Abstract
Catechins are a major constituent of green tea. For green tea to have cancer therapeutic benefit, catechin concentrations in the range of 100 nM are required continuously until apoptosis (programmed cell death) is induced. To prolong elevated plasma and interstitial concentrations of catechins, a sustained-release formulation of green tea extract was tested and compared to a commercial green tea extract (Tegreen97®). Sustained-release formulations are usually developed in the pharmaceutical industry to slowly deliver the compound over a period of time and increase the dosing interval. Plasma and interstitial fluid (ISF) pharmacokinetics of catechins were determined following an oral dose in the rat. The sustained-release formulation profile included multiple smaller peaks of total catechins in both plasma and ISF. Interstitial fluid profiles of green tea extract indicate that higher catechins concentration and longer duration in tissue than in blood may make a sustained-release form unnecessary.
Keywords: Green tea pharmacokinetics, sustained release
INTRODUCTION
Much attention to tea catechins and health has focused on its effects on cancer (Dreosti, 1996). Drinking tea has been regarded traditionally in Asia as a generally healthful practice. Polyphenols are a class of aromatic compounds containing several phenolic groups. They are found in fruits and vegetables and confer colors. Catechins, many of which are thought to have health benefits, are flavan-3-ols, a subset of the class of polyphenols. Health benefits of tea catechins in cancer have been broadly attributed to one of the three areas: antioxidant properties, effects on intestinal microorganisms and nutrient absorption, and effects on metabolism and metabolic enzymes and on growth, especially of cancer cells (Dreosti, 1996). Anticancer effects for tea are indicated both from animal in vivo studies (Ahmad, Feyes, Nieminen, Agarwal, & Mukhtar, 1997; Chen, Schell, Ho, & Chen, 1998;Fujiki et al., 1999; Liao, Umekita, Guo, Kokontis, & Hiipakka, 1995) and from human epidemiological observations (Fujiki et al., 1999; Katdare, Osborn, & Telang, 1998). In general, these effects have been attributed to the principal green tea catechin epigallocatechin-3-gallate (EGCg) (Chen et al., 1998) but other catechins and polyphenols present in tea act synergistically with EGCg in their anticancer properties (Morre et al., 2003).
Human studies show that cancer onset of patients in Japan who have consumed 10 cups of green tea per day was 8.7 years later among females and 3 years later among males, compared with patients who had consumed under three cups per day (Liao et al., 1995). Thus, a possible relationship between high consumption of green tea and the low incidence of prostate and breast cancers in some Asian countries has been postulated (Chen et al., 1998).
A total of 31 studies and four reviews were examined by Bushman (1998), the majority of which showed an inverse association between tea consumption and cancers of the colon, urinary bladder, stomach, esophagus, lung, and pancreas. A negative association between green tea consumption and cancer incidence, especially among females drinking more than 10 cups per day was reported (Imai, Litt, Suga, & Nakachi, 1997).
A large case-controlled study conducted in Shanghai, China, involving 2266 cancer patients and 1552 controls suggested that green tea consumption lowered the risk of cancer of the colon, rectum, and pancreas by 18%, 28%, and 37%, respectively in men, and by 33%, 43%, and 47% respectively in women (Yu et al., 1995). Other studies suggested protective effects of green tea of similar magnitude against esophageal and stomach cancers (Guo et al., 1994). Other smaller studies have revealed a significantly reduced risk of colon cancer and precancerous adenomas (tumors of glandular origin) of the colon and rectum in tea drinkers (Ji et al., 1996; Kono, Ikada, Tokudome, & Kuratsune, 1988). Five out of six cohort studies reported lower risks of pancreatic cancer among tea drinkers (Ji et al., 1997).
Animal studies involving known chemical carcinogens as well as transplanted tumor cells indicate reduced cancer risk from consumption of green or black tea catechins. These numerous animal studies have implicated cancer protective responses that span the entire process of carcinogenesis including the formation and activation of carcinogens, cancer initiation, promotion and progression, as well as diminished tumor growth and metastatic spread. Prevention is most often attributed to antioxidant effects whereas the diminished tumor growth and metastatic spread may be related to more specific mechanisms of cancer cell growth inhibition (Cooper, Morré, & Morré, 2005).
An autochthonous transgenic mouse prostate model spontaneously develops metastatic prostate carcinoma in 100% of the male mice by 24–28 weeks of age (Gupta, Hastak, Ahmad, Lewin, & Mukhtar, 2001). These mice have a 3-fold increase in ornithine decarboxylase activity and a 4-fold increase in protein expression over nontransgenic littermates. In this mouse model, infusion of green tea polyphenols at a human achievable dose equivalent to 6 cups of green tea per day significantly inhibited tumor development and increased survival (Gupta et al., 2001). Oral administration of green tea, black tea or epigallocatechin-3-gallate inhibited the growth of well-established skin tumors and, in some cases, regression was observed (Conney, Lu, Lou, Xie, & Huang, 1999). The human prostate cancer cell lines, PC-3 (androgen-insensitive), and LNCaP 104-R (androgen repressed) were inoculated subcutaneously into nude mice to produce prostate tumors. Intraperitoneal injection of green tea epigallocatechin-3-gallate, inhibited the growth and rapidly reduced the size of human prostate tumors (Liao et al., 1995).
