Summary
The ability to adapt to changing environmental conditions is essential to the fitness of organisms. In some cases, adaptation of the parent alters the offspring’s phenotype[1-10]. Such parental effects are adaptive for the offspring if the future environment is similar to the current one, but can be maladaptive otherwise[11]. One mechanism by which adaptation occurs is altered provisioning of embryos by the parent[12-16]. Here we show that exposing adult Caenorhabditis elegans to hyperosmotic conditions protects their offspring from the same, but causes sensitivity to anoxia exposure. We show that this alteration of survival is correlated to changes in the sugar content of adults and embryos. In addition, mutations in gene products which alter sugar homeostasis also alter the ability of embryos to survive in hyperosmotic and anoxic conditions and engage in the adaptive parental effect. Our results indicate that there is a physiological trade-off between the presence of glycerol, which protects animals from hyperosmotic conditions, and glycogen, which is consumed during anoxia. These two metabolites play an essential role in the survival of worms in these adverse environments, and the adaptive parental effect we describe is mediated by the provisioning of these metabolites to the embryo.
Results
C. elegans embryos can survive a 24-hour bout of anoxia at 20-23°C with 95% or greater viability (Table 1) [17, 18]. We performed experiments to determine if survival could be altered by changing the parental environment. We found that exposing wild-type (N2) L4 larvae to 300mM sodium chloride for 24 hours as they progressed to adulthood (hyperosmotic preconditioning or OPC) reduced survival of their embryos in anoxia to a mean of 42% (Table 1; P<0.001). However, embryos from OPC mothers hatched in normoxia and were resistant to 500mM sodium chloride, a concentration which would kill embryos which did not have parental OPC (Table 1; P=0.01). This effect persists for at least four hours after the adults are removed from the high salt environment (Table 1; P<0.001). These results demonstrate that the parental environment adapts offspring to their probable future environment in an environment-specific fashion. They also show that the embryos are not generally ‘sick’ as a result of parental exposure to hyperosmotic conditions, but are actually adapted to the salt in a way that makes them more sensitive to anoxia.
Table 1.
An adaptive parental effect protects C. elegans from hyperosmotic stress and requires daf-2 in a daf-16 dependent manner
| Strain | Adult treatmenta |
Embryo treatmentb |
Survival of embryosc |
SDd | # of trials |
Ne | p-value vs control |
|---|---|---|---|---|---|---|---|
| N2 | 50mM NaCl | 50mM NaCl | 98.6% | 1.4% | 9 | 768 | |
| 50mM NaCl | Anoxia 23°C | 95.7% | 3.2% | 15 | 1382 | ||
| N2 | 300mM NaCl | 50mM NaCl | 95.6% | 1.7% | 7 | 505 | |
| 300mM NaCl | Anoxia 23°C | 41.6% | 21.1% | 14 | 741 | <0.001$ | |
| N2 | 50mM NaCl | 500mM NaCl | 0.4% | 5.2% | 18 | 1005 | |
| 300mM NaCl | 500mM NaCl | 26.2% | 22.0% | 17 | 789 | 0.010* | |
| 300mM NaCl + 4hrs 50mM |
500mM NaCl | 10.3% | 7.9% | 7 | 282 | <0.001* | |
| daf-2(e1370) | 50mM NaCl | 500mM NaCl | 1.1% | 2.5% | 8 | 475 | |
| 300mM NaCl | 500mM NaCl | 0.0% | 0.0% | 6 | 179 | 0.491* | |
|
daf-16(m26)/ daf-2(e1370) |
50mM NaCl | 500mM NaCl | 7.2% | 7.4% | 5 | 417 | |
| 300mM NaCl | 500mM NaCl | 65.5% | 15.4% | 5 | 316 | <0.001$ | |
| daf-16(m26) | 50mM NaCl | 500mM NaCl | 1.1% | 3.1% | 7 | 532 | |
| 300mM NaCl | 500mM NaCl | 23.3% | 22.1% | 7 | 442 | 0.001* | |
Adult treatment consisted of: growing animals from L4 to adulthood on 50mM NaCl (50mM NaCl) or 300mM NaCl (300mM NaCl).
Embryo treatment consisted of: exposing embyros to 50mM NaCl (50mM NaCl) or 500mM NaCl (500mM NaCl) in normoxia, or to anoxia for 24 hrs (Anoxia).
Mean percent of embryos hatched 24 hours after embryo treatment
Standard deviation of the sample mean
Number of embryos assayed in all trials
Mann-Whitney Rank Sum test was performed
Two-tailed t-test was performed
We next tested daf-2(e1370) (GI175410) adult nematodes, which carry a hypomorphic allele of the C. elegans insulin/IGF receptor, for its ability to engage in OPC, and found that e1370 animals are unable to adapt their embryos to survive in hyperosmotic conditions. No embryos from OPC e1370 mothers hatched on 500mM salt, while ∼30% of wild-type hatched in the same situation (Table 1). These results suggest that the worm insulin receptor plays a role in regulating the adaptive parental effect. To further investigate this, we analyzed a downstream target of daf-2 signalling, the FOXO transcription factor daf-16. We determined that the deficit in OPC found in e1370 animals was hypostatic to the daf-16 reference allele m26. daf-16(m26) mutant embryos performed very similarly to wild type, while daf-2(e1370);daf-16(m26) double mutants performed better than wild-type (Table 1, P<0.001 for improvement of daf-16/daf-2over N2).
