Greater than 80% of the world's new cases and deaths due to cervical cancer occur in the developing world [1]. No more than 5% of women in these settings are screened for cervical cancer even once in their lifetimes [2]. Earlier attempts to establish population-based cervical cancer prevention programs using cytology screening in resource-limited settings have inevitably fallen short or failed [3–5]. Although many of the reasons for failure can be attributed to lack of resources and trained manpower, the multiple visit requirements of cytology-based screening programs jeopardizes success and sustainability.
HIV infection is associated with higher incidence, more rapid progression, and increased recurrence rates of human papillomavirus (HPV)-associated cervical intraepithelial neoplasia (CIN) and invasive cervical cancer, an AIDS-defining disease [6–14]. The last decade has seen a global push for increasing access to affordable antiretroviral therapy (ART) for HIV-infected individuals in the developing world. Programs such as the US President's Emergency Plan for AIDS Relief (PEPFAR) and the Global Fund to Fight AIDS, Tuberculosis, and Malaria, have substantially upgraded healthcare infrastructures in many parts of sub-Saharan Africa and elsewhere at a scale never experienced in history [15,16]. Zambia has one of the world's highest cervical cancer incidence rates (53.7/100 000 women/year) and also a national adult HIV seroprevalence rate of 16% (23% in Lusaka, the capital city) [1,17]. Through PEPFAR-supported programs, greater than 100 000 people (50% women) are now (September 2008) accessing ART in Zambia [18]. This care is largely delivered through partnerships between the public sector, nonprofit organizations, development agencies, and academic institutions.
Through support from the US Centers for Disease Control and Prevention (CDC)-PEPFAR program and other charitable resources, we launched an innovative program for cervical cancer prevention targeting, but not limited to, HIV-infected women in Lusaka, Zambia [19,20]. To our knowledge, this is the first effort within PEPFAR-supported programs to undertake cervical cancer prevention as a component of HIV care. We describe some of the major lessons learned through our experiences in the field operationalization of this program:
-
Integration of the program within preexisting healthcare infrastructures ensures access to the target population and sustainability.
Government-operated clinics provide healthcare for a majority of Lusaka's residents who cannot afford private sector care. We integrated cervical cancer prevention services into these public sector health clinics as a routine healthcare service for women. Significant advantages of integration include access to physical space, preexisting utilities, maintenance and toxic waste disposal services, and other medical/pharmacy services for patients and ease of referral to and from other departments within the health center. Improvements to the infrastructure from our program's funds benefited the general healthcare infrastructure, and vice-versa. Such circumstances facilitated the initiation and roll-out of our program, and soon changed the ‘on the ground’ view of our activities from ‘special project’ to ‘routine service.’
-
Piggybacking on infrastructure established by the HIV/AIDS care and treatment program ensures optimization of resources and additional efficiencies for both programs.
PEPFAR-sponsored HIV care and treatment services supported by our institution (UAB-CIDRZ) were already tightly integrated into 53 government-operated public health clinics throughout Zambia [21]. By collaborating with the HIV care and treatment leadership, we were able to learn from the organizational history of the HIV treatment and care program roll out. Piggybacking on the program's healthcare delivery infrastructure and administrative resources provided us an affordable opportunity to utilize their information technology system, office space, management expertise, data management capabilities, and approaches to problem solving.
-
Shifting of tasks usually performed by physicians to nurses helps to overcome the critical historic barrier of manpower shortage.
The nationwide physician/population density in Zambia is only 0.12 physicians per 1000 population [22], with only nine gynecologists located in the capital city of Lusaka (population two million). On the contrary, nurses are in relative greater availability (reported 8859 nurses compared with 706 doctors in the country in 2005) [23]. Government health officials supported our plan to use female nurses as primary providers because of their certification by the General Nursing Council of Zambia to perform certain low-risk surgical procedures, familiarity with pelvic anatomy and reproductive tract physiology and pathology, ability to perform pelvic examination, acceptance as providers of obstetric and gynecologic healthcare by women and men, and willingness to undergo rigorous training and ongoing quality monitoring and retraining as needed [24].
