Abstract
Formation of heteromeric complexes of ion channels via co-assembly of different subunit isoforms provides an important mechanism for enhanced channel diversity. We have previously demonstrated co-association of the hyperpolarization activated cyclic-nucleotide gated (HCN1/HCN2) channel isoforms that was regulated by network (seizure) activity in developing hippocampus. However, the mechanisms that underlie this augmented expression of heteromeric complexes have remained unknown. Glycosylation of the HCN channels has been implicated in the stabilization and membrane expression of heteromeric HCN1/HCN2 constructs in heterologous systems. Therefore, we used in vivo and in vitro systems to test the hypothesis that activity modifies HCN1/HCN2 heteromerization in neurons by modulating the glycosylation state of the channel molecules. Seizure-like activity (SA) increased HCN1/HCN2 heteromerization in hippocampus in vivo as well as in hippocampal organotypic slice cultures. This activity increased the abundance of glycosylated HCN1 but not HCN2-channel molecules. In addition, glycosylated HCN1 channels were preferentially co-immunoprecipitated with the HCN2 isoforms. Provoking SA in vitro in the presence of the N-linked glycosylation blocker tunicamycin abrogated the activity-dependent increase of HCN1/HCN2 heteromerization. Thus, hippocampal HCN1 molecules have a significantly higher probability of being glycosylated after SA, and this might promote a stable heteromerization with HCN2.
Keywords: glycosylation, hyperpolarization activated cyclic-nucleotide gated, Ih, ion channel, post-translational modification, seizure activity
Ion channels govern the firing properties of neurons (Catterall 1988; Yaari and Beck 2002). The hyperpolarization-activated, cyclic-nucleotide gated (HCN) channels conduct Ih, a current important in maintenance of neuronal resting membrane potential and in governing neuronal response to network input (Maccaferri and McBain 1996; Lüthi and McCormick 1998; Magee 1999; Santoro and Baram 2003). The functions of these channels are regulated at several levels: In addition to cAMP-mediated short-term alteration of biophysical properties of existing channels, activity-dependent transcriptional changes of these channels have been delineated. Thus, normal and pathological network activity increased (Fan et al. 2005) or reduced (Bräuer et al. 2001; Brewster et al. 2002) HCN1 expression, and increased HCN2 mRNA levels (Brewster et al. 2002). At the posttranslational level, activity has been shown to influence trafficking of the HCN1 channel to axonal compartments (Bender et al. 2007), as well as to the distal dendrites (Shin and Chetkovich 2007). HCN channel molecules themselves contain sites for glycosylation (Proenza et al. 2002; Much et al. 2003) and phosphorylation (Poolos et al. 2006), and modulation of channel phosphorylation and glycosylation influence, respectively, their biophysical properties and their membrane insertion in heterologous systems (Much et al. 2003; Poolos et al. 2006).
An additional means for modulating the function of the HCN channels is via molecular rearrangements of channel isoforms (Much et al. 2003; Robinson and Siegelbaum 2003; Santoro and Baram 2003; Strauss et al. 2004; Brewster et al. 2005). HCN channels are tetramers and, in heterologous systems, HCN1, 2, 3, and 4 co-associate widely in almost all possible combinations (Much et al. 2003). In contrast to heterologous systems, heteromerization seems to be restricted in hippocampal tissue (Brewster et al. 2005) and is markedly enhanced by network activity bursts (seizures). This apparent increased formation of heteromeric channels is associated with altered properties of the resulting Ih, significantly enhancing network excitability (Chen et al. 2001a; Simeone et al. 2005). Therefore, it is important to understand how activity promotes HCN1 isoform heteromerization with HCN2 channels, yet the means by which activity enhances formation or stabilization of heteromeric HCN1/HCN2 complexes have remained unclear. One broad possibility is that the reduced HCN1/HCN2 ratio generated by the transcriptional down-regulation of HCN1 channels discussed above (Brewster et al. 2002, 2005) increases the stochastic probability that an HCN1 channel will interact with an HCN2 isoform, as described for other members of the voltage-gated K+ channel superfamily (Levitan and Takimoto 1998). Alternatively, activity-dependent modification of the HCN1 and/or HCN2 channel molecules might govern the formation, stability, trafficking, or membrane insertion of heteromeric channels.
Here, we studied activity-dependent heteromerization, focusing on the potential role of N-glycosylation in the expression of heteromeric HCN1/HCN2 tetramers in the hippocampus.
Experimental procedures
Animals
Rats used in both in vivo and in vitro experiments were products of Sprague–Dawley derived pregnant dams that were maintained in a federally approved animal facility under a 12 h light/dark cycle (light on at 07:00) with unlimited food and water. On postnatal day 2 (P2) (date of birth = day 0), litters were adjusted to 12 pups. All experimental procedures were approved by the Animal Care Committee (University of California, Irvine, CA, USA) and carried out in accordance to National Institutes of Health (NIH) guidelines.
In vivo induction of abnormal network activity (seizures)
Seizures were induced in immature (P10) Sprague–Dawley rats using either kainic acid (KA; 1.2 mg/kg, i.p.) or hyperthermia, as previously described (Dubé et al. 2000, 2006; Brewster et al. 2002; Dubé and Baram 2006). Briefly, KA was given into the peritoneal cavity, and animal behavior observed for the following 3 h; hyperthermia was induced using a stream of warm air directed ~30 cm above the animals. The core temperature of pups was measured prior to hyperthermia induction, at 2 min intervals and at the onset of hyperthermia-provoked seizures. Hyperthermia was maintained for 30 min resulting in seizure duration of ~24 min. Following hyperthermia, animals were moved to a cool surface for 10–15 min and then returned to their mothers. Experimental animals were compared with littermate controls.