Green tea has been shown by Morre, Bridge, Wu, & Morre (2000) to inhibit the growth of HeLa (human cervical cancer) and BT-20 (human mammary cancer) cells in vitro by interaction with tumor-associated Nicotinamide Adenine Dinucleotide (NADH) oxidase (tNOX), a cancer specific variant of a family of cell surface proteins with both the reduced form of NADH or hydroquinone oxidative activity that also carry out protein disulfide-thiol interchange. Epigallocatechin gallate is the most potent catechin for the inhibition of growth of cancer cells but the other catechins in green tea act synergistically to enhance the inhibitory effect of EGCg (Morre et al., 2003). Epicatechin (EC) is another catechin found in tea. In brewed tea it is usually about one fourth the concentration of EGCg (Green, Murphy, Schulz, Watkins, & Ferruzzi, 2007). In in vitro studies EC alone at 10−4M concentration has no killing effect on HeLa cells but when added to EGCg it potentiated the inhibitory effect of EGCg. Without EC, EGCg reduced the cell number by 50% at a concentration of 10−5M. In the presence of 10−4M EC, EGCg reduced the cell number by 50% at a concentration of 10−7M (Morre et al., 2003).
The green tea catechins prevent cell enlargement by a specific interaction with tNOX (Morre et al., 2003; Morre et al., 2000). Without enlargement there is no cell division and cell death ensues. The concentrations and ratios of catechins needed to inhibit cancer cells in vitro have been determined (Morre et al., 2003). The catechins must be continually present until cell death occurs (Morre et al., 2000). If the catechins degrade or are metabolized, the cell growth starts again. In order for green tea catechins to effectively inhibit cancer in vivo, catechins will need to reach effective concentrations and these concentrations will have to be maintained. For orally consumed green tea, catechins reach a peak blood concentration in 1–2 hr and are eliminated by 5–6 hr (Liao, Kao, & Hiipakka, 2001; Zhu, Chen, & Li, 2000). For green tea catechins to maintain high blood levels, they would need to be administered at least every 4 hr including during the night.
Perhaps more important than the plasma catechin concentrations are the concentrations in the interstitial fluid (ISF) at the tissue level where the interaction with cancer cells occurs. In most cases when the bioavailability of a bioactive compound or drug is being assessed, the blood levels of the compound are determined and the area under the time vs. concentration curve (AUC) is used to evaluate the amount of the compound available for therapeutic efficacy. However, to have a therapeutic effect the bioactive substance must leave the blood compartment and move into ISF surrounding the cancer cells. Therefore ISF concentrations may be more indicative of therapeutic potential than blood concentrations. In vitro studies indicate that an effective dose of EGCg in green tea for inhibiting cancer is 100 nM (Morre et al., 2003; Morre et al., 2000). It may be more appropriate to choose dosing intervals based on achieving these concentrations in ISF rather than in blood.
In order to achieve a longer duration of catechin elevation, a sustained-release green tea extract was formulated. It is hypothesized that to be effective in controlling cancer, the concentration of catechins in ISF will need to be equivalent to the concentrations found effective in vitro. The plasma and ISF pharmacokinetics of EGCg and EC in green tea extract and a sustained-release formulation of green tea extract were determined after a single oral dose in rats.
MATERIALS AND METHODS
Animals and Procedures
Ten male Sprague Dawley rats between 250 and 300 g were obtained from Harlan (Indianapolis, IN). The rats were anesthetized with a mixture of ketamine and xylazine (10:1 100 mg/ml) at a dose of 0.1 ml/100 g and implanted with jugular catheters and subcutaneous ultrafiltration probes (PN MF-7023, Bioanalytical Systems, INC, West Lafayette, IN) under aseptic conditions as previously described (Janle, Portocarrero, Zhu, & Zhou, 2005). The rats were placed in a Culex-automated blood sampling system (Bioanalytical Systems, INC. West Lafayette, IN) and allowed to recover from surgery for 2 days. During the recovery period the Culex was placed on the “Tend” protocol which flushes the catheter with small amounts of sterile saline at 10 minute intervals to maintain patency. The ultrafiltration probe was connected to a needle hub which was inserted into a Vacutainer® tube to maintain flow until the dosing study. Before dosing, the ultrafiltration probe was attached to a mini peristaltic pump and fractions were collected in a fraction collector (BAS, West Lafayette, IN).
