Abstract
Human pancreatic cancer is a malignant disease with almost equal incidence and mortality. Effective diagnostic and therapeutic strategies are still urgently needed to improve its survival rate. With advances in structural and functional genomics, recent work has focused on targeted molecular therapy using monoclonal antibodies. This review summarizes the target molecules on the tumor cell surface and normal tissue stroma, which are related to pancreatic cancer oncogenesis, tumor growth or resistance to chemotherapy, as well as molecules involved in regulating inflammation and host immunoresponses. Targeted molecules include cell-surface receptors, such as the EGF receptor, HER2, death receptor 5 and IGF-1 receptor. Effects of monoclonal antibodies against these target molecules alone or in combination with chemotherapy, small-molecule signal transduction inhibitors, or radiation therapy are also discussed. Also discussed are the use of toxin or radioisotope conjugates, and information relating to the use of these targeting agents in pancreatic cancer clinical trials. Although targeted molecular therapy with monoclonal antibodies has made some progress in pancreatic cancer treatment, especially in preclinical studies, its clinical application to improve the survival rate of pancreatic cancer patients requires further investigation.
Keywords: cancer therapy, immunotherapy, monoclonal antibody, pancreatic cancer
Pancreatic cancer remains a disease with high mortality despite numerous efforts that have been made to improve its survival rates. In a recent analysis using a database from 1973 to 2003 based on modeled period analysis, 5-year survival of pancreatic cancer patients was 7.1% and 10-year survival was below 5% [1]. The survival rate is apparently related to the disease stage with a low rate at 1.6–3.3% among patients with distant metastases [1,2]. Early diagnosis and effective treatment to control the advanced stages of disease may prolong the survival rate of pancreatic cancer [3]. The search for effective treatment of pancreatic cancer has involved in-depth translational research for decades. With the achievements made in molecular biology and cell biology, studies have focused on structural genomics, which have evolved to functional genomics (proteomics) in recent years [4]. Extensive studies to explore molecular mechanisms involved in pancreatic cancer oncogenesis, cancer stem cells, cell proliferation control and metastasis supplied valuable information and directed the treatment strategies towards targeted therapies. In targeted therapies, tumor-associated or tumor-specific proteins were targeted and their roles in corresponding signal transduction pathways were investigated [5]. Targeted therapies could be accomplished by using specific monoclonal antibodies (mAbs) against these proteins or by pathway-specific small-molecule inhibitors [5]. In this review, we focus on the application of mAbs in human pancreatic cancer treatment.
Ideally, the cellular targets in the targeted therapies should be expressed in high concentration on the surface of tumor cells. They might be required for tumor cell survival or play an important role in maintaining tumor cell proliferation. The targets should not be shed/secreted from the cell surface and their concentration or function should not be modulated after binding to the administered antibodies [4,6,7]. The targets are generally classified into three major categories: cell surface proteins; antigens associated with the tumor stroma; and antigens on tumor-associated vasculature and angiogenic ligands [8].
Monoclonal antibodies against human tumor targets were initially raised in rodents, most of them from mice. However, the immunogenicity of the mouse antibodies induced immunologic responses from patients, which produced human antimouse immunoglobulin antibodies. This immune response exacerbates after multiple administrations of mouse antibodies, which subsequently induces their rapid clearance from the bloodstream. Recombinant DNA technology was used to overcome such immune responses against mouse antibodies. Chimeric antibodies (consisting of variable regions from mouse antibodies and constant regions of human immunoglobulin), antibody fragments or intact fully human antibodies were produced and tested clinically [8]. Immunoconjugation with chemotherapy agents, radioisotopes or toxins directly to targeted antibodies enhanced their efficacy [9,10]. Recently, recombinant techniques were used to generate multivalent antibodies to optimize the targeting and to create bispecific antibody molecules [11].
Many antibodies binding different target molecules have been tested in clinical trials to treat pancreatic cancer (Table 1) [201]. In most of the clinical trials, antibodies were combined with other chemotherapy agents, small molecule signal transduction inhibitors, or radiation. In some studies, radioisotopes were conjugated with antibodies before administration (Table 2).
Table 1.
Monoclonal antibodies investigated in Phase I clinical trials of pancreatic cancer.
| Target molecule | Antibody | Drugs in combined therapy | Ref. |
|---|---|---|---|
| EGFR | Cetuximab | Bortezomib | [124] |
| Erlotinib + bevacizumab | [125] | ||
| Gemcitabine + radiation | [17,126] | ||
| ABX-EGFR | Panitumumab | [23] | |
| HER2 | Trastuzumab | Tipifarnib | [33] |
| IL-12 | [35] | ||
| VEGF | Bevacizumab | Sorafenib | [127,128] |
| RAAG12 | RAV 12 | [129] | |
| MUC1 | HuPAM 4 | Y-90 radiolabeled | [89] |
| Glucoprotein with mucin properties | CC49 | Radioisotope conjugated to mAb | [130] |
| CanAg | HuC242-DM4 | Maytansinoid DM4 conjugated mAb | [131] |
| Mesothelin | MORAb-009 | [70] | |
| SS1(dsFv) PE38 | Immunotoxin conjugated | [69] | |
| Lewis Y | LMB-9 | Immunotoxin conjugated | [118] |
EGFR: EGF receptor; mAb: Monoclonal antibody; MUC: Mucin.
Table 2.
Monoclonal antibodies investigated in Phase II and III clinical trials of pancreatic cancer.
| Target molecule | Antibody | Drugs in combined therapy | Ref. |
|---|---|---|---|
| EGFR | Cetuximab | Gemcitabine | [14,18] |
| Irinotecan | [15] | ||
| Docetaxel | [15] | ||
| Cyclophosphamide/GM-CSF tumor cell vaccine | [132] | ||
| Bevacizumab/gemcitabine | [133] | ||
| Gemcitabine/oxaliplatin | [134] | ||
| Radiation | [126] | ||
| Gemcitabine/radiation | [126] | ||
| Bevacizumab/gemcitabine/capecitabine/radiation | [201] | ||
| Bortezomib | [124,135] | ||
| Bevacizumab/erlotinib | [201] | ||
| Erlotinib | [136] | ||
| EGFR | Nimotuzumab | [27] | |
| ABX-EGFR | Panitumumab | Radiation/fluorouracil/capecitabine | [137,138] |
| Gemcitabine/erlotinib | [139] | ||
| HER2 | Trastuzumab | Gemcitabine | [31] |
| Vorinostat | [34] | ||
| Tipifarnib | [33] | ||
| IL-12 | [35] | ||
| VEGF | Bevacizumab | Gemcitabine | [99,140] |
| Gemcitabine/cisplatin | [100] | ||
| Gemcitabine/oxaliplatin | [141] | ||
| Gemcitabine/capecitabine | [142] | ||
| Gemcitabine/radiation | [143–145] | ||
| Docetaxel | [146] | ||
| Fluorouracil/leucovorin/oxaliplatin | [147] | ||
| Capecitabine/radiation | [98] | ||
| Gemcitabine/oxaliplain/fluorouracil | [201] | ||
| Erlotinib/cisplatin | [201] | ||
| DR5 | CS-1008 | Gemcitabine | [44] |
| AMG 655 | Gemcitabine | [50,148] | |
| IGF-1R | IMC-A12 | Erlotinib | [59] |
| Gemcitabine | [59] | ||
| AMG 479 | Gemcitabine | [60,149] | |
| MUC1 | HuPAM 4 | Y-90 radiolabeled | [89,150] |
| Glycoprotein with property of mucin | CC49 | Radioisotope conjugated to mAb | [130] |
| CEA | MN-14 | Filgrastim | [72] |
| RAAG12 | RAV12 | Gemcitabine | [63,64] |
| Mesothelin | SS1(dsFv) PE38 | Immunotoxin conjugate | [68,69] |
| α5-β1 intergrin | Volociximab | [104] | |
| CTLA-4 | Ipilimumab | [112] | |
| TNF | Infliximab | Gemcitabine | [109] |
| CanAg | HuC242-DM4 | [92,151] | |
CEA: Carcinoembryonic antigen; CTLA-4: Cytotoxic T-lymphocyte antigen-4; DR: Death receptor; EGFR: EGF receptor; IGF-1R: IGF-1 receptor; mAb: Monoclonal antibody; MUC: Mucin; RAAG12: N-linked carbohydrate antigen.
Target molecules on the cell surface
EGF receptor
The EGF receptor (EGFR) belongs to a large family of cell surface receptors, which comprise four isotypes EGFR/ErbB1, HER2/ErbB2, HER3/ErbB3 and HER4/ErbB4. Ligands for these receptors include EGF, TGF-α, heparin-binding EGF-like growth factor and neuregulins. EGFRs are dimerized upon binding by ligands and induce activation of Ras-MAPK and PLC-γ pathways. The EGFR signal transduction pathway is essential for tissue and organ development, and regulation of cell migration, adhesion and proliferation. In many cancers, including pancreatic cancer, activated EGFR has been demonstrated to be related with many pathophysiology mechanisms, including oncogenesis, angiogenesis, apoptosis inhibition and tumor metastasis.
Cetuximab is a chimeric mouse–human antibody against an epitope located in the extra-celluar part of EGFR. Preclinical studies revealed cetuximab could decrease cell proliferation and phosphorylation of EGFR, and blocked the binding of the adaptor protein Grb2 to EGFR upon activation by EGF [12]. Clinically, cetuximab was administered to patients at an initial dose of 400mg/m 2 body area followed by weekly doses of 250 mg/m2 [13]. Synergistic effects were observed using combination therapy of cetuximab and chemotherapy agents. In a multicenter Phase II trial, patients were treated with cetuximab and gemcitabine. The results showed the median survival duration was 7.1 months [14]. In a Phase II trial, patients with pancreatic adenocarcinoma were treated with irinotecan/docetaxel with or without cetuximab. The results showed median overall survival of 7.4 months in the group treated with irinotecan/docetaxel and cetuximab, compared with 6.5 months in the group treated with irinotecan or docetaxel only [15]. Recently, Kullmann et al. reported the results of combination treatment with cetuximab and gemcitabine/oxaliplatin from a multicenter Phase II study. The overall response rate from 34 evaluable patients was 38% (with one complete and 12 partial remissions). There were 24% of patients with stable disease and 38% with progressive disease (Box 1). Median time to progression was 155 days with a preliminary 6-month survival estimate of 54% (95% CI: 37–78%) [16]. In one preclinical study reported recently, the combination of cetuximab together with gemcitabine and radiation effectively prolonged the tumor xenograft volume doubling time (30.1± 3.3 days), compared with gemcitabine monotherapy (11.6 ± 3.1 days), radiation monotherapy (16.7 ± 3.1 days), cetuximab with gemcitabine (20.1± 3.1 days) or cetuximab with radiation (22.5 ± 3.3 days) [17]. However, cetuximab failed to show a synergistic effect in combination with gemcitabine/cisplatin treatment in a Phase II trial. No significant differences were found in objective response rate (17.5% in cetuximab group, 12.2% in noncetuximab group), median progression-free survival (3.4 months in cetuximab group, 4.2 months in noncetuximab group) and median overall survival (7.5 months in cetuximab group, 7.8 months in noncetuximab group) [13]. Similarly, a Phase III study of gemcitabine plus cetuximab versus gemcitabine alone showed median survival of 6.5 months and progression-free survival of 3.5 months in the combination treatment group, versus 6 months and 3 months in the gemcitabine and cetuximab monotherapy groups, respectively [18]. Whether the sensitivity to cetuximab is related to the level of EGFR expression is uncertain. Affinity of expressed EGFR to cetuximab may be an important parameter to consider. Other factors have been reported to be related to cetuximab sensitivity, such as mutation of KRAS, PTEN expression, or host complement level [19–21]. Mutation of EGFR was a topic many investigators focused on regarding the relationship to sensitivity to cetuximab. A recent review summarized that in pancreatic cancer, the EGFR tyrosine kinase domain is highly conserved and EGFR mutation may not be predictive of sensitivities to EGFR tyrosine kinase inhibitors [22].
