Abstract
Background
We hypothesize that functional control of the serotonergic system is regulated in part by differential expression of the serotonin (5-HT) transporter (5-HTT). Alcohol-dependent individuals with the LL/LS genotype (L-carriers), compared with those with the SS genotype, have a lower 5-HT neurotransmission, which we hypothesize would be associated with higher craving for alcohol among L-carriers. We hypothesize further that acute peripheral depletion of tryptophan (5-HT’s precursor), while further reducing 5-HT function, might decrease auto-inhibition of 5-HT neuronal firing, thereby increasing 5-HT neurotransmission transiently and lowering alcohol craving.
Methods
We tested these hypotheses by examining whether in 34 Hispanic alcohol-dependent individuals subjective and physiological cue craving for alcohol differed by genotype, age of problem drinking onset, and tryptophan availability.
Results
On subjective “urge to drink” and “crave for a drink”, we found a significant (p<0.05) main effect of genotype and cue, as well as an interaction among genotype, age of problem drinking onset, and tryptophan depletion. For the physiological measure of pulse, there was a main effect of genotype. L-carriers had higher craving than their SS counterparts, an effect that decreased under tryptophan depletion. While craving in L-carriers increased the earlier the age of problem drinking onset, the opposite effect was seen in those with the SS genotype.
Conclusion
These results not only provide support for the hypothesis that alcoholics who are L-carriers have greater alcohol craving and possibly greater propensity for drinking but also propose that there is an important 5-HTT gene-by-environment interaction that alters cue craving response for alcohol.
Keywords: alcohol dependence, serotonin transporter, genotype, cue craving, tryptophan depletion
INTRODUCTION
Serotonergic function is an important determinant of alcohol consumption. Several studies have demonstrated that lower function of serotonin (5-HT), as evidenced by reduced levels of its primary metabolite — 5-hydroxyindoleacetic acid (5-HIAA) — in the nucleus accumbens, is associated with greater alcohol consumption (Balldin et al., 1994; LeMarquand et al., 1994).
Of the mechanisms that control central serotonergic function, perhaps the most influential relates to the functional state of the 5-HT transporter (5-HTT) (Lesch et al., 2002). The 5′-regulatory promoter region of SLC6A4 contains a known functional polymorphism in the serotonergic system (5-HTT-linked polymorphic region; 5-HTTLPR) (Heils et al., 1996, 1997). The polymorphism consists of two forms: a 44-base-pair insertion/long (L) variant and a deletion/short (S) variant. This gene is responsible for encoding the 5-HTT in all tissues where it is expressed (Esterling et al., 1998; Ramamoorthy et al., 1993). Allelic variants of the 5-HTT gene have been shown to alter transcription and function of the 5-HTT.
Among healthy individuals without alcohol dependence, the long (LL) genotype, compared with the short (SS) and heterozygous (LS) forms, is associated with greater 5-HT uptake from synapses (Greenberg et al., 1999) and, therefore, reduced intrasynaptic 5-HT levels and 5-HT neurotransmission (Heils et al., 1996; Lesch et al., 1996). This may render such individuals more vulnerable to a range of impulse-dyscontrol behaviors including an earlier onset of alcoholism and antisocial behavior (Johnson, 2000; LeMarquand et al., 1994).
Among individuals with alcohol dependence, the relationship between drinking behavior and the expression of 5-HT genotype is different from, and more complex than, that in normal individuals. In a study of 5-HTT binding in human raphe brain areas, healthy individuals with the LL genotype showed the expected increases in binding compared with those who were S-carriers (Heinz et al., 2000). In contrast, alcoholics with the LL genotype displayed 5-HT binding density that was lower than those with the SS genotype. This and other findings led Heinz et al. (2000) to suggest that alcoholics with the LL genotype might have a greater propensity toward alcohol-induced neurotoxic damage of the 5-HTT. Presumably, this neurotoxic damage will be influenced by the duration of alcohol exposure. Therefore, it would be reasonable to suggest that individuals with an earlier onset of problem drinking and, consequently, greater years of drinking might be expected to have greater functional dysregulation.
We have extended these findings of Heinz et al. (2000) by showing that among alcohol-dependent individuals, those with the LL/LS genotype (L-carriers), compared with their SS counterparts, had lower 5-HT binding and uptake in platelets (Javors et al., 2005). Furthermore, among L-carriers, more lifetime drinking was associated with lower levels of 5-HT binding and uptake (Johnson et al., 2008). Thus, in L-carriers, 5-HTT expression in platelets is influenced by years of problem drinking. Assuming a similar effect in the raphe nucleus of the brain, reduced somatodendritic 5-HTT would decrease 5-HT firing rates because the raised extracellular 5-HT levels would increase autoreceptor inhibition (Little et al., 1998).
We hypothesize that this relatively lower 5-HT neurotransmission among L-carriers, compared with their SS counterparts, would be associated with increased craving for alcohol. Therefore, our first aim, as an extension of our proof-of-concept analysis of the in vivo findings in neuronal tissue and platelets, was to study the relationship — at the level of the organism — between cue-induced propensity for drinking and the expression of 5-HTT genotype by age of problem drinking onset.