In vitro induction of network activity bursts and organotypic slice cultures
Hippocampal slice cultures were prepared and maintained using the interface technique (Stoppini et al. 1991; Chen et al. 2004; Bender et al. 2007). Briefly, hippocampi from P8 rat pups were dissected and cut into 400-µm slices using a McIlwain tissue chopper (Mickle Laboratory Engineering, Guilford, UK). Slices were collected in ice-cold preparation buffer (100% Minimal Essential Medium, containing 30 mmol/L glucose and 3 mmol/L glutamine, pH 7.3; Life Technologies, Rockville, MD, USA), then placed onto moistened membrane inserts (Millicell-CM, 30 mm, 0.4-µm pore diameter; Millipore, Bedford, MA, USA), transferred to sterile six-well plates filled with 1 mL pre-warmed culture medium (50% Minimal Essential Medium, 25% Hank’s balanced salt solution, 20% heat-inactivated horse serum, 30 mmol/L HEPES, 30 mmol/L glucose, 3 mmol/L glutamine, 0.5 mmol/L ascorbic acid, 1 mg/mL insulin, and 5 mmol/L NaHCO3, pH 7.3) and incubated in a humidified, CO2-enriched atmosphere at 36°C. Seizure-like events were induced after 3 days in vitro by incubating cultures for 3 h in medium containing KA (6 µmol/L; Sigma, St Louis, MO, USA). Seizure-like events were terminated by returning cultures to normal medium. Controls were treated identically, but media were devoid of convulsants. To prevent N-linked glycosylation of proteins, tunicamycin (10 µg/mL; Sigma-Aldrich, St. Louis, MO, USA) was applied together with KA or alone, and maintained for 72 h. Tunicamycin doses were determined following a dose response as described later. Cultures were harvested by freezing on dry ice for western blot analyses, or by fixing in 4% buffered p-formaldehyde (2 h) followed by cryoprotection (25% sucrose, 12 h), and freezing, for immunocytochemistry.
Membrane preparation and de-glycosylation assay
For in vivo experiments, each sample consisted of a hippocampal extract from an individual rat. Rats were decapitated rapidly, and dissected hippocampi were immediately frozen on dry ice and homogenized in glass/Teflon homogenizers in an ice-cold solution containing 0.32 mol/L sucrose, 0.1 mol/L Tris–HCl (pH 7.4), and Protease Inhibitor Cocktail (PIC Complete; diluted according to the manufacturer’s instructions; Roche, Alameda, CA, USA). For organotypic hippocampal slice cultures, each sample consisted of combined 9–12 slice cultures of the same treatment group. After homogenization, samples were centrifuged at 800 g for 10 min at 4°C and pellet discarded. The resulting supernatant was centrifuged at 16 000 g for 60 min at 4°C and the pellet containing membrane fractions resuspended in radio-immuno-precipitation assay (RIPA) buffer (50 mmol/L Tris–HCl, pH 7.4, 1% NP-40 (Fluka Chemicals, Buchs, Switzerland), 1% Triton X-100, 1 mmol/L EDTA, 150 mmol/L NaCl, and 1x PIC). Protein concentration was determined using Bio-Rad Protein assay (Bio-Rad, Hercules, CA, USA).
De-glycosylation was carried out by first denaturing the membrane protein fraction in 50 mmol/L β-mercaptoethanol and 0.1% sodium dodecyl sulfate (SDS) for 5 min. The mixture was then transferred to a solution composed of 20 mmol/L sodium phosphate, pH = 7.5, and 0.75% NP-40 (Fluka Chemicals). Finally 5 U of N-glycanase (EC 3.5.1.52; Prozyme, San Leandro, CA, USA) were added, and the reaction was incubated at 37°C for 2 h.
Immunoprecipitation
Equal amounts of protein from each hippocampal sample (~1 mg/mL diluted in RIPA buffer) or organotypic slice culture was pre-cleared with anti-rabbit IgG beads (eBioscience, San Diego, CA, USA) for 30 min on ice. The samples were then centrifuged at 10 000 g for 3 min. The supernatant was incubated with rabbit anti-HCN1 or anti-HCN2 serum (5 µL of 0.8 µg/µL; Chemicon, Temecula, CA, USA) or rabbit IgG at 4°C for 1 h on a rotating device. Following incubation with primary antibodies, samples were incubated with 60 µL anti-rabbit IgG beads for 1 h at 4°C and collected in spin-filter columns. Samples were washed with RIPA + PIC (2x) followed by phosphate-buffered saline (PBS) + PIC (3x), eluted with 50 µL of 2x Laemmli buffer and processed for western blot analyses.
Western blotting
Protein samples were denatured at 70°C for 5 min in Laemmli buffer, separated on a 7.5% SDS–polyacrylamide gel electrophoresis, and visualized using the enhanced chemiluminescence ECL-Plus kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA) as described elsewhere (Brewster et al. 2005). Briefly, following SDS–polyacrylamide gel electrophoresis, samples were transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech) and non-specific binding was blocked by incubation with 10% non-fat milk in PBS overnight at 4°C. Membranes were probed with rabbit anti-HCN1 or anti-HCN2 serum (1 : 1000 and 1 : 2000, respectively; Chemicon) in PBS with 5% non-fat milk overnight at 4°C followed by washes in PBS-0.1% Tween (3× 5 min). Membranes were then incubated with anti-rabbit IgG horseradish peroxidase (1 : 10 000; Amersham Pharmacia Biotech) in PBS for 1 h at 21°C. Membranes were incubated with ECL-Plus for 5 min and immunoreactive bands visualized by apposing the membranes to hyperfilm™ ECL.
Quantitative correlation of the western blots and co-immunoprecipitation of the same samples
Hippocampal culture homogenates were divided (after protein determination), and 15 µg protein was loaded for direct western blot analyses, whereas 40 µg protein was utilized for co-immunoprecipitation, to allow quantitative comparison of total HCN channels to the fraction co-immunoprecipitated with HCN2 channels. The optical density (OD) of HCN1 bands on the western blots was normalized to β-actin of the same sample and the same exposure time, thus providing the input for the portion of the HCN1 that co-immunoprecipitated with HCN2 antiserum.
Immunocytochemistry
Immunocytochemistry was performed as described previously (Brewster et al. 2002, 2007; Chen et al. 2004). Briefly, free-floating brain or culture sections (40 µm) were collected in PBS + 0.3% Triton X-100, pre-incubated for 1 h with 3% normal goat serum/PBS + 0.3% Triton X-100, followed by incubation with a polyclonal rabbit anti-HCN1 (1 : 2500; Chemicon), a polyclonal rabbit anti-HCN2 (1 : 1500; a kind gift of Dr R. Shigemoto), or a polyclonal rabbit anti-c-fos (1 : 30 000; Calbiochem, San Diego, CA, USA) for 48 h at 4°C. After several washes, sections were transferred to secondary antibody solution (biotinylated goat-anti-rabbit IgG, 1 : 250 for HCN1/2 or 1 : 400 for c-fos) for 3 h, followed by additional washes and avidin–biotin-peroxidase complex solution (Vector, Burlingame, CA, USA) for 2 h at 21°C. Antibody binding was visualized by incubating sections in a solution containing 0.04% 3,3′ diaminobenzidine, 0.01% H2O2, 0.01% NiCl2, and 0.01% CoCl2. HCN channel-antisera specificity was determined by testing against tissue from mice lacking a given HCN channels isoform (see Results). Additional control experiments included treatment of sections as above, but without primary antibodies. No immunoreactivity was observed under these conditions.
