Abstract
Background
Skeletal muscle (SM) is an important compartment but is difficult to quantify in children and adolescents.
Objective
We investigated the potential of dual-energy X-ray absorptiometry (DXA) for measuring total-body SM in pediatric subjects.
Design
A previously published adult DXA SM prediction formula was evaluated in children and adolescents aged 5–17 y (n=99) who varied in pubertal maturation stage. SM estimated by whole-body magnetic resonance imaging (MRI) was used as the reference. The adult SM model was not accurate for subjects below Tanner stage 5 (n = 65; aged 5–14 y). New pediatric SM prediction models were therefore developed and validated in a separate group (n = 18).
Results
The adult DXA SM prediction model was valid in subjects at Tanner stage 5 but significantly (P<0.001) overestimated SM in subjects below Tanner stage 5. New SM prediction formulas were developed with appendicular lean soft tissue (ALST) estimates by DXA as the main predictor variable (eg, model 1, ALST alone: R2=0.982, SEE = 0.565 kg, P<0.001). The new models were validated by the leave-one-out method and were cross-validated in a separate validation group.
Conclusions
A previously reported adult DXA SM prediction model is applicable in children and adolescents late in pubertal development (Tanner stage 5). A new DXA SM prediction model was developed for prepubertal and pubertal subjects (Tanner stage ≤4) aged ≥5 y. DXA thus provides an important opportunity for quantifying total-body SM mass across most of the human life span.
Keywords: Body composition, growth and development, magnetic resonance imaging, skeletal muscle, pediatric prediction models, dual-energy X-ray absorptiometry
INTRODUCTION
Skeletal muscle (SM) is a large and important body-composition compartment in children and adolescents. SM mass increases during growth, and a sexual dimorphism appears during adolescence that persists throughout adult life (1-5).
Although central to growth, development, and nutrition, the SM compartment remains difficult or impractical to quantify in children and adolescents. Available methods for pediatric use are generally of value only for regional measurements, are inaccurate or impractical, or pose undue risk for young subjects (6-8).
The introduction of dual-energy X-ray absorptiometry (DXA) has offered a practical new approach for estimating SM in pediatric subjects. The DXA method provides lean soft tissue estimates of the extremities, and a large proportion of total-body SM is within the fat-free appendicular compartment (9). The capability of measuring appendicular lean soft tissue (ALST) by DXA therefore provides an opportunity for developing whole-body SM prediction formulas. We recently reported an adult whole-body DXA SM prediction model based on reference SM estimates obtained by magnetic resonance imaging (MRI) (10, 11).
With growth, development, and maturation come changes in body proportions, including the relations between extremity length and stature (12). Accordingly, the present study was designed as a linked two-phase investigation. In the first phase, we tested the validity of the adult DXA SM prediction model reported by Kim et al (11) in children and adolescents with the aim of establishing the level of agreement with SM measured by MRI. If we failed to validate the adult model, our next step was to develop and validate a standalone SM prediction formula for use in pediatric research and clinical practice.
SUBJECTS AND METHODS
Protocol and subjects
In the first study phase, the previously developed adult DXA SM prediction formula (11) was evaluated in the pediatric sample:
| (1) |
Pediatric subjects with Tanner pubertal stages ranging from 1 to 5 had measurements similar to those in the adult model-development group: ALST was estimated by DXA and total-body SM was quantified by whole-body MRI. Children and adolescents in the pediatric sample were recruited through schools, newspaper advertisements, and flyers posted in the local community.
In the second study phase, we developed pediatric SM prediction formulas based on DXA ALST in subjects at and below Tanner stage 4 and internally validated the new prediction models by use of the leave-one-out method. In addition, we cross-validated the newly developed formulas in a separate pediatric validation group. Children who had participated in previous cross-sectional investigations of body composition served as the child validation sample (13).
The pediatric samples included healthy children and adolescents with body mass indexes (BMI; in kg/m2)<35. The Tanner stage of each subject was determined by a pediatrician according to breast stage and pubic hair development in girls and genitalia development in boys (5). Subjects taking medications that could affect appetite, metabolism, or growth were excluded from the study.
Each subject completed a medical examination that included screening blood tests. Only healthy adult and pediatric subjects, without any diagnosed medical conditions, were enrolled in the study. The Institutional Review Board of St Luke’s-Roosevelt Hospital Center approved the study, and all subjects gave written consent before participation.
