Abstract
Multipotent mesenchymal stromal cells (MSC) are increasingly used to treat refractory graft-versus-host-disease and other complications following hematopoietic stem cell transplantation (HSCT). We evaluated immunogenicity of HLA-mismatched MSC infused post-transplant to HSCT recipients. Recipient lymphocyte response to MSC and peripheral blood lymphocytes (PBL) from the MSC or third party donors was measured before and after infusion. In vitro primary and rechallenge lymphocyte responses of healthy individuals to MSC and to PBL from the MSC donor were similarly studied. HSCT recipients given MSC responded to third party allostimuli, but showed no response to infused MSC before and up to 6 months after infusion, while maintaining an alloresponse to the MSC donor. This indicates immune unresponsiveness restricted to MSC, since the HSCT recipient was not tolerized to the MSC donor. In vitro we confirmed that MSC failed to prime responder lymphocytes to rechallenge with PBL from the MSC donor, and lymphocytes primed with MSC donor PBL and rechallenged with MSC only showed weak responses at high stimulator-responder ratios. Although MSC upregulated lymphocyte gene expression of CD25, IFN-γ, FoxP3, CTLA-4 and IL-10, they failed in both unprimed and primed responders to induce CD25+ (activated) or CD57+ (effector) CD4+ or CD8+ T lymphocyte subsets and only inconsistently induced FoxP3+ regulatory T lymphocytes. These results show for the first time that infused MSC are only weakly immunogenic in humans and validate the clinical use of MSC from HLA-mismatched donors.
Keywords: immunogenicity, HLA-match, stem cell transplantation, mesenchymal stromal cells
INTRODUCTION
In the last few years, human multipotent mesenchymal stromal cells (MSC) have been increasingly used in a variety of experimental cell therapy approaches evaluating their immunosuppressive and anti-inflammatory properties. Intravenous administration of MSC improve healing of renal, neural and lung injury in experimental animal models [1–3]. In clinical trials in hematopoietic stem cell transplant (HSCT) recipients, MSC have improved hematopoietic engraftment and the outcome of therapy-refractory graft-versus-host disease (GvHD), while reducing regimen-related organ damage [4–8].
It has been assumed that MSC are transplantable across HLA barriers but the nature of such tolerance has not been studied in human MSC recipients. In vitro, MSC (in contrast to renal tubular epithelial cells and skin fibroblasts) do not induce proliferation of allogeneic lymphocytes, even when costimulated by an anti-CD28 antibody or after transfection with B7-1 or B7-2 genes [9, 10]. MSC are resistant to lysis by both alloreactive and peptide-specific MHC class I restricted cytotoxic T lymphocyte clones [11–13]. Furthermore, MSC are not lysed by cytotoxic T lymphocytes generated by stimulating with peripheral blood mononuclear cells from the MSC donor [11].
In vivo evidence for tolerance to MSC comes from the observation that mismatched MSC engraft in adult rodents, dogs, pigs and baboons [14–16]. Furthermore, human MSC infused in utero persist in fetal sheep, although at low numbers [17, 18]. It has been suggested that MSC can tolerize the recipient to cells and tissues from the MSC donor, but only preliminary data exists in man [19]. Most clinical studies have used MSC from HLA matched or related donors, but mismatched MSC are increasingly used since MSC are easily expanded ex vivo and large numbers of cells derived from a single donor can be cryobanked for treatment of multiple recipients. The relevance of HLA matching of allogeneic MSC with recipient is not known and data on the survival of infused MSC mismatched with recipient and HSCT donor is limited to anecdotal reports of low levels of engraftment [5, 6, 20–22]. It is therefore of practical importance to explore the clinical feasibility of HLA mismatched MSC infusions.
Here we investigate immune responses to allogeneic MSC infused into HSCT recipients. We show that patients infused with MSC that are HLA haploidentical or completely HLA mismatched with the stem cell donor and recipient show no immunological memory to the infused MSC. Similarly, on rechallenge experiments in healthy individuals in vitro, MSC fail to induce T lymphocyte responses against themselves. Furthermore, post-MSC infusion, recipients maintain an alloresponse to the MSC donor, indicating an immune unresponsiveness restricted to MSC rather than tolerance to the MSC donor.
SUBJECTS, MATERIAL AND METHODS
MSC recipients
Eighteen MSC recipients underwent HSCT at the Karolinska University Hospital, Huddinge, Sweden, between 2003 and 2005. Patients received myeloablative (n=13) or reduced intensity conditioning (n=5) and GvHD prophylaxis, mainly cyclosporine and four doses of methotrexate, according to previously published procedures [23–27]. Before HSCT, patients with unrelated donors (n=10) were treated with anti-thymocyte globulin or alemtuzumab, in patients with chronic lymphatic leukemia or melodysplastic syndromes, to further prevent graft rejection and GvHD[28].
MSC were incorporated in clinical trials at our institutions from 2003. The indications for MSC administration were failure of standard treatment approaches for: (1) treatment of acute GvHD refractory to standard therapy in 9 patients [5, 8], (2) tissue injury after HSCT (hemorrhagic cystitis and pneumomediastinum) in 4 patients [6], (3) to support hematopoietic engraftment in patients with previous graft rejections or severe immunodeficiency requiring prompt immunohematopoietic recovery in 5 patients [29]. When available, all patients with these indications transplanted between 2003–2005 were offered this experimental therapy. All patients have previously been reported [5, 6, 8, 29]. Eight patients received MSC from mismatched third party donors, 7 from haploidentical related donors and 3 from HLA identical siblings. Patients received MSC from passage 1–3 in doses of ~1–2×106/kg. Peripheral blood samples were collected and cryopreserved for lymphocyte studies pre-infusion, 1–2 weeks, and 1, 3 and 6 months post-MSC infusion. Recipients gave informed consent and the study was approved by the Regional Ethics Review Board.
Clinical expansion of MSC
All MSC donors (n=18) were considered healthy after assessment of medical history, physical examination and serological screening for HIV and hepatitis viruses. To isolate MSC, bone marrow aspirates of 50 (range 32–80) mL were taken from the iliac crest of healthy donors (median age 37, range 24–58 years). The expansion of clinical grade MSC was performed according to the guidelines of the MSC consortium of the European Blood and Marrow Transplantation Group (EBMT), and was approved by the Swedish Medical Products Agency, as previously described in detail [29]. Briefly, mononuclear cells were separated over a gradient of Redigrad (GE Health Care, Uppsala, Sweden), washed and resuspended in human MSC medium consisting of Dulbecco’s Modified Eagles Medium-Low Glucose (DMEM-LG; Life Technologies, Gaithersburg, MD, USA), supplemented with 10% fetal calf serum (FCS; National Veterinary Institute, Uppsala, Sweden) and plated at a density of 1.6×105 cells/cm2. When cultures were near confluence, the cells were detached by trypsin and EDTA (Invitrogen Corp., Grand Island, NY, USA), and replated/passaged at a density of 4×103 cells/cm2.