Assessment of cell injury using fluorojade
Cultures were exposed to 6 µmol/L KA for 3 h, and fixed 24 or 48 h later, sectioned (50 µm), mounted on slides, and dried. Sections from adult rats subjected to prolonged KA seizures were used as positive controls. The Fluorojade method was performed as described previously (Schmued et al. 1997; Dube et al. 2004). Briefly, fixed, cut, and mounted organotypic slice cultures were rinsed and dehydrated in 100% and then in 70% ethanol, and pre-treated with 0.06% potassium permanganate. After rinsing in distilled water, sections were exposed to the fluorojade solution (0.001% in 0.1% acetic acid) for 30 min at 45°C. Sections were rinsed thrice in acetic acid, then dried, and coverslipped using Permount. The visualization of individual neurons with fluorojade, generally accepted to denote injury (Schmued et al. 1997; Dube et al. 2004) was accomplished using a fluorescence microscope and an FITC filter.
Analysis
All analyses were performed without knowledge of treatment group. Western blot data acquisition and analysis were accomplished by measuring OD of immunoreactive bands from the ECL films using the ImageTool software (UTHSCA, San Antonio, TX, USA). Significance level for unpaired t-tests and anova was set at 0.05 (GraphPad Prism, San Diego, CA, USA), and data are presented as mean with standard errors.
Results
Seizures increase heteromerization of hippocampal HCN1 and HCN2 channels in vivo
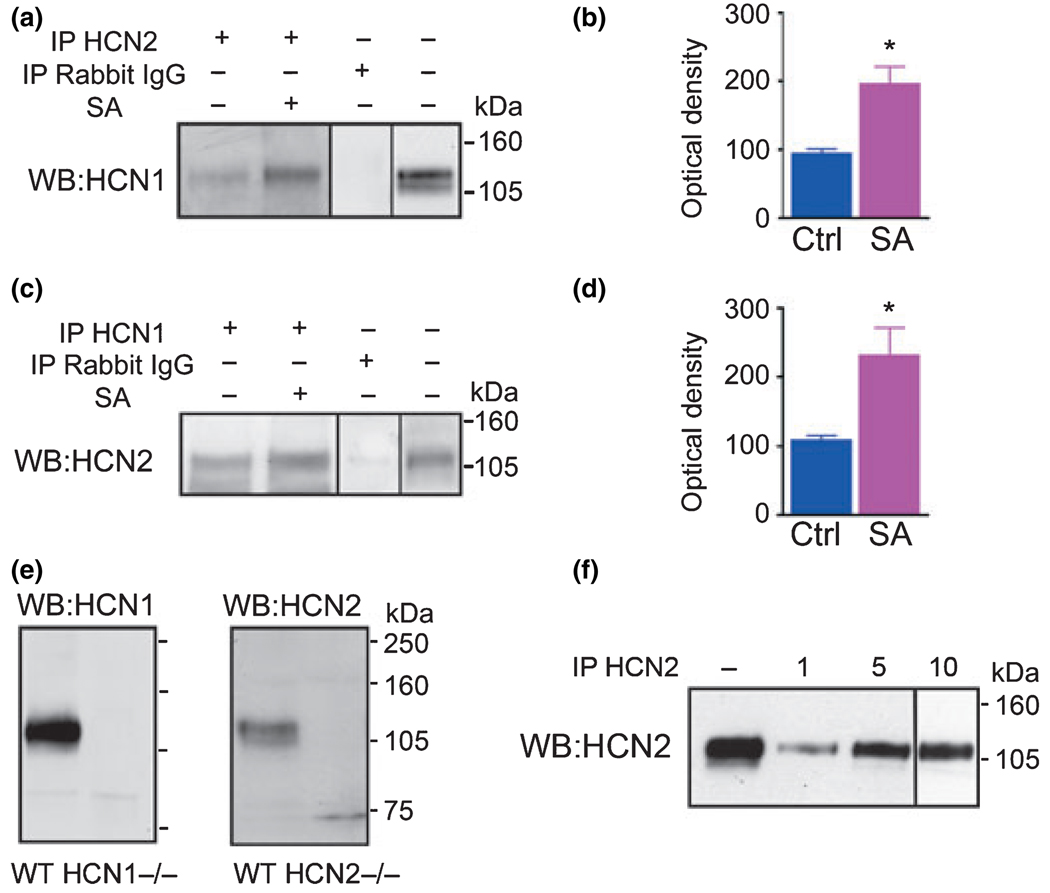
In hippocampi of immature rats, co-assembly of hippocampal HCN1 and HCN2 channel subunits was increased after experimental seizures provoked by either hyperthermia or the glutamate receptor agonist KA (Brewster et al. 2005). As shown in Fig. 1a and b, immunoprecipitation of HCN1 molecules with an antiserum directed at HCN2 was more than double in samples from seizure-experiencing rats (increased by 115.4 ± 20.4%; n = 10), although HCN2 expression levels did not change (Brewster et al. 2005). The increased interaction among HCN1 and HCN2 molecules after seizure activity was evident also when evaluated by using an antiserum directed against HCN1 channel molecules for precipitation, and blotting for co-immunoprecipitated HCN2 channels (Fig. 1c and d). Seizures increased this interaction more than twofold (by 123.9 ± 48.8%; n = 5, Fig. 1d). Note that this occurred despite a reduction in the overall expression of HCN1 channels: intensity of HCN1 immunoreactive band after seizure activity was 113.9 ± 13.9 OD, and in controls 165.6 ± 14 OD; n = 14; p < 0.01 (not shown; see also Brewster et al. 2005). The specificity of the antisera against the HCN1 and HCN2 channels was demonstrated by the absence of immunoreactive bands of the appropriate molecular weight (MW) in hippocampal extracts from mice lacking HCN1 (Fig. 1e, left) and HCN2 (Fig. 1e, right) channel isoforms, respectively.
Fig. 1.