Rats were assigned to two groups (n = 5) and dosed with either green tea extract (Tegreen97®, Pharmanex, Provo, Utah) or a sustained-release form of green tea extract prepared by Pharmanex. Baseline blood and ultrafiltrate samples were obtained. Each rat received an amount of product to provide 50 mg/kg of EGCg, the major catechin in green tea. The powder was suspended in 1.5 ml of water and was delivered by gavage. For the rats receiving green tea extract, the Culex was programmed to take 150 μL blood samples every hour for the first 8 hr, every two hours until 12 hr, and at 24 hr post-dose. For the sustained-release green tea extract the blood samples were taken at 1 and 2 hr and at 2-hr intervals after that until 24 hr post-dose. Blood withdrawn was automatically replaced by the Culex with an equal volume of saline after each withdrawal. Blood samples were centrifuged and plasma was stored at −80°C until analyzed. Ultrafiltrate was collected continuously at 1-hour intervals. Ultrafiltrate samples were stored at −80°C. All animal protocols were approved by the Bioanalytical Systems Animal Care and Use Committee.
Sustained-Release Green Tea
The sustained-release formulation of Tegreen97® (P-3069) containing 28.5% EGCg (Morré, Morré, Cooper, & Chang, 2002) was donated by Pharmanex (Provo, Utah). It was synthesized with a Capsudar®SR microencapsulation to achieve a slow or sustained-release effect. In addition to the green tea extract, the sustained-release formulation also contained the active ingredients microcrystalline cellulose, maltodextrine, ethylcellulose, and magnesium stearate.
Sample Analysis
Samples were analyzed for the catechins EGCg and EC using HPLC. A Bioanalytical Systems, 480e chromatograph with a multichannel amperometric detector (Epsilon™, BAS) with a 4 × 2 mm glassy carbon electrodes in an arc flow cell was used with a C18 microbore (100 × 1 mm, 5 μm) column. Output was analyzed with Chromgraph 2.0 software (BAS, West Lafayette, IN). The mobile phase was 0.6% formic acid in water and acetonitrile (92:8, v/v). The flow rate was 130 μL/min. Four channels of the electrochemical detector with potentials of +800, +700, +600, and +500 mV vs. Ag/AgCl reference electrode were used. The limit of detection for EGCg was 0.67 ng/ml at a signal-to-noise ratio of 4:1 and the limit of quantification was 1 ng/ml. The linearity of the calibration curve was obtained over the range of 1 to 100 ng/ml EGCg.
Analysis
Mean and standard errors were calculated for each point of the plasma and ISF profile. The area under the curve was calculated for plasma and ISF EGCg and EC. Differences between groups were analyzed by Student’s t-test procedure.
RESULTS
The plasma profile of EGCg in rats dosed with green tea extract exhibited elevated concentrations between 1 and 3 hr with a peak concentration of 57 ng/ml at 2 hr (Figure 1(a)). By 5 hr the plasma levels had fallen to 7 ng/ml. However, amounts between 4 and 9 ng/ml were detectable in plasma for as long as 12 hr. The concentration of EGCg in the ISF was 54 ng/ml by 2 hr and reached a peak concentration of 85 ng/ml at about 4 hr. Interstitial concentrations of EGCg remained elevated up to 9 hr.
FIGURE 1.
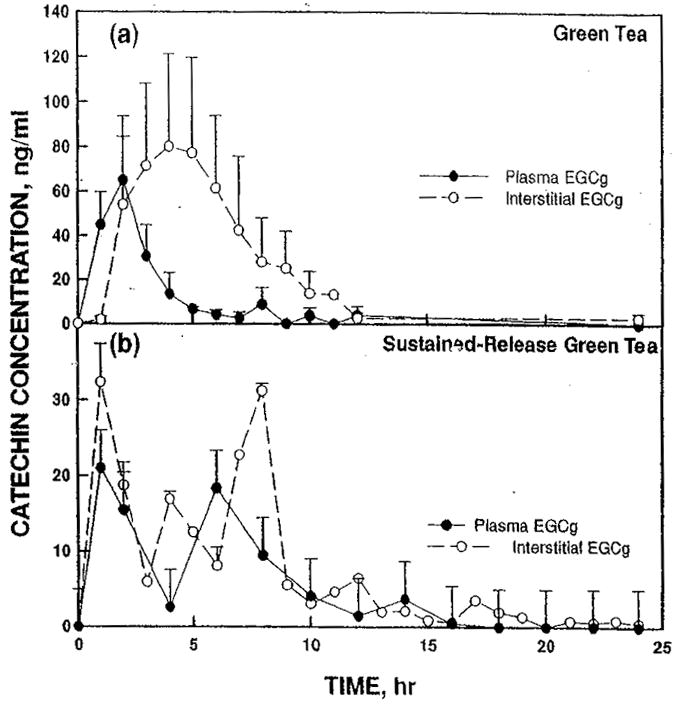
Concentrations of EGCg (a) after dosing with green tea extract or (b) with sustained-release green tea extract comparing plasma (solid circles, solid line) and interstitial fluid (open circles, dashed line).