-
It is essential to choose a prevention modality that is cost-effective and locally appropriate.
Our goal was to select a prevention modality that was low cost, optimally sensitive and specific, acceptable to women and healthcare providers, and cognizant of resource constraints in the Zambian healthcare environment. Our emphasis was on higher population coverage rather than increased frequency of examinations. In Zambia, resources for establishing a patient-recall system are limited. There are no formally trained and certified cytotechnologists, only one full-time pathologist is available for the public sector, and there is no functioning colposcopy facility.
Cervical cancer screening using visual inspection with acetic acid (VIA) has proven to be a realistic and simple intervention that gives on-the-spot results and is suitable for large-scale implementation at a population level. VIA linked with same-visit cryotherapy can be implemented in field settings by trained paramedical health workers, including nurses and midwives. Studies, including community randomized trials, have documented the efficacy, safety, acceptability, and cost-effectiveness of a single-visit see-and-treat methodology based on VIA and same-visit cryotherapy of eligible lesions [25–33].
With this substantial evidence supporting the benefits of single-visit VIA and cryotherapy, the Zambian Ministry of Health endorsed it fully, and we selected it as our principal prevention approach (Figs. 1 and 2). We also developed a referral center (Gynecologic Cancer Prevention Clinic) for performing histopathologic evaluation, using punch biopsy or loop electrosurgical excision procedure (LEEP), for women who were VIA positive, but ineligible for cryotherapy (e.g. lesions occupying >75% of transformation zone, lesions extending into endocervical canal that cannot be completely visualized, the presence of abnormal vasculature, and lesions too large to fit the largest available cryotherapy probe) [19,20]. For women with invasive cancer, we utilized the surgical and radio-therapic facilities already in place at the University Teaching Hospital, Zambia's principal public sector tertiary referral center.
-
Using low-cost digital cervicography as an adjunct to VIA facilitates patient education, documentation of lesions, and monitoring and evaluation of quality of care.
Our nurses use a commercially available digital camera to capture images during the VIA examination (Fig. 1). Projection of the image of the cervix to a bedside monitor/television screen allows: opportunity for immediate education of patients about examination results, printing and documentation in the medical records of the actual results of the visual examination, quality assurance of the treatment decisions made by nurses through a periodic review of images, distance consultation by electronic transmission of digital images between nurses and consultants, and continued education. Through ongoing training and structural changes in the clinics, we ensure a high level of privacy for patients and their data.
-
Training of nurses is an ongoing critical need, and utilizing innovative teaching and continuing education models enhances program effectiveness.
We combined both a training-of-trainers approach and systematic, ongoing monitoring and individual mentoring of nurses to ensure competency-based targets in speculum examination and VIA, visual detection skills, risk communication to patients, and appropriate treatment and referral decisions. All nurses met weekly to review and discuss the digital images captured during the week with a supervising consultant gynecologist (M.H.M. or G.P.P.).
-
Using peer educators as health promotion advocates and patient navigators enhances program uptake and reduces loss to follow-up.
From the experiences of the HIV/AIDS program, we realized the need for a strong community outreach component to deal with myths and misunderstandings surrounding the disease. We selected women from the community with an aptitude for voicing health concerns and trained them for conducting community-based cervical health promotion talks. We engaged an acting troupe to perform sensitizing street dramas in conjunction with the peer educators when possible. The peer educators informed women of free, walk-in/same-day see-and-treat cervical cancer prevention services in their respective clinic catchment areas. Women requesting more information or expressing desires to be screened were ‘navigated’ by the peer educators to the cervical cancer prevention clinics. Women attending cervical cancer prevention clinics who had not been tested for HIV were educated by nurses regarding HPVand HIV transmission and offered HIV counseling and testing services. HIV-seropositive women were escorted to the nearby HIV care/treatment clinic for further evaluation. To minimize concerns of stigma, we nested our screening clinics in the same government-operated public health center near to, but not directly within the HIV clinic. HIV-seronegative women or those not yet aware of their HIV status were offered the same screening and treatment services.