Neuronal activity bursts (seizures) increase the membrane expression of heteromeric HCN1/HCN2 channels. (a) Immunoprecipitation (IP) with an antiserum to the HCN2 channel isoform followed by western blot (WB) analysis for HCN1 shows enhanced co-association of HCN1/HCN2 in hippocampi from animals subjected to experimental seizures (SA), as quantified in (b). The immunoreactive HCN1 band has an apparent molecular weight of ~125 kDa. The specificity of the immunoprecipitation is supported by the absence of immunoreactive band when IgG is substituted for anti-HCN2. The correspondence of the co-precipitated band to native HCN1 is demonstrated by comparing it to a WB from the same tissue. (c and d) The increased co-association of HCN1 and HCN2 isoforms is also apparent when immunoprecipitation (IP) is performed using an antiserum to HCN1 and the precipitated channels probed by WB analysis for HCN2. (e) The specificity of the HCN1 and HCN2 antisera is evident from the absence of an immunoreactive band of the appropriate molecular weight in brains from mice lacking these channels (HCN1−/− and HCN2−/−). (f) The efficiency of precipitating HCN channels with the antisera used was evaluated using escalated amounts of each anti-serum. Input and HCN2 immunoreactive bands precipitated with 1, 5, and 10 µL anti-HCN2 are shown. Efficiency for this antibody was 20.4 ± 2.8%, 66.1 ± 8.1%, and 36% (n = 2), respectively. Efficiency of HCN1 antiserum in precipitating the same isoform was 9.7%, 30.5%, and 26.5%, respectively (not shown).
The efficiency of the antisera to precipitate their target HCN channel isoform was evaluated using increasing amounts of anti-HCN2 and HCN1, to determine the saturating dose. As shown in Fig. 1f, anti-HCN2 efficiency demonstrated a dose–response: using 1, 5, and 10 µL of HCN2 antiserum resulted in precipitation efficiency of the HCN2 channels of 20.4 ± 2.8%, 66.1 ± 8.1%, and 36% (n = 2), respectively. Based on this, 5 µL of this antiserum was used in these experiments.
Seizures increase the high MW fraction of HCN1-, but not of HCN2-channels, and this is a result of increased relative abundance of glycosylated channel species
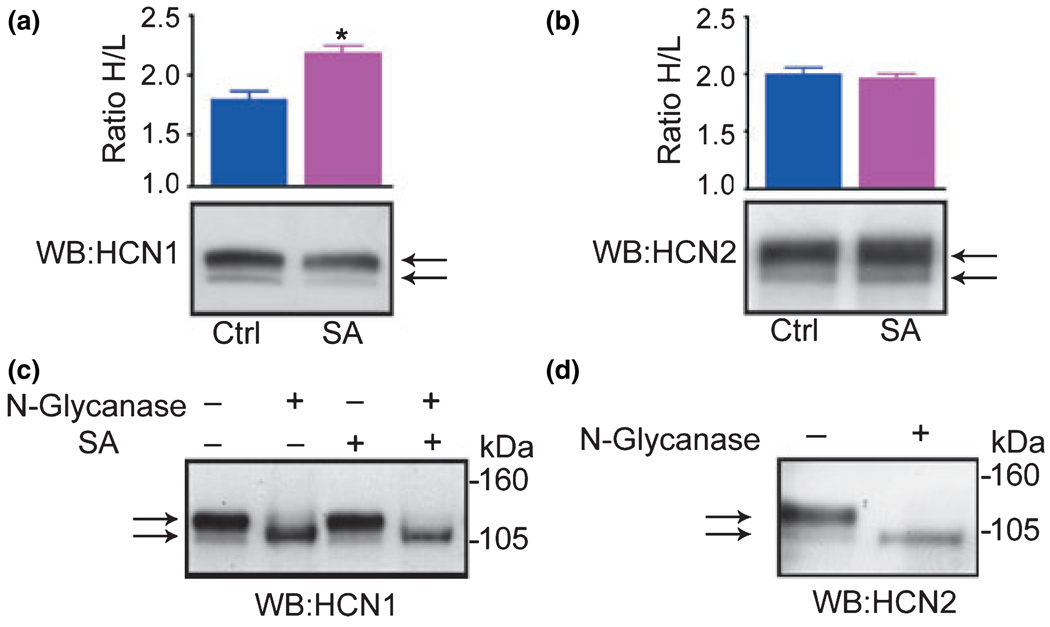
Western blots of rat hippocampal extracts typically demonstrate two bands that are immunoreactive with anti-HCN1 and anti-HCN2 channel antisera (Fig 1a, Fig 2a, and 2b; see also Brewster et al. 2005). For HCN1, the band with a lower apparent MW (~105 kDa), corresponds to the predicted MW of the protein core, and for HCN2 channels this lower MW band migrates at an apparent MWof 100–104 kDa. For both channel isoforms, a second, ‘heavier’ immunoreactive band migrates at the apparent MW of ~125 kDa. Following seizure activity, the relative abundance of the apparently ‘heavier’ band of HCN1 was significantly increased (Fig. 2a; the heavy/light ratio was 1.79 ± 0.07 in controls and 2.17 ± 0.26 after seizure activity, n = 14; p < 0.01) note the lower intensity of the combined HCN1 bands after seizures, consistent with the reduced expression of this isoform (Brewster et al. 2002, 2005). In contrast to HCN1 channels, no significant change in the proportion of higher-MW HCN2 channels was noted after seizures (Fig. 2b; 2.0 ± 0.16 in controls and 1.9 ± 0.11 after seizure activity, n = 6; p = 0.6).
Fig. 2.
Seizure activity increases the proportion of glycosylated HCN1 channel molecules, but not of the HCN2 isoform. (a) Western blot (WB) analysis of HCN1 reveals two distinct HCN1-immunoreactive bands of different molecular weights (MW): The lower (L) band, migrating at ~105 kDa is the predicted MW of the protein moiety, and the higher band (H) migrates at ~125 kDa. Quantification of the optical density of the HCN1 bands demonstrates an increased H/L ratio from 1.8 in control tissue to 2.2 (n = 14, p < 0.001). (b) Seizure activity does not influence the ratio of the high and low apparent MW bands. (c and d) Treatment with N-glycanase eliminates the high MW band immunoreactive for HCN1 (c), or HCN2 (d), demonstrating that the high MW band is a result of N-glycosylation of the channel molecules.
All HCN channel isoforms contain one potential N-linked glycosylation consensus sequence in the extracellular domain between trans-membrane domains S5 and S6 (Asn-X-Ser/Thr), and are known to be glycosylated in heterologous systems and in total brain extracts (Much et al. 2003). Therefore, we subjected the hippocampal extracts to the de-glycosylating enzyme N-glycanase, to examine whether the apparent increase in MW of the HCN1 channel isoform following experimental seizures was a result of increased glycosylation. Treatment with this enzyme eliminated the 125 kDa band, demonstrating that the high apparent MW band was due to N-glycosylation of the channel molecules (Fig. 2c). A similar analysis demonstrated glycosylation also of the HCN2 isoform (Fig. 2d). Because the increase in glycosylation provoked by seizure activity was confined to the HCN1 channel isoform, we focused the next set of experiments on this subunit.