Methodologically, measurement of cue-induced propensity to drink can be elicited in the human laboratory, using a conditioned-appetitive motivational model (Childress et al., 1988; Meyer, 1988; Niaura et al., 1988), whereby anticipatory cravings are provoked by the presentation of visual, olfactory, and tactile conditioned stimuli (Kaplan et al., 1985; Walitzer and Sher, 1990) that have been associated with alcohol consumption.
Of course, a study of the expression of 5-HTT genotype would be incomplete without examining the effects of manipulating 5-HT function directly. One pharmacological strategy for manipulating brain 5-HT function directly is to deplete the brain of 5-HT’s precursor, tryptophan (Delgado et al., 1991; Neumeister, 2003; Young and Leyton, 2002), through a procedure described as tryptophan depletion. Tryptophan depletion involves the administration of a drink that is rich in large neutral amino acids, which compete preferentially with tryptophan for entry into the brain and subsequent synthesis of 5-HT. Tryptophan depletion can lead to profound decreases in plasma and cerebrospinal fluid (CSF) tryptophan levels and decreased CSF levels of the 5-HT metabolite, 5-HIAA (Carpenter et al., 1998; Salomon et al., 2003; Williams et al., 1999). Therefore, tryptophan depletion can be used as a pharmacological probe to manipulate the differences in the functional expression of 5-HTT genotype.
Acute peripheral depletion of tryptophan would be expected to reduce tryptophan transport into the brain and lower extracellular 5-HT. It is, therefore, reasonable for us to hypothesize further that, as a counter-regulatory response, this transient decrease in extracellular 5-HT would reduce autoreceptor inhibition, thereby increasing 5-HT neuronal firing transiently and lowering the craving for alcohol.
Therefore, our second aim was to examine whether acute tryptophan depletion would alter cue-induced propensity to drink among alcohol-dependent individuals who vary in 5-HTT genotype and age of problem drinking onset.
MATERIALS AND METHODS
Participants
Thirty-four male and female alcohol-dependent individuals (aged between 21 and 65 yr) were enrolled in the study. All participants were Hispanic and in good health, as determined by a complete physical examination, electrocardiogram, and laboratory screening tests within an acceptable range. Only one female participant was enrolled, and she was not pregnant at intake. She was instructed to use an acceptable form of contraception, which included oral contraceptive pill, barriers and spermicide, or hormonal implants. Men drank ≥21 standard drinks/wk whereas women drank ≥14 standard drinks/wk in the 30 d prior to enrollment; these data were collected using the timeline follow-back method (Sobell and Sobell, 1992). All participants were recruited and studied at the University Clinical Psychopharmacology Laboratory at University Hospital in San Antonio, Texas.
Using the Structured Clinical Interview for DSM-IV (First et al., 1994), we excluded individuals seeking treatment for alcohol dependence and those with a current axis I Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association, 1994) diagnosis of mental illness other than alcohol and nicotine dependence. Also, we excluded individuals with a lifetime history of major depression, panic disorder, bipolar disorder, or eating disorder or a positive parental history of major depression. All participants were otherwise currently healthy as determined by history, complete physical examination, and electrocardiogram.
Experimental Design and General Procedures
The study design was a 3×2×2 mixed factorial design accounting for three genotype groups (LL, SS, and LS individuals balanced for age and drinking level) and the two-factor within-participant combination of the tryptophan manipulation (sham drink or amino acid drink) and the presentation of alcohol vs. neutral cues. Each participant had four test sessions, in which the 2×2 factorial combinations of tryptophan and cue conditions were varied in an order determined by a Latin Square design that counterbalanced the sequence of the four experimental conditions. Each session was separated by at least 24 h to allow tryptophan reserves to be replenished. Both participants and staff were blinded as to the genotype and tryptophan condition. Testing was preceded by a familiarization day to train participants on the rating scales and expose them to the experimental environment. Therefore, all participants resided on the inpatient unit for a minimum of 8 d to participate in this study.
All participants admitted to our inpatient research unit had a negative urine drug screen and a breath alcohol level of 0 at check-in and prior to each experimental session. Participants were not allowed to proceed with testing if these criteria were not met. On the morning of each test day, participants were asked to consume either the amino acid drink or the sham beverage. Subjective and physiological measures of craving were collected at the following scheduled intervals throughout the experiment: thrice prior to cue exposure (t1, t2, and t3) and twice after the cue exposure (t4 and t5). Time points t1, t2, and t3 occurred at 400 min, 120 min, and 5 min, respectively, before the cue exposure. Time points t4 and t5 occurred just after the cue exposure and 30 min later, respectively. Exposure to the cues took place 5.5 h after consumption of the sham or Tryp–beverage, which allowed sufficient time for alterations in tryptophan availability to occur.
Cue Craving Method
Alcohol cue exposure procedure
During the initial screening, we determined each participant’s choice of alcoholic drink (i.e. beer, liquor, or wine). At the time of the cue exposure, participants were seated in a private room. Then, they were connected to a Spacelabs Ultraview® 1050, Module 90496 cardiac monitor (Spacelabs Medical Inc., Issaquah, WA, USA) for measurement of heart rate and blood pressure. At that time, a can or bottle of the participant’s preferred drink was presented under a cloth, along with a glass and a medicine cup containing 2 ml of the drink. At the beginning of the cue exposure, the cloth was removed. Next, the participant was instructed to pick up the can or bottle, open it, pour a small amount into the glass, and hold the glass against his or her chin to smell the drink. Participants were asked to pour the contents of the medicine cup into their mouth and swirl it around before swallowing. Objective measures (i.e. blood pressure and heart rate) and subjective measures of craving were collected. At the end of the session, participants were allowed to consume the drink to avoid habituation and extinction to the craving cues. The alcohol cue exposure task took approximately 5 min to perform.