With sustained-release green tea extract, a much broader distribution of EGCg was observed over an 8-hr time course (Figure 1(b)). The distribution in plasma was bimodal with peaks of about 20 ng/ml at 1 and 6 hr. For ISF there were three peaks at 1, 4, and 8 hr with maximum concentrations of EGCg reaching 32 ng/ml. Both the plasma and the ISF concentrations of EGCg remained slightly elevated at similar levels for at least 8 hr.
The pharmacokinetic pattern of EC in the serum and ISF with green tea extract was similar but not identical to those for EGCg (Figure 2(a)). The plasma concentrations of EC were elevated for about 4 hr reaching a peak of 12 ng/ml at 2 hr. Interstitial concentrations remain elevated for 6 to 7 hr reaching a peak concentration of 10 ng/ml at 3 hr.
FIGURE 2.
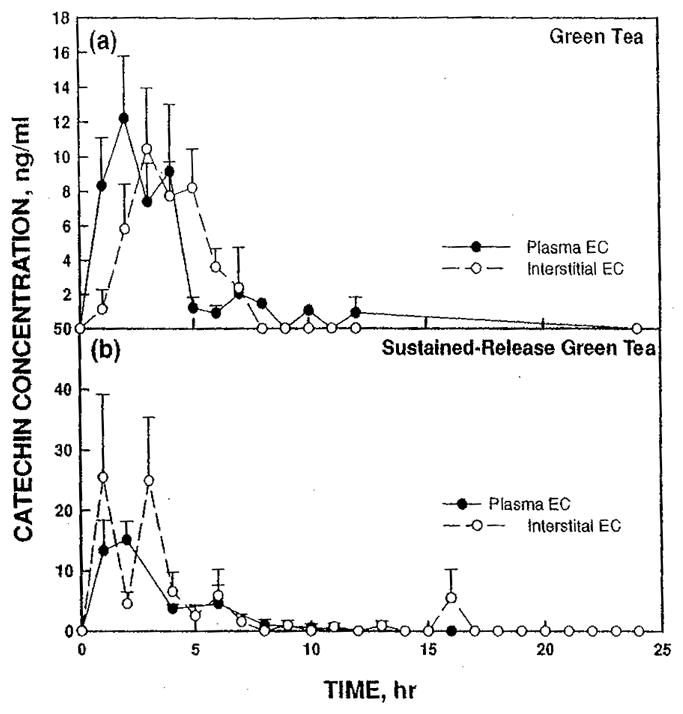
Concentration of EC (a) after dosing with green tea extract or (b) with sustained-release green tea extract comparing plasma (solid circles, solid line) and interstitial fluid (open circles, dashed line).
Sustained-release green tea extract had a peak plasma concentration of 15 ng/ml EC at 2 hr, comparable to tea green extract of 12 ng/ml (Figure 2(b)). Plasma concentrations remained elevated longer with the sustained-release product. At 8 hr the sustained-release product was 4.5 ng/ml vs. 1.5 ng/ml for green tea extract. The interstitial concentrations of EC after administration of sustained-release green tea extract (Figure 2(b)) were 25 ng/ml at 2 and 4 hr, nearly twice those found in the plasma. Additionally, the interstitial levels showed a definite trimodal pattern as seen with EGCg, although the third peak was much smaller compared to EGCg.
The relative amounts of plasma and interstitial EGCg and EC for green tea extract and the sustained-release formulation were estimated from the areas under the curves shown in Figures 1 and 2 (Table 1). The AUC of EGCg in plasma for green tea extract (216 ng · h/ml) was greater than the plasma AUC for the sustained-release formulation (129 ng · h/ml). However, because of the variability, the difference did not reach statistical significance. For ISF EGCg, there was a trend (p = .07) for a greater AUC for green tea extract (462 ng · h/ml) than for the sustained-release form (186 ng · h/ml). For EC plasma, differences in AUC between green tea extract (52 ng. h/ml) and sustained-release green tea (56 ng. h/ml) were not significantly different. In ISF, the AUC for the EC from the sustained-release formulation (79 ng · h/m) was significantly (p < .05) greater than the green tea extract (39 ng · h/ml).
TABLE 1.
Area Under the Curve ± SE (ng·h/ml) for Green Tea Extract and Sustained-Release Green Tea Extract
| Plasma |
Interstitial Fluid |
|||
|---|---|---|---|---|
| EGCg | EC | EGCg | EC | |
| Green tea extract | 216 ± 63 | 52 ± 13 | 462 ± 254 | 39 ± 6 |
| Sustained Release | 83 ± 11 | 56 ± 9 | 186 ± 71 | 79 ± 23 |
When represented as total catechins measured (EGCg + EC) (Figure 3(a)), the sustained-release formulation (Figure 3(b)) continued to show a multimodal distribution with bioavailability extending to 8 hr at a relatively uniform ISF level after 2 hr of between 20 and 30 ng/ml. The green tea extract profile for the combined catechins shows a plasma peak of 40 ng/ml at 2 hr and 88 ng/ml at 4 hr.