Glycosylated HCN channel molecules are preferentially heteromerized
The concomitant increase in heteromerization and in glycosylation of HCN1 channel molecules after abnormal network activity (seizures), suggested that glycosylated channel molecules may be more likely to form heteromeric complexes. We tested this possibility by comparing the MWs of HCN1 channel molecules that were engaged in homomeric versus heteromeric complexes. Figure 3 demonstrates that both low and high MW HCN1 channels were precipitated by an antiserum to HCN1 in two independent samples of hippocampal homogenates (left panels). In contrast, an antiserum selective for the HCN2 isoform precipitated preferentially the high MW HCN1 isoform (right panels). These data are consistent with the notion that glycosylated species of HCN1 engage in stable co-assembly with HCN2 channel molecules, and are thus co-precipitated with this subunit.
Fig. 3.
The high MW species of HCN1 isoforms is preferentially co-immunoprecipitated with the HCN2 isoform. Two samples of hippocampal homogenates (S1 and S2) have been subjected to immunoprecipitation either with anti-HCN1 (left panels) or with anti-HCN2 sera. The precipitate was then probed for HCN1 immunoreactivity using western blots. Two species of the HCN1 channels, of low and high apparent MWs, were precipitated with anti-HCN1, as expected. In contrast, the HCN1 species that co-immunoprecipitated with anti-HCN2 was primarily of a higher MW, i.e. glycosylated. This indicates that stable complexes (heteromeric channels) with HCN2 are formed mainly by glycosylated HCN1 channel molecules.
Whereas the data shown so far demonstrate a good correlation of glycosylation and stable heteromerization of HCN1 channels, they did not address the issue of whether glycosylation was required for the augmented number of stable heteromeric HCN1/HCN2 complexes evoked by seizure activity. In order to address this question, an in vitro system for activity-evoked heteromerization was employed.
Seizure activity increases HCN1/HCN2 heteromerization in hippocampal organotypic slice cultures
To manipulate the state of glycosylation of the HCN channels during seizure-like network activity, we employed hippocampal organotypic slice cultures. These cultures recapitulate many feature of the developing hippocampus (Stoppini et al. 1991; Bender et al. 2007). In the context of the current studies, the expression patterns of HCN1 and HCN2 channel isoforms in the hippocampal formation were preserved in hippocampal cultures at an equivalent developmental age (Bender et al. 2007). For example, Fig. 4a shows the similar regional and subcellular distribution pattern of the HCN1 channels in native hippocampus and hippocampal organotypic cultures. In both systems, the expression of the channel in dendritic fields of area CA1 is evident. Previous work has found that seizure-like events can be generated within the preserved tri-synaptic hippocampal circuit in these cultures (Routbort et al. 1999; Bender et al. 2007; Richichi et al. 2007), and does not lead to cell death (Richichi et al. 2007). Therefore, we examined the effects of seizure-like activity (SA) on the heteromerization of HCN1/HCN2 channels, and whether eliminating channel glycosylation in this system altered this molecular rearrangements.
Fig. 4.
The distribution of HCN1 channels and the augmentation of their heteromerization with HCN2 isoforms are preserved in the hippocampal organotypic slice culture. (a): Immunocytochemistry demonstrates the similarity of HCN1 expression pattern in vivo and in the organotypic hippocampal slice cultures (in vitro). Note the strong immunoreactive signal in the distal dendritic fields of area CA1 in both preparations (white arrows). (b and c) Seizure-like activity generated by exposing the cultures to a low convulsant dose of the glutamate receptor agonist, kainic acid (see main text), increases the co-immunoprecipitation of HCN1 with the HCN1 isoform, as quantified in (c) (n = 4 per group). This recapitulates the findings in vivo, suggesting that the in vitro system can be used to study the underlying mechanisms. (d) HCN1 channels exist in species of different apparent MW (left gel panel), and the higher MW species are a result of glycosylation. Treatment with N-glycanase eliminated the ‘higher MW’ HCN1-immunoreactive band. In addition, treatment with the enzyme endo H, that preferentially cleaves mannose-type glycosyl moieties, failed to eliminate the ‘heavier’ band, suggesting that HCN1 glycosylation is not of the high-mannose type. DG denotes dentate gyrus.
Seizure-like activity, induced in organotypic hippocampal slice cultures by the application of 6 µmol/L KA for 3 h, significantly increased the co-immunoprecipitation of HCN1/HCN2 channels studied 48 h later (Fig. 4b and c; 96.8 ± 11.0 OD in controls and 138.1 ± 12.3 OD after seizure activity, n = 4; p < 0.05). This is consistent with the seizure-induced increased HCN1/HCN2 heteromeric complexes observed in vivo. As in the native hippocampus, co-immunoprecipitated HCN1 species were of an apparent MW (~125 kDa) consistent with their glycosylation. Indeed, treatment with N-glycanase shifted the mobility of the HCN1 immunoreactive bands to ~105 kDa (Fig. 4d). Further dissection of the type of glycosylation moieties attached to the HCN1 protein core was carried out using Endo H (EC 3.2.1.96), an enzyme cleaving specifically mannose chains (Plummer and Tarentino 1991). This treatment failed to eliminate the 125 kDa band, suggesting that the majority of the glycosyl residues attached to the HCN1 protein do not consist of high mannose chains (Fig. 4d). We then queried whether glycosylation was necessary for the enhancing effects of SA on HCN1 heteromerization.
Blocking glycosylation in vitro eliminates activity-dependent increase in HCN1/HCN2 heteromerization
To eliminate HCN channel glycosylation, cultures were grown in the presence of 10 µg/mL tunicamycin. Using the fluorojade method to assess neuronal injury (Schmued et al. 1997; Dube et al. 2004), Fig. 5a shows that tunicamycin did not make cultures more vulnerable upon exposure to KA, compared with cultures not treated with tunicamycin. Tunicamycin reduced HCN1 channel glycosylation in a dose-dependent manner (Fig. 5b), and the dose used here eliminated the high MW band of HCN1 channels.
Fig. 5.