Characterized by flow cytometry, the MSC were CD73+, CD90+, CD105+ but negative for CD34, CD45 and CD14. Adipogenic and osteogenic differentiation after induction was evaluated as previously described [30]. The MSC suspensions were culture-negative for bacteria and fungi, and PCR negative for Mycoplasma pneumoniae [29].
Lymphocyte proliferation assays
Peripheral blood lymphocytes (PBL) were isolated from whole blood by centrifuging on a Ficoll-hypaque gradient (1077 g/cm3; Axis-Shield PoC AS, Oslo, Norway). In co-culture experiments, triplicates of 1 × 105 PBL from the recipients, MSC donors or healthy volunteers were cultured in RPMI 1640 medium supplemented with 2 mM HEPES, 100 units/ml penicillin, 100 µg/ml streptomycin, 2 mM L-glutamine (Gibco-BRL, Life Technologies, Paisley, UK), and 10% heat-inactivated pooled human AB-serum, in microtitre plates (Nunclon, Copenhagen, Denmark), as previously described [31]. Irradiated unfractionated PBL (1×105/well) or MSC (1×104/well) were added as stimulators. For larger experiments, PBL were stimulated with PBL (ratio 1:1) or MSC (ratio 10:1) and cultured in tissue culture flasks. In primary co-culture, proliferation peaked on day 6, which was chosen as the harvest time point. In secondary co-culture, cells from primary co-culture were washed, renumerated and added together with stimulators to microtitre plates in fresh medium. Proliferation in secondary co-culture was maximal on day 3. On the day before peak of proliferation, 1 µCi/mL 3H-thymidine (Amersham Biosciences, Little Chalfont, United Kingdom) was added for incorporation in DNA. The cells were harvested on glass fibre filters (Wallac, Turku, Finland) using a harvesting machine (Tomtec, Orange, CT, USA). Radioactivity was determined using a β-counter (Wallac) [31, 32]. Proliferation data, in counts per minute (CPM), were calculated as mean of triplicates after subtraction of autologous counts (background control).
Flow cytometric profile of T lymphocyte subsets
Responder PBL were labeled with 0.5 µM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen, Carlsbad, CA, USA), according to manufacturer’s instructions, and subsequently relabeled in secondary cultures, at restimulation. At termination of cultures, PBL were collected and washed in phosphate buffered saline (PBS) and stained with CD4-Pacific Blue/CD8-AlexaFluor 647/CD25-PE-Cy7 (BD Biosciences, San Jose, CA, USA) and CD4-Pacific Blue/CD8-AlexaFluor 647/CD27-PE (BD Biosciences)/CD57-PE-Cy5 (Abcam, Cambridge, UK). Cells stained with the first set of antibodies were fixed in 4% formaldehyde, washed in 0.5% saponin and incubated with antibodies for human fork head box P3 (FoxP3-PE; eBioscience, San Diego, CA, USA) and CD152-PE-Cy5 (BD Biosciences). Single-stained controls were used. Washed cells were assayed in a multicolor flow cytometer (FACS Aria; BD Biosciences). Fluorescence signals from 5×104 cells were counted and analyzed.
Gene expression in PBL
To investigate how MSC affect gene expression in PBL, 106 PBL were cultured in 12-well plates (Nunclon). As indicated, 106 irradiated PBL from a pool of 5 allogeneic donors were added as stimulators. When present, 105 MSC were added in transwell inserts for 24 and 48 hours. Gene expression in resting PBL was used as reference.
RNA prepared from PBL was subjected to RT-qPCR (ABI 7000 Sequence Detection System; Applied Biosystems, Foster City, CA, USA), as previously described [33]. Primer and probe sequences used for CD25, interferon-γ (IFN-γ), FoxP3, interleukin-10 (IL-10) and cytotoxic T lymphocyte antigen 4 (CTLA-4/CD152) are shown in Table 1.
Table 1.
Sequences of primers and probes used in real-time quantitative-PCR analysis
| Gene: | Sequences: | |
|---|---|---|
| CD25 | Forward: | AATGCAGCCAGTGGACCAA |
| Reverse: | TGATAAATTCTCTCTGTGGCTTCATTT | |
| Probe: | CCAGGTCACTGCAGGGAACCTCCAC | |
| IFN-γ | Forward: | GAAAAGCTGACTAATTATTCGGTAACTG |
| Reverse: | GTTCAGCCATCACTTGGATGAG | |
| Probe: | TTGAATGTCCAACGCAAAGCAATACATGA | |
| FoxP3 | Forward: | AACAGCACATTCCCAGAGTTCCT |
| Reverse: | CATTGAGTGTCCGCTGCTTCT | |
| Probe: | CCTTTCACCTACGCCACGCTCATCC | |
| IL-10 | Forward: | GAGGCTACGGCGCTGTCAT |
| Reverse: | AGATGCCTTTCTCTTGGAGCTTATT | |
| Probe: | CAAGAGCAAGGCCGTGGAGCAGG | |
| CTLA-4 | Forward: | CGCCATACTACCTGGGCATAG |
| Reverse: | AGGATCCAGAGGAGGAAGTCAGA | |
| Probe: | AGATTTATGTAATTGATCCAGAACCGTG | |
Statistical analysis
Data from lymphocyte proliferation assays were reported as mean CPM of triplicates. Wilcoxon’s signed rank test was used to compare results at each time point. Friedman test and Kruskal-Wallis test were used to compare paired and non-paired groups, respectively, followed by Dunn’s multiple comparison test. Statistical significance was considered at the level p<0.05.
RESULTS
Patient outcome
Eighteen patients received MSC infusions as treatment of life-threatening complications to HSCT. Patient characteristics are shown in Table 2. No adverse events during or after the MSC infusion were observed. Seven patients (39%) are alive >1 to 4 years post-transplant.
Table 2.
