Abstract
Purpose
To examine the efficacy of a sequential tube shunt versus transscleral diode cyclophotocoagulation following failure of an initial tube shunt on maximal medical therapy in treatment of refractory childhood glaucoma.
Methods
A nonrandomized retrospective chart review was conducted of 17 eyes of 14 pediatric patients (less than 18 years old) with refractory glaucoma treated with either sequential tube shunt (Group A) or diode cyclophotocoagulation (Group B) following initial failed tube shunt. Success was defined as an intraocular pressure (IOP) ≤22 mm Hg on medical therapy, no visually devastating complications, and no further glaucoma surgery performed or recommended.
Results
Of the 17 eyes, 8 had a sequential tube shunt and 9 underwent diode cyclophotocoagulation as a secondary procedure. Kaplan-Meier analysis demonstrated a successful outcome of 75% and 62.5% at 12 months and 24 months, respectively, for Group A, and 66.7% at both 12 and 24 months for Group B (p = 0.48) Corneal decompensation or graft failure was noted in 3/8 eyes (38%) in Group A. Cataract surgery was performed in 2/5 phakic eyes (40%) in Group B. One eye in each group progressed to no light perception.
Conclusions
Diode cyclophotocoagulation and sequential tube shunt following primary tube shunt failure in childhood glaucoma showed similar efficacy and complication rates. However, the small sample size of this study warrants further evaluation of these two procedures following failure of a tube shunt device in pediatric glaucoma.
Introduction
Treatment of glaucoma in the pediatric population is frequently challenging and may require multiple surgical interventions. Ten to fifty percent of patients with primary congenital glaucoma fail goniotomy surgery and require further surgical intervention.1,2 Childhood glaucoma associated with systemic or ocular anomalies and secondary glaucoma such as that associated with congenital aphakia or pseudophakia have a worse surgical prognosis compared to primary congenital glaucoma.2,3 Trabeculectomy surgery with adjunctive mitomycin has a lower chance of successful control of children less than two years of age and in children who have had congenital cataract surgery.4,5 Additionally, compared to adults, the adjunctive use of anti-fibrotic agents with trabeculectomy in children may be associated with a greater risk of late bleb-related infections.6-8
Due to these factors, an increasing number of tube shunts are being used for control of various pediatric glaucomas with success rates of 50%-90%, depending on length of follow-up and definition of success.4,9-12 When a tube shunt fails to adequately control the intraocular pressure, limited treatment options remain. These options include a sequential tube shunt in another quadrant of the eye,13-14 revision or replacement of the existing tube shunt,14-16 or a cyclodestructive procedure (usually transscleral or endoscopic diode cyclophotocoagulation or cyclocryotherapy).17-22 Both tube shunts and transscleral diode cyclophotocoagulation have been examined for treatment of refractory pediatric glaucoma, but we are unaware of any studies comparing these two treatments following primary tube shunt failure. Sequential tubes shunt procedures have been noted to have a success rate of 62%-63% in adult patients with glaucoma, with corneal decompensation commonly noted.13,14 Transscleral diode laser cyclophotocoagulation following failure of aqueous shunt surgery has been noted to have 78% success (7 of 9 eyes) after mean follow-up of 30 months in childhood glaucoma, with cataract formation and retinal detachment noted in one eye each.23 The purpose of this study was to determine whether sequential tube shunt or diode cyclophotocoagulation is superior following an initial failed tube shunt in childhood glaucoma.
Methods
All patients enrolled in this nonrandomized, retrospective study were 18 years old or less, failed an initial tube shunt, and then were treated with either sequential tube shunt (Group A) or a cyclophotocoagulation procedure (Group B) between the years 1999 to 2006. The Human Investigation Committee at Emory University approved this study, which conformed to the United States Health Insurance Portability and Privacy Act.
