Abstract
Leptin is a hormone whose central role is to regulate endocrine functions and to control energy expenditure. After the discovery that leptin can also have pro-inflammatory effects, several studies have tried to address - at the molecular level - the pathways involved in leptin-induced modulation of the immune functions in normal and pathologic conditions. The signaling events influenced by leptin after its binding to the leptin receptor have been under scrutiny in the past few years, and considerable experimental work has elucidated the consequences of leptin effects on immune cells. This review examines the biochemistry, function and regulation of leptin signaling in view of possible intervention on this molecule for a better management and therapy of immune-mediated diseases.
Introduction
The past few years of research on leptin have provided important insights into the intricate network that links metabolism and immune homeostasis. Leptin is a 16-kD hormone mainly produced by the adipose tissue that has the peculiarity of also acting as a cytokine (1).
As hormone, leptin helps monitoring body weight (as fat content) to adjust the metabolic rate, whereas as cytokine leptin can exert a prominent action on innate and adaptive immune responses (2, 3). The signalling events that follow the binding of leptin to its receptors have been studied extensively and characterized at the biochemical and molecular levels in many systems and, more recently, in relation to immune responses. This review highlights the most recent advances in this field and discusses the newly envisioned possibilities of a modulation of leptin signalling for immunotherapeutic intervention.
Leptin receptors
In addition to its hormonal activity, leptin effects on the immune system can be explained by the fact that leptin is a cytokine with a long-chain four-helical bundle typical of the type I cytokine family (4). Moreover, the pleiotropic biological effects of leptin can be explained by the wide distribution of leptin receptors on different types of cells, including those in extraneural tissues.
Structurally, leptin receptors (OB-R) belong to class I cytokine family receptors (which include the receptors of IL-2, IL-3, IL-4, IL-6, IL-7 and granulocyte colony-stimulating factor (GM-CSF) (5–11).
Leptin receptors have characteristic extracellular motifs of four cysteine residues and contain the aminoacid sequence WSXWS (Trp-Ser-Xaa-Trp-Ser) (12) and fibronectin type III domains (13, 14). These receptors are produced as isoforms derived from alternative splicing of RNA transcripts of the db gene, and are designated OB-Ra, OB-Rb, OB-Rc, OB-Rd, OB-Re and OB-Rf (15). OB-Rb is expressed at high levels in the hypothalamus and, at lower levels, in many peripheral tissues (16, 17). OB-Ra and OB-Rc are highly expressed in choroid plexus and microvessels, where they may play a role in leptin uptake or efflux from the cerebrospinal fluid, as well as in receptor-mediated transport of leptin across the blood–brain barrier (18).
All isoforms have a common extracellular domain of more than 800 amino acids, a transmembrane domain of 34 amino acids, and a variable intracellular domain characteristic of each isoform. Among the six isoforms, the main differences are in intracellular amino acids: 301 for OB-Rb, and 34, 32 and 40, respectively, for the short forms OB-Ra, OB-Rc and OB-Rd. The OB-Re isoform lacks the transmembrane and cytoplasmic parts, acting as a soluble receptor (19, 6, 15) which likely contributes to the regulation of plasma leptin levels by binding with high affinity free circulating leptin (20, 21).
Another classification of OB-R isoforms is in three subclasses: short, long and secreted receptors. The long form of OB-R seems to be the only functional (signalling) isoform, while the lack of a full-length leads to the obese phenotype in db/db mice (22) – a phenotype rescued by neuronal transgenic expression of the long-form of OB-R (23).
The short and long isoforms share the extracellular and transmembrane domains as well as the same first 29 intracellular amino acids, diverging in sequence following alternative splicing of the 3′ exons. Moreover, the OB-R extracellular domain has two cytokine-like receptor motifs and four fibronectin type III domains (5, 8, 9). Studies with mutant receptors have revealed that only the second putative binding domain mediates leptin binding and receptor activation, with an affinity in the nanomolar range (24).
Both long and short OB-R isoforms are capable to form homodimers in the absence of ligand (25–27) and the formation of dimers seems necessary for signalling (28). Considering that each OB-R binds one molecule of leptin, the result of dimerization is the formation of a tetrameric receptor/ligand complex (27) that induces a conformational change in the receptor structure which is critical for activation (24).
Intracellularly, the OB-R does not have an intrinsic tyrosine kinase domain, therefore it binds cytoplasmic kinases - mainly Janus tyrosine kinase (Jak)2 (29).
Like other cytokine receptors, OB-R contains a highly conserved, proline-rich box1 (30) and two putative, less conserved, box2 motifs (29, 31, 32). Box1 and box2 motifs are considered important in recruiting and binding Jaks (33, 34), yet recent studies showed that only box1 and surrounding amino acids (31–36) were essential for full Jak activation (32, 35), being two conserved aminoacids (Leu896, Phe897) crucial for signalling (35). However, although an intact box2 motif seems not required to activate Jaks (32, 35), it is nonetheless important for full induction of the signal transducer and activator of transcription (STAT) signalling pathway (36).
Jak/STATs
The finding of a homology of OB-R with other class I cytokine receptors has long suggested the possibility that leptin binding might mediate cytokine receptor-like signals, including the activation of Jaks and STATs (37–39).
The Jak/STAT pathway is mostly activated by interferons, interleukins or other cytokines whose receptors lack intrinsic kinase activity, and comprises a family of four non-receptor Jaks and seven 85–95 kDa transcription factors (STATs) that are regulated by phosphorylation on specific serine and tyrosine residues.
Of the four known members of the Jak family, Jak1, Jak2 and tyrosine kinase 2 (Tyk2) are widely expressed, while Jak3 is found only in cells of the haematopoietic system (40). Recent studies indicate that, under physiological conditions, only Jak2 is activated during OB-R signaling (32).
Activated Jaks transphosphorylate each other, as well as other tyrosine residues (Tyr985, Tyr1138 and Tyr 1077) of the OB-R (41, 42), providing docking sites for downstream molecules such as STATs. At the end, OB-R signalling by leptin results in STAT3 binding (37, 43–48), and activation of STAT1 (37, 43), STAT5 (37, 43, 45) and STAT6 (43). Then, recruited STATs become tyrosine-phosphorylated by Jaks (14), with subsequent dissociation from the receptor and the formation of homo- or heterodimers. STAT dimers translocate into the nucleus and act as transcription factors by binding specific response elements in the promoter region of their target genes, such as sis-inducible-element (SIE), acute-phase-response-element (APRE) and GAS-like elements (14, 37, 43,).
Notably, the three intracellular tyrosine residues of OB-Rb exhibit different capabilities for downstream activation signalling. Tyr985 is required for the activation of the Ras/Raf/ERK pathway. The phosphorylation of Tyr985 creates a binding site for the C-terminal SH2 domain of the tyrosine phosphatase SHP-2, leading to the activation of the canonical p21Rasρ/extracellular regulated kinase (ERK) signalling cascade through the recruitment of the SH2-domain-containing adapter protein, growth factor receptor binding-2 (Grb-2) (9). While either Tyr1077 or Tyr1138 are required for leptin-induced tyrosine phosphorylation of STAT5, Tyr1138 is essential for the activation of STAT1 and STAT3 (49, 50).
Also, Tyr1077 appears as the only intracellular tyrosine residue sufficient to induce tyrosine phosphorylation of STAT5 and STAT5-driven reporter gene activity in vitro [50]. In db/db mice lacking the long form of the leptin receptor, impaired STAT signalling has been demonstrated (38), suggesting that db phenotypes are caused by a failure of STAT signalling. Confirming a role of STAT signalling in the control of body weight, leptin-deficient ob/ob mice show significantly lower STAT3 in the hypothalamic arcuate nucleus (51).