Characteristics of the HSCT patients receiving MSC
| Patient | Hematopoietic stem cell transplantation | Mesenchymal stromal cell transplantation: | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN: | Sex: | Age: | Diagnosis: | Match: | Cell source: | Conditioing: | Anti-T-lymph: | GvHD prophyl: | aGvHD: | Indication: | Match: | Day: | Passage: | Dose × 106/kg: | Effect of MSC: | Outcome: |
| 995 | M | 59 | AML | Sibling | PBSC | Bu+Cy | X | CsA+MTX | III | aGvHD | Sibling | 57 | 2 | 1.4 | CR | alive, 3 y and 11 m, bronchiolitis, status post-HD |
| 1020 | M | 38 | ALL | Sibling | PBSC | fTBI+Cy | CsA+MTX | II | Engraftm | Sibling | 0 | 2 | 1.0 | n/a | A & W, 4 y and 1 m | |
| 1110 | F | 12 | SAA | Sibling | BM | Flu | X | CsA | - | Engraftm | Sibling | 0 | 2 | 1.1 | n/a | A & W, 2 y and 9 m |
| 868 | M | 27 | CML | MUD | BM | Flu+TBI | X | CsA+MMF | II | cGvHD | Haplo | 153 | 1 | 0.6 | NR | died, 1 y and 0 m, PTLD |
| 917 | M | 61 | Renal cancer | MUD | PBSC | Flu+TBI | X | CsA+MTX | IV* | aGvHD | Haplo | 242 | 3 | 1.0 | NR | died, 0 y and 9 m, progression, aspergillosis, adenovirus- and CMV- disease |
| 924 | M | 9 | ALL | MUD | PBSC | fTBI+Cy | X | CsA+MTX | IV | aGvHD | Haplo | 73 | 1 | 2.0 | CR | died, 1 y & 7 m, penumonitis |
| 994 | F | 34 | AML | MUD | PBSC | Bu+Cy | X | CsA+MTX | I | Engraftm | Haplo | 0 | 2 | 1.4 | n/a | A & W, 4 y and 6 m |
| 1033 | M | 22 | ALL | MMUDm | CB | fTBI+Cy | X | CsA+Pred | III | Hem cyst | Haplo | 81 | 1 | 0.7 | PR | died, 0 y and 3 m, MOF |
| 1047 | M | 9 | SAA | MMUDs | BM | fTBI+Cy | X | Tacro+Siro | II | Engraftm | Haplo | 0 | 2 | 1.0 | n/a | A & W, 4 y 2 m |
| 1126 | M | 1 | SCID | MMUDm | CB | Bu+Cy | X | CsA+Pred | I | Engraftm | Haplo | 0 | 3 | 1.0 | n/a | died, 0 y and 8 m, aspergillosis |
| 981 | F | 21 | ALL | MUD | PBSC | fTBI+Cy | X | CsA+MTX | I | Pneumo | MM | 474 | 2 | 0.7 | CR | died, 1 y & 4 m, relapse |
| 1007 | M | 59 | Prostate cancer | MUD | PBSC | Flu+Cy | X | CsA+MTX | III* | aGvHD | MM | 251 | 3 | 0.7 | PR | died, 1 y and 7 m, disseminated VZV |
| 1044 | M | 60 | Myeloma | Sibling | PBSC | Flu+TBI | CsA+MMF | III | aGvHD | MM | 77 | 2 | 0.9 | CR | died, 2 y and 2 m, relapse |
|
| 1068 | F | 34 | ALL | Sibling | PBSC | fTBI+Cy | CsA+MTX | III | aGvHD | MM | 32 | 2 | 2.0 | PR | died, 0 y and 6 m, penumonia sepsis |
|
| 1082 | F | 64 | CLL | Sibling | PBSC | Flu+TBI | CsA+MTX | III | aGvHD | MM | 49 | 2+3 | 1.7 | PR | died, 0 y and 4 m, invasive fusarium, aspergillosis |
|
| 1098 | M | 13 | Thalassemia | Sibling | BM | Bu+Cy | Tacro+Siro | - | Hem cyst | MM | 24 | 2 | 1.6 | CR | A & W, 3 y and 0 m | |
| 1115 | M | 55 | CML | MUD | BM | Bu+Cy | X | CsA+MTX | III | aGvHD | MM | 103 | 3 | 1.1 | n/a | died, 0 y and 10 m, MOF |
| 1118 | M | 54 | AML | Sibling | PBSC | Bu+Cy | CsA+MTX | I | Hem cyst | MM | 48 | 3 | 0.75 | CR | alive, 1 y and 10 m, cGvHD in eyes |
|
UPN, unique patient number; M, male; F, female; CML, chronic myeloid leukemia; ALL, acute lymphatic leukemia; AML, acute myeloid leukemia; SAA, severe aplastic anemia; CLL, chronic lymphatic leukemia; SCID, severe combined immunodeficiency disorder; MUD, HLA matched unrelated donor; MMUDm; major HLA mismatched unrelated donor; MMUDs, subtype HLA mismatched unrelated donor; BM, bone marrow; PBSC, peripheral blood stem cells; CB, cord blood; Flu, fludarabine; TBI, total body irradiation; fTBI, fractionated total body irradiation; Cy, cyclophosphamide; Bu, busulphan; Anti-T-lymphocyte; anti-T-lymphocyte prophylaxis; GvHD phophyl, graft-versus-host-disease prophylaxis; CsA, cyclosporine A; MMF, mycophenolate mofetil; MTX, methorexate; Pred, predisolone; Tacro, tacrolimus; Siro, sirolimus; aGvHD, acute graft-versus-host-disease; cGVHD, chronic graft-versus-host-disease; Penumo, penumomediastinum; Engraftm, engraftment; Hem cyst, hemorrhagic cystitis; Haplo, HLA halploidentical; MM, mismatched; NR, no response; CR, complete remission; PR, partial response; n/a, not applicable; y, years; m, months; PTLD, post-transplant lymphoproliferative disorder; CMV, cytomegalovirus; A & W, alive and well; HD, Hodgkin’s disease; VZV; varicella zoster virus infection; MOF, multiorgan failure.
HSCT recipients have specific tolerance to MSC but not to the MSC donor
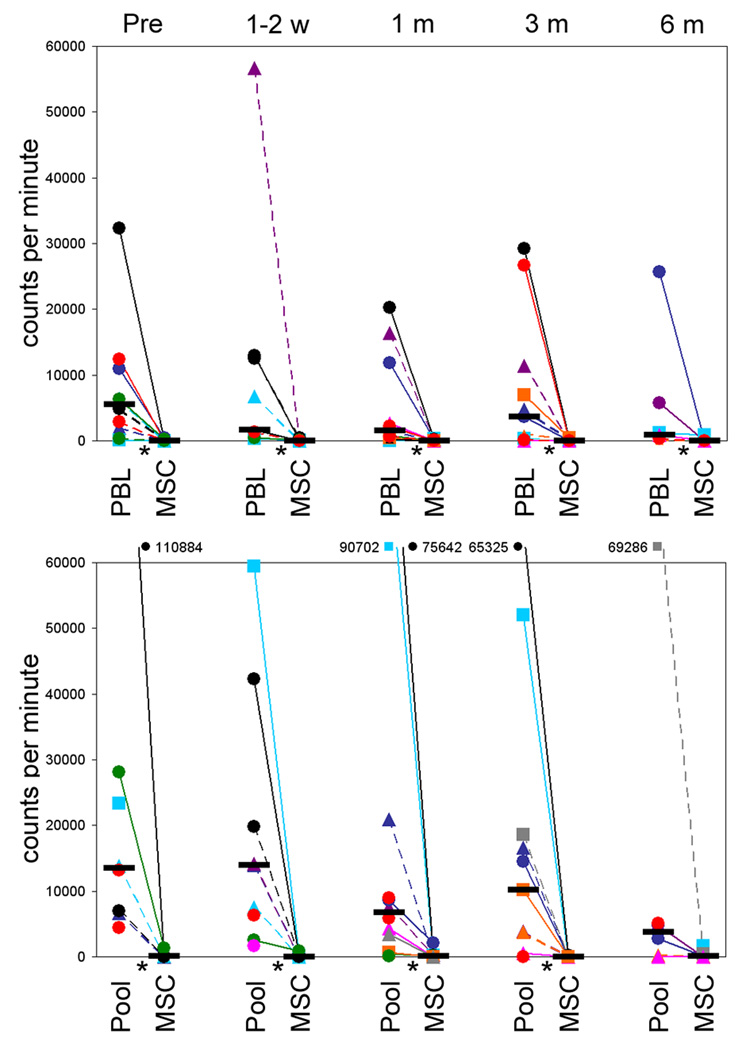
Patient PBL, harvested prior to MSC infusion, were stimulated with PBL or MSC from the MSC donor, or with other third party MSC or PBL from healthy volunteers (Figure 1). All recipients were immunocompetent, responding equally to allogeneic PBL both before and after infusion. However, pre-infusion the recipients showed no proliferative response to the donated MSC (nor to MSC from other donors), while responding fully to PBL from the MSC donor (p<0.05). Proliferation assays were repeated using responder PBL obtained from the recipient 1 week to 6 months after the MSC infusion. At no time after infusion were alloresponses against the donated MSC or other third party MSC detected. However at all time points studied the recipient showed proliferative responses to PBL from the MSC donor, comparable in magnitude to responses to the third party lymphocyte pool, indicating that MSC donor tolerance was not induced and that the absence of immunological memory was restricted to MSC. Moreover, there were no significant differences in proliferative responses between patients infused beyond 6 months from transplant (UPN 917, 981, and 1007) compared to those treated earlier, suggesting unresponsiveness to MSC was independent of the time-point of infusion.
