Abstract
Ischemic heart disease is characterized chronically by a healed infarct, foci of myocardial scarring, cavitary dilation and impaired ventricular performance. These alterations can only be reversed by replacement of scarred tissue with functionally-competent myocardium. We tested whether cardiac progenitor cells (CPCs) implanted in proximity of healed infarcts or resident CPCs stimulated locally by HGF and IGF-1 (GFs) invade the scarred myocardium and generate myocytes and coronary vessels improving the hemodynamics of the infarcted heart. HGF is a powerful chemoattractant of CPCs and IGF-1 promotes their proliferation and survival. Injection of CPCs or GFs led to the replacement of ~42% of the scar with newly-formed myocardium, attenuated ventricular dilation and prevented the chronic decline in function of the infarcted heart. Cardiac repair was mediated by the ability of CPCs to synthesize MMPs that degraded collagen proteins, forming tunnels within the fibrotic tissue during their migration across the scarred myocardium. New myocytes had a 2n karyotype and possessed two sex chromosomes, excluding cell fusion. Clinically, CPCs represent an ideal candidate cell for cardiac repair in patients with chronic heart failure. CPCs may be isolated from myocardial biopsies and, following their expansion in vitro, administered back to the same patients avoiding the adverse effects associated with the use of non-autologous cells. Alternatively, GFs may be delivered locally to stimulate resident CPCs and promote myocardial regeneration. These forms of treatments could be repeated over time to reduce progressively tissue scarring and expand the working myocardium.
Keywords: cardiac-repair, cardiac-stem-cells, chronic-myocardial-infarct
Coronary artery disease, hypertension, idiopathic dilated cardiomyopathy and the unsuccessful repair of a valvular defect lead with time to severe ventricular dysfunction. Cavitary dilation is a common characteristic but the phenotypic architecture and loading of the heart vary significantly among these conditions. If we consider the evolution of the post-infarcted heart, the size of the infarct is not an infallible predictor of the short-, mid- and long-term outcome of the disease. Negative remodeling and accumulation of damage in the spared myocardium may become critical determinants of cardiac decompensation and its progression to terminal failure.1 The number of events differs in the patient population and segmental losses of myocardium are by far more complex to reconstitute than small foci of injury or scattered myocyte death across the wall. Although areas of spontaneous regeneration have been found,2 these regions are minute and do not reduce significantly infarct size. Myocyte formation occurs acutely3 and chronically4 but the addition of new cells is mostly restricted to the viable myocardium.
The identification of cardiac progenitor cells (CPCs) in the adult heart5–10 has challenged the generally accepted but never proven paradigm that the heart is a postmitotic organ, but has not answered the question why CPCs do not reach spontaneously an infarcted area and rebuild the lost myocardium completely or in part. This is not a particular behavior of the heart but a common defect of proliferating organs. In all cases, occlusion of a supplying artery results in infarcts and scar formation in the skin, intestine, liver, spleen, brain and bone marrow.1 Although the administration of CPCs locally or their activation by growth factors regenerates infarcted myocardium acutely,5,8,11 whether the same protocols can rescue old scarred infarcts remains an important unanswered question. This possibility would make cellular therapy more relevant to the management of chronic human heart failure.
Therefore, in this study, rats with a healed myocardial infarct were treated with implantation of clonogenic CPCs or with intramyocardial delivery of hepatocyte growth factor (HGF) and insulin-like growth factor-1 (IGF-1). These growth factors were employed because CPCs express c-Met and IGF-1-receptors and HGF is a powerful chemoattractant of CPCs while IGF-1 promotes their division and survival.8,11
Materials and Methods
Adult rats were injected with CPCs or GFs 20 days after infarction and scar formation. Hearts were evaluated 20-days later to establish the efficacy of these interventions. Experimental protocols are described at http://circres.ahajournals.org.
Results
Myocardial Regeneration
Myocardial infarction was produced in rats and 20-days later animals were randomly injected with EGFP-tagged-clonogenic-CPC s (CPC-treated), HGF and IGF-1 (GF-treated) or saline (untreated). Rats were exposed to BrdU to detect newly formed myocardial structures. In GF-treated rats, BrdU-labeling identified regenerated myocytes and vessels while in CPC-treated animals EGFP and BrdU were both utilized to recognize the CPC progeny. Animals were sacrificed 20-days after treatment.
Employing fluorescein-conjugated HGF we have previously shown that fluorescence intensity is higher adjacent to the infarct than in the distant myocardium up to 1 hour after its injection preserving the expected chemotactic gradient between these two regions of the damaged heart. Most importantly, HGF quantity measured by ELISA 24 hours after infarction was confirmed to be higher in the border than in the remote myocardium.11 Additionally, the effects of HGF and IGF-1 on CPCs were measured biochemically; the autocatalytic phosphorylation of c-Met and IGF-1R, phospho-Akt, phospho-IRS-1 and phospho-FAK increased in CPCs isolated from the infarcted heart 12, 24 and 72 hours following coronary ligation and GF-administration.11
Only animals with infarcts involving more than 45% of left ventricular (LV) myocytes were studied to establish the effects of CPCs and GFs on infarcts associated with LV failure.12 Infarct size averaged 67% in all animals (supplemental Figure I). In treated-rats, the infarcted region showed a thicker wall with a mixture of scarred and new myocardium. Myocardial regeneration was absent in untreated-infarcts; the LV wall was mostly replaced by collagen bundles (Figure 1A through 1C). Infarct thickness was 0.26±0.05, 0.52±0.11 and 0.45±0.08 mm in untreated, CPC-treated and GF-treated rats, respectively. The increased thickness in treated rats was significant (P < 0.001).