For the purposes of this study, failure of the initial tube shunt was based on inability to adequately control the intraocular pressure (IOP) on maximally tolerated medical therapy. There was no evidence of blockage of the tube by iris, fibrin or vitreous, and a clinically apparent bleb over the implant was present. Given that the tube-shunt procedure was still apparently functioning and outflow was likely reduced by progressive fibrosis of the bleb capsule, revision of the original shunt was not attempted in the belief that the success rate would be low.16 Initially, a sequential tube shunt was considered the next favored step in the treatment algorithm, prior to consideration of a cyclophotocoagulation procedure. However, the outcomes of the sequential tube shunt procedures were frequently not ideal (need for multiple medications and a complication rate that was higher than with the initial tube shunt), leading to more frequent consideration of cyclophotocoagulation prior to a sequential tube shunt. For that reason, the sequential tube shunt (Group A) had a longer follow-up or time-to-failure interval as compared to the cyclophotocoagulation arm (Group B) (Table 1). The surgical technique for the Ahmed S2 (New World Medical, Rancho Cucamonga, CA) and Baerveldt® implants (Advanced Medical Optics, Santa Ana, CA) has previously been described.10-12 The decision between a Baerveldt® versus an Ahmed implant was based on individual ocular factors such as adequate space in another quadrant to place the implant, size of the eye, and prior ocular surgeries.
Table 1.
| Group A | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # |
Age months |
Glc Type |
1st surgery |
2nd surgery |
pre-op IOP |
meds | Vision | post-op IOP |
meds | Vision | Success | follow- up months |
| 1 | 43 | aphakic | Ahmed | Ahmed | 34 | 3 | NA | 18 | 3 | 20/200 | yes | 42 |
| 1 | 51 | aphakic | Ahmed | Ahmed | 48 | 3 | NA | 22 | 3 | CF | yes | 34 |
| 2 | 12 | ant seg | Ahmed | BGI | 32 | 3 | LP | 20 | 0 | NLP | no | 13 |
| 3 | 96 | cong | BGI | BGI | 28 | 2 | 20/300 | 26 | 4 | LP | no | 24 |
| 3 | 98 | cong | BGI | BGI | 30 | 2 | 20/40 | 13 | 4 | 20/100 | yes | 22 |
| 4 | 95 | Lowe | BGI | Ahmed | 23 | 4 | NA | 12 | 3 | NA | yes | 32 |
| 5 | 13 | aphakic | Ahmed | Ahmed | 34 | 3 | NA | 8 | 3 | NA | yes | 30 |
| 6 | 184 | cong | Molteno | BP | 30 | 3 | 20/200 | 38 | 3 | 20/200 | no | 13 |
| mean | 74 | 32.4 | 2.9 | 19.6 | 2.9 | 26.2 | ||||||
| SD | 53.2 | 6.8 | 0.6 | 8.8 | 1.2 | 9.5 | ||||||
| Group B | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # |
Age months |
Glc Type |
1st surgery |
2nd surgery |
pre-op IOP |
meds | Vision | post-op IOP |
meds | Vision | success | follow- up months |
| 7 | 12 | ant seg | Ahmed | CPC | 34 | 3 | NA | 30 | 3 | NA | no | 26 |
| 8 | 59 | cong | BGI | CPC | 35 | 3 | NA | 13 | 1 | 20/200 | yes | 53 |
| 8 | 65 | cong | BGI | CPC | 28 | 3 | CF | 0 | 0 | NLP | no | 5 |
| 9 | 78 | cong | Ahmed | CPC | 34 | 4 | 20/70 | 17 | 4 | 20/50 | yes | 23 |
| 10 | 84 | cong | Ahmed | CPC | 36 | 4 | CF | 21 | 4 | 20/200 | yes | 13 |
| 11 | 124 | cong | BGI | CPC | 38 | 4 | CF | 18 | 3 | CF | yes | 11 |
| 12 | 205 | trauma | BGI | CPC | 26 | 4 | 20/400 | 14 | 4 | 20/200 | yes | 33 |
| 13 | 16 | ant seg | Ahmed | CPC | 31 | 3 | NA | 31 | 3 | NA | no | 7 |
| 14 | 15 | cong | Ahmed | CPC | 23 | 3 | NA | 34 | 3 | NA | no | 7 |
| mean | 73.1 | 31.7 | 3.4 | 19.8 | 2.8 | 19.8 | ||||||
| SD | 58.5 | 4.7 | 0.5 | 10.1 | 1.3 | 14.9 | ||||||
Glc, glaucoma; IOP, intraocular pressure; meds, medications; ant seg, anterior segment dysgenesis; cong, congenital glaucoma; trauma, traumatic glaucoma; Lowe, Lowe syndrome; BGI, Baerveldt Glaucoma Implant; BP, Ahmed bi-plate implant; CPC, cyclophotocoagulation; NA, not available due to age, developmental issues; CF, counting fingers; LP, light perception; NLP, no light perception; SD, standard deviation.