Neutral cue exposure procedure
This was similar to the procedure for the alcohol cue exposure described above except that participants were asked to choose between iced tea and lemonade during screening and the beverage of their choice was given during the session.
Tryptophan Depletion Method
Consumption of a tryptophan-depleted beverage (see details below) results in preferential uptake of large neutral amino acids, thereby reducing the availability of tryptophan in the brain. Tryptophan depletion causes about a 30% reduction in central 5-HT levels as estimated by CSF levels of 5-HIAA (Carpenter et al., 1998). Generally, however, tryptophan depletion studies have shown varying depletion effects that range from −33% to −94%, depending upon the measure being used (Benkelfat et al., 1994; Carpenter et al., 1998; Ellenbogen et al., 1996; Kaye et al., 2000; Park et al., 1994; Porter et al., 2000; Weltzin et al., 1995; Williams et al., 1999; Young et al., 1985, 1989). Tryptophan depletion has been used widely as a research tool for uncovering underlying depression in those with a personal or family history, while individuals without a personal or family history of depression do not show significant reductions in mood (Moreno et al., 1999). It also has been used in alcoholics undergoing alcohol cue exposure to test the effect of tryptophan depletion on mood and urge to drink (Pierucci-Lagha et al., 2004).
Tryptophan-depleted (Tryp–) beverage
The Tryp– beverage contained L-isoleucine (4.2 g), L-leucine (6.6 g), L-lysine (4.8 g), L-phenylalanine (6.6 g), L-threonine (3.0 g), and L-valine (4.8 g). To make the mixture more palatable, some grapefruit concentrate was added. L-methionine (1.5 g) was given separately in gelatin capsule because of its sour taste.
Sham beverage
The sham beverage contained a mixture of cornstarch and grapefruit concentrate. One capsule containing only cornstarch was provided to match the administration of L-methionine for the Tryp– beverage procedure. This ensured that participants and staff remained blind to the beverage- and pill-taking condition.
End of experiments
At the end of each experimental session, participants were provided with a meal rich in proteins that included tryptophan.
Craving Instruments
Subjective measures
Visual analogue scales (VAS) of alcohol craving were adapted from the Tiffany Craving Questionnaire. We modified this self-report scale to assess alcohol craving in our laboratory. Such an adaptation has been described previously (Petrakis et al., 2004). The VAS of interest included 100-mm lines with adjectival descriptions of “I have an urge for a drink” and “I crave a drink right now” that were anchored on the left by “not at all” and on the right by “extremely”.
Physiological measures
Assessments of pulse and blood pressure.
Age of Problem Drinking Onset
Classifying alcoholics into clinically meaningful subtypes provides better delineation of the psychopathological factors most associated with the manifestations and course of the disease. Of these classification systems, age of onset of problem drinking (Buydens-Branchey et al., 1989) has emerged as an important factor for segregating subtypes of alcoholics. Briefly, there are at least two types of alcoholics (Babor et al., 1992; Brown et al., 1994; Litt et al., 1992; Tarter et al., 1977). Early-onset alcoholics develop problem drinking during youth (i.e. <25 yr), experience severe behavioral problems, and have a high familial or biological propensity toward alcoholism. In contrast, late-onset alcoholics develop drinking problems in adulthood (i.e. ≥25 yr), often as a consequence of psychosocial stressors, and have little familial or genetic predisposition to alcoholism. For this study, age of problem drinking onset, which we used as a continuous variable, was derived from the answer to the following question on the Structured Clinical Interview for DSM-IV (First et al., 1994): “At what age did alcohol drinking become a problem for you?” Early-onset alcoholics were those who started their problem drinking before the age of 25 years.
Ethical Approval
Ethical approval was provided by the institutional review board at The University of Texas Health Science Center at San Antonio.
Statistical Analysis
The general timeline for the assessments was as follows: t1 to t3 (pre-cue), t4 (cue), and t5 (post-cue). Cue exposure occurred 5 min after time point t3. The VAS (i.e. “I have an urge for a drink” and “I crave a drink right now”) were administered at all time points described above for the four different sessions. The VAS measures were described as their mean and standard error or 95% confidence interval. For the VAS, we selected two outcomes: the difference between the after-cue time points (t4 to t5) and the before-cue time points (t1 to t3) (i.e. post-cue minus pre-cue scores); t4 to t5 represents the average VAS craving score after the cue exposure, and t1 to t3 represents the average VAS craving score before the exposure. The formula (t4 to t5) – (t1 to t3) represents the change from baseline. A mixed-model (SAS® PROC MIXED; SAS Institute Inc., Cary, NC, USA) approach was used to study the effects of treatment, genotype, and cue on the two outcomes for the VAS. The analytical model was adjusted for covariates that included the participant’s age of problem drinking onset, average drinking level in the 90 d prior to enrollment, and alcohol severity prior to enrollment measured on the subscales of severity and frequency of withdrawal and delirium tremens on the addiction severity index (McLellan et al., 1980). The correlation structure for the repeated measures (i.e. four different sessions) was taken to be AR(1). Interactions of treatment (i.e. sham or Tryp–), genotype, and cue first were included in the model but were excluded from the final model if they were not significant.