Box 1.
National Cancer Institute response evaluation criteria in solid tumors
Complete response: disappearance of all target lesions
Partial response: 30% decrease in the sum of the longest diameter of target lesions
Progressive disease: 20% increase in the sum of the longest diameter of target lesions
Stable disease: small changes that do not meet above criteria
Other monoclonal antibodies against EGFR
Panitumumab
Panitumumab is a fully human IgG2 antibody against EGFR. It blocked the binding of EGF and TGF-α to EGFR and subsequent phosphorylation of EGFR tyrosine kinase. Clinical efficacy of panitumumab to treat pancreatic cancer is under investigation in various clinical trials [23].
Matuzumab (EMD 72000)
This is a humanized IgG1 mAb against EGFR. Phase I studies showed this antibody significantly inhibited EGFR downstream signal transduction and was well tolerated by patients. In a Phase I clinical trial, matuzumab was given to pancreatic cancer patients at a dose of 400–800 mg once weekly for 8 weeks, followed by gemcitabine 1000 mg/m2 weekly for two cycles (3 weeks of treatment with 1 week rest in one cycle). The partial response or stable disease in 12 evaluated advanced pancreatic cancer patients was 66.7% [24]. The partial response or stable disease in 22 evaluated advanced solid tumors was 50% in another study [25]. In an early report by Burris et al. in 1997, gemcitabine was administered as a monotherapy at a similar dose for up to two cycles. Of 56 patients, 44% had partial response or stable disease [26].
Nimotuzumab
Another humanized antibody against EGFR, nimotuzumab, is currently in a clinical trial to treat pancreatic cancer [201]. In a preclinical study using non-small-cell lung cancer (NSCLC), this antibody enhanced antitumor efficacy of radiation [27].
CH 806
A chimeric mAb against mutant EGFR, CH 806, has been tested in patients with solid tumors [28]. The advantage of using this antibody is its very low normal tissue distribution. Currently, there is no information available for this antibody in pancreatic cancer treatment.
ErbB2/HER2
One isotype of the EGFR family, ErbB2/HER2, has been found to be overexpressed in many tumors, including pancreatic cancer. A recent report using improved techniques showed about 42% HER2-positive staining and 16% HER2 gene amplification in pancreatic cancer tissue samples [29]. Preclinical studies using the humanized mAb trastuzumab (Herceptin®) showed significant growth inhibition of a pancreatic cancer cell line and xenografts established with that cell line. Additive effects were observed when trastuzumab was combined with fluoropyrimidine S-1 both in vitro and in vivo (fluoropyrimidine S-1 is an oral preparation of 5-fluorouracil [5-FU] that consists of tegafur [a prodrug of 5-FU], 5-chloro-2,4-dihydoxypyridine and potassium oxonate, where the latter two can inhibit degradation of 5-FU and reduce gastrointestinal toxicity). In addition to the inhibition of the HER2 signal transduction pathway, antibody-dependent cellular cytotoxicity (ADCC) induced by trastuzumab contributed to cell growth inhibition [30]. Many effector cells in the immune system express IgG Fc receptors, which may activate the effector cells upon binding with specific antibodies. Activated effector cells may possess the ability of cytolytic, endocytic or phagocytic functions. This function of Fc receptors plays a key role in immune defense and immune surveillance. Kimura et al. tested cytotoxicity of trastuzumab in four pancreatic cancer cell lines and reported no inhibitory effect and no synergistic or additive effect of this antibody when combined with gemcitabine [31]. However, ADCC was observed in three of four cell lines and was proportional to HER2 expression [31]. Trastuzumab administration significantly inhibited tumor growth in Capan-1 xenografted mice and prolonged survival. This inhibitory effect was enhanced by the addition of gemcitabine [31]. Dual inhibition of EGFR and HER2 has been reported. Larbouret et al. showed that combination of matuzumab (anti-EGFR) and trastuzumab enhanced the inhibitory effect on HER2 phosphorylation. Combination treatment with both antibodies significantly decreased xenograft tumor sizes or induced more complete remissions as compared with either antibody alone, and prolonged survival in mice inoculated with BxPC-3 or MIA PaCa-2 pancreatic cancer cells [32]. Combination treatment with trastuzumab and other tumor inhibitory agents, such as the histone deacetylase inhibitor vorinostat, the farnesyltransferase inhibitor tipifarnib or IL-12, are being tested in clinical trials [33–35].
Pertuzumab, another antibody against HER2, is a recombinant humanized mAb. The binding site of this antibody on HER2 is different from that of trastuzumab. The binding of this antibody to HER2 blocked the receptor dimerization and subsequent signaling [36]. In a group of 21 incurable, locally advanced, recurrent or metastatic solid tumors patients, including two pancreatic cancer patients, pertuzumab was administered (0.5–15 mg/kg) every 3 weeks. Two of the patients (one pancreatic cancer patient with stable disease 15.3 months) showed partial responses. Histochemistry did not show overexpression of HER2 and HER2 gene amplification. Stable disease of greater than 2.5-month duration was observed in six patients (29%). Three patients with prostate cancer had stable disease for 2.6, 2.7 and 5.5 months. Three others who had lung cancer, colorectal cancer and ovarian cancer had stable disease for 4.1, 2.8 and 4.0 months, respectively [37].
Death receptor 5
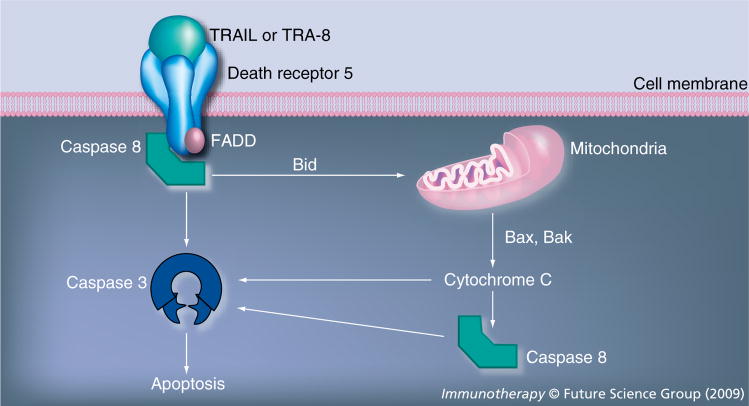
Death receptor (DR)5 is a transmembrane protein which belongs to the TNF receptor (TNFR) superfamily. It contains an extracellular domain and a cytosolic death domain. Upon binding by its ligand, TNF-related apoptosis-inducing ligand (TRAIL), the receptor forms a homotrimer which induces the formation of a death-inducing signaling complex (DISC). The trimerization of the receptor death domain recruits adaptor molecules, including Fas-associated death domain (FADD) protein in the DISC, which subsequently recruits and activates caspase 8-triggered apoptosis (Figure 1) [38–41]. Apoptosis is also induced by activation of the intrinsic mitochondrial pathway [38,42]. The intrinsic pathway is mediated by caspase 8 cleavage of the protein Bcl-2 homology domain 3 (BH3)-interfering domain death agonist (Bid). Cleaved Bid triggers mitochondrial cytochrome C release through the proapoptotic protein Bax and Bak [38,42]. Although DR5 is present in a variety of normal tissues as well as tumors, normal cells are in general resistant to TRAIL induced apoptosis. By contrast, tumor cell lines of diverse origins are sensitive to TRAIL [43].
Figure 1. Death receptor 5 activation pathway.
Death receptor 5 is a member of the TNF-receptor superfamily. When the TRAIL (or TRA-8) binds to receptor, the receptor forms a homotrimer, which subsequently induces the death-inducing signaling complex formation. DISC includes FADD, caspase 8 and cIAP or FLIP. Apoptosis is induced by the activation of caspase 8/caspase 3 as well as activation of the intrinsic mitochondrial pathway.
FADD: Fas-associated death domain protein; TRAIL: TNF-related apoptosis-inducing ligand.
CS-1008 is a humanized antihuman DR5 receptor antibody derived from the mouse antihuman DR5 antibody TRA-8 [44]. TRA-8 (IgG1κ) binds to the extracellular domain of human DR5 and competes with TRAIL for binding to DR5. TRA-8 has been shown to inhibit the proliferation of a variety of tumor cell lines and induce apoptosis both in vitro and in vivo [45,46]. We tested five human pancreatic cancer cell lines and found they had different levels of DR5 expression and different sensitivities to TRA-8. The sensitivities to TRA-8 did not correlate with the expression levels of DR5 [47]. Preclinical studies using an orthotopic pancreatic cancer mouse model (MIA PaCa-2) showed synergistic effects when gemcitabine was used together with TRA-8 [47]. In a subcutaneous mouse model (MIA PaCa-2), gemcitabine plus TRA-8 prolonged tumor-size doubling time from untreated (38 days), monotherapy with TRA-8 (49 days), or with gemcitabine (32 days) to 64 days [48]. Preclinical studies in this orthotopic mouse tumor model (MIA PaCa-2) also showed 3 weeks of treatment with TRA-8 (200 μg twice a week) and irinotecan (25 mg/kg twice a week) prolonged survival time. Mean survival was 70 ± 6.2 days in untreated mice, 84.3 ± 3.8 days in mice treated with CPT-11 alone, 90.4 ± 8.2 days in mice treated with TRA-8 and 120.6 ± 6.6 days in those receiving combination therapy. A 6-week treatment prolonged survival time by 169 days [49].
Another human antibody against DR5 (AMG 655) was developed recently by Amgen, Inc. (CA, USA), and is being evaluated in clinical trials. AMG 655 induced activation of caspase 3/7 in MIA PaCa-2 cells 3 h after treatment and decreased cell viability at 18 h. In the MIA PaCa-2 xenograft mouse model, AMG 655 significantly inhibited tumor growth at doses of 3 μg or greater biweekly. This effect was enhanced by combination treatment with gemcitabine [50].
Adams et al. reported on a human single chain antibody against DR5 (Apomab®). This antibody bound specifically to DR5 and had an overlapping DR5 binding site with the ligand TRAIL. It caused DR5 internalization and association of FADD and caspase 8. In a mouse model injected with MIA PaCa-2 cells, tumor sizes were followed for 32days after a single injection of Apomab (10 mg/kg). Complete remission was observed in ten of ten mice. On day 32, nine of the ten mice remained tumor free. In mice bearing BxPC-3 pancreatic cancer xenografts, a single treatment of Apomab induced a partial remission (reduction of tumor volume between 50–100% of its original volume) in five of ten mice. Combination of this antibody with gemcitabine (160 mg/kg, once every 3 days for four-times) caused partial remission in seven and a complete remission in one of ten mice [51]. Data from preclinical studies using TRA-8 and Apomab provide encouraging results in xenograft models of pancreatic cancer treatment. However, the pharmacologic information and toxicity of these products, especially liver damage assessment, and whether these products produce similar effects in patients need to be evaluated by clinical trials.
Other anti-DR5 antibodies including lexatumumab, HGS-ETR2, HW1 and WD1 have been reported and tested in preclinical or clinical trials [52–55]. No information is available in regard to pancreatic cancer treatment using these antibodies.