The tube was placed in the anterior chamber in all cases, except for the Ahmed B4 implant. The Ahmed B4 implant (unvalved implant with surface area of 180 mm2) was placed into the superior nasal quadrant, using an Ahmed tube extender TE (New World Medical, Rancho Cucamonga, CA) to place the tube into the existing superior temporal bleb of the previously placed implant, and ligation of the tube extender with a 6-0 polyglactin suture. The Ahmed B4 implant was used in the case of a monocular patient with a corneal transplant to avoid the potential effect of a second tube on the function of the graft.
In the transscleral diode cyclophotocoagulation treatment arm (Group B), an OcuLight SLx diode laser (Iris Medical Instruments, Mountain View, CA) with an Iris G-probe (Iris Medical Instruments, Mountain View, CA) was used. Power ranged from 1000-1500 mW with a duration time of 3000-4000 ms, treating 180° to 270°. The probe was placed at the limbus with adjustment of treatment parameters based on the occurrence of “pops” with the Iris-G probe (adjustment downward on power for consecutive “pops”).24
Success was defined as an IOP ≤22 mm Hg on medical therapy, no visually devastating complications, and no further glaucoma surgery being performed or recommended. Intraocular pressure was assessed in the operating room at initial induction of general anesthesia with pneumatonometry (Reichert, Depew, NY) or by applanation tonometry in the clinic (hand-held or slit lamp depending on the age and cooperation of the patient. Preoperative and postoperative information evaluated were patient age, type of glaucoma, type of tube shunt utilized for the initial surgery, visual acuity, IOP, medications, follow-up interval, and complications. Curves showing the cumulative probability of success versus time were determined using the product-limit method. The time to failure was compared in the two groups using the log rank test. An unpaired t-test was used to compare preoperative to postoperative IOP reduction.
Results
Seventeen eyes of 14 patients with pediatric glaucoma were included in this nonrandomized, retrospective study. In Group A, 8 eyes of 6 patients (average age 73.1 months; range, 12-205 months) had a sequential tube shunt and in Group B, 9 eyes of 8 patients (average age 74 months; range, 12-184 months) had diode CPC as a secondary procedure (Table 1). In Group A, 8 tube shunts were performed on 8 eyes of 6 patients using 4 Ahmed model S2, 1 Ahmed model B4, and 3 Baerveldt® BG-101-350. In Group B, a total of 9 diode laser cyclophotocoagulation procedures were performed on 9 eyes of 8 patients with an average of 17.4 applications (range, 14-22) over 180° to 270°. The amount of treatment with the laser was influenced by the one case of phthisis which received 22 applications over 270° (patient 8 OS). Subsequent treatments were 14-16 applications over 180°-210°.
The average follow-up or time to failure of Group A was 26.3 months (range, 13-42 months) and 19.8 months (range, 5-53 months) in Group B. Initial diagnoses were similar in the two groups: 7 eyes with congenital glaucoma, 2 eyes with glaucoma associated with aphakia, three eyes with glaucoma associated with anterior segment anomalies, and 1 eye each with traumatic glaucoma and Lowe syndrome (Tables 1). One of 8 (12.5%) eyes was phakic in Group A, and 5 of 9 eyes (55.6%) were phakic in Group B.