Leptin seems to exert its effects through the Jak/STAT pathway not only in the hypothalamus to control body weight, but also on immune cells. In blood mononuclear cells, leptin increases Jak2/3 and STAT3 phosphorylation, which promote proliferation and activation of T lymphocytes upon PHA-stimulation. On anti-CD3/CD28 stimulated T regulatory cells, leptin neutralization results in the phosphorylation of STAT3 associated with degradation of p27kip1 (52).
Suppressors of Cytokine Signalling (SOCS)
The Jak/STAT pathway of cytokine signalling is under the negative-feedback control of suppressors of cytokine signalling (SOCS) proteins (53, 54). SOCS proteins are induced upon cytokine stimulation and attenuate signalling by various cytokine receptors, allowing possible cross-regulation among several cytokine systems.
Members of the SOCS family, which contain an SH2 domain, are induced by a variety of cytokines, acting as negative regulators of signalling by binding to phosphorylated Jak proteins or by direct interaction with tyrosine phosphorylated receptors. The family of SOCS proteins consists of 8 members: cytokine inducible Src-like homology 2 (SH-2) protein (CIS) and SOCS1 through SOCS7. Structurally, SOCS proteins are characterized by a central SH-2 domain, an N-terminal preSH-2 domain, with an ESS (extended SH-2 subdomain) region and in some cases a kinase inhibitory region (KIR) domain, which abolishes the kinase activity of the Jaks, and a more conserved C-terminal SOCS-box (55)- a key mediator of proteasomal degradation (by linking ubiquitin to the substrate).
Only SOCS1 and 3 carry a KIR domain in their N-terminal region involved in the inhibition of the Jak activity. They both inhibit Ob-R signalling, using different mechanisms. SOCS1 directly interacts with the kinase domain of Jak2 by targeting the phosphotyrosine Y1007 in the Jak2 activation loop (56, 57). The KIR domain association with the catalytic groove of Jak2 suggests that it might act as a pseudosubstrate mimicking the activation loop that regulates the access to the catalytic groove (it could obstruct the ATP binding pocket and hinder accessibility for substrates) (56, 57). Differently from SOCS1, SOCS3 has only weak affinity for Jak2. It is thought to inhibit kinase activity through its KIR domain after the binding through its SH-2 domain with phosphotyrosine motifs in the receptor in the proximity of the Jaks (58).
Interestingly, leptin can induce SOCS3 expression (59–63) and the Tyr985 of OB-Rb is a high-affinity binding site for SOCS3 (19, 41, 59, 62, 64). Endogenous SOCS3 expression inhibits tyrosine phosphorylation of OB-R, thus providing an important feedback mechanism for receptor signalling at the transcriptional level (62). In this context, the participation of SOCS3 in the negative-feedback mechanism of leptin signalling has been proposed to underlie the development of leptin resistance in relation to the hyperleptinaemia observed in the context of the majority of obesity cases (41).
Other SOCS proteins, in addition to the negative regulatory effects, can have positive effects on cytokine signalling (65, 66). For example, SOCS2 can interfere with other SOCS proteins on several cytokine receptors, including OB-R signalling (65, 67–69). SOCS2 can impair the inhibitory effect of SOCS1 or SOCS3 on leptin-induced signalling (69), and it can interfere with the association of CIS to the OB-R membrane proximal tyrosine (70). Also SOCS6 and SOCS7 can interact with the OB-R. SOCS7 may be implicated in OB-R signalling termination (71), i.e. by inhibiting STAT3 activation and/or by interacting with activated STAT3 to prevent translocation to the nucleus (72).
PTP 1B
PTP1B (protein tyrosine phosphatase 1B) is another negative regulator of leptin signalling (and also insulin signalling (73, 74) both in vivo and in vitro via dephosphorylation of Jak2 (75–77). Under physiological conditions, the effects of PTP1B are likely to be exerted via central and peripheral actions. Mice lacking PTP1B are resistant to developing diet-induced obesity and do not exhibit hyperphagia despite a clear hypoleptinemia (75). It appears that PTP1B-mediated hypophosphorylation of Jak2 can abrogate leptin-dependent induction of the STAT3 and MAPK-inducible SOCS3 and c-fos genes, respectively (76).
Src-like homology 2 (SH2) domain containing protein tyrosine phosphatase (SHP-2)
Kinases are typically considered “dominant” regulators of intracellular signalling. However, in the past few years there has been growing attention on the study of phosphatases, which have opposite effects to kinases.
SH-2 domain-containing phosphatase-2 (SHP-2) is a constitutively expressed tyrosine phosphatase that regulates leptin signalling (78). SHP-2 is involved in the dephosphorylation of Jaks in vitro and down-modulates Jak2 and STAT3 activation in vivo (79).
SHP-2 carries two tandem SH-2 domains followed by a tyrosine phosphatase catalytic domain (80). When one SH-2 domain interacts with a tyrosine phosphorylated ligand, a conformational change occurs and brings this phosphatase to activation of OB-R at position Y985 (indicated by the fact that in 293T cells SHP-2 binding to OB-Rb is prevented by the Y985 mutation) (80).
The role of SHP-2 in OB-R signalling has been matter of debate, and initially suggested as inhibitor of OB-R signalling (mutation of the Y986 position in the human OB-R leads to increased STAT3 signalling) (81). It is known that SHP-2 strongly activates the MAPK pathway and the SHP-2 induction of the ERK pathway can enhance leptin signalling, switching towards the MAPK signalling. ERK activation occurs predominantly through SHP-2 recruitment at tyrosine Y985 via its C-terminal SH-2 domain. SHP-2 can be phosphorylated by Jak2 to form a docking site for the adaptor protein Grb2, leading to the activation of the ERK signalling cascade (41). Alternatively, ERK can be activated directly by Jak2, but still requires the intervention of SHP-2 (19).
Cyclic AMP and PDE
Cyclic AMP activates protein kinase A (PKA) and plays a pivotal role in the crosstalk between many signalling systems (82, 83). Metabolism of cAMP, and cGMP, is controlled by a family of PDE enzymes (84).
Leptin decreases cAMP levels in pancreatic β-cells via the activation of PDE3B (85), and the inhibition of GLP-1-stimulated insulin secretion (85) is one of the most evident physiological consequence of the response to leptin.
5′-AMP-activated protein Kinase (AMPK)
Leptin stimulates fatty acid oxidation to exert protective effects against lipotoxicity in the non-adipose tissues (86). Leptin selectively activates the α2 catalytic subunit of AMPK in skeletal muscle, which stimulates fatty-acid oxidation by blocking the effect of ACC (acetyl-CoA carboxylase) (87). AMPK represents a heterotrimeric enzyme that functions as a ‘fuel gauge’ to monitor cellular energy status (88, 89). AMPK regulates food intake by responding to hormonal and nutrient signals in the hypothalamus (88). Activation of AMPK represents a signal to shut down anabolic pathways and to promote catabolic processes in response to a decrease in the ATP/AMP ratio by phosphorylating key enzymes of intermediary metabolism. In parallel with AMPK activation, leptin suppresses ACC activity, thereby stimulating β-oxidation in muscle by disinhibiting carnitine palmitoyltransferase 1 (CTP1). The direct activation of AMPK by leptin can explain, at least in part, the findings that leptin increases both in vitro and in vivo glucose uptake and metabolism.
Phosphatidylinisitol (PI) 3-kinase (PI3K)
PI 3-kinase activity is regulated by a wide spectrum of ligands, in particular growth factors such as insulin (90). The binding of PI3K regulatory subunit to tyrosine-phosphorylated proteins induces a conformational change allowing the activation of its catalytic subunit and consequent full activation of PI3K. This event leads this kinase to add a phosphate to the 3′ position of the inositol ring of phosphatidylinositols, allowing switching of protein kinase-dependent cascades to lipid-dependent signalling cascades (90). PI 3-kinase products typically stimulate protein kinases such as Akt, also called protein kinase B (PKB) and protein kinase C (PKC) (90).
