neuronal marker 4 days later (n=6, P<0.01, Mann Whitney U, fig. 6). No difference was seen in the rate of expression of the astrocyte marker GFAP (n=6, P=0.94, Mann Whitney U, fig. 6).
Figure 6. Isoflurane increased neuronal fate selection.
Proliferating neural precursor cells were plated on coated glass slides in differentiation media and immediately exposed to isoflurane for 4 hours. The percentage of cells positive for neuron-specific class III beta tubulin (Tuj1) 4 days later was increased in isoflurane exposed cultures but no difference was seen in glial fibrillary acidic protein (GFAP) positive cells (Mann-Whitney ** P<0.01, IQR=interquartile range).
DISCUSSION
Multiple anesthetics have previously been reported to lead to caspase activation and neuronal cell death throughout the brain of postnatal day 7 animals1. We report in an accompanying article in this issue that isoflurane decreases NPC proliferation (BrdU uptake) in vivo in adults and neonates4. This could be due to isoflurane mediated decreases in neuronal firing in neonatal and adult animals29 or to direct toxicity in neonates where cell death has been observed3. Here we have attempted to determine whether this effect is independent of the actions of isoflurane on the surrounding brain by growing NPCs in an in vitro environment. Cultures of hippocampal precursor cells were isolated from postnatal day 2 rats in order to maximize the contribution of cells from the DG and minimize the contribution from the sub-ventricular zone and other brain regions. Embryonic animals are often used for NPC cultures, however in rats the hippocampus is poorly defined or absent until birth making isolation nearly impossible. We are most interested in NPCs specifically from the dentate in order to correlate with in vivo studies of this region and therefore obtained our cultures from postnatal day 2 animals.
Translating anesthetic studies from animals (let alone cell culture) to humans is at least flawed if not impossible. In order to minimize differences between and in vitro and in vivo models of anesthesia a comparable dose must be used. One can use an equal dose such as 1% isoflurane that is not clinically equivalent across species or one can choose to measure a clinical endpoint such as minimum alveolar concentration and use a dose that produces an equivalent endpoint between species. We have chosen to base our in vivo studies on minimum alveolar concentration and therefore use the same dose for the in vitro studies presented here. Fentanyl was used as a control in these studies because both GABAA and N-methyl-D-aspartate receptors mediate signals that are known to influence proliferation and differentiation of NPCs13–16 and most anesthetic drugs act at one or both of these receptors29. Opiates do not act at GABAA or N-methyl-D-aspartate receptors and fentanyl is a commonly used opiate and was chosen as a control for that reason.
In the experiments presented here, with isoflurane as the sole anesthetic, we were unable to induce cell death, caspase activation, or LDH release in cultured NPCs. This suggests that NPCs do not undergo apoptosis or necrosis following isoflurane exposure, consistent with limited cell death being reported in the DG of the hippocampus (the source of these cultured cells) compared with other regions of the brain3. Decreased proliferation 48 hours after isoflurane exposure combined with a decrease in the number of cells in S-phase and loss of the cell cycle regulator Ki-67 suggests the cells are either in growth phase arrest or have exited the cell cycle as would occur with differentiation. Cell cycle exit and differentiation is further suggested by the loss of the stem cell gene Sox2, and increased neuronal fate selection.
Culturing cells in different states can change the characteristics of those cells and how they respond to different signals or stresses and represents a potential limitation of this study. These experiments were performed on cells in two different states: floating as neurospheres or adherent to glass slides but undifferentiated. The results we observed, however, are similar in both cases. Growth inhibition, loss of Ki-67, and lack of caspase activation in floating neurospheres and decreased ratio of cells in S-phase and lack of nuclear cleaved caspase 3 in adherent cells, are all consistent with a lack of cell death and a decrease in proliferation. Similar effects on proliferation in vivo are also reported in this issue4.
Isoflurane facilitates opening of the GABAA receptor, and has previously been shown to raise the intracellular calcium concentration in neurons and slice cultures by liberating it from endoplasmic reticulum30–33. GABAA receptor opening, and increased intracellular calcium decrease proliferation of precursor cells in sub ventricular zone13,34 and increase differentiation and selection of a neuronal fate in hippocampal progenitors of the DG14,16,35. Isoflurane may lead to cell cycle exit and differentiation of hippocampal NPCs by facilitating GABAA receptor opening and increasing intracellular calcium. Future studies are necessary to determine the exact mechanism of isoflurane mediated growth inhibition and increased neuronal differentiation we have observed in NPCs, but GABAA receptor activation and changes in calcium concentration are likely targets.
