Abstract
Generalized whole brain volume loss has been well documented in moderate-to-severe traumatic brain injury (TBI), as has diffuse cerebral atrophy based on magnetic resonance imaging (MRI) volumetric methods where white matter may be more selectively affected than gray matter. However, specific regional differences in gray matter thickness of the cortical mantle have not been previously examined. As such, cortical thickness was assessed using FreeSurfer® software to identify regions of significant gray matter cortical thinning in MRI scans of 16 young TBI subjects (age range, 9–16 years) compared to 16 demographically matched controls. Significant cortical thinning was observed globally in the TBI group compared to the cohort of typically developing children. Reduced cortical thickness was related to reported deficits in working memory. TBI-induced cortical thickness reductions are probably due to a combination of focal and diffuse effects and have implications for the neurobehavioral sequelae of TBI.
Key words: MRI, neural injury, other tools of modern imaging, pediatric brain injury, traumatic brain injury
Traumatic brain injury (TBI) is known to produce both focal and diffuse damage with generalized atrophic changes resulting in reduced overall brain volume (Levine et al., 2008). Given these nonspecific effects, it would be anticipated that TBI would also affect cortical thickness, but this has not been examined to date. Despite the heterogeneity of TBI, including severity and the relative contributions of focal and diffuse injury, common areas of residual injury have been observed, suggesting the likelihood that TBI would result in some characteristic regional changes in cortical thickness, including frontotemporal and limbic regions (Gale et al., 2005; Levine et al., 2008). Accordingly, in the current study, we compared the MRI-derived cortical thickness of 16 children aged 9–16 years who had sustained moderate-to-severe TBI to a comparison group of 16 demographically matched, normally developing children.
Given previous reports of diffuse white matter atrophy following TBI (Gale et al., 1995a; Levine et al., 2008), we hypothesized that significant differences in cortical thickness would also be apparent globally between the TBI and comparisons cohorts in our sample, including areas such as the frontal and temporal cortex, which have been identified previously using volumetric approaches (Gale et al., 2005; Wilde et al., 2005; Yount et al., 2002).
The TBI group consisted of 16 children (eight male, eight female) who had sustained moderate-to-severe injury (initial Glasgow Coma Scale [GCS] score of 3–12). Mean post-injury interval was 3.1 ± 2.4 years, and mean age at the time of scanning was 12.9 ± 2.5 years (Table 1). Sixteen typically developing children were selected to demographically match the TBI patients. All subjects underwent magnetic resonance imaging (MRI) without sedation on 1.5-Tesla Intera scanners (Philips, Cleveland, OH). A T1-weighted (15 msec TR, 4.6 msec TE, 1.0-mm slices) three-dimensional (3D) sagittal acquisition series with a 256-mm field of view (FOV) was used for cortical thickness analysis.
Table 1.
Demographic Characteristics of Traumatic Brain Injury (TBI) and Typically Developing Children
| TBI group (n = 16) | Typically developing children (n = 16) | |
|---|---|---|
| Age at testing (years) | 12.9 ± 2.5 | 12.8 ± 2.4 |
| Age at injury (years) | 9.75 ± 3.0 | N/A |
| Time post-injury (years) | 3.1 ± 2.4 | N/A |
| Gender distribution (M/F) | 8/8 | 8/8 |
| Mechanism of injury (accident type) | 6 auto-pedestrian, 1 bicycle, 6 MVA, 1 motorcycle, 2 RV | N/A |
| Glasgow Coma Scale score | 5.7 ± 2.8 (range, 3–11) | N/A |
M, male; F, female; MVA, motor vehicle accident; RV, recreation vehicle accident; N/A, not applicable.
Cortical surfaces were reconstructed using FreeSurfer® v4.0.4 software (Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA; https://surfer.nmr.mgh.harvard.edu), which has been described by others (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 1999). Briefly, representations of the gray and white matter boundary and the pial surface were reconstructed for each subject using both intensity and continuity information from the MRI scan. The results for each subject were visually inspected and corrected where necessary (e.g., removal of dura and skull, accurate delineation of pial and white matter surfaces). Cortical thickness was measured as the distance between these two surfaces at each point on the cortical mantle. The procedure has been shown to be capable of detecting sub-millimeter differences between groups (Fischl and Dale, 2000). Surface smoothing was performed using a 10-mm full-width half-maximum Gaussian kernel.