Figure 1.
Myocardial regeneration. A: Healed myocardial infarct (collagen accumulation, blue). A thin layer of spared myocytes is present in the subendocardium. B and C: Band of regenerated myocytes within the infarct (MHC: myosin-heavy-chain, red; arrowheads) following GF-activation of resident CPCs (B) or treatment with EGFP-positive-CPCs (C: EGFP, green). The areas included in the rectangles are shown at higher magnification in the adjacent panels.
Regeneration accounted for 50 and 56 mm3 of myocardium, which resulted in a 40±12% and 44±5% recovery of the scar in CPC- and GF-treated rats, respectively. Additionally, the injection of CPCs reduced infarct size by 39%, from 69% to 42%, while GFs decreased infarct dimension by 43%, from 67% to 39% (supplemental Figure I).
Cardiac Anatomy and Ventricular Function
Large myocardial infarcts resulted in a 2.2-fold increase in LV cavitary volume and a 56% decrease in ventricular-mass-to-chamber-volume ratio. Treatment with CPCs or GFs attenuated ventricular dilation by ~21% and the change in ventricular-mass-to-chamber-volume ratio by ~34% (Figure 2A). Ejection fraction (EF) was obtained 2-days before treatment in 18-days old-infarcts, and 14-days after treatment in 34-days old-infarcts. At 18-days, EF was decreased in all animals (Figure 2B). The absence of contraction in the infarcted region was apparent and persisted in untreated-infarcts at 34-days (supplemental Figure II). Conversely, contraction reappeared in treated-infarcts. From 18 to 34-days, treated-animals showed an increase in EF while EF was further reduced in untreated-rats (Figure 2B); the latter was dictated partly by progressive ventricular dilation typical of this model.12 Hemodynamically, treated-infarcts showed a lower increase in LVEDP, and a lower decrease in LVSP, LVDP and dP/dt. These factors, together with the attenuation in ventricular dilation, resulted in a relative reduction in diastolic stress (Figure 2C).
Figure 2.
Cardiac anatomy and function. A: Myocardial regeneration attenuates cavitary dilation. SO: sham-operated; MI: infarcted-untreated; MI-T: infarcted-treated. *P<0.05 vs SO. †P<0.05 vs MI. B: EF decreased in infarcted animals at 18 days; in untreated-infarcts EF decreased further from 18 to 34 days. Conversely, EF increased from 18 to 34 days in infarcts treated with CPCs or GFs. C: Ventricular function. For symbols, see A.
Regenerated Myocardium
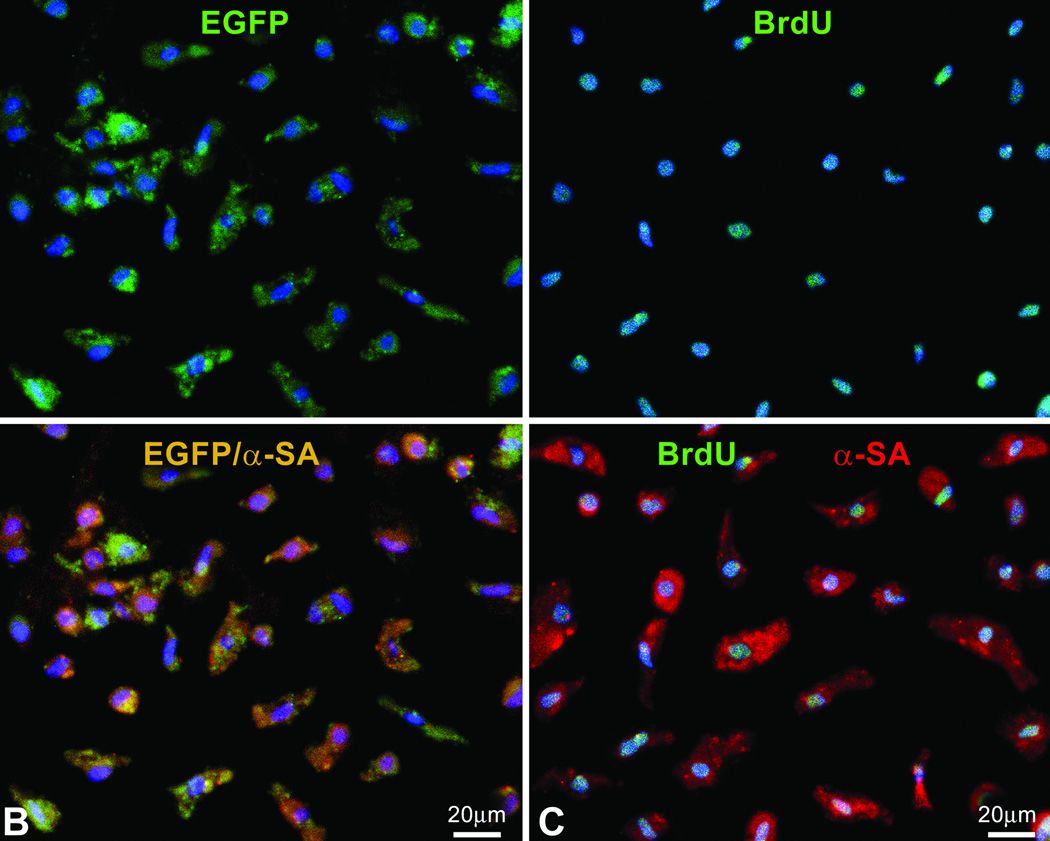
The new myocardium consisted of myocytes and vessels which collectively comprised ~90% of the regenerated tissue (supplemental Figure III). CPCs led to the formation of a larger number of arterioles while the number of capillaries and myocytes was comparable in treated-hearts. The number of new myocytes markedly exceeded the number of myocytes lost but they had volumes varying from ~300 to ~10,000 µm3 (supplemental Figure III). Myocyte regeneration was confirmed by FACS analysis of cells isolated by enzymatic digestion from the scarred region of treated- and untreated-hearts (Figure 3A through 3C; supplemental Figure IV). EGFP-positive/α-sarcomeric-actin-positivemyocytes and BrdU-positive/α-sarcomeric-actin-positive-myocytes obtained from infarcted-hearts injected respectively with CPCs and GFs constituted ~26% of the cell pool. Conversely, in untreated-infarcts, BrdU-positive/α-sarcomeric-actin-positive-myocytes represented 1% of the cell population. Several BrdU-positive/α-sarcomeric-actin- negative cells reflected replicating fibroblasts. This population of fibroblasts was markedly decreased in treated-infarcts.