Body-composition analysis
The subject’s body mass and height were measured with a digital scale (Weight Tronix, New York, NY) and stadiometer (Holtain, Crosswell, United Kingdom), respectively.
Dual-energy X-ray absorptiometry
Whole-body and regional body composition were estimated in adults with a Lunar DPX scanner (GE Medical, Madison, WI) with software version 3.6. Two different DXA scanners were used in the pediatric sample: Lunar Prodigy (GE Medical) in the pediatric model development and Tanner 5 groups and Lunar DPX with pediatric software version 3.8G in the pediatric model-validation group. Estimates of ALST are comparable across DXA systems, and only small differences were detected between Lunar’s Prodigy and DPX systems (14).
ALST was considered equivalent to the sum of lean soft tissue in both the right and left arms and legs. Appendages were isolated from the trunk and head by using regional computer-generated default lines, with manual adjustment, on the anterior view planogram. Specific anatomical landmarks were used to define the legs (ie, soft tissue extending from a line drawn through and perpendicular to the axis of the femoral neck and angled with the pelvic brim to the phalange tips) and arms (ie, soft tissue extending from the center of the arm socket to the phalange tips) (10, 15). The system software provided the total mass, fat, lean soft tissue, and bone mineral mass for each of the selected regions.
Repeated daily measurements over 5 d in 4 adult subjects showed a CV of 1.5% for leg lean soft tissue and 2.2% for arm lean soft tissue (15). DXA scan reproducibility in children has been reported in prepubertal girls measured 6 wk apart with repeat CVs of 2.3% for fat-free mass, 4.1% for arm mass, and 2.8% for leg mass (16).
Magnetic resonance imaging
Total-body SM and intermuscular adipose tissue (IMAT) were measured by using whole-body multislice MRI. Subjects were placed on the 1.5-T scanner (6X Horizon; General Electric, Milwaukee, WI) platform with their arms extended above their heads. The images were created by using a T1-weighted spin-echo sequence with a 210-ms repetition time and an echo time of 17 ms. The intervertebral space between the fourth and fifth lumbar vertebrae (L4—L5) was set as the point of origin for all scans. Transverse images (10 mm slice thickness) were then obtained across the whole body. The between-slice gap in taller pediatric subjects and adults was 40 mm, whereas 35-mm or 25-mm gaps were empirically applied for shorter pediatric subjects (13, 17). Each whole-body scan thus included ≈30–40 cross-sectional images.
Images were by analyzed using SLICEOMATIC software (TomoVision Inc, Montreal, Canada) for segmentation and calculation of cross-sectional tissue areas (18). The IMAT component was separated from SM in each image slice as described previously (11, 13).
The MRI scans were reanalyzed after phase 1 for appendicular SM in 160 adults and all the children and adolescents. This procedure was carried out to further explore the differences between adults and children in the relations between ALST and SM observed in the phase 1 analyses. Appendicular SM as defined by MRI was considered equivalent to the sum of SM in both right and left arms and legs where separated from the torso. Extremity SM mass as measured by MRI is smaller than the amount of SM present in ALST as defined by DXA because of between-method differences in appendicular cutoffs. Total-body SM and IMAT volume estimates were converted to mass by using the assumed density of 1.04 kg/L for skeletal muscle and 0.92 kg/L for adipose tissue (19).
The technical error for repeated readings of the same adult whole-body scans by the same analyst of MRI-derived SM and IMAT volumes in our laboratory are 1.4% and 5.9%, respectively (11). The intraclass correlation coefficient between analysts for total-body MRI-derived SM from the same adult subjects in our laboratory is 0.99 (11).
Statistical analysis
Descriptive statistics of between-group differences in subject characteristics were tested for significance by using Student’s t tests. Data were analyzed by using SPSS for WINDOWS version 10.0 (SPSS Inc, Chicago, IL), and statistical significance was set at P < 0.05. Group data are expressed as means ± SDs.
In the first phase of the study, the predicted value for total-body SM mass was calculated for each child and adolescent by using the adult prediction equation reported by Kim et al (equation 1 in reference 11). We reasoned that if the adult model could predict total-body SM mass in children and adolescents, then one model could serve for all ages. The differences between predicted and actual total-body SM mass were tested for significance by using Student’s paired t tests. Although we initially pooled data from all children and adolescents, we subsequently found that stage of maturation was critical in the ALST-SM relation and used Tanner stage for grouping. The presence of a significant difference between predicted and MRI-measured SM mass in subjects at and below Tanner stage 4 indicated that a child-specific SM prediction formula was needed. Pearson’s correlation coefficients were used to examine the associations between ALST and total-body SM in the adult and Tanner stage 5 groups and in subjects ≤ Tanner stage 4.
