Although phosphines serve as nucleophilic catalysts for an array of useful transformations, comparatively few highly enantioselective variants in the presence of chiral phosphines have been described.[1,2] In 1994, Trost discovered a novel dppp-catalyzed (dppp=1,3-bis(diphenylphosphino)propane) cyclization of hydroxy-2-alkynoates that generates saturated oxygen heterocycles.[3] Interestingly, despite the importance of such structures, due to their presence in a wide range of bioactive molecules,[4] there has been no progress toward the development of an asymmetric version of the Trost cyclization. In this report, we establish that a chiral spiro phosphepine (1) can achieve this objective with a variety of hydroxy-2-alkynoates with good enantiomeric excess (eq 1).
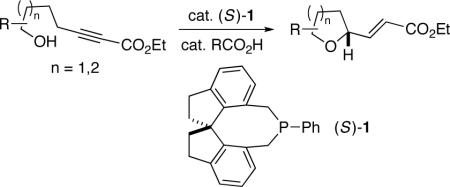 |
(1) |
A plausible pathway, originally suggested by Trost,[3] for the phosphine-catalyzed cyclization of hydroxy-2-alkynoates is illustrated in Scheme 1. On the basis of this mechanism, it seemed reasonable to anticipate that the catalytic asymmetric synthesis of oxygen heterocycles might be achieved through the use of an appropriate chiral phosphine. In our initial studies, we investigated the cyclization of hydroxy-2-alkynoate 2 to form tetrahydofuran 3 in the presence of an array of chiral bisphosphines (for a sampling, see entries 1−4 of Table 1), since Trost had observed that dppp is significantly more effective than PPh3 for non-asymmetric processes.[3] Because the results were not especially promising, we turned our attention to monophosphines (e.g., entries 5−9). Phosphepines emerged as the most promising catalysts,[5,6] with the spiro phosphepine of Zhou (1)[7] accomplishing the desired cyclization with particularly good ee and yield (entry 9).[8]
Scheme 1.
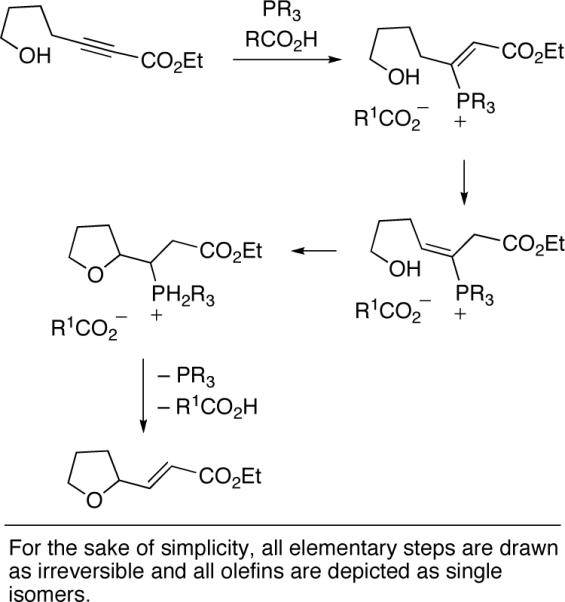
Outline of a possible pathway for the phosphinecatalyzed synthesis of oxygen heterocycles from hydroxy-2-alkynoates.
Table 1.
Catalytic enantioselective synthesis of oxygen heterocycles by chiral bidentate and monodentate phosphines.
 | |||
|---|---|---|---|
| entry | cat. | ee (%)[a] | yield (%)[b] |
| 1 | (S,S)-CHIRAPHOS | - | <2 |
| 2 | (R,R)-DIPAMP | 22 | 70 |
| 3 | (R,R)-Me-DUPHOS | - | <2 |
| 4 | (R,R)-BINAPHANE | 17 | 9 |
| 5 | (R)-MOP | - | <2 |
| 6 | (S)-MONOPHOS | - | <2 |
| 7 | (S)-4 | −66 | 72 |
| 8 | (S)-5 | −45 | 65 |
| 9 | (S)-1 | 87 | 80 |
All data are the average of two experiments.
A negative value for the ee signifies that the enantiomer of 3 is formed preferentially.
The yield was determined by GC analysis with the aid of a calibrated internal standard.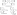
The conditions that we developed for the cyclization of hydroxy-2-alkynoate 2 can be applied to a variety of substrates (Table 2), providing not only tetrahydrofurans (entries 1−3), but also tetrahydropyrans (entries 4−8), in high ee and generally good yield. Substituents can be present α, β, or γ to the hydroxyl group.