Figure 3.
Regenerated myocytes. A: Scatter plot of cells isolated from the scarred region of untreated, CPC-treated and GF-treated infarcts. Newly formed myocytes express EGFP and α-sarcomeric-actin (α-SA) or BrdU and α-SA. The values of these cell populations are listed. B and C: Sorted regenerated myocytes are positive for EGFP and α-SA (B), and BrdU and α-SA (C). D: Border zone between surviving (SM) and regenerated (RM) myocardium in an infarcted-heart treated with CPCs. The area included in the rectangle is shown at higher magnification in the lower panel. Connexin 43 (white, arrowheads) is present between EGFP-positive regenerated myocytes and EGFP-negative pre-existing myocytes. E: Ki67- and BrdU-labeled regenerated myocytes.
Myocyte Phenotype and Mechanics
New myocytes with a volume of 2,000 µm3 or larger comprised ~40% of the population; they expressed cardiac myosin heavy chain (MHC), α-sarcomeric actin (α-SA), α-actinin and troponin I (TnI). Connexin 43 (Cnx43) and N-cadherin (N-cadh) were present between new myocytes and preexisting and regenerated myocytes (Figure 3D; supplemental Figure V). To determine whether small myocytes represented dividing amplifying cells, Ki67 was evaluated; ~10% of myocytes were Ki67 positive. They corresponded to the fraction of replicating cells at sacrifice. Additionally, ~83% of myocytes were BrdU-positive (Figure 3E), indicating that myocytes were continuously added over the 20-day period to the developing myocardium. Apoptosis comprised ~0.4% of new myocytes.
To evaluate whether the newly formed myocytes actively contributed to ventricular performance, myocytes were enzymatically dissociated from the regenerated and surviving myocardium and their mechanical properties were determined. This analysis was restricted to hearts (n=4) treated with EGFP-positive CPCs. The detection of EGFP by fluorescence microscopy in freshly isolated myocytes was confirmed by immunolabeling and confocal microscopy of formalin-fixed cells (not shown). EGFP-positive myocytes and EGFP-negative myocytes had a volume of ~7,500 and 57,000 µm3, respectively. CPC-derived myocytes contracted in response to electrical stimulation and exhibited higher cell shortening than resident cells (Figure 4A). Newly-formed smaller myocytes embedded within the scar did not tolerate well enzymatic digestion; they lacked an intact membrane and could not be employed in this analysis. Therefore, these results have this inherent limitation.
Figure 4.
