Abstract
We evaluated the association between two antiretroviral therapy (ART) adherence measurements—the medication possession ratio (MPR) and patient self-report—and detectable HIV viremia in the setting of rapid service scale-up in Lusaka, Zambia. Drug adherence and outcomes were assessed in a subset of patients suspected of treatment failure based on discordant clinical and immunologic responses to ART. A total of 913 patients were included in this analysis, with a median time of 744 days (Q1, Q3: 511, 919 days) from ART initiation to viral load (VL) measurement. On aggregate over the period of follow-up, 531 (58%) had optimal adherence (MPR ≥95%), 306 (34%) had suboptimal adherence (MPR 80–94%), and 76 (8%) had poor adherence (MPR <80%). Of the 913 patients, 238 (26%) had VL ≥400 copies/ml when tested. When compared to individuals with optimal adherence, there was increasing risk for virologic failure in those with suboptimal adherence [adjusted relative risk (ARR): 1.3; 95% confidence interval (CI): 1.0, 1.6] and those with poor adherence (ARR: 1.7; 95% CI: 1.3, 2.4) based on MPR. During the antiretroviral treatment course, 676 patients (74%) reported no missed doses. The proportion of patients with virologic failure did not differ significantly among those reporting any missed dose from those reporting perfect adherence (26% vs. 26%, p = 0.97). Among patients with suspected treatment failure, a lower MPR was associated with higher rates of detectable viremia. However, the suboptimal sensitivity and specificity of MPR limit its utility as a sole predictor of virologic failure.
Introduction
Adherence to combination antiretroviral therapy (ART) reduces viral replication1 and prevents the emergence of drug-resistant strains of HIV.2 Numerous studies have demonstrated that high levels of adherence can be achieved in Africa—even in the face of resource limitations—but adherence measures remain inconsistent and vary greatly from program to program.3 Although several methods have been validated, no “gold standard” for adherence measurement has been identified for use in an African public health setting.4
Two measures that hold potential for widespread use in resource-constrained settings are the medication possession ratio5 (MPR) and patient self-report. MPR is derived from pharmacy refill data and describes the proportion of days a patient on a treatment regimen has medication on hand. Good adherence as determined by MPR has been correlated with virologic suppression6–10 and improved patient survival.11,12 Patient self-report of drug adherence is commonly obtained in the context of clinical care; however, its utility for predicting patient outcomes is poorly validated in resource-constrained settings. We evaluated the performance of both measures to predict virologic suppression among individuals suspected of treatment failure in a large public sector program in Lusaka, Zambia.
Materials and Methods
We used longitudinal clinical care and pharmacy data from patients receiving care in the Lusaka, Zambia public health sector. This program is supported by the Zambian Ministry of Health; patient care and clinic dynamics have been described previously.13,14 Briefly, known HIV-seropositive patients are screened and deemed eligible to initiate ART based on clinical staging and CD4+ T-lymphocyte (CD4) criteria.15 Eligible patients receive monthly dispensations of ART based on two nucleoside reverse transcriptase inhibitor (NRTI) drugs and one non-NRTI by pharmacy technicians; an extra 3-day supply of medications is provided to safeguard against missed drug doses if patients were to arrive late to their next visit. All patients are encouraged to identify a neighbor or family member who acts as an “adherence supporter.” This individual lends social support and may retrieve pharmacy refills when the patient is unable to make appointments. Medical information is entered into an electronic database for program monitoring and reporting.16
Clinical evaluations are scheduled every 3 months, while CD4 testing is performed every 6 months. Measurement of plasma viral load (VL) is available in our setting, but is too costly to be used routinely. Instead, we have devised an algorithm to guide its judicious use, modified from the WHO guidelines.15 A diagnosis of treatment failure is made presumptively when both clinical and immunologic criteria are met. Clinical failure is defined as a new or recurrent World Health Organization (WHO) stage 3 or 4 condition after 6 months. Immunologic failure is defined in any one of four ways: a CD4 count rise of <50 cells/μl during the first 6 months of therapy, a persistent CD4 count <100 cells/μl after 12 months of ART, a decline in CD4 count to a value below that of pretreatment baseline, or a CD4 count decline of >30% from the on-treatment peak. When clinical and immunologic criteria are discordant (i.e., one suggests failure and the other does not), then a VL test is performed to guide care. Once a diagnosis of treatment failure is made, based either on virologic or nonvirologic criteria, patients are switched to second-line therapy.