Insulin-like growth factor-1 receptor
Insulin-like growth factor-1 receptor (IGF-1R) is a transmembrane receptor tyrosine kinase and has been reported to be overexpressed in many types of tumors, including human pancreatic cancer. Activation of IGF-1R has been shown to enhance cell growth and inhibit apoptosis. In a majority of pancreatic cancers (64%), IGF-1R expression was accompanied by expression of c-Src [56] and its antiapoptotic effect was mediated by activation of MAPK and phosphatidylinositol 3-kinase (PI3K). Neid et al. reported that IGF-1R signaling played an important role in pancreatic cancer angiogenesis [57]. IGF-1R physically interacts with focal adhesion kinase (FAK) leading to proliferation and cell survival. Blocking this interaction can inhibit cell viability and increase apoptosis [58].
IMC-A12, a human IgG1 antibody (ImClone Systems Incorporated, Branchburg, NJ, USA) was tested in preclinical studies [59] and is now under clinical investigation. Blockage of activation of PI3K/AKT, p38 pathway via IGF-1R signaling may be the main mechanism for its antitumor activity. In mice bearing BxPC-3 tumors, IMC-A12 used as a single agent at 1 mg/kg every 3 days inhibited xenograft growth by 80%. A Phase I clinical trial showed two patients among 11 with refractory solid tumors had stable disease after treatment with 3 mg/kg of IMC-A12. One of these two patients had stable disease for 9 months and circulating cancer markers decreased in another patient [59].
AMG 479, a human anti-IGF-1R antibody is now under investigation in a clinical trial to treat pancreatic cancer and has been tested against Ewing’s sarcoma xenografts in mice [60]. Two other antibodies against human IGF-1R, CP-751,871 and h7C10 were tested in preclinical studies of other tumors [61,62].
A chimeric antibody against an N-linked carbohydrate antigen (RAAG12), RAV12, is now in a clinical trial in pancreatic cancer patients. RAAG12 was found to selectively coexist with many membrane proteins, including IGF-1R, ALCAM, IFN-γR1, TfR, IR, EphA2 and EGFR [63]. RAV12 induced enhanced IGF-1 mediated IGF-1R phosphorylation followed by quick desensitization, as well as phosphorylation of downstream signaling components IRS1, Erk/MAPK1/2, Akt/PKB and p70s6K [63]. Cell growth was inhibited by RAV12 only and additive or synergistic effects from MAPK inhibitors were observed in vitro. In a colon cancer xenograft model, RAV12 showed a dose-dependent inhibition of tumor growth. Tumor sizes were inhibited by 60% at 1 mg/kg intraperitroneally, three-times per week for 2 weeks. The inhibition reached 92–100% at a dose 25 mg/kg or 50 mg/kg [64]. Tumors derived from cells expressing less RAAG12, such as A549 NSCLC cells, had no responses to RAV12 treatment. Modulation of IGF-1R-activity by RAV12 is considered to be the major mechanism of this antibody [63].
Mesothelin
Mesothelin is a 40-kDa glycosyl phosphatidylinositol anchored cell surface protein. It is a cleaved product from a precursor protein encoded by the Mesothelin gene (MSLN). Another part of the cleaved product is a soluble 31-kDa megakaryocyte potentiating factor [65]. The physiologic roles of these proteins have not been identified completely. Normally, expression of mesothelin is only seen in mesothelial cells lining peritoneal, pleural and pericardial cavities. Overexpression of mesothelin protein as well as MSLN gene were found in about one third of cancers, including ovarian, pancreatic, biliary and gastroesophageal cancers [65]. One study showed that in pancreaticobiliary adenocarcinomas, mesothelin was expressed in 100% of pancreatic adenocarcinomas and bile duct adenocarcinomas, and in 89% of ampullar adenocarcinoma, but not in normal pancreas and chronic pancreatitis [66]. Overexpression of MSLN is regulated by transcription enhancer factor (TEF)-1 and its cofactor [65]. Due to its unique tissue distribution pattern in cancers and normal tissues, mesothelin has become an important target in cancer diagnosis and treatment.
Single-chain Fv against mesothelin and immunotoxin SS1(dsFv) PE38 (SS1P)
Single-chain Fv (scFv) against mesothelin was isolated from a phage display library obtained from the spleen of mice immunized with mesothelin-expression plasmid. The scFv was then fused genetically with a truncated mutant of Pseudomonas exotoxin A to obtain recombinant immunotoxin. In one preclinical study using SS1P plus radiation in treating mesothelin-expressing tumor xenografts, combination treatment significantly prolonged the doubling time of tumors [67]. Similarly, synergy was observed when gemcitabine was used together with SS1P and this treatment induced complete regression of xenografts [68]. In a Phase I clinical study, SS1P was found to be well tolerated. SS1P was administered by intravenous infusion in 34 patients with mesothelin-expressing tumors, including two pancreatic cancer patients. The dose was 8–28 μg/kg in the groups receiving the drug every other day for six doses followed by 25–60 μg/kg every other day for three doses. Of the patients tested, 12% showed minor responses (tumor size decreased between 20–50% and lasted for more than 4 weeks). In addition, 56% of the patients showed stable disease and 29% of the patients had progressive disease [69].
MORAb-009
This antibody is a chimeric of a mouse and human mAb derived from a phage-display library as described above and re-engineered [70]. In a preclinical mesothelin expressing xenograft mouse model, monotherapy using antibody alone moderately inhibited tumor growth. Combination therapy with gemcitabine significantly enhanced the effects of chemotherapy. Blockage of binding between MUC16 and tumor cells by this antibody may be a possible mechanism for this synergistic effect [70].
Carcinoembryonic antigen
Carcinoembryonic antigen (CEA) has been recognized as an overexpressed cell surface antigen in many cancers, mainly in gastrointestinal tract malignancy. The pathophysiological role in cancer growth is not well defined. It was reported to possess antiapoptotic function and may be involved in cancer invasion, adhesion and metastasis [71]. A humanized anti-CEA antibody MN-14 (labetuzumab) is now under clinical trial to treat pancreatic cancer together with filgrastim (G-CSF). It has been demonstrated that MN-14 increased median survival of lung cancer xenograft mice by 10.7–42.7 weeks when combined with G-CSF, indicating an ADCC mechanism [72]. Enhanced antitumor effect was observed when MN-14 was combined with 5-FU or CPT-11 [72]. MN-14 was labeled with 131I for radioimmunotherapy (RIT) and was combined with gemcitabine in a preclinical colon cancer xenograft study [73]. BALB/c mice bearing peritoneal tumors from LS174T cells were treated with gemcitabine (0.11 or 0.33 mg/mouse × 4), RIT monotherapy (20 μg/mouse × 1) or RIT plus gemcitabine. Median survival was 39 days in untreated mice, 52 and 57 days in gemcitabine monotherapy groups, 66 days in RIT monotherapy group, 73 days (0.11 mg/mouse of gemcitabine) and 94 days (0.33 mg/mouse) for RIT combined with gemcitabine. Another humanized anti-CEA antibody, hPRIA3, has been shown to induce tumor cells lysis in vitro, mainly via ADCC [74].
Epithelial cell adhesion molecule
Epithelial cell adhesion molecule (EpCAM) is a 40-kDa transmembrane glycoprotein normally expressed on the basolateral surface of a majority of epithelial cells. The function of this protein is not fully understood and was considered as a Ca2+-independent homophilic intercellular adhesion molecule. EpCAM was found to be overexpressed in many types of cancers. Human antibodies against EpCAM have been tested in preclinical as well as clinical trials and were shown to produce antitumor activity mediated via ADCC [75–77]. EpCAM overexpression was observed in 56% of pancreatic cancers [78]. No information is available regarding clinical trials using antibodies against EpCAM in pancreatic cancer patients.
MUC1
MUC1 is a heavily glycosylated type I membrane protein with several extracellular tandem repeat domains. These domains undergo a proteolytic cleavage from the full length of MUC1 between amino acid 1097 and 1098 after translation. However, they remain associated with the transmembrane region and cytoplasmic tail and are transported to the cell surface. The diassociation of these domains from MUC1 may be achieved by mechanical stress and may be related to the protection of the apical cell membrane of the epithelial cells from rupture [79]. MUC1 is expressed by nearly all human glandular epithelial tissues and throughout all regions of the gastrointestinal tract and its expression is largely limited to the apical membrane of the cells [80]. MUC1 expression is upregulated in almost all human adenocarcinomas with an expression pattern over the entire cell surface [80–82]. The expression of MUC1 in cancer cells was regulated by DNA methylation and histone H3 lysine 9 modification of the MUC1 promoter. Inhibition of DNA methylation enhanced MUC1 gene expression [83]. MUC1 serves as a counter-receptor for myelin-associated glycoprotein in pancreatic cancer and their interaction is considered to be related to pancreatic cancer perineural invasion [84]. The intact tandem repeats and cytoplasmic tail of MUC1 may play roles in preventing tumor invasion and metastasis [85]. Recently, Ahmad et al. reported that MUC1 was recruited to the TNF-R1 complex upon stimulation by TNF-α and interacted with the IκB kinase complex resulting in phosphorylation and degradation of IκBα, which favored cell survival [86]. MUC1 was found to block death receptor-mediated apoptosis by binding to caspase 8 and FADD [87]. Downregulation of MUC1 by RNA interference upregulated β-catenin and E-cadherin expression, promoting E-cadherin/catenin complex formation which decreased in vitro cell invasion [88].
PAM4 is a murine antibody obtained from mice immunized with purified mucin from a human pancreatic cancer xenograft sample. The binding of this antibody to MUC1 is conformation dependent and its epitope may contain carbohydrate. The humanized form, HuPAM4, has been radiolabeled with 90Y and is now under investigation in a clinical trial of pancreatic cancer patients. In a Phase I study, 15 patients with advanced pancreatic cancer received 90Y-HuPAM4 at 15–25 mCi/m2. At 4 weeks after treatment, three patients showed 32–51% tumor shrinkage, and three patients had stable disease. The major toxicity was hematologic at 25 mCi/m2 [89]. Recently, a humanized bispecific mAb – TF10 – was reported that is diavalent for mAb PAM4 and monovalent for mAb 679. mAb 679 is reactive against the histamine-succinyl-glycine hapten (HSG-hapten peptide). Pretargeting TF10 in the CaPan1 human pancreatic cancer xenograft model followed by 111In-labeled peptide IMP-288 (containing HSG groups) showed high 111In-IMP-288 uptake in tumors [90].
CC49 is a mouse mAb against a tumor-associated antigen TAG-72. TAG-72 is a cell surface glycoprotein and has characteristics of a mucin. It is expressed in many types of malignancies, including pancreas, colon, ovary, prostate, lung and esophagus, while absent in normal tissues. The antibody has been conjugated to radioisotopes for diagnosis and treatment purposes. A sFv of CC49 was developed and was shown to localize in tumors. Humanized CC49 was engineered to decrease its immunogenicity and increase the retention time in circulation after it was labeled with radioisotope. In a recent report by Baranowska-Kortylewicz et al., 131I-CC49 significantly prolonged pancreatic cancer xenograft quadrupling time and this effect was enhanced by administration of a tyrosine kinase inhibitor, imatinib [91]. The therapeutic effect of imatinib was considered to be related to inhibition of phosphorylation of platelet-derived growth factor receptor (PDGFR), a reduction in the tumor interstitial fluid pressure, and an increase in the absorbed radiation dose in tumor by 60%.
A humanized mAb HuC242 against CanAg (a glycoform of MUC1) was conjugated with an antimicrotuble agent DM1 (N2′-deacetyl-N2′-[3-mercapto-1-oxopropyl]-maytansine; cantuzumab mertansine) and tested in a Phase I clinical trial of 37 patients with solid tumors [92]. The results showed two patients with chemotherapy resistance had minor regression and four patients had stable disease during treatment. Conjugation of huC242 to another maytansinoid derivative (huC242-DM4) is now in a clinical trial of pancreatic cancer treatment.