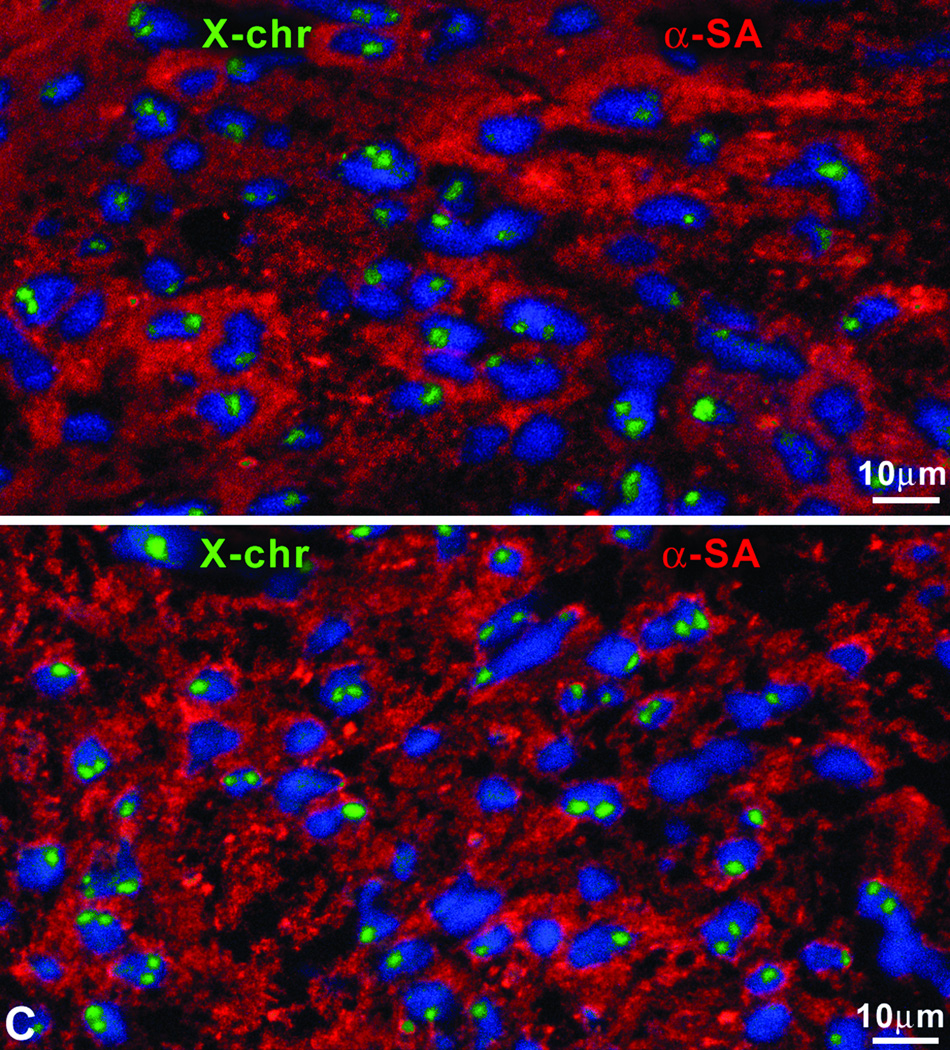
Properties of regenerated myocytes. A: Regenerated (left panels) and surviving (right panels) myocytes. Green: native EGFP fluorescence. Representative tracings of EGFP-positive and EGFP-negative myocytes, volume of myocytes measured physiologically and myocyte fractional shortening. B: DNA content of regenerated and spared myocytes. Shaded bars correspond to cycling Ki67-positive myocytes. C: Nuclei of newly formed myocytes exhibit at most two X chromosomes (green).
Cell Fusion
We then determined whether the regenerated myocytes were the product of cell fusion; measurements of DNA content in mono- and bi-nucleated myocytes showed diploid DNA content per nucleus (Figure 4B) excluding polyploidization or fusion. Higher DNA values were found only in Ki67-positive cycling myocytes. Since female rats were used and endogenous CPCs were mobilized by GFs or female CPCs were locally injected, the number of X chromosomes was evaluated. In all cases, at most two X chromosomes were found in newly formed myocytes further excluding fusion events (Figure 4C).
Surviving Myocardium
To determine the response of the surviving myocardium, tissue volume composition, myocyte cell volume, arteriole and capillary density, BrdU-labeling of myocytes and endothelial cells (ECs), and Ki67 expression in myocytes were measured. The proportion of myocytes, coronary vessels and other interstitial structures was comparable in untreated- and treated-infarcts (supplemental Figure VI). Moreover, myocyte volume increased 70%, 53% and 49% in untreated-, CPC-treated-, and GF-treated-infarcts, respectively. However, these differences were not significant. The decrease in arteriole and capillary density was also similar in untreated- and treated-infarcts (Figure 5).
Figure 5.
Characteristics of the surviving myocardium. Myocyte volume, arteriole and capillary density, and proliferation of myocytes and endothelial cells (ECs). SO: sham-operated; MI: infarcted-untreated; MI-T: infarcted-treated. *P<0.05 vs SO.
BrdU-positive myocytes and ECs and Ki67 labeling of myocytes were comparable in all infarcted-hearts (Figure 5). However, the presence of similar levels of myocyte and EC formation in treated hearts with decreased loading conditions suggests that the injected CPCs may have exerted a paracrine effect activating resident CPCs. A similar mechanism of growth has previously been shown.13
Invasive Properties of CPCs
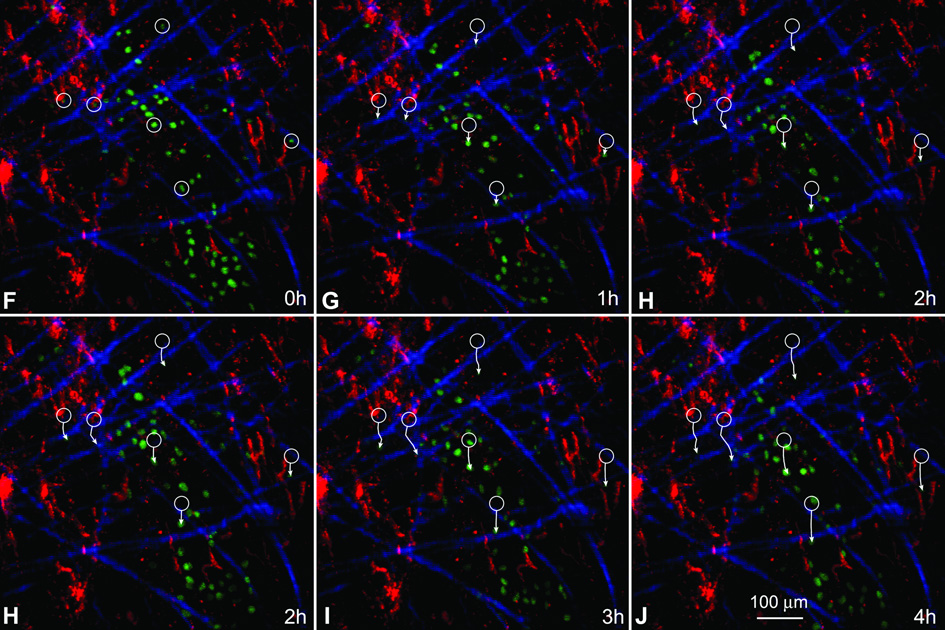
EGFP-tagged-CPCs were injected in proximity of chronic infarcts and their ability to spread across the scar was evaluated ex vivo by two-photon microscopy 4 and 24 hours after cell implantation. The coronary vasculature was perfused with rhodamine-labeled dextran and collagen was detected by second harmonic generation.14 At 4 and 24 hours after cell delivery, microscopic fields were examined continuously for 5–7 hours to measure the movement of EGFP-positive-cells. CPCs migrated through collagen bundles and accumulated within the scar. While single EGFP-positive-CPCs were detected at 4 hours, clusters of moving cells were present at 24 hours (Figure 6A through 6E), suggesting that these cells possessed the ability to permeate scarred myocardium.