We measured adherence in two ways: MPR and patient recall. To calculate MPR, we divided the cumulative number of days late to pharmacy visits by the cumulative number of days on ART, and then subtracted this proportion from 100%. Patients were not counted as late to pharmacy appointments until after 3 days, to account for routine provision of extra drug doses. Patients in this analysis were categorized according to previously published conventions for adherence by MPR: optimal (≥95%), suboptimal (80–94%), and poor (<80%).10,11 We also measured adherence based on 3-day recall of missed doses. Because most patients in our setting report perfect adherence by this measure, we categorized individuals into one of two groups: those with any reported missed doses while on therapy and those with no reported missed doses. Adherence measurements prior to the patient's first VL measurement were included. VL concentration was determined using the Roche COBAS Ampliprep/COBAS Amplicor HIV-1 Monitor Test, version 1.5 standard format (Roche Molecular Systems, Branchburg, NJ). Virologic failure was defined as VL ≥400 copies/ml, the limit of detection of our assay.
Individuals included in this analysis were >16 years of age, initiated ART based on the local eligibility criteria, were ART naive at the time of treatment initiation, and met criteria for VL measurement >100 days on treatment. Baseline characteristics were compared across each of the adherence categories using Pearson's Chi-square test (categorical variables) and the Wilcoxon rank sum test (continuous variables). We included all factors associated with adherence at a p ≤ 0.05 level in a multivariable log Poisson model to estimate relative risk (RR).17 Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for MPR thresholds of <95% and <80%, and for any missed doses via patient self-report. We performed statistical analyses using SAS v.9.1.3 (SAS Institute, Cary, NC). This study was approved by the University of Zambia Research Ethics Committee (Lusaka, Zambia) and the University of Alabama Institutional Review Board (Birmingham, AL).
Results
Cohort description
From June 1, 2006 to November 30, 2007, 913 HIV-infected adults were found to have discordant clinical and immunologic responses after at least 100 days on ART and were tested via VL. At time of enrollment, 69% (635/910) were WHO stage III or IV, 48% (439/913) were male, and 15% (134/913) had a diagnosis of active tuberculosis. The median baseline CD4 count was 115 cells/μl (Q1, Q3: 58, 182) and median baseline hemoglobin was 11.0 g/dl (Q1, Q3: 9.7, 12.5). An adherence supporter had been identified by 68% (621/913), but only 27% (243/913) reported disclosure of their HIV status to a partner or spouse.
MPR adherence and virologic suppression
Of the 913 adults, 531 (58%) had optimal adherence, 306 (34%) had suboptimal adherence, and 76 (8%) had poor adherence. Individuals with an MPR <80% had a higher median BMI when compared to those with MPR ≥95% (19.9 vs. 21.1; p = 0.03). Patients with suboptimal and poor adherence did not otherwise differ from patients with optimal adherence according to baseline characteristics (data not shown). The median time from ART initiation to VL measurement was 744 days (Q1, Q3: 511, 919 days).
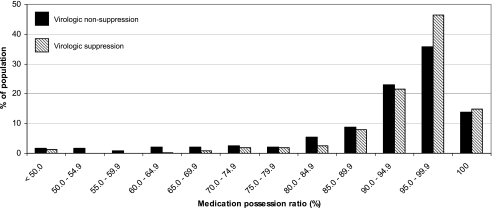
Overall, 238 of 913 (26%) had detectable viremia (i.e., ≥400 copies/ml). The distribution of adherence according to virologic suppression status appears in Fig. 1. The proportion with detectable VL increased according to MPR-based adherence categories from optimal [118/531 (22%)], suboptimal [89/306 (29%)], to poor [31/76 (41%); p for trend = 0.0002; Table 1]. This trend remained consistent in a multivariable model adjusting for age and the presence of an adherence supporter. When compared to those with optimal adherence, the risk for virologic failure was higher among those with suboptimal [adjusted RR (ARR): 1.7; 95% CI: 1.0, 1.6] and poor (ARR: 1.8; 95% CI: 1.3, 2.4) adherence. When we added baseline CD4+ lymphocyte count, WHO stage, hemoglobin, and BMI to the model, results did not change (data not shown).