Target molecules in tumor stroma & vasculature
VEGF
The VEGF family consists of VEGF-A, -B, -C, -D and placenta growth factor (PLGF). The receptors for the VEGF family are VEGFR-1 (Flt-1), VEGFR-2 (KDR) and VEGFR-3. The VEGF (usually referred to as VEGF-A) family as well as its receptors have been studied intensively and have been found to be very important molecules in tumor angiogenesis [93]. VEGF was found to be expressed in 93% of ductal pancreatic adenocarcinoma samples and was correlated with liver metastasis and prognosis. VEGF was not only found to be expressed in tumor cells but also in endothelial cells. It was also detected in bone marrow derived cells or mesenchymal stem cells [94,95]. Expression of VEGF-C and -D in pancreatic adenocarcinoma was found to be correlated with lymph node meastasis, lymphatic invasion and venous invasion [96]. Dineen et al. reported recently that tumor-associated macrophage infiltration was mediated through VEGF–VEGFR-2 [97]. Blocking the VEGF signals is an important antiangiogenesis approach.
Bevacizumab is a humanized murine anti-human VEGF-A mAb and has been used in the treatment of colon and breast cancer, among others, together with different chemotherapy agents [95]. A Phase I study of bevacizumab plus radiation and capecitabine in pancreatic cancer patients showed some side effect of gastrointesintal bleeding. Approximately 20% of the assessable patients had partial responses. Overall median survival was 11.6 months [98]. A PhaseII trial with bevacizumab plus gemcitabine in 52 patients showed 21% of patients had partial responses and 46% had stable disease. Median survival was 8.8 months [99]. Ko et al. reported the results of a Phase II study with bevacizumab together with fixed-dose rate gemcitabine and low-dose cisplatin. Approximately 19.2% of patients had unconfirmed response, and 57.7% had stable disease with a median survival of 8.2 months [100]. In the 2007 American Society of Clinical Oncology’s Gastrointestinal Cancer Symposium, Kindler et al. reported the CALGB study results using bevacizumab and gemcitabine from 602 patients with advanced pancreatic cancer. Combination therapy with bevacizumab plus gemcitabine did not show any beneficial effect compared with gemcitabine alone [101]. It seems that bevacizumab did not improve the response to chemotherapeutic agents in pancreatic cancer treatment [101]. Only a small portion of patients had experienced benefit from anti-VEGF therapy. Attempts to identify markers that may predict the response to bevacizumab, including plasma angiogenic factors, baseline plasma VEGF levels, as well as circulating tumor cells before and after bevacizumab treatment, were unsuccessful [100].
Integrin α5β1
Integrins are heterodimeric receptors. Binding of integrins to their ligands can induce morphological, motility and growth rate changes in endothelial cells. Ligation of integrin α5β1 promotes endothelial cell survival, migration and proliferation rates, thus inducing angiogenesis [102]. A chimeric mAb – volociximab – has been demonstrated to inhibit tumor growth in a preclinical study [103]. A Phase II clinical trial using this antibody together with gemcitabine in 20 pancreatic cancer patients (volociximab 10 mg/kg every 2 weeks plus gemcitabine 1000 mg/m2 on dayS 1, 8 and 15 of a 28-day cycle) showed one patient (5%) with partial response, and ten patients (50%) with stable disease, with an overall survival of 34% at 1 year. In another group of 20 patients who received volociximab 15 mg/kg/week on days 1, 8, 15 and 22 with gemcitabine on days 1, 8 and 15, preliminary results showed two patients with partial response (10%), seven patients with stable disease (35%), and overall survival was 4.8 months. It seems too early to evaluate the objective response of this mAb. Overall survival did not benefit from this regimen. Most frequent toxicities observed were gastrointestinal with nausea (75%), constipation (60%), diarrhea (55%) and abdominal pain, (50%). Four patients were found to have pulmonary embolism [104].
Other target molecules studied in pancreatic cancer treatment are connective tissue growth factor (CTGF), extradomain (ED)-B of fibronectin and prostate-specific membrane antigen. A human IgG1 mAb against CTGF, FG-3019, inhibited orthotopic pancreatic tumor growth in nude mice, enhanced apoptosis and decreased lymph node metastasis of tumor cells [105,106]. Combination of FG-3019 with gemcitabine had an additional effect in decreasing tumor growth [105]. Wagner et al. recently reported that IL-2-conjugated human scFv (L19) antibody against ED-B in an orthotopic pancreatic cancer mouse model inhibited tumor growth in a dose-dependent manner [107]. Tumor volumes were reduced up to 2.7% of their original size and lymph node metastasis was diminished [107]. An antibody J591 against the extracellular domain of prostate-specific membrane antigen (BZL Biologics Inc., Framingham, MA, USA) was conjugated to 111In and tested in a clinical trial of 27 patients with advanced solid tumors, including three pancreatic cancer patients [108]. The results showed the conjugated antibody successfully targeted the vascular endothelium of metastatic sites. No objective regression was observed. Three of 27 patients had stable disease [108].
Other target molecules
TNF-α
TNF-α is a cytokine that induces an inflammatory process, and has been shown to be elevated in pancreatic cancer samples. Overexpressed TNF-α either from endogenous cancer cells or exogenous sources (from immune or stromal cells) is recognized to play a role in pancreatic cancer development and progression [109]. It is also considered to be related with surgery-associated tumor recurrence and metastasis [109]. The chimeric monoclonal IgG1κ antibody against human TNF-α, infliximab, has been tested in a preclinical orthotopic xenograft model of human pancreatic cancer and is now in a clinical trial in pancreatic cancer patients [110,111]. In mice bearing xenografts, tumor volumes were inhibited with infliximab treatment by 20–30% [109]. In a Phase II clinical trial to evaluate the safety and efficacy of infliximab given together with gemcitabine to treat cancer cachexia, lean body mass was measured in the groups treated with gemcitabine plus placebo, gemcitabine plus 3mg/kg of infliximab or gemcitabine plus 5 mg/kg of the antibody. The differences among these three groups were not statistically significant. Even the infliximab (5 mg/kg, together with gemcitabine) treated group showed a higher lean body mass at 1.7 kg compared with 0.4 kg in a placebo group and 0.3 kg in a group that received 3 mg/kg of antibody [111].
Cytotoxic T-lymphocyte antigen-4
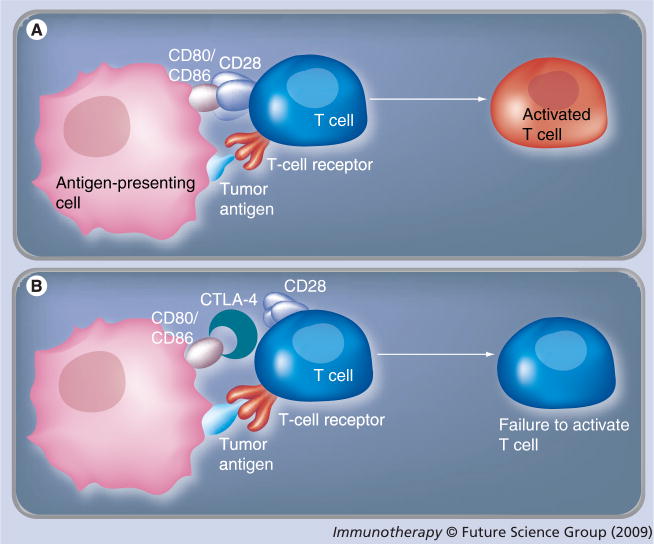
Tumor antigens can induce host immunosurveillance and initiate activation of cytotoxic T lymphocytes through the reaction between tumor antigens presented by antigen-presenting cells (APCs) with the T-cell receptor. The further activation of T cells requires binding of CD28 of T cells with CD80 (B7-1) or CD86 (B7-2) from APCs. Cytotoxic T-lymphocyte antigen (CTLA)-4, a homolog of CD28, can bind to CD80 or CD86 with a much higher affinity than CD28. This binding prevents further activation of T cells and reduces the T-cell population to a small pool of memory cells [112]. The ability of host immunosurveillance is thus compromised (Figures 2A & B ). Besides CTLA-4, a similar molecule programmed death (PD)-1 was discovered, which binds to B7-H1 to prevent activation of T cells [113]. CTLA-4 polymorphism has been detected in many diseases. Expression of CTLA-4 and PD-1 was found in tumor-infiltrating immune cells in human pancreatic cancers [113]. Thus, CTLA-4 or PD-1 blockade with antibodies may serve as an important regimen for cancer treatment.
Figure 2. T-cell activation in immunosurveillance.
Tumor antigens may be recognized and expressed by antigen-presenting cells (APCs). (A) T cells are activated by the binding of expressed tumor antigen to T-cell receptor. This activation also requires the binding of CD80 or CD86 from APCs to CD28 on T cells. (B) CTLA-4, a homolog of CD28 expressed on T cells, can bind to CD80 or CD86 with a higher affinity. This binding prevents the activation of T cells despite the binding of tumor antigen to T-cell receptor. Blocking the binding between CTLA-4 with CD80 or CD86 with antibodies against CTLA-4 may promote T-cell activation and enhance immunosurveillance.
CTLA: Cytotoxic T-lymphocyte antigen.
Ipilimumab and tremelimumab are two human anti-CTLA-4 mAbs that have been tested in clinical trials in patients with melanoma [114–116]. The treatment included monotherapy of antibody or combination therapy with chemotherapy agents, vaccines or cytokines. Immune-related adverse events (IRAEs) such as enterocolitis, dermatitis or hepatitis were reported. Ipilimumab is currently in a clinical trial of pancreatic cancer.
In addition to blockade of CTLA-4 to optimize the activation of T cells, efforts have been made to directly activate T cells by stimulating a member of the TNF superfamily, TNFRSF9 (4-1BB). Ligation of this receptor by either its ligand (TNFSF9 or 4-1BBL) or agonistic antibodies has been shown to provide a potent signal in activation of T cells [117]. It is thus proposed that combination treatment with antibodies against CTLA-4 and 4-1BB may result in an enhanced immunomodulation effect in cancer treatment [117].
Monoclonal antibodies conjugated with protein toxins in pancreatic cancer therapy
Monoclonal antibodies have been conjugated to radioisotopes, chemotherapy drugs or ribonucleases and used in cancer treatment. mAbs conjugated to modified protein toxins, such as pseudomonas exotoxin and diphtheria toxin, have been used in solid tumor treatment after successful application in hematologic malignancy therapy. These products possess specific tumor tissue binding from the specific target antibodies, as well as cytotoxic activities. A mAb against the carbohydrate moiety Lewis Y was conjugated with Pseudomonas aeruginosa exotoxin A (LMB-9) and is in Phase I clinical trials for pancreatic, esophageal, stomach, colon and rectal cancer. The patients received LMB-9 4–12 μg/kg/day for 10 days. Allergic skin reaction, reversible renal function damage and proteinuria were reported. Most patients developed antitoxin antibodies [118]. No objective responses were reported. Antibody or antibody fragment against EpCAM has been conjugated with a molecularly engineered, truncated Pseudomonas exotoxin A (ETA 252–608). The internal furin site of the toxin was replaced by a cleavage site that can be cleaved by cancer-associated proteases, gelainase A and B (MMP-2 and MMP-9) to increase the cancer tissue specificity [119]. Modified P. aeroginosa exotoxin A was also conjugated to a single chain variable fragment against human EGFR. Preclinical studies in disseminated human pancreatic cancer mouse models showed that treatment with this immunotoxin reduced the average number of lung metastases from 56.25 per mouse to 0.875 per mouse in single injection group and 0.286 per mouse in a multiple injection group [120]. Although the preclinical studies have showed encouraging results, this regimen still faces several obstacles to be used effectively. Immunogenicity from the toxin, unwanted toxicity, limited half-life and subsequently developed resistance need to be solved for the future application of immunotoxins clinically [121].