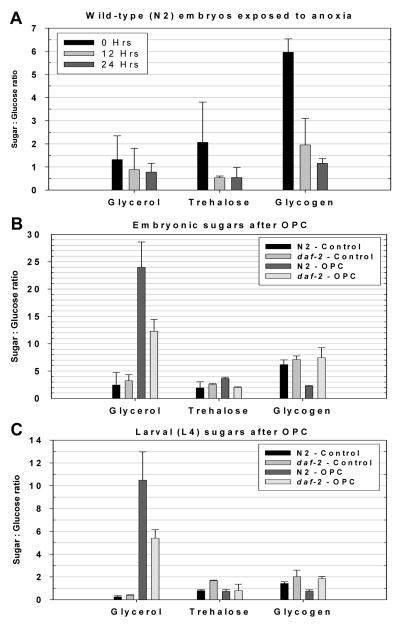
Given the known importance of glycerol and trehalose to osmotic resistance in the nematode[19-22], and glycogen to the survival of a wide variety of animals in anoxia[23, 24] we tested how OPC alters the sugar homeostasis in C. elegans. We chose to investigate a broad range of sugars using a gas chromatography-mass spectrometry (GC/MS) technique[25, 26]. In wild-type embryos, glycogen levels fell to approximately 20% of their initial levels after a 24 hour exposure to anoxia, indicating that fermentative metabolism is continuing in the arrested embryos (Fig. 1A; P<0.01). This is consistent with work done in mixed stage animals showing a similar reduction in glycogen stores over a 24-hour period of anoxia[27]. Next, we treated wild-type and e1370 adults with OPC and looked for alterations in sugar homeostasis in their embryos using the GC/MS technique. Embryos from OPC adults had 10x the glycerol content, nearly 2x the trehalose and only 1/3 of the glycogen content of control embryos (Fig. 1B; P<0.01 for glycerol and glycogen; P<0.05 for trehalose). Glycerol is a non-fermentable sugar and therefore cannot be used for energy during anoxia. Trehalose has also been implicated in salt resistance in C. elegans, possibly as a cytoprotectant[22]. daf-2(e1370) embryos from OPC adults, on the other hand, only have a 4x increase in glycerol levels, no change in their glycogen content, and a decrease in trehalose levels (Fig. 1B, P<0.01 for trehalose). The deficiency in either glycerol or trehalose may explain why e1370 embryos are not adapted to their hyperosmotic environment.
Figure 1.
OPC mediated adaptive sugar provisioning in C. elegans requires daf-2
The graphs display the mass of each of the sugars relative to the mass of glucose in the sample, as described in the materials and methods section. The values represented are the mean of 3-6 replicates, +/- SD.
(A) Young embryos were collected and placed into anoxia, with samples taken for sugar analysis at T=0; 12 and 24 hours. Over the 24 hour incubation, glycogen levels fall to 1/5 of their initial level (P<0.01).
(B) Wild-type (N2) Embryos from OPC adults have much more glycerol, near twice the amount of trehalose, and 1/3 of the glycogen of control animals (P<0.01 for glycerol and glycogen; P<0.05 for trehalose). daf-2 embryos only accumulate ½ of the glycerol of wild-type after OPC, have decreased trehalose levels and no change in glycogen storage (P<0.01 for trehalose).
(C) Changes in larval sugar levels largely reiterate the observation in embryos, with the exception of the fact that trehalose levels do not increase in wild-type larvae treated with OPC.
We next examined the larval response to preconditioning by GC/MS. These results largely reiterate the observations made in embryos, with the exception of the fact that trehalose levels did not rise in wild-type larvae treated with OPC. Glycerol rose in both wild-type and e1370 after OPC, but only reached half of the wild-type level in the e1370 adult. Glycogen levels dropped by half in wild-type (P<0.01), but were unchanged in e1370 (Fig. 1C). Glycerol accumulation in high salt requires the glycerol-3-phosphate dehydrogenase enzymes gpdh-1 (GI173272) and gpdh-2 (GI176399)[21]. In order to better understand the relationship between glycogen, glycerol, and trehalose, we exposed gpdh-1(ok1558);gpdh-2(kb33) to OPC and analyzed their sugar profile. As expected, adults treated in this fashion had only a minor increase in glycerol. However, like wild-type, they still utilized two thirds of their glycogen, and trehalose abundance increased 2.3-fold over its initial level (Fig. S1).