Figure 6.
Migration of CPCs. A–E: Translocation of EGFP-positive-CPCs at 24 hours after cell implantation in a chronic infarct. The same field, examined at one-hour intervals is illustrated. Green: EGFP-positive-cells; red: coronary vasculature perfused with rhodamine-labeled-dextran. Blue: collagen. White circles in A indicate the position of selected cells at the beginning of observation. White arrows reflect the direction of migration and the distance covered by the cells in 1–4 hours. F–J: Translocation of EGFP-positive-CPCs at 24 hours after cell implantation in an acute infarct. The movement of cells is illustrated as described above.
EGFP-positive-CPCs moved at a speed of 14±4 µm/hour and there were 520±360 cells/mm3 of scarred myocardium. Corresponding values in 12–24 hours acute infarcts were 21±5 µm/hour and 2,300±600 cells/mm3 of infarcted myocardium (Figure 6F through 6J).
To study the effects of GFs, a lentivirus-carrying-EGFP was injected 20-days after infarction and 2-days later GFs were given. After 4 hours, the translocation of cells from the border to the infarct was analyzed for 5–10 hours. EGFP-labeled-cells entered the scar and moved within the scar (not shown). EGFP-positive-cells were characterized by confocal microscopy; they corresponded to c-kit-positive CPCs. Non-moving EGFP-positive- cells represented fibroblasts, ECs and myocytes. EGFP-tagged-CPCs whether locally injected or activated by GFs created tunnels surrounded by thick bundles of collagen type-1 and type-III (not shown).
In chronic infarcts, GF-activated CPCs moved at 30±12 µm/hour and there were 270±150 cells/mm3 of scarred myocardium. Corresponding values in 12–24 hours acute infarcts were 62±23 µm/hour and 450±230 cells/mm3 of infarcted myocardium.
Matrix Metalloproteinases (MMPs)
MMPs degrade the extracellular matrix and play a key role in invasive growth suggesting that intracellular or membrane-bound MMPs favor CPC translocation. Importantly, HGF increases the function of MMP-2 and MMP-9 in CPCs.11 Therefore, the expression of MMP-2, MMP-9 and MMP-14 was evaluated in samples of infarcted scarred myocardium 1, 2 and 3 days after the injection of CPCs. MMP-2 and MMP-9 cleave interstitial collagen and MMP-14 belongs to the group of membrane-bound MMPs which digest several interstitial proteins and activate other MMPs.15,16 The catalytic activity of MMPs is blocked by the tissue inhibitors of MMPs (TIMPs); TIMP-4 is highly expressed in the adult heart.17
In the presence of CPCs, MMP-2, MMP-9 and MMP-14 were significantly increased and TIMP-4 was markedly decreased (Figure 7A; supplemental Figure VII). Importantly, the activity of MMP-9 was 10-fold higher in CPC-treated than in untreated infarcted hearts (Figure 7B and 7C). However, MMP-2 activity was similar in the two groups of rats. Additionally, MMP-14 was not evaluated in this assay because it does not possess gelatinase activity. Since MMP-14 activates MMP-2 and MMP-9, we cannot exclude that MMP-14 contributed to the enhanced function of MMP-9.
Figure 7.
Expression and activity of MMPs and TIMP-4. A: Expression of MMP-2, MMP-9, MMP-14 and TIMP-4 in treated (T) and untreated (C) infarcts at 1, 2 and 3 days (d).B: Activity of MMP-9 and MMP-2. Gelatinase-activity appears as clear bands against a blue background. C: OD. *P<0.05 vs untreated control.