FIGURE 3.
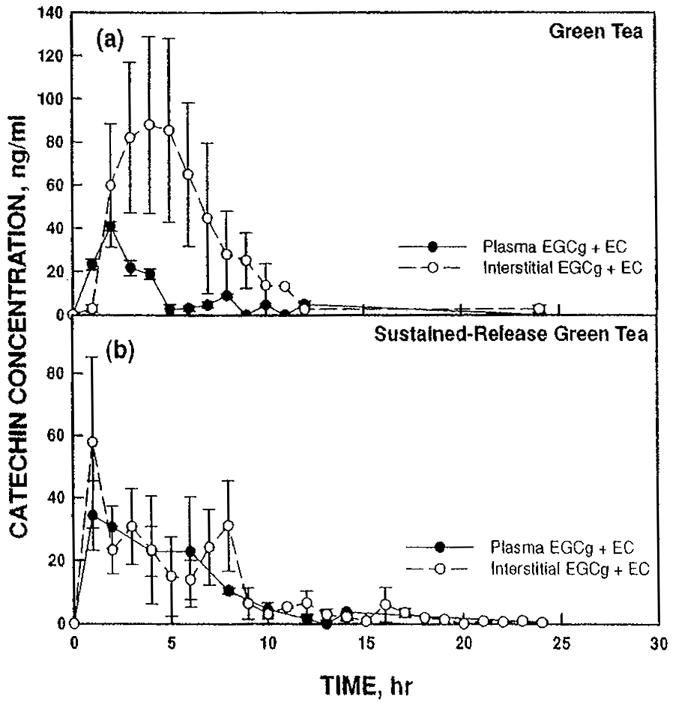
Concentration of EGCg + EC (a) after dosing with green tea extract or (b) with sustained-release green tea extract comparing plasma (solid circles, solid line) and interstitial fluid (open circles, dashed line).
DISCUSSION
Previous in vitro studies have demonstrated that green tea can effectively inhibit the growth and division of cancer cells if the EGCg concentrations is maintained at or near 100 nM (Morre et al, 2000). Based on the short half-life of less than 6 hr that green tea catechins in plasma have, it was expected that doses every 4–5 hr would be needed for green tea to be used therapeutically. A sustained-release formulation was prepared and tested with the goal of providing an effective level of catechins over a longer period and facilitating the therapeutic use of green tea by increasing the dosing interval.
Most pharmacokinetic studies measure blood concentrations. However, since the catechins would need to achieve a critical concentration at the level of the cancerous tissue, the ISF concentrations are probably more indicative of the therapeutic efficacy of a given dose. In this study, we compared the plasma and ISF levels of EGCg and EC after a single oral dose of either a commercial green tea extract (Tegreen 97®) or a sustained-release formulation of the same green tea extract. The concentrations of both EGCg and EC are important because they have been demonstrated to work synergistically (Morre et al., 2003). Therefore the profile of the sum of the catechins along with the profiles of individual catechins were determined.
After an oral dose of green tea extract, plasma concentrations of EGCg showed the expected pharmacokinetic profile with peak concentration of 65 ng/ml (140 nM) occurring at 2 hr and falling to low levels by 5 hr. The peak concentrations in the ISF (80 ng/ml) occurred later than in the plasma. Peak ISF concentrations were higher and remained elevated longer than plasma concentrations. Interstitial fluid concentrations were elevated from 2 to 9 hr with the peak occurring at 4 hr (Figure 1(a)). The effective concentration of 100 nM EGCg (46 ng/ml) was maintained in the ISF from 2–7 hr after dosing.
With the sustained-release green tea extract, there were two distinct points of elevated plasma EGCg, but the peak plasma concentrations were only about one third of those reached with the green tea extract. The ISF concentrations of EGCg were elevated longer with the sustained-release product but only reached 32 ng/ml (70 mM), which was 40% of the ISF levels achieved with green tea extract. At this dose, the catechin levels are likely insufficient to halt the growth of cancer cells. To achieve therapeutic levels of EGCg with the sustained-release form of green tea larger doses or a different formulation, which provided greater bioavailability of catechins, would be needed. A number of factors affect the bioavailability of bioactive compounds in a product. Particle size determines how quickly compound dissolves and become available for absorption. An important factor is how well compounds are absorbed by the small intestine. However, compounds can be lost before they can be absorbed. They may be consumed and metabolized by gut microflora, they may be degraded due to instability in the pH of the gastric or intestinal phases of absorption. Formulations can be designed to minimize these losses.
In both plasma and ISF there was a trend for a greater amount of EGCg with the green tea extract than for the sustained-release product. It is possible that the longer residence time in the gastrointestinal tract could have resulted in increased degradation of the EGCg. Ferruzzi et al (Green et al., 2007) have shown in an in vitro model of digestion, which simulated the gastric and small intestinal phases of digestion, that 80% of all catechins were lost and EGCg was the most sensitive one. With a sustained-release product, increased time in the gastrointestinal track could have been responsible for increased degradation.