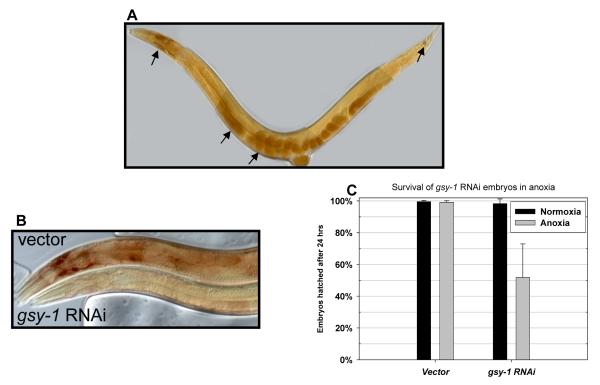
We next stained animals with iodine in order to further analyze how glycogen contributes to survival in adverse environments. Adult worms stained with iodine reveal major glycogen stores just anterior to the posterior bulb of the pharynx, in a region of the tail near the dorso-rectal ganglion, and in the most proximal two oocytes of the gonad arm (Fig. 2A). Iodine staining largely disappears when glycogen synthase (gsy-1 (GI174924)) is knocked down by RNAi (Fig. 2B). The small amount of remaining stain may be due to incomplete knockdown or the presence of a small quantity of another substance that stains with iodine. Embryos from adults treated with gsy-1 RNAi are sensitive to anoxia exposure, further verifying the importance of glycogen to survival of embryos in anoxia (Fig. 2C; P<0.01). Iodine staining also disappears upon anoxia or salt exposure, consistent with the decrease in glycogen we observed by GC/MS in these situations. daf-2(e1370) adults have more glycogen than wild-type by iodine staining (Fig. S2C), which is consistent with our GC/MS results and their known resistance to anoxia[28]. For a more complete description of the appearance and dynamics of glycogen in the worm, see the supplementary materials.
Figure 2.
Iodine staining reveals glycogen storage in C. elegans.
(A) Wild-type (N2) adult animal stained with iodine vapor. Arrows denote the primary sites of glycogen deposition. From left to right they are: anterior to the posterior bulb of the pharynx, the proximal oocytes; embryos in utero and the tail hypodermis.
(B) A wild-type (N2) animal fed with empty vector RNAi control food (top) stained with iodine simultaneously with an N2 animal treated with glycogen synthase RNAi (bottom).
(C) gsy-1 RNAi renders embryos sensitive to 24hrs of anoxia at 23°C. Values are mean survival rate from 14 trials, +SD.
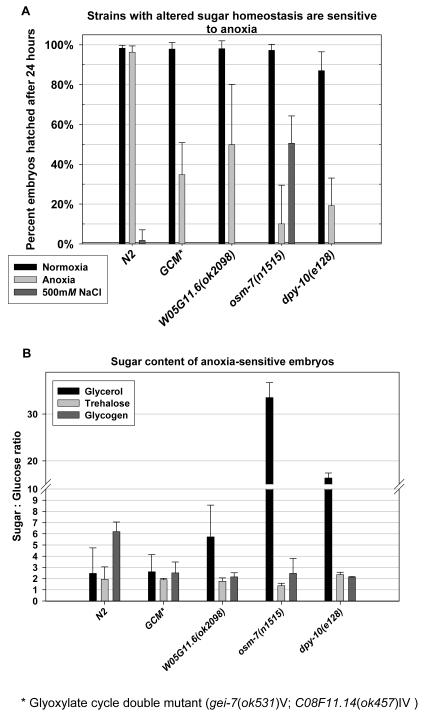
We wished to extend our understanding of how sugars affect survival in adverse environments by studying mutants with altered sugar homeostasis. First we tested the mutant osm-7(n1515) (GI176790). While the molecular function of the osm-7 gene remains obscure, mutant animals are constitutively adapted to high salt and are known to accumulate glycerol[20]. We found that osm-7 mutant animals also had low glycogen content and that their embryos were very sensitive to anoxia exposure (average survival 10%), but resistant to 500mM sodium chloride (Fig. 3A and 3B). dpy-10(e128) (GI174106) mutant animals, which have a defect in a cuticle collagen, are also known to be osmotic resistant and to accumulate glycerol[20]. These mutants were also found to be deficient in glycogen and sensitive to anoxia (Fig. 3A and 3B). The phenotypes of these mutants reiterate the relationship between glycerol and glycogen as well as survival in hyperosmotic and anoxic conditions that was previously demonstrated by environmental manipulation in wild-type. Neither of the mutants had increased trehalose relative to wild-type, implying that increased glycerol is sufficient for resistance to high salt in embryos.
Figure 3.
Mutations that alter sugar homeostasis affect the survival of embryos in adverse environments.
(A) This graph displays the mean percent of embryos hatching 24 hours after the treatments described, with error bars representing the standard deviation from the mean (N=number of embryos assayed).
N2 embryos survive 24hrs of anoxia, but die when exposed to 500mM NaCl (P<0.01). Glyoxylate cycle double mutant (GCM, gei-7(ok531); C08F11.14(ok457) embryos are sensitive to anoxia (N=277 normoxia; N= 1026 anoxia, P<0.01). W05G11.6 (ok2098) embryos are sensitive to anoxia (N=150 normoxia; N=299 anoxia, P<0.01). osm-7 embryos are sensitive to anoxia (N=572 normoxia; N=600 anoxia, P<0.01). osm-7 embryos are resistant to 500mM NaCl compared to wild-type (N=252 500mM NaCl, P<0.01). dpy-10(e128) animals are sensitive to anoxia (N=83 normoxia; N=100 anoxia, P<0.01).