Cytokine and Growth Factor Profile
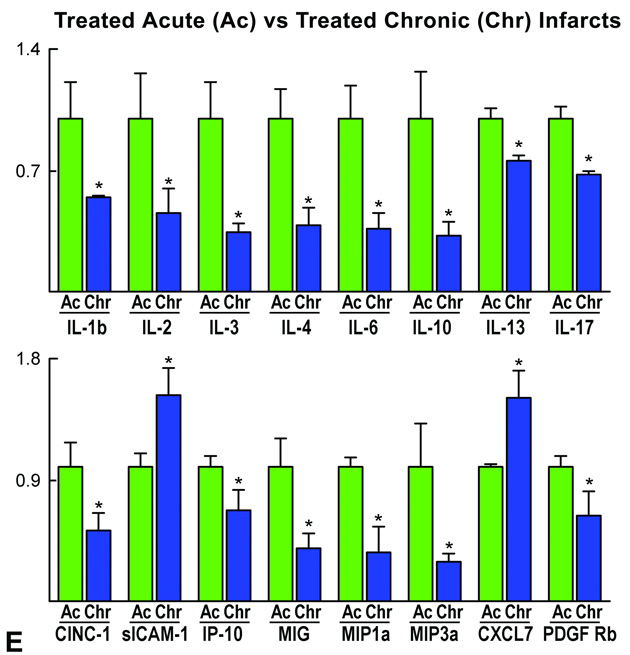
In an attempt to identify the signals involved in the migration and homing of CPCs in the infarcted heart and determine whether substantial differences existed between acute and chronic infarcts, a cytokine and growth factor array was performed (Supplemental Table I). In comparison with sham-operated (SO) rats, the scarred myocardium in untreated-chronic infarcts contained higher quantities of the cell adhesion molecule sICAM-1, the chemo-attractant CXCL7, bFGF and the MMP inhibitor TIMP-1 (Figure 8). The latter promotes cell division, opposes apoptosis and participates in vessel remodeling.18 Conversely, the inhibitor of cytokine synthesis IL-10 was downregulated. Untreated-chronic infarcts also had greater levels of sICAM-1 and bFGF than untreated-acute infarcts. However, the acutely infarcted myocardium possessed significantly higher amounts of proteins involved in the early phases of the inflammatory response (CINC-1, CINC-2α/β, TNF-α) together with chemokines associated with acute tissue injury (LIX, MIP-3α). IP-10 was more abundant in untreated-acute than in untreated-chronic infarcts (Figure 8). This CXC3 receptor ligand is implicated in vessel homeostasis and repair.19
Figure 8.
Cytokine and growth factor array. The content of cytokines and growth factors was analyzed in myocardial tissue samples. Only differences in expression that were statistically significant are shown. For a complete list of cytokines and growth factors, see Supplemental Table I. A–E: Fold changes in protein quantities. Data are mean±SD; *P<0.05. sICAM-1, soluble intercellular adhesion molecule-1; CXCL7, CXC chemokine ligand 7; bFGF, basic fibroblast growth factor; TIMP-1, tissue inhibitor of MMPs-1; IL, interleukin; CINC, cytokine induced-neutrophil-chemoattractant; TNF-α, tumor necrosis factor α; LIX, LPS-induced CXC chemokine; MIP, macrophage inflammatory protein; IP-10, interferon-γ-inducible protein 10; PDGF-R, platelet-derived growth factor-receptor; AR, amphiregulin; G-CSF, granulocyte-colony stimulating factor; IL-1Ra, interleukin-1 receptor antagonist; TGF-β, transforming growth factor-β; MIG, migration-inducing protein.
With cell treatment, there was an upregulation of several cytokines and growth factors in the scarred myocardium including CINC-3, PDGF-Rα, IP-10, LIX, L-selectin, TIMP-1 and the member of the EGF family amphiregulin AR. Cell treatment in acute infarcts increased a variety of chemokines which, with the exception of IP-10, differed from those in chronic infarcts (Figure 8).
DISCUSSION
The results of the current study demonstrate that CPCs locally activated by GFs or injected directly in proximity of a healed infarct can rescue nearly 45% of the infarct by replacing fibrotic tissue with functionally competent myocardium. Myocardial regeneration protects the infarcted heart from the progressive increase in cavitary dilation, decrease in wall thickness and deterioration in ventricular function with time. These findings, together with previous observations in the acutely infarcted heart,5,8,10,12 strongly suggest that CPCs are a powerful form of cell therapy for ischemic cardiomyopathy. CPCs are effective whether administered intramyocardially,5,10 via the coronary route20 or activated in situ with HGF and IGF-1, which trigger their growth and mobilization shortly after an ischemic event8,11 and, as shown here, chronically at the completion of healing. CPCs migrate through the myocardial interstitium reaching areas of necrotic and scarred myocardium where they home, divide and differentiate into myocytes and vascular structures. From a clinical perspective, CPCs appear to represent an ideal candidate for cardiac repair in patients with chronic heart failure in which discrete areas of damage are present in combination with multiple foci of replacement fibrosis across the ventricular wall.1 Potentially, CPCs may be isolated from endomyocardial biopsy or surgical samples and, following their expansion in vitro, administrated back to patients avoiding the inevitable and threatening adverse effects of rejection and other complications with non-autologous transplantation. Alternatively, GFs can be delivered locally to stimulate resident CPCs and promote myocardial regeneration. Importantly, these strategies may be repeated to reduce further myocardial scarring and expand the working myocardium.