Conclusion
Immunotherapy using mAbs to treat human pancreatic adenocarcinoma has shown promising results in preclinical studies as well as in some clinical trials. A variety of antibodies have been developed to block the cancer cell growth or promote apoptosis. Targeted therapy with mAbs has established a new strategy in cancer treatment and has the potential to increase the tumor tissue specificity of chemotherapy as well as radiation therapy. Although monotherapy with mAbs alone could achieve significant effects, combination therapy together with classical chemotherapy agents or radiation has been proven more potent and is highly recommended. However, the clinical efficacy of mAb products available so far in human pancreatic cancer therapy trials is not satisfactory. Despite in-depth mechanistic studies in vitro and excellent efficacy of treatment in xenograft models, mixed results were obtained in clinical trials. Preclinical efficacy in pancreatic cancer therapy studies apparently were not translated perfectly to clinical application as expected. The mechanisms responsible for the discrepancy between clinical trials and preclinical studies are unknown. Sufficient drug delivery to tumor sites may be a critical factor that needs to be considered. Fast growth of tumor cells causes reduced vascular density and increases the space between vessels and cells. This may be more critical for protein products with large molecules, such as mAbs. A Phase III trial with the EGFR tyrosine kinase inhibitor erlotinib plus gemcitabine achieved a better overall survival rate than cetuximab plus gemcitabine [122]. Whether the small molecular inhibitor of EGFR signal transduction could be more efficiently delivered to tumor sites than cetuximab needs to be further studied. Some ligands of receptors, such as TNF-α or TRAIL, can induce dual effects in apoptosis after binding to receptors. An antiapoptotic process can be activated through the NF-κB pathway at the same time as activation of apoptosis. The fate of the cells is dependent on the net effects of pro- and anti-apoptotic balance. The net effects of mAbs on tumor cells in vivo needs more investigation. Orthotopic xenograft models or molecular analysis from fine needle aspiration samples of patients may supply valuable information in future clinical trials. It will be too naive to consider that targeted therapy would eliminate all cancer cells from the body. Cancer is not a mass of autonomous malignant cells but is more like an ‘organ’ with its repertoire of cellular matrix, vasculature and immune cells, growth factors and other secreted molecules [123]. Even if an effective therapy induced massive destruction of cancer tissue, there may still remain a possible drug-resistant subpopulation, or possibly cancer stem cells that could escape the killing. Targeted therapy with mAbs must be applied in combination with not only traditional chemotherapy or radiation therapy but other regimens, including adjustment of immune surveillance and inflammation control, among others.
Future perspective
With the identification of the molecular mechanisms associated with pancreatic cancer, more target molecules which play critical roles in tumor pathophysiology will be identified. This may enable the molecular targeting techniques using mAbs to become more specific and potent. A combination therapy protocol using mAbs and conventional chemotherapy or radiotherapy will be established clinically for pancreatic cancer. The combination may also comprise more than one mAb. Further efforts to investigate target molecule expression, mutations and dynamic changes in cancer development may help to identify reliable biomarkers in order to guide the application of mAbs with other cytotoxic agents. DNA recombinant techniques to generate multivalent antibodies as well as bispecific antibodies may help to generate new targeting molecules. Advanced nanotechnology may develop more mAb preparations with different conjugates and different functions. This may simplify the treatment, decrease the systemic side effects, and enhance the efficacy. Although mAbs have great potential in cancer treatment, molecular targeted therapy with mAbs must be combined with other regimens aimed at multiple targets to improve the treatment efficacy.
Executive summary
Molecular targeting with monoclonal antibodies
Monoclonal antibodies (mAbs) against EGF receptor (EGFR), HER2, VEGF and death receptor (DR)5 have been investigated and have produced promising results in preclinical studies. These antibodies are under extensive clinical investigation in a variety of human cancers, including pancreatic adenocarcinoma.
Combination therapy with mAbs & chemotherapy or radiotherapy
There are reports that combination therapy with mAbs against EGFR, HER2, VEGF, DR5 and chemotherapy produces increased inhibition of tumor growth in preclinical and some clinical studies. Investigation of mAbs with different chemotherapy agents is highly recommended.
Combination therapy with mAbs & chemotherapy may not have synergistic or additive effects
No synergistic or additive effect was found in a Phase II study with cetuximab and gemcitabine/cisplatin.
Combination therapy of bevacizumab and gemcitabine did not show any benefit compared with monotherapy in one clinical trial.
Lack of treatment efficacy using mAbs may be related to the special clinical & molecular characteristics of pancreatic cancer patients
Despite the promising results obtained in vitro or in preclinical animal model studies, clinical efficacy using mAbs in pancreatic cancer is still a challenge.
Further research on the clinical and molecular characteristics of pancreatic cancer may help to identify biomarkers and to establish their relationship to mAbs and combination treatment. This may promote therapy with mAbs and result in a more potent and specific pancreatic cancer treatment.
DNA recombinant technology & nanotechnology are two important tools in developing more potent mAb preparations
DNA recombinant technology to generate multivalent or bispecific antibodies and nanotechnology to conjugate mAb and cytotoxic agents with multiple functions are expected to produce more potent anticancer effects with less-systemic side effects in the future.
Acknowledgments
The authors wish to thank Sally B Lagan for manuscript preparation.
Financial & competing interests disclosure
The authors are supported in part by NCI SPORE in Pancreatic Cancer grant P20 CA10195. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
• of interest
• of considerable interest
- 1.Brenner H, Gondos A, Arndt V. Recent major progress in long-term cancer patient survival disclosed by modeled period analysis. J Clin Oncol. 2007;25:3274–3280. doi: 10.1200/JCO.2007.11.3431. [DOI] [PubMed] [Google Scholar]
- 2.Shaib YH, Davila JA, El-Serag HB. The epidemiology of pancreatic cancer in the United States: changes below the surface. Aliment Pharmacol Ther. 2006;24:87–94. doi: 10.1111/j.1365-2036.2006.02961.x. [DOI] [PubMed] [Google Scholar]
- 3.Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nat Clin Pract Oncol. 2008;5:206–219. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalofonos HP, Grivas PD. Monoclonal antibodies in the management of solid tumors. Curr Top Med Chem. 2006;6:1687–1705. doi: 10.2174/156802606778194208. [DOI] [PubMed] [Google Scholar]
- 5▪▪.Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5:147–159. doi: 10.1038/nrd1957. General review of targeted therapy with antibodies for cancer treatment. [DOI] [PubMed] [Google Scholar]
- 6.Di Cristina M, Minenkova O, Pavoni E, et al. A novel approach for identification of tumor-associated antigens expressed on the surface of tumor cells. Int J Cancer. 2007;120:1293–1303. doi: 10.1002/ijc.22395. [DOI] [PubMed] [Google Scholar]
- 7.Wu AA, Niparko KJ, Pai SI. Immunotherapy for head and neck cancer. J Biomed Sci. 2008;15:275–289. doi: 10.1007/s11373-008-9247-x. [DOI] [PubMed] [Google Scholar]
- 8.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 9.Albrecht H, DeNardo SJ. Recombinant antibodies: from the laboratory to the clinic. Cancer Biother Radiopharm. 2006;21:285–304. doi: 10.1089/cbr.2006.21.285. [DOI] [PubMed] [Google Scholar]
- 10.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 11.Karacay H, Brard PY, Sharkey RM, et al. Therapeutic advantage of pretargeted radioimmunotherapy using a recombinant bispecific antibody in a human colon cancer xenograft. Clin Cancer Res. 2005;11:7879–7885. doi: 10.1158/1078-0432.CCR-05-1246. [DOI] [PubMed] [Google Scholar]
- 12.Huang Z-q, Buchsbaum DJ, Raisch KP, et al. Differential responses by pancreatic carcinoma cell lines to prolonged exposure to erbitux (IMC–C225) anti-EGFR antibody. J Surg Res. 2003;111:274–283. doi: 10.1016/s0022-4804(03)00076-3. [DOI] [PubMed] [Google Scholar]
- 13▪▪.Cascinu S, Berardi R, Labianca R, et al. Cetuximab plus gemcitabine and cisplatin compared with gemcitabine and cisplatin alone in patients with advanced pancreatic cancer: a randomised, multicentre, Phase II trial. Lancet Oncol. 2008;9:39–44. doi: 10.1016/S1470-2045(07)70383-2. Negative Phase II study results. [DOI] [PubMed] [Google Scholar]
- 14.Xiong HQ, Rosenberg A, LoBuglio A, et al. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter Phase II trial. J Clin Oncol. 2004;22:2610–2616. doi: 10.1200/JCO.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 15.Burtness BA, Powell M, Berlin J, et al. Phase II trial of irinotecan/docetaxel for advanced pancreatic cancer with randomization between irinotecan/docetaxel and irinotecan/docetaxel plus C225, a monoclonal antibody to the epidermal growth factor receptor (EGF-r) J Clin Oncol. 2007;25:4519. [Google Scholar]
- 16▪▪.Kullmann F, Hollerbach S, Dollinger M, et al. Cetuximab plus gemcitabine/oxaliplatin (GEMOXCET) in 1st line metastatic pancreatic cancer, first results from a multicenter Phase II study. Presented at: 2007 Gastrointestinal Cancers Symposium. (Abstract128); 2007; Phase II study from a large population. [Google Scholar]
- 17.Morgan MA, Parsels LA, Kollar LE, et al. The combination of epidermal growth factor receptor inhibitors with gemcitabine and radiation in pancreatic cancer. Clin Cancer Res. 2008;14:5142–5149. doi: 10.1158/1078-0432.CCR-07-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philip P, Benedetti J, Fenoglio–Preiser C, et al. Phase III study of gemcitabine (G) plus cetuximab (C) versus gemcitabine in patients (pts) with locally advanced or metastatic pancreatic adenocarcinoma (PC): SWOG S0205 study. J Clin Oncol. 2007;25:LBA 4509. [Google Scholar]
- 19.Diaz RM, Bateman A, Emiliusen L, et al. A lentiviral vector expressing a fusogenic glycoprotein for cancer gene therapy. Gene Ther. 2000;7:1656–1663. doi: 10.1038/sj.gt.3301277. [DOI] [PubMed] [Google Scholar]