(B) Embryos from each of the strains that has decreased survival in anoxia also has decreased glycogen storage compared to wild-type (N2) embryos (P<0.01 for all comparisons) Both osm-7 and dpy-10 embryos accumulate glycerol, as well as being deficient in glycogen. Glycerol accumulation is associated with resistance to hyperosmotic conditions. Values are the mean of three replicates, +/- SD.
We also analyzed strains with defects in glucose metabolism in order to determine how this affected survival in anoxic and hyperosmotic environments. Three mutants involved in gluconeogenesis were identified that were sensitive to anoxia and hyperosmotic conditions (Fig. 3A). One strain has a mutated phosphoenolpyruvate carboxykinase enzyme (PEPCK/W05G11.6 (ok2098) (GI175171)). This strain lays embryos that are sensitive to anoxia (average survival 50%; P<0.01), though with high variability. We could not test the adaptation of PEPCK mutant embryos to salt because, unlike wild-type, very few ok2098 L4 animals develop into egg-laying adults when placed on 300mM NaCl. The other two strains have mutations in the bifunctional isocitrate lyase/malate synthase enzymes of the worm (gei-7(ok531) (GI178583); C08F11.14(ok457) (GI178328)), key components of the glyoxylate cycle. Each of the individual glyoxylate cycle mutants has a weaker anoxia sensitivity phenotype (50% and 85% survival, respectively (DNS)) than the double mutant strain (Fig 4A, 35% survival; P<0.01). The embryos from glyoxylate cycle double mutants treated with OPC were not adapted to high salt and were even worse off in anoxia after OPC. No glyoxylate cycle mutant embryos survived in 500mM salt after OPC and 1.3% survived in anoxia after OPC, compared to 26% and 42% for wild-type, respectively (P<0.05 for both). While the exact reason that these mutants are sensitive to both salt and anoxia is not fully elucidated, the sum of the data from these experiments suggest a strong correlation between the ability to store glycogen and survival in both anoxia and high salt environments.
Discussion
We have provided the first evidence that C. elegans adults are capable of preparing their embryos to survive in a specific environment. APE mediated by hyperosmotic preconditioning is associated with increased glycerol and decreased glycogen in the adult worm and the same changes in the embryo. We find it particularly interesting that trehalose levels remain constant in the OPC adult but rise in the embryo, as this suggests an alteration in embryonic provisioning that is not merely reflective of parental physiology. Both glycerol and trehalose have previously been shown to be important for survival in hyperosmotic conditions[19, 21, 22].
daf-2(e1370) adults are unable to engage APE in response to OPC in a daf-16(m26) dependent manner. The evidence suggests that daf-2 animals are incapable of correctly provisioning their embryos with the level of glycerol or trehalose required to survive in high salt environments. The metabolic origin of these defects are difficult to hypothesize a priori, as gene expression analysis and other studies have suggested that daf-2 adults have very different metabolic activity from wild-type[29-35]. The high level of glycerol accumulated by wild-type animals upon OPC is correlated with a decrease in glycogen storage, which also did not occur in OPC e1370 adults. Glycerol accumulation in high salt requires gpdh-1 and gpdh-2; this strongly suggests that the source of the glycerol is the glycolytic intermediate dihydroxyacetone phosphate (DHAP) which may be produced from either gluconeogenesis or glycolysis. Triglyceride catabolism, which produces glycerol without glycerol-3-phosphate dehydrogenase, is not likely to be an important source for glycerol upon hyperosmotic exposure. Trehalose was still produced and glycogen consumed when we exposed gpdh-1;gpdh-2 mutants to OPC, so trehalose production and glycogen consumption are most likely independent of glycerol production. Among the possible explanations for the lack of glycerol and trehalose accumulation in daf-2 animals is that these worms have a dampened environmental response, and as a result do not adequately shunt glycolytic intermediates into either glycerol or trehalose, thereby avoiding the need to consume their glycogen stores. Another possibility is that either trehalose or glycerol is produced directly from glycogen and that this function is deficient in daf-2 animals, though the quantity of glycerol produced seems to be far in excess of glycogen stores. Metabolic tracer studies will likely be required in order to successfully disentangle these possibilities. Because daf-2 adults are known to be long-lived and resistant to a variety of stresses, including hyperosmotic stress and anoxia, we wonder if the improved stress resistance of the adult may be detrimental to their offspring[22, 28, 36].
In order to learn more about the relationship between sugars and survival in adverse conditions, we investigated several mutants with defects in sugar homeostasis. Based upon these studies and our work in wild-type animals, we suggest that survival in hyperosmotic and anoxic conditions is antithetical for C. elegans, and that the mechanistic basis for this may be the apparently obligatory trade-off between the abundance of glycerol and glycogen. RNAi of gsy-1, which encodes the worm glycogen synthase gene, results in low glycogen and sensitivity of embryos to anoxia. Mutants that are known to have increased osmotic resistance and to accumulate glycerol, such as osm-7(n1515) and dpy-10(e128) are also sensitive to anoxia and low in glycogen. We hypothesize that many strains that accumulate glycerol as a means of compensating for defects in protein homeostasis or collagen structure will be sensitive to anoxia. Worms with defects in genes required for gluconeogenesis (glyoxylate cycle genes gei-7 and C08F11.14 as well as PEPCK/W05G11.6) have low glycogen content and are sensitive to both anoxic and hyperosmotic environments, implying that glycogen itself or functional sugar metabolism in general is required for survival in both situations. These conclusions are supported by our biochemical analysis of carbohydrate changes upon anoxia and hyperosmotic stress in wild-type, which show that glycerol is produced and glycogen consumed as a response to hyperosmotic environments, and that glycogen is consumed in anoxia.