-
Constantly refining program operations, with active community feedback and participation enhances program effectiveness.
Periodic meetings with nurses, peer educators, and community stakeholders were important to get feedback about the impact and community perceptions of the program. On the basis of these suggestions, many changes were made early in the course of program delivery and expansion.
-
Seemingly, daunting resource limitations may be amenable to modern technological solutions.
About 15 months through the program, the increased number of tissue specimens resulting from excisional biopsies at the Gynecologic Cancer Prevention Clinic had exceeded the capacity of the University's pathology department. As a temporary measure, we subsidized supplies and materials. Plans for a centralized automated and digitized pathology consultation are in progress. Another challenge has been an inconsistent supply of compressed gas for cryotherapy. Negotiations are presently ongoing with a local beverage company for their surplus compressed gas. We are working with other partners towards the construction of a manufacturing plant to produce compressed gas in Zambia.
Fig. 1. Nurse performing visual inspection with acetic acid (VIA) with simultaneous digital cervicography.
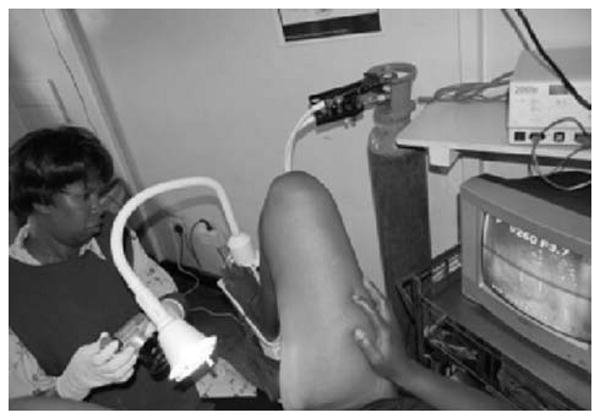
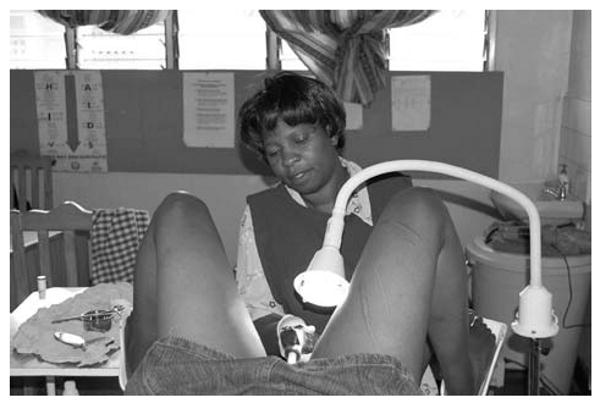
Fig. 2. Nurse performing cryotherapy.