Tunicamycin can be used in organotypic hippocampal cultures to prevent HCN channels glycosylation, and the treatment permit evaluation of seizure-like activity (SA). (a) Exposure of cultures to 10 µg/mL of the glycosylation blocker tunicamycin does not compromise their viability. Using fluorojade as a sensitive method for neuronal injury and death, no increase in cell death was found in treated cultures compared with controls. (b) Tunicamycin applied for 72 h prevents glycosylation of HCN1 channels in a dose-dependent manner. The large majority of HCN1 channels are de-glycosylated at the dose used here 10 µg/mL. (c) Activation of the principal hippocampal neurons by SA takes place also in the presence of tunicamycin. The immediate early gene c-fos, an established marker of neuronal activation (Labiner et al. 1993) is minimally expressed in control hippocampal cultures (ctrl, left panel). A 3 h exposure to KA leads to robust expression of c-fos, and this has been found to correlate with electrophysiological seizures (Labiner et al. 1993; Richichi et al. 2007). c-fos is expressed to a similar degree in hippocampal cultures maintained in the absence (SA; middle panel) or presence (SA + Tu; right panel) of tunicamycin (10 µg/mL).
Seizure-like activity was induced in control-and tunicamycin-treated cultures, and neuronal activation was apparent from the induction of the immediate-early gene, c-fos (Fig. 5c). Fos expression has been shown to correlate well with network activity (Labiner et al. 1993), including seizure-like events induced by KA in hippocampal organotypic cultures (Richichi et al. 2007). Compared with cultures that were not treated by the glutamate receptor agonist (Fig. 5c, left panel), both tunicamycin-treated and control cultures exposed to KA exhibited robust fos expression in hippocampal principal cell layers (Fig. 5c middle and right panels), indicating that network activation was not compromised by the exposure to tunicamycin.
The effect of blocked HCN1 channel glycosylation on seizure-induced heteromerization are shown in Fig. 6. Co-immunoprecipitation of HCN1/HCN2 was compared among cultures not exposed to KA and those that were treated with 6 µmol/L KA in the absence or presence of tunicamycin. In control hippocampal slice cultures, ~29% of total HCN1 channels were immunoprecipitated with HCN2 channels (Fig. 2a and b). The percentage of total HCN1 channels that heteromerized with HCN2 channels after 3 h of KA induced SA was increased to 38%. Tunicamycin treatment alone reduced the amount of detectable HCN1, as shown by the ‘input’ bands in Fig. 6a, likely because reduced glycosylation affected channel stability. Tunicamycin reduced the percentage of HCN1 channels associated with the HCN2 isoform to 21%. Importantly, treatment with tunicamycin abolished the seizure-induced increase in HCN1/HCN2 heteromerization (from 38.0 ± 2.7 in SA to 21.1 ± 3.7 in SA + tunicamycin, n = 4; p < 0.05; Fig. 6a and b). These data demonstrate that N-glycosylation may be required for seizure-induced stable heteromerization of HCN1 channels with HCN2 isoforms, an effect significantly altering the properties of the resulting h current (Chen et al. 2001a).
Fig. 6.
Inhibition of N-linked glycosylation abolishes the increased heteromerization of HCN1 channels by seizure-like network activity. (a) Quantitative analysis of the extent of heteromerization of HCN1 channels in organotypic hippocampal slice cultures was carried out using co-immunoprecipitation (IP), calibrated to the total amount of the HCN1 channels in the tissue. Actin was used to compare protein content among groups. As shown in the representative gels, in the absence of tunicamycin seizure-like activity (SA) increased the optical density of the HCN1 immunoreactive band that precipitated with antiserum to HCN2. However, after cultures were maintained in Tunicamycin (see text and Fig. 5), SA no longer enhanced the co-association of HCN1/HCN2 channels. (b) Quantitative analysis of the percentage of heteromerization (n = 5 per group).
Discussion
The main findings of the current set of experiments are (i) HCN1 and HCN2 are glycosylated in brain, specifically in hippocampus; (ii) glycosylated HCN1 channels are those that heteromerize with the HCN2 subunit; (iii) SA increases heteromerization of hippocampal HCN1 and HCN2 channels in vivo and in vitro, concurrent with augmentation of the relative abundance of the glycosylated form of HCN1; and (iv) blocking HCN channel glycosylation eliminates the ability of SA to increase HCN1/HCN2 heteromerization. These data are consistent with a role for glycosylation in promoting the expression of stable heteromeric HCN channels within hippocampus, with consequences for the properties of the h current and neuronal excitability.
The studies reported here evaluated the potential role of glycosylation in the seizure-induced formation of stable heteromeric HCN channels. Glycosylation of extracellular domains of membrane proteins is common, may commence already in the endoplasmic reticulum or Golgi apparatus, and may affect protein folding, stability, routing to the cell surface, as well as function and turnover (Lennarz 1983). The overall importance of glycosylation of membrane and secretory proteins is evident from the fact that abnormal glycosylation may lead to disease (e.g. carbohydrate-deficient glycoprotein syndromes) that provokes mental retardation or death (Carchon et al. 1999).
Glycosylation of ion channels, molecules that govern the firing properties of neurons and neuronal networks (Yaari and Beck 2002), has been demonstrated. For example, several members of the voltage gated K+ channel superfamily, which encompasses the HCN channels, are known to be glycosylated (Shi and Trimmer 1999; Much et al. 2003). Glycosylation of ion channels may influence their function at several levels (Thornhill et al. 1996; Bennett et al. 1997; Shi and Trimmer 1999). In the case of the HCN channels, a role for glycosylation in trafficking and membrane insertion has been illustrated in heterologous systems (Much et al. 2003). Indeed, it is interesting that glycosylation of a single channel isoform can rescue a non-glycosylated isoform that is engaged with the glycosylated one in a heteromeric complex, increasing its stability and membrane insertion (Much et al. 2003; Whitaker et al. 2007). These findings led us to query whether glycosylation played a role in the established activity-dependent augmentation of stable heteromerization of the HCN1/HCN2 channel isoforms.
Hyperpolarization activated cyclic-nucleotide gated 1/2 heteromerization is restricted in native hippocampus (estimated at ~20% (Amy L. Brewster, Qinqin Zha, Tallie Z. Baram, unpublished and see Fig. 1). Seizure-activity markedly enhances the formation of heteromeric HCN1/HCN2 channels, and these are associated with altered properties of the h current, and hyperexcitability of the hippocampal circuit. Heteromeric HCN1/HCN2 channels may also contribute to the generation of abnormal network activity of the thalamocortical circuit, contributing to absence epilepsy (Kuisle et al. 2006). Therefore, understanding the mechanisms of seizure-induced enhancement of HCN channel heteromerization is important. Increased heteromerization may be a passive process: seizures reduce HCN1 protein expression, increasing the ratio of HCN2/HCN1, and thus increasing the stochastic probability that an HCN1 molecule will encounter and interact with an HCN2 channel molecule. Alternatively, seizure-induced augmented HCN1/HCN2 heteromerization might be regulated, for example via increased glycosylation of the HCN1 channels, as shown here (Fig. 2). The glycosylated species of HCN1 channels seem to be preferentially heteromerized with the HCN2 isoform (Fig. 3). Importantly, blocking HCN channel glycosylation by tunicamycin abrogated the increased expression of heteromeric HCN1/HCN2 complexes in hippocampal tissue after seizures. This suggests that glycosylation is mechanistically involved in this process. Glycosylation may not influence the initial, co-translational interaction of HCN1 and HCN2 molecules (Deutsch 2002), a process dependent on the amino acid sequence of the N-terminus of the channels (Proenza et al. 2002). However, glycosylation probably influences the stability of HCN1/HCN2 complexes and their membrane insertion, consistent with the rescue of a non-glycosylated HCN isoform by its glycosylated heteromeric partner (Much et al. 2003; Whitaker et al. 2007).