Figure 1.
MSC recipient lymphocyte proliferative responses to MSC donor PBL and MSC (upper panel) and to a pool of allogeneic PBL and to third party MSC (lower panel); at pre-MSC infusion (pre), 1–2 weeks (1–2 w), 1 month (1 m), 3 months (3 m) and 6 months (6 m) post-MSC infusion. Unfractionated PBL were cultured in microtitre plates with irradiated PBL (ratio 1:1) or MSC (ratio 10:1) for 6 days. The MSC were derived from HLA identical siblings (■), or from haploidentical (▲) and mismatched donors (●). Data shown for UPN: 1118, black plain; 1068, red plain; 1044, blue plain; 1115, green plain; 995, light-blue plain; 1098, purple plain; 1110, orange plain; 1047, pink plain; 1126, gray plain; 981, black dashed; 1007, red dashed; 924, blue dashed; 1082, green dashed; 917, light-blue dashed; 868, purple dashed; 994, orange dashed; 1033, pink dashed; 1020, gray dashed (see table 2 for details). Horizontal black bar denotes median proliferation and statistical significance (p<0.05) is indicated by *.
Priming induces weak responses against MSC in vitro
As further confirmation of the inability of responders to be sensitized by MSC we performed experiments where responders were stimulated in vitro either with MSC or PBL from the MSC donor and then rechallenged with PBL or MSC. When either PBL or MSC were used as stimulators in these co-culture experiments, lymphocyte proliferation peaked on day 6 after primary challenge and on day 3 after rechallenge. These time points were therefore used in subsequent analysis.
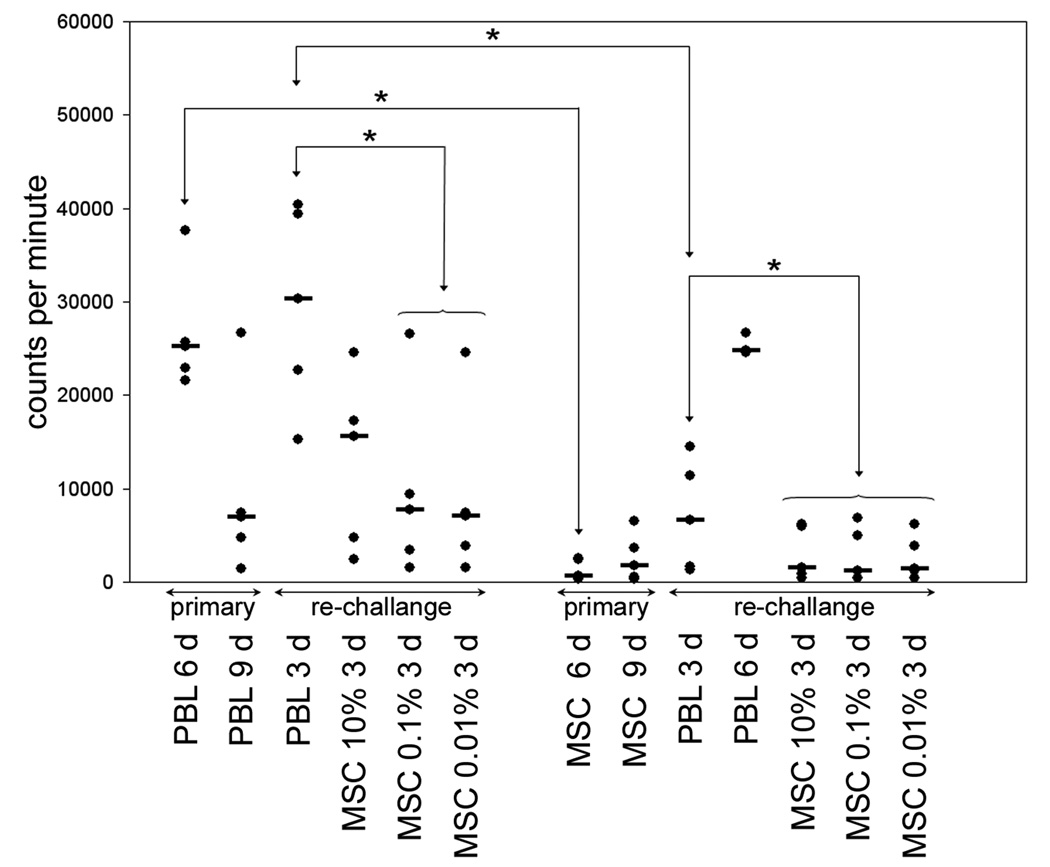
Alloresponses induced in PBL from healthy individuals in primary culture were significantly higher than those induced by MSC from the same donor (Figure 2). Proliferation of lymphocytes on day 3, stimulated with the same PBL in rechallenge cultures, was comparable to the day 6 proliferative response seen in primary culture. MSC derived from the same donor added to PBL-primed lymphocytes in rechallenge culture, predictably reduced proliferation at concentrations of 0.01 and 0.1%. These responses were no greater than the proliferation seen in primary PBL-stimulated cultures at the equivalent 9 day time point. Thus, when added at low ratios in rechallenge cultures, MSC did not evoke an immune response in primed lymphocytes. However, proliferation in response to 10% MSC was higher, suggesting a weak capacity of MSC to provide an allogeneic stimulus in primed responders at high stimulator responder ratios.