Experimental Procedures
Nematode culture
Unless otherwise mentioned, nematodes were grown at 20°C on NGM-lite agar seeded with OP-50 bacteria using standard protocols[37]. Worms were only used from populations that had not experienced a starvation event in the last week. The following strains were acquired from the Caenorhabditis Genetics center: N2 (wild-type var. Bristol); MT3564(osm-7(n1515)III); CB1370(daf-2(e1370)III); DR26(daf-16(m26)I); DR1309(daf-16(m26)I; daf-2(e1370)III); CB128(dpy-10(e128)II); RB1688(W05G11.6(ok2098)III); RB766(gei-7(ok531)V); RB692(C08F11.14(ok457)IV). The glyoxylate cycle double mutant (gei-7(ok531)V; C08F11.14(ok457)IV) was a gift from Heather Thieringer and the glycerol-3-phosphate dehydrogenase double mutant (gpdh-1(ok1588)I;gpdh-2(kb33)III) was a gift from Kevin Strange.
Salt and Anoxia Exposure
Hyperosmotic preconditioning
L4 nematodes were picked from populations maintained at 20°C on NGM-lite plates and moved to another NGM-lite plate or a plate supplemented with sodium chloride to a concentration of 300mM. These worms were grown for 24 hours (to young adulthood) at 20-23°C. To determine if OPC lasted 4 hours after removal from salt, adult worms preconditioned as described above were moved back to standard NGM-lite plates for four hours before exposure of embryos to 500mM NaCl as described below.
Anoxia exposure of embryos
Young adult nematodes were moved to a new plate with a spot of OP50 ringed with palmitic acid and allowed to lay eggs for 1-2 hours. Adults were then aspirated and the plates were placed in a 350mL airtight bowl which was flushed with 100mL·min-1 of either 100% nitrogen gas (Airgas, Seattle) or with room air. The gas was hydrated by passage through a 125mL gas washing bottle filled with distilled water. Fresh water was used for each experiment. The worms were exposed to the gases for 24 hours at 23°C, after which the percent of embryos hatched on the plates exposed to room air was counted. The embryos exposed to anoxia were allowed to recover for 24 hours at 23°C before counting the number of embryos hatched.
gsy-1 knockdown
RNAi interference experiments were conducted by feeding worms E. coli strain HT115 that express double-stranded RNA corresponding to the gsy-1 gene (GenePairs name Y46G5.kk). The strain was obtained from a commercially available C. elegans RNAi library (MRC Geneservice, UK). Synchronized worms were grown on gsy-1 RNAi or empty vector control food as described[38] from L1 to young adult at 20°C and their embryos exposed to anoxia as described above.
Exposure of embryos to 500mM salt
Adult nematodes grown as described above were picked into a drop of water and cut open with a razor blade. Embryos were quickly mouth-pipetted onto a NGM plate supplemented with sodium chloride to a concentration of 500mM. The percent of embryos hatched was determined after 24 hours at 23°C.
Statistical analysis
Two-tailed Student’s T-test’s were used to evaluate statistical differences between treatments. If the data failed to pass a normality test, Mann-Whitney Rank sum test was used to evaluate statistical differences between treatments.
Sugar biochemistry
Collection of nematodes
Collection of larvae
∼6000 starved L1 larvae hatched overnight from bleached adults were grown to early L4 or young adult stage at 20°C on 15cm NGM-lite plates. For OPC, early L4 nematodes were split to a fresh NGM-lite plate and a NGM-lite plate supplemented with sodium chloride to a concentration of 300mM. The worms were grown an additional 12 hours at 20°C before harvest. gpdh-1/gpdh-2 larvae were only exposed to salt for 7 hours before harvest because they do not adapt to salt well and we wished to avoid starving them on the plate due to their inability to move. Worms were washed off in M9, centrifuged at 1500RCF for 1 min, washed with water and centrifuged again. 350μL of concentrated worms were moved to a screw-top 1.5ml centrifuge tube (VWR). 35μL of these worms were removed to another tube for later counting, and the remainder was frozen in liquid nitrogen. In order to improve accuracy of worm transfer and counting, we used pipette tips with a wide opening that were blocked with 1% BSA before use. Each sample used for extraction contained 1500-3000 L4 larvae/adults.
Measurement of sugars in wild-type embryos during anoxia exposure
Synchronized young adult worms grown on peptone-enriched plates seeded with NA22 bacteria were washed off of the plates with M9, washed with water and chopped with razor blades before filtering through 43μM and 15μM nytex filters to collect embryos. Chopping and filtering was employed because wild-type embryos will not survive anoxia after being bleached as is described in the next section. Embryos were aliquoted in 250μL M9 onto a small unseeded plate and exposed to anoxia as described in the preceding section; however the temperature was kept at 20°C. For each time point, 400μL water was added to the plates and the embryos collected with the aid of a rubber policeman. The volume of embryos suspension obtained was determined and 10% removed for counting of embryos, after which the remained was frozen in liquid nitrogen. Each sample used for sugar profiling contained 30,000-60,000 embryos.
