Abstract
We have previously demonstrated that 40%–70% of elf+/− mice spontaneously develop hepatocellular cancer (HCC) within 15 months, revealing the importance of the transforming growth factor-beta (TGF-β) signaling pathway in suppressing tumorigenesis in the liver. The current study was carried out to investigate mechanisms by which embryonic liver fodrin (ELF), a crucial Smad3/4 adaptor, suppresses liver tumor formation. Histological analysis of hyperplastic liver tissues from elf+/− mice revealed abundant newly formed vascular structures, suggesting aberrant angiogenesis with loss of ELF function. In addition, elf+/− mice displayed an expansion of endothelial progenitor cells. Ectopic ELF expression in fetal bovine heart endothelial (FBHE) cells resulted in cell cycle arrest and apoptosis. Further analysis of developing yolk sacs of elf−/− mice revealed a failure of normal vasculature and significantly decreased endothelial cell differentiation with embryonic lethality. Immunohistochemical analysis of hepatocellular cancer (HCC) from the elf+/− mice revealed an abnormal angiogenic profile, suggesting the role of ELF as an angiogenic regulator in suppressing HCC. Lastly, acute small interfering RNA (siRNA) inhibition of ELF raised retinoblastoma protein (pRb) levels nearly fourfold in HepG2 cells (a hepatocellular carcinoma cell line) as well as in cow pulmonary artery endothelial (CPAE) cells, respectively. Conclusion: Taken together these results, ELF, a TGF-β adaptor and signaling molecule, functions as a critical adaptor protein in TGF-β modulation of angiogenesis as well as cell cycle progression. Loss of ELF in the liver leads the cancer formation by deregulated hepatocyte proliferation and stimulation of angiogenesis in early cancers. Our studies propose that ELF is potentially a powerful target for mimetics enhancing the TGF-β pathway tumor suppression of HCC.
Hepatocellular cancer (HCC) is one of the most common, aggressive malignancies and the third leading cause of cancer-related deaths (World Health Organization Report, 2006). Its prognosis remains extremely poor, with a 5-year survival rate of less than 5% and a rising incidence in the United States.1 Currently, the only curative therapeutic option for the early stages of HCC is surgical intervention, including hepatic resection and liver transplantation.2,3 The development of HCC is a multi-step process often beginning with cirrhosis, progressing to adenoma and dysplastic nodule formation.4 HCC is typically a hypervascular tumor, dependent on neo-angiogenesis, the formation of new blood vessels from preexisting vascular beds, to receive an adequate supply of oxygen and nutrients.5–7 Moreover, angiogenesis is a characteristic hallmark for tumor invasiveness and metastasis.8 The balance between stimulatory and inhibitory factors of angiogenesis, the so-called angiogenic switch, is often a rate-limiting step in the tumoral development, and imbalance of this process has been tightly associated with tumor development and growth.9
The transforming growth factor-beta (TGF-β) signaling pathway has been known to play an important role in cellular development, cell differentiation, proliferation, migration, and neoplasia.3,10 Frequent inactivation of the TGF-β pathway components in tumorigenesis demonstrates a powerful tumor suppressor role of the TGF-β pathway, partially through control of normal epithelial cell proliferation.11–14 TGF-β pathway members can also act as regulators of endothelial cells and vascular smooth muscle cells, as well as in the maintenance of vascular homeostasis.15,16 Knockout mice for the several components of the TGF-β signaling pathway have shown that TGF-β is indispensable for angiogenesis.15 Moreover, hereditary hemorrhagic telangiectasia, a human vascular disorder, results from mutations of TGF-β receptors, ALK1 and endoglin.16
The multifunctional effects of TGF-β in cellular actions occur through binding its receptors, TGF-β receptor II and receptor I, activation of intrinsic kinase activity, and phosphorylation and translocation of mediators, Smads, followed by TGF-β target gene activation.17,18 Embryonic liver fodrin (ELF), a β-spectrin, is a stem cell adaptor protein that has been recently found to play a pivotal role in TGF-β signaling and is required for colocalization of Smad3 and Smad4.19 This β-spectrin is a major dynamic scaffolding molecule involved in generating functionally distinct membrane protein domains, conferring cell polarity, and regulating endocytic traffic.20 Our previous analysis revealed that mice with complete loss of ELF (elf−/−) displayed similar phenotypes to Smad2+/−/Smad3+/− mutant mice with midgestational death, severely defective livers, as well as gastrointestinal, neural, and heart defects.21,22 Elf+/− heterozygotes develop liver fibrosis and dysplasia,23 and 40% to 70% of these mice spontaneously develop hepatocellular cancers with markedly increased expression of several oncogenes.24
In view of the phenotype of the elf−/− null mice, we speculated a role for ELF in modulating angiogenesis. The current study was carried out to investigate the potential involvement of ELF in hepatocyte and endothelial cell regulation in human HCCs and mutant mice. We demonstrate that ELF plays a role in cell-cycle deregulation in not only hepatocytes but also endothelial cells through the modulation of CDK4, cyclin D1, and phosphorylated Rb levels. Elf-deficient embryos reveal abnormalities in blood vessel formation with expansion of endothelial precursor cells and abnormal distribution of smooth muscle cells. Moreover, histological analysis of liver tumors from elf+/− mice revealed increased angiogenesis according to the tumor stages. Lastly, acute inhibition of ELF by small interfering RNAs (siRNAs) displayed a marked accumulation of phospho-Rb in hepatocyte and endothelial cells. Thus, our findings suggest that loss of ELF, a mediator of TGF-β signaling, results in liver cancer formation through hypervascularization in addition to hepatocyte deregulation. These studies also imply that loss of ELF could serve as a primary event in progression toward a fully transformed phenotype, and its recovery could hold promise for new therapeutic approaches in human cancers of the liver.
Materials and Methods
Elf Mutant Mice and Analysis
The generation of elf+/− knockout in mice has been described previously.21 Timed matings between heterozygous elf+/− mice were set up to yield litters for harvest at 10.5 and 11.5 days past coitum. Part of each embryo was removed for polymerase chain reaction genotyping, using primers and conditions as described previously.21 Elf+/− mutant mice were maintained on a mixed 129SvEv/Black Swiss background. Mice were monitored at least twice per week for possible symptoms of illness and tumor formation. When the mice demonstrated phenotypical abnormalities, tissue and adjacent normal tissue were collected for further analysis. All animal procedures were approved by the Institutional Animal Care and Use Committee of Georgetown University Medical Center, Washington, DC.
Cell Culture and Transfection
HepG2 (hepatocellular carcinoma cells), FBHE (fetal bovine heart endothelial), and CPAE (cow pulmonary artery endothelial) cells were obtained from the American Type Culture Collection (Manassas, VA). The cells were plated at a concentration of 105 cells/mL in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum, 2 mM L-glutamine, 100 units/mL penicillin, 10 µg/mL streptomycin (Invitrogen). Twenty percent fetal bovine serum was used for culture of FBHE and CPAE cells. Heparin 0.1 µg/mL and 2 ng/mL fibroblast growth factor-2 (Upstate) were supplemented for culture of FBHE cells. Human HCC cell lines SNU-398, SNU-449, and SNU-475 (American Type Culture Collection) were grown in Roswell Park Memorial Institute 1640 supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 µ/mL streptomycin. Human ELF was cloned by polymerase chain reaction from the reverse transcribed complementary DNA of Hela cell RNA. The sequences of the primers were as following: forward primer; 5-ACC ATG GAA TTG CAG AGG ACG TCT AG-3, reverse primer; 5-CAG TCC AGA CCA TGG CTG GTC-3. The polymerase chain reaction products were cleaned and cloned into pcDNA3.1/V5-HisTOPO TA plasmid (Invitrogen). Cells were plated and transfected on the next day with the ELF plasmid or empty vector using Lipofectamine 2000 (Invitrogen).