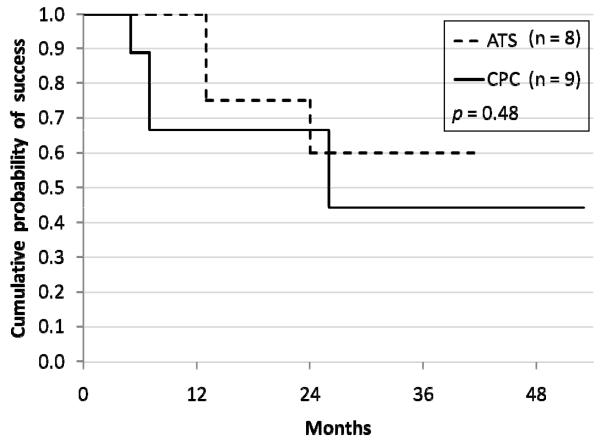
Cumulative probability of success in Group A was 75% at 12 months and 62.5% at 24 months, as compared to 66.7% for both 12 month and 24 month intervals in Group B (p = 0.48) (Figure 1). Average IOP in the tube shunt group (Group A) was 32.3 ± 6.8 mm Hg prior and 19.6 ± 8.8 mm Hg at last follow-up or time of failure. Average IOP reduction in Group A was 12.8 mm Hg (p = 0.009). Mean number of medications did not change from 2.9 (range, 2 to 4) preoperative to 2.9 (range, 0 to 4) postoperative in this group. Average IOP in the cyclophotocoagulation group (Group B) before surgery was 31.7 ± 4.7 mm Hg and it was 19.8 ± 10.1 mm Hg at last follow-up or time of failure, or 22.5 ± 8.2 mm Hg (censoring the one case of phthisis with an IOP of zero). Average IOP reduction was 11.9 mm Hg (p = 0.008), or 9.9 mm Hg (p = 0.01) censoring the one case of phthisis. The mean number of medications in Group B decreased from 3.4 (range, 3 to 4) to 2.8 (range, 0 to 4) (Table 1).
FIG 1.
Cumulative probability of success of Group A sequential tube shunt (dashed line) compared to Group B cyclophotocoagulation (solid line). The x-axis shows months of follow-up.
In Group A, preoperative visual acuity could be obtained in 4 eyes of 3 patients (remaining 4 eyes of 3 patients were either too young or developmental delay precluded standardized vision testing). Visual acuity worsened in 3 of the 4 eyes (75%) due to one case each of corneal decompensation, retinal detachment, and cataract formation. In Group B preoperative vision could be obtained in 5 eyes of 5 patients (remaining 4 eyes of 3 patients could not be obtained due to age or developmental issues). Visual acuity worsened in 1 eye (count fingers to no light perception due to phthisis), was unchanged in 3 eyes, and improved in 1 eye, compared to preoperative acuity. One eye from each group progressed to no light perception (retinal detachment and phthisis) (Table 1).
Corneal decompensation or graft failure was noted in 3/8 eyes (37.5%) in Group A. Cataract surgery was performed in 2/5 phakic eyes (40%) of Group B, while the only phakic patient in Group A was noted to have cataract formation (Table 2). Surgical revision of the tube shunt was required in 2/8 eyes (25%) in Group A, one case for tube repositioning and one case for bleb failure. No strabismus complications were noted in either group.
Table 2.
Complications
| Group A | ||
|---|---|---|
| Corneal decompensation/graft failure | 3/8 eyes | 37.5% |
| Shunt revision | 2/8 eyes | 25% |
| Suprachoroidal hemorrhage | 1/8 eyes | 12.5% |
| Retinal detachment | 1/8 eyes | 12.5% |
| Cataract (one phakic eye in group) | 1/1 eyes | 100% |
| Group B | ||
|---|---|---|
| Cataract (5 phakic eyes in group) | 2/5 eyes | 40% |
| Vitreous hemorrhage | 1/9 eyes | 11.1% |
| Phthisis | 1/9 eyes | 11.1% |
Discussion
The use of tube shunt surgery for childhood glaucoma has provided greater success than previous surgical approaches to this group of diseases, and may be the most predictable and safest surgery after angle surgery has failed.25 Well-known complications are associated with tube shunt surgery in children, including tube malposition or extrusion, tube/implant exposure, corneal decompensation, and endophthalmitis.4,10-12,26 As with any glaucoma surgery, failure rates of tube shunt surgery increase with length of follow-up,28 with no standard approach to the next step in treatment. The decision to perform another surgery in a child who may have had multiple prior ocular surgeries is difficult. The surgical options from which to choose include a sequential tube shunt, revision or replacement of the existing tube shunt, and cyclodestruction (external or endoscopic approaches).