The residual error variance of the total-body SM-ALST ratio was not equal across the adult, Tanner stage 5, and pediatric model-development groups. Therefore, between-group differences in the total-body SM-ALST ratio were tested by using Kruskal-Wallis one-way analysis of variance (ANOVA) on rank. Between-group differences in the ratio of appendicular to total-body SM and in the ratio of appendicular SM to ALST were tested for statistical significance by using one-way ANOVA.
In the second phase of the study, prediction equations for total-body SM were developed by using general linear regression model analysis in the pediatric model-development group. MRI-measured total-body SM was set as the dependent variable. Potential independent variables included ALST, age, body weight, height, Tanner stage, sex, and race. All main effects and all possible two-way interactions were investigated to find the best-fitting model with the lowest SEE. The adjusted R2 and SEE values were used to quantify model-fitting performance. The developed models were then validated by using the leave-one-out method (20). The value for total-body SM was also calculated for each child in the separate model-validation group by using the developed pediatric prediction equations. The differences between predicted and actual total-body SM mass were tested for significance by using Student’s paired t tests, and the level of agreement was assessed according to the method of Bland and Altman (21).
RESULTS
Subject characteristics
The characteristics of the adult model-development group and the 3 pediatric groups (ie, Tanner stage 5, pediatric model development, and model validation groups) are presented in Table 1. The total sample of 270 healthy subjects in the adult model-development group was ethnically diverse (11) and ranged in age, BMI, and SM mass from 18 to 88 y, 15.9 to 34.8, and 11.7 to 46.1 kg, respectively.
TABLE 1.
Subject characteristics and body composition1
| Pediatric |
||||||||
|---|---|---|---|---|---|---|---|---|
| Adult model development2 |
Tanner stage 5 |
Model development |
Model validation |
|||||
| Group | Men (n = 96) |
Women (n = 174) |
Boys (n = 17) |
Girls (n = 17) |
Boys (n = 36) |
Girls (n = 29) |
Boys (n = 10) |
Girls (n = 8) |
| Age (y) | 46.4 ± 19.0 | 45.2 ± 17.6 | 14.9 ± 2.0 | 15.1 ± 1.3 | 9.6 ± 2.7 | 9.0 ± 2.0 | 8.2 ± 2.0 | 10.0 ± 1.13 |
| Body weight (kg) | 79.2 ± 12.2 | 64.5 ± 13.64 | 68.6 ± 15.1 | 60.1 ± 14.5 | 37.8 ± 12.5 | 38.1 ± 11.8 | 27.8 ± 8.15 | 31.7 ± 8.1 |
| Height (cm) | 176.4 ± 7.9 | 162.3 ± 7.54 | 173.0 ± 7.6 | 161.7 ± 5.64 | 140.8 ± 15.4 | 140.7 ± 13.0 | 129.8 ± 12.15 | 138.6 ± 9.8 |
| BMI (kg/m2) | 25.4 ± 3.0 | 24.4 ± 4.43 | 22.8 ± 4.2 | 22.9 ± 4.8 | 18.5 ± 3.0 | 18.9 ± 3.7 | 16.4 ± 4.0 | 16.2 ± 2.15 |
| Body fat (%) | 20.2 ± 7.6 | 31.4 ± 9.14 | 18.0 ± 10.9 | 32.7 ± 8.74 | 21.1 ± 9.1 | 27.1 ± 11.33 | 14.7 ± 8.9 | 16.8 ± 4.06 |
| ALST (kg) | 28.1 ± 4.6 | 18.0 ± 3.24 | 24.4 ± 4.9 | 16.5 ± 2.64 | 12.1 ± 4.3 | 11.3 ± 3.1 | 9.2 ± 2.35 | 10.7 ± 2.8 |
| Total-body SM (kg) | 31.7 ± 5.9 | 19.8 ± 3.94 | 27.4 ± 5.9 | 18.2 ± 2.94 | 12.4 ± 4.7 | 11.5 ± 3.5 | 8.9 ± 2.05 | 10.5 ± 3.5 |
| IMAT (kg) | 0.8 ± 0.5 | 1.0 ± 0.67 | 0.5 ± 0.4 | 0.7 ± 0.5 | 0.4 ± 0.2 | 0.4 ± 0.3 | 0.4 ± 0.6 | 0.3 ± 3.5 |
All values are . ALST, appendicular lean soft tissue; IMAT, intermuscular adipose tissue; SM, skeletal muscle.