Histological Analysis
Embryonic yolk sacs and liver specimens were fixed in 10% formalin, blocked in paraffin, sectioned, stained with hematoxylin-eosin (HE), and examined by light microscopy. Immunohistochemical analysis was performed by using the ZYMED Histomouse Kit (Zymed) as described previously.25 Antibodies against alpha-smooth muscle actin (α-SMA, Sigma), CD34 (Abcam), Hep Par1 (Dako), Ki67 (Novus), vascular endothelial growth factor receptor 2 (VEGFR2, Cell Signaling), and von Willebrand Factor (vWF, Chemicon) were applied according to the manufacturers’ instructions.
Western Blot Analysis
Western blot analysis was carried out according to standard procedures using enhanced chemiluminescence detection (Amersham). In brief, cells were washed three times with ice-cold phosphate-buffered saline and harvested with a cell scraper. The cells were lysed in the buffer consisting of 30 mM 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (pH 7.5), 100 mM NaCl, 10% glycerol, 2% Triton X-100, and 1 × protease inhibitor cocktail (Roche). Protein concentrations were determined by the method of Bradford,26 and bovine serum albumin was used as a standard. The following primary antibodies were used: peptide-specific antibody, VA-1, to ELF (Santa Cruz, or VA-127) V5 (Invitrogen), proliferating cell nuclear antigen (Pharmingen), CDK4, cyclin D1, retinoblastoma protein (pRb), mitogen-activated protein kinase, and cleaved caspase-3 (Cell Signaling Technologies), p53, Rb, Smad3, TGF-β receptor I, TGF-β receptor II, and α-Tubulin (Santa Cruz). Horseradish peroxidase–conjugated donkey antirabbit or sheep anti-mouse antibodies (Jackson Immuno Research) were used as secondary antibodies. The results were quantified by using Multi Gauge (ver. 3.0, Fuji film).
Cell Analysis
HepG2 and FBHE cells were transfected with ELF, further incubated for 48 hours, and harvested by trypsin. The collected cells were resuspended, and fixed in 70% ethanol. The resulting cells were washed by phosphate-buffered saline, resuspended in propidium iodide solution containing RNase A, and the cellular fluorescence measured by using FACSCalibur flow cytometer (BD Biosciences). DNA content and the cell cycle distribution of those cells were analyzed by CellQuest (BD Biosciences).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)-based cell growth determination kit (Sigma) was used to measure the proliferation of HepG2 and FBHE cells. Proliferation was measured 2 days after transfection at optical density 570 nm by subtraction of readings at optical density 690 nm.
Knockdown of ELF, β-Spectrin
HepG2 and CPAE cells were transfected with 100 nM control siRNA (Dharmacon) or ELF siRNA (Santa Cruz) by using Lipofectamine 2000 (Invitrogen). The ELF siRNA was the pool of three target-specific 20-nucleotide to 25-nucleotide siRNAs for knockdown of ELF, and control siRNA was the mixture of four mismatches. Cells were harvested for western blotting at 48 hours after transfection. ELF antibody was used to confirm the knockdown of ELF expression. Fifty micrograms protein was loaded, and α-tubulin was used as loading control.
Statistical Analyses
T test (http://www.physics.csbsju.edu/stats/t-test.html) was used to compare the differences as specified in the text. P ≤ 0.05 was considered statistically significant.
Results
Role of ELF in Hepatocyte Proliferation
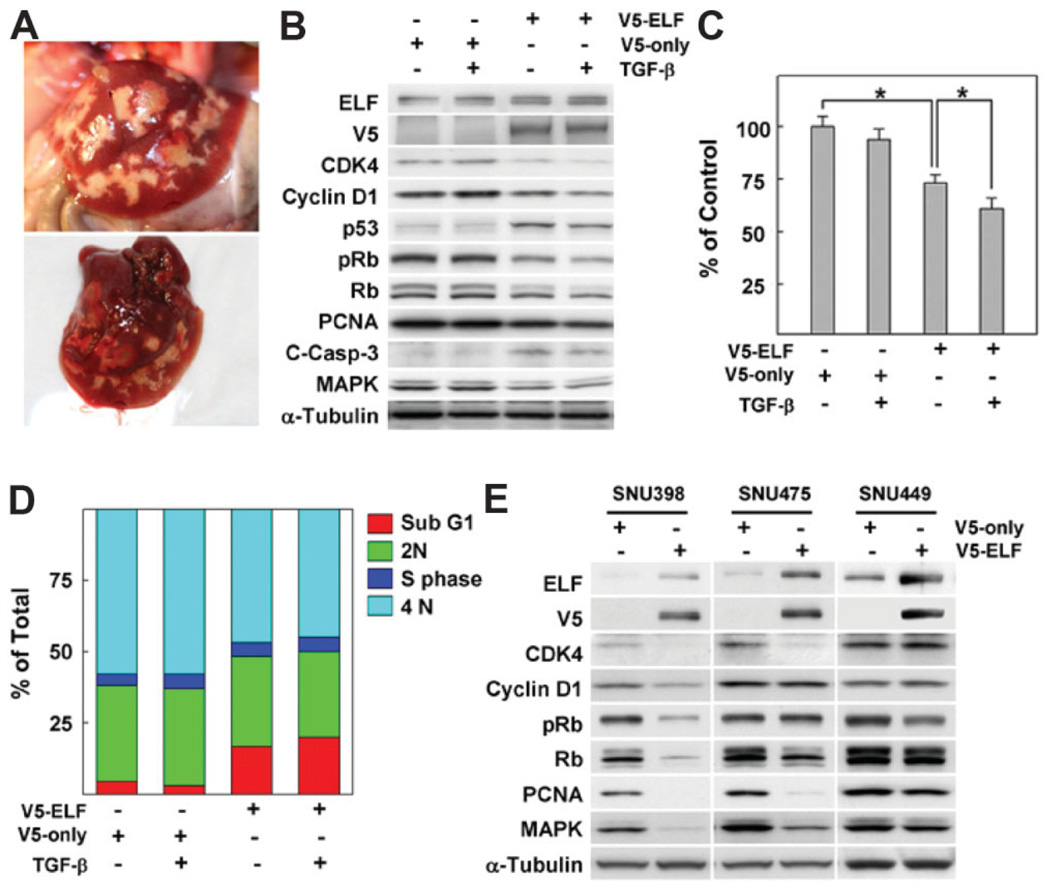
We have previously reported that 40% of elf heterozygous mutant mice spontaneously developed HCCs as early as 15 months of age, whereas none of the age-matched wild-type mice developed similar abnormalities (Fig. 1A).24 Spontaneous tumor formation from heterozygous loss of elf suggests that decreasing the level of ELF is sufficient to result in malignant transformation of the liver. To investigate the relationship between the level of ELF and hepatocyte proliferation (the main cell-type in the liver), we examined the expression patterns of proteins responsible for cell cycle regulation in transient overexpression of ELF in HepG2 cells in the absence or presence of TGF-β. As shown in Fig. 1B, we observed that overexpression of ELF markedly decreased expression of proteins responsible for the G1/S cell cycle checkpoint such as CDK4, cyclin D1, and pRb and, at the same time, stabilized p53 (Fig. 1B). In particular, these three proteins responsible for G1/S transition were reduced down to a third of normal values by ectopic ELF overexpression, significantly greater than in the controls, in the presence of TGF-β.
Fig. 1.