- 20.Jimeno A, Tan AC, Coffa J, et al. Coordinated epidermal growth factor receptor pathway gene overexpression predicts epidermal growth factor receptor inhibitor sensitivity in pancreatic cancer. Cancer Res. 2008;68:2841–2849. doi: 10.1158/0008-5472.CAN-07-5200. [DOI] [PubMed] [Google Scholar]
- 21.Rivera F, Vega-Villegas ME, Lopez-Brea MF. Cetuximab, its clinical use and future perspectives. Anticancer Drugs. 2008;19:99–113. doi: 10.1097/CAD.0b013e3282f23287. [DOI] [PubMed] [Google Scholar]
- 22.Kang SP, Saif MW. Pharmacogenomics and pancreatic cancer treatment. Optimizing current therapy and individualizing future therapy. JOP. 2008;9:251–266. [PubMed] [Google Scholar]
- 23.Weiner LM, Belldegrun AS, Crawford J, et al. Dose and schedule study of panitumumab monotherapy in patients with advanced solid malignancies. Clin Cancer Res. 2008;14:502–508. doi: 10.1158/1078-0432.CCR-07-1509. [DOI] [PubMed] [Google Scholar]
- 24.Graeven U, Kremer B, Sudhoff T, et al. Phase I study of the humanised anti-EGFR monoclonal antibody matuzumab (EMD 72000) combined with gemcitabine in advanced pancreatic cancer. Br J Cancer. 2006;94:1293–1299. doi: 10.1038/sj.bjc.6603083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanhoefer U, Tewes M, Rojo F, et al. Phase I study of the humanized antiepidermal growth factor receptor monoclonal antibody EMD72000 in patients with advanced solid tumors that express the epidermal growth factor receptor. J Clin Oncol. 2004;22:175–184. doi: 10.1200/JCO.2004.05.114. [DOI] [PubMed] [Google Scholar]
- 26.Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 27.Akashi Y, Okamoto I, Iwasa T, et al. Enhancement of the antitumor activity of ionising radiation by nimotuzumab, a humanised monoclonal antibody to the epidermal growth factor receptor, in non-small cell lung cancer cell lines of differing epidermal growth factor receptor status. Br J Cancer. 2008;98:749–755. doi: 10.1038/sj.bjc.6604222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott AM, Lee FT, Tebbutt N, et al. A Phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc Natl Acad Sci USA. 2007;104:4071–4076. doi: 10.1073/pnas.0611693104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsiambas E, Karameris A, Dervenis C, et al. HER2/neu expression and gene alterations in pancreatic ductal adenocarcinoma: a comparative immunohistochemistry and chromogenic in situ hybridization study based on tissue microarrays and computerized image analysis. JOP. 2006;7:283–294. [PubMed] [Google Scholar]
- 30.Saeki H, Yanoma S, Takemiya S, et al. Antitumor activity of a combination of trastuzumab (Herceptin) and oral fluoropyrimidine S-1 on human epidermal growth factor receptor 2-overexpressing pancreatic cancer. Oncol Rep. 2007;18:433–439. [PubMed] [Google Scholar]
- 31.Kimura K, Sawada T, Komatsu M, et al. Antitumor effect of trastuzumab for pancreatic cancer with high HER-2 expression and enhancement of effect by combined therapy with gemcitabine. Clin Cancer Res. 2006;12:4925–4932. doi: 10.1158/1078-0432.CCR-06-0544. [DOI] [PubMed] [Google Scholar]
- 32.Larbouret C, Robert B, Navarro-Teulon I, et al. In vivo therapeutic synergism of anti-epidermal growth factor receptor and anti-HER2 monoclonal antibodies against pancreatic carcinomas. Clin Cancer Res. 2007;13:3356–3362. doi: 10.1158/1078-0432.CCR-06-2302. [DOI] [PubMed] [Google Scholar]
- 33.Cengel KA, Voong KR, Chandrasekaran S, et al. Oncogenic K-Ras signals through epidermal growth factor receptor and wild-type H-Ras to promote radiation survival in pancreatic and colorectal carcinoma cells. Neoplasia. 2007;9:341–348. doi: 10.1593/neo.06823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumagai T, Wakimoto N, Yin D, et al. Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (Vorinostat®, SAHA) profoundly inhibits the growth of human pancreatic cancer cells. Int J Cancer. 2007;121:656–665. doi: 10.1002/ijc.22558. [DOI] [PubMed] [Google Scholar]
- 35.Parihar R, Nadella P, Lewis A, et al. A Phase I study of interleukin 12 with trastuzumab in patients with human epidermal growth factor receptor-2-overexpressing malignancies: analysis of sustained interferon-γ production in a subset of patients. Clin Cancer Res. 2004;10:5027–5037. doi: 10.1158/1078-0432.CCR-04-0265. [DOI] [PubMed] [Google Scholar]
- 36.Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2–pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 37.Agus DB, Gordon MS, Taylor C, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. 2005;23:2534–2543. doi: 10.1200/JCO.2005.03.184. [DOI] [PubMed] [Google Scholar]
- 38.Aggarwal BB, Bhardwaj U, Takada Y. Regulation of TRAIL–induced apoptosis by ectopic expression of antiapoptotic factors. Vitam Horm. 2004;67:453–483. doi: 10.1016/S0083-6729(04)67023-3. [DOI] [PubMed] [Google Scholar]
- 39.Debatin K–M, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23:2950–2966. doi: 10.1038/sj.onc.1207558. [DOI] [PubMed] [Google Scholar]
- 40.Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell. 2004;116:491–497. doi: 10.1016/s0092-8674(04)00166-7. [DOI] [PubMed] [Google Scholar]
- 41.Yagita H, Takeda K, Hayakawa Y, Smyth MJ, Okumura K. TRAIL and its receptors as targets for cancer therapy. Cancer Sci. 2004;95:777–783. doi: 10.1111/j.1349-7006.2004.tb02181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudner J, Jendrossek V, Lauber K, et al. Type I and type II reactions in TRAIL-induced apoptosis – results from dose-response studies. Oncogene. 2005;24:130–140. doi: 10.1038/sj.onc.1208191. [DOI] [PubMed] [Google Scholar]
- 43.Buchsbaum DJ, Zhou T, LoBuglio AF. TRAIL receptor-targeted therapy. Future Oncol. 2006;2:493–508. doi: 10.2217/14796694.2.4.493. [DOI] [PubMed] [Google Scholar]
- 44.Yada A, Yazawa M, Ishida S, et al. A novel humanized anti-human death receptor 5 antibody CS-1008 induces apoptosis in tumor cells without toxicity in hepatocytes. Ann Oncol. 2008;19:1060–1067. doi: 10.1093/annonc/mdn015. [DOI] [PubMed] [Google Scholar]
- 45.Buchsbaum DJ, Zhou T, Grizzle WE, et al. Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clin Cancer Res. 2003;9:3731–3741. [PubMed] [Google Scholar]
- 46.Ohtsuka T, Buchsbaum D, Oliver P, et al. Synergistic induction of tumor cell apoptosis by death receptor antibody and chemotherapy agent through JNK/p38 and mitochondrial death pathway. Oncogene. 2003;22:2034–2044. doi: 10.1038/sj.onc.1206290. [DOI] [PubMed] [Google Scholar]
- 47.DeRosier LC, Vickers SM, Zinn KR, et al. TRA-8 anti-DR5 monoclonal antibody and gemcitabine induce apoptosis and inhibit radiologically validated orthotopic pancreatic tumor growth. Mol Cancer Ther. 2007;6:3198–3207. doi: 10.1158/1535-7163.MCT-07-0299. [DOI] [PubMed] [Google Scholar]
- 48.DeRosier LC, Huang Z–Q, Sellers JC, Buchsbaum DJ, Vickers SM. Treatment with gemcitabine and TRA-8 anti- death receptor-5 mAb reduces pancreatic adenocarcinoma cell viability in vitro and growth in vivo. J Gastrointest Surg. 2006;10:1291–1300. doi: 10.1016/j.gassur.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 49.DeRosier LC, Buchsbaum DJ, Oliver PG, et al. Combination treatment with TRA–8 anti-death receptor-5 antibody and CPT–11 induces tumor regression in an orthotopic model of pancreatic cancer. Clin Cancer Res. 2007;13:S5535–S5543. doi: 10.1158/1078-0432.CCR-07-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaplan-Lefko P, Bush T, Belmontes B, et al. AMG 655, a fully human agonistic antibody against death receptor 5, enhances the anti-tumor activity of gemcitabine in MiaPaCa2/T2, a pancreatic cancer model. Presented at: American Association for Cancer Research Annual Meeting Proceedings; San Diego, CA, USA. 2008. [Google Scholar]
- 51.Adams C, Totpal K, Lawrence D, et al. Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5. Cell Death Differ. 2008;15:751–761. doi: 10.1038/sj.cdd.4402306. [DOI] [PubMed] [Google Scholar]
- 52.Park KJ, Lee SH, Kim TI, et al. A human scFv antibody against TRAIL receptor 2 induces autophagic cell death in both TRAIL-sensitive and TRAIL-resistant cancer cells. Cancer Res. 2007;67:7327–7334. doi: 10.1158/0008-5472.CAN-06-4766. [DOI] [PubMed] [Google Scholar]
- 53.Plummer R, Attard G, Pacey S, et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–6194. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 54.Shimada O, Wu X, Jin X, et al. Human agonistic antibody to tumor necrosis factor-related apoptosis-inducing ligand receptor 2 induces cytotoxicity and apoptosis in prostate cancer and bladder cancer cells. Urology. 2007;69:395–401. doi: 10.1016/j.urology.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Lin Z, Qiao CX, et al. Characterization of a novel anti-DR5 monoclonal antibody WD1 with the potential to induce tumor cell apoptosis. Cell Mol Immunol. 2008;5:55–60. doi: 10.1038/cmi.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hakam A, Fang Q, Karl R, Coppola D. Coexpression of IGF-1R and c-Src proteins in human pancreatic ductal adenocarcinoma. Dig Dis Sci. 2003;48:1972–1978. doi: 10.1023/a:1026122421369. [DOI] [PubMed] [Google Scholar]
- 57.Neid M, Datta K, Stephan S, et al. Role of insulin receptor substrates and protein kinase C-ζ in vascular permeability factor/vascular endothelial growth factor expression in pancreatic cancer cells. J Biol Chem. 2004;279:3941–3948. doi: 10.1074/jbc.M303975200. [DOI] [PubMed] [Google Scholar]
- 58.Liu W, Bloom DA, Cance WG, et al. FAK and IGF-IR interact to provide survival signals in human pancreatic adenocarcinoma cells. Carcinogenesis. 2008;29:1096–1107. doi: 10.1093/carcin/bgn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rowinsky EK, Youssoufian H, Tonra JR, et al. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res. 2007;13:5549s–5555s. doi: 10.1158/1078-0432.CCR-07-1109. [DOI] [PubMed] [Google Scholar]
- 60.Beltran PJ, Cajulis E, Moody G, et al. AMG 479, a fully human anti-IGF1R monoclonal antibody, Inhibits IGF-1 phospho-Akt and enhances the antineoplastic activity of cyclophosphamide in vivo(abstract) Proc Am Assoc Cancer Res nr 4001. 2008 [Google Scholar]
- 61.Cohen BD, Baker DA, Soderstrom C, et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP–751,871. Clin Cancer Res. 2005;11:2063–2073. doi: 10.1158/1078-0432.CCR-04-1070. [DOI] [PubMed] [Google Scholar]
- 62.Pandini G, Wurch T, Akla B, et al. Functional responses and in vivo anti-tumour activity of h7C10: a humanised monoclonal antibody with neutralising activity against the insulin-like growth factor-1 (IGF-1) receptor and insulin/IGF-1 hybrid receptors. Eur J Cancer. 2007;43:1318–1327. doi: 10.1016/j.ejca.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 63.Li JC, Li R. RAV12 accelerates the desensitization of Akt/PKB pathway of insulin-like growth factor I receptor signaling in COLO205. Cancer Res. 2007;67:8856–8864. doi: 10.1158/0008-5472.CAN-07-0971. [DOI] [PubMed] [Google Scholar]