These subjects were included in an earlier report (11).
Significantly different by from men or boys (Student’s t test): P < 0.05
Significantly different by from men or boys (Student’s t test): P < 0.001
Significantly different from pediatric model-development group (Student’s t test): P < 0.05
Significantly different from pediatric model-development group (Student’s t test): P < 0.001.
Significantly different by from men or boys (Student’s t test): P < 0.01.
The Tanner stage 5 group (n = 34, aged 11–17 y) was ethnically diverse with 13 African Americans, 4 white, 12 Hispanics, and 5 classified as other. There were no significant differences in age, body weight, BMI, or IMAT between the boys and girls in the Tanner stage 5 group. However, the boys were taller (P < 0.001) than the girls and had a lower percentage of body fat and a greater ALST and total-body SM mass (all P < 0.001).
The 65 subjects aged 5–14 y in the pediatric model-development group (37 Tanner stage 1; 8 Tanner stage 2; 11 Tanner stage 3; and 9 Tanner stage 4) were ethnically diverse with 27 African Americans, 2 Asians, 9 whites, 22 Hispanics, and 5 classified as other. There were no between-sex group differences in age, body mass, height, ALST, total-body SM, or IMAT in the pediatric model-development group. However, the boys had a lower percentage of body fat than did the girls (P = 0.02)
The 18 subjects aged 5–12 y in the pediatric model-validation group (8 Tanner stage 1; 8 Tanner stage 2; and 2 Tanner stage 3) were also ethnically diverse with 3 African Americans, 13 whites, and 2 Hispanics. There was no significant difference in body mass, height, percentage body fat, ALST, total-body SM, or IMAT between the boys and girls in the pediatric model-validation group. However, the boys were significantly younger than the girls (P = 0.04).
Application of the adult model in children and adolescents
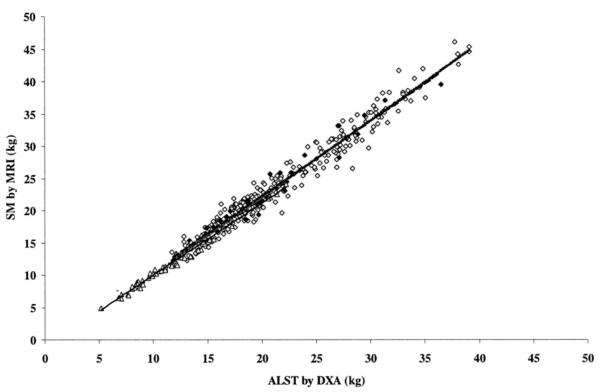
The applicability of the adult DXA model (11) was evaluated in children and adolescents with the aim of developing a combined DXA model for estimating SM in all age groups. When the adult SM prediction model (equation 1) (11) was applied to all of the subjects below the age of 18 y, predicted (15.9±7.2 kg) and measured SM (15.7 ± 7.3 kg) were highly correlated (r = 0.99, P < 0.001). However, the adult model significantly (P = 0.04) overestimated SM by 0.2 ± 0.9 kg in children and adolescents. Although we initially pooled all children and adolescents regardless of their maturational stage and attempted to test the adult model in a single group of nonadult subjects, we eventually realized that maturational level is critical to the relation between ALST and SM. Using subsets of children and adolescents in our sample, who were divided into subgroups according to Tanner pubertal stage, we found that the SM of subjects at Tanner stage 5 was predicted well (P=0.54) by the adult equation. However, the adult equation tended to overestimate SM at Tanner stage 4 by a mean difference of 0.5 kg (P = 0.08). In addition, the adult model significantly overestimated SM in boys and girls below Tanner stage 4 by a mean difference of 0.3 kg (P < 0.001). In phase 2 of the study, a separate pediatric equation was therefore developed for children and adolescents at and below Tanner stage 4. ALST alone was highly correlated with total-body SM in adults (r = 0.97) and subjects at and below Tanner stage 5 (r = 0.98 and 0.99, respectively; all P < 0.001) (Figure 1).
FIGURE 1.