Figure 2.
Lymphocyte proliferation to PBL and MSC in primary and rechallenge co-cultures. Responder PBL were cultured with irradiated, allogeneic PBL or MSC from the same donor for 6–9 days. In restimulation experiments responder PBL were resuspended and 10×105 cells transferred to microtitre plates and rechallenged with PBL or MSC from the original stimulator for 3 days. Proliferation to PBL peaked at 6 days in primary culture and at 3 days on rechallenge (columns 1–3). When responders were rechallenged with MSC from the same donor there was only modest proliferation at 3 days to the highest MSC concentration (columns 3–6). When MSC were used as primary challenge there was minimal proliferation compared with PBL (columns 7–8) and these MSC primed responders mounted a proliferative response to corresponding PBL that followed primary challenge kinetics, i.e. maximum at 6 days, (columns 9–10). Similarly, responders primed with MSC failed to proliferate on rechallenge with MSC (columns 11–13). Horizontal black bars denote median proliferation and statistical significance (p<0.05) is indicated by *.
Predictably, MSC induced only minimal proliferation above autologous control in primary cultures and no response was detected when PBL were rechallenged with the same MSC, irrespective of the number of MSC added to the culture. Finally, when PBL primed with MSC were rechallenged with PBL from the MSC donor, proliferation followed the 6-day kinetics of the primary response to PBL indicating that MSC neither primed nor subsequently blocked the response of PBL to lymphocytes from the MSC donor.
MSC interactions with T lymphocytes
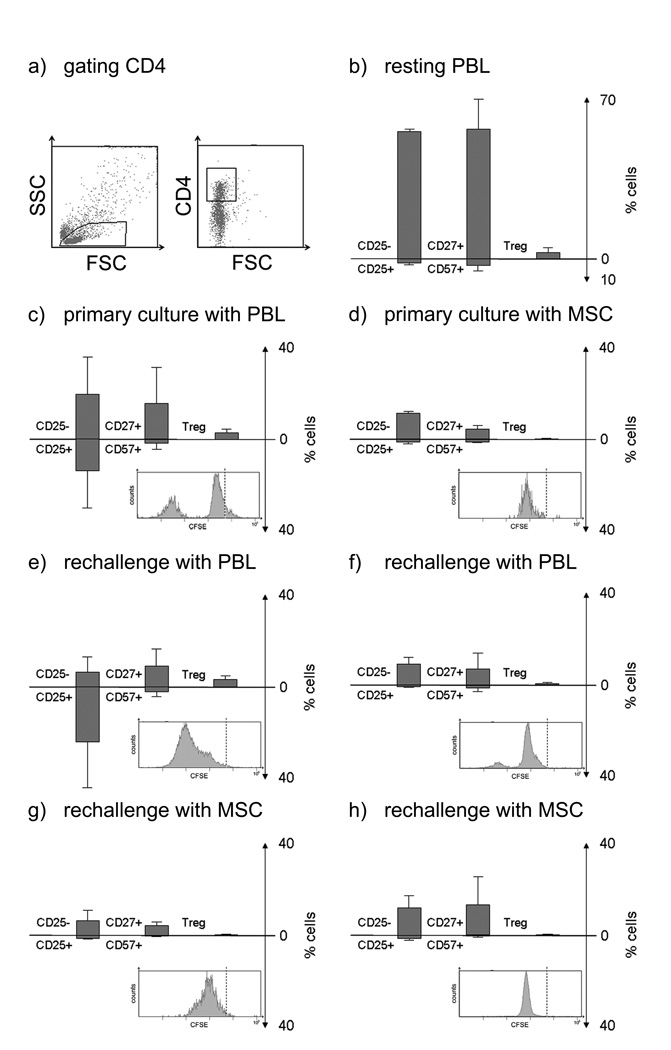
CD4+ cells (Figure 3): Flow cytometric measurement of proliferation by CFSE-labelling produced comparable results to the lymphocyte proliferation assays with much greater proliferation occurring in PBL- versus MSC-primed cultures and in PBL- versus MSC-rechallenged cultures. Proliferation on rechallenge was always inferior in cultures primed with MSC, as expected, confirming the low immunogenicity of MSC and implying MSC mediated immunosuppression. On primary challenge with PBL, proliferation was associated with an increase in the CD25+ subset. Rechallenge of PBL-primed cells with PBL, induced expansion in all CD4+ subsets, with increased proportions of CD25+ (activated) versus CD25- cells and maintained frequencies CD57+ (effector/terminally differentiated) versus CD27+ (memory/naïve) and CTLA-4+ FoxP3+ (regulatory T lymphocyte phenotype; Treg). When PBL primed cultures were rechallenged with MSC, weak proliferation occurred, and only low frequencies of lymphocytes with activated, effector and Treg phenotype were found. Similarly, PBL cultured with MSC displayed low proliferation and a small CD25+ subset. On rechallenge of MSC primed cultures with PBL some proliferation was seen, but the relative proportions of CD25+ versus CD25− and CD57+ versus CD57− did not alter significantly from the primary culture and did not match the expansion of activated cells seen in the rechallenge of cells primed with PBL. The cells primed with MSC and rechallenged with MSC displayed the lowest proliferation, with almost absent levels of activated or effector cells. Cultures either primed or rechallenged with MSC did not show an increase in the Treg cells. In conclusion, these data suggest that MSC possess low immunogenicity in regard to CD4+ T lymphocytes, irrespective of priming by MSC or highly immunogenic PBL.
Figure 3.
Flow cytometric analysis of CD4+ lymphocytes primed and rechallenged with cells from the same donor. In primary cultures responder PBL were cultured with PBL (ratio 1:1) or MSC (ratio 10:1) for 6 days (top panel). For rechallenge, responder cells were recovered and stimulated with PBL or MSC from the original stimulator for 3 days (bottom two panels). Each panel shows the proliferation as histogram of CFSE labelled cells (the broken line indicates CFSE level for resting cells), and shows the percentages of CD25− versus CD25+ (activated) CD4+ cells, CD27+/CD57− (memory/naïve) versus CD57+/CD27-(effector) cells, and CD25+ CTLA-4+ FoxP3+ (Treg) cells. (a) Representative dot plot with gating for CD4+ cells. (b) Levels in resting PBL. (c) PBL primed with PBL. (d) PBL primed with MSC. (e) PBL primed and rechallenged with PBL. (f) PBL primed with MSC rechallenged with PBL (g) PBL primed with PBL and rechallenged with MSC. (h) PBL primed with MSC and rechallenged with MSC. Figures represent mean ± SD of three experiments using stimulators and responders from different individuals.