- 64.Loo D, Pryer N, Young P, et al. The glycotope-specific RAV12 monoclonal antibody induces oncosis in vitro and has antitumor activity against gastrointestinal adenocarcinoma tumor xenografts in vivo. Mol Cancer Ther. 2007;6:856–865. doi: 10.1158/1535-7163.MCT-06-0581. [DOI] [PubMed] [Google Scholar]
- 65▪▪.Hucl T, Brody JR, Gallmeier E, et al. High cancer-specific expression of mesothelin (MSLN) is attributable to an upstream enhancer containing a transcription enhancer factor dependent MCAT motif. Cancer Res. 2007;67:9055–9065. doi: 10.1158/0008-5472.CAN-07-0474. Regulation of unique expression of mesothelin in cancer. [DOI] [PubMed] [Google Scholar]
- 66.Hassan R, Laszik ZG, Lerner M, et al. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124:838–845. [PubMed] [Google Scholar]
- 67.Hassan R, Williams-Gould J, Steinberg SM, et al. Tumor-directed radiation and the immunotoxin SS1P in the treatment of mesothelin-expressing tumor xenografts. Clin Cancer Res. 2006;12:4983–4988. doi: 10.1158/1078-0432.CCR-06-0441. [DOI] [PubMed] [Google Scholar]
- 68.Hassan R, Broaddus VC, Wilson S, Liewehr DJ, Zhang J. Anti-mesothelin immunotoxin SS1P in combination with gemcitabine results in increased activity against mesothelin-expressing tumor xenografts. Clin Cancer Res. 2007;13:7166–7171. doi: 10.1158/1078-0432.CCR-07-1592. [DOI] [PubMed] [Google Scholar]
- 69.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 70.Hassan R, Ebel W, Routhier EL, et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20. [PMC free article] [PubMed] [Google Scholar]
- 71.Blumenthal RD, Hansen HJ, Goldenberg DM. In vitro and in vivo anticancer efficacy of unconjugated humanized anti-CEA monoclonal antibodies. Br J Cancer. 2008;99:837–838. doi: 10.1038/sj.bjc.6604548. author reply 839–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blumenthal RD, Osorio L, Hayes MK, et al. Carcinoembryonic antigen antibody inhibits lung metastasis and augments chemotherapy in a human colonic carcinoma xenograft. Cancer Immunol Immunother. 2005;54:315–327. doi: 10.1007/s00262-004-0597-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koppe MJ, Oyen WJ, Bleichrodt RP, et al. Combination therapy using gemcitabine and radioimmunotherapy in nude mice with small peritoneal metastases of colonic origin. Cancer Biother Radiopharm. 2006;21:506–514. doi: 10.1089/cbr.2006.21.506. [DOI] [PubMed] [Google Scholar]
- 74.Conaghan P, Ashraf S, Tytherleigh M, et al. Targeted killing of colorectal cancer cell lines IgG1 monoclonal antibody by a humanised that binds to membrane-bound carcinoembryonic antigen. Br J Cancer. 2008;98:1217–1225. doi: 10.1038/sj.bjc.6604289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lutterbuese P, Brischwein K, Hofmeister R, et al. Exchanging human Fcα1 with murine Fcα2a highly potentiates anti-tumor activity of anti-EpCAM antibody adecatumumab in a syngeneic mouse lung metastasis model. Cancer Immunol Immunother. 2007;56:459–468. doi: 10.1007/s00262-006-0218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oberneder R, Weckermann D, Ebner B, et al. A Phase I study with adecatumumab, a human antibody directed against epithelial cell adhesion molecule, in hormone refractory prostate cancer patients. Eur J Cancer. 2006;42:2530–2538. doi: 10.1016/j.ejca.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 77.Ruan HH, Scott KR, Bautista E, Ammons WS. ING-1(heMAb), a monoclonal antibody to epithelial cell adhesion molecule, inhibits tumor metastases in a murine cancer model. Neoplasia. 2003;5:489–494. doi: 10.1016/s1476-5586(03)80033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fong D, Steurer M, Obrist P, et al. Ep-CAM expression in pancreatic and ampullary carcinomas: frequency and prognostic relevance. J Clin Pathol. 2008;61:31–35. doi: 10.1136/jcp.2006.037333. [DOI] [PubMed] [Google Scholar]
- 79.Macao B, Johansson DG, Hansson GC, Hard T. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat Struct Mol Biol. 2006;13:71–76. doi: 10.1038/nsmb1035. [DOI] [PubMed] [Google Scholar]
- 80.Levi E, Klimstra DS, Andea A, Basturk O, Adsay NV. MUC1 and MUC2 in pancreatic neoplasia. J Clin Pathol. 2004;57:456–462. doi: 10.1136/jcp.2003.013292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patel KN, Maghami E, Wreesmann VB, et al. MUC1 plays a role in tumor maintenance in aggressive thyroid carcinomas. Surgery. 2005;138:994–1001. doi: 10.1016/j.surg.2005.09.030. discussion 1001–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zaretsky JZ, Barnea I, Aylon Y, et al. MUC1 gene overexpressed in breast cancer: structure and transcriptional activity of the MUC1 promoter and role of estrogen receptor α (ERα) in regulation of the MUC1 gene expression. Mol Cancer. 2006;5:57. doi: 10.1186/1476-4598-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamada N, Nishida Y, Tsutsumida H, et al. MUC1 expression is regulated by DNA methylation and histone H3 lysine 9 modification in cancer cells. Cancer Res. 2008;68:2708–2716. doi: 10.1158/0008-5472.CAN-07-6844. [DOI] [PubMed] [Google Scholar]
- 84.Swanson BJ, McDermott KM, Singh PK, et al. MUC1 is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Res. 2007;67:10222–10229. doi: 10.1158/0008-5472.CAN-06-2483. [DOI] [PubMed] [Google Scholar]
- 85.Kohlgraf KG, Gawron AJ, Higashi M, et al. Contribution of the MUC1 tandem repeat and cytoplasmic tail to invasive and metastatic properties of a pancreatic cancer cell line. Cancer Res. 2003;63:5011–5020. [PubMed] [Google Scholar]
- 86▪▪.Ahmad R, Raina D, Trivedi V, et al. MUC1 oncoprotein activates the IκB kinase κ complex and constitutive NF-κB signalling. Nat Cell Biol. 2007;9:1419–1427. doi: 10.1038/ncb1661. New role for an old molecule MUC1 in a signal transduction pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agata N, Ahmad R, Kawano T, et al. MUC1 oncoprotein blocks death receptor-mediated apoptosis by inhibiting recruitment of caspase-8. Cancer Res. 2008;68:6136–6144. doi: 10.1158/0008-5472.CAN-08-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yuan Z, Wong S, Borrelli A, Chung MA. Down-regulation of MUC1 in cancer cells inhibits cell migration by promoting E-cadherin/catenin complex formation. Biochem Biophys Res Commun. 2007;362:740–746. doi: 10.1016/j.bbrc.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 89.Gulec SC, Cohen SJ, Zuckier LS, et al. First clinical experience with 90Y–radiolabeled humanized anti-MUC1 antibody (hPAM4) in patients with advanced pancreatic cancer: a Phase I study. J Clin Oncol. 2007;25:15034. [Google Scholar]
- 90.Gold DV, Goldenberg DM, Karacay H, et al. A novel bispecific, trivalent antibody construct for targeting pancreatic carcinoma. Cancer Res. 2008;68:4819–4826. doi: 10.1158/0008-5472.CAN-08-0232. [DOI] [PubMed] [Google Scholar]
- 91.Baranowska-Kortylewicz J, Abe M, Nearman J, Enke CA. Emerging role of platelet-derived growth factor receptor-α inhibition in radioimmunotherapy of experimental pancreatic cancer. Clin Cancer Res. 2007;13:299–306. doi: 10.1158/1078-0432.CCR-06-1702. [DOI] [PubMed] [Google Scholar]
- 92.Mita M, Ricart A, Mita A, et al. A Phase I study of a CanAg-targeted immunoconjugate, huC242-DM4, in patients with CanAg-expressing solid tumors. J Clin Oncol. 2007;25 (Abstract 3062) [Google Scholar]
- 93.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 94.Beckermann BM, Kallifatidis G, Groth A, et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. 2008;99:622–631. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shojaei F, Ferrara N. Antiangiogenic therapy for cancer: an update. Cancer J. 2007;13:345–348. doi: 10.1097/PPO.0b013e31815a7b69. [DOI] [PubMed] [Google Scholar]
- 96.Zhang B, Zhao WH, Zhou WY, et al. Expression of vascular endothelial growth factors-C and -D correlate with evidence of lymphangiogenesis and angiogenesis in pancreatic adenocarcinoma. Cancer Detect Prev. 2007;31:436–442. doi: 10.1016/j.cdp.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 97.Dineen SP, Lynn KD, Holloway SE, et al. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res. 2008;68:4340–4346. doi: 10.1158/0008-5472.CAN-07-6705. [DOI] [PubMed] [Google Scholar]
- 98.Crane CH, Ellis LM, Abbruzzese JL, et al. Phase I trial evaluating the safety of bevacizumab with concurrent radiotherapy and capecitabine in locally advanced pancreatic cancer. J Clin Oncol. 2006;24:1145–1151. doi: 10.1200/JCO.2005.03.6780. [DOI] [PubMed] [Google Scholar]
- 99.Kindler HL, Friberg G, Singh DA, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033–8040. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- 100.Ko AH, Dito E, Schillinger B, et al. A Phase II study evaluating bevacizumab in combination with fixed-dose rate gemcitabine and low-dose cisplatin for metastatic pancreatic cancer: is an anti-VEGF strategy still applicable? Invest New Drugs. 2008 doi: 10.1007/s10637-008-9127-2. [DOI] [PubMed] [Google Scholar]
- 101▪▪.Gemcitabine plus bevacizumab no better than gemcitabine alone in clinical study of 600 patients with pancreatic cancer. Cancer Biol Ther. 2007;6:137 . Important clinical report from a large population. [PubMed] [Google Scholar]
- 102.Bhaskar V, Zhang D, Fox M, et al. A function blocking anti-mouse integrin α5β1 antibody inhibits angiogenesis and impedes tumor growth in vivo. J Transl Med. 2007;5:61. doi: 10.1186/1479-5876-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhaskar V, Fox M, Breinberg D, et al. Volociximab, a chimeric integrin α5β1 antibody, inhibits the growth of VX2 tumors in rabbits. Invest New Drugs. 2008;26:7–12. doi: 10.1007/s10637-007-9078-z. [DOI] [PubMed] [Google Scholar]
- 104.Evans T, Valle J, Berlin J, et al. Interim results from a Phase II study of volociximab in combination with gemcitabine (GEM) in patients (pts) with metastatic pancreatic cancer (MPC). Presented at: 2008 Gastrointestinal Cancers Symposium.; 2008. Abstract 142. [Google Scholar]
- 105.Aikawa T, Gunn J, Spong SM, Klaus SJ, Korc M. Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Mol Cancer Ther. 2006;5:1108–1116. doi: 10.1158/1535-7163.MCT-05-0516. [DOI] [PubMed] [Google Scholar]
- 106.Dornhofer N, Spong S, Bennewith K, et al. Connective tissue growth factor-specific monoclonal antibody therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2006;66:5816–5827. doi: 10.1158/0008-5472.CAN-06-0081. [DOI] [PubMed] [Google Scholar]
- 107.Wagner K, Schulz P, Scholz A, Wiedenmann B, Menrad A. The targeted immunocytokine L19–IL2 efficiently inhibits the growth of orthotopic pancreatic cancer. Clin Cancer Res. 2008;14:4951–4960. doi: 10.1158/1078-0432.CCR-08-0157. [DOI] [PubMed] [Google Scholar]