Skeletal muscle (SM) measured by magnetic resonance imaging (MRI) versus appendicular lean soft tissue (ALST) measured by dual-energy X-ray absorptiometry (DXA) in the adult model-development group (◇, gray line; r = 0.97, P < 0.001, n = 270), in adolescents and children at Tanner stage 5(◆, dashed line; r = 0.98, P < 0.001, n = 34), and in children below Tanner stage 5 (△, black line; r = 0.99, P < 0.001, n = 65).
Three associations encompass the DXA-ALST prediction of SM: total-body SM and ALST; appendicular SM and total-body SM; and appendicular SM and ALST. We examined each of these associations after reviewing the phase 1 results and we present here a brief descriptive overview of the findings. We expand on these findings in the Discussion.
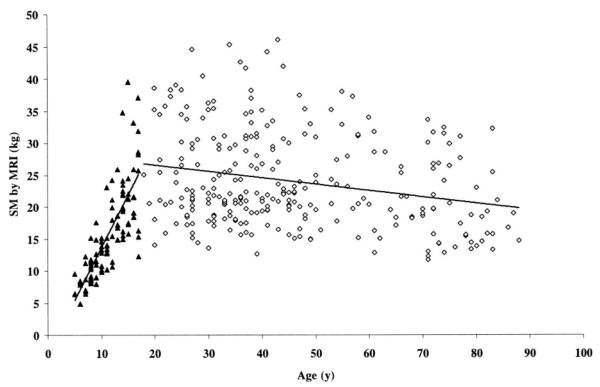
Compared with adults, children and adolescents below Tanner stage 5 had a lower ratio of total-body SM to ALST (1.01±0.05 compared with 1.11±0.07, P<0.001), a higher ratio of appendicular SM to total-body SM (0.56±0.03 compared with 0.53±0.03, P<0.001), and a lower ratio of appendicular SM to ALST (0.56 ± 0.03 compared with 0.59 ± 0.04, P < 0.001). These differing relations between prepubertal and pubertal subjects below Tanner stage 5 and adults reveal fundamental maturation-related variation in SM and ALST distribution. Additionally, total-body SM mass was larger in older children and adolescents (SM=1.6×age - 2.8; R2=0.62, P<0.001), whereas SM mass was smaller in older adults (SM=-0.1×age+28.6;R2=0.06, P < 0.001; Figure 2).
FIGURE 2.
Skeletal muscle (SM) mass measured by magnetic resonance imaging (MRI) as a function of age in the adult model-development group (◇; r=-0.24, P < 0.001, n = 270) and in adolescents and children (▲; r = 0.79, P < 0.001, n = 99).
Pediatric prediction models
Model development
Appendicular lean soft tissue was the strongest predictor (P<0.001) of total-body SM in the pediatric model-development group, explaining 98.2% of the variance in MRI-measured SM (model 1 in Table 2). The ratio of total-body SM to ALST in the boys (1.01 ± 0.05) did not differ significantly from that in the girls (1.01 ± 0.05).
TABLE 2.
Developed models for predicting total-body skeletal muscle mass in children and adolescents below Tanner stage 5 (n = 65)1
| Model | ALST (kg) | Weight (kg) | Height (cm) | ALST × height | Intercept | Adjusted R2 | SEE (kg) |
|---|---|---|---|---|---|---|---|
| 1 | 1.115 ± 0.0192 | -1.135 ± 0.2312 | 0.982 | 0.565 | |||
| 2 | 1.003 ± 0.0372 | 0.039 ± 0.0123 | -1.315 ± 0.2212 | 0.985 | 0.524 | ||
| 3 | 0.483 ± 0.1954 | 0.042 ± 0.0122 | -0.015 ± 0.015 | 0.003 ± 0.0014 | 1.734 ± 1.930 | 0.986 | 0.502 |
Values are estimates of the regression coefficient ± SEE. ALST, appendicular lean soft tissue. Models were developed by using general linear regression analysis.
P < 0.001.
P < 0.001.
P < 0.05.
The inclusion of weight along with ALST in a general linear regression model explained an additional 0.3% of the variance in measured total-body SM mass (model 2 in Table 2).
Height and the interaction of height with ALST were also significant predictors in the model, increasing to 98.6% the variance explained in measured total-body SM (P < 0.001) with an SEE of 0.50 kg (model 3 in Table 2). The addition of race (African American, Hispanic, or white), age, sex, and Tanner stage along with the model 3 predictors failed to contribute significantly to the developed model.