Leptin can act through some of the components of the insulin signalling cascade, as most insulin-dependent actions involve the activation of PI3k (91). The binding of insulin to its receptor recruits several IRSs (insulin receptor substrates) that are tyrosine-phosphorylated by the intrinsic kinase activity of the receptor. This suggests a potential crosstalk between insulin and leptin signalling pathways (although the magnitude of PI3k stimulation in response to leptin is less than with insulin) (92).
The stimulation of PI3K leads to activation of PtdIns(3,4,5)P3-dependent serine/threonine kinases such as PDK1 (phosphoinositide-dependent kinase 1), which can activate Akt, a key serine/threonine kinase in subsequent downstream signalling. Although the contribution of insulin versus leptin-induced PI3K stimulation to signalling has to be better clarified, the current findings suggest that the PI3K/PDE3B/cAMP pathway interacting with the Jak2/STAT3 cascade represents a critical component of leptin signalling (53).
Protein kinase B (PKB or Akt)
PKB is a serine/threonine kinase that plays an important role in many cellular processes including cell survival and carbohydrate metabolism (93)The binding of its PH domain to D3-phosphorylated phosphoinositides leads to PKB activation and subsequent phosphorylation on T308 and S473 residues (93).
Cell treatment with leptin induces Akt phosphorylation (94), although - as for PI-3K - the magnitude of this effect is small and much less than that produced by insulin.
Other studies suggest that the ability of leptin to stimulate Akt may depend on high intracellular cAMP levels, and this mediator might inhibit a phosphatase resulting in leptin-induced increase Akt phosphorylation (53).
Protein kinase C (PKC)
Leptin has been shown to have both stimulatory and inhibitory effects on PKC, a serine/threonine kinase implicated, like PKB, in a wide range of cellular effects (95). Leptin seems to decrease insulin release from pancreatic islets of ob/ob mice in response to PKC stimulation (96), leptin action in pancreatic islets may involve inhibition of the PKC-regulated component of the phospholipase C (PLC)–PKC signalling system (that normally elicits insulin secretion) (97).
MAPK
ERK members of the MAPK family are serine/threonine kinases of 44 and 42 kDa activated by a wide range of stimuli, including leptin, and are components of the Ras/MAPK signalling cascade (98).
The MAPK pathway can be stimulated by either the long or the short isoform of OB-R (at lesser extent by the former) (19, 41) - an observation that supports the idea that the distal portion of the OB-R may not be essential for activation of MAPK.
It is possible that leptin can stimulate the MAPK pathway in two different ways: via tyrosine phosphorylation of Jak2 receptor-associated activation, or independently of receptor phosphorylation.
In any case, Tyr985 of the long OB-R isoform has an important role in leptin-induced ERK activation (41). As a result of leptin administration, Tyr985 becomes phosphorylated by recruited Jaks (mainly Jak2 and Jak1), and provides a docking site for SHP-2. After binding to that specific tyrosine residue, SHP-2 is phosphorylated at the C-terminus. This phosphorylated form, together with its adapter molecule Grb-2, activates downstream signalling, leading to the activation of the p21Ras/ERK signalling cascade (41). Subsequent steps lead to the activation of ras and raf molecules, followed by the activation of MEK1 (99). The final consequences of ERK activation by leptin are many and include induction of specific target genes expression, such as c-fos or egr-1, a zinc-finger transcription factor that influences the initiation of growth and differentiation (100, 101).
The activation of the MAPK signalling cascade by leptin has been observed both in vitro (30, 41) and in vivo, both centrally and peripherally (101, 102, 103).
In monocytes, leptin induces expression and secretion of the IL-1 receptor antagonist (IL-1Ra) using the MAPK pathway that activates NF-κB (104). Also, the expansion of T regulatory cells following anti-leptin neutralization is mediated by the induction of ERK1/2 phosphorylation (52).
Leptin has also been shown to induce apoptosis through the MAPK pathway in precursor cells of the osteoblastic lineage. In this case, ERK1/2 activates cytosolic phospholipase A2 (cPLA2) that leads to cytochrome c release and caspase-3 and -9 activation, which coordinate cell death (105).
Finally, a wide range of stimuli, including cytokines, heat shock, osmotic stress, and ultraviolet light are can activate another member of the MAP kinase family, p38 MAPK (106). Treatment of human mononuclear cells with leptin increases p38 MAPK phosphorylation (107). However, a study in L6 muscle cells suggests that leptin does not alter p38 MAP kinase phosphorylation per se but rather reduces insulin-stimulated p38 MAP kinase phosphorylation (108).
Finally, leptin shares with other cytokines, growth factors and stressors, the ability to activate the stress-activated protein kinase c-Jun N-terminal kinase (JNK). For example, leptin enhances tumour necrosis factor (TNF)-α production via p38 and JNK MAPK (109). Among the possible downstream targets of leptin-induced activation of p38 and JNK MAPK pathways, the regulation of the transcription factor NF-κB appears important for the transcriptional regulation of pro-inflammatory cytokines such as TNFα and IL-1β.
Leptin signalling in immune cells
OB-R lacks an intrinsic tyrosine kinase activity, and requires activation of receptor-associated kinases of the Jaks (38), which initiate downstream signalling and activate STATs (37) which dimerize and translocate to the nucleus, where specific gene responses are elicited (110).
Leptin stimulates and promotes the proliferation of human peripheral blood mononuclear cells (PBMC) (4). The presence of OB-R on monocytes and lymphocytes has been shown in mice (2, 111) and confirmed in human peripheral blood T lymphocytes (both CD4 and CD8) (112).
The Jak–STAT signalling pathway triggered by leptin stimulation has been studied in human PBMC (47). It was shown that Jak2/3 are activated by tyrosine phosphorylation, and this effect is transient. Both Jak isoforms are physically associated with OB-R, as indicated by the co-immunoprecipitation of OB-R with Jak2 or Jak3. A preassociation of Jak2 with OB-R has also been described (38, 40).
In PBMC, leptin stimulation induces tyrosine phosphorylation and translocation to the nucleus of STAT3 molecules (47, 110, 113), in addition to the phosphorylation of the STAT3 associated RNA binding protein Sam68 (39, 40, 114, 115) (a tyrosine phosphorylated adaptor protein in T cell receptor activation that is associated with the SH2 and SH3 domains of Src and other signalling momlecules, such as Grb2, PLC-γ-1, and PI3K) (116–119). Since Sam68 is an RNA-binding protein and tyrosine phosphorylation is known to modulate RNA binding activity, the data implicate possible regulation of RNA as a component of tyrosine kinases signalling pathways (117–119), i.e. through modification of the mRNA stability and/or translation.
This PI3K pathway has also been explored in PBMC responses to human leptin. PI3K activity associated with tyrosine phosphorylated proteins is increased more than 3-fold after leptin stimulation (120–122). Leptin treatment, similarly to insulin response, induced tyrosine phosphorylation of Sam68 and IRS-1, which associated with p85 (123, 124), the regulatory subunit of PI3K via the SH2 domain, recruiting and leading to stimulation of PI3K activity (122). It is not yet known whether Tyr-phosphorylated Sam68 contributes to the increase in PI3K activity along with IRS-1, or whether it is only working as a docking protein.
Recently, leptin was shown to inhibit apoptosis in the thymus through an IRS-1/PI3K-dependent and Jak2-indipendent pathway (125). Treatment with leptin reduced thymic apoptosis, an effect that was not inhibited by the JAK inhibitor AG490 but that was inhibited by the PI3K inhibitor LY294002 and by antisense oligonucleotides to IRS-1 (125).
