Abstract
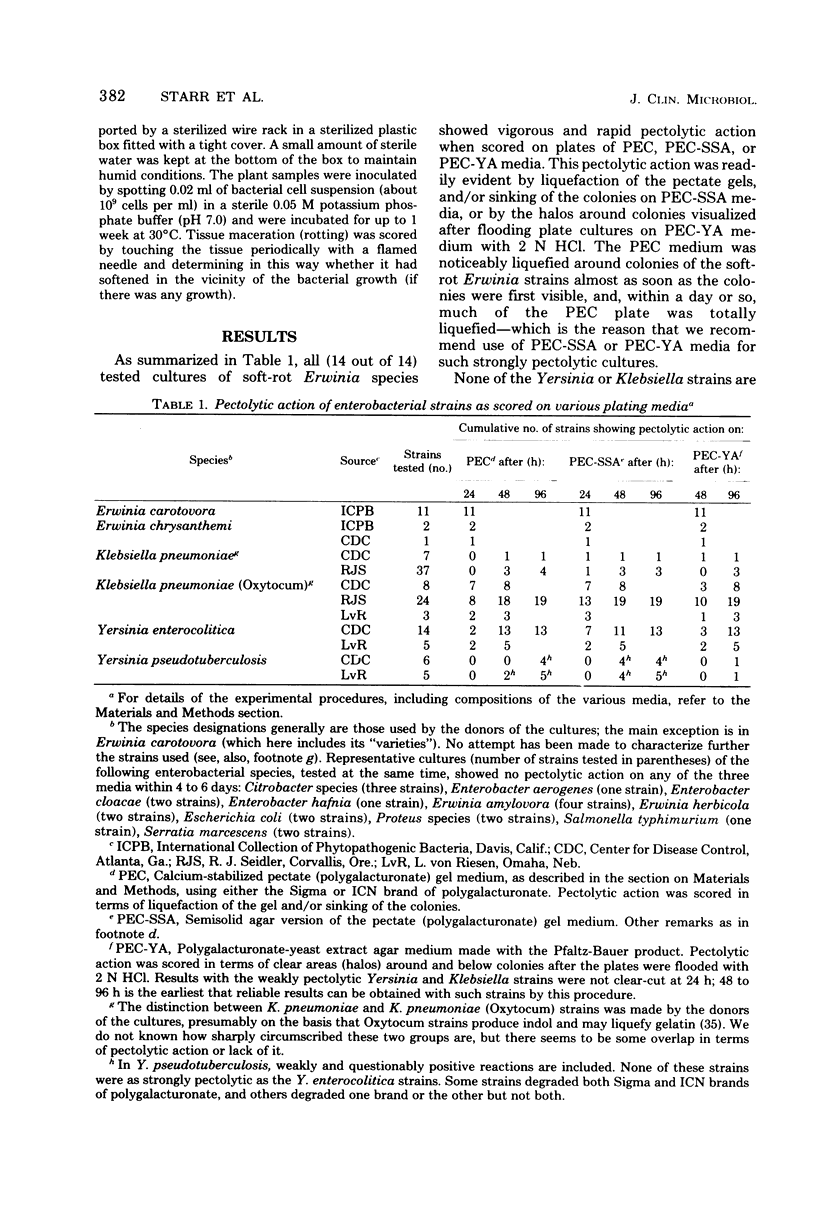
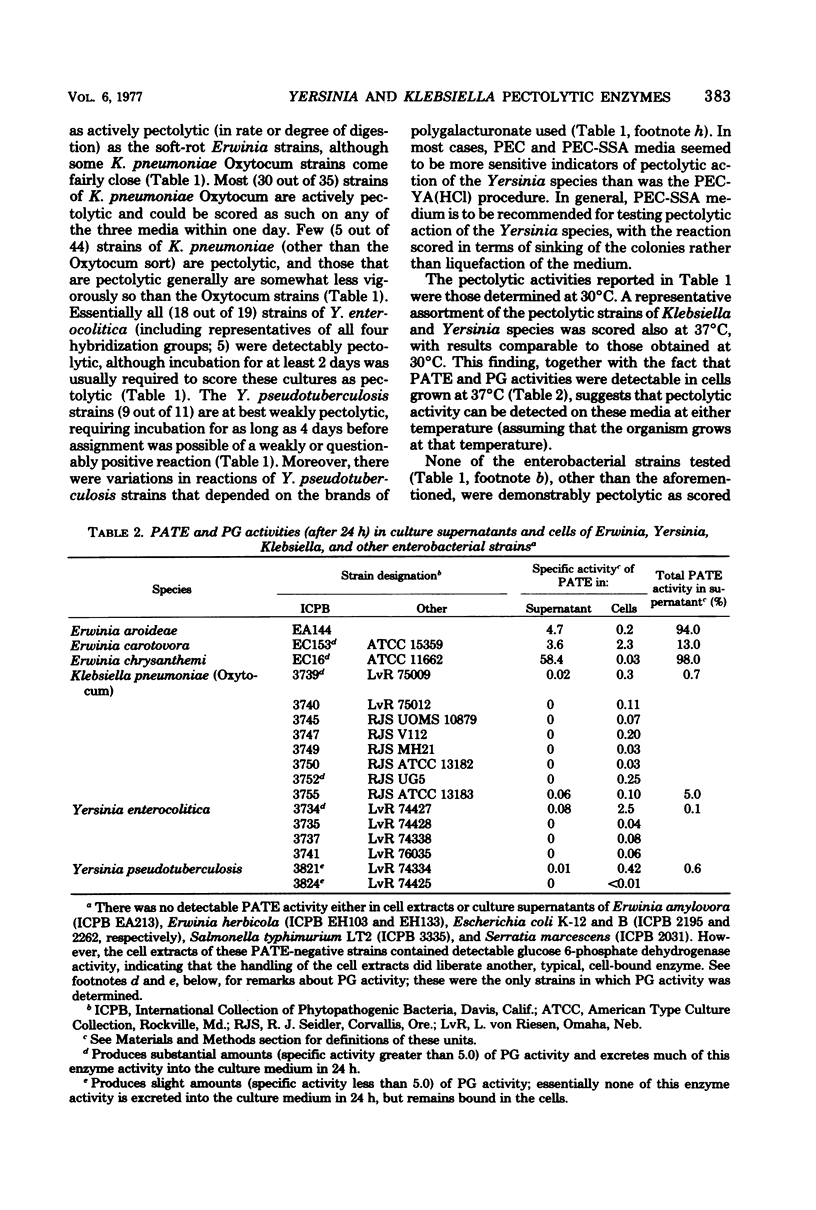
As scored by several specified plating procedures, clinical and environmental strains of Yersinia enterocolitica, Yersinia pseudotuberculosis, and Klebsiella pneumoniae “Oxytocum” showed detectable, albeit generally weak, ability to digest polygalacturonic (pectic) acid. None of these bacterial strains had the vigorous and rapid pectolytic activity on these polygalacturonic acid-containing media that is typical of soft-rot Erwinia species, although some of the Oxytocum strains came fairly close. Analyses of the pectolytic enzyme contents of the cells and culture supernatants of the Yersinia and Klebsiella species revealed that readily detectable quantities of cell-bound polygalacturonic acid trans-eliminase and hydrolytic polygalacturonase were formed by the Yersinia and Klebsiella species; however, the total units of enzyme activity produced by these bacteria were, in general, lower than were produced by soft-rot Erwinia species. Furthermore, unlike the situation in soft-rot Erwinia cultures, these pectolytic enzymes of Yersinia and Klebsiella species were not excreted rapidly and massively into the growth medium. Cultures of other enterobacteria (Citrobacter species, Enterobacter species, Erwinia amylovora, Erwinia herbicola, Escherichia coli, Proteus species, Salmonella typhimurium, and Serratia marcescens) showed no pectolytic ability whatsoever by any of the plating procedures used and (to the extent they were so examined) produced no pectolytic enzymes detectable either in their cells or culture supernatants. This slow or weak release of pectolytic enzymes by Yersinia and Klebsiella species has a bearing on clinical laboratory procedures suitable for detecting their pectolytic activity; methods adequate for this purpose are detailed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown C., Seidler R. J. Potential pathogens in the environment: Klebsiella pneumoniae, a taxonomic and ecological enigma. Appl Microbiol. 