Abstract
Despite progress made over the past 25 years, existing immunotherapies have limited clinical effectiveness in patients with cancer. Immune tolerance consistently blunts the generated immune response, and the largely solitary focus on CD8+ T cell immunity has proven ineffective in the absence of CD4+ T cell help. To address these twin-tier deficiencies, we developed a translational model of melanoma immunotherapy focused on the exploitation of high avidity CD4+ T cells that become generated in germline antigen deficient mice. We had previously identified a TRP-1 specific HLA-DRB1*0401-restricted epitope. Using this epitope in conjunction with a newly described TRP-1 germline-knockout, we demonstrate that endogenous TRP-1 expression alters the functionality of the auto-reactive T cell repertoire. More importantly, we show, by using MHC-mismatched combinations, that CD4+ T cells derived from the self-antigen deficient host indirectly triggers the eradication of established B16 lung metastases. We demonstrate that the treatment effect is mediated entirely by endogenous CD8+ T cells and is not affected by the depletion of host Tregs. These findings suggest that high avidity CD4+ T cells can overcome endogenous conditions and mediate their anti-tumor effects exclusively through the elicitation of CD8+ T cell immunity.
Keywords: CD4 T cells, Tolerance/Suppression/Anergy, Tumor Immunity, Knockout Mice, Melanoma
Introduction
Over the past 25 years, cancer immunotherapy has undergone tremendous innovation and progress with developments running parallel to advances in basic immunology, molecular genetics and gene therapy. Many of these treatments, which have included potent cytokine infusions, cancer vaccines, and the adoptive transfer of gene-modified lymphocytes, have been applied to patients with metastatic melanoma (1-3). These efforts, however, have been consistently hampered by poor immunogenicity, limited immune durability, immune tolerance and tumor escape (4-6). Moreover, many of these treatment strategies have focused on the activation of tumor-specific CD8+ T cells. Although this approach has produced striking effects in a wide array of animal models, it has also yielded limited effectiveness as a one-dimensional treatment strategy in patients with metastatic melanoma.
Despite emphasis on CD8+ T cell responses, there has been growing evidence to support the critical role for CD4+ T-helper cells in antitumor immunity. Murine studies have demonstrated that CD4+ T cells exert their effects through the induction and maintenance of B-cells and CD8+ T cells, and appear indispensable for the long-term maintenance of antigen-activated memory CD8+ T cells (7, 8). Several lines of evidence also suggest that CD4+ T cells have other direct and indirect effects on tumor cells, including those deficient in MHC class II, through the induction of a DTH-like reaction, attracting inflammatory cells (macrophages, granulocytes, eosinophils, and natural killer cells) to the tumor microenvironment (9, 10). The generalized production of IFN-γ made by activated CD4+ T cells has also been shown to have multiple effects, including direct tumor cytotoxicity, the upregulation of MHC molecules thus increasing tumor recognition, the enhancement of antigen processing, and the inhibition of tumor-induced angiogenesis (11-13).
Given the obvious need to develop more effective treatments for patients with cancer, we sought to develop a new translational-based model of immunotherapy that would more comprehensively evaluate the mechanistic effects of CD4+ T cells. Most proof-of-principle animal models of CD4 T cell immunotherapy involve the recognition of ubiquitous and highly immunogenic foreign or surrogate antigens (14-16), employ TCR transgenics with artificially high clonal T cell frequency (17), or overexpress potent cytokines (18). We chose to target the melanocyte-differentiation-antigen (MDA) TRP-1, an abundantly expressed melanosomal protein present in normal melanocytes and in melanomas, and partially secreted into the peripheral circulation (19, 20). We had previously described a novel DR4-restricted epitope from human TRP-1 (positions 277-297, an epitope contained within the secreted form of the protein) and shown that specific CD4+ T cells were capable of reacting to specific peptide, recombinant protein, lysate and whole tumor (21). Other investigators have shown that immune responses against TRP-1 are associated with autoimmune vitiligo (22-24) and the treatment of established tumors (17).