Myocardial Regeneration
Experimental and clinical studies have documented that bone marrow progenitor cells (BMPCs), administered acutely and chronically after infarction, improve ventricular function in animals and humans.21 Based on these initial observations, double-blind clinical trials have been conducted and, with one exception,22 the actual efficacy of BMPCs in human heart failure has been shown.23,24 These findings show rather convincingly that BMPCs have a potential therapeutic effect on the infarcted heart but the mechanism involved remains unclear. The ability of BMPCs to transdifferentiate and form cardiomyocytes and coronary vessels has been controversial and data in favor and against this possibility have been reported.1 Recently, four laboratories have addressed this issue in a collective manner and provided strong evidence in support of the notion of BMPC plasticity and the therapeutic import of BMPCs for myocardial regeneration.25 The implementation of BMPCs in patients has a six years history and any other form of cell therapy will have to be compared with that of BMPCs. And this will be inevitable for CPCs as well.
The identification of CPCs has challenged the generally accepted but never proven paradigm that the heart is a postmitotic organ, but has not answered the question why CPCs do not reach spontaneously an infarcted area rebuilding completely or in part the lost myocardium. This was in the past and continues today to be interpreted as a growth limitation of the adult heart. Conversely, the current results document that there is a significant pool of functionally competent CPCs located in the surviving myocardium adjacent to healed infarcts that, following activation with GFs, infiltrate the scarred tissue and generate a remarkable number of cardiomyocytes and coronary vessels. Similarly, the injection of adult CPCs leads rapidly to extensive myocardial regeneration. CPCs are programmed to acquire the myocyte, and vascular smooth muscle cell (SMC) and EC lineages and, in contrast to BMPCs, do not have to transdifferentiate to give rise to cardiac cell categories. Transdifferentiation is a time consuming process that necessitates the reorganization of chromatin through activation and silencing of specific transcription factors together with epigenetic changes.26 Nevertheless, BMPCs are easily accessible and this is a critical factor clinically.
Based on the current results, the possibility is advanced that CPCs may be superior to BMPCs for myocardial regeneration. However, control animal studies addressing this question have yet to be performed. CPCs are well equipped to sustain the physiological turnover of myocytes, SMCs and ECs of the adult heart but are not programmed to sense distant damage, translocate to this site and activate regenerative growth in response to injury. This limitation, together with the death of CPCs in the ischemic area,8,11 precludes an effective reconstitution of the infarcted myocardium. Importantly, this is not a unique deficiency or a peculiar behavior of the heart but a common deficiency of proliferating organs; artery occlusion results in tissue necrosis and scarring in the skin, intestine, liver, spleen, kidney, brain and bone marrow, regardless of whether they are self-renewing organs regulated by a stem cell compartment.1
CPC Migration
Observations here indicate that myocardial scarring interferes with the migration and engraftment of locally injected or GF-activated CPCs. Acute infarcts are more amenable to CPC translocation and homing providing a milieu in which CPCs rapidly accumulate and generate a committed progeny. In spite of the less favorable environment dictated by collagen deposition chronically after infarction, CPCs retained the ability to infiltrate the scar, digest part of the connective tissue and form cardiomyocytes and coronary vessels. The comparable effects on myocardial regeneration obtained by local CPC-delivery and GF-activation of resident CPCs may be explained by the pattern of migration of these cells within the scar; the higher number of cells found with intramyocardial injection of CPCs was compensated by the faster speed of migration of GF-activated CPCs. When these two variables are considered, i.e., speed and cell number, the accumulation of cells under these conditions is remarkably similar. However, this was not the case in acute infarcts in which cell infiltration was more efficient following CPC-implantation than after CPC-activation by GFs.
Whether these results have implications for the treatment of chronic human heart failure is difficult to predict. Risk factor such as aging and diabetes are frequently present in patients with ischemic cardiomyopathy and they have profound negative consequences on the number and function of CPCs.27 However, functionally-competent human CPCs have been isolated from a variety of patients undergoing open-heart surgery10 and the activation of CPCs by GFs in senescent rats with severe ventricular decompensation reverses the cardiac phenotype and prolongs lifespan.28 Collectively, these findings raise the possibility that the strategies utilized here in an animal model may be relevant to the management of human heart failure.
CPCs and Cytokine Milieu
The invasion of the scarred tissue by CPCs appeared to be mediated by enhanced activity of MMP-9 and possibly MMP-14. MMP-9 is critical for the recruitment of bone marrow stem cells and their mobilization from quiescent to proliferative niches;29 and a similar mechanism may be operative in the translocation to the chronically infarcted heart of GF-treated resident CPCs or delivered CPCs. The upregulation of MMP-9 expression and activity in CPCs is dependent on HGF.11 Additionally, SDF-1 which is highly expressed in myocytes and endothelial cells after ischemic injury acts on MMP-9 and promotes the differentiation of CPCs into vascular cells and cardiomyocytes.14 The lack of increase in MMP-2 activity observed here may favor the stability of SDF-1 which is degraded by this protease.30
Importantly, with respect to the intact heart, the content of cytokines and growth factors differed in the scarred myocardium. The enhanced expression of the chemotactic factors sICAM-1, CXCL7 and bFGF in chronic infarcts may have created a condition facilitating migration and homing of CPCs.31 Additionally, the presence of sICAM-1, CXCL7 and TIMP-1 in the scar could have promoted CPC mobilization, EC migration and differentiation and vessel formation.32 The increases in CXCL7 and TIMP-1 were restricted to chronic infarcts. As expected, the acutely infarcted myocardium displayed a cytokine profile that reflected an inflammatory response and tissue reaction to acute injury. However, the higher levels of sICAM-1 and bFGF within the scar are particularly relevant for myocardial regeneration since sICAM-1 may have created a niche structure that supported the engraftment of CPCs and bFGF is critical for the differentiation of resident CPCs into myocytes.33
The analysis of cytokine and growth factor expression in acute and chronic infarcted hearts offered the possibility to establish whether the delivery of CPCs participated in the synthesis and secretion of soluble proteins which exerted an autocrine or paracrine effect on myocardial regeneration. The presence of CPCs in the scar attenuated, at least in part, the differences in cytokine profile with acute infarcts by increasing the quantities of LIX that is involved in stem cell maintenance and proliferation34 and IP-10 that modulates vessel homeostasis and growth.19 Thus, acute and chronic myocardial damage lead to the expression of genes that create a microenvironment that conditions the activation, migration and growth of CPCs ultimately controlling the regenerative response of the pathologic heart.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by NIH grants. JF-M: supported by Foundation for Science and Technology, Ministry of Science, Technology and Higher Education, Portugal.