Measurement of sugars in mutant embryos
Synchronized young adult worms grown on peptone-enriched plates seeded with NA22 bacteria were washed off of the plates, washed once in M9 and bleached in alkaline hypochlorite according to standard procedures[37]. Bleached embryos were washed once with M9, once with water and collected as described for L4 larvae. Each sample used for extraction contained 50,000-100,000 embryos.
Measurement of sugars in embryos after OPC
These experiments were carried out like the L4-larvae preconditioning experiments described above, but with 10x as many adult worms, by growing these animals on peptone-enriched plates seeded with NA22. Preconditioned animals were bleached and their embryos collected as in the procedure for mutant embryos immediately preceding.
Extraction and derivatization
This extraction procedure was adapted from Pellerone et al. (2003)[25]. Frozen samples were brought to 400μl of 20% ethanol spiked with 2μg (10μl of 0.2mg/ml) each of the standards: butanetriol, mannose and sucrose. The sample was then heated to 110°C for 1 hour. The samples were centrifuged 2 min at 14,000RCF and the supernatant containing the free sugars removed to a glass screw-top tube for derivatization. 300μl of 30% sodium hydroxide was added to the pellet and heated for an additional hour at 110°C. After centrifugation the supernatant was moved to a new tube and 900μL ice-cold ethanol was added to precipitate the glycogen. The tubes were centrifuged and the supernatant aspirated. 400μl of 5% HCl with 4μg mannose was added and the samples heated to 110°C for an additional half-hour before being moved to a glass tube for derivatization. The derivatization protocol was adapted from Medeiros and Simoneit (2007)[26]. Supernatants from previous steps were evaporated under a stream of nitrogen followed by addition of 200μl of BSFTA +1% TMS and 80μl pyridine (Sigma). These samples were heated to 70°C for two hours. Following heating, the reaction mixture was evaporated by a nitrogen stream and 200μl of hexane was added to dissolve the TMS-derivatives from the free sugars. 400μl of hexane was added to dissolve the TMS-derivatives in the glycogen sample. This was washed once with 1mL water and analyzed via GC/MS.
GC/MS analysis
GC/MS settings were adapted from Medeiros and Simoneit (2007)[26]. 1μL of sample was injected in splitless mode into an Agilent 6890N gas chromatograph operated with a HP-5MS column coupled to an Agilent 5975 mass spectrometer. The injector temperature was set to 280°C, the source to 230°C with a helium flow of 1.3mL·min-1. The oven was programmed to rest at 65°C for two minutes, followed by an increase of 6°C per minute to 300°C. This temperature was held for 15 minutes. The mass spectrometer was operated in scan mode with a range of 50-650 Daltons and 1.27 scans·s-1. A single ion representing each sugar was extracted and quantified from the GC/MS spectra using the program Metaquant[39]. The retention time and m/z of each of the species verified by a purchased standard was as follows: TMS-Glycerol (12.72; 205); TMS-Butanetriol (14.68; 219); TMS-α-D(+)-Mannose (23.58; 204); TMS-β-D(+)-Mannose (25.21; 204); TMS-α-D(+)-Glucose (25.01; 204); TMS-β-D(+)-Glucose (26.50; 204); TMS-D-Sorbitol (25.81; 205); TMS-D(+)-Sucrose (35.89; 361); TMS-D(+)-Trehalose (37.12; 361). The mass of each sugar present was then determined based upon a standard curve, adjusted for losses during processing to the appropriate internal standard (butanetriol for glycerol, sucrose for trehalose, mannose for glucose), and represented as a ratio of that sugar to the quantity of glucose in the sample. For mannose, only the α peak was quantified because the β peak overlaps with an unidentified sugar in the worm samples. The α peak value was divided by 0.69 to determine the total quantity of mannose in the sample, assuming the α and β anomers are in equilibrium. While the quantity of sugars in the sample can be expressed per worm based upon the counting we did before extraction, we found that the trends were the same when normalized to free glucose, and that the normalization to free glucose eliminated error in counting animals and did not introduce error due to worms being different sizes. The values from multiple replicates evaluated for statistical significance using two-tailed, unpaired, Student’s t-test.
Glycogen Staining
Live nematodes on 3% agarose pads were inverted over the opening in a 100g bottle of iodine crystals (Sigma) and allowed to sit for 40-60 seconds before viewing. For the photographs presented here, worms were stained simultaneously and manipulated on the pad for DIC microscopy. Photographs were taken with a Zeiss MRc color camera. For staining with Best’s carmine, worms were fixed in 70% ethanol; 20% glacial acetic acid and 10% concentrated formalin for 90 minutes before proceeding with Best’s carmine staining as described in Humason (1979)[40]. Worms were mounted in glycerol for microscopy and photography.