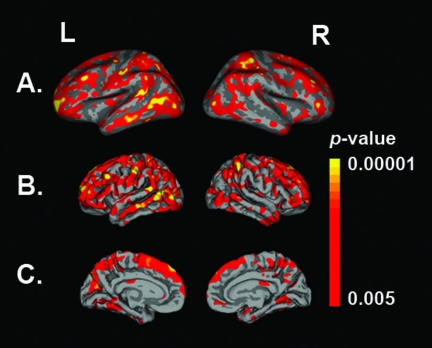
The cortical thickness analysis revealed a diffuse pattern of significant group difference (p < 0.005 to p < 0.00001) in cortical thickness (Fig. 1). For reporting group differences by cortical region, we selected a threshold of p < 0.0008, which corresponds to a threshold of p < 0.05 Bonferroni-corrected for the number of multiple comparisons. After adjusting for the effects of age and gender, highly significant mean cortical loss was observed in the TBI group as compared to the typically developing children, including left superior frontal (p = 0.0002), right pars opercularis (p = 0.0005), right frontal pole (p = 0.0002), rostral middle frontal (left p = 0.0001, right p = 0.0004), caudal middle frontal (left p = 0.0001, right p = 0.0004), left precentral (p = 0.0007), supramarginal (left p = 0.0001, right p = 0.0003), left middle temporal (p = 0.0001), inferior temporal (left p = 0.0006, right p = 0.0002), left fusiform (p = 0.0001), postcentral (left p = 0.0001, right p = 0.0001), superior parietal (left p = 0.0001, right p = 0.0001), inferior parietal (left p = 0.0001, right p = 0.0001), and precuneus (left p = 0.0002, right p = 0.0003) regions.
FIG. 1.
Regions of significant cortical loss in pediatric traumatic brain injury (TBI) as compared to typically developing children, reflecting adjustments made for age and gender. The p-value color scale indicates group differences ranging from dark red (p < 0.005) to yellow (p < 0.00001). Results are displayed on a customized averaged pediatric subject. (A) Lateral view (with surfaces inflated to reveal the extent of significant regions) showing group differences bilaterally for temporal and frontal lobe (p < 0.005). (B) Lateral view (now shown as pial surfaces) indicating the same significant regions as displayed in A. (C) Midsagittal pial surfaces showing significant cortical regional differences.
While cortical thickness was not related to the Glasgow Outcome Scale (GOS) (Jennett and Bond, 1975), cortical thickness was highly correlated with the working memory index of the Behavior Rating Inventory of Executive Function (BRIEF) (Gioia et al., 2000). Working memory is believed to be mediated by a distributed network of cortical regions (Postle, 2006a,b), and working memory impairment is common subsequent to TBI (Christodoulou et al., 2001). Significant correlations with working memory performance were observed in key regions that have been reported to subserve working memory function (Postle, 2006a,b), including inferior temporal (left p < 0.035, right p < 0.017), left fusiform (p < 0.005), superior parietal (left p < 0.001, right p < 0.003), and inferior parietal (left p < 0.001, right (p < 0.005).
To our knowledge, this is the first study to specifically investigate cortical thickness changes in children who have sustained TBI. While previous studies have implicated greater white matter vulnerability in TBI (Anderson and Bigler, 1994; Bigler, 2001; Gale et al., 1995a,b), the current findings indicate concomitant changes in cortical gray matter thickness as well. These changes likely result from multiple etiologies including focal cortical injury from impact compression and contusion, and deafferentation and defferentation secondary to diffuse axonal injury and Wallerian degeneration (Bigler, 2007).
Limitations of the study include its small sample size and cross-sectional design. While the procedure for estimating cortical thickness has been shown to be highly reliable and accurate when used with high-quality data (Fischl and Dale, 2000), the nature of traumatic lesions in TBI may cause distortion in MRI signal characteristics and thus result in abnormalities of the parenchymal surface reconstruction. This could result in some bias because the semi-automated procedure incorporates atlas-based steps which may not take into account the degree of deformation in an individual subject's brain with focal damage, even though we carefully examined data from each subject and applied the appropriate correction as necessary.