Our use of TRP-1 as a model antigen was further strengthened by the availability of a TRP-1 germline-deficient mouse (25). The immunologic effects of self-antigen expression on MHC class I and II-restricted T cell frequency, avidity and function have been demonstrated in murine models involving germline antigen loss to the MDA's gp100 and Tyrosinase (26, 27), and in a human model involving the MDA OA1 (ocular albinism I) (28). Based on the compelling data shown by these antigen knockout studies, we felt that TRP-1 was a highly relevant experimental self-antigen, uniquely suitable for experiments focused on CD4-based tumor immunity, autoimmunity and self-tolerance.
The present study was conducted to investigate the mechanistic effects and clinical utility of DR4-restricted TRP-1-specific CD4+ T cells. Our data indicate that high avidity CD4+ T cells generated in TRP-1-deficient mice successfully eradicate established melanoma tumors. To elucidate the mechanism of the anti-tumor effect, we utilized different genetic combinations of MHC expression present on both tumor cells and host APCs. We then partnered these MHC combinations with different restriction elements that mediate T cell reactivity. In so doing, we demonstrate that the tumor treatment effect is directly mediated by endogenous host melanoma-specific CD8+ T cells. We complete our studies by showing that depletion of host regulatory T cells (Tregs) have no demonstrable impact on tumor treatment efficacy. This final result appears to run in contrast with the prevailing school of thought that Tregs inhibit the anti-tumor effects of auto-reactive T cells.
Materials and Methods
Animals
TRP-1B-w, alternatively named TRP1-/- and described previously (17, 29, 30), were fully backcrossed onto a C57BL/6 background (31) and found to accept syngeneic tumor implants and skin grafts from C57BL/6 mice (data not shown). Murine class II-deficient, DR4-IE transgenic mice (DR4 Tg) (32), fully backcrossed onto a C57BL/6, were purchased from Taconic Farms. To create a double-mutant strain expressing the TRP-1B-w mutation and the DR4-IE transgene and appropriate control littermates, TRP1-/- mice were bred with DR4 Tg mice. Mice were confirmed to have two copies of the TRP-1B-w mutation based on phenotype (cappuccino colored, see Fig. 2A) and confirmed to have at least one allelic copy of the DR4-IE chimeric transgene using PCR amplification of genomic DNA (oligonucleotide primers previously described (32)). The F2 progeny yielded two experimental groups designated as TRP1-/-//DR4+and TRP1+/-//DR4+. C57BL/6 (BL/6), OT-I, and OT-II transgenic (33, 34) mice were purchased from Jackson Laboratories. All mice were 6-10 weeks old for experiments, and housed and bred at the IU LARC barrier facility under an established study.
Figure 2.