Expression of ELF is critical for proliferation of hepatocytes. (A) Macroscopic images of liver cancer from 1-year-old elf+/− mouse. (B) pcDNA3.1 vectors expressing V5-tagged ELF (V5-ELF) or empty vector (V5-only) were transfected into HepG2 cells. Two days later, the cells were incubated with 100 pM TGF-β for 2 hours, then harvested, and we analyzed the patterns of cell cycle regulatory protein by western blot as described in Materials and Methods. α-Tubulin was used as loading control. (C) Cell viability assay based on MTT showed significant decreases in cell viability by ELF transfection and TGF-β treatment. Cells were treated with TGF-β for 16 hours after 2 days’ ELF transfection. P > 0.01 are indicated by asterisks. (D) Flow cytometry revealed the DNA content of cells after ELF transfection and TGF-β treatment. Percentages of cell distribution in each phase are shown on the histogram. (E) Expression patterns of the regulatory protein for cell cycle and proliferation by transfection of ELF in several HCC cell lines. Restoration of ELF resulted in the dramatic decrements in SNU398, and SNU475 but not in SNU449.
Results of flow cytometry and MTT assays also showed that ELF levels are critical for survival in TGF-β–dependent HepG2 cells. To test whether expression of ELF is involved in survival and proliferation of liver tumor cells, we performed MTT assays in HepG2 cells on ELF transfection in the absence or presence of TGF-β. Survival of HepG2 cells after introducing exogenous ELF showed statistically significant reduction in the absence and presence of TGF-β treatment (26.3% and 39% of normal levels, respectively) (Fig. 1C). After ELF transfection, sub-G1 peaks representing apoptotic populations were dramatically increased by 5.5 and 7.1 times in the absence or presence of TGF-β, respectively, whereas no induction was detected in TGF-β treatment without exogenous ELF (Fig. 1D). Next, we tested the effects of ELF overexpression on cell-cycle–related proteins in additional human HCC cell lines (Fig. 1E). In SNU398, which has a near complete loss of ELF, reductions of CDK4, cyclin D1, pRb, proliferating cell nuclear antigen, and mitogenactivated protein kinase expressions were detected after rescue of ELF. Similarly, reduced CDK4, proliferating cell nuclear antigen, and mitogen-activated protein kinase were observed in SNU475 cells. However, in SNU449, which has high expression of ELF, there was no significant change in the expressions of these regulatory proteins. These results suggest that ELF is a critical regulator of transition of G1/S cell cycle and apoptosis in the response to TGF-β in HCC cells.
Role of ELF in Angiogenic Regulation of Liver Neoplasia
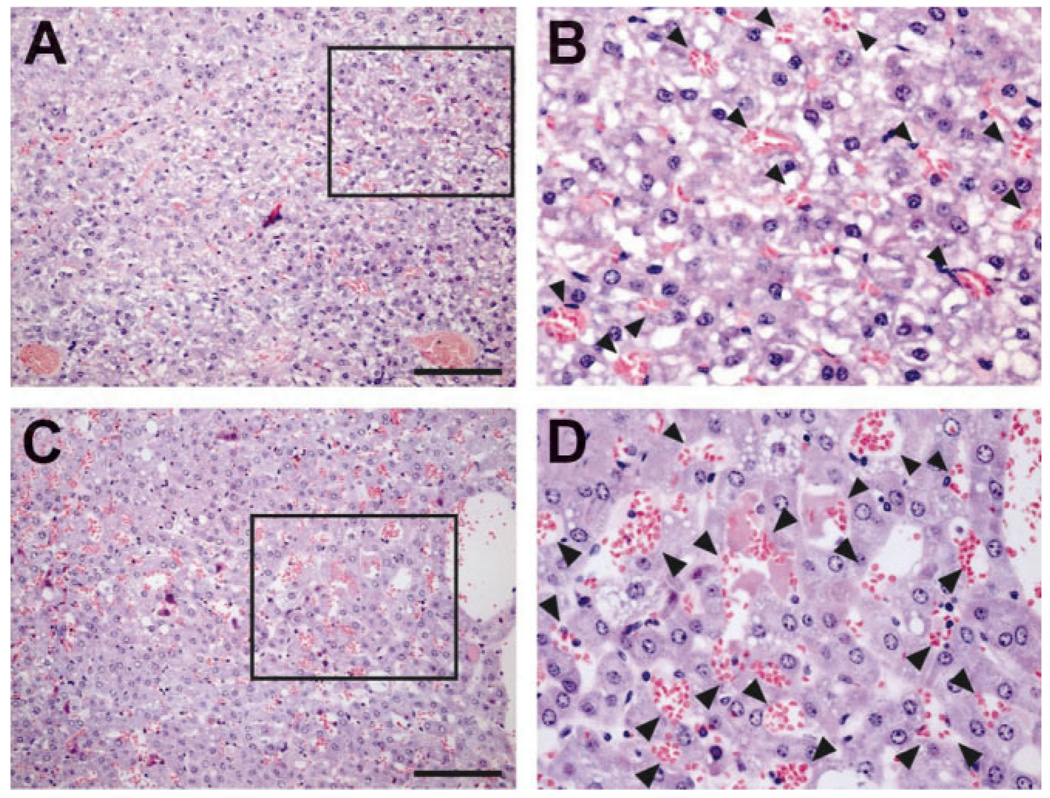
To detail the liver abnormalities from ELF insufficiency, we performed a histological analysis of 10 abnormal livers from elf+/− mice. The abnormal liver sections from elf+/− mice displayed abundant new blood vessels independent of the degree of other histopathological abnormalities. Hepatocytes appeared large and hyperplastic, with moderately enlarged nuclear size, resulting in an increased nucleo-cytoplasmic ratio. These nuclei were also characterized by a hyperchromatic pattern. Loss of liver cell plate architecture with proliferation of small blood-filled vascular channels was identified. As a result, the hepatic sinusoids that intervene between liver plates were not discernible. There were no portal triads or central veins (Fig. 2A–D). Some hepatocytes were swollen and showed vacuolated cytoplasm (Fig. 2B) or microvesicular steatosis (Fig. 2D). These findings led us to test whether insufficiency of ELF is involved in angiogenic stimulation in addition to the hyperproliferation of hepatocytes.
Fig. 2.
Angiogenic stimulation in elf+/− mice liver. HE stainings for abnormal liver tissues from elf+/− mice at 18 months revealed the newly formed abnormal blood vessels (arrows). The hepatocytes of elf+/− mice appeared hyperplastic with characteristic nucleo-cytoplasmic ratio. The hepatic sinusoids that intervene between liver plates are not discernible. (B, D) Magnifications of the boxed area of A and C, respectively. Scale bar; 100 µm.