This study was designed to evaluate if either a sequential tube shunt or diode cyclophotocoagulation, following failure of an initial tube shunt procedure, offers an advantage in terms of IOP control, visual acuity, number of medications, and complication rate. Surgical revision of the tube shunt was not attempted as the tube showed no evidence of blockage and a clinically apparent bleb was present over the plate of the device. Adult studies of revision of a tube shunt for bleb encapsulation have noted low success rates of 25%-42%.13,15 Revision of tube shunts due to tube malposition, extrusion, or a flat bleb over the implant have demonstrated higher success rates.15-16 Placement of a sequential tube shunt in adult patients has demonstrated success rates of 62%-63% at two years of follow-up, with corneal edema a common complication.13,14,27 Our study noted a success rate of 62.5% at 24 months, and corneal decompensation or graft failure was noted in 3/8 eyes (37.5%).
Cyclodestruction is generally regarded as a treatment of last resort, due to low success rates, vision loss, phthisis, chronic inflammation, and reports of sympathetic ophthalmia.17-19,22,29 With the advent of external and internal laser approaches, cyclocryotherapy has been replaced by transscleral diode cyclophotocoagulation and endoscopic cyclophotocoagulation. Pediatric studies of transscleral diode cyclophotocoagulation have noted success rates of 37%-75%, but with frequent need for re-treatment.17-19,22 Endoscopic cyclophotocoagulation has reduced the energy required to perform the procedure, with the advantage of direct visualization of the ciliary processes in cases with corneal opacities.30 However, success rates with endocyclophotocoagulation have been similar to transscleral diode cyclophotocoagulation, with single treatment success rates of 34%-38% noted with endocylophotocoagulation.20-21
Our study found similar success rates for sequential tube shunt surgery as compared to diode laser cyclophotocoagulation, following failure of an initial tube shunt surgery in childhood glaucoma. Different complications were noted in the two groups, with corneal decompensation or graft failure noted in 37.5% of the sequential tube shunt patients, and 40% of phakic patients requiring cataract surgery in the CPC group. Limitations of the study include a low statistical power to determine whether the two groups are actually equivalent, as well as the nonrandomized, retrospective nature of this study. Visual acuity data was too limited to make a meaningful comparison between the two groups.
Based on the findings in this study, intervention should be tailored according to the individual patient with specific attention to the number of prior surgeries, anatomy and visual prognosis. With the long potential lifespan of the pediatric glaucoma patient, pursuing other surgical options and holding cyclodestruction in reserve is a reasonable option. Further study of the surgical treatment of pediatric patients who fail tube shunt surgery is needed to better delineate the risks and benefits. The vision loss noted in this study with either procedure highlights the need for careful consideration and consent prior to proceeding with another surgical intervention for refractory pediatric glaucoma.
Acknowledgments
The authors wish to acknowledge the contributions of Michael Lynn, MS, for statistical assistance with this study (P30 EY06360, National Institutes of Health Departmental Core Grant, Bethesda, Maryland).