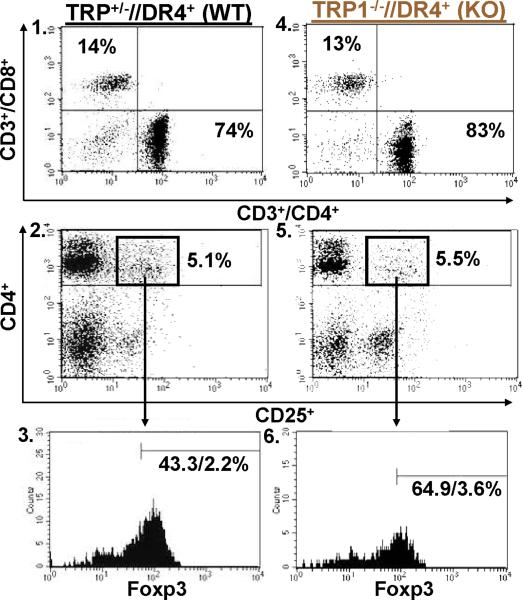
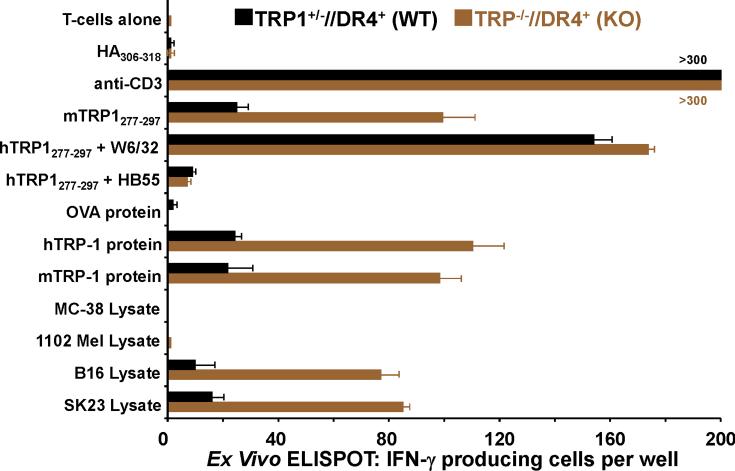
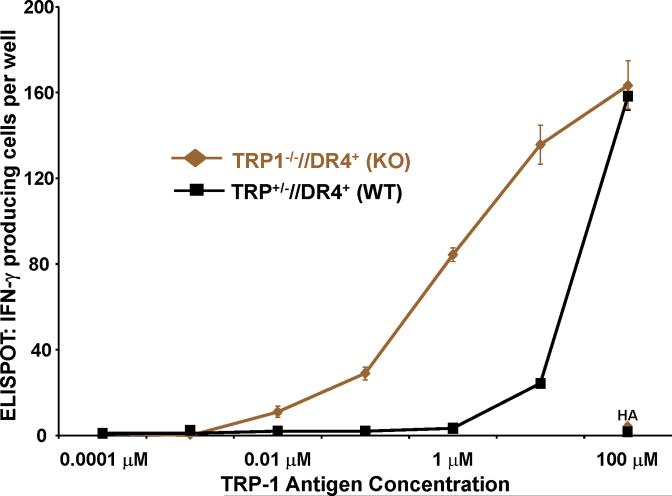
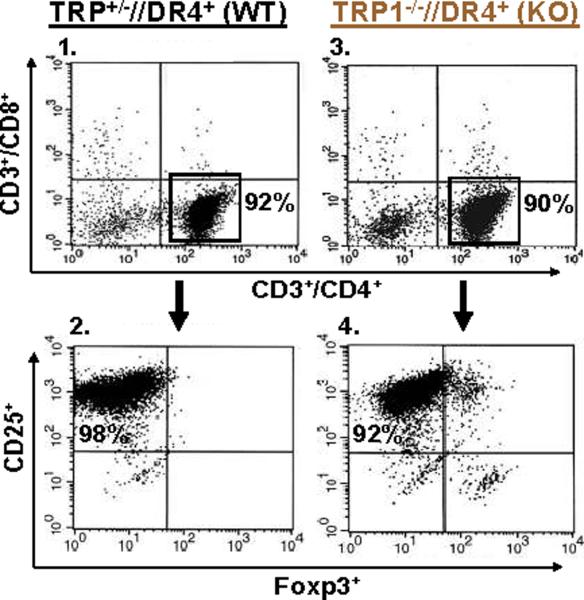
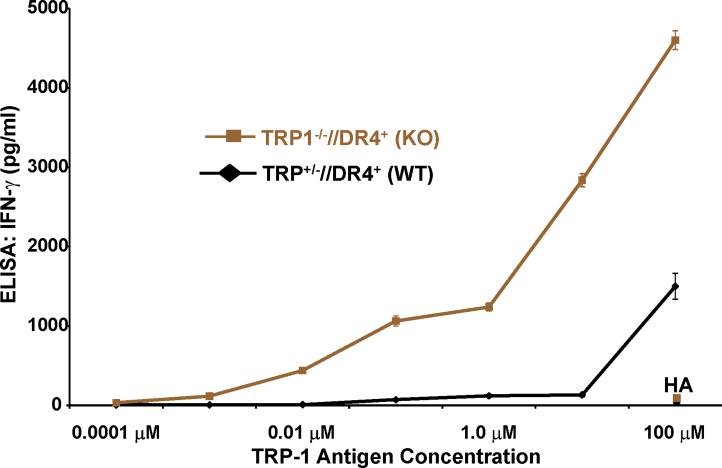
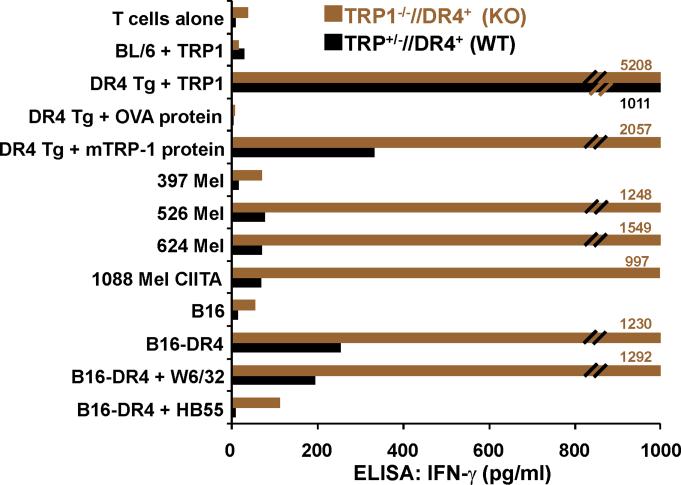
T cells from TRP1-/-//DR4+ Tg mice react more strongly to TRP-1 than wild-type controls. (A) TRP-1B-w (TRP-1-/-) mice were bred with DR4 Tg mice to generate TRP1-/-//DR4+ and control TRP1+/-//DR4+ littermates. (B) Flow cytometry analysis: LN populations obtained after vaccination are phenotypically equivalent. TRP1-/-//DR4+ (KO) and TRP1+/-//DR4+ (WT) littermates were immunized twice with recombinant hTRP-1. 14 days after the second immunization, LN cells (WT, panels 1-3; KO, panels 4-6) were harvested and stained with antibodies specific for murine CD4, CD8, CD3, CD25, and Foxp3. The panels are representative of three distinct experiments. (C) LN cells obtained from TRP-1 KO mice are more potent than WT controls. Following immunization, LN cells (4×105 per well) were stimulated ex vivo in ELISPOT plates with soluble anti-CD3 (2 μg/ml) or