The mechanisms by which activity regulates glycosylation are not well established. N-glycosylation of HCN channels is a co-translational process, so that altered glycosylation of fully mature channel molecules is unlikely. In addition, the glycosylation takes place on the ‘extracellular’ domain of the channel, and is thus less likely to be rapidly influenced by cellular processes such as neuronal activity. A plausible alternative suggests that the seizure-induced reduction of HCN1 transcription and translation (Brewster et al. 2002, 2005) increases the availability of glycosylating enzymes for each nascent HCN1 protein molecule. In other words, reduction of the number of HCN1 molecules going through the translation process ‘frees up’ glycosylation enzymes, to enhance the glycosylation of the channels that are being synthesized. In general, the overall glycosylation process is controlled by the amount and sequential availability of a large number of glycosylating enzymes (e.g. oligosaccharyl-transferase [EC 2.4.1.119], Golgi mannosidase I [EC 3.2.1.113]) in the different Golgi compartments.
Even moderate changes in the degree of HCN1 glycosylation may impact the abundance of heteromeric HCN1 channels: The authors speculate that HCN1 glycosylation increases the stability of HCN1/HCN2 heteromers and the half-life of heteromeric channels. Therefore, overtime, even a modest increase in glycosylated HCN1 channels will lead to a cumulative, robust increase of the overall percentage of HCN1 channels that are engaged in heteromeric complexes, and conduct an h current with distinctive biophysical properties.
In summary, heteromerization of HCN1 channels leads to currents with different biophysical properties (Chen et al. 2001b; Ulens and Tytgat 2001; Simeone et al. 2005; Kuisle et al. 2006). These changes alter the excitability of neurons and their responses to input from the neuronal network (Chen et al. 2001a), so that the molecular pathways by which activity alters the co-association and molecular rearrangement of these ion channels is important for our understanding of neuronal function. The current studies show that heteromerization of HCN1/HCN2 channels is increased by seizure activity concurrently with enhanced glycosylation of the HCN1 channels. Importantly, blocking HCN1 glycosylation abrogates seizure-evoked augmentation of heteromerization, implicating glycosylation of this channel in the activity-dependent expression of stable heteromeric HCN1/HCN2 complexes.
Acknowledgements
The authors thank Professor R. Shigemoto for antiserum to HCN1 (made in guinea pig), Drs. A. Ludwig and M. Biel for brains of HCN2–null mice, and J. Calara for excellent editorial help. This study was supported by NIH grant NS 35439.
Abbreviations used
- HCN
hyperpolarization activated cyclic-nucleotide gated
- KA
kainic acid
- MW
molecular weight
- OD
optical density
- P2
postnatal day 2
- PBS
phosphate-buffered saline
- PIC
protease inhibitor cocktail
- RIPA
radio-immuno-precipitation assay
- SA
seizure-like activity
- SDS
sodium dodecyl sulfate
References
- Bender RA, Kirschstein T, Kretz O, et al. Localization of HCN1 channels to presynaptic compartments: novel plasticity that may contribute to hippocampal maturation. J. Neurosci. 2007;27:4697–4706. doi: 10.1523/JNEUROSCI.4699-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett E, Urcan MS, Tinkle SS, Koszowski AG, Levinson SR. Contribution of sialic acid to the voltage dependence of sodium channel gating. A possible electrostatic mechanism. J. Gen. Physiol. 1997;109:327–343. doi: 10.1085/jgp.109.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräuer AU, Savaskan NE, Kole MH, et al. Molecular and functional analysis of hyperpolarization-activated pacemaker channels in the hippocampus after entorhinal cortex lesion. FASEB J. 2001;15:2689–2701. doi: 10.1096/fj.01-0235com. [DOI] [PubMed] [Google Scholar]
- Brewster AL, Bender RA, Chen Y, Dubé CM, Eghbal-Ahmadi M, Baram TZ. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform and cell-specific manner. J. Neurosci. 2002;22:4591–4599. doi: 10.1523/JNEUROSCI.22-11-04591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster AL, Bernard JA, Gall CM, Baram TZ. Formation of heteromeric hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the hippocampus is regulated by developmental seizures. Neurobiol. Dis. 2005;19:200–207. doi: 10.1016/j.nbd.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster AL, Chen Y, Bender RA, Yeh A, Shigemoto R, Baram TZ. Quantitative analysis and subcellular distribution of mRNA and protein expression of the hyperpolarization-activated cyclic nucleotide-gated channels throughout development in rat hippocampus. Cereb. Cortex. 2007;17:702–712. doi: 10.1093/cercor/bhk021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carchon H, Van Schaftingen E, Matthijs G, Jaeken J. Carbohydrate-deficient glycoprotein syndrome type IA (phosphor-mannomutase-deficiency) Biochim. Biophys. Acta. 1999;1455:155–165. doi: 10.1016/s0925-4439(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and function of voltage-sensitive ion channels. Science. 1988;242:50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Chen K, Aradi I, Thon N, Eghbal-Ahmadi M, Baram TZ, Soltesz I. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat. Med. 2001a;7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wang J, Siegelbaum SA. Properties of hyper-polarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J. Gen. Physiol. 2001b;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Brunson KL, Pomper J, Grigoriadis DE, Wurst W, Baram TZ. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc. Natl Acad. Sci. USA. 2004;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch C. Potassium channel ontogeny. Annu. Rev. Physiol. 2002;64:19–46. doi: 10.1146/annurev.physiol.64.081501.155934. Review. [DOI] [PubMed] [Google Scholar]
- Dubé CM, Baram TZ. Complex febrile seizures – an experimental model in immature rodent. In: Pitkanen A, Schwartzkroin PA, Moshe SL, editors. Models of Seizures and Epilepsy. San Diego: Elsevier; 2006. pp. 333–340. [Google Scholar]