Physiological measures (i.e. pulse, systolic pressure, and diastolic pressure) were described as their mean and standard error or 95% confidence interval. The general statistical approach was similar to that for the VAS.
Because of limited sample sizes in this study, and as a data reduction technique, contrasts on genotype were simplified to comparisons between the L-carriers and those with the SS genotype. This simplified model for genetic analysis is further justified by our previous findings showing that L-carriers differed primarily from SS homozygotes (Johnson et al., 2008).
All analyses were carried out using SAS® 9.1 (SAS Institute Inc.).
RESULTS
This human laboratory study included 34 participants. Twelve of them had the LL genotype, 9 had SS, and 13 had LS. All were male except for one female, who had the LS genotype. Their mean±SD age, mean±SD age of problem drinking onset, and mean±SD baseline drinking levels were as follows: LL (33.6±7.8 yr, 19.6±7.0 yr, and 11.6±7.4 drinks/d, respectively), SS (38.3±8.8 yr, 21.8±5.7 yr, and 8.3±2.3 drinks/d, respectively), and LS (33.5±8.5 yr, 22.7±5.8 yr, and 5.8±1.7 drinks/d, respectively).
The experiments were conducted safely with 24-h nurse monitoring. There was no report of alcohol withdrawal symptoms in any of the participants. All participants tolerated well their drinks, and no one experienced any adverse events. With the tryptophan depletion method described above, we confirmed a tryptophan depletion rate of 71% in 5.5 h based on measurement of plasma tryptophan level (data not shown). Although testing days were only separated by 24 h, baseline total tryptophan levels were not affected irrespective of the order of the tryptophan depletion or placebo sessions that preceded the measurement. More specifically, at the end of the study, there was an increase of 2.48 points in the total tryptophan level when compared with baseline, which constitutes a percentage change from baseline of +6%. When we looked at the total tryptophan value in the sessions that were preceded by tryptophan depletion, we found an average increase in total tryptophan of 1.42±6.28 after allowing only 1 day between the two sessions for the tryptophan level to replenish.
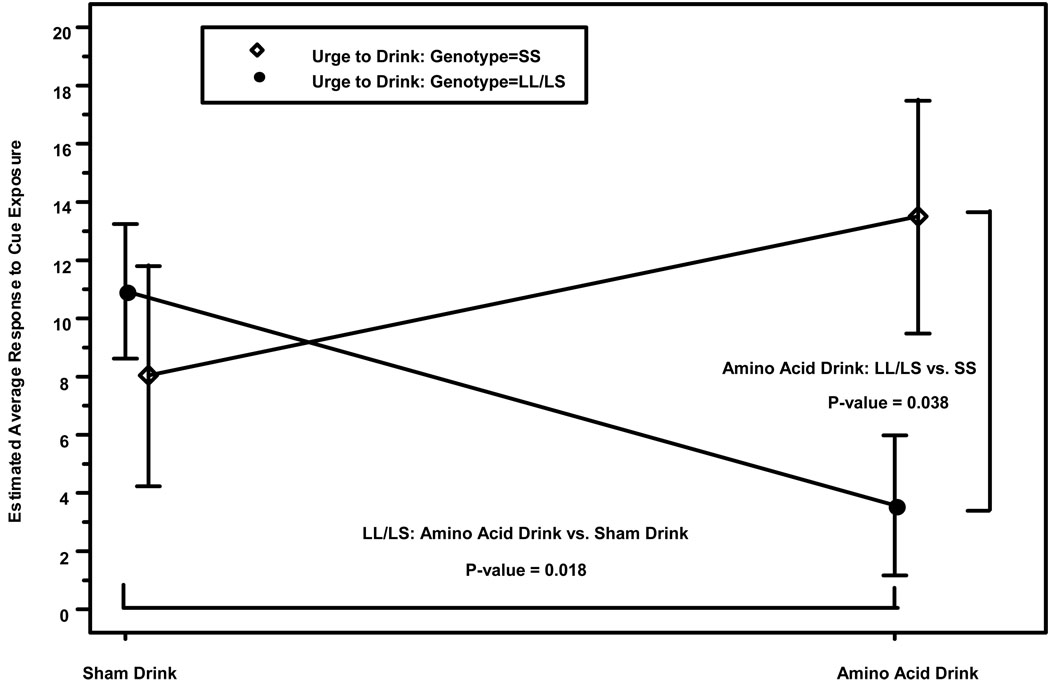
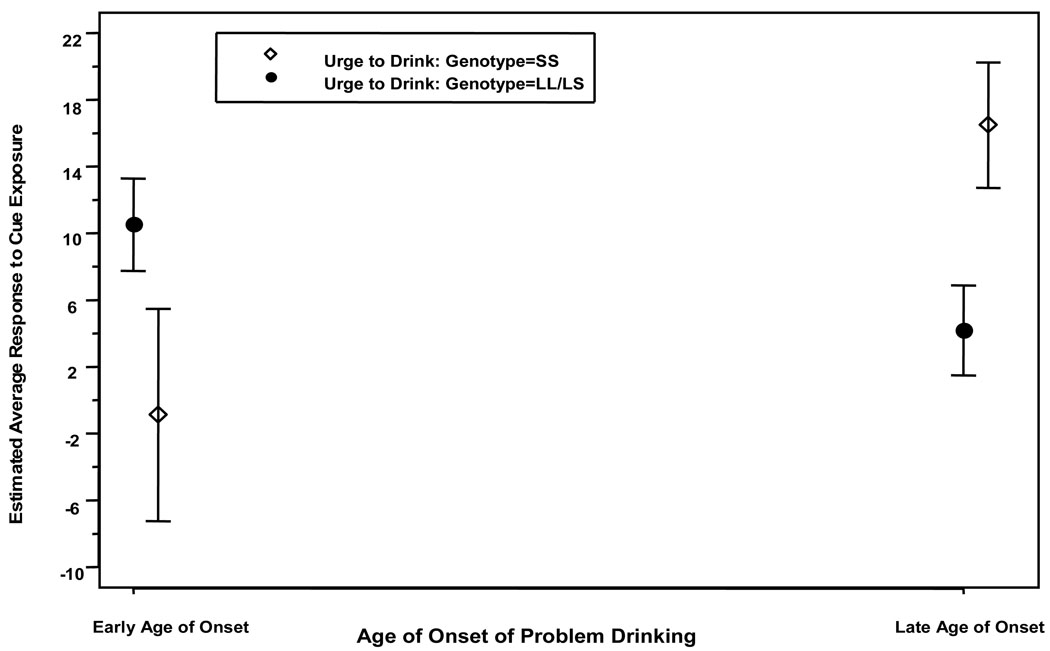
Table 1 shows that on “urge to drink”, there was a significant (all p values <0.05) main effect of genotype, cue, and age of problem drinking onset, and an interaction between genotype and the treatment and age of problem drinking onset variables. The contrasts for “urge to drink” showed that L-carriers, compared with their SS counterparts, had higher scores (12.89±95% CI 1.15 to 24.63; p=0.03), and the alcohol cue elicited greater scores compared with the neutral cue (7.20±95% CI 2.07 to 12.34; p=0.007). For the interactions (Figure 1), L-carriers had lower “urge to drink” scores during tryptophan depletion compared with the normal tryptophan condition (−7.36±95% CI −13.43 to −1.29; p=0.018), but the tryptophan depletion effects were not significant for those with the SS genotype. Furthermore, during the completion of tryptophan depletion, L-carriers, compared with their SS counterparts, had lower “urge to drink” scores (−9.91±95% CI −19.26 to −0.56; p=0.038). The direction of the genotype-by-age-of-problem-drinking-onset interaction was for lower age of problem drinking onset to be associated with increased “urge to drink” among L-carriers but not among those with the SS genotype, who showed the opposite effect (Figure 2).