DR4+ EBV-B cells (1×105 per well; 1088 EBV-B) pulsed with peptide (100 □M) or protein (50 μg/ml) or lysate (105 cell equivalents). LN cells from both groups produce IFN-γ to anti-CD3 and EBV-B cells pulsed with mTRP-1277-297 or hTRP-1277-297 peptide, hTRP-1 or mTRP-1 protein, and to B16 and SK23 Mel lysate. Specific reactivity from both groups was blocked with anti-DR antibody HB55, but not with anti-MHC class I antibody W6/32. No reactivity observed to either the control peptide (HA306-318), protein (OVA) or lysates (MC-38, or 1102 Mel, both negative for TRP-1). (D) Ex Vivo ELISPOT titration assay: hTRP-1277-297-specific T cells from TRP1-/-//DR4+ mice are present at a higher frequency. TRP1-/-//DR4+ and TRP1+/-//DR4+ Tg littermates were immunized with hTRP-1 protein using the same immunization regimen. LN cells were harvested and stimulated (4×105 per well in triplicates) ex vivo with hTRP-1277-297 pulsed onto DR4+ EBV-B cells (1×105 per well; 1088 EBV-B) at titrating (100 to 0.0001 mM) peptide concentrations. All experiments were performed 2-3 times with similar results.