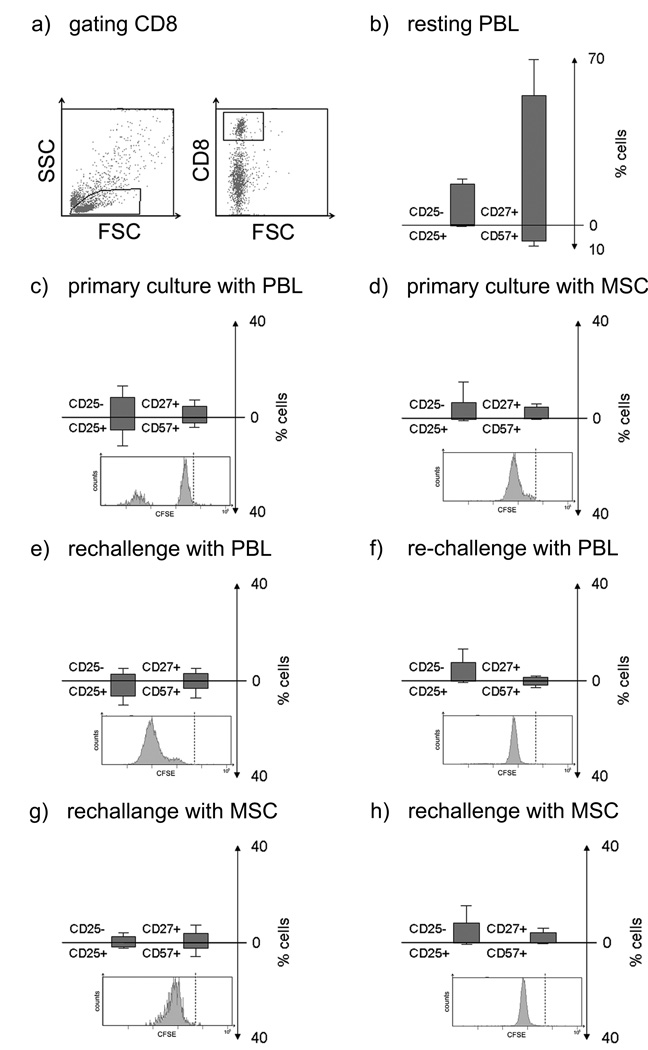
CD8+ cells (Figure 4): On primary challenge with PBL, proliferation of CD8+ cells was associated with an increase in the CD25+ subset compared with unstimulated cultures. Rechallenge of PBL-primed cells with PBL induced expansion in all CD8+ subsets, but with similar proportions of each subset observed in primary culture. When PBL-primed cultures were rechallenged with MSC, low proliferation with decreased frequencies of CD25+ cells were found. PBL cultured with MSC displayed low proliferation with almost no CD25+ cells. Low proliferation of responder PBL was seen in the CD25+ and terminally differentiated/effector subsets of the CD8+ population on rechallenge of MSC-primed cultures with PBL, without increase in frequencies compared to the corresponding primary culture. The frequency of CD25+ cells was markedly lower in cells primed with MSC and rechallenged with PBL in comparison to PBL-primed cells rechallenged with PBL. The PBL-primed with MSC and rechallenged with MSC displayed the least proliferation. To summarize, these data uniformly suggest low immunogenicity in regard to CD8+ T lymphocytes, irrespective of priming by MSC or highly immunogenic PBL.
Figure 4.
Flow cytometric analysis of CD8+ lymphocytes primed with PBL or MSC and rechallenged with either MSC or PBL. In primary cultures responder PBL were cultured with PBL (ratio 1:1) or MSC (ratio 10:1) for 6 days (top panel). For rechallenge, responder cells were recovered and stimulated with PBL or MSC from the original stimulator for 3 days (bottom two panels). Each panel shows the proliferation as histogram of CFSE labelled cells (the broken line indicates CFSE level for resting cells), and shows the percentages of CD25− versus CD25+ (activated) CD4+ cells, CD27+/CD57− (memory/naïve) versus CD57+/CD27− (effector) cells. (a) Representative dot plot with gating for CD8+ cells. (b) Levels in resting PBL. (c) PBL primed with PBL. (d) PBL primed with MSC. (e) PBL primed with PBL and rechallenged with PBL. (f) PBL primed with MSC rechallenged with PBL (g) PBL primed with PBL and rechallenged with MSC. (h) PBL primed with MSC and rechallenged with MSC. Figures represent mean ± SD of three experiments using stimulators and responders from different individuals.
Gene expression in lymphocytes after exposure to MSC
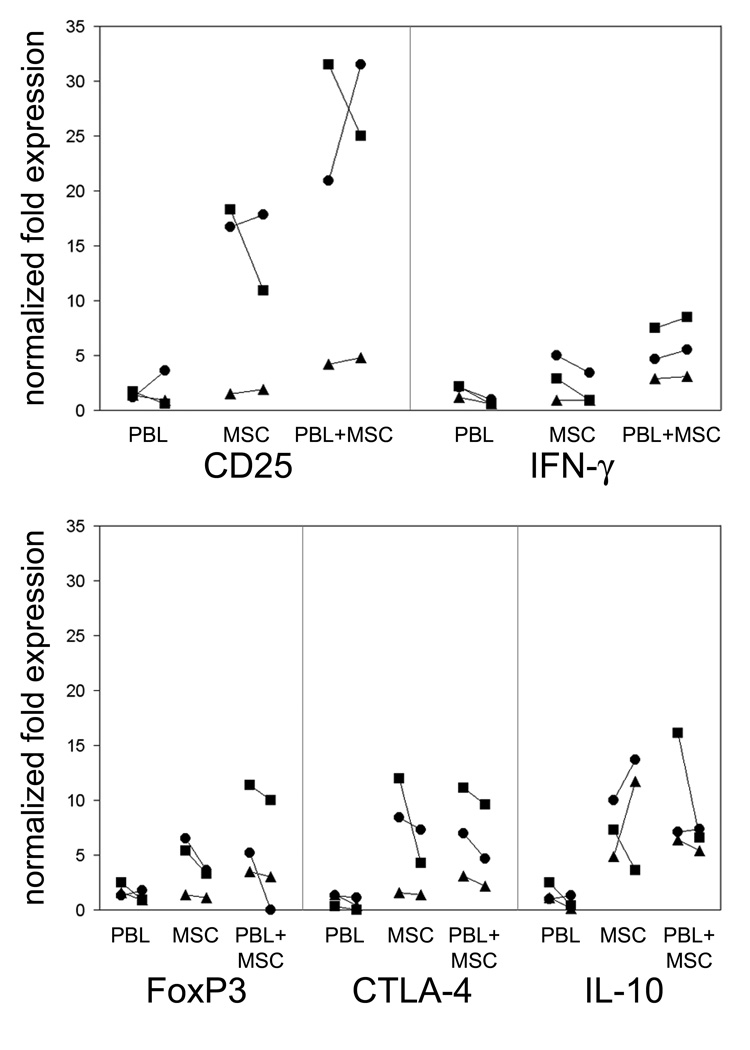
Gene expression of CD25, IFN-γ, FoxP3, CTLA-4 and IL-10 increased slightly in responder PBL stimulated with allogeneic lymphocytes for 24 and 48 h (Figure 5). Addition of MSC in transwell inserts to cultures of resting PBL resulted in considerably higher expression of CD25, IFN-γ, FoxP3, CTLA-4 and IL-10. This increased gene expression induced in resting PBL by MSC was similar to that induced by MSC on responding PBL with concurrent simulation of allogeneic lymphocytes. These data imply a MSC induced activation of genes associated to immunity and immune regulation.