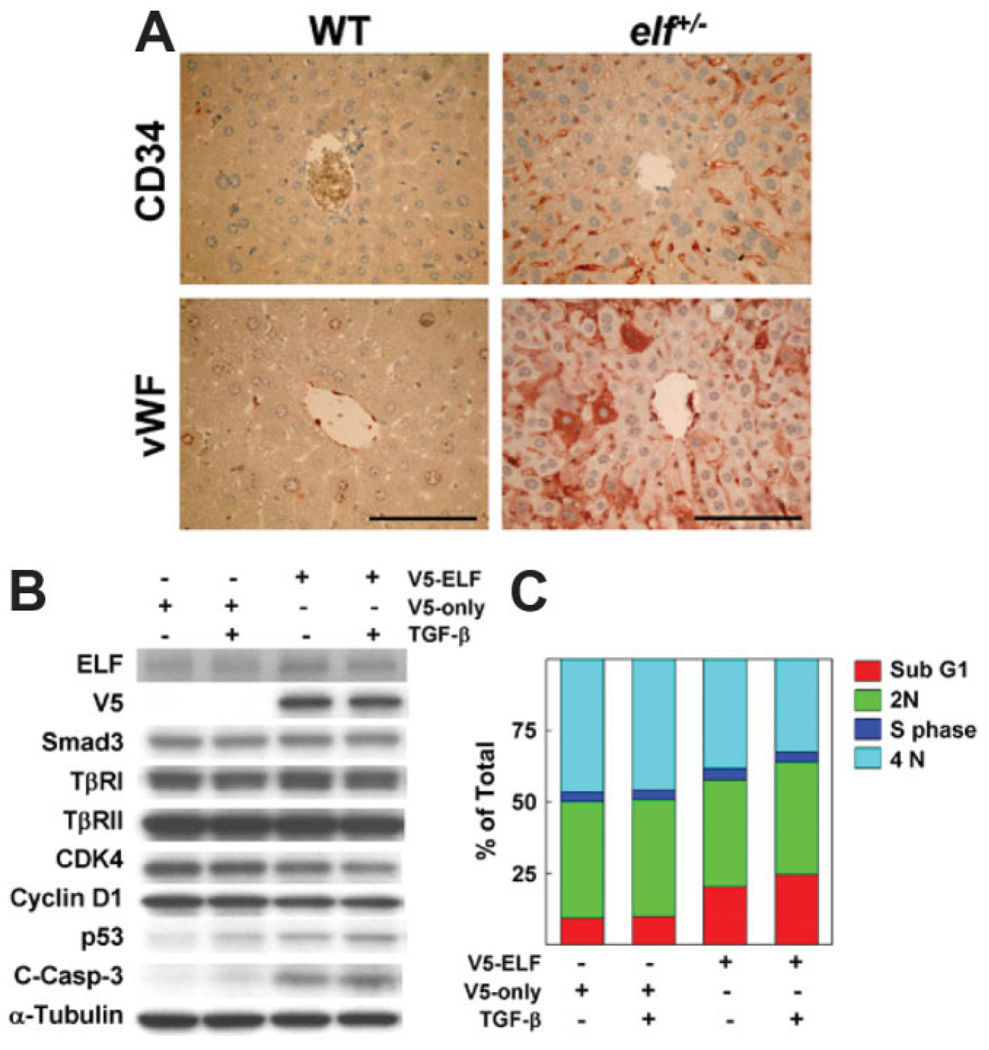
To determine whether the angiogenic stimulation in elf mutant livers resulted from insufficiency of ELF or alterations in the microenvironment from hepatocyte hyperproliferation, we investigated angiogenic stimulation in normal-appearing livers from 1-year-old elf+/− mice, comparing the profile with that of age-matched controls. HE labeling did not reveal any obvious differences (data not shown). However, our immunohistochemical analyses using the antibodies against CD34 and vWF, markers of endothelial progenitor cell, demonstrated amplification of endothelial cells in liver tissues of elf+/− mice with no detectable signals in age-matched wild type (Fig. 3A). Results from the histological analysis of liver from elf+/− mice suggest that insufficiency of ELF results in increased angiogenesis in liver tissues regardless of hepatocyte status.
Fig. 3.
ELF is a critical regulator of cell cycle progression and survival of endothelial cells. (A) Normal livers from wild-type and elf+/− mice were stained with antibodies against CD34 and vWF. Scale bar; 100 µm. (B) Pattern of cell cycle regulatory proteins by transfection of exogenous V5 tagged-ELF in the absence or presence of TGF-β is shown. Exogenous ELF expression was monitored by V5 antibody, and α-tubulin was used as loading control. Immunoblot analysis reveals decreased levels of CDK4 and cyclin D1 by overexpression of ELF in FBHE cells. p53 and cleaved caspase-3 levels were increased by ELF. TβRI; TGF-β receptor I, TβRII; TGF-β receptor II, C-Casp-3; cleaved caspase-3. (C) Percentages of FBHE cell distribution in each phase are shown on the histogram on transfection of exogenous ELF expression in the absence or presence of TGF-β. ELF-transfected FBHE cells were treated with TGF-β for 16 hours, harvested by trypsin, and analyzed by flow cytometry with 4′,6-diamidino-2-phenylindole (DAPI) staining.
Next, we tested whether ectopic expression of ELF could modulate endothelial cell proliferation. As shown in Fig. 3B, overexpression of ELF in endothelial FBHE cells markedly decreased levels of cell cycle promoting proteins (CDK4 and cyclin D1) as well as increased levels of proteins responsible for cell cycle arrest and apoptosis (p53, and cleaved caspase-3) such as hepatic HepG2 cells. However, any detectable alteration was not identified in other TGF-β signaling components and α-Tubulin as loading control. Next, we examined whether alteration of the above proteins by ELF expression influenced cell cycle distribution or survival of FBHE cells (Fig. 3C). Our flow cytometric analysis showed that the induction of ELF with TGF-β treatment dramatically increased the sub-G1 population from 9% to 25%, whereas no increments were detected in TGF-β treatment alone. Taken together, these results suggest that ELF expression is a critical determinant of endothelial cell as well as hepatocyte proliferation.
Defects in Blood Vessel Formation in elf−/− Embryos by Hyperproliferation of Endothelial Cells
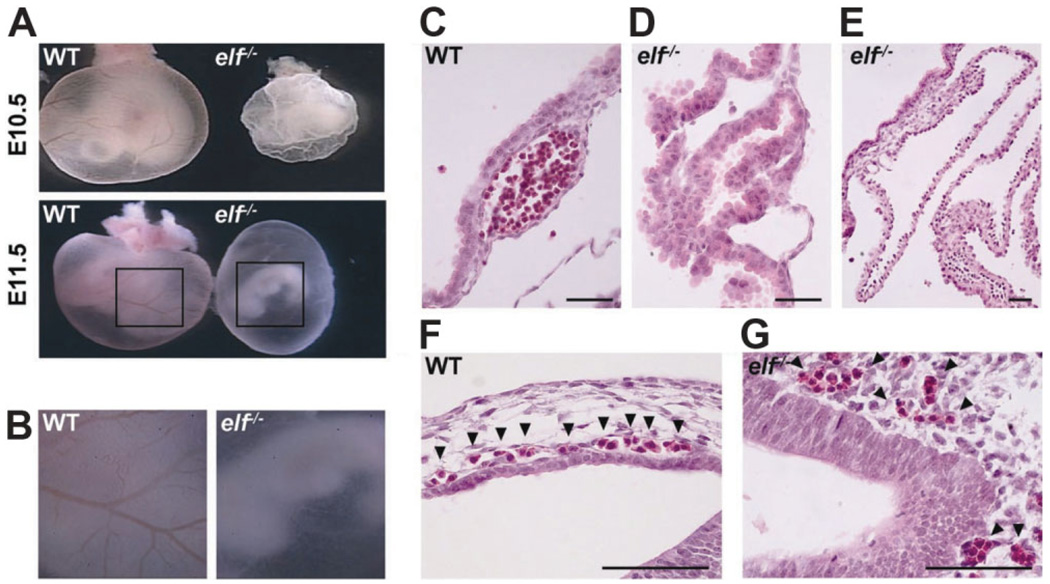
Mice heterozygous for elf mutant alleles appeared phenotypically normal in development, but homozygous mutant elf−/− mice were not detected at birth, indicating that the complete loss of elf is a recessive embryonic lethal. Our previous study showed abnormal or degenerating embryos that were recovered between embryonic day 8.5 (E8.5) and 16.5 (E16.5).21 At the same time, elf−/− embryos displayed multiple defects that included liver abnormalities. Therefore, we determined whether loss of elf in mouse embryos contributed to angiogenic defect in elf−/− yolk sacs, a widely accepted model tissue for analyzing angiogenesis. As expected, abnormal embryos of elf−/− in early embryonic days (E8.5-E11.5) were notable for the lack of a clear branching network of vessels in the yolk sac (Fig. 4A,B). Yolk sacs of wild-type embryos developed a well-formed vascular network filled with blood cells, whereas mutant yolk sacs were pale with no evident blood vessel structures. Moreover, mutant embryos with angiogenic defects began to degenerate with easily breakable yolk sacs and shrinking body size at E11.5. Histological analysis of yolk sac of wild-type embryos showed capillary-like vessels filled with blood cells between mesothelial and endodermal layers, whereas yolk sac of mutants showed a series of large cavities with scattered blood cells and an expansion of extra-embryonic mesoderm or endoderm (Fig. 4C–E). The blood vessels of the mutant embryo did not appear well formed or connected, whereas normal wild-type blood vessels were clearly delineated and lined by endothelial cells (Fig. 4F,G).