Model validation
The SD of the prediction error for model 1 derived by the leave-one-out method was 0.58 kg. For models 2 and 3, the SDs of the prediction error by the leave-one-out method were 0.54 and 0.52 kg, respectively. These are estimates of the SD error if the developed regression equations are applied to samples of subjects similar to the one in which they were developed.
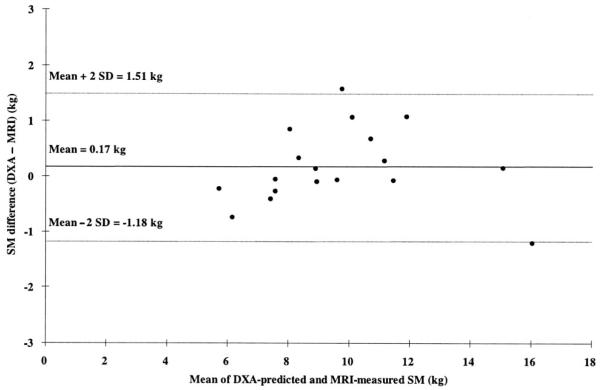
Predicted total-body SM values derived from all 3 pediatric models did not differ significantly from measured SM in the model-validation group. Predicted total-body SM was highly correlated with measured SM for all 3 models in the validation group (r = 0.962 and 0.969, all P < 0.001). Bland-Altman analysis did not disclose a significant bias between predicted and measured SM values (eg, model 3 in Figure 3).
FIGURE 3.
Skeletal muscle (SM) difference [predicted by dual-energy X-ray absorptiometry (DXA) model 3 — measured by magnetic resonance imaging (MRI)] versus mean of DXA-predicted and MRI-measured SM in the child model-validation group. The dashed lines indicate 95% CIs. n = 18.
DISCUSSION
In the present study, we tested in children and adolescents the validity of a previously reported adult SM prediction model based on ALST as measured by the widely available and practical DXA method (11). The adult model was valid in children and adolescents who were at a late stage of maturation but overestimated SM in subjects below Tanner stage 5. We also observed differing relations between ALST and SM in adults and prepubertal and pubertal subjects below Tanner stage 5. Accordingly, we developed and then validated a new series of DXA SM prediction formulas for use in children and adolescents at and below Tanner pubertal stage 4.
Application of the adult model in children and adolescents
The current study extends the earlier investigation of Kim et al (11), which reported DXA whole-body SM prediction models in adults. More advanced DXA models reported by Kim et al (11) that included ALST and age as predictors were not considered for testing the applicability of adult models in children and adolescents. These more advanced models were not evaluated because the relation between age and SM differs among the 3 age groups, with SM being larger with greater age in children and adolescents but smaller with greater age in adults.
Kim’s adult SM prediction model was developed on the basis of empirical and assumed stable relations between ALST, appendicular SM, and total-body SM. Our results indicate that these relations differ between adults and children and adolescents, and we computed some simple ratios to explore these differences. Specifically, children and adolescents below Tanner stage 5 had a smaller total-body SM relative to ALST (ie, SM/ALST ≈ 1.01) than did the adult group (SM/ALST ≈ 1.11). At a later stage of puberty, the SM-ALST ratio was larger and approached the adult mean value in subjects at Tanner stage 5, who were mostly adolescents and close to adult characteristics in terms of maturation (≈1.11).
Two separate relations determine the SM-ALST ratio: the association between appendicular SM and total-body SM and the association between appendicular SM and ALST. First, we observed that a higher percentage of total-body SM is appendicular SM in children and adolescents below Tanner stage 5 than in adults (ie, 56% compared with 53%). Although we expressed these relations as a single ratio, it is likely that the association between appendicular SM and total-body SM changes dynamically during growth and maturation. Skeletal muscle distribution is therefore likely to vary with maturation level in pediatric subjects.
Second, we found that a smaller percentage of ALST is appendicular SM in children and adolescents below Tanner pubertal stage 5 than in adults (ie, 56% compared with 59%). Because ALST is composed of muscle, skin, connective tissue, and the lean portion of adipose tissue, the present study suggests that subjects at and below Tanner stage 4 have a higher proportion of nonmuscle ALST than do adults.