Figure 5.
Gene expression in lymphocytes challenged with PBL and MSC. Responder PBL (n=3; ■, ▲, and ●) were co-cultured with a pool of allogeneic PBL (PBL), allogeneic MSC (MSC) and pool of allogeneic PBL with addition of MSC (PBL+MSC) for 24 and 48 h. Gene expression of CD25, IFN-γ, FoxP3, CTLA-4 and IL-10 were determined by real-time quantitative PCR and are reported as times the expression in a resting PBL.
DISCUSSION
Previous studies have shown engraftment of allogeneic MSC in transplant-conditioned and non-conditioned humans [5, 22, 34, 35]. The present study provides the first clear evidence in humans for the weak allogenicity of HLA-mismatched MSC, which we confirmed in vitro in proliferation assays and by phenotypic analysis. In this unique cohort of 18 HSCT patients receiving MSC infusions, patient PBL proliferated normally to stimulation with PBL from healthy donors or the MSC donor, confirming that these HSCT recipients were immunocompetent to alloimmune stimuli at the time of MSC infusion. Most notable was the absence in our patients of any response to MSC on rechallenge: proliferation against donor or third party MSC was 2–3 log less, with no accelerated proliferation kinetics in MSC-exposed recipients. At the same time a primary response to lymphocytes of the MSC donor was retained on repeated challenge of the MSC recipient with PBL from the MSC donor suggesting that infused MSC failed to induce memory T lymphocyte responses. Thus, a recipient of HLA mismatched MSC neither develops immunological memory to the infused MSC, nor acquires tolerance to other cells from the same donor. Furthermore, there were no responses observed to superior matched (HLA identical or -haploidentical) MSC, suggesting absence of immunization to minor histocompatibility antigens and possible MSC specific epitopes.
We recapitulated these observations from our patients using healthy donors in vitro: PBL from healthy volunteers, stimulated in primary cultures with MSC, failed to demonstrate accelerated proliferation kinetics upon rechallenge with the MSC donor's PBL, implying that MSC did not prime the responder lymphocytes. This finding is in contrast to our previous experience, injecting human MSC to rat with ischemic myocardium [36]. Significant rat lymphocyte proliferation was observed when human MSC were added to PBL from rats, previously exposed to human MSC. No reactivity was seen in lymphocytes from untreated rats and athymic rats. Human MSC could only be identified in the myocardium of athymic rats, suggesting rejection. Thus, although MSC can induce xenoreactivity, they fail to induce alloreactivity in man. MSC could also block, but to a lesser extent, the rechallenge response to PBL primed with MSC donor's PBL indicating that MSC were less effective at blocking an established alloresponse. These in vitro findings support the use of allogeneic MSC to HLA disparate HSCT recipients without risking allosensitization of the recipient to the MSC donor. Our results contrast with an in vitro study by Klyushnenkova et al, which concluded that MSC induce priming and development of secondary responses to MSC [10], but corroborate findings in primate models indicating that MSC can be administered without inducing immune sensitization [37]. In a study, designed to investigate MSC immunogenicity, non-conditioned baboons received multiple high-dose infusions of allogeneic MSC. None of the animals displayed enhanced T lymphocyte responses against donor alloantigens, but showed a progressive loss of alloreactivity with time, while maintaining normal responses against the mitogen concanavalin A [37].
It should be noted, however, that when MSC were used as a secondary challenge following induction of a robust T lymphocyte response to a primary challenge with PBL, the MSC at a 10% concentration did induce an alloreaction, albeit somewhat smaller than that seen after rechallenge with PBL. In clinical practice, it is therefore possible that HSCT recipients already sensitized to other individuals by transfusion, transplantation, or prior pregnancy might reject infused MSC by cross-reactivity. The high stimulator-responder ratios required to trigger an alloresponse are not easily achieved in vivo. Nevertheless, our results argue for minimizing the size of mismatched MSC infusions to reduce their rejection risk. Our findings suggest that rejection would be more likely if autologous MSC (from the HSCT patient) were infused into the recipient with an established immunocompetent stem cell allograft. For this reason third party MSC may appear to be more acceptable for transfusion into an allogeneic HSCT recipient. Use of third party MSC would also have a practical advantage: while culture of autologous MSC requires about four weeks, stored third party MSC could be immediately available.
Consistent with our previous findings, both CD4+ and CD8+ T lymphocyte subsets proliferated poorly after challenge or rechallenge with MSC [32]. When PBL were used to rechallenge MSC stimulated cultures, proliferation was slightly increased in comparison to MSC as secondary stimulus, confirming the potency of the blockade by MSC of both CD4+ and CD8+ T lymphocytes. Nevertheless, MSC did not completely block the induction of the alloresponse, since terminally differentiated CD4+ and CD8+ T lymphocytes expanded after MSC priming and PBL rechallenge. While, the in vitro experiments suggest MSC may be weakly immunogenic, it should be noted that we did not observe in vivo sensitization to MSC donor-derived cells in the recipients in proliferative assays (although the HSCT recipients responded normally in third party lymphocyte proliferation assays). Our findings contrast with a murine model reported by Nauta et al, indicating that MSC induce memory T lymphocyte responses [38]. Although species-specific differences remain the more likely explanation for the discrepant findings, more sensitive methods of detecting alloreactivity, such as the use of T lymphocyte precursor frequency assays, might reveal recipient memory cells to antigens of the MSC donor also after infusion in humans. In contrast to reports by Maccario et al [39] and Prevosto et al [40], we only occasionally found expansion of T regulatory lymphocytes upon exposure to MSC.
The gene expression studies reveal that, even when present in transwell inserts, MSC induce lymphocyte expression of activation markers and molecules associated with immune regulation. Gene expression of CD25, IFN-γ and IL-10 RNA was notably increased in PBL after challenge with both lymphocytes and MSC, corroborating our previous finding that although MSC reduce surface expression of CD25 on PBL, IL-2 secretion is increased [32, 41]. MSC will also induce earlier and higher lymphocyte secretion of the anti-inflammatory cytokine IL-10 [41].
In conclusion, this is the first study of immunogenicity to MSC performed in a patient cohort treated with infusions of HLA disparate MSC. Our results indicate that MSC can be transplanted successfully into allogeneic HSCT recipients across HLA barriers with little evidence that they would be rejected or sensitize the recipient to other cells of the same HLA type. The inability of MSC to induce donor-specific tolerance is a critical observation since it suggests that cotransplantation of solid organs with MSC could only facilitate engraftment through the well-described non-specific immunosuppressive action of MSC and not by inducing specific tolerance to the transplanted organ. In vitro studies confirm that MSC powerfully limit the alloresponse, but suggest that MSC are more susceptible to allogeneic attack by individuals already sensitized to cells bearing antigens common to the infused MSC.