Table 1.
Analysis of variance table for the mean difference between time points post-cue (i.e. t4 to t5) vs. pre-cue (i.e. t1 to t3)
| “Urge to Drink” | “Crave for a Drink” | |||
|---|---|---|---|---|
| F value | p value | F value | p value | |
| Treatment (Tryp–) vs. | 0.11 | 0.75 | 0.25 | 0.62 |
| placebo (sham drink) | ||||
| Genotype | 5.01 | 0.032 | 4.31 | 0.045 |
| Genotype by treatment | 4.90 | 0.030 | 2.74 | 0.10 |
| Cue (alcohol vs. neutral) | 7.79 | 0.007 | 7.16 | 0.009 |
| Age of problem drinking | 4.51 | 0.042 | 1.22 | 0.13 |
| onset | ||||
| Genotype by age of | 14.31 | 0.007 | 10.17 | 0.003 |
| problem drinking onset | ||||
Figure 1.
Effect of genotype on the mean difference ± standard error in “urge to drink” for response to cue exposure. Estimated average cue exposure response is calculated using the following formula: (t4 to t5) – (t1 to t3), or post-cue minus pre-cue craving scores between the alcohol and control cues, which represents the change from baseline both with and without tryptophan depletion.
Figure 2.
Effect of genotype on the mean difference ± standard error on “urge to drink” for response to cue exposure. Estimated average cue exposure response is calculated using the following formula: (t4 to t5) – (t1 to t3), or post-cue minus pre-cue craving scores between the alcohol and control cues, which represents the change from baseline by age of problem drinking onset. Participants comprised: 11 LL early-onset alcoholics (EOAs), 1 LL late-onset alcoholic (LOA), 6 SS EOAs, 3 SS LOAs, 9 LS EOAs, and 4 LS LOAs. This figure is shown to describe the direction of the interaction between genotype and age of problem drinking onset. Inferential statistics were conducted using age of problem drinking onset as a continuous, not a dichotomous, variable. The purpose of the figure dichotomizing early onset from late onset is for visual display only.
Table 1 also shows that on “crave for a drink”, there was a significant (all p values <0.05) main effect of genotype and cue but not age of problem drinking onset, as well as an interaction between genotype and age of problem drinking onset. The contrasts for “crave for a drink” showed that L-carriers, compared with their SS counterparts, had higher craving scores (12.11±95% CI 0.26 to 23.96; p=0.04) and that the alcohol cue elicited greater scores compared with the neutral cue (7.18±95% CI 1.84 to 12.51; p=0.009). The direction of the genotype-by-age-of-problem-drinking-onset interaction was for lower age of problem drinking onset to be associated with increased “crave for a drink” among L-carriers but not among those with the SS genotype (data not shown).