Moreover, the activation of MAPK by leptin in PBMC has also been assessed. Both ERK-1 and ERK-2 were found phosphorylated in a dose-dependent fashion in PBMC after incubation with human leptin (122). It was also found that leptin could induce sustained phosphorylation of p38 MAPK in human PBMCs (126), and the phosphorylation of the ribosomal protein S6 - the only protein in the large 40S subunit that has been shown to be phosphorylated in response to growth factors and mitogens (126). One route of leptin-induced S6 phosphorylation in human PBMCs is via MEK and p42/p44 MAPK (19, 127–129), which activate MAPK-dependent S6 Kinase p90 RSK and S6. The other route seems to be mediated via activation of p70 S6 kinase, since it has been shown that leptin phosphorylates p70 S6 kinase at Thr389, the mammalian target of rapamycin (mTOR) target residue in the linker domain - an event that plays a central role in p70 activation (130). Accordingly, pre-tratment of cells with rapamycin (inhibitor of mTOR) abolished this phosphorylation and substantially reduced S6 phosphorylation (130). Strikingly, the MEK inhibitor PD98059 not only inhibited p90 RSK phosphorylation, as expected, but also abolished p70 S6 Kinase and S6 phosphorylation, suggesting an essential role of MEK activation in a full induction of p70 S6 kinase activity in human PBMC (131, 132).
In CD4+CD25− T cells, leptin induced strong STAT3 phosphorylation, while stimulation of CD4+CD25+ T cells was not associated with a marked increase of phosphorylated STAT3 (52). SOCS3, a negative regulator of cytokine signaling, was activated by leptin blockade in CD4+CD25+ T cells, in which the stimulation with anti-CD3/28 induced phosphorylation of ERK1/2 and subsequent cell proliferation (52). In the same subset of cells, the cyclin-dependent kinase inhibitor p27 (p27kip1, a molecule involved in the control of cell cycle and T cell anergy) was elevated before and after anti-CD3/28 stimulation, and leptin neutralization induced degradation of this molecule, partly explaining the reversal of the anergic state and proliferation of these cells.
In macrophages, ERK-mediated phosphorylation of Ser727 was found required for full stimulation of STAT3 by leptin (133). Macrophages express high levels of OB-Rb, and leptin stimulates STAT3 phosphorylation on both Tyr705 and Ser727 (133). While the MEK-1 inhibitor PD98059 had no effect on leptin-stimulated phosphorylation of STAT3 Tyr705, it greatly attenuated leptin’s effects on STAT3 Ser727 phosphorylation (133). Leptin-induced ERK activation in macrophages showed a biphasic pattern, with an initial reduction in ERK phosphorylation and a subsequent increased phosphorylation of ERK (133) paralleled by phosphorylation of Ser727 and STAT3 DNA binding activity.
In human neutrophils, leptin was found to have activity as chemoattractant devoid of secretagogue properties but capable of inhibiting chemotaxis to classical neutrophilic chemoattractants (134). This effect was dependent on the activation of intracellular kinases involved in F-actin polymerization and neutrophil locomotion (134). Indeed, p38 MAPK and Src kinase, but not ERK, were activated by short-term incubation with leptin (135). Moreover, the p38 MAPK inhibitor SB203580 and Src kinase inhibitor PP1, but not the MEK inhibitor PD98059, blocked neutrophils chemotaxis toward leptin (ref.).
In neutrophils, the ability of leptin to delay apoptosis seemed secondary to effects on the PI3K and MAPK-dependent pathways (136). Additionally, leptin delayed the cleavage of Bid and Bax, the mitochondrial release of cytocrome c and mitochondria-derived activator of caspase, as well as the activation of both caspase-8 and caspase-3 in neutrophils (136). The use of the specific inhibitors SB203580 and LY294002 (respectively a selective inhibitor of p38 MAPK and PI3K) blocked the anti-death effects of leptin, suggesting the key role of these pathways in transducing leptin-mediated antiapoptotic signals in neutrophils (136). The same effects of leptin mediated by PI3K and MAPK were observed on eosinophils (137).
It is possible that leptin signaling can also play a role in dendritic cell (DC) development and function. Optimal level of STAT3 activation induced by leptin are critically important for normal DC differentiation (138), and PI3K/Akt, p38 MAPK and NF-kB signalling pathways seem to have a central role in the survival of DC. In immature and LPS-induced matured DC in db/db mice, markedly reduced levels of active Akt and active PDK1 were observed (139.). In line with those findings, active PTEN (a major negative regulator of PI3K/Akt signaling pathway) was also found up-regulated (139). Finally, both immature and LPS-matured db/db DC had decreased level of c-Raf phosphorylation at the inhibitory binding site (ser 259) (139), and db/db DC had decreased level of active p38 and phosphorylated IkB-α and high level of IkB-α (139). Taken together, these findings suggest that OB-R deficiency can impair signal transduction - including the Akt, MAPK and NF-kB pathways – in DC, affecting their survival and maturation.
Conclusions
Leptin is a multifunctional hormone cytokine that is involved in processes as disparate yet intimate as metabolism and immune response. Because of its broad action, signalling pathways triggered by the OB-R can affect intracellular transduction pathways in different types of cells and/or promote cross talking among different cells.
Although many effects of leptin have been elucidated in recent times, the details of the signalling events that govern the response to leptin need further investigation to understand how the different pathways downstream of leptin are ultimately integrated. It will be also worthwhile to focus in the future on how leptin signalling integrates with the intracellular cascades activated by other factors in the immune cells, to have a better view of leptin’s actions - ultimately allowing new strategies of therapeutic targeting.
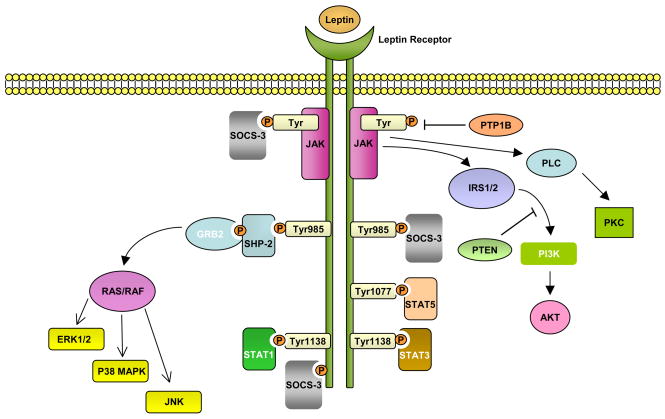
Figure Schematic diagram of the pathways influenced by leptin.
After leptin binds to the long isoform of the leptin receptor (OB-Rb), Jak2 is activated at the box1 motif, resulting in the autophosphorylation of tyrosine residues and phosphorylation of tyrosines that provide docking sites for signalling proteins containing src homology 2 (SH2) domains. The autophosphorylated Jak2 at the box 1 motif can phosphorylate insulin receptor substrate1/2 (IRS1/2) that leads to activation of phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Akt can regulate a wide range of targets including FOXO1 and NF-κB. Activation of NF-κB after leptin binding has been shown to induce Bcl-2 and Bcl-XL expressions. Leptin binding to OB-Rb can also activate the phospholipase C (PLC) for stimulation of c-jun N-terminal protein kinase (JNK) via protein kinase C (PKC).