Acknowledgements
This work was supported by grants from the Swedish Cancer Society, the Swedish Research Council, the Tobias Foundation, the Cancer Society in Stockholm, the Swedish Society of Medicine, the Stockholm County Council, the Sven and Ebba-Christina Hagbergs Foundation (KLB), the Children’s Cancer Foundation (KLB), Signe och Olof Wallenius stiftelse, Stiftelsen Sigurd och Elsa Goljes Minne and the Claes Högman’s SAGMAN-scholarship/Fenwal Blood Technologies Inc. (MS), Karolinska Institutet (MS, KLB), and Intramural Research Program of the National Heart, Lung and Blood Institute, National Institutes of Health (AJB).
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work
Conflict-of-interest disclosure: The authors declare no competing financial interests.
REFERENCES
- 1.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100(14):8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Togel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289(1):F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 3.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106(5):1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 4.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 5.Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81(10):1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 6.Ringden O, Uzunel M, Sundberg B, et al. Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia. 2007;21(11):2271–2276. doi: 10.1038/sj.leu.2404833. [DOI] [PubMed] [Google Scholar]
- 7.Ball LM, Bernardo ME, Roelofs H, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110(7):2764–2767. doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- 8.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 9.Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75(3):389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 10.Klyushnenkova E, Mosca JD, Zernetkina V, et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12(1):47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 11.Rasmusson I, Ringden O, Sundberg B, et al. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76(8):1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 12.Angoulvant D, Clerc A, Benchalal S, et al. Human mesenchymal stem cells suppress induction of cytotoxic response to alloantigens. Biorheology. 2004;41(3–4):469–476. [PubMed] [Google Scholar]
- 13.Rasmusson I, Uhlin M, Le Blanc K, et al. Mesenchymal stem cells fail to trigger effector functions of cytotoxic T lymphocytes. J Leukoc Biol. 2007;82(4):887–893. doi: 10.1189/jlb.0307140. [DOI] [PubMed] [Google Scholar]
- 14.Pochampally RR, Neville BT, Schwarz EJ, et al. Rat adult stem cells (marrow stromal cells) engraft and differentiate in chick embryos without evidence of cell fusion. Proc Natl Acad Sci U S A. 2004;101(25):9282–9285. doi: 10.1073/pnas.0401558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devine SM, Cobbs C, Jennings M, et al. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101(8):2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 16.Kraitchman DL, Heldman AW, Atalar E, et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107(18):2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 17.Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6(11):1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 18.Airey JA, Almeida-Porada G, Colletti EJ, et al. Human mesenchymal stem cells form Purkinje fibers in fetal sheep heart. Circulation. 2004;109(11):1401–1407. doi: 10.1161/01.CIR.0000124222.16321.26. [DOI] [PubMed] [Google Scholar]
- 19.Ikehara S. A novel method of bone marrow transplantation (BMT) for intractable autoimmune diseases. J Autoimmun. 2008;30(3):108–115. doi: 10.1016/j.jaut.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Fouillard L, Bensidhoum M, Bories D, et al. Engraftment of allogeneic mesenchymal stem cells in the bone marrow of a patient with severe idiopathic aplastic anemia improves stroma. Leukemia. 2003;17(2):474–476. doi: 10.1038/sj.leu.2402786. [DOI] [PubMed] [Google Scholar]
- 21.Horwitz EM, Prockop DJ, Gordon PL, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97(5):1227–1231. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- 22.Le Blanc K, Gotherstrom C, Ringden O, et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005;79(11):1607–1614. doi: 10.1097/01.tp.0000159029.48678.93. [DOI] [PubMed] [Google Scholar]
- 23.Ringden O, Remberger M, Persson U, et al. Similar incidence of graft-versus-host disease using HLA-A, -B and -DR identical unrelated bone marrow donors as with HLA-identical siblings. Bone Marrow Transplant. 1995;15(4):619–625. [PubMed] [Google Scholar]
- 24.Ringden O, Ruutu T, Remberger M, et al. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: a report from the Nordic Bone Marrow Transplantation Group. Blood. 1994;83(9):2723–2730. [PubMed] [Google Scholar]
- 25.Storb R, Deeg HJ, Fisher L, et al. Cyclosporine v methotrexate for graft-v-host disease prevention in patients given marrow grafts for leukemia: long-term follow-up of three controlled trials. Blood. 1988;71(2):293–298. [PubMed] [Google Scholar]
- 26.Sundin M, Ringden O, Sundberg B, et al. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007;92(9):1208–1215. doi: 10.3324/haematol.11446. [DOI] [PubMed] [Google Scholar]
- 27.Cutler C, Kim HT, Hochberg E, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2004;10(5):328–336. doi: 10.1016/j.bbmt.2003.12.305. [DOI] [PubMed] [Google Scholar]
- 28.Remberger M, Svahn BM, Mattsson J, et al. Dose study of thymoglobulin during conditioning for unrelated donor allogeneic stem-cell transplantation. Transplantation. 2004;78(1):122–127. [PubMed] [Google Scholar]
- 29.Le Blanc K, Samuelsson H, Gustafsson B, et al. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21(8):1733–1738. doi: 10.1038/sj.leu.2404777. [DOI] [PubMed] [Google Scholar]
- 30.Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57(1):11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 31.Moller G. Induction of DNA synthesis in human lymphocytes: interaction between non-specific mitogens and antigens. Immunology. 1970;19(4):583–598. [PMC free article] [PubMed] [Google Scholar]
- 32.Le Blanc K, Rasmusson I, Gotherstrom C, et al. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutin-inactivated lymphocytes. Scand J Immunol. 2004;60(3):307–315. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- 33.Uzunel M, Ringden O. Poor correlation of kinetics between BCR-ABL and WT1 transcript levels after allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;33(1):47–52. doi: 10.1038/sj.bmt.1704296. [DOI] [PubMed] [Google Scholar]
- 34.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99(13):8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koc ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18(2):307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 36.Grinnemo KH, Mansson A, Dellgren G, et al. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg. 2004;127(5):1293–1300. doi: 10.1016/j.jtcvs.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 37.Beggs KJ, Lyubimov A, Borneman JN, et al. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006;15(8–9):711–721. doi: 10.3727/000000006783981503. [DOI] [PubMed] [Google Scholar]
- 38.Nauta AJ, Westerhuis G, Kruisselbrink AB, et al. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108(6):2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maccario R, Podesta M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90(4):516–525. [PubMed] [Google Scholar]
- 40.Prevosto C, Zancolli M, Canevali P, et al. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92(7):881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 41.Rasmusson I, Ringden O, Sundberg B, et al. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305(1):33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]