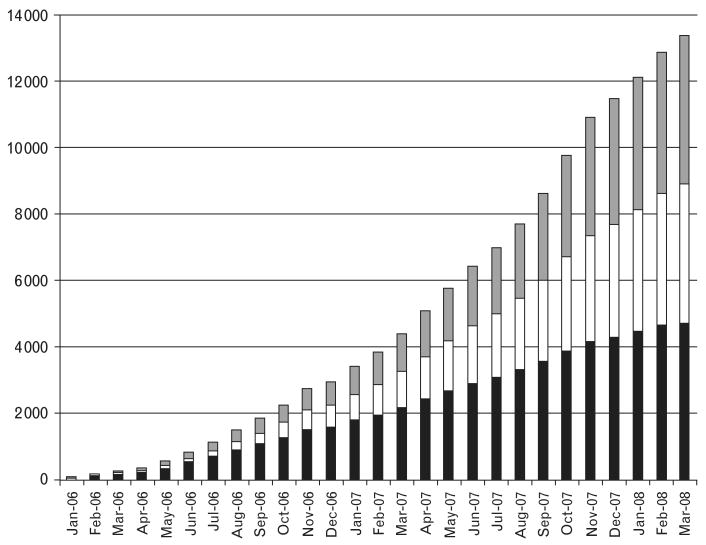
Over the past two and a half years, our program has screened over 20 000 women through services offered in 15 primary care clinics and one referral Gynecologic Cancer Prevention Clinic (Fig. 3). Our experience has shown that linking cervical cancer prevention services with HIV care and treatment services has potentiated the impact of initiatives such as PEPFAR by preventing cervical cancer in women living longer on ART and who have never been screened. We believe that HIV-targeted funds can and should address closely allied health concerns whose neglect may reduce the long-term success of HIV programs. This holds the promise of both saving lives and ensuring the strengthening of the broader primary care context so essential for sustaining HIV programs beyond the PEPFAR/Global Fund era.
Fig. 3. Cumulative enrolment (per month) in the cervical cancer prevention program between January 2006 and March 2008 stratified by HIV serostatus.
 , HIV serostatus unknown; □, HIV seronegative; ■, HIV seropositive.
, HIV serostatus unknown; □, HIV seronegative; ■, HIV seropositive.
Acknowledgments
The authors wish to acknowledge and thank Dr Moses Sinkala, Dr Bushimbwa Tambatamba, Dr Velepi Mtonga, Dr Ann Chao, and Mr Fresher Mapiri for their contributions to the program described herein; and Mr. Alain Degroot (CIDRZ) for his contribution to the data collection system. None of them received compensation.
The program was conceptualized and designed by G.P.P., M.H.M., V.V.S., S.H.V. and J.S.A.S. Clinical/laboratory training was provided by G.P.P., M.H.M., K.S.P., V.M. and M.L.H. Data were analyzed, interpreted, and managed by V.V.S., G.P.P., M.H.M., S.H.V. and J.S.A.S. The manuscript was drafted by M.H.M., V.V.S. and G.P.P., and critical revision of the manuscript for important intellectual content was provided by S.H.V., K.S.P., J.S.A.S., V.M. and M.L.H. Funding for the program was obtained by J.S.A.S. and G.P.P. Administrative and technical support was provided by J.S.A.S., G.P.P. and M.H.M. The program was led and supervised by G.P.P. and M.H.M.
This study was supported by the President's Emergency Plan for AIDS Relief grant through the Elizabeth Glaser Pediatric AIDS Foundation from the Centers for Disease Control and Prevention Global AIDS Program with additional support from the National Institutes of Health (NIH) through the Center for AIDS Research at UAB (NIH grant P30AI027767), the International Clinical Research Scholars/Vanderbilt-UAB AIDS International Training and Research Program (NIH grant D43TW001035 and R24TW007988), Career Development award (NIH grant K23AI01411), and an Elizabeth Glaser Pediatric AIDS Foundation Clinical Scholars Award.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006;20:207–225. doi: 10.1016/j.bpobgyn.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Miller AB, Nazeer S, Fonn S, Brandup-Lukanow A, Rehman R, Cronje H, et al. Report on consensus conference on cervical cancer screening and management. Int J Cancer. 2000;86:440–447. doi: 10.1002/(sici)1097-0215(20000501)86:3<440::aid-ijc22>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 4.Miller AB, Sankaranarayanan R, Bosch FX, Sepulveda C. Can screening for cervical cancer be improved, especially in developing countries? Int J Cancer. 2003;107:337–340. doi: 10.1002/ijc.11388. [DOI] [PubMed] [Google Scholar]
- 5.Monsonego J, Bosch FX, Coursaget P, Cox JT, Franco E, Frazer I, et al. Cervical cancer control, priorities and new directions. Int J Cancer. 2004;108:329–333. doi: 10.1002/ijc.11530. [DOI] [PubMed] [Google Scholar]