Both Tyr1077 and Tyr1138 bind to STAT5, whereas only Tyr1138 recruits STAT1 and STAT3. STAT3 proteins form dimers and translocate to the nucleus to induce expression of genes such as c-fos, c-jun, egr-1, activator protein-1 (AP-1) and suppressors of cytokine signaling 3 (SOCS3). SOCS3 negatively regulates signal transduction by leptin by binding to phosphorylated tyrosines on the receptor, to inhibit the binding of STAT proteins and the SH2 domain-containing phosphatase 2 (SHP2). SHP2 activates the mitogen-activated protein kinase (MAPK) pathways including extracellular signal-regulated kinase (ERK1/2), p38 MAPK and p42/44 MAPK through an interaction with the adaptor protein growth factor receptor-bound protein 2 (GRB2), to induce cytokine and chemokine expression in immune cells. SOCS2 binds to Tyr1077 and might interfere with STAT5 binding. After stimulation with leptin, Src associated in mitosis protein 68 (Sam68) can form a complex with activated STAT3, leading to its dissociation from RNA. Sam68 can also be directly activated by Jak2 to phosphorylate IRS1/2 for Akt activation.
References
- 1.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 2.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Margalet V, Martin-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clin Exp Immunol. 2003;133:11–9. doi: 10.1046/j.1365-2249.2003.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matarese G, La Cava A, Sanna V, et al. Balancing susceptibility to infection and autoimmunity: a role for leptin? Trends Immunol. 2002;23:182–7. doi: 10.1016/s1471-4906(02)02188-9. [DOI] [PubMed] [Google Scholar]
- 5.Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 6.Lee GH, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–5. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 7.Lollmann B, Gruninger S, Stricker-Krongrad A, Chiesi M. Detection and quantification of the leptin receptor splice variants Ob-Ra, b, and e in different mouse tissues. Biochem Biophys Res Commun. 1997;238:648–52. doi: 10.1006/bbrc.1997.7205. [DOI] [PubMed] [Google Scholar]
- 8.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 9.Myers MG. Leptin receptor signaling and the regulation of mammalian physiology. Rec Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 10.Bazan JF. A novel family of growth factor receptors: a common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor β chain. Biochem Biophys Res Commun. 1989;164:788–95. doi: 10.1016/0006-291x(89)91528-3. [DOI] [PubMed] [Google Scholar]
- 11.Hegyi K, Fülöp K, Kovács K, Tóth S, Falus A. Leptin-induced signal transduction pathways. Cell Biol Int. 2004;28:159–69. doi: 10.1016/j.cellbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Bazan JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci USA. 1990;87:6934–8. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243–1254. [PubMed] [Google Scholar]
- 14.Heim MH. The Jak-STAT pathway: specific signal transduction from the cell membrane to the nucleus. Eur J Clin Invest. 1996;26:1–12. doi: 10.1046/j.1365-2362.1996.103248.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang MY, Zhou YT, Newgard CB, Unger RH. A novel leptin receptor isoform in rat. FEBS Lett. 1998;392:87–90. doi: 10.1016/0014-5793(96)00790-9. [DOI] [PubMed] [Google Scholar]
- 16.Siegrist-Kaiser CA, Pauli V, Juge-Aubry CE, et al. Direct effects of leptin on brown and white adipose tissue. J Clin Invest. 1997;100:2858–64. doi: 10.1172/JCI119834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kielar D, Clark JS, Ciechanowicz A, Kurzawski G, Sulikowski T, Naruszewicz M. Leptin receptor isoforms expressed in human adipose tissue. Metabolism. 1998;47:844–7. doi: 10.1016/s0026-0495(98)90124-x. [DOI] [PubMed] [Google Scholar]
- 18.Hileman SM, Pierroz DD, Masuzaki H, et al. Characterization of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology. 2002;143:775–83. doi: 10.1210/endo.143.3.8669. [DOI] [PubMed] [Google Scholar]
- 19.Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–95. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 20.Lammert A, Kiess W, Bottner A, Glasow A, Kratzsch J. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem Biophys Res Commun. 2001;283:982–988. doi: 10.1006/bbrc.2001.4885. [DOI] [PubMed] [Google Scholar]
- 21.Chan JL, Bluher S, Yiannakouris N, Suchard MA, Kratzsch J, Mantzoros CS. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes. 2002;51:2105–12. doi: 10.2337/diabetes.51.7.2105. [DOI] [PubMed] [Google Scholar]
- 22.Chua SC, Chung WK, Wu-Peng XS, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–6. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 23.Kowalski TJ, Liu SM, Leibel RL, Chua SC. Transgenic complementation of leptin-receptor deficiency: Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes. 2001;50:425–35. doi: 10.2337/diabetes.50.2.425. [DOI] [PubMed] [Google Scholar]
- 24.Fong TM, Huang RR, Tota MR, et al. Localization of leptin binding domain in the leptin receptor. Mol Pharmacol. 1998;53:234–40. doi: 10.1124/mol.53.2.234. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima K, Narazaki M, Taga T. Leptin receptor (OB-R) oligomerizes with itself but not with its closely related cytokine signal transducer gp130. FEBS Lett. 1997;403:79–82. doi: 10.1016/s0014-5793(97)00013-6. [DOI] [PubMed] [Google Scholar]
- 26.White DW, Tartaglia LA. Evidence for ligand-independent homo-oligomerization of leptin receptor (OB-R) isoforms: A proposed mechanism permitting productive long-form signaling in the presence of excess short-form expression. J Cell Biochem. 1999;73:278–88. [PubMed] [Google Scholar]
- 27.Devos R, Guisez Y, Van der Heyden J, et al. Ligand-independent dimerization of the extracellular domain of the leptin receptor and determination of the stoichiometry of leptin binding. J Biol Chem. 1997;272:18304–10. doi: 10.1074/jbc.272.29.18304. [DOI] [PubMed] [Google Scholar]
- 28.White DW, Kuropatwinski KK, Devos R, Baumann H, Tartaglia LA. Leptin receptor (OB-R) signaling. Cytoplasmic domain mutational analysis and evidence for receptor homo-oligomerization. J Biol Chem. 1997;272:4065–71. doi: 10.1074/jbc.272.7.4065. [DOI] [PubMed] [Google Scholar]
- 29.Ghilardi N, Skoda RC. The leptin receptor activates Janus kinase 2 and signals for proliferation in a factor-dependent cell line. Mol Endocrinol. 1997;11:393–9. doi: 10.1210/mend.11.4.9907. [DOI] [PubMed] [Google Scholar]
- 30.White DW, Wang DW, Chua SC, Jr, et al. Constitutive and impaired signaling of leptin receptors containing the Gln -> Pro extracellular domain fatty mutation. Proc Natl Acad Sci USA. 1997;94:10657–62. doi: 10.1073/pnas.94.20.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chua SC, Koutrs IK, Han L, et al. Fine structure of the murine leptin receptor gene: splice site suppression is required to form two alternatively spliced transcripts. Genomics. 1997;45:264–70. doi: 10.1006/geno.1997.4962. [DOI] [PubMed] [Google Scholar]
- 32.Kloek C, Haq AK, Dunn SL, Lavery HJ, Banks AS, Myers MG. Regulation of Jak kinases by intracellular leptin receptor sequences. J Biol Chem. 2002;277:41547–55. doi: 10.1074/jbc.M205148200. [DOI] [PubMed] [Google Scholar]
- 33.Jiang N, He TC, Miyajima A, Wojchowski DM. The box1 domain of the erythropoietin receptor specifies Janus kinase 2 activation and functions mitogenically within an interleukin 2 beta-receptor chimera. J Biol Chem. 1996;271:16472–6. doi: 10.1074/jbc.271.28.16472. [DOI] [PubMed] [Google Scholar]