Supplementary Material
Acknowledgments
We gratefully acknowledge Heather Thieringer for sharing with us the glyoxylate cycle double mutant (gei-7(ok531); C08F11.14(ok457)) and Kevin Strange for sharing the gpdh-1(ok1588)I;gpdh-2(kb33)III double mutant. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). Jesse P. Goldmark originally observed that dpy-10 mutant embryos were sensitive to anoxia. Critical review of the manuscript was provided by members of the Roth lab, Marc Van Gilst and Jeffery Rasmussen. Members of the Van Gilst lab assisted with GC/MS troubleshooting. This work was funded by NIH grant GM048435 to MBR. The authors have no conflicting interests to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Horton TH. Fetal origins of developmental plasticity: animal models of induced life history variation. Am J Hum Biol. 2005;17:34–43. doi: 10.1002/ajhb.20092. [DOI] [PubMed] [Google Scholar]
- 2.Stetson MH, Elliott JA, Goldman BD. Maternal transfer of photoperiodic information influences the photoperiodic response of prepubertal Djungarian hamsters (Phodopus sungorus sungorus) Biol Reprod. 1986;34:664–669. doi: 10.1095/biolreprod34.4.664. [DOI] [PubMed] [Google Scholar]
- 3.Horton TH. Cross-fostering of voles demonstrates in utero effect of photoperiod. Biol Reprod. 1985;33:934–939. doi: 10.1095/biolreprod33.4.934. [DOI] [PubMed] [Google Scholar]
- 4.Horton TH. Growth and reproductive development of male Microtus montanus is affected by the prenatal photoperiod. Biol Reprod. 1984;31:499–504. doi: 10.1095/biolreprod31.3.499. [DOI] [PubMed] [Google Scholar]
- 5.Shaw D, Goldman BD. Developmental changes in male Siberian hamsters (Phodopus sungorus) exposed to different gestational and postnatal photoperiods. J Pineal Res. 2007;43:25–34. doi: 10.1111/j.1600-079X.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- 6.Fox CW, Thakar Monica S, Mousseau Timothy A. Egg Size Plasticity in a Seed Beetle: An Adaptive Maternal Effect. The American Naturalist. 1997;149:149–163. [Google Scholar]
- 7.Galloway LF. Maternal effects provide phenotypic adaptation to local environmental conditions. New Phytol. 2005;166:93–99. doi: 10.1111/j.1469-8137.2004.01314.x. [DOI] [PubMed] [Google Scholar]
- 8.Maternal Effects as Adaptations. Oxford University Press; New York: 1998. [Google Scholar]
- 9.Wells JC. The thrifty phenotype as an adaptive maternal effect. Biol Rev Camb Philos Soc. 2007;82:143–172. doi: 10.1111/j.1469-185X.2006.00007.x. [DOI] [PubMed] [Google Scholar]
- 10.Wells JC. The thrifty phenotype hypothesis: thrifty offspring or thrifty mother? J Theor Biol. 2003;221:143–161. doi: 10.1006/jtbi.2003.3183. [DOI] [PubMed] [Google Scholar]
- 11.Marshall DJ, Uller Tobias. When is a maternal effect adaptive? Oikos. 2007;116:1957–1963. [Google Scholar]
- 12.Fox CW, Czesak ME, Mousseau TA, Roff DA. The Evolutionary Genetics of an Adaptive Maternal Effect: Egg Size Plasticity in a Seed Beetle. Evolution. 1999;53:552–560. doi: 10.1111/j.1558-5646.1999.tb03790.x. [DOI] [PubMed] [Google Scholar]
- 13.Hassall M, Walters RJ, Telfer M, Hassall MR. Why does a grasshopper have fewer, larger offspring at its range limits? J Evol Biol. 2006;19:267–276. doi: 10.1111/j.1420-9101.2005.00967.x. [DOI] [PubMed] [Google Scholar]
- 14.Czesak ME, Fox CW. Evolutionary ecology of egg size and number in a seed beetle: genetic trade-off differs between environments. Evolution. 2003;57:1121–1132. doi: 10.1111/j.0014-3820.2003.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 15.Horie Y, Kanda T, Mochida Y. Sorbitol as an arrester of embryonic development in diapausing eggs of the silkworm, Bombyx mori. J Insect Physiol. 2000;46:1009–1016. doi: 10.1016/s0022-1910(99)00212-7. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita O. Diapause hormone of the Silkworm, Bombyx mori: Structure, Gene Expression and Function. Journal of Insect Physiology. 1996;42:669–679. [Google Scholar]
- 17.Van Voorhies WA, Ward S. Broad oxygen tolerance in the nematode Caenorhabditis elegans. J Exp Biol. 2000;203:2467–2478. doi: 10.1242/jeb.203.16.2467. [DOI] [PubMed] [Google Scholar]
- 18.Padilla PA, Nystul TG, Zager RA, Johnson AC, Roth MB. Dephosphorylation of cell cycle-regulated proteins correlates with anoxia-induced suspended animation in Caenorhabditis elegans. Mol Biol Cell. 2002;13:1473–1483. doi: 10.1091/mbc.01-12-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamitina ST, Morrison R, Moeckel GW, Strange K. Adaptation of the nematode Caenorhabditis elegans to extreme osmotic stress. Am J Physiol Cell Physiol. 2004;286:C785–791. doi: 10.1152/ajpcell.00381.2003. [DOI] [PubMed] [Google Scholar]