- 6.Harris TG, Burk RD, Palefsky JM, Massad LS, Bang JY, Anastos K, et al. Incidence of cervical squamous intraepithe-lial lesions associated with HIV serostatus, CD4 cell counts, and human papillomavirus test results. JAMA. 2005;293:1471–1476. doi: 10.1001/jama.293.12.1471. [DOI] [PubMed] [Google Scholar]
- 7.Heard I, Palefsky JM, Kazatchkine MD. The impact of HIV antiviral therapy on human papillomavirus (HPV) infections and HPV-related diseases. Antivir Ther. 2004;9:13–22. [PubMed] [Google Scholar]
- 8.Heard I, Schmitz V, Costagliola D, Orth G, Kazatchkine MD. Early regression of cervical lesions in HIV-seropositive women receiving highly active antiretroviral therapy. AIDS. 1998;12:1459–1464. doi: 10.1097/00002030-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Heard I, Tassie JM, Schmitz V, Mandelbrot L, Kazatchkine MD, Orth G. Increased risk of cervical disease among human immunodeficiency virus-infected women with severe immunosuppression and high human papillomavirus load (1) Obstet Gynecol. 2000;96:403–409. doi: 10.1016/s0029-7844(00)00948-0. [DOI] [PubMed] [Google Scholar]
- 10.Minkoff H, Ahdieh L, Massad LS, Anastos K, Watts DH, Melnick S, et al. The effect of highly active antiretroviral therapy on cervical cytologic changes associated with oncogenic HPV among HIV-infected women. AIDS. 2001;15:2157–2164. doi: 10.1097/00002030-200111090-00011. [DOI] [PubMed] [Google Scholar]
- 11.Palefsky JM, Minkoff H, Kalish LA, Levine A, Sacks HS, Garcia P, et al. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. J Natl Cancer Inst. 1999;91:226–236. doi: 10.1093/jnci/91.3.226. [DOI] [PubMed] [Google Scholar]
- 12.Parham GP, Sahasrabuddhe VV, Mwanahamuntu MH, Shepherd BE, Hicks ML, Stringer EM, Vermund SH. Prevalence and predictors of squamous intraepithelial lesions of the cervix in HIV-infected women in Lusaka, Zambia. Gynecol Oncol. 2006;103:1017–1022. doi: 10.1016/j.ygyno.2006.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strickler HD, Burk RD, Fazzari M, Anastos K, Minkoff H, Massad LS, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97:577–586. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 14.Tate DR, Anderson RJ. Recrudescence of cervical dysplasia among women who are infected with the human immunodeficiency virus: a case-control analysis. Am J Obstet Gynecol. 2002;186:880–882. doi: 10.1067/mob.2002.123607. [DOI] [PubMed] [Google Scholar]
- 15.Gilks CF, Crowley S, Ekpini R, Gove S, Perriens J, Souteyrand Y, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–510. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 16.United States. Congress House Committee on Foreign Affairs. PEPFAR: an assessment of progress and challenges: hearing before the Committee on Foreign Affairs, House of Representatives. One Hundred Tenth Congress, first session; 24 April 2007; Washington, DC: U.S. G.P.O.; 2007. [Google Scholar]
- 17.Central Intelligence Agency. The World Factbook 2008. Washington, DC, United States: Central Intelligence Agency; 2008. [Google Scholar]
- 18.United States. Congress House Committee on Foreign Affairs. PEPFAR reauthorization: from emergency to sustainability: hearing before the Committee on Foreign Affairs, House of Representatives. One Hundred Tenth Congress, first session; 25 September 2007; Washington, DC: U.S. G.P.O.; 2007. [Google Scholar]