- 34.Murakami M, Narazaki M, Hib M, et al. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc Natl Acad Sci USA. 1991;88:11349–53. doi: 10.1073/pnas.88.24.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bahrenberg G, Behrmann I, Barthel A, et al. Identification of the critical sequence elements in the cytoplasmic domain of leptin receptor isoforms required for Janus kinase/signal transducer and activator of transcription activation by receptor heterodimers. Mol Endocrinol. 2002;16:859–72. doi: 10.1210/mend.16.4.0800. [DOI] [PubMed] [Google Scholar]
- 36.Murakami T, Yamashita T, Iida M, Kuwajima M, Shima K. A short form of leptin receptor performs signal transduction. Biochem Biophys Res Commun. 1997;231:26–9. doi: 10.1006/bbrc.1996.6030. [DOI] [PubMed] [Google Scholar]
- 37.Baumann H, Morella KK, White DW, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA. 1996;93:8374–8. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci USA. 1996;93:6231–5. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2002;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ihle IN. Cytokine receptor signalling. Nature. 1995;377:591–4. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 41.Banks AS, Davis SM, Bates SH, Myers MG. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–72. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 42.Eyckerman S, Waelput W, Verhee A, Broekaert D, Vandekerckhove J, Tavernier J. Analysis of Tyr to Phe and fa/fa leptin receptor mutations in the PC12 cell line. Eur Cytokine Netw. 1999;10:549–56. [PubMed] [Google Scholar]
- 43.Bendinelli P, Maroni P, Pecori Giraldi F, Piccoletti R. Leptin activates Stat3, Stat1 and AP-1 in mouse adipose tissue. Mol Cell Endocrinol. 2000;168:11–20. doi: 10.1016/s0303-7207(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 44.Briscoe CP, Hanif S, Arch JR, Tadayyon M. Fatty acids inhibit leptin signalling in BRIN-BD11 insulinoma cells. J Mol Endocrinol. 2001;26:145–54. doi: 10.1677/jme.0.0260145. [DOI] [PubMed] [Google Scholar]
- 45.Briscoe CP, Hanif S, Arch JR, Tadayyon M. Leptin receptor long-form signalling in a human liver cell line. Cytokine. 2001;14:225–9. doi: 10.1006/cyto.2001.0871. [DOI] [PubMed] [Google Scholar]
- 46.Goiot H, Attoub S, Kermorgant S, et al. Antral mucosa expresses functional leptin receptors coupled to STAT-3 signaling, which is involved in the control of gastric secretions in the rat. Gastroenterology. 2001;121:1417–27. doi: 10.1053/gast.2001.29581. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez-Margalet V, Martin-Romero C. Human leptin signaling in human peripheral blood mononuclear cells: activation of the JAK-STAT pathway. Cell Immunol. 2001;211:30–6. doi: 10.1006/cimm.2001.1815. [DOI] [PubMed] [Google Scholar]
- 48.Tsumanuma I, Jin L, Zhang S, Bayliss JM, Scheithauer BW, Lloyd RV. Leptin signal transduction in the HP75 human pituitary cell line. Pituitary. 2000;3:211–20. doi: 10.1023/a:1012994712851. [DOI] [PubMed] [Google Scholar]
- 49.Hekerman P, Zeidler J, Bamberg-Lemper S, et al. Pleiotropy of leptin receptor signalling is defined by distinct roles of the intracellular tyrosines. FEBS J. 2005;272:109–19. doi: 10.1111/j.1742-4658.2004.04391.x. [DOI] [PubMed] [Google Scholar]
- 50.Münzberg H, Björnholm M, Bates SH, Myers MG., Jr Leptin receptor action and mechanisms of leptin resistance. Cell Mol Life Sci. 2005;62:642–52. doi: 10.1007/s00018-004-4432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hakansson-Ovesjo ML, Collin M, Meister B. Down-regulated STAT3 messenger ribonucleic acid and STAT3 protein in the hypothalamic arcuate nucleus of the obese leptin-deficient (ob/ob) mouse. Endocrinology. 2000;141:3946–55. doi: 10.1210/endo.141.11.7779. [DOI] [PubMed] [Google Scholar]
- 52.De Rosa V, Procaccini C, Cali G, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–55. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 53.Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol. 2004;24:225–53. doi: 10.1016/j.yfrne.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Müller P, Kuttenkeuler D, Gesellchen V, Zeidler MP, Boutros M. Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature. 2005;436:871–5. doi: 10.1038/nature03869. [DOI] [PubMed] [Google Scholar]
- 55.Starr R, Willson TA, Viney EM, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–21. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 56.Giordanetto F, Kroemer RT. A three-dimensional model of Suppressor Of Cytokine Signalling 1 (SOCS-1) Protein Eng. 2003;16:115–24. doi: 10.1093/proeng/gzg015. [DOI] [PubMed] [Google Scholar]
- 57.Yasukawa H, Misawa H, Sakamoto H, et al. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 1999;18:1309–20. doi: 10.1093/emboj/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki R, Sakamoto H, Yasukawa H, et al. CIS3 and JAB have different regulatory roles in interleukin-6 mediated differentiation and STAT3 activation in M1 leukemia cells. Oncogene. 1998;17:2271–8. doi: 10.1038/sj.onc.1202143. [DOI] [PubMed] [Google Scholar]
- 59.Eyckerman S, Broekaert D, Verhee A, Vandekerckhove J, Tavernier J. Identification of the Y985 and Y1077 motifs as SOCS3 recruitment sites in the murine leptin receptor. FEBS Lett. 2000;486:33–7. doi: 10.1016/s0014-5793(00)02205-5. [DOI] [PubMed] [Google Scholar]
- 60.Bjørbæck C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–25. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 61.Bjørbæck C, El Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–65. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 62.Bjørbæck C, Lavery HJ, Bates SH, et al. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem. 2000;275:40649–57. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 63.Münzberg H, Myers MG. Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;5:566–70. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 64.Fairlie WD, De Souza D, Nicola NA, Baca M. Negative regulation of gp130 signalling mediated through tyrosine-757 is not dependent on the recruitment of SHP2. Biochem J. 2003;372:495–502. doi: 10.1042/BJ20030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Favre H, Benhamou A, Finidori J, Kelly PA, Edery M. Dual effects of suppressor of cytokine signaling (SOCS-2) on growth hormone signal transduction. FEBS Lett. 1999;453:63–6. doi: 10.1016/s0014-5793(99)00681-x. [DOI] [PubMed] [Google Scholar]
- 66.Greenhalgh CJ, Metcalf D, Thaus AL, et al. Biological evidence that SOCS-2 can act either as an enhancer or suppressor of growth hormone signaling. J Biol Chem. 2002;277:40181–4. doi: 10.1074/jbc.C200450200. [DOI] [PubMed] [Google Scholar]
- 67.Dif F, Saunier E, Demeneix B, Kelly PA, Edery M. Cytokine-inducible SH2-containing protein suppresses PRL signaling by binding the PRL receptor. Endocrinology. 2001;142:5286–93. doi: 10.1210/endo.142.12.8549. [DOI] [PubMed] [Google Scholar]
- 68.Tannahill GM, Elliott J, Barry AC, Hibbert L, Cacalano NA, Johnston JA. SOCS2 can enhance interleukin-2 (IL-2) and IL-3 signaling by accelerating SOCS3 degradation. Mol Cell Biol. 2005;25:9115–26. doi: 10.1128/MCB.25.20.9115-9126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piessevaux J, Lavens D, Montoye T, et al. Functional cross-modulation between SOCS proteins can stimulate cytokine signalling. J Biol Chem. 2006;281:32953–66. doi: 10.1074/jbc.M600776200. [DOI] [PubMed] [Google Scholar]