For the ISF, the AUC represents the total exposure of the tissue to EGCg. In both the green tea and the sustained-release formulation, the AUC was greater for the ISF than for plasma indicating that the catechins are more stable or are not as easily eliminated from the ISF. The higher ISF, AUC, and the longer period of elevated concentrations indicate that the therapeutic effect of EGCg may have been greater and longer in the tissue than indicated by the plasma profile of EGCg.
The relationship between plasma and ISF concentrations was different for EC (Figure 2) from that of EGCg after dosing with green tea concentrate. The maximum concentration of EC in plasma occurred at 2 hr and the ISF peak occurred 1 hr later. The peak ISF concentration was less than the plasma concentration. The ISF concentrations remained elevated longer than the plasma concentrations (Figure 2(a)). EC area under the curve for the plasma was similar in the sustained-release product and green tea extract indicating that bioavailability of EC was approximately the same for each form. The sustained-release product delivered more EC to the ISF than the green tea extract. For both green tea extract and sustained-release green tea extract, the ISF concentrations were not detectable by 8 hr.
These results suggest that there is less degradation of EC in the gut than EGCg. These results are consistent with the work of Ferruzzi (Green et al., 2007) who demonstrated with an in vitro model of digestion that during the digestive phase 90% of EGCg and only 32% of EC are degraded. The ISF accumulated more EC with the sustained-release green tea extract than with the green tea extract. However, only with EGCg did the sustained-release green tea extract show a longer duration of catechins in the plasma than with the green tea extract.
The bimodal distribution of EGCg and EC for the sustained-release green tea concentrate most likely was the result of the heterogeneous nature of the sustained-release preparation, which consisted of a mixture of fines (small particles) as well as larger particles. For maximum effectiveness, a range of particle sizes spanning release over an 8 hr period would provide one approach to a solution for extending the release time of green tea catechins.
The interstitial concentrations are probably more indicative of the therapeutic value of the catechins than the plasma levels, because it is the catechin concentration at the tumor level which will determine efficiency of inhibition of tumor growth. The in vitro concentration of EGCg, which was effective alone in killing HeLa cells as a single dose, was 46 μg/ml (10−4 M). However, if the EGCg was renewed every 2 hr to maintain a constant external concentration, the effective dose to prevent the growth of HeLa cells was 100 nM (0.046 μg/ml) (Morre et al., 2000). This amount is well within the range of interstitial concentrations achieved in this study with rats. Moreover, there was a synergy between EC and EGCg where the presence of EC greatly enhanced EGCg effectiveness (Morre et al., 2003). This could be very important especially in ISF where EC levels remain elevated longer than those of EGCg.
The rationale for developing a sustained-release form of green tea is predicated on the knowledge that the effect of EGCg is reversible. In order for cathechins to have therapeutic efficacy in selective killing of cancer cells, our findings show that the cathchins must be present in the blood at a level of about 100 nM and to inhibit tNOX continuously at that level for a period of 48 to 72 hr (Morre et al., 2000). If the EGCg is removed even after 8 hr, cancer cells in vivo resume normal rates of growth as EGCg is cleared from the blood and/or metabolized. Even in cell culture it may not survive in the media more than few hours at nanomolar concentrations. The cancer cells in vitro must be inhibited from growing for at least 48 and perhaps up to 72 hr in order for programmed cell death (apoptosis) to be induced by EGCg.
When cancer cells in vitro are challenged with nanomolar concentrations of catechins every 2 hr during the day, growth is stopped, but during the night after the catechins are taken up or lost from the medium, normal growth resumes. Feasibility of an efficacious dosing schedule is indicated from our studies with rats and is consistent with epidemiological studies in humans and animal experiments where cancer benefit has been ascribed to drinking 12 cups of tea per day without adverse effects. Tea polyphenols are absorbed after oral administration and reach their highest plasma levels about 1 to 2 hr after dosing both in rats (Unno & Takeo, 1995; Zhu et al., 2000) and in humans (Warden, Smith, Beecher, Balentine, & Clevidence, 2001). In the rat, the level of EGCg reached 12.3 nmoles/ml, in plasma (12.3 μmolar) 60 min after a single oral administration of 500 mg/kg body weight (Nakagawa, 1997), which is more than 100 times the effective dose to stop the growth of tumor cells. The studies by Yang (1997) indicated that the concentration of EGCg in the blood after 2–3 cups of green tea could reach a maximum of 0.6 μM.