Cell Lines
Murine (B16.F10, MC-38, E.G7; ATCC, Manassas, VA), human tumor lines (397, 526, 624, 1088, 1102 and SK23 Mel, provided as a gift from S.A. Rosenberg, Surgery Branch, NCI/NIH) and human EBV-B cell line (1088 EBV-B) were maintained in complete media (CM), as previously described (35). 1088 Mel stably transduced with CIITA was prepared as previously described (21). HLA-DRB1* genotypes of tumor lines used in the following experiments included 397 Mel (0404, 0408); 526 Mel (0401,1401); 624 Mel (0401,0701); 1088 Mel and EBV-B (0301,0401), as previously described (36). Murine tumors B16.F10 (B16) and MC38 were transduced with the retrovirus pLXSN (Clontech, Mountain View, CA) expressing the DR4 transgene (provided as a gift from P. Robbins, Surgery Branch, NCI/NIH).
HLA Class II peptide-binding assays
HLA class II molecules were purified from Epstein-Barr virus (EBV) transformed homozygous B lymphoblastoid cell lines or transfected fibroblasts by affinity chromatography. Peptide binding assays were performed by incubating purified human Class II molecules (5 to 500 nM) with various concentrations of unlabeled peptide inhibitors and 0.1-1nM 125I-radiolabeled probe peptides for 48h in PBS containing 0.05-0.15% Nonidet P-40 (NP40) in the presence of a protease inhibitor cocktail. MHC binding of the radiolabeled peptide was determined by capturing MHC/peptide complexes on LB3.1 (anti-HLA-DRA) antibody coated Lumitrac 600 plates (Greiner Bio-One, Frickenhausen, Germany), and measuring bound cpm using the TopCount (Packard Instrument Co., Meriden, CT) microscintillation counter. The concentration of peptide yielding 50% inhibition of the binding of the radiolabeled probe peptide (IC50) was then calculated. Peptides were typically tested at six different concentrations covering a 100,000-fold dose range, and in three or more independent assays. Under the conditions utilized, where (label) < (MHC) and IC50 ≥ (MHC), the measured IC50 values are reasonable approximations of the KD values.
Immunizations
Experimental mice (TRP1-/-//DR4+ (KO) and TRP1+/-//DR4+ (WT)) or control mice (OT-I or OT-II) were immunized with 50 μg of protein (mTRP-1, hTRP-1 or OVA). Preparation of recombinant mTRP-1 and h-TRP-1 has been previously described (21). Recombinant full-length OVA protein was purchased from Sigma; St. Louis, MO. Proteins were emulsified in CFA (final volume 100 μL), divided equally and administered subcutaneously into the rear footpads. Fourteen days after primary vaccination, mice were boosted with same amount of protein in IFA in the same location. Two weeks after the second immunization, inguinal lymph node (LN) cells were extracted and assessed for ex vivo reactivity or expanded in culture for subsequent experimental assays.
Ex vivo assays
Two weeks after the second immunization, LN cells were extracted and assessed for phenotypic expression using fluorochrome-conjugated antibodies specific for murine CD4 (GK1.5, APC, BD Bioscience, San Diego, CA), CD8 (53-6.7, PE, BD Bioscience), CD3 (17A2, PE-Cy5, BD Bioscience), CD25 (7D4, FITC, BD Bioscience), and Foxp3 (FJK-16s, Pacific Blue, eBioscience, San Diego, CA). ELISPOT ex vivo assays were performed in the following manner: M200 plates (CTL, Shaker Heights, OH) were precoated with IFN-γ antibody (R46A2; BD Biosciences) at 4 μg/ml and incubated overnight; LN cells (4×105/well) and targets (pulsed 1×105 1088 EBV-B cells) were added to wells in triplicate in 200 μL of HL-1 CM (HL-1 Media, Biowhittaker, Walkersville, MD, L-glutamine 200 mM; and Gentamycin sulfate 50 mg/ml); EBV-B cells were pulsed the day before (for 12-18 hours) with peptide (50 μM), protein (50 μg/ml) or lysate (105 cell equivalents/well; and prepared as previously described (21)); Following 24-hour coculture, plates were incubated with biotinylated IFN-γ antibody (XMG1.2; BD Biosciences) at 2 μg/ml; The next day plates were incubated with Streptavidin-AP (Southern Biotechnology, Birmingham, AL) at a 1:1000 dilution for 2 hours, followed by the addition of NBT-BCIP (Thermo Scientific, Rockford, IL); the resulting spots were counted on a computer-assisted ELISPOT image analyzer (Immunospot Series I Analyzer, CTL). Monoclonal antibodies (mAbs) used (at 50 μg/ml) to inhibit recognition by T cells included HB55 (against HLA-DR; IgG2a; ATCC) and W6/32 (against HLA-A, B, C; IgG2a; ATCC).