Footnotes
Disclosures: None.
References
- 1.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 2.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci USA. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. New Engl J Med. 2001;334:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 4.Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci USA. 1998;95:8801–8805. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beltrami AP, Barlucchi L, Torella D, Baker M, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–766. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 6.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman NL, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, Komuro I. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 8.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci USA. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31− but not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 10.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Cascapera S, Beltrami AP, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that following activation regenerate the infarcted myocardium improving ventricular function and long-term survival. Circ Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer JM, Pfeffer MA, Fletcher PJ, Braunwald E. Progressive ventricular remodeling in rat after myocardial infarction. Am J Physiol. 1991;260:1H1406–1H1414. doi: 10.1152/ajpheart.1991.260.5.H1406. [DOI] [PubMed] [Google Scholar]
- 13.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 14.Tillmanns J, Rota M, Hosoda T, Misao Y, Esposito G, Gonzalez A, Vitale S, Parolin C, Yasuzawa-Amano S, Muraski J, De Angelis A, LeCapitaine N, Siggins RW, Loredo M, Bearzi C, Bolli R, Urbanek K, Leri A, Kajstura J, Anversa P. Formation of large coronary arteries by cardiac stem cells. Proc Natl Acad Sci USA. 2008;105:1668–1673. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res. 2001;89:201–210. doi: 10.1161/hh1501.094396. [DOI] [PubMed] [Google Scholar]
- 16.Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995;270:5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- 17.Liu YE, Wang M, Greene J, Su J, Ullrich S, Li H, Sheng S, Alexander P, Sang QA, Shi YE. Preparation and characterization of recombinant tissue inhibitor of metalloproteinase 4 (TIMP-4) J Biol Chem. 1997;272:20479–20483. doi: 10.1074/jbc.272.33.20479. [DOI] [PubMed] [Google Scholar]
- 18.Boulday G, Fitau J, Coupel S, Soulillou JP, Charreau B. Exogenous tissue inhibitor of metalloproteinase-1 promotes endothelial cell survival through activation of the phosphatidylinositol 3-kinase/Akt pathway. Ann NY Acad Sci. 2004;1030:28–36. doi: 10.1196/annals.1329.004. [DOI] [PubMed] [Google Scholar]
- 19.Rosenkilde MM, Schwartz TW. The chemokine system - a major regulator of angiogenesis in health and disease. APMIS. 2004;112:481–495. doi: 10.1111/j.1600-0463.2004.apm11207-0808.x. [DOI] [PubMed] [Google Scholar]
- 20.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa P, Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci USA. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005;96:151–163. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- 22.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grøgaard HK, Bjørnerheim R, Brekke M, Müller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 23.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hammc CW, Süselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. REPAIR-AMI Investigators. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 24.Assmus B, Honold J, Schächinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 25.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Ireguas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pomerantz J, Blau HM. Nuclear reprogramming: a key to stem cell function in regenerative medicine. Nat Cell Biol. 2004;6:810–816. doi: 10.1038/ncb0904-810. [DOI] [PubMed] [Google Scholar]
- 27.Dimmeler S, Leri A. Aging and disease as modifiers of cell therapy efficacy. Circ Res. 2008 doi: 10.1161/CIRCRESAHA.108.175943. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Müller P, Esposito G, Cascapera S, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, Leri A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 29.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt A, Ladage D, Schinköthe T, Klausmann U, Ulrichs C, Klinz FJ, Brixius K, Arnhold S, Desai B, Mehlhorn U, Schwinger RH, Staib P, Addicks K, Bloch W. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells. 2006;24:1750–1758. doi: 10.1634/stemcells.2005-0191. [DOI] [PubMed] [Google Scholar]
- 32.Gho YS, Kim PN, Li HC, Elkin M, Kleinman HK. Stimulation of tumor growth by human soluble intercellular adhesion molecule-1. Cancer Res. 2001;61:4253–4257. [PubMed] [Google Scholar]
- 33.Rosenblatt-Velin N, Lepore MG, Cartoni C, Beermann F, Pedrazzini T. FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J Clin Invest. 2005;115:1724–1733. doi: 10.1172/JCI23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choong ML, Yong YP, Tan AC, Luo B, Lodish HF. LIX: a chemokine with a role in hematopoietic stem cells maintenance. Cytokine. 2004;25:239–245. doi: 10.1016/j.cyto.2003.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.