This study was supported in part by an unrestricted grant from Research to Prevent Blindness Inc., New York, New York, and by P30 EYO6360 (a National Institutes of Health Departmental Core Grant, Bethesda, Maryland).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ishida K, Mandal AK, Netland PA. Glaucoma drainage implants in pediatric patients. Ophthalmol Clin N Am. 2005;18:431–42. doi: 10.1016/j.ohc.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Taylor RH, Ainsworth JR, Evans AR, et al. The epidemiology of pediatric glaucoma: The Toronto experience. J AAPOS. 1999;3:308–15. doi: 10.1016/s1091-8531(99)70028-5. [DOI] [PubMed] [Google Scholar]
- 3.Wallace DK, Plager DA, Synder SK, et al. Surgical results of secondary glaucomas in childhood. Ophthalmology. 1998;105:101–11. doi: 10.1016/s0161-6420(98)91519-6. [DOI] [PubMed] [Google Scholar]
- 4.Beck AD, Freedman SF, Kammer J, Jin J. Aqueous shunt devices compared with trabeculectomy with mitomycin-C for children in the first two years of life. Am J Ophthalmology. 2003;136:994–1000. doi: 10.1016/s0002-9394(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 5.Beck AD, Wilson WR, Lynch MG, Lynn MJ, Noe R. Trabeculectomy with adjunctive mitomycin C in pediatric glaucoma. Am J Ophthalmol. 1998;126:648–57. doi: 10.1016/s0002-9394(98)00227-x. [DOI] [PubMed] [Google Scholar]
- 6.Waheed S, Ritterband DC, Greenfield DS, et al. Bleb-related ocular infection in children after trabeculectomy with mitomycin C. Ophthalmology. 1997;104:2117–20. doi: 10.1016/s0161-6420(97)30051-7. [DOI] [PubMed] [Google Scholar]
- 7.Sidoti PA, Belmonte SJ, Liebmann JM, et al. Trabeculectomy with mitomycin-C in the treatment of pediatric glaucomas. Ophthalmology. 2001;107:422–9. doi: 10.1016/s0161-6420(99)00130-x. [DOI] [PubMed] [Google Scholar]
- 8.Beck AD, Freedman SF. Trabeculectomy with mitomycin C in the treatment of pediatric glaucoma [Letter] Ophthalmology. 2001;108:835–6. doi: 10.1016/s0161-6420(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 9.Molteno ACB, Ancker E, Van Biljon G. Surgical technique for advanced juvenile glaucoma. Arch Ophthalmol. 1984;102:51–7. doi: 10.1001/archopht.1984.01040030035030. [DOI] [PubMed] [Google Scholar]
- 10.Coleman Al, Smyth RJ, Wilson MR, et al. Initial clinical experience with the Ahmed Glaucoma Valve implant in pediatric patients. Arch Ophthalmol. 1997;115:186–91. doi: 10.1001/archopht.1997.01100150188007. [DOI] [PubMed] [Google Scholar]
- 11.Englert JA, Freedman SF, Cox TA. The Ahmed Valve in refractory pediatric glaucoma. Am J Ophthalmol. 1999;127:32–4. doi: 10.1016/s0002-9394(98)00292-x. [DOI] [PubMed] [Google Scholar]
- 12.Budenz DL, Gedde SJ, Brandt JD, et al. Baerveldt glaucoma implant in the management of refractory childhood glaucomas. Ophthalmology. 2004;111:2204–10. doi: 10.1016/j.ophtha.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Burgoyne JK, WuDunn D, Lakhani V, Cantor LB. Outcomes of sequential tube shunts in complicated glaucoma. Ophthalmology. 2000;107:309–14. doi: 10.1016/s0161-6420(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 14.Shah AA, WuDunn D, Cantor LB. Shunt revision versus additional tube shunt implantation after failed tube shunt surgery in refractory glaucoma. Am J Ophthalmol. 2000;129:455–60. doi: 10.1016/s0002-9394(99)00410-9. [DOI] [PubMed] [Google Scholar]
- 15.Tsai JC, Grajewski AL, Parrish RK., 2nd Surgical revision of glaucoma shunt implants. Ophthalmic Surg Lasers. 1999;30:41–6. [PubMed] [Google Scholar]