Despite these limitations, the results make heuristic sense and demonstrate global cortical change. The analytic techniques presented in this initial study could be used to examine the longitudinal effects of TBI on cortical thinning and its impact on cognition and emotional regulation in children as they mature.
Acknowledgments
This research was supported by grants NS-21889 and NIH R01 HD048946, by the Ira Fulton Foundation, and a grant from the College of Family, Home and Social Science, Brigham Young University. The technical assistance of Tracy J. Abildskov and the editorial assistance of Jo Ann Petrie are gratefully acknowledged.
Author Disclosure Statement
No competing financial interests exist.
References
- Anderson C.V. Bigler E.D. The role of caudate nucleus and corpus callosum atrophy in trauma-induced anterior horn dilation. Brain Inj. 1994;8:565–569. doi: 10.3109/02699059409151008. [DOI] [PubMed] [Google Scholar]
- Bigler E.D. Quantitative magnetic resonance imaging in traumatic brain injury. J. Head Trauma Rehabil. 2001;16:117–134. doi: 10.1097/00001199-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Bigler E.D. Anterior and middle cranial fossa in traumatic brain injury: relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology. 2007;21:515–531. doi: 10.1037/0894-4105.21.5.515. [DOI] [PubMed] [Google Scholar]
- Christodoulou C. DeLuca J. Ricker J.H. Madigan N.K. Bly B.M. Lange G. Kalnin A.J. Liu W.C. Steffener J. Diamond B.J. Ni A.C. Functional magnetic resonance imaging of working memory impairment after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry. 2001;71:161–168. doi: 10.1136/jnnp.71.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M. Fischl B. Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Fischl B. Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. Sereno M.I. Dale A.M. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Gale S.D. Baxter L. Roundy N. Johnson S.C. Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. J. Neurol. Neurosurg. Psychiatry. 2005;76:984–988. doi: 10.1136/jnnp.2004.036210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale S.D. Johnson S.C. Bigler E.D. Blatter D.D. Nonspecific white matter degeneration following traumatic brain injury. J. Int. Neuropsychol. Soc. 1995a;1:17–28. doi: 10.1017/s1355617700000060. [DOI] [PubMed] [Google Scholar]
- Gale S.D. Johnson S.C. Bigler E.D. Blatter D.D. Trauma-induced degenerative changes in brain injury: a morphometric analysis of three patients with preinjury and postinjury MR scans. J. Neurotrauma. 1995b;12:151–158. doi: 10.1089/neu.1995.12.151. [DOI] [PubMed] [Google Scholar]
- Gioia G.A. Isquith P.K. Guy S.C. Kenworthy L. Behavior Rating Inventory of Executive Function. Child Neuropsychol. 2000;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Jennett B. Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- Levine B. Kovacevic N. Nica E.I. Cheung G. Gao F. Schwartz M.L. Black S.E. The Toronto Traumatic Brain Injury Study: injury severity and quantified MRI. Neurology. 2008;70:771–778. doi: 10.1212/01.wnl.0000304108.32283.aa. [DOI] [PubMed] [Google Scholar]
- Postle B.R. Distraction-spanning sustained activity during delayed recognition of locations. Neuroimage. 2006a;30:950–962. doi: 10.1016/j.neuroimage.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Postle B.R. Working memory as an emergent property of the mind and brain. Neuroscience. 2006b;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde E.A. Hunter J.V. Newsome M.R. Scheibel R.S. Bigler E.D. Johnson J.L. Fearing M.A. Cleavinger H.B. Li X. Swank P.R. Pedroza C. Roberson G.S. Bachevalier J. Levin H.S. Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. J. Neurotrauma. 2005;22:333–344. doi: 10.1089/neu.2005.22.333. [DOI] [PubMed] [Google Scholar]
- Yount R. Raschke K.A. Biru M. Tate D.F. Miller M.J. Abildskov T. Gandhi P. Ryser D. Hopkins R.O. Bigler E.D. Traumatic brain injury and atrophy of the cingulate gyrus. J. Neuropsychiatry Clin. Neurosci. 2002;14:416–423. doi: 10.1176/jnp.14.4.416. [DOI] [PubMed] [Google Scholar]