- 70.Lavens D, Montoye T, Piessevaux J, et al. A complex interaction pattern of CIS and SOCS2 with the leptin receptor. J Cell Sci. 2006;119:2214–24. doi: 10.1242/jcs.02947. [DOI] [PubMed] [Google Scholar]
- 71.Montoye T, Piessevaux J, Lavens D, et al. Analysis of leptin signalling in hematopoietic cells using an adapted MAPPIT strategy. FEBS Lett. 2006;580:3301–7. doi: 10.1016/j.febslet.2006.04.094. [DOI] [PubMed] [Google Scholar]
- 72.Martens N, Uzan G, Wery M, Hooghe R, Hooghe-Peters EL, Gertler A. Suppressor of cytokine signaling 7 inhibits prolactin, growth hormone, and leptin signaling by interacting with STAT5 or STAT3 and attenuating their nuclear translocation. J Biol Chem. 2005;280:13817–23. doi: 10.1074/jbc.M411596200. [DOI] [PubMed] [Google Scholar]
- 73.Elchebly M, Payette P, Michaliszyn E, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–8. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 74.Klaman LD, Boss O, Peroni OD, et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–89. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zabolotny JM, Bence-Hanulec KK, Stricker-Kongrad A, et al. PTB1 regulates leptin signal transduction in vivo. Dev Cell. 2002;2:489–95. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 76.Kaszubska W, Falls HD, Schaefer VG, et al. Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol Cell Endocrinol. 2002;195:109–18. doi: 10.1016/s0303-7207(02)00178-8. [DOI] [PubMed] [Google Scholar]
- 77.Cook WS, Unger RH. Protein tyrosine phosphatase 1B: a potential leptin resistance factor of obesity. Dev Cell. 2002;2:385–7. doi: 10.1016/s1534-5807(02)00158-2. [DOI] [PubMed] [Google Scholar]
- 78.Neel BG, Tonks NK. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 79.Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci USA. 2004;101:16064–8. doi: 10.1073/pnas.0405041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li C, Friedman JM. Leptin receptor activation of SH2 domain containing protein tyrosine phosphatase 2 modulates Ob receptor signal transduction. Proc Natl Acad Sci USA. 1999;96:9677–11. doi: 10.1073/pnas.96.17.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carpenter LR, Farruggella TJ, Symes A, Karow ML, Yancopoulos GD, Stahl N. Enhancing leptin response by preventing SH2-containing phosphatase 2 interaction with Ob receptor. Proc Natl Acad Sci USA. 1998;95:6061–5. doi: 10.1073/pnas.95.11.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Houslay MD. Compartmentalization of cyclic AMP phosphodiesterases, signalling ‘crosstalk’, desensitization and the phosphorylation of Gi-2 add cell specific personalization to the control of the levels of the second messenger cyclic AMP. Adv. Enzyme Regul. 1995;35:303–38. doi: 10.1016/0065-2571(94)00012-r. [DOI] [PubMed] [Google Scholar]
- 83.Houslay MD, Milligan G. Tailoring cAMP-signalling responses through isoform multiplicity. Trends Biochem Sci. 1997;22:217–24. doi: 10.1016/s0968-0004(97)01050-5. [DOI] [PubMed] [Google Scholar]
- 84.Soderling SH, Beavo JA. Regulation of cAMP and cGMP signaling: New phosphodiesterases and new functions. Curr Opin Cell Biol. 2000;12:174–9. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 85.Zhao AZ, Bornfeldt KE, Beavo JA. Leptin inhibits insulin secretion by activation of phosphodiesterase 3B. J Clin Invest. 1998;102:869–73. doi: 10.1172/JCI3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Unger RH. The hyperleptinemia of obesity – regulator of caloric surpluses. Cell. 2004;117:145–51. doi: 10.1016/s0092-8674(04)00339-3. [DOI] [PubMed] [Google Scholar]
- 87.Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–43. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 88.Minokoshi Y, Alquier T, Furukawa N, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–74. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 89.Ceddia RB. Direct metabolic regulation in skeletal muscle and fat tissue by leptin: implications for glucose and fatty acid homeostasis. Int J Obes. 2005;29:1175–83. doi: 10.1038/sj.ijo.0803025. [DOI] [PubMed] [Google Scholar]
- 90.Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–54. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 91.Shepherd PR, Withers DJ, Siddle K. Phosphoinositide 3-kinase: The key switch mechanism in insulin signalling. Biochem J. 1998;333:471–90. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim YB, Uotani S, Pierroz DD, Flier JS, Kahn BB. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues: Overlapping but distinct pathways from insulin. Endocrinology. 2001;141:2328–39. doi: 10.1210/endo.141.7.7536. [DOI] [PubMed] [Google Scholar]
- 93.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: Kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 94.Szanto I, Kahn CR. Selective interaction between leptin and insulin signaling pathways in a hepatic cell line. Proc Natl Acad Sci USA. 2000;97:2355–60. doi: 10.1073/pnas.050580497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dempsey EC, Newton AC, Mochly-Rosen D, et al. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung: Cell Mol Physiol. 2000;279:L429–38. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- 96.Chen NG, Swick AG, Romsos DR. Leptin constrains acetylcholine-induced insulin secretion from pancreatic islets of ob/ob mice. J Clin Invest. 1997;100:1174–9. doi: 10.1172/JCI119629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ookuma M, Ookuma K, York DA. Effects of leptin on insulin secretion from isolated rat pancreatic islets. Diabetes. 1998;47:219–23. doi: 10.2337/diab.47.2.219. [DOI] [PubMed] [Google Scholar]
- 98.Cobb MH, Hepler JE, Cheng M, Robbins D. The mitogen-activated protein kinases, ERK1 and ERK2. Semin Cancer Biol. 1994;5:261–8. [PubMed] [Google Scholar]
- 99.Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci USA. 1993;90:5889–92. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahima RS, Flier J. Leptin. Annu Rev Physiol. 2000;62:413–37. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 101.Bjorbaek C, Buchholz RM, Davis SM, et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–55. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 102.Figenschau Y, Knutsen G, Shahazeydi S, Johansen O, Sveinbjornsson B. Human articular chondrocytes express functional leptin receptors. Biochem Biophys Res Commun. 2001;287:190–7. doi: 10.1006/bbrc.2001.5543. [DOI] [PubMed] [Google Scholar]
- 103.Machinal-Quelin F, Dieudonne MN, Leneveu MC, Pecquery R, Giudicelli Y. Proadipogenic effect of leptin on rat preadipocytes in vitro: activation of MAPK and STAT3 signaling pathways. Am J Physiol Cell Physiol. 2002;282:C853–63. doi: 10.1152/ajpcell.00331.2001. [DOI] [PubMed] [Google Scholar]
- 104.Dreyer MG, Juge-Aubry CE, Gabay C, et al. Leptin activates the promoter of the interleukin-1 receptor antagonist through p42/44 mitogen-activated protein kinase and a composite nuclear factor kappa B/PU.1 binding site. Biochem J. 2003;370:591–9. doi: 10.1042/BJ20021270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim GS, Hong JS, Kim SW, et al. Leptin induces apoptosis via ERK/cPLA2/cytochrome c pathway in human bone marrow stromal cells. J Biol Chem. 2003;278:21920–9. doi: 10.1074/jbc.M204598200. [DOI] [PubMed] [Google Scholar]