1973 Jun;25(6):900–904. doi: 10.1128/am.25.6.900-904.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. M., Michaels G., Klein R. D., Roth I. L. Isolation of Klebsiella pneumoniae from lake water. Can J Microbiol. 1976 Dec;22(12):1762–1767. doi: 10.1139/m76-260. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. K., Buss R. F., Starr M. P. Unusual susceptibility of Erwinia amylovora to antibacterial agents in relation to the barrier function of its cell envelope. Antimicrob Agents Chemother. 1977 May;11(5):897–905. doi: 10.1128/aac.11.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Genetic transfer of episomic elements among Erwinia species and other enterobacteria: F'Lac+. J Bacteriol. 1972 Jul;111(1):169–176. doi: 10.1128/jb.111.1.169-176.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. R., EWING W. H. LIPOLYTIC, PECTOLYTIC, AND ALGINOLYTIC ACTIVITIES OF ENTEROBACTERIACEAE. J Bacteriol. 1964 Jul;88:16–19. doi: 10.1128/jb.88.1.16-19.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S., Greenwood J. R., Pickett M. J., Mah R. A. Recovery of Yersinia enterocolitica from streams and lakes of California. Appl Environ Microbiol. 1976 Sep;32(3):352–354. doi: 10.1128/aem.32.3.352-354.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILGORE W. W., STARR M. P. Catabolism of galacturonic and glucuronic acids by Erwinia carotovora. J Biol Chem. 1959 Sep;234:2227–2235. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lakso J. U., Starr M. P. Comparative injuriousness to plants of Erwinia spp. and other enterobacteria from plants and animals. J Appl Bacteriol. 1970 Dec;33(4):692–707. doi: 10.1111/j.1365-2672.1970.tb02252.x. [DOI] [PubMed] [Google Scholar]