Generation of established CD4+T cell lines
Extracted LN cells from immunized mice were cultured in 24-well plates at 5×106 cells per well with peptide hTRP-1277-297 at 50 μM (or control class I OVA257-264 at 10 μM or control class II OVA323-339 50 μM). Twelve days after the first ex vivo stimulation, both lines were restimulated with peptide-pulsed, irradiated, syngeneic DR4 Tg splenocytes. Splenocytes were pulsed with hTRP-1277-297 at 50 μM for 3 hours at 37 degrees, washed, irradiated with 3000 rads, then added to each T cell culture at a 10:1 ratio (5×106 APCs per well). CM containing IL-2 (Chiron, Emeryville, CA) at 7.5 CU/ml was added 2 days after the stimulation (day 14). Multi-color flow cytometry for CD4, CD8, CD25 and Foxp3 T cell expression was performed using mAb's, described above. Lines were stimulated and maintained using the same methods every 10-15 days. To evaluate specific T cell reactivity, splenocytes or 1088 EBV-B cells were pulsed for 3 hours with peptide (at various concentrations) or with recombinant protein overnight. Whole tumor cells (pre-treated with IFN-γ × 48 hours at 200 U/ml) or pulsed APCs (105 cells per well) were then co-cultured with 105 T cells per well in U-bottom 96-well plates for 24 hours. mAb's HB55 and W6/32 were again used to block T cell interactions. Culture supernatants were assayed for IFN-γ, IL-4 or IL-17 using commercially available ELISA kits (BD Bioscience).
Tumor treatment and antibody depletion assays
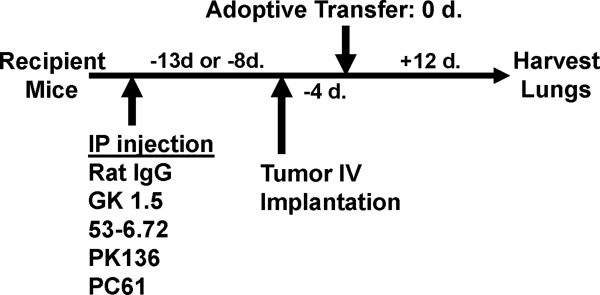
Six- to eight-week-old female C57BL/6 and DR4 Tg mice were injected IV through the tail vein with 5×105 B16 melanoma cells, MC-38 or E.G7 tumor cells at 5 animals per group (day -4). Four days later mice were injected IV (on day 0) with various quantities of T cells generated from immunized OT-1, OT-II, TRP1-/-//DR4+ and TRP1+/-//DR4+ mice. Twelve days later (day +12), lungs were countered stained with India ink, removed, and metastases enumerated in a blinded fashion (15). For tumor treatment experiments involving antibody depletion, monoclonal antibodies to murine CD4 (GK1.5), CD8 (53-6.7), and NK and NKT (PK136) were purified from hybridoma culture supernatants (ATCC), and injected (100 μg) intraperitoneally (IP) into host mice at 5 animals per group every 3 days beginning 9 days (day -13) before tumor injection. Treg depletion was performed with a single IP injection of 400 μg (anti-CD25 PC61 or control rat IgG) 4 days prior to tumor implantation (day -8) into 5 experimental mice per group. Depletion analysis by FACS was performed on peripheral blood on the day of adoptive transfer (day 0). Treatment results are shown as SEM and statistical significance between groups was determined by Student T-test.