Figure 1 shows a significant main effect (p<0.05) of genotype on the mean difference in “urge to drink” after cue exposure as well as an interaction between genotype and tryptophan condition. L-carriers had higher craving than their SS counterparts under the normal physiological condition, an effect that was reversed under the tryptophan depletion condition. Figure 2 shows a significant (p<0.05) genotype-by-age-of-problem-drinking-onset interaction on “urge to drink” and “crave for a drink”. L-carriers who started their problem drinking earlier than 25 years of age had higher craving than their SS counterparts with the same age of onset of problem drinking.
On the physiological measure of pulse, there was a significant main effect (p<0.05) only on genotype, and there were no interactions. The contrast for pulse showed that L-carriers, compared with their SS counterparts, had higher rates on the mean difference before and after the cue presentation (2.35±95% CI 0.04 to 4.67; p=0.047). There were no significant main effects or interactions on any other physiological measures.
DISCUSSION
As predicted by our hypotheses, alcohol-dependent individuals who were L-carriers, compared with their counterparts with the SS genotype, had higher scores on both subjective craving measures, an effect that increased with lower age of problem drinking onset. Furthermore, on “urge to drink”, L-carriers, compared with their SS counterparts, had lower (i.e. improved) scores following tryptophan depletion. A higher pulse rate in response to the presentation of the cues was associated with being an L-carrier compared with having the SS genotype. We, therefore, propose that there is an important 5-HTT gene-by-environment interaction that alters cue craving for alcohol.
Our findings may have at least two potential consequences. First, we expect that because alcohol-dependent L-carriers appear to have relatively low 5-HT neurotransmission, this would result in post-synaptic receptor upregulation. Thus, we would hypothesize that a 5-HT3 post-synaptic receptor blocker such as ondansetron would be an efficacious treatment for this type of alcoholic. Second, we also would hypothesize that chronic treatment with a selective reuptake inhibitor would exacerbate craving among L-carriers with alcohol dependence. This is because, even though a selective reuptake inhibitor might “normalize” 5-HT firing rate and 5-HT release, the accumulated effect would be a further marked reduction in 5-HTT density and function, a loss of 5-HTT from the cell surface, and increased activation of post-synaptic 5-HT receptors (Owens and Nemeroff, 2003).
We hypothesize further that the reduction in “urge to drink” among alcohol-dependent L-carriers following acute tryptophan depletion would probably be transient and that a prolonged reduction in tryptophan availability would eventually result in reduced 5-HT turnover and neurotransmission and would rebound to higher levels of craving. Therefore, tryptophan depletion is unlikely to be of any clinical value as a treatment strategy but can serve as a pharmacological probe with which to understand further the neurobiology of the 5-HT system.
There are six important caveats that need to be considered in the evaluation of our results. First, this is a relatively small-scale pharmacogenetic study, and we had insufficient statistical power to detect other important group differences. Second, because the frequency of early- vs. late-onset alcoholism may not be distributed equally between 5-HTT genotypes in the general population and we did not oversample, we did not achieve an equal balance between early- and late-onset alcoholics, which could have enhanced the observed interaction with genotype or led to additional interactions being detected. Third, due to the multiple presentations of the cues, some habituation might have occurred, thereby limiting the size of the differences between groups that we observed. Fourth, due to the inclusion of only one female in the study, we could not examine for gender effects. Fifth, our human laboratory experiment was done in a controlled environment. Thus, further studies are needed to understand how our findings might translate to a more naturalistic clinical setting. Finally, although low 5-HT neurotransmission in LL alcoholics is hypothesized, we did not measure directly serotonergic transmission. Future studies should include functional neuroimaging such as single photon emission computerized tomography to corroborate some of the findings.
While our genotyping strategy included only the L and S alleles for the 5-HTTLPR of the 5-HTT gene (SLC6A4), we are well aware of the functional single nucleotide polymorphism rs25531 (A/G) located within the 5-HTTLPR of the SLC6A4 gene (Hu et al., 2006) and of the previous studies conducted with combined 5-HTTLPR-L/S and rs25531 alleles. Although such an analysis with combined 5-HTTLPR-L/S and rs25531 polymorphisms may have better characterized the SLC6A4 genetic effects on 5-HTT expression differences, given our relatively small sample size, we were not able to include rs25531 polymorphisms in our analyses with sufficient statistical power.
Nevertheless, an important strength of our study is that it was prospective, parametric, and an evidence-based approach to delineate the effects of 5-HTT genotype and age of problem drinking onset on the cue response to alcohol in alcohol-dependent individuals. Furthermore, the study with tryptophan depletion enables us to provide an additional neuropharmacological rationale as to why different types of serotonergic agents might have varying clinical effects among alcohol-dependent individuals who are L-carriers compared with their SS counterparts.