- 19.Pfaendler KS, Mwanahamuntu MH, Sahasrabuddhe VV, Mudenda V, Stringer JS, Parham GP. Management of cryotherapy-ineligible women in a “screen-and-treat” cervical cancer prevention program targeting HIV-infected women in Zambia: lessons from the field. Gynecol Oncol. 2008;110:402–407. doi: 10.1016/j.ygyno.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parham G, Mwanahamuntu M, Pfaendler K, Mkumba G, Sahasrabuddhe V, Hicks M, et al. Building a cervical cancer prevention program into an HIV care and treatment infrastructure. In: Marlink R, Teitelman S, Brandt A, editors. From the ground up: a guide to building comprehensive HIV/AIDS Care Programs in resource-limited settings. Washington, DC: Elizabeth Glaser Pediatric AIDS Foundation; 2008. [Google Scholar]
- 21.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 22.Mullan F, Frehywot S. Nonphysician clinicians in 47 sub-Saharan African countries. Lancet. 2007;370:2158–2163. doi: 10.1016/S0140-6736(07)60785-5. [DOI] [PubMed] [Google Scholar]
- 23.Bowa K. Zambia's health-worker crisis. Lancet. 2008;371:1577–1578. doi: 10.1016/S0140-6736(08)60686-8. [DOI] [PubMed] [Google Scholar]
- 24.Samb B, Celletti F, Holloway J, Van Damme W, De Cock KM, Dybul M. Rapid expansion of the health workforce in response to the HIV epidemic. N Engl J Med. 2007;357:2510–2514. doi: 10.1056/NEJMsb071889. [DOI] [PubMed] [Google Scholar]
- 25.Sankaranarayanan R, Basu P, Wesley RS, Mahe C, Keita N, Mbalawa CC, et al. Accuracy of visual screening for cervical neoplasia: results from an IARC multicentre study in India and Africa. Int J Cancer. 2004;110:907–913. doi: 10.1002/ijc.20190. [DOI] [PubMed] [Google Scholar]
- 26.Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 27.Sankaranarayanan R, Nene BM, Dinshaw KA, Mahe C, Jayant K, Shastri SS, et al. A cluster randomized controlled trial of visual, cytology and human papillomavirus screening for cancer of the cervix in rural India. Int J Cancer. 2005;116:617–623. doi: 10.1002/ijc.21050. [DOI] [PubMed] [Google Scholar]
- 28.Denny L, Kuhn L, De Souza M, Pollack AE, Dupree W, Wright TC., Jr Screen-and-treat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. JAMA. 2005;294:2173–2181. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- 29.Denny L, Kuhn L, Pollack A, Wainwright H, Wright TC., Jr Evaluation of alternative methods of cervical cancer screening for resource-poor settings. Cancer. 2000;89:826–833. doi: 10.1002/1097-0142(20000815)89:4<826::aid-cncr15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahe C, Wright TC. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353:2158–2168. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 31.Goldie SJ, Kuhn L, Denny L, Pollack A, Wright TC. Policy analysis of cervical cancer screening strategies in low-resource settings: clinical benefits and cost-effectiveness. JAMA. 2001;285:3107–3115. doi: 10.1001/jama.285.24.3107. [DOI] [PubMed] [Google Scholar]
- 32.Sankaranarayanan R, Rajkumar R, Esmy PO, Fayette JM, Shanthakumary S, Frappart L, et al. Effectiveness, safety and acceptability of ‘see and treat’ with cryotherapy by nurses in a cervical screening study in India. Br J Cancer. 2007;96:738–743. doi: 10.1038/sj.bjc.6603633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaffikin L, Blumenthal PD, Emerson M, Limpaphayom K. Safety, acceptability, and feasibility of a single-visit approach to cervical-cancer prevention in rural Thailand: a demonstration project. Lancet. 2003;361:814–820. doi: 10.1016/s0140-6736(03)12707-9. [DOI] [PubMed] [Google Scholar]