- 16.Trigler L, Proia AD, Freedman SF. Fibrovascular ingrowth as a cause of Ahmed Glaucoma Valve failure in children. Am J Ophthalmol. 2006;141:388–9. doi: 10.1016/j.ajo.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 17.Kirwan JF, Shah P, Khaw PT. Diode laser cyclophotocoagulation: Role in the management of refractory pediatric glaucoma. Ophthalmology. 2002;109:316–23. doi: 10.1016/s0161-6420(01)00898-3. [DOI] [PubMed] [Google Scholar]
- 18.Izgi B, Demirci H, Demirci YK, Rurker G. Diode laser cyclophotocoagulation in refractory glaucoma: Comparison between pediatric and adult glaucoma. Ophthalmic Surgery and Lasers. 2001;32:100–07. [PubMed] [Google Scholar]
- 19.Bock CJ, Freedman SF, Buckley EG, Shields MB. Transscleral diode laser cyclophotocoagulation for refractory pediatric glaucomas. J Pediatr Ophthalmol Strabismus. 1997;34:235–9. doi: 10.3928/0191-3913-19970701-11. [DOI] [PubMed] [Google Scholar]
- 20.Neely DE, Plager DA. Endocyclophotocoagulation for management of difficult pediatric glaucomas. JAAPOS. 2001;5:221–9. doi: 10.1067/mpa.2001.116868. [DOI] [PubMed] [Google Scholar]
- 21.Carter BC, Plager DA, Neely DE, et al. Endoscopic laser cyclophotocoagulation in the management of aphakic and pseudophakic glaucoma in children. JAAPOS. 2007;11:34–40. doi: 10.1016/j.jaapos.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Wagle NS, Freedman SF, Buckley EG, et al. Long-term outcome of cyclocryotherapy for refractory pediatric glaucoma. Ophthalmology. 1998;105:1921–7. doi: 10.1016/S0161-6420(98)91042-9. [DOI] [PubMed] [Google Scholar]
- 23.Semchyshyn TM, Tsai JC, Joos KM. Supplemental transscleral diode laser cyclophotocoagulation after aqueous shunt placement in refractory glaucoma. Ophthalmology. 2002;109:1078–84. doi: 10.1016/s0161-6420(02)01019-9. [DOI] [PubMed] [Google Scholar]
- 24.Gaasterland D, Pollack I. Initial experience with a new method of laser transscleral cyclophotocoagulation for ciliary ablation in severe glaucoma. Trans Am Ophthalmol Soc. 1992;90:225–46. [PMC free article] [PubMed] [Google Scholar]
- 25.Tanimoto SA, Brandt JD. Options in pediatric glaucoma after angle surgery has failed. Curr Opin Ophthalmol. 2006;17:132–7. doi: 10.1097/01.icu.0000193091.60185.27. [DOI] [PubMed] [Google Scholar]
- 26.Trzcinka A, Soans FP, Archer SM, et al. Late onset Haemophilus Influenza endophthalmitis in an immunized child after Baerveldt implant. J AAPOS. 2008;12:412–14. doi: 10.1016/j.jaapos.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Godfrey DG, Krishna R, Greenfield DS, et al. Implantation of second glaucoma drainage devices after failure of primary devices. Ophthalmic Surgery & Lasers. 2002;33:37–43. [PubMed] [Google Scholar]
- 28.O’Malley Schotthoefer E, Yanovitch TL, Freedman SF. Aqueous drainage device surgery in refractory pediatric glaucomas: I. Long-term outcomes. J AAPOS. 2008;12:33–9. doi: 10.1016/j.jaapos.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Bechrakis NE, Muller-Stolzenburg NW, Helbis H, et al. Sympathetic ophthalmia following laser cyclocoagulation. Arch Ophthalmol. 1994;112:80–84. doi: 10.1001/archopht.1994.01090130090024. [DOI] [PubMed] [Google Scholar]
- 30.Al-Haddad CE, Freedman SF. Endoscopic laser cyclophotocoagulation in pediatric glaucoma with corneal opacities. J AAPOS. 2007;11:23–8. doi: 10.1016/j.jaapos.2006.08.005. [DOI] [PubMed] [Google Scholar]