Decreased proliferation and increased neuronal differentiation caused by isoflurane could lead to cognitive dysfunction in neonates by permanently disrupting the architecture of the hippocampus during a critical period of development or by depleting the pool of precursor cells present for the duration of the animals life. The same mechanism in an adult might not cause any cognitive dysfunction when the hippocampus is already fully developed and connected and can more easily accept an increased number of cells choosing to become neurons at any given time. The adult hippocampus routinely integrates new neurons into its circuitry with learning10,36.
Elsewhere in this issue we report isoflurane-mediated changes in NPC proliferation in vivo4 and the present experiments demonstrate an effect of isoflurane on hippocampal neural precursor cells grown in culture, isolated from the DG demonstrating that this effect is on the cells themselves rather than being mediated by the surrounding tissue of the neonatal brain or specifically the DG. In addition to suggesting a possible mechanism for isoflurane-induced hippocampal dysfunction, the role of anesthesia in the field of neural stem cells and neural stem cell transplantation remains largely unexplored. The results reported here show that isoflurane may have a direct impact on the proliferation and fate selection of transplanted stem cells. Future studies on the effect of volatile and non-volatile anesthetics on NPCs are important for our understanding of how these drugs effect the biology of NPCs and eventually for choosing the appropriate anesthetic for trials of stem cell transplantation in animals and someday humans.
This model system can be used to efficiently screen many anesthetics that have been previously reported to cause neurodegeneration to determine if they have an effect on NPC proliferation and differentiation. This system may also be used to address questions of mechanism that are difficult to answer with in vivo studies. Understanding how anesthetics interact with the complex system of neurogenesis in the neonatal and adult brain may provide some insight into normal development and will guide our choice of drugs for future studies related to both anesthetic mediated cognitive dysfunction and transplantation of NPCs.
Acknowledgments
Foundation for Anesthesia Education and Research, 200 1st street SW, Rochester Minnesota USA
Anesthesia Patient Safety Foundation, 8007 South Meridian Street, Indianapolis Indiana USA
Footnotes
Portions of this publication were previously presented at:
Society of Neuro-Surgical Anesthesia and Critical Care 34th Annual Meeting, October 13, 2006, Atlanta, GA
Society for Neuroscience, 36th Annual Meeting, October 14–18, 2006, Chicago, IL
Western Anesthesia Residents Conference, May 4–6, 2007, Sacramento, CA
America Society of Anesthesiologist 2008 Annual Meeting, October 18–22, 2008, Orlando, FL
Society for Neuroscience 38th Annual Meeting, November 15–19, 2008, Washington, DC
References
- 1.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681–707. doi: 10.1213/ane.0b013e318167ad77. [DOI] [PubMed] [Google Scholar]
- 2.Ma D, Williamson P, Januszewski A, Nogaro MC, Hossain M, Ong LP, Shu Y, Franks NP, Maze M. Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology. 2007;106:746–53. doi: 10.1097/01.anes.0000264762.48920.80. [DOI] [PubMed] [Google Scholar]
- 3.Stratmann G, May LV, Sall JW, Alvi RS, Bell JS, Ormerod BK, Rau V, Hilton JF, Dai R, Lee MT, Visrodia KH, Ku B, Zusmer EJ, Guggenheim J, Firouzian A. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day old rats. Anesthesiology. 2009;110:xxx–xxx. doi: 10.1097/ALN.0b013e31819c7140. [DOI] [PubMed] [Google Scholar]
- 4.Stratmann G, Sall JW, May LV, Bell JS, Magnusson KR, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day old and 7-day old rats. Anesthesiology. 2009;110:xxx–xxx. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 5.Johnson SA, Young C, Olney JW. Isoflurane-induced neuroapoptosis in the developing brain of nonhypoglycemic mice. J Neurosurg Anesthesiol. 2008;20:21–8. doi: 10.1097/ANA.0b013e3181271850. [DOI] [PubMed] [Google Scholar]