Fig. 4.
Elf−/− embryos exhibited defects of angiogenesis in embryonic yolk sac. (A) Whole-mount view of elf−/− homozygous mutant mice at E10.5 and E11.5 days past coitum were shown. The mutant embryos were smaller and pale compared with wild-type. (B) Magnified images of yolk sac at E11.5 (boxed areas of E11.5-day embryos) were shown. Large blood vessels in wild-type (normal) were seen, but not in elf−/− mice. (C) HE stainings for day 10.5 embryonic yolk sac showed the well-made blood vessel in the normal embryo. (D, E) Mutant yolk sacs lacked the normal vasculature with widened cavities, and blood cells were irregularly distributed. Embryonic vascular structures from wild-type (F) and elf−/− (G) at E10.5 days past coitum are displayed. Distribution of wild-type blood cells is confined by a barrier, whereas mutant blood cells are mixed with neighboring cells. Arrows indicate the blood cells. Scale bar; 50 µm.
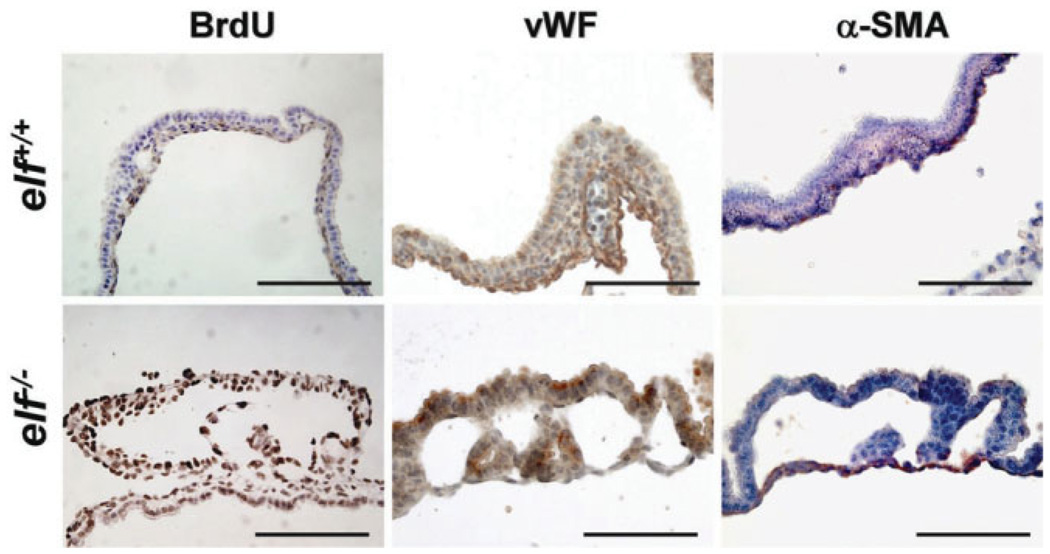
We next investigated the vascular endothelium in the developing yolk sacs using antibodies against bromodeoxyuridine (BrdU), a proliferation marker, vWF, a marker of endothelial cell, and α-SMA, a marker for vascular smooth muscle cells (Fig. 5). BrdU-positive proliferating cells in the yolk sac of wild-type mice were mainly located in endodermal layer, whereas elf mutant cells appeared aberrantly at the both sides with stronger labeling, indicating that loss of ELF results in the aberrant and uncontrolled proliferation of these yolk sac vascular endothelial cells. At the same time, the endothelial cells of wild-type mice displayed a narrow distribution with an elongated shape around the BrdU-negative mesodermal cells. However, elf mutant yolk sacs showed a broad distribution of endothelial cells appearing as a highly proliferating round shape in both layers of the yolk sac. Interestingly, the distribution of vascular smooth muscle cells, labeled by α-SMA, appeared predominantly in the endodermal layer of day 10.5 mutant yolk sac compared with the tightly closed circle around the blood cells in normal wild type. These observations suggest that the failure of proper blood vessel formation in the elf−/− mutant yolk sac resulted from maturation defects in endothelial cells.
Fig. 5.
Embryonic endothelial cells of elf−/− showed hyperproliferation and defects of differentiation. Immunohistochemical analysis of normal and mutant yolk sacs of day 10.5 embryos using antibodies against BrdU, vWF, and α-SMA are shown. Notably BrdU-positive cells were located in endodermal cells, whereas they appeared in both sides of the elf mutant. The distribution of endothelial cells, labeled by antibody against vWF, also appeared in a narrow distribution around the blood cell with elongated shape in wild-type, but in a broad distribution with a round shape in mutant. The α-SMA stainings, representing vascular smooth muscle cells, were mainly located in the endodermal layer of mutant yolk sac but formed the tightly closed circle around the blood cells in wild-type. Scale bar: 100 µm.
ELF Status Is Critical for Angiogenic Stimulation
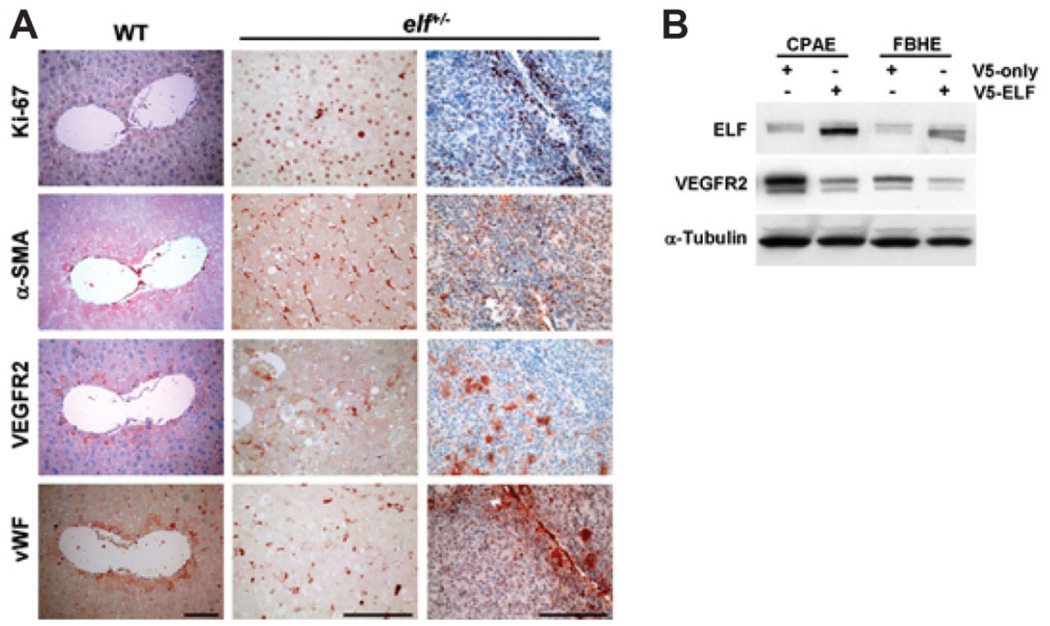
To determine whether angiogenesis is activated and abnormal in liver neoplasia caused by insufficiency of ELF, we performed immunohistochemical analysis of various stages of liver tissues from elf+/− mice. Immunohistochemical analysis showed that hyperproliferation in the livers is accompanied by overexpression of angiogenic markers at different stages of cancer formation (Fig. 6). Hyper-proliferation of elf+/− mutant livers, stained with Ki-67 antibodies, is also accompanied by activated networks of vascular structures, identified by higher expressions of α-SMA, and vWF, representing vascular muscle cells and endothelial cells, respectively. At the same time, mutant livers from elf+/− mice also displayed accumulation of vascular endothelial growth factor receptor (VEGFR), required for vascular endothelial growth factor (VEGF)-stimulated proliferation, chemotaxis, and sprouting, as well as survival of cultured endothelial cells in vitro and angiogenesis in vivo.28,29 Interestingly, early hyperplastic hepatocytes also displayed an activation of VEGFR2, suggesting a direct relationship between the extent of the vascular network and progression of HCC from even early stages.