Results
Despite differences between human and murine TRP-1, the 277-297 epitope is a potent auto-antigen
We previously described a HLA-DRB1*0401-restricted epitope from hTRP-1, using a strategy that combined the immunization of DR4 Tg mice with candidate peptide epitopes generated from a computer-based prediction algorithm (21). Antigen-specific T cells from a melanoma patient were then raised with serial in vitro stimulation and found to react with specific peptide, recombinant protein, lysate and whole tumor. These findings suggested that endogenous and exogenous antigen processing pathways presented the specific epitope to available CD4+ T cells, a finding also shown to be dramatically more pronounced from the PBMC of melanoma patients than those from normal volunteers (21).
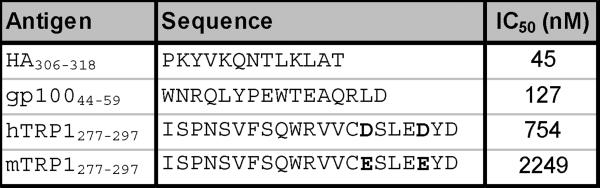
To begin exploring the functional effects of TRP-1 specific CD4+ T cell immunity within a purely autoimmune setting we initially sought to determine if the human TRP-1277-297 epitope was cross-reactive with its murine counterpart. Amino acid sequences of murine and human TRP-1 sequences are more than 80% identical with the greatest degree of dissimilarity found at both NH2 and COOH termini (37). In addition, two conservative amino acid substitutions within the human epitope at positions 291 and 295 (aspartic acid - D to glutamic acid - E; mTRP-1277-297 ISPNSVFSQWRVVCESLEEYD) (Fig. 1A) were identified. When the relative affinity (IC50) of the two peptides was measured in a competitive binding assay, the concentration of the human variant needed to inhibit recognition of the standard peptide was nearly three times less than that of its murine counterpart, yet both were significantly weaker binders than control peptides from other well established DR4-epitopes (HA306-318 and gp10044-59 (35)) (Fig. 1B).
Figure 1.
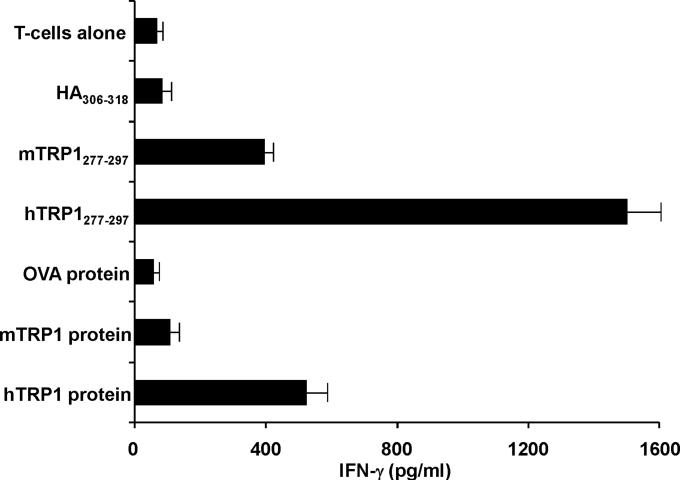
Despite differences between human and murine TRP-1, TRP-1277-297 is a potent self-antigen. (A) Sequence comparison analysis between human and murine TRP-1277-297 demonstrate conserved differences in positions 291 and 295, that involve aspartic acid (D) to glutamic acid (E) changes in both positions. (B) The IC50 nM concentration of the human TRP-1 variant is nearly one-third that of the murine form, but greater than epitopes that HA306-318 and