Table 2.
Catalytic enantioselective synthesis of tetrahydrofurans and tetrahydropyrans.
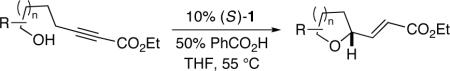 | |||
|---|---|---|---|
| entry | substrate | ee (%) | yield (%)[a] |
| 1 |  |
87 | 78 |
| 2 | 94 | 90 | |
| 3 | 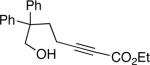 |
87 | 63 |
| 4 | 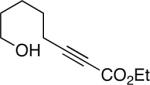 |
92 | 90 |
| 5 | 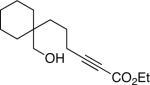 |
94 | 85 |
| 6 | 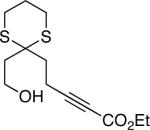 |
92 | 72 |
| 7 | 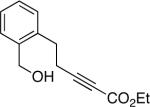 |
94 | 82 |
| 8 | 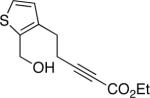 |
91 | 80 |
All data are the average of two experiments.
Yield of purified product.
To date, phenols have not been employed as nucleophiles in phosphine-catalyzed syntheses of oxygen heterocycles from 2-alkynoates. We have determined that, under similar conditions as for aliphatic alcohols,[9] spiro phosphepine 1 catalyzes the cyclization of 2-alkynoates that bear pendant phenols, thereby providing access to enantioenriched dihydrobenzopyrans[10] (Table 3). Phenols with ortho substituents or that are fused to nitrogen heterocycles are suitable substrates.
Table 3.
Catalytic enantioselective synthesis of dihydrobenzopyrans.
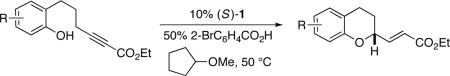 | |||
|---|---|---|---|
| entry | substrate | ee (%) | yield (%)[a] |
| 1 | 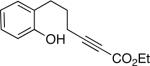 |
88 | 86 |
| 2 | 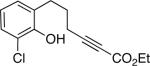 |
63 | 82 |
| 3 | 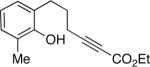 |
84 | 89 |
| 4 | 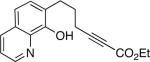 |
84 | 79 |
All data are the average of two experiments.
Yield of purified product.
We have not yet pursued extensive mechanistic studies of this phosphine-catalyzed method for the enantioselective synthesis of oxygen heterocycles. According to 31P NMR spectroscopy, when benzoic acid is added to a solution of spiro phosphepine 1 in THF, proton transfer to form an ion pair does not occur. Furthermore, the resting state of the phosphepine during the catalytic cycle is free phosphepine 1 (rather than, for example, one of the phosphonium salts illustrated in Scheme 1). Spiro phosphepine 1 is reasonably air-stable (after exposure of the solid to air for three days at room temperature, no phosphine oxide is observed by 1H NMR spectroscopy). In addition, the phosphine oxide does not serve as a catalyst for the cyclization.
Prior to this study, three types of phosphine-catalyzed processes had been described that furnish very good enantioselectivity with some generality: acylations of alcohols, Morita-Baylis-Hillman reactions, and couplings of allenes with an unsaturated partner (e.g., an alkene or imine).[2] The current process, adding to some promising earlier results with carbon nucleophiles,[11] represents a fourth class of asymmetric transformations that can be effectively catalyzed by chiral phosphines: γ additions of nucleophiles to unsaturated carbonyl compounds.
In conclusion, we have established that a chiral phosphine can catalyze the transformation of an array of hydroxyl-bearing 2-alkynoates into saturated oxygen heterocycles with good enantioselectivity. In particular, we have demonstrated that spiro phosphepine 1, which had previously proved effective as a chiral ligand in transition-metal chemistry, catalyzes the synthesis of tetrahydrofurans, tetrahydropyrans, and dihydrobenzopyrans with high efficiency. Additional studies are underway that exploit the rich potential of chiral phosphines as asymmetric nucleophilic catalysts.