Fig. 6.
ELF status is critical for angiogenic stimulation. (A) Immunohistochemical analysis of various abnormal liver tissues from elf+/− mice and normal liver from age-matched wild-type mice. The antibody stainings against Ki-67, α-SMA, VEGFR2, and vWF are shown. The activated networks of vascular structures were found in hyperplastic liver tissues of elf+/− mice, which were monitored by expressions of α-SMA and vWF, representing vascular muscle cells and endothelial cells, respectively. The accumulation of VEGFR2 was also identified in same tissues, suggesting VEGF-stimulated proliferation of endothelial cells in hyperproliferating liver of elf+/− mice. Scale bar: 100 µm. (B) Expressions of VEGFR2 on transfection of exogenous V5-tagged ELF in endothelial FBHE and CPAE cells. α-Tubulin was used as a loading control.
Increased expression of angiogenic markers in HCCs derived from elf+/− mice suggest that the loss of ELF protein might result in angiogenic stimulation. To examine this hypothesis, we transfected an ELF expression vector into the two endothelial cell lines and examined the expression of VEGFR2. The induction of exogenous ELF transfection dramatically reduced the expression of VEGFR2 by 2.4 and 3.1 times in CPAE, and FBHE, respectively. Taken together, these results suggest that expression of ELF is a strong determinant of angiogenic stimulation in endothelial cells.
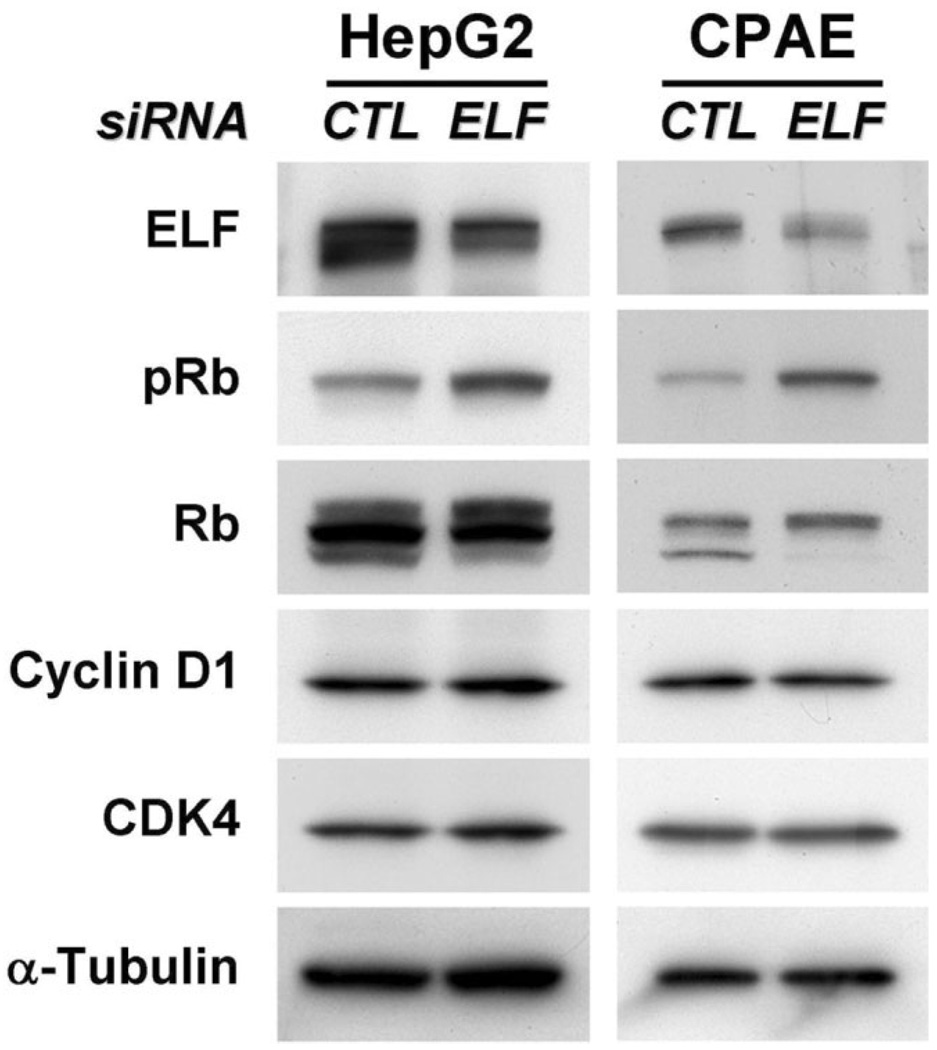
Knockdown of ELF Decreasing Phospho-Rb Protein
To confirm the relationship between insufficiency of ELF and hyperproliferation of hepatocytes and endothelial cells, we knocked down endogenous ELF by transfecting with the ELF siRNA and examined the expression of proteins responsible for cell cycle regulation (Fig. 7). Our western blot analysis of protein patterns responsible for cell cycle displayed an approximately two-fold to fourfold increase in pRb levels by ELF knockdown in HepG2 and CPAE cells, respectively. In contrast, levels of cyclin D1 and CDK4, which decreased with exogenous ELF transfection, did not display detectable differences. These results suggest that the role of ELF in cell cycle arrest occurs through modulation of Rb protein phosphorylation.
Fig. 7.
Increased level of pRb by ELF siRNA in HepG2 and CPAE cells. (A) Expression levels of cell cycle regulatory proteins are shown on transfection of control siRNA or siRNA for ELF in HepG2 and CPAE cells. One hundred nM control siRNA and ELF siRNA were transfected into HepG2 and CPAE cells for 2 days. The cells were harvested, and then the patterns of cell cycle regulatory protein were analyzed by western blot. The expression levels of pRb are dramatically increased on decrement of ELF. 50 µg proteins were loaded, and α-Tubulin was used as a loading control.