- Moran F., Nasuno S., Starr M. P. Extracellular and intracellular polygllacturonic acid trans-eliminases of Erwinia carotovora. Arch Biochem Biophys. 1968 Feb;123(2):298–306. doi: 10.1016/0003-9861(68)90138-0. [DOI] [PubMed] [Google Scholar]
- Moran F., Nasuno S., Starr M. P. Oligogalacturonide trans-eliminase of Erwinia carotovora. Arch Biochem Biophys. 1968 Jun;125(3):734–741. doi: 10.1016/0003-9861(68)90508-0. [DOI] [PubMed] [Google Scholar]
- Moran F., Starr M. P. Metabolic regulation of polygalacturonic acid trans-eliminase in Erwinia. Eur J Biochem. 1969 Dec;11(2):291–295. doi: 10.1111/j.1432-1033.1969.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Murata N., Starr M. P. Intrageneric clustering and divergence of Erwinia strains from plants and man in the light of deoxyribonucleic acid segmental homology. Can J Microbiol. 1974 Nov;20(11):1545–1565. doi: 10.1139/m74-242. [DOI] [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Polygalacturonase of Erwinia carotovora. J Biol Chem. 1966 Nov 25;241(22):5298–5306. [PubMed] [Google Scholar]
- STARR M. P., MORAN F. Eliminative split of pectic substances by phytopathogenic soft-rot bacteria. Science. 1962 Mar 16;135(3507):920–921. doi: 10.1126/science.135.3507.920. [DOI] [PubMed] [Google Scholar]
- Starr M. P., Chatterjee A. K. The genus Erwinia: enterobacteria pathogenic to plants and animals. Annu Rev Microbiol. 1972;26:389–426. doi: 10.1146/annurev.mi.26.100172.002133. [DOI] [PubMed] [Google Scholar]
- von Riesen V. L. Polypectate digestion by Yersinia. J Clin Microbiol. 1975 Dec;2(6):552–553. doi: 10.1128/jcm.2.6.552-553.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