- 6.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredriksson A, Ponten E, Gordh T, Eriksson P. Neonatal Exposure to a Combination of N-Methyl-d-aspartate and gamma-Aminobutyric Acid Type A Receptor Anesthetic Agents Potentiates Apoptotic Neurodegeneration and Persistent Behavioral Deficits. Anesthesiology. 2007;107:427–36. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- 8.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–7. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 9.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–60. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 10.Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–42. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 12.Czurko A, Czeh B, Seress L, Nadel L, Bures J. Severe spatial navigation deficit in the Morris water maze after single high dose of neonatal x-ray irradiation in the rat. Proc Natl Acad Sci U S A. 1997;94:2766–71. doi: 10.1073/pnas.94.6.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–98. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 14.Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–32. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- 15.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–15. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Aberg MA, Aberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, Bang P, Rosengren LE, Olsson T, Gage FH, Eriksson PS. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci. 2003;24:23–40. doi: 10.1016/s1044-7431(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 18.Ray J, Raymon HK, Gage FH. Generation and culturing of precursor cells and neuroblasts from embryonic and adult central nervous system. Methods Enzymol. 1995;254:20–37. doi: 10.1016/0076-6879(95)54004-0. [DOI] [PubMed] [Google Scholar]
- 19.Palmer TD, Ray J, Gage FH. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;6:474–86. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 20.Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995;18:159–92. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- 21.Merkel G, Eger EI., 2nd A comparative study of halothane and halopropane anesthesia including method for determining equipotency. Anesthesiology. 1963;24:346–57. doi: 10.1097/00000542-196305000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Vansteensel MJ, Magnone MC, van Oosterhout F, Baeriswyl S, Albrecht U, Albus H, Dahan A, Meijer JH. The opioid fentanyl affects light input, electrical activity and Per gene expression in the hamster suprachiasmatic nuclei. Eur J Neurosci. 2005;21:2958–66. doi: 10.1111/j.1460-9568.2005.04131.x. [DOI] [PubMed] [Google Scholar]
- 23.McDowell TS. Exogenous nerve growth factor attenuates opioid-induced inhibition of voltage-activated Ba2+ currents in rat sensory neurons. Neuroscience. 2004;125:1029–37. doi: 10.1016/j.neuroscience.2004.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001;21:7598–607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzi S, Carter LB, Ori C, Jevtovic-Todorovic V. Clinical anesthesia causes permanent damage to the fetal guinea pig brain. Brain Pathol. 2008;18:198–210. doi: 10.1111/j.1750-3639.2007.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Shetty AK, Turner DA. In vitro survival and differentiation of neurons derived from epidermal growth factor-responsive postnatal hippocampal stem cells: inducing effects of brain-derived neurotrophic factor. J Neurobiol. 1998;35:395–425. doi: 10.1002/(sici)1097-4695(19980615)35:4<395::aid-neu7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 28.Campos LS. Neurospheres: insights into neural stem cell biology. J Neurosci Res. 2004;78:761–9. doi: 10.1002/jnr.20333. [DOI] [PubMed] [Google Scholar]
- 29.Sonner JM, Antognini JF, Dutton RC, Flood P, Gray AT, Harris RA, Homanics GE, Kendig J, Orser B, Raines DE, Trudell J, Vissel B, Eger EI., II Inhaled Anesthetics and Immobility: Mechanisms, Mysteries, and Minimum Alveolar Anesthetic Concentration. Anesth Analg. 2003;97:718–740. doi: 10.1213/01.ANE.0000081063.76651.33. [DOI] [PubMed] [Google Scholar]
- 30.Kindler CH, Eilers H, Donohoe P, Ozer S, Bickler PE. Volatile anesthetics increase intracellular calcium in cerebrocortical and hippocampal neurons. Anesthesiology. 1999;90:1137–45. doi: 10.1097/00000542-199904000-00029. [DOI] [PubMed] [Google Scholar]
- 31.Bickler PE, Fahlman CS. The inhaled anesthetic, isoflurane, enhances Ca2+-dependent survival signaling in cortical neurons and modulates MAP kinases, apoptosis proteins and transcription factors during hypoxia. Anesth Analg. 2006;103:419–29. doi: 10.1213/01.ane.0000223671.49376.b2. [DOI] [PubMed] [Google Scholar]
- 32.Bickler PE, Zhan X, Fahlman CS. Isoflurane preconditions hippocampal neurons against oxygen-glucose deprivation: role of intracellular Ca2+ and mitogen-activated protein kinase signaling. Anesthesiology. 2005;103:532–9. doi: 10.1097/00000542-200509000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Gray JJ, Bickler PE, Fahlman CS, Zhan X, Schuyler JA. Isoflurane neuroprotection in hypoxic hippocampal slice cultures involves increases in intracellular Ca2+ and mitogen-activated protein kinases. Anesthesiology. 2005;102:606–15. doi: 10.1097/00000542-200503000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–87. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–52. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 36.Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–6. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]