- 20.Wheeler JM, Thomas JH. Identification of a novel gene family involved in osmotic stress response in Caenorhabditis elegans. Genetics. 2006;174:1327–1336. doi: 10.1534/genetics.106.059089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamitina T, Huang CG, Strange K. Genome-wide RNAi screening identifies protein damage as a regulator of osmoprotective gene expression. Proc Natl Acad Sci U S A. 2006;103:12173–12178. doi: 10.1073/pnas.0602987103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamitina ST, Strange K. Transcriptional targets of DAF-16 insulin signaling pathway protect C. elegans from extreme hypertonic stress. Am J Physiol Cell Physiol. 2005;288:C467–474. doi: 10.1152/ajpcell.00451.2004. [DOI] [PubMed] [Google Scholar]
- 23.Dawes GS, Mott JC, Shelley HJ. The importance of cardiac glycogen for the maintenance of life in foetal lambs and newborn animals during anoxia. J Physiol. 1959;146:516–538. doi: 10.1113/jphysiol.1959.sp006208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stafford A, Weatherall JA. The survival of young rats in nitrogen. J Physiol. 1960;153:457–472. doi: 10.1113/jphysiol.1960.sp006547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellerone FI, Archer SK, Behm CA, Grant WN, Lacey MJ, Somerville AC. Trehalose metabolism genes in Caenorhabditis elegans and filarial nematodes. Int J Parasitol. 2003;33:1195–1206. doi: 10.1016/s0020-7519(03)00173-5. [DOI] [PubMed] [Google Scholar]
- 26.Medeiros PM, Simoneit BR. Analysis of sugars in environmental samples by gas chromatography-mass spectrometry. J Chromatogr A. 2007;1141:271–278. doi: 10.1016/j.chroma.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Foll RL, Pleyers A, Lewandovski GJ, Wermter C, Hegemann V, Paul RJ. Anaerobiosis in the nematode Caenorhabditis elegans. Comp Biochem Physiol B Biochem Mol Biol. 1999;124:269–280. doi: 10.1016/s0305-0491(99)00130-3. [DOI] [PubMed] [Google Scholar]
- 28.Scott BA, Avidan MS, Crowder CM. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science. 2002;296:2388–2391. doi: 10.1126/science.1072302. [DOI] [PubMed] [Google Scholar]
- 29.Burnell AM, Houthoofd K, O’Hanlon K, Vanfleteren JR. Alternate metabolism during the dauer stage of the nematode Caenorhabditis elegans. Exp Gerontol. 2005;40:850–856. doi: 10.1016/j.exger.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 30.McElwee JJ, Schuster E, Blanc E, Thornton J, Gems D. Diapause-associated metabolic traits reiterated in long-lived daf-2 mutants in the nematode Caenorhabditis elegans. Mech Ageing Dev. 2006;127:458–472. doi: 10.1016/j.mad.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Houthoofd K, Fidalgo MA, Hoogewijs D, Braeckman BP, Lenaerts I, Brys K, Matthijssens F, De Vreese A, Van Eygen S, Munoz MJ, Vanfleteren JR. Metabolism, physiology and stress defense in three aging Ins/IGF-1 mutants of the nematode Caenorhabditis elegans. Aging Cell. 2005;4:87–95. doi: 10.1111/j.1474-9726.2005.00150.x. [DOI] [PubMed] [Google Scholar]
- 32.Houthoofd K, Braeckman BP, Lenaerts I, Brys K, Matthijssens F, De Vreese A, Van Eygen S, Vanfleteren JR. DAF-2 pathway mutations and food restriction in aging Caenorhabditis elegans differentially affect metabolism. Neurobiol Aging. 2005;26:689–696. doi: 10.1016/j.neurobiolaging.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Vanfleteren JR, De Vreese A. The gerontogenes age-1 and daf-2 determine metabolic rate potential in aging Caenorhabditis elegans. Faseb J. 1995;9:1355–1361. doi: 10.1096/fasebj.9.13.7557026. [DOI] [PubMed] [Google Scholar]
- 34.Van Voorhies WA, Ward S. Genetic and environmental conditions that increase longevity in Caenorhabditis elegans decrease metabolic rate. Proc Natl Acad Sci U S A. 1999;96:11399–11403. doi: 10.1073/pnas.96.20.11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez CL, Van Gilst MR. A 13C isotope labeling strategy reveals the influence of insulin signaling on lipogenesis in C. elegans. Cell Metab. 2008;8:266–274. doi: 10.1016/j.cmet.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 37.C. elegans: A Practical Approach. Oxford University Press; Oxford: 1999. [Google Scholar]
- 38.Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2001;2 doi: 10.1186/gb-2000-2-1-research0002. RESEARCH0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bunk B, Kucklick M, Jonas R, Munch R, Schobert M, Jahn D, Hiller K. MetaQuant: a tool for the automatic quantification of GC/MS-based metabolome data. Bioinformatics. 2006;22:2962–2965. doi: 10.1093/bioinformatics/btl526. [DOI] [PubMed] [Google Scholar]
- 40.Humason GL. Animal Tissue Techniques. 4th Edition WH Freeman and Co; San Francisco: 1979. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.