Acknowledgments
We are grateful to the National Institute on Alcohol Abuse and Alcoholism for their generous support through grants 7 U10 AA011776-10, 1 N01 AA001016-000, 2 R01 AA010522-13, 5 R01 AA013964-05, 5 R01 AA014628-04, and 5 R01 AA012964-06 awarded to Prof. Bankole A. Johnson and grant 5 K23 AA000329-06 awarded to Dr. Nassima Ait-Daoud. We also thank Robert H. Cormier, Jr. for his assistance with manuscript preparation. The authors are entirely responsible for the scientific content of this paper.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Babor TF, Dolinsky ZS, Meyer RE, Hesselbrock M, Hofmann M, Tennen H. Types of alcoholics: concurrent and predictive validity of some common classification schemes. Br J Addict. 1992;87:1415–1431. doi: 10.1111/j.1360-0443.1992.tb01921.x. [DOI] [PubMed] [Google Scholar]
- Balldin J, Berggren U, Engel J, Eriksson M. Neuroendocrine evidence for reduced serotonergic neurotransmission during heavy drinking. Alcohol Clin Exp Res. 1994;18:822–825. doi: 10.1111/j.1530-0277.1994.tb00045.x. [DOI] [PubMed] [Google Scholar]
- Benkelfat C, Ellenbogen MA, Dean P, Palmour RM, Young SN. Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective disorders. Arch Gen Psychiatry. 1994;51:687–697. doi: 10.1001/archpsyc.1994.03950090019003. [DOI] [PubMed] [Google Scholar]
- Brown J, Babor TF, Litt MD, Kranzler HR. The type A/type B distinction: subtyping alcoholics according to indicators of vulnerability and severity. Ann N Y Acad Sci. 1994;708:23–33. doi: 10.1111/j.1749-6632.1994.tb24695.x. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey MH, Noumair D. Age of alcoholism onset. I. Relationship to psychopathology. Arch Gen Psychiatry. 1989;46:225–230. doi: 10.1001/archpsyc.1989.01810030031004. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Anderson GM, Pelton GH, Gudin JA, Kirwin PD, Price LH, Heninger GR, McDougle CJ. Tryptophan depletion during continuous CSF sampling in healthy human subjects. Neuropsychopharmacology. 1998;19:26–35. doi: 10.1016/S0893-133X(97)00198-X. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O'Brien CP. Classically conditioned responses in opioid and cocaine dependence: a role in relapse? NIDA Res Monogr. 1988;84:25–43. [PubMed] [Google Scholar]
- Delgado PL, Price LH, Miller HL, Salomon RM, Licinio J, Krystal JH, Heninger GR, Charney DS. Rapid serotonin depletion as a provocative challenge test for patients with major depression: relevance to antidepressant action and the neurobiology of depression. Psychopharmacol Bull. 1991;27:321–330. [PubMed] [Google Scholar]
- Ellenbogen MA, Young SN, Dean P, Palmour RM, Benkelfat C. Mood response to acute tryptophan depletion in healthy volunteers: sex differences and temporal stability. Neuropsychopharmacology. 1996;15:465–474. doi: 10.1016/S0893-133X(96)00056-5. [DOI] [PubMed] [Google Scholar]
- Esterling LE, Yoshikawa T, Turner G, Badner JA, Bengel D, Gershon ES, Berrettini WH, Detera-Wadleigh SD. Serotonin transporter (5-HTT) gene and bipolar affective disorder. Am J Med Genet. 1998;81:37–40. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0) New York: New York State Psychiatric Institute, Biometrics Research Department; 1994. [Google Scholar]
- Greenberg BD, Tolliver TJ, Huang SJ, Li Q, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am J Med Genet. 1999;88:83–87. [PubMed] [Google Scholar]
- Heils A, Mossner R, Lesch KP. The human serotonin transporter gene polymorphism — basic research and clinical implications. J Neural Transm. 1997;104:1005–1014. doi: 10.1007/BF01273314. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger DR. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 2000;47:643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javors MA, Seneviratne C, Roache JD, Ait-Daoud N, Bergeson SE, Walss-Bass MC, Akhtar FZ, Johnson BA. Platelet serotonin uptake and paroxetine binding among allelic genotypes of the serotonin transporter in alcoholics. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:7–13. doi: 10.1016/j.pnpbp.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA. Serotonergic agents and alcoholism treatment: rebirth of the subtype concept — an hypothesis. Alcohol Clin Exp Res. 2000;24:1597–1601. [PubMed] [Google Scholar]
- Johnson BA, Javors MA, Roache JD, Seneviratne C, Bergeson SE, Ait-Daoud N, Dawes MA, Ma JZ. Can serotonin transporter genotype predict serotonergic function, chronicity, and severity of drinking? Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:209–216. doi: 10.1016/j.pnpbp.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RF, Cooney NL, Baker LH, Gillespie RA, Meyer RE, Pomerleau OF. Reactivity to alcohol-related cues: physiological and subjective responses in alcoholics and nonproblem drinkers. J Stud Alcohol. 1985;46:267–272. doi: 10.15288/jsa.1985.46.267. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Gendall KA, Fernstrom MH, Fernstrom JD, McConaha CW, Weltzin TE. Effects of acute tryptophan depletion on mood in bulimia nervosa. Biol Psychiatry. 2000;47:151–157. doi: 10.1016/s0006-3223(99)00108-0. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biol Psychiatry. 1994;36:326–337. doi: 10.1016/0006-3223(94)90630-0. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Greenberg BD, Higley JD. In: Serotonin transporter, personality, and behavior: toward dissection of gene-gene and gene-environment interaction, in Molecular Genetics and the Human Personality. Benjamin J, Ebstein RP, Belmaker RH, editors. Washington, DC: American Psychiatric Publishing; 2002. pp. 109–136. [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Litt MD, Babor TF, DelBoca FK, Kadden RM, Cooney NL. Types of alcoholics, II. Application of an empirically derived typology to treatment matching. Arch Gen Psychiatry. 1992;49:609–614. doi: 10.1001/archpsyc.1992.01820080017003. [DOI] [PubMed] [Google Scholar]
- Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, DelProposto ZS, Hill E, Cassin BJ, Watson SJ, Cook EH. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am J Psychiatry. 1998;155:207–213. doi: 10.1176/ajp.155.2.207. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Meyer RE. Conditioning phenomena and the problem of relapse in opioid addicts and alcoholics. NIDA Res Monogr. 1988;84:161–179. [PubMed] [Google Scholar]
- Moreno FA, Gelenberg AJ, Heninger GR, Potter RL, McKnight KM, Allen J, Phillips AP, Delgado PL. Tryptophan depletion and depressive vulnerability. Biol Psychiatry. 1999;46:498–505. doi: 10.1016/s0006-3223(99)00095-5. [DOI] [PubMed] [Google Scholar]
- Neumeister A. Tryptophan depletion, serotonin, and depression: where do we stand? Psychopharmacol Bull. 2003;37:99–115. [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Neuropharmacology of paroxetine. Psychopharmacol Bull. 2003;37 Suppl 1:8–18. [PubMed] [Google Scholar]
- Park SB, Coull JT, McShane RH, Young AH, Sahakian BJ, Robbins TW, Cowen PJ. Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology. 1994;33:575–588. doi: 10.1016/0028-3908(94)90089-2. [DOI] [PubMed] [Google Scholar]
- VA Naltrexone Study Collaboration Group. Petrakis IL, O'Malley S, Rounsaville B, Poling J, McHugh-Strong C, Krystal JH. Naltrexone augmentation of neuroleptic treatment in alcohol abusing patients with schizophrenia. Psychopharmacology. 2004;172:291–297. doi: 10.1007/s00213-003-1658-9. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Feinn R, Modesto-Lowe V, Swift R, Nellissery M, Covault J, Kranzler HR. Effects of rapid tryptophan depletion on mood and urge to drink in patients with co-morbid major depression and alcohol dependence. Psychopharmacology. 2004;171:340–348. doi: 10.1007/s00213-003-1588-6. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Lunn BS, Walker LL, Gray JM, Ballard CG, O'Brien JT. Cognitive deficit induced by acute tryptophan depletion in patients with Alzheimer's disease. Am J Psychiatry. 2000;157:638–640. doi: 10.1176/appi.ajp.157.4.638. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U S A. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon RM, Kennedy JS, Johnson BW, Schmidt DE, Kwentus J, Gwirtsman HE, Ebert MH. Association of a critical CSF tryptophan threshold level with depressive relapse. Neuropsychopharmacology. 2003;28:956–960. doi: 10.1038/sj.npp.1300098. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. In: Timeline follow-back: a technique for assessing self-reported alcohol consumption, in Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Litten RZ, Allen JP, editors. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Tarter RE, McBride H, Buonpane N, Schneider DU. Differentiation of alcoholics: childhood history of minimal brain dysfunction, family history, and drinking pattern. Arch Gen Psychiatry. 1977;34:761–768. doi: 10.1001/archpsyc.1977.01770190023002. [DOI] [PubMed] [Google Scholar]
- Walitzer KS, Sher KJ. Alcohol cue reactivity and ad lib drinking in young men at risk for alcoholism. Addict Behav. 1990;15:29–46. doi: 10.1016/0306-4603(90)90005-i. [DOI] [PubMed] [Google Scholar]
- Weltzin TE, Fernstrom MH, Fernstrom JD, Neuberger SK, Kaye WH. Acute tryptophan depletion and increased food intake and irritability in bulimia nervosa. Am J Psychiatry. 1995;152:1668–1671. doi: 10.1176/ajp.152.11.1668. [DOI] [PubMed] [Google Scholar]
- Williams WA, Shoaf SE, Hommer D, Rawlings R, Linnoila M. Effects of acute tryptophan depletion on plasma and cerebrospinal fluid tryptophan and 5-hydroxyindoleacetic acid in normal volunteers. J Neurochem. 1999;72:1641–1647. doi: 10.1046/j.1471-4159.1999.721641.x. [DOI] [PubMed] [Google Scholar]
- Young SN, Leyton M. The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels. Pharmacol Biochem Behav. 2002;71:857–865. doi: 10.1016/s0091-3057(01)00670-0. [DOI] [PubMed] [Google Scholar]
- Young SN, Smith SE, Pihl RO, Ervin FR. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology. 1985;87:173–177. doi: 10.1007/BF00431803. [DOI] [PubMed] [Google Scholar]
- Young SN, Ervin FR, Pihl RO, Finn P. Biochemical aspects of tryptophan depletion in primates. Psychopharmacology. 1989;98:508–511. doi: 10.1007/BF00441950. [DOI] [PubMed] [Google Scholar]