Discussion
In response to TGF-β treatment, Smad2 and Smad3 are phosphorylated by the TGF-β receptor at the C-terminus, forming heteromeric complexes with Smad4, nuclear translocation, and regulation of target genes that include cell cycle regulators. The TGF-β signaling pathway plays a critical role in diverse cell functions, including inhibition of cell growth, cell migration, and differentiation. Thus, loss of TGF-β responsiveness results in deregulated cellular growth, which is considered a crucial step in the development of various tumors, including liver cancer.30,31 In particular, Smad4, also known as DPC4 (deleted in pancreatic carcinoma 4) in humans, is commonly inactivated in human pancreatic and gastrointestinal tumors.32–34 ELF, an Smad adaptor protein, has been identified to play a critical role in localizing Smads and facilitating tumor suppressor functions of the TGF-β pathway.21,27
Our previous studies have demonstrated a functional contribution of ELF in tumor suppression from enhanced susceptibility of elf heterozygous mutant mice to the development of cancers.24,35,36 We have also elucidated a higher rate of cell proliferation in elf+/− mutant mice, suggesting that the abnormalities of TGF-β signaling in ELF-insufficient mice results in the deregulation of growth arrest and subsequent tumor formation.35,36 Recently, we observed that 40% of elf+/− mice spontaneously develop HCCs within 15 months.24 Importantly, we also show that statistically significant reductions of ELF expression but not TGF-β receptor II, or Smad4 were observed in human HCCs.24 Deficiency of the ELF protein has been shown to result in mislocalization of Smad3 and Smad4 as well as loss of the TGF-β–dependent transcriptional response. These functions could be rescued by restoration of ELF.21
Deregulation of the cell cycle has been recognized as an important factor in tumorigenesis, and TGF-β inhibits the growth of cells by preventing cell cycle progression during the G1 phase. In mammalian cells, several regulators of the G1/S transition have been implicated in TGF-β–induced cell cycle arrest.37 To provide further insights into the relationship between TGF-β signaling and G1 checkpoint regulation, we analyzed the critical regulators of the G1/S transition in several cells from HCCs as well as endothelia under modulation of ELF expression. Levels of CDK4, cyclin D1, and pRb were decreased by ELF induction, whereas a rise in pRb was observed on reduction of ELF by siRNA. These results suggest that expression of ELF is involved in control of G1/S cell cycle transition through the modulation of CDK4 and cyclin D1 and phosphorylation of Rb. In addition to the regulation of cell cycle, the levels of p53 and cleaved caspase-3 were dramatically increased by ELF expression. Moreover, ELF expression and TGF-β treatment synergistically increased the population of sub-G1 phase cells. Thus, disruption of the TGF-β signaling pathway through insufficiency of ELF results in deregulated proliferation of hepatocytes with common secondary genetic alterations, such as mutation of p53 and p21.38,39
Initiation, progression, and metastasis of tumors are dependent on angiogenesis.40 Inhibition of angiogenesis has become a promising approach for the treatment of many human malignancies.41 Angiogenesis is also regarded as a marker for invasiveness and metastasis. The balance between stimulatory and inhibitory factors of angiogenesis is important for tumor development, and an imbalance of this process has been associated with cancer.7,9 It is well known that HCC is typically a hypervascular tumor, with a radiological arterial hypervascular pattern which serves as an important diagnostic criterion for liver cancer.42 The results of this study show that insufficiency of elf (elf+/−) and loss of elf (elf−/−) in mice lead to amplification of endothelial progenitor cells in the liver tissues and embryonic yolk sac, respectively. In the neoplastic liver tissue of elf+/− mice, we identified an abundance of newly formed blood vessels in disarrayed lobular architecture of the liver with hyperplastic hepatocytes. Blood vessels of elf−/− mice in the developing yolk sac also exhibited immature large blood vessels surrounded by undifferentiated and hyperproliferating endothelial progenitor cells. Further analysis on the endothelial cells by transfection and knockdown of ELF revealed that ELF is critical for cell cycle arrest, stimulation of an angiogenic switch, and survival of these endothelial cells.
In general, VEGF is a critical regulator of angiogenesis, and its induction has been reported in liver cancer and in the surrounding liver.43–46 Augmenting VEGF increases liver cancer formation and metastasis.47,48 Furthermore, VEGF levels are a significant prognostic indicator for HCC patients, suggesting that progression and metastasis of liver tumors rely on VEGF-dependent angiogenesis.49 Our immunopathological analysis of liver from elf+/− mice exhibited a significant induction of VEGFR2 with newly formed blood vessel in hyperplastic regions. Taken together, angiogenic stimulation is an important phenotype of liver cancer from inactivation of ELF, and this feature will be the critical determinant of prognosis by influencing tumor progression and metastasis.
In summary, loss of ELF, which is frequently found in human HCC, leads the deregulation of cell cycle by disrupting the TGF-β pathway and results in the development of liver cancer with activated vasculogenesis, an important factor of poor prognosis. Thus, our study provides intriguing and potentially important insights into tumor biology of liver cancers, and in the future we may use this tumor suppressor protein for the diagnosis, prognosis, and targeted therapeutics of cancers of the liver.
Acknowledgments
Supported by National Cancer Center, Korea, grant 0610660, and 0610270 (H.J.B., K.H.C., S.S.K.), U.S. National Institutes of Health grants R01 CA106614A (L.M.), R01 DK56111 (L.M.), R01 CA4285718A (L.M.), R01 DK58637 (B.M.), VA Merit Award (L.M.), R. Robert and Sally D. Funderburg Research Scholar (L.M.), and the Benn Orr Scholar Award (L.M.).
Abbreviations
- α-SMA
alpha-smooth muscle actin
- BrdU
bromodeoxyuridine
- CPAE
cow pulmonary artery endothelial
- ELF
embryonic liver fodrin
- FBHE
fetal bovine heart endothelial
- HCC
hepatocellular cancer
- HE
hematoxylin-eosin
- HepG2
hepatocellular carcinoma cells
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- pRb
retinoblastoma protein
- siRNA
small interfering RNA
- TGF-β
transforming growth factor beta
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
- vWF
von Willebrand Factor
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Roberts LR, Gores GJ. Hepatocellular carcinoma: molecular pathways and new therapeutic targets. Semin Liver Dis. 2005;25:212–225. doi: 10.1055/s-2005-871200. [DOI] [PubMed] [Google Scholar]
- 4.Feitelson MA, Sun B, Satiroglu Tufan NL, Liu J, Pan J, Lian Z. Genetic mechanisms of hepatocarcinogenesis. Oncogene. 2002;21:2593–2604. doi: 10.1038/sj.onc.1205434. [DOI] [PubMed] [Google Scholar]
- 5.Sun HC, Tang ZY. Angiogenesis in hepatocellular carcinoma: the retrospectives and perspectives. J Cancer Res Clin Oncol. 2004;130:307–319. doi: 10.1007/s00432-003-0530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 7.Semenza GL. Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med. 2003;54:17–28. doi: 10.1146/annurev.med.54.101601.152418. [DOI] [PubMed] [Google Scholar]
- 8.Lien WH, Chen CK, Lai LY, Chen YH, Wu MP, Wu LW. Participation of cyclin D1 deregulation in TNP-470-mediated cytostatic effect: involvement of senescence. Biochem Pharmacol. 2004;68:729–738. doi: 10.1016/j.bcp.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 10.Kaminska B, Wesolowska A, Danilkiewicz M. TGF beta signalling and its role in tumour pathogenesis. Acta Biochim Pol. 2005;52:329–337. [PubMed] [Google Scholar]
- 11.Villanueva A, Garcia C, Paules AB, Vicente M, Megias M, Reyes G, et al. Disruption of the antiproliferative TGF-beta signaling pathways in human pancreatic cancer cells. Oncogene. 1998;17:1969–1978. doi: 10.1038/sj.onc.1202118. [DOI] [PubMed] [Google Scholar]