These 2 associations, appendicular SM to total SM and appendicular SM to ALST, thus account for the smaller SM/ALST of 1.0 in children and adolescents at and below Tanner stage 4 than in adults (SM/ALST of 1.1). Subtle maturity-related compartment distribution differences may therefore provide a basis for why the adult SM prediction formula proved less accurate when applied in prepubertal subjects and in subjects at pubertal stages 2–4.
Pediatric skeletal muscle prediction models
Our measurements show that ALST alone explains 98.2% of the observed between-individual variation in MRI-measured IMAT-free SM mass with a low SEE (ie, 0.57 kg), which indicates high estimation accuracy. An additional significant factor was noted during model development. At a similar ALST, a child with greater weight had more SM than did a child with a lower weight. Accordingly, the additional developed model controlled for weight after adjustment for ALST, with a lower SEE than the other model (0.52 kg) and explaining 98.5% of the between-subject variance in MRI-measured SM mass (model 2). Inclusion of height and the interaction between height and ALST along with ALST and weight in model 3 further lowered the SEE to 0.50 kg, explaining 98.6% of the variance in MRI-measured SM mass. However, the height effect on SM mass was dependent on ALST. Race, sex, age, and Tanner stage were not significant predictors of SM after the other 3 predictor variables were controlled for.
The newly developed pediatric SM DXA-prediction models have high R2 values (0.98–0.99). The pediatric models also have low SEEs (0.6–0.5 kg), which indicate high estimation accuracy. These low SEEs can be compared with the corresponding SM prediction model SEEs for the adult DXA-ALST models (ie, 1.5–1.1 kg) (11). All 3 models were validated with the leave-one-out method, which indicates that these models would accurately predict SM when applied to samples of subjects similar to our pediatric model development sample. The pediatric DXA-SM models accurately predicted total-body SM compared with the MRI reference method in the pediatric model-validation group.
Study limitations
Our subject group was not sufficiently large enough or diverse enough to fully encompass all race groups (eg, Asians). We therefore cannot fully discount the possibility of a race effect, although our models accounted for a large percentage of the between-subject variance in MRI-measured SM (ie, 98.6% in model 3). However, the database presented in this report is the largest reported pediatric sample (n = 117) evaluated for total-body muscle mass by use of a highly accurate reference method (ie, whole-body MRI) for SM measurement. Several years were required to evaluate the age, sex, and race-heterogeneous sample at various pubertal stages. The reported equations should thus provide an operational basis for SM prediction until it becomes practical to obtain much larger pediatric samples for study.
Estimates of ALST are central to our models, and a key assumption is that estimates are comparable across DXA systems. In an earlier report, we detected only small differences in ALST between Lunar’s Prodigy and DPX systems (14). However, some reports have been made of between-manufacturer differences in DXA body-composition estimates, and these measurement differences could introduce bias in SM predictions. A need exists to develop and apply standard DXA body-composition phantoms and to continue between-instrument evaluations. We accept these concerns as limitations of our models with the tradeoff of providing investigators with relatively simple and applicable prediction formulas.
Conclusions
In the present study, we observed that an adult DXA SM prediction formula could be accurately applied to children and adolescents at a late stage of puberty (Tanner 5), but not to prepubertal children or to children in earlier puberty (Tanner stage ≤4). Development-related varying relations between key model components likely cause the model inaccuracy in children and adolescents at and below Tanner pubertal stage 4. New empirical SM prediction formulas were therefore developed and validated for prepubertal and pubertal subjects over the age of 5 y. ALST mass estimated by DXA is highly predictive of total SM with the use of different equations in children at or below Tanner pubertal stage 5. The new pediatric DXA prediction models should prove practical and useful in assessing the growth and development of the skeletal muscle compartment in vivo.
Footnotes
Supported by National Institutes of Health grant PPG PO1 DK 42618.
The authors had no conflicts of interest to report.