In human studies of ingested catechins, 0.2% to 2% of the ingested EGCg and 0.2% to 1.3% of ingested (−)-epigallocatechin (EGC) were found in plasma 90 min after ingestion (Nakagawa, Okuda, & Miyazawa, 1997). After ingestion of green tea by human volunteers (Morre et al., 2000), Cmax values were observed 1.4 to 2.4 hr after ingestion with a half-life for EGCg of 5 to 5.5 hr. This provided the rational basis for dosing at regular 4-hr intervals with green tea extract. The goal of this study was to provide a dosing regimen with a dosing interval long enough that patients would not need to interrupt their sleep at night to take medication. When examining the plasma, the sustained-release product tested did not provide any advantage over the green tea extract. However, the interstitial concentration is more likely to determine effectiveness of treatment. If one considers the ISF patterns, it is possible that repeated dosing with the green tea extract would only be required at 7–8-hr intervals. In this case, in order for a sustained-release product to provide a therapeutic improvement over the extract, it would need to provide a therapeutic dose for at least 12 hr.
CONCLUSIONS
If a sustained-release product was to be used, either higher doses would be required to obtain therapeutic concentrations or a reformulation of the product with different particle sizes or stabilizing compounds would be necessary to enhance stability and/or absorption of catechins. It may be possible to reduce the losses in EGCg due to extended exposure to digestion by formulations which protect catechins. A sustained-release green tea formulation is potentially useful but will require further development. However, ISF catechins profiles indicate that green tea extract may provide effective concentrations at the tissue level over a period of 7–8 hr and a sustained-release form may not be necessary to achieve an acceptable therapeutic regimen. If green tea were used to treat cancer patients effective levels of the catechins would be need to be maintained at all times to prevent the cancer cells from enlarging and dividing. It had previously been assumed that since blood levels of catechins reached their peaks between 1 and 2 hr and fell to low levels by 4–5 hr that dosing would need to occur at 4-hr intervals. This would interrupt the sleep schedules of the patients. This was the motivation to try to develop a sustained-release formulation. However, since the interstitial concentrations of catechins remain elevated for a longer period of 7–8 hr, patients may be able to take the extract on an 8-hr schedule and need not to interrupt their sleep.
Acknowledgments
This work was supported by the Purdue University, University of Alabama at Birmingham Botanical Center for Age-Related Diseases, which is funded by The Office of Dietary Supplements and NCCAM Grant P50 AT 00477.
The authors thank. Ms Carla Portocarrero and Hwa Chiang for technical assistance.
Contributor Information
Elsa M. Janle, Botanical Center In Vivo Core, Purdue University, Department of Foods and Nutrition, West Lafayette, IN.
Dorothy M. Morré, Purdue University, Department of Foods and Nutrition, West Lafayette, IN.
D. James Morré, Hansen Life Sciences Research Building, Purdue University, West Lafayette, IN.
Qin Zhou, Chemistry Instructor in Ventura College, Ventura, CA.
Yongxin Zhu, Senior Scientist, JNJ, Welsh and McKean Roads, Spring House, PA.
References
- Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Instit. 1997;89:1881–86. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- Bushman JL. Green tea and cancer in humans: a review of the literature. Nutr Cancer. 1998;31:151–59. doi: 10.1080/01635589809514697. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Schell JB, Ho CT, Chen KY. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998;129:173–79. doi: 10.1016/s0304-3835(98)00108-6. [DOI] [PubMed] [Google Scholar]
- Conney AH, Lu Y-P, Lou Y-R, Xie J-G, Huang M-T. Inhibition effect of green and black tea on tumor growth. Pro Soc Exper Biol Med. 1999;220:229–33. doi: 10.1046/j.1525-1373.1999.d01-39.x. [DOI] [PubMed] [Google Scholar]
- Cooper R, Morré DJ, Morré DM. Medicinal benefits of green tea: Part II. Review of anticancer properties. J Altern Complement Med. 2005;11:639–52. doi: 10.1089/acm.2005.11.639. [DOI] [PubMed] [Google Scholar]
- Dreosti IE. Bioactive ingredients: antioxidants and polyphenols in tea. Nutr Rev. 1996;54:S51–S58. doi: 10.1111/j.1753-4887.1996.tb03819.x. [DOI] [PubMed] [Google Scholar]
- Fujiki H, Suganuma M, Okabe S, Sueoka N, Imai K, Nakachi S, et al. Mechanistic findings of green tea as cancer preventive for humans. Proc Soc Exp Biol Med. 1999;220:225–28. doi: 10.1046/j.1525-1373.1999.d01-38.x. [DOI] [PubMed] [Google Scholar]