- 12.Xu G, Chakraborty C, Lala PK. Expression of TGF-beta signaling genes in the normal, premalignant, and malignant human trophoblast: loss of smad3 in choriocarcinoma cells. Biochem Biophys Res Commun. 2001;287:47–55. doi: 10.1006/bbrc.2001.5533. [DOI] [PubMed] [Google Scholar]
- 13.Grady WM, Myeroff LL, Swinler SE, Rajput A, Thiagalingam S, Lutterbaugh JD, et al. Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res. 1999;59:320–324. [PubMed] [Google Scholar]
- 14.Woodford-Richens KL, Rowan AJ, Poulsom R, Bevan S, Salovaara R, Aaltonen LA, et al. Comprehensive analysis of SMAD4 mutations and protein expression in juvenile polyposis: evidence for a distinct genetic pathway and polyp morphology in SMAD4 mutation carriers. Am J Pathol. 2001;159:1293–1300. doi: 10.1016/S0002-9440(10)62516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertolino P, Deckers M, Lebrin F, ten Dijke P. Transforming growth factor-beta signal transduction in angiogenesis and vascular disorders. Chest. 2005;128:585S–590S. doi: 10.1378/chest.128.6_suppl.585S. [DOI] [PubMed] [Google Scholar]
- 16.Marchuk DA, Srinivasan S, Squire TL, Zawistowski JS. Vascular morphogenesis: tales of two syndromes. Hum Mol Genet. 2003;12(Spec No 1):R97–R112. doi: 10.1093/hmg/ddg103. [DOI] [PubMed] [Google Scholar]
- 17.Feng XH, Derynck R. A kinase subdomain of transforming growth factor-beta (TGF-beta) type I receptor determines the TGF-beta intracellular signaling specificity. EMBO J. 1997;16:3912–3923. doi: 10.1093/emboj/16.13.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimchi A, Wang XF, Weinberg RA, Cheifetz S, Massague J. Absence of TGF-beta receptors and growth inhibitory responses in retinoblastoma cells. Science. 1988;240:196–199. doi: 10.1126/science.2895499. [DOI] [PubMed] [Google Scholar]
- 19.Mishra L, Derynck R, Mishra B. Transforming growth factor-beta signaling in stem cells and cancer. Science. 2005;310:68–71. doi: 10.1126/science.1118389. [DOI] [PubMed] [Google Scholar]
- 20.De Matteis MA, Morrow JS. Spectrin tethers and mesh in the biosynthetic pathway. J Cell Sci. 2000;113(Pt 13):2331–2343. doi: 10.1242/jcs.113.13.2331. [DOI] [PubMed] [Google Scholar]
- 21.Tang Y, Katuri V, Dillner A, Mishra B, Deng CX, Mishra L. Disruption of transforming growth factor-beta signaling in ELF beta-spectrin-deficient mice. Science. 2003;299:574–577. doi: 10.1126/science.1075994. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein M, Monga SP, Liu Y, Brodie SG, Tang Y, Li C, et al. Smad proteins and hepatocyte growth factor control parallel regulatory pathways that converge on beta1-integrin to promote normal liver development. Mol Cell Biol. 2001;21:5122–5131. doi: 10.1128/MCB.21.15.5122-5131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra B, Tang Y, Katuri V, Fleury T, Said AH, Rashid A, et al. Loss of cooperative function of transforming growth factor-beta signaling proteins, smad3 with embryonic liver fodrin, a beta-spectrin, in primary biliary cirrhosis. Liver Int. 2004;24:637–645. doi: 10.1111/j.1478-3231.2004.0958.x. [DOI] [PubMed] [Google Scholar]
- 24.Kitisin K, Ganesan N, Tang Y, Jogunoori W, Volpe EA, Kim SS, et al. Disruption of transforming growth factor-beta signaling through betaspectrin ELF leads to hepatocellular cancer through cyclin D1 activation. Oncogene. 2007;26:7103–7110. doi: 10.1038/sj.onc.1210513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SS, Cao L, Lim SC, Li C, Wang RH, Xu X, et al. Hyperplasia and spontaneous tumor development in the gynecologic system in mice lacking the BRCA1-Delta11 isoform. Mol Cell Biol. 2006;26:6983–6992. doi: 10.1128/MCB.00796-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Mishra L, Cai T, Yu P, Monga SP, Mishra B. Elf3 encodes a novel 200-kD beta-spectrin: role in liver development. Oncogene. 1999;18:353–364. doi: 10.1038/sj.onc.1202313. [DOI] [PubMed] [Google Scholar]
- 28.Rahimi N, Dayanir V, Lashkari K. Receptor chimeras indicate that the vascular endothelial growth factor receptor-1 (VEGFR-1) modulates mitogenic activity of VEGFR-2 in endothelial cells. J Biol Chem. 2000;275:16986–16992. doi: 10.1074/jbc.M000528200. [DOI] [PubMed] [Google Scholar]
- 29.Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem Soc Trans. 2003;31:20–24. doi: 10.1042/bst0310020. [DOI] [PubMed] [Google Scholar]
- 30.Gold LI. The role for transforming growth factor-beta (TGF-beta) in human cancer. Crit Rev Oncog. 1999;10:303–360. [PubMed] [Google Scholar]
- 31.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 32.Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 33.Miyaki M, Kuroki T. Role of Smad4 (DPC4) inactivation in human cancer. Biochem Biophys Res Commun. 2003;306:799–804. doi: 10.1016/s0006-291x(03)01066-0. [DOI] [PubMed] [Google Scholar]
- 34.Thiagalingam S, Lengauer C, Leach FS, Schutte M, Hahn SA, Overhauser J, et al. Evaluation of candidate tumour suppressor genes on chromosome 18 in colorectal cancers. Nat Genet. 1996;13:343–346. doi: 10.1038/ng0796-343. [DOI] [PubMed] [Google Scholar]
- 35.Tang Y, Katuri V, Srinivasan R, Fogt F, Redman R, Anand G, et al. Transforming growth factor-beta suppresses nonmetastatic colon cancer through Smad4 and adaptor protein ELF at an early stage of tumorigenesis. Cancer Res. 2005;65:4228–4237. doi: 10.1158/0008-5472.CAN-04-4585. [DOI] [PubMed] [Google Scholar]
- 36.Katuri V, Tang Y, Li C, Jogunoori W, Deng CX, Rashid A, et al. Critical interactions between TGF-beta signaling/ELF, and E-cadherin/beta-catenin mediated tumor suppression. Oncogene. 2006;25:1871–1886. doi: 10.1038/sj.onc.1209211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu F, Matsuura I. Inhibition of Smad antiproliferative function by CDK phosphorylation. Cell Cycle. 2005;4:63–66. doi: 10.4161/cc.4.1.1366. [DOI] [PubMed] [Google Scholar]
- 38.Kim SS, Shetty K, Katuri V, Kitisin K, Baek HJ, Tang Y, et al. TGF-beta signaling pathway inactivation and cell cycle deregulation in the development of gastric cancer: role of the beta-spectrin, ELF. Biochem Biophys Res Commun. 2006;344:1216–1223. doi: 10.1016/j.bbrc.2006.03.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baek HJ, Kim SS, da Silva FM, Volpe EA, Evans S, Mishra B, et al. Inactivation of TGF-beta signaling in lung cancer results in increased CDK4 activity that can be rescued by ELF. Biochem Biophys Res Commun. 2006;346:1150–1157. doi: 10.1016/j.bbrc.2006.05.195. [DOI] [PubMed] [Google Scholar]
- 40.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 41.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 42.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 43.Chow NH, Hsu PI, Lin XZ, Yang HB, Chan SH, Cheng KS, et al. Expression of vascular endothelial growth factor in normal liver and hepatocellular carcinoma:an immunohistochemical study. Hum Pathol. 1997;28:698–703. doi: 10.1016/s0046-8177(97)90179-9. [DOI] [PubMed] [Google Scholar]
- 44.Miura H, Miyazaki T, Kuroda M, Oka T, Machinami R, Kodama T, et al. Increased expression of vascular endothelial growth factor in human hepatocellular carcinoma. J Hepatol. 1997;27:854–861. doi: 10.1016/s0168-8278(97)80323-6. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi R, Yano H, Iemura A, Ogasawara S, Haramaki M, Kojiro M. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology. 1998;28:68–77. doi: 10.1002/hep.510280111. [DOI] [PubMed] [Google Scholar]
- 46.El-Assal ON, Yamanoi A, Soda Y, Yamaguchi M, Igarashi M, Yamamoto A, et al. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology. 1998;27:1554–1562. doi: 10.1002/hep.510270613. [DOI] [PubMed] [Google Scholar]
- 47.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Yanase K, et al. Involvement ofthe vascular endothelial growth factor receptor-1 in murine hepatocellular carcinoma development. J Hepatol. 2004;41:97–103. doi: 10.1016/j.jhep.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, et al. Halting the interaction between vascular endothelial growth factor and its receptors attenuates liver carcinogenesis in mice. Hepatology. 2004;39:1517–1524. doi: 10.1002/hep.20218. [DOI] [PubMed] [Google Scholar]
- 49.Kong SY, Park JW, Lee JA, Park JE, Park KW, Hong EK, et al. Association between vascular endothelial growth factor gene polymorphisms and survival in hepatocellular carcinoma patients. Hepatology. 2007;46:446–455. doi: 10.1002/hep.21720. [DOI] [PubMed] [Google Scholar]