REFERENCES
- 1.Kanehisa H, Ikegawa S, Tsunoda N, Fukunaga T. Cross-sectional areas of fat and muscle in limbs during growth and middle age. Int J Sports Med. 1994;15:420–5. doi: 10.1055/s-2007-1021081. [DOI] [PubMed] [Google Scholar]
- 2.Kanehisa H, Yata H, Ikegawa S, Fukunaga T. A cross-sectional study of the size and strength of the lower leg muscles during growth. Eur J Appl Physiol Occup Physiol. 1995;72:150–6. doi: 10.1007/BF00964130. [DOI] [PubMed] [Google Scholar]
- 3.Malina RM. Growth of muscle tissue and muscle mass. In: Falkner F, Tanner JM, editors. Human growth: a comprehensive treatise. Plenum Press; New York, NY: 1986. [Google Scholar]
- 4.Malina RM, Bouchard C. Growth, maturation, and physical activity. Human Kinetics; Champaign, IL: 1991. [Google Scholar]
- 5.Tanner JM. Growth at adolescence. Blackwell Scientific; Oxford, United Kingdom: 1962. [Google Scholar]
- 6.Boye KR, Dimitriou T, Manz F, et al. Anthropometric assessment of muscularity during growth: estimating fat-free mass with 2 skinfold-thickness measurements is superior to measuring mid-upper arm muscle area in healthy prepubertal children. Am J Clin Nutr. 2002;76:628–32. doi: 10.1093/ajcn/76.3.628. [DOI] [PubMed] [Google Scholar]
- 7.Fuller NJ, Fewtrell MS, Dewit O, Elia M, Wells JC. Segmental bioelectrical impedance analysis in children aged 8–12 y: 2. The assessment of regional body composition and muscle mass. Int J Obes Relat Metab Disord. 2002;26:692–700. doi: 10.1038/sj.ijo.0801989. [DOI] [PubMed] [Google Scholar]
- 8.Schonau E, Schwahn B, Rauch F. The muscle-bone relationship: methods and management—perspectives in glycogen storage disease. Eur J Pediatr. 2002;161:S50–2. doi: 10.1007/s00431-002-1003-z. [DOI] [PubMed] [Google Scholar]
- 9.Heymsfield SB, Smith R, Aulet M, et al. Appendicular skeletal muscle mass: measurement by dual photon absorptiometry. Am J Clin Nutr. 1990;52:214–8. doi: 10.1093/ajcn/52.2.214. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76:378–83. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Heshka S, Gallagher D, et al. Intermuscular adipose tissue-free skeletal muscle mass: estimation by dual-energy X-ray absorptiometry in adults. J Appl Physiol. 2004;97:655–60. doi: 10.1152/japplphysiol.00260.2004. [DOI] [PubMed] [Google Scholar]
- 12.Bayer LM, Bayley L. Growth diagnosis. University of Chicago Press; Chicago, IL: 1959. [Google Scholar]
- 13.Hsu A, Heshka S, Janumala I, et al. Larger mass of high-metabolic-rate organs does not explain higher resting energy expenditure in children. Am J Clin Nutr. 2003;77:1506–11. doi: 10.1093/ajcn/77.6.1506. [DOI] [PubMed] [Google Scholar]
- 14.Ioannidou E, Padilla J, Wang J, et al. Pencil-beam versus fan-beam dual-energy X-ray absorptiometry comparisons across four systems: appendicular lean soft tissue. Acta Diabetol. 2003;40:S83–5. doi: 10.1007/s00592-003-0034-x. [DOI] [PubMed] [Google Scholar]
- 15.Song MY, Kim J, Horlick M, et al. Prepubertal Asians have less limb skeletal muscle. J Appl Physiol. 2002;92:2285–91. doi: 10.1152/japplphysiol.01066.2001. [DOI] [PubMed] [Google Scholar]
- 16.Figueroa-Colon R, Mayo MS, Treuth MS, Aldridge RA, Weinsier RL. Reproducibility of dual-energy X-ray absorptiometry measurements in prepubertal girls. Obes Res. 1998;6:262–7. doi: 10.1002/j.1550-8528.1998.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 17.Ross R. Magnetic resonance imaging provides new insights into the characterization of adipose and lean tissue distribution. Can J Physiol Pharmacol. 1996;74:778–85. [PubMed] [Google Scholar]
- 18.Lee RC, Wang Z, Heo M, Ross R, Janssen I, Heymsfield SB. Total-body skeletal muscle mass: development and cross-validation of anthropometric prediction models. Am J Clin Nutr. 2000;72:796–803. doi: 10.1093/ajcn/72.3.796. [DOI] [PubMed] [Google Scholar]
- 19.Snyder WS, Cook MJ, Nasset ES, Karhansen LR, Howells GP, Tipton IH. Report of the Task Group on Reference Men. Pergamon; Oxford, United Kingdom: 1975. [Google Scholar]
- 20.Stone M. Cross-validatory choice and assessment of statistical prediction. J R Stat Soc. 1974;36:111–47. [Google Scholar]
- 21.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]