- 106.Sweeney G. Leptin signalling. Cell Signalling. 2002;14:655–63. doi: 10.1016/s0898-6568(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 107.van den Brink GR, O’Toole T, Hardwick JC, et al. Leptin signaling in human peripheral blood mononuclear cells, activation of p38 and p42/44 mitogen-activated protein (MAP) kinase and p70 S6 kinase. Mol Cell Biol Res Commun. 2000;4:144–50. doi: 10.1006/mcbr.2001.0270. [DOI] [PubMed] [Google Scholar]
- 108.Sweeney G, Keen J, Somwar R, Konrad D, Garg R, Klip A. High leptin levels acutely inhibit insulin-stimulated glucose uptake without affecting glucose transporter 4 translocation in L6 rat skeletal muscle cells. Endocrinology. 2001;142:4806–12. doi: 10.1210/endo.142.11.8496. [DOI] [PubMed] [Google Scholar]
- 109.Shen J, Sakaida I, Uchida K, Terai S, Okita K. Leptin enhances TNF-α production via p38 and JNK MAPK in LPS-stimulated Kupffer cells. Life Sci. 2005;77:1502–15. doi: 10.1016/j.lfs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 110.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Stoffel M, Friedman JM. Leptin activation of STAT3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–7. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 111.Loffreda S, Rai R, Yang SQ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 112.Martín-Romero C, Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 113.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–6. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taylor SJ, Shalloway D. An RNA-binding protein associated with Src through its SH2 and SH3 domains in mitosis. Nature. 1994;368:867–71. doi: 10.1038/368867a0. [DOI] [PubMed] [Google Scholar]
- 115.Fumagalli S, Totti N, Hsuan JJ, Coutneidge SA. A target of Src in mitosis. Nature. 1994;368:871–4. doi: 10.1038/368871a0. [DOI] [PubMed] [Google Scholar]
- 116.Richard S, Yu D, Blummer KJ, Hausladen D, Olszowy MW, Connelly PA, Shaw AS. Association of p62, a multifunctional SH2- and SH3-domain binding protein, with src family tyrosine kinases, Grb2, and phospholipase Cγ1. Mol Cell Biol. 1995;15:186–97. doi: 10.1128/mcb.15.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fusaki N, Iwamatsu A, Iwashima M, Fujisawa JI. Interaction between Sam68 and src family tyrosine kinases Fyn and Lck, in T cell receptor signalling. J Biol Chem. 1995;272:6214–9. doi: 10.1074/jbc.272.10.6214. [DOI] [PubMed] [Google Scholar]
- 118.Jabado N, Pallier A, LeDeist F, Bernard F, Fischer A, Hivroz C. CD4 ligands inhibit the formation of multifunctioonal transduction complexes involved in T cell activation. J Immunol. 1997;158:94–103. [PubMed] [Google Scholar]
- 119.Jabado N, Jauliac S, Pallier A, Bernard F, Fischer A, Hivroz C. Sam68 association with p120GAP in CD4+ T cells is dependent on CD4 molecule expression. J Immunol. 1998;161:2798–803. [PubMed] [Google Scholar]
- 120.Kellerer M, Koch M, Metzinger E, Mushack J, Capp E, Haring HU. Leptin activates PI-3 kinase in C2C12 myotubes via janus kinase-2 (JAK-2) and insulin receptor substrate-2 (IRS-2) dependent pathways. Diabetologia. 1997;40:1358–62. doi: 10.1007/s001250050832. [DOI] [PubMed] [Google Scholar]
- 121.Harvey J, McKay NG, Walker KS, Van der Kaay J, Downes CP, Ashford MLJ. Essential role of phosphoinositide 3-kinase in leptin-induced KATP channel activation in the rat CRI-G1 insulinoma cell line. J Biol Chem. 2000;275:4660–9. doi: 10.1074/jbc.275.7.4660. [DOI] [PubMed] [Google Scholar]
- 122.Martín-Romero C, Sanchez-Margalet V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells. Possible role of Sam68. Cell Immunol. 2001;212:83–91. doi: 10.1006/cimm.2001.1851. [DOI] [PubMed] [Google Scholar]
- 123.Sánchez-Margalet V, Najib S. Sam68 is a substrate of the insulin receptor and associates with the SH2 domains of p85 PI3K. FEBS Lett. 1999;455:307–10. doi: 10.1016/s0014-5793(99)00887-x. [DOI] [PubMed] [Google Scholar]
- 124.Sung CK, Sanchez-Margalet V, Goldfine ID. Role of p85 subunits of phosphatidylinositol-3-kinase as an adaptor molecule linking the insulin receptor, p62 and GTPase-activating protein. J Biol Chem. 1994;269:12503–7. [PubMed] [Google Scholar]
- 125.Mansour E, Fereira FG, Araújo EP, et al. Leptin inhibits apoptosis in thymus through a janus kinase-2-independent, insulin receptor substrate-1/phosphatidylinositol-3 kinase-dependent pathway. Endocrinology. 2006;147:5470–9. doi: 10.1210/en.2006-0223. [DOI] [PubMed] [Google Scholar]
- 126.Gressner AM, Wool IG. The phosphorylation of liver ribosomal proteins in vivo. Evidence that only a single small subunit is phosphorylated. J Biol Chem. 1974;249:6917–25. [PubMed] [Google Scholar]
- 127.Takahashi Y, Okimura Y, Mizuno I, et al. Leptin induces mitogen-activated protein kinase-dependent proliferation of C3H10T1/2 cells. J Biol Chem. 1997;272:12897–900. doi: 10.1074/jbc.272.20.12897. [DOI] [PubMed] [Google Scholar]
- 128.Tanabe K, Okuya S, Tanizawa Y, Matsutani A, Oka Y. Leptin induces proliferation of pancreatic β cell line MIN6 through activation of mitogen-activated protein kinase. Biochem Biophys Res Commun. 1997;241:765–8. doi: 10.1006/bbrc.1997.7894. [DOI] [PubMed] [Google Scholar]
- 129.Bouloumié A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999;13:1231–8. [PubMed] [Google Scholar]
- 130.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA. 1998;95:1432–6. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lenormand P, Brondello JM, Brunet A, Pouyssegur J. Growth factor-induced p42/p44 MAPK nuclear translocation and retention requires both MAPK activation and neosynthesis of nuclear anchoring proteins. J Cell Biol. 1998;142:625–33. doi: 10.1083/jcb.142.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eguchi S, Iwasaki H, Ueno H, et al. Intracellular signaling of angiotensin II-induced p70 S6 kinase phosphorylation at Ser(411) in vascular smooth muscle cells. Possible requirement of epidermal growth factor receptor, Ras, extracellular signal-regulated kinase, and Akt. J Biol Chem. 1999;274:36843–51. doi: 10.1074/jbc.274.52.36843. [DOI] [PubMed] [Google Scholar]
- 133.O’Rourke L, Sheperd P. Biphasic regulation of extracellular-signal-regulated protein kinase by leptin in macrophages: role in regulating STAT3 Ser727 phosphorylation and DNA binding. Biochem J. 2002;364:875–9. doi: 10.1042/BJ20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Caldefie-Chezet F, Poulin A, Tridon A, Sion B, Vasson MP. Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? J Leukoc Biol. 2001;69:414–8. [PubMed] [Google Scholar]
- 135.Montecucco F, Bianchi G, Gnerre P, Bertolotto M, Dallegri F, Ottonello L. Induction of neutrophil chemotaxis by leptin: crucial role for p38 and Src kinases. Ann N Y Acad Sci. 2006;1069:463–71. doi: 10.1196/annals.1351.045. [DOI] [PubMed] [Google Scholar]
- 136.Bruno A, Conus S, Schmid I, Simon HU. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J Immunol. 2005;174:8090–6. doi: 10.4049/jimmunol.174.12.8090. [DOI] [PubMed] [Google Scholar]
- 137.Conus S, Bruno A, Simon HU. Leptin is an eosinophil survival factor. J Allergy Clin Immunol. 2005;116:1228–34. doi: 10.1016/j.jaci.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 138.Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–8. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- 139.Lam QL, Liu S, Cao X, Lu L. Involvement of leptin signaling in the survival and maturation of bone marrow-derived dendritic cells. Eur J Immunol. 2006;36:3118–30. doi: 10.1002/eji.200636602. [DOI] [PubMed] [Google Scholar]