- 108.Milowsky MI, Nanus DM, Kostakoglu L, et al. Vascular targeted therapy with anti-prostate-specific membrane antigen monoclonal antibody J591 in advanced solid tumors. J Clin Oncol. 2007;25:540–547. doi: 10.1200/JCO.2006.07.8097. [DOI] [PubMed] [Google Scholar]
- 109.Egberts JH, Cloosters V, Noack A, et al. Anti-tumor necrosis factor therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2008;68:1443–1450. doi: 10.1158/0008-5472.CAN-07-5704. [DOI] [PubMed] [Google Scholar]
- 110.Robinson DW, Jr, Eisenberg DF, Cella D, et al. The prognostic significance of patient-reported outcomes in pancreatic cancer cachexia. J Support Oncol. 2008;6:283–290. [PubMed] [Google Scholar]
- 111.Wiedenmann B, Malfertheiner P, Friess H, et al. A multicenter, Phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. J Support Oncol. 2008;6:18–25. [PubMed] [Google Scholar]
- 112.O’Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer. 2007;110:2614–2627. doi: 10.1002/cncr.23086. [DOI] [PubMed] [Google Scholar]
- 113.Loos M, Giese NA, Kleeff J, et al. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008;268:98–109. doi: 10.1016/j.canlet.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 114.Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a Phase I/II study. Ann Surg Oncol. 2005;12:1005–1016. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Phan GQ, Weber JS, Sondak VK. CTLA -4 blockade with monoclonal antibodies in patients with metastatic cancer: surgical issues. Ann Surg Oncol. 2008 doi: 10.1245/s10434-008-0104-y. [DOI] [PubMed] [Google Scholar]
- 116.Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864–872. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- 117.Lynch DH. The promise of 4 -1BB (CD137)-mediated immunomodulation and the immunotherapy of cancer. Immunol Rev. 2008;222:277–286. doi: 10.1111/j.1600-065X.2008.00621.x. [DOI] [PubMed] [Google Scholar]
- 118.Hausner P, Karp J, Edelman M, et al. Phase I study of LMB-9 (B3(dsFv)PE38), a recombinant disulfide stabilized anti-Lewis Y immunotoxin administered by continuous infusion. Proc Am Soc Clin Oncol. 2003;22 [Google Scholar]
- 119.Zangemeister-Wittkke U, Di Paolo C. Methods for treating cancer using an immunotoxin comprising an exotoxin A moiety having a furin cleavage site replaced with a cancer-associated protease site cleaved by MMP-2 or MMP-9. 2006 16.02.2006. (patent) [Google Scholar]
- 120.Bruell D, Bruns CJ, Yezhelyev M, et al. Recombinant anti-EGFR immunotoxin 425(scFv)-ETA’ demonstrates anti-tumor activity against disseminated human pancreatic cancer in nude mice. Int J Mol Med. 2005;15:305–313. [PubMed] [Google Scholar]
- 121.Kreitman RJ. Immunotoxins for targeted cancer therapy. AAPS J. 2006;8:E532–E551. doi: 10.1208/aapsj080363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a Phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 123.Mohla S. Tumor microenvironment. J Cell Biochem. 2007;101:801–804. doi: 10.1002/jcb.21320. [DOI] [PubMed] [Google Scholar]
- 124.Ryan DP, O’Neil BH, Supko JG, et al. A Phase I study of bortezomib plus irinotecan in patients with advanced solid tumors. Cancer. 2006;107:2688–2697. doi: 10.1002/cncr.22280. [DOI] [PubMed] [Google Scholar]
- 125.Sloss CM, Wang F, Liu R, et al. Proteasome inhibition activates epidermal growth factor receptor (EGFR) and EGFR-independent mitogenic kinase signaling pathways in pancreatic cancer cells. Clin Cancer Res. 2008;14:5116–5123. doi: 10.1158/1078-0432.CCR-07-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Krempien R, Muenter MW, Huber PE, et al. Randomized Phase II study evaluating EGFR targeting therapy with cetuximab in combination with radiotherapy and chemotherapy for patients with locally advanced pancreatic cancer–PARC: study protocol (ISRCTN56652283) BMC Cancer. 2005;5:131. doi: 10.1186/1471-2407-5-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Minami H, Kawada K, Ebi H, et al. Phase I and pharmacokinetic study of sorafenib, an oral multikinase inhibitor, in Japanese patients with advanced refractory solid tumors. Cancer Sci. 2008;99:1492–1498. doi: 10.1111/j.1349-7006.2008.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Siu LL, Awada A, Takimoto CH, et al. Phase I trial of sorafenib and gemcitabine in advanced solid tumors with an expanded cohort in advanced pancreatic cancer. Clin Cancer Res. 2006;12:144–151. doi: 10.1158/1078-0432.CCR-05-1571. [DOI] [PubMed] [Google Scholar]
- 129.Molckovsky A, Siu LL. First-in-class, first-in-human Phase I results of targeted agents: highlights of the 2008 American Society of Clinical Oncology meeting. J Hematol Oncol. 2008;1:20. doi: 10.1186/1756-8722-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kennel SJ, Brechbiel MW, Milenic DE, Schlom J, Mirzadeh S. Actinium-225 conjugates of MAb CC49 and humanized delta CH2CC49. Cancer Biother Radiopharm. 2002;17:219–231. doi: 10.1089/108497802753773847. [DOI] [PubMed] [Google Scholar]
- 131.Erickson HK, Park PU, Widdison WC, et al. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 2006;66:4426–4433. doi: 10.1158/0008-5472.CAN-05-4489. [DOI] [PubMed] [Google Scholar]
- 132.Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kindler H, Bylow K, Hochster H, et al. A randomized Phase II study of bevacizumab (B) and gemcitabine (G) plus cetuximab (C) or erlotinib (E) in patients (pts) with advanced pancreatic cancer (PC): a preliminary analysis. J Clin Oncol. 2006;24 Abstract 4040. [Google Scholar]
- 134.Merchan J, Venkatraman A, Macintyre J, et al. A pilot study of gemcitabine (g), oxaliplatin (o), cetuximab (c) for locally advanced or metastatic pancreatic cancer. J Clin Oncol. 2008;26 Abstract 11510. [Google Scholar]
- 135.Tang ZY, Wu YL, Gao SL, Shen HW. Effects of the proteasome inhibitor bortezomib on gene expression profiles of pancreatic cancer cells. J Surg Res. 2008;145:111–123. doi: 10.1016/j.jss.2007.03.061. [DOI] [PubMed] [Google Scholar]
- 136.Preston G, Calvo E, Papadopoulos K, et al. Multi-targeted inhibition of the epidermal growth factor (EGFR) and vascular endothelial growth factor receptor (VEGFR) pathways: a Phase I study of cetuximab (C), erlotinib (E), and bevacizumab (B) in patients with solid tumors. J Clin Oncol. 2006;24 Abstract 3005. [Google Scholar]
- 137.Chu E. Dual biologic therapy in the first-line mCRC setting: implications of the CAIRO2 study. Clin Colorectal Cancer. 2008;7:226. doi: 10.3816/CCC.2008.n.030. [DOI] [PubMed] [Google Scholar]
- 138.Lee JJ, Chu E. An update on treatment advances for the first-line therapy of metastatic colorectal cancer. Cancer J. 2007;13:276–281. doi: 10.1097/PPO.0b013e3181570062. [DOI] [PubMed] [Google Scholar]
- 139.Rocha-Lima CM, Soares HP, Raez LE, Singal R. EGFR targeting of solid tumors. Cancer Control. 2007;14:295–304. doi: 10.1177/107327480701400313. [DOI] [PubMed] [Google Scholar]
- 140.Kindler H, Niedzwiecki D, Hollis D, et al. A double-blind, placebo-controlled, randomized Phase III trial of gemcitabine (G) plus bevacizumab (B) versus gemcitabine plus placebo (P) in patients (pts) with advanced pancreatic cancer (PC); a preliminary analysis of cancer and Leukemia Group B (CALGB) J Clin Oncol. 2007;25 doi: 10.1200/JCO.2010.28.1386. Abstract 4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim G, Oberg A, Foster N, et al. Phase II trial of bevacizumab, gemcitabine, oxaliplatin in patients with metastatic pancreatic adenocarcinoma. 2007 ASCO Annual Meeting Proceedings Part I. J Clin Oncol. 2007;25 Abstract 4553. [Google Scholar]
- 142.Iyer R, Yu J, Garrett C, et al. Multicenter phase II study of gemcitabine, capecitabine, and bevacizumab in patients with advanced pancreatic cancer (APC): final analysis of clinical and quality of life endpoints. J Clin Oncol. 2008;26 Abstract 4616. [Google Scholar]
- 143.Moser A, Zeh H, Ramanathan R, et al. Preoperative treatment of potentially–resectable pancreatic adenocarcinoma with fixed–dose rate gemcitabine (GEM), bevacizumab (BEV), and 30 Gy radiotherapy (RT) J Clin Oncol. 2008;26:4631. doi: 10.1245/s10434-013-3161-9. Abstract number. [DOI] [PubMed] [Google Scholar]
- 144.Small W, Mulcahy M, Benson B, et al. A Phase II trial of weekly gemcitabine and bevacizumab in combination with abdominal radiation therapy in patients with localized pancreatic cancer. J Clin Oncol. 2007;25 Abstract 15043. [Google Scholar]
- 145.Varadhachary G, Wolff R, Crane CH, et al. Preoperative gemcitabine (gem) and bevacizumab (bev)-based chemoradiation for resectable pancreatic adenocarcinoma. J Clin Oncol. 2008;26 doi: 10.1200/JCO.2007.15.8642. Abstract 4630. [DOI] [PubMed] [Google Scholar]
- 146.Astsaturov I, Meropol N, Alpaugh R, et al. A randomized Phase II andcoagulation study of bevacizumab alone or with docetaxel in patients with previously treated metastatic pancreatic adenocarcinoma. J Clin Oncol. 2007;25 doi: 10.1097/COC.0b013e3181d2734a. Abstract 4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Allegra C, Yothers G, O’connell M, Sharif S, Wolmark N. Initial safety report of NSABP C-08, a randomized Phase III study of modified 5-fluorouracil (5-FU)/leucovorin (LCV) and oxaliplatin (OX) (mFOLFOX6) with or without bevacizumab (bev) in the adjuvant treatment of patients with stage II/III colon cancer. J Clin Oncol. 2008;26 doi: 10.1200/JCO.2009.21.9220. Abstract 4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wall J, Colloton M, Huard J, et al. AMG 655, a monoclonal antibody agonist directed against death receptor 5, induces apoptosis in human colon carcinoma cell lines and its therapeutic potential is enhanced in combination with chemotherapeutic agents. [abstract]. Proceedings of the 99th Annual Meeting of the American Association for Cancer Research; 12–16 April; San Diego, CA, USA. Philadelphia (PA): AACR; 2008. Abstract 1326. [Google Scholar]
- 149.Calzone F, Chaung Y, Cajulis E, et al. Domain-specific mechanisms of receptor inhibition by AMG 479, a fully human IGF1R targeted antibody. Proceedings of the 99th Annual Meeting of the American Association for Cancer Research; 12–16 April; San Diego, CA, USA. Philadelphia (PA): AACR; 2008. Abstract 3994. [Google Scholar]
- 150.Gold DV, Karanjawala Z, Modrak DE, Goldenberg DM, Hruban RH. PAM4-reactive MUC1 is a biomarker for early pancreatic adenocarcinoma. Clin Cancer Res. 2007;13:7380–7387. doi: 10.1158/1078-0432.CCR-07-1488. [DOI] [PubMed] [Google Scholar]
- 151.Qin A, Watermill J, Mastico R, et al. The pharmacokinetics and pharmacodynamics of IMGN242 (huC242-DM4) in patients with CanAg-expressing solid tumors. J Clin Oncol. 2008;26 Abstract 3066. [Google Scholar]
Website
- 201.Clinical Trials.gov, a service of the US NIH . www.ckubucaktruaks.gov.