- Green RJ, Murphy AS, Schulz B, Watkins BA, Ferruzzi MG. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Molecular Nutrition and Food Research. 2007;51:1152–62. doi: 10.1002/mnfr.200700086. [DOI] [PubMed] [Google Scholar]
- Guo Y, McLaughlin JK, Blot WJ, Ji BT, Dai Q, Fraumeni JR., Jr Reduced risk of esophageal cancer associated with green tea consumption. J Nat Can Inst. 1994;86:855–58. doi: 10.1093/jnci/86.11.855. [DOI] [PubMed] [Google Scholar]
- Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Nat Acad Sci USA. 2001;98:10350–55. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Litt O, Suga K, Nakachi K. Cancer preventative effects of drinking green tea among a Japanese population. Prevent Med. 1997;26:769–75. doi: 10.1006/pmed.1997.0242. [DOI] [PubMed] [Google Scholar]
- Janle EM, Portocarrero C, Zhu Y, Zhou Q. Effect of long-term oral administration of green tea extract on weight gain and glucose tolerance in zucker diabetic (ZDF) rats. J Herbal Pharmacother. 2005;5:55–64. [PubMed] [Google Scholar]
- Ji B-T, Chow WH, Hsing AW, McLaughlin JK, Dai Q, Guo YT, et al. Green tea consumption and the risk of pancreatic and colorectal cancers. Int J Cancer. 1997;70:255–58. doi: 10.1002/(sici)1097-0215(19970127)70:3<255::aid-ijc1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Ji B-T, Chow WH, Yang G, McLaughlin JK, Gao RN, Zheng W, et al. The influence of cigarette smoking, alcohol and green tea consumption on the risk of carcinoma of the cardia and distal stomach in Shanghai, China. Cancer. 1996;77:2449–57. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2449::AID-CNCR6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Katdare M, Osbom MP, Telang NT. Inhibition of aberrant proliferation and induction of apoptosis in pre-neoplastic human mammary epithelial cells by natural phytochemicals. Oncol Repts. 1998;5:311–15. doi: 10.3892/or.5.2.311. [DOI] [PubMed] [Google Scholar]
- Kono S, Ikada M, Tokudome S, Kuratsune M. A case control study of gastric cancer and diet in Northern Kyishu, Japan. Jpn J Cancer Res. 1988;79:1067–74. doi: 10.1111/j.1349-7006.1988.tb01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Kao Y-H, Hiipakka RA. Green tea:biochemical and biological basis for health benefits vitamins and hormones. Vitam Horm. 2001;62:1–94. doi: 10.1016/s0083-6729(01)62001-6. [DOI] [PubMed] [Google Scholar]
- Liao S, Umekita Y, Guo J, Kokontis JM, Hiipakka RA. Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Lett. 1995;96:239–43. doi: 10.1016/0304-3835(95)03948-v. [DOI] [PubMed] [Google Scholar]
- Morre DJ, Bridge A, Wu LY, Morre DM. Preferential inhibition by (−)-epigallocatechin-3-gallate of the cell surface NADH oxidase and growth of transformed cells in culture. Biochem Pharmacol. 2000;60:937–46. doi: 10.1016/s0006-2952(00)00426-3. [DOI] [PubMed] [Google Scholar]
- Morré D, Morré DJ, Cooper R, Chang MM. United States Patent Number 6,410,052. Tea catechins in sustained release formulations as cancer-specific proliferation inhibitors. 2002 June 25;
- Morre DJ, Morre DM, Sun H, Cooper R, Chang J, Janle EM. Tea catechin synergies in inhibition of cancer cell proliferation and of a cancer specific cell surface oxidase (ECTO-NOX) Pharmacol Toxicol. 2003;92:234–41. doi: 10.1034/j.1600-0773.2003.920506.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa KMT. Absorption and distribution of tea catechin, (−)-epigallocatechin-3-gallate, in the rat. J Nutr Sci Vitaminol. 1997;43:679–84. doi: 10.3177/jnsv.43.679. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Okuda S, Miyazawa T. Dose-dependent incorporation of tea catechins, (−)-epigallocatechin-3-gallate and (−)-epigallocatechin, into human plasma. Biosci Biotechnol Biochem. 1997;61:1981–85. doi: 10.1271/bbb.61.1981. [DOI] [PubMed] [Google Scholar]
- Unno T, Takeo T. Absorption, distribution, elimination of tea polyphenols in rats. Absorption of (−)-epigallocatechin gallate into the circulation system of rats. Biosci Biotechnol Biochem. 1995;59:1558–59. doi: 10.1271/bbb.59.1558. [DOI] [PubMed] [Google Scholar]
- Warden BA, Smith LS, Beecher GR, Balentine DA, Clevidence BA. Catechins are bioavailable in men and women drinking black tea throughout the day. J Nutr. 2001;131:1731–37. doi: 10.1093/jn/131.6.1731. [DOI] [PubMed] [Google Scholar]
- Yang CS. Inhibition of carcinogenesis by tea. Nat Clin Prac Cardiovasc Med. 1997;389:134–35. doi: 10.1038/38154. [DOI] [PubMed] [Google Scholar]
- Yu GP, Hsich CC, Wang LY, Yu SZ, Li XL, Jin TH. Green tea consumption and risk of stomach cancer: a population-based case-control study in Shanghai, China. Cancer Causes Control. 1995;6:532–38. doi: 10.1007/BF00054162. [DOI] [PubMed] [Google Scholar]
- Zhu M, Chen Y, Li RC. Oral absorption and bioavailability of tea catechins. Planta Med. 2000;66:444–47. doi: 10.1055/s-2000-8599. [DOI] [PubMed] [Google Scholar]