- Dubé CM, Yu H, Nalcioglu O, Baram TZ. Serial MRI after experimental febrile seizures: altered T2 signal without neuronal death. Ann. Neurol. 2004;56:709–714. doi: 10.1002/ana.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé CM, Chen K, Eghbal-Ahmadi M, Brunson K, Soltesz I, Baram TZ. Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long term. Ann. Neurol. 2000;47:336–344. [PMC free article] [PubMed] [Google Scholar]
- Dubé CM, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, Johnston D. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in Ih. Nat. Neurosci. 2005;8:1542–1551. doi: 10.1038/nn1568. [DOI] [PubMed] [Google Scholar]
- Kuisle M, Wanaverbecq N, Brewster AL, Frere SG, Pinault D, Baram TZ, Luthi A. Functional stabilization of weakened thalamic pacemaker channel regulation in rat absence epilepsy. J. Physiol. 2006;15:83–100. doi: 10.1113/jphysiol.2006.110486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labiner DM, Butler LS, Cao Z, Hosford DA, Shin C, McNamara JO. Induction of c-fos mRNA by kindled seizures: complex relationship with neuronal burst firing. J. Neurosci. 1993;13:744–751. doi: 10.1523/JNEUROSCI.13-02-00744.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennarz W. Role of intracellular membrane systems in glycosylation of proteins. Methods Enzymol. 1983;98:91–97. doi: 10.1016/0076-6879(83)98142-9. [DOI] [PubMed] [Google Scholar]
- Levitan ES, Takimoto K. Dynamic regulation of K+ channel gene expression in differentiated cells. J. Neurobiol. 1998;37:60–68. doi: 10.1002/(sici)1097-4695(199810)37:1<60::aid-neu5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Lüthi A, McCormick DA. H-current: properties of a neuronal and network pacemaker. Neuron. 1998;21:9–12. doi: 10.1016/s0896-6273(00)80509-7. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneruons. J. Physiol. 1996;497:119–130. doi: 10.1113/jphysiol.1996.sp021754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat. Neurosci. 1999;2:508–514. doi: 10.1038/12229. [DOI] [PubMed] [Google Scholar]
- Much B, Wahl-Schott C, Zong X, Schneider A, Baumann L, Moosmang S, Ludwig A, Biel M. Role of subunit heteromerization and N-linked glycosylation in the formation of functional hyperpolarization-activated cyclic nucleotide-gated channels. J. Biol. Chem. 2003;278:43781–43786. doi: 10.1074/jbc.M306958200. [DOI] [PubMed] [Google Scholar]
- Plummer TH, Tarentino AL. Purification of the oligosachharide-cleaving enzymes of Flavobacterium meningosepticum. Glycobiology. 1991;1:257–263. doi: 10.1093/glycob/1.3.257. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Bullis JB, Roth MK. Modulation of h-channels in hippocampal pyramidal neurons by p38 mitogen-activated protein kinase. J. Neurosci. 2006;26:7995–8003. doi: 10.1523/JNEUROSCI.2069-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proenza C, Tran N, Angoli D, Zahynacz K, Balcar P, Accili EA. Different roles for the cyclic nucleotide binding domain and amino terminus in assembly and expression of hyperpolarization-activated, cyclic nucleotide-gated channels. J. Biol. Chem. 2002;277:29634–29642. doi: 10.1074/jbc.M200504200. [DOI] [PubMed] [Google Scholar]
- Richichi C, Brewster AL, Bender RA, et al. Mechanisms of seizure-induced ‘transcriptional channelopathy’ of hyperpolarization-activated cyclic nucleotide gated (HCN) channels 2007. Neurobiol. Dis. 2007 doi: 10.1016/j.nbd.2007.09.003. (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu. Rev. Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- Routbort MJ, Bausch SB, McNamara JO. Seizures, cell death, and mossy fiber sprouting in kainic acid-treated organotypic hippocampal cultures. Neuroscience. 1999;94:755–765. doi: 10.1016/s0306-4522(99)00358-9. [DOI] [PubMed] [Google Scholar]
- Santoro B, Baram TZ. The multiple personalities of h-channels. Trends Neurosci. 2003;26:550–554. doi: 10.1016/j.tins.2003.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Shi G, Trimmer JS. Differential asparagine-linked glycosylation of voltage-gated K+ channels in mammalian brain and in transfected cells. J. Membr. Biol. 1999;168:265–273. doi: 10.1007/s002329900515. [DOI] [PubMed] [Google Scholar]
- Shin M, Chetkovich DM. Activity-dependent regulation of h channel distribution in hippocampal CA1 pyramidal neurons. J. Biol. Chem. 2007;282:33168–33180. doi: 10.1074/jbc.M703736200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone TA, Dube C, Rho MJ, Baram TZ. Properties of neuronal single channel hyperpolarization-activated cation currents (IH) are altered after experimental febrile seizures. Soc. Neurosci. 2005;35:377.6. [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods. 1991;31:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Strauss U, Kole MH, Brauer AU, Pahnke J, Bajorat R, Rolfs A, Nitsch R, Deisz RA. An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur. J. Neurosci. 2004;19:3048–3058. doi: 10.1111/j.0953-816X.2004.03392.x. [DOI] [PubMed] [Google Scholar]
- Thornhill WB, Wu MB, Jiang X, Wu X, Morgan PT, Margiotta JF. Expression of Kv1.1 delayed rectifier potassium channels in Lec mutant Chinese hamster ovary cell lines reveals a role for sialidation in channel function. J. Biol. Chem. 1996;271:19093–19098. doi: 10.1074/jbc.271.32.19093. [DOI] [PubMed] [Google Scholar]
- Ulens C, Tytgat J. Functional heteromerization of HCN1 and HCN2 pacemaker channels. J. Biol. Chem. 2001;276:6069–6072. doi: 10.1074/jbc.C000738200. [DOI] [PubMed] [Google Scholar]
- Whitaker GM, Angoli D, Nazzari H, Shigemoto R, Accili EA. HCN2 and HCN4 isoforms self-assemble and co-assemble with equal preference to form functional pacemaker channels. J. Biol. Chem. 2007;282:22900–22909. doi: 10.1074/jbc.M610978200. [DOI] [PubMed] [Google Scholar]
- Yaari Y, Beck H. ‘Epileptic neurons’ in temporal lobe epilepsy. Brain Pathol. 2002;12:234–239. doi: 10.1111/j.1750-3639.2002.tb00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]