Supplementary Material
Footnotes
We thank Prof. Qi-Lin Zhou, Kodak, and Degussa for generous gifts of catalysts and catalyst precursors. Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences, grant R01-GM57034), the Croucher Foundation (postdoctoral fellowship for Y.K.C.), Merck Research Laboratories, and Novartis.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.For reviews of phosphine-catalyzed reactions, see: Methot JL, Roush WR. Adv. Synth. Catal. 2004;346:1035–1050.;Ye L-W, Zhou J, Tang Y. Chem. Soc. Rev. 2008;37:1140–1152. doi: 10.1039/b717758e..
- 2.For some key studies of asymmetric catalysis, see: kinetic resolutions of alcohols: Vedejs E, Daugulis O, Diver ST. J. Org. Chem. 1996;61:430–431. doi: 10.1021/jo951661v.;Vedejs E, Daugulis O. J. Am. Chem. Soc. 1999;121:5813–5814.;Morita-Baylis-Hillman reactions: Hayase T, Shibata T, Soai K, Wakatsuki Y. Chem. Commun. 1998:1271–1272.;Shi M, Chen L-H, Li C-Q. J. Am. Chem. Soc. 2005;127:3790–3800. doi: 10.1021/ja0447255.;couplings of allenes with an unsaturated partner: Zhu G, Chen Z, Jiang Q, Xiao D, Cao P, Zhang X. J. Am. Chem. Soc. 1997;119:3836–3837.;Wurz RP, Fu GC. J. Am. Chem. Soc. 2005;127:12234–12235. doi: 10.1021/ja053277d.;Wilson JE, Fu GC. Angew. Chem., Int. Ed. 2006;45:1426–1429. doi: 10.1002/anie.200503312.;Cowen BJ, Miller SJ. J. Am. Chem. Soc. 2007;129:10988–10989. doi: 10.1021/ja0734243.;Fang Y-Q, Jacobsen EN. J. Am. Chem. Soc. 2008;130:5660–5661. doi: 10.1021/ja801344w.;Voituriez A, Panossian A, Fleury-Bregeot N, Retailleau P, Marinetti A. J. Am. Chem. Soc. 2008;130:14030–14031. doi: 10.1021/ja806060a.
- 3.Trost BM, Li C-J. J. Am. Chem. Soc. 1994;116:10819–10820. [Google Scholar]
- 4.For example, saturated five- and six-membered oxygen heterocycles are found in gingkolide B, monensin, morphine, and palytoxin. For some leading references to the synthesis of saturated oxygen heterocycles, see: Elliott MC. J. Chem. Soc., Perkin Trans. 1. 2002:2301–2323.
- 5.For pioneering studies of phosphepines 4 and 5, see: Gladiali S, Dore A, Fabbri D, De Lucchi O, Manassero M. Tetrahedron: Asymmetry. 1994;5:511–514.;Junge K, Hagemann B, Enthaler S, Oehme G, Michalik M, Monsees A, Riermeier T, Dingerdissen U, Beller M. Angew. Chem., Int. Ed. 2004;43:5066–5069. doi: 10.1002/anie.200460190..
- 6.For our efforts to employ phosphepine 4 as a chiral nucleophilic catalyst, see Reference 2c.
- 7.Zhu S-F, Yang Y, Wang L-X, Liu B, Zhou Q-L. Org. Lett. 2005;7:2333–2335. doi: 10.1021/ol050556x.;Xie J-H, Zhou Q-L. Acc. Chem. Res. 2008;41:581–593. doi: 10.1021/ar700137z.. To the best of our knowledge, until now, spiro phosphepine 1 had been used exclusively as a ligand for transition-metal catalyzed asymmetric processes, not as an enantioselective nucleophilic catalyst.
- 8.Notes: a) In the absence of benzoic acid, under otherwise identical conditions, spiro phosphepine 1 generates the tetrahydrofuran in 80% ee and 13% yield. An array of other acids, including chiral acids, furnish lower ee and/or yield; b) If 5% catalyst 1/25% benzoic acid is employed, the reaction proceeds more slowly (e.g., for the substrate illustrated in entry 1 of Table 2, the product is generated in 86% ee and 58% yield after four days).
- 9.For the substrate illustrated in entry 1 of Table 3, we obtain 65% ee and 86% yield under the conditions employed in Table 2.
- 10.An array of natural products, including vitamin E, siccanin, and tazettine, bear a dihydrobenzopyran subunit.
- 11.Chen Z, Zhu G, Jiang Q, Xiao D, Cao P, Zhang X. J. Org. Chem. 1998;63:5631–5635. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