FIG. 1.
Distribution of adherence by medication possession ratio, stratified according to viral suppression or nonsuppression, among adults on antiretroviral therapy suspected of treatment failure in Lusaka, Zambia.
Table 1.
Risk of Virologic Failure for ART-Naive Adults Suspected of Treatment Failure in Lusaka, Zambia
| Crude RR (95% CI) | ||
|---|---|---|
| Medication possession ratio | ≥95% | 1.0 |
| 80–94% | 1.3 (1.0–1.7) | |
| <80% | 1.8 (1.3–2.5) | |
| Self-reported missed doses | None | 1.0 |
| Any | 1.0 (0.8–1.3) | |
| Sex | Female | 1.0 |
| Male | 0.9 (0.7–1.1) | |
| Disclosed HIV status to spouse/partner | No | 1.0 |
| Yes | 0.7 (0.6–1.0) | |
| Spouse/partner tested for HIV | No | 1.0 |
| Yes | 0.7 (0.5–0.9) | |
| Adherence supporter | No | 1.0 |
| Yes | 0.7 (0.6–0.9) | |
| CD4+ lymphocyte count | ≥200 cells/μl | 1.0 |
| 50–199 cells/μl | 0.9 (0.7–1.3) | |
| <50 cells/μl | 1.2 (0.9–1.7) | |
| WHO stage | I or II | 1.0 |
| III or IV | 1.0 (0.8–1.2) | |
| Hemoglobin | ≥8.0 g/dl | 1.0 |
| <8.0 g/dl | 0.9 (0.6–1.6) | |
| Body mass index | ≥16 kg/m2 | 1.0 |
| <16 kg/m2 | 1.4 (0.8–2.4) | |
| Tuberculosis (active) | No | 1.0 |
| Yes | 1.0 (0.7–1.3) |
We assessed the performance of two MPR threshold in predicting virologic failure. When we used a <95% MPR threshold, the sensitivity was 0.50 (95% CI: 0.44, 0.57), the specificity was 0.61 (95% CI: 0.57, 0.65), the PPV was 0.31 (95% CI: 0.27, 0.36), and the NPV was 0.78 (95% CI: 0.74, 0.81). When we used a <80% MPR threshold, the sensitivity was 0.13 (95% CI: 0.09, 0.18), the specificity was 0.93 (95% CI: 0.91, 0.95), the PPV was 0.41 (95% CI: 0.30, 0.53), and the NPV was 0.75 (95% CI: 0.72, 0.78).
Patient report and virologic suppression
Of the 913 patients, 676 (74%) reported never missing a dose in the 3 days prior to clinic visits. The proportion of patients with virologic failure did not differ significantly among those who did (176/676, 26%) and did not (62/237, 26%; p = 0.97) report ever missing a dose. Inclusion of age and adherence supporter to a multivariable model did not change these results when those reporting at least one missed dose were compared to those reporting no missed doses (ARR: 1.0; 95% CI: 0.8, 1.3). The sensitivity of reporting a missed ART dose was 0.26 (95% CI: 0.21, 0.32), the specificity was 0.74 (95% CI: 0.71, 0.77), the PPV was 0.26 (95% CI: 0.21, 0.32), and the NPV was 0.74 (95% CI: 0.71, 0.77).
Discussion
In the setting of rapid service scale-up, we observed a dose–response relationship between MPR-based adherence measures and virologic outcomes among individuals with discordant clinical and immunologic responses to ART. However, the poor sensitivity and specificity of MPR limits its utility to predict virologic failure in this population. While some investigators have found patient self-report to be reliable,18 our self-report data based on 3-day recall had little variability and performed poorly.
We found MPR to be more reliable than patient self-report in predicting HIV treatment outcomes. Others have reported similar dose-dependent relationships between MPR adherence and virologic suppression.6–10 However, our observations demonstrate that MPR measurements of adherence can predict increased risk for virologic failure in a busy public sector clinical environment, as they do in public sector programs in Australia,8 managed care settings in urban South Africa,11 and research settings in rural Uganda.10
We studied a select group of patients with discordant clinical and immunologic response to ART. Unlike other settings,6–10 VL was not routinely incorporated into long-term treatment monitoring in our program, but instead was used selectively due to resource constraints.19 We found performance of our a priori MPR thresholds to be suboptimal for predicting virologic failure. Both PPV and NPV at the <95% and <80% MPR thresholds were inadequate to justify the sole use of this measure to presume virologic failure; incorporation of VL is still required for diagnosis. However, given the demonstrated association between MPR and virologic failure, this may have a role in multiple-indicator clinical algorithms to diagnose treatment failure without routine use of VL measurements.
Identification of an adherence supporter, disclosure of one's HIV status to a spouse or partner, and knowledge of a partner's or spouse's HIV testing history were all protective for treatment failure in our analysis. Each is a correlate of social support, which is widely acknowledged to contribute positively to patient adherence,20 retention,21 and treatment outcomes.22 Although an interesting and important ancillary finding, at present we are unclear whether such social structures provide therapeutic benefits for patients on ART, or whether reports of such support are merely indicators of an individual's health-seeking behavior. Despite this uncertainty, community education programs designed to increase HIV awareness and to reduce stigma are clearly needed, given their broad potential to impact prevention, care, and treatment.
The primary limitation of this analysis was the imprecision inherent in these surrogate markers for adherence. Although valuable as an indicator for health-seeking behavior, MPR represents a best-case scenario in terms of ART adherence and likely overestimates actual patient ingestion of drugs. Our ability to obtain only a single VL measurement may lead to misclassification via intermittent viremic spikes (i.e., “blips”) among patients. Since VL testing is a surrogate for clinical outcome, the addition of antiretroviral resistance measurements would add to the precision of the VL predictive value in guiding the decision to switch antiretroviral therapy. We suggest the need for including viral drug resistance in future studies.23
In summary, while low MPR was associated with poor virologic outcomes, when used alone it is an inadequate measure to predict treatment failure. At present, we are working to incorporate this metric into larger models that may have improved prognostic implications. We intend to continue to use MPR to help identify persons at risk for failure on their given ART regimen, until there is a superior approach suitable for resource-constrained settings.
Acknowledgments
The authors thank the patients and health personnel from the participating Lusaka clinics for providing this important information. We also acknowledge the Zambian Ministry of Health for consistent and high-level support of operations research surrounding its national HIV care and treatment program. Investigator support was provided by the Fogarty International Clinical Research Scholars Program (D43 TW001035, R24 TW007988), the National Institutes of Health (K23 AI01411, K01 TW06670), the University of Alabama at Birmingham Center for AIDS Research (P30 AI27767-20), and the Doris Duke Clinical Scientist Development Award (2007061). The clinical program described in this paper was supported by a multicountry grant to the Elizabeth Glaser Pediatric AIDS Foundation from the U.S. Centers for Disease Control and Prevention (U62/CCU12354). Data monitoring and quality improvement were supported in part by a Doris Duke Charitable Foundation grant for Operations Research for AIDS Care and Treatment in Africa (2005047).
References
- 1.Paterson DL. Swindells S. Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Oyugi JH. Byakika-Tusiime J. Ragland K, et al. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007;21:965–971. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- 3.Mills EJ. Nachega JB. Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: A meta-analysis. JAMA. 2006;296:679–690. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 4.Chesney MA. The elusive gold standard. Future perspectives for HIV adherence assessment and intervention. J Acquir Immune Defic Syndr. 2006;43(Suppl. 1):S149–155. doi: 10.1097/01.qai.0000243112.91293.26. [DOI] [PubMed] [Google Scholar]
- 5.Dezii CM. Persistence with drug therapy: A practical approach using administrative claims data. Manag Care. 2001;10:42–45. [PubMed] [Google Scholar]
- 6.Grossberg R. Zhang Y. Gross R. A time-to-prescription-refill measure of antiretroviral adherence predicted changes in viral load in HIV. J Clin Epidemiol. 2004;57:1107–1110. doi: 10.1016/j.jclinepi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Gross R. Yip B. Lo Re V, 3rd, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis. 2006;194:1108–1114. doi: 10.1086/507680. [DOI] [PubMed] [Google Scholar]
- 8.Fairley CK. Permana A. Read TR. Long-term utility of measuring adherence by self-report compared with pharmacy record in a routine clinic setting. HIV Med. 2005;6:366–369. doi: 10.1111/j.1468-1293.2005.00322.x. [DOI] [PubMed] [Google Scholar]
- 9.Nachega JB. Hislop M. Dowdy DW. Chaisson RE. Regensberg L. Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146:564–573. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 10.Weidle PJ. Wamai N. Solberg P, et al. Adherence to antiretroviral therapy in a home-based AIDS care programme in rural Uganda. Lancet. 2006;368:1587–1594. doi: 10.1016/S0140-6736(06)69118-6. [DOI] [PubMed] [Google Scholar]
- 11.Nachega JB. Hislop M. Dowdy DW, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006;43:78–84. doi: 10.1097/01.qai.0000225015.43266.46. [DOI] [PubMed] [Google Scholar]
- 12.Hogg RS. Heath K. Bangsberg D, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16:1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 13.Stringer JS. Zulu I. Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: Feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 14.Chi BH. Sinkala M. Stringer EM, et al. Early clinical and immune response to NNRTI-based antiretroviral therapy among women with prior exposure to single-dose nevirapine. AIDS. 2007;21:957–964. doi: 10.1097/QAD.0b013e32810996b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: Towards universal access. Recommendations for a public health approach. WHO; Geneva, Switzerland: 2006. [Google Scholar]
- 16.Fusco H. Hubschman T. Mweeta V, et al. Electronic patient tracking supports rapid expansion of HIV care and treatment in resource-constrained settings. Third IAS Conference on HIV Pathogenesis and Treatment; Rio de Janeiro, Brazil. Jul 25–28;2005 ; (Abstract MoPe11.2C37.) [Google Scholar]
- 17.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 18.Nieuwkerk PT. Oort FJ. Self-reported adherence to antiretroviral therapy for HIV-1 infection and virologic treatment response: A meta-analysis. J Acquir Immune Defic Syndr. 2005;38:445–448. doi: 10.1097/01.qai.0000147522.34369.12. [DOI] [PubMed] [Google Scholar]
- 19.Colebunders R. Moses KR. Laurence J, et al. A new model to monitor the virological efficacy of antiretroviral treatment in resource-poor countries. Lancet Infect Dis. 2006;6:53–59. doi: 10.1016/S1473-3099(05)70327-3. [DOI] [PubMed] [Google Scholar]
- 20.Catz SL. Kelly JA. Bogart LM. Benotsch EG. McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychol. 2000;19:124–133. [PubMed] [Google Scholar]
- 21.Nash D. Korves C. Saito S, et al. Characteristics of facilities and programs delivering HIV care and treatment services are associated with loss to follow-up rates in programs from sub-Saharan African countries. Fifteenth Conference on Retroviruses and Opportunistic Infections; Boston, MA. Feb 3–6;2008 ; (Abstract #838.) [Google Scholar]
- 22.Zachariah R. Teck R. Buhendwa L, et al. Community support is associated with better antiretroviral treatment outcomes in a resource-limited rural district in Malawi. Trans R Soc Trop Med Hyg. 2007;101:79–84. doi: 10.1016/j.trstmh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Bangsberg DR. Kroetz DL. Deeks SG. Adherence-resistance relationships to combination HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4:65–72. doi: 10.1007/s11904-007-0010-0. [DOI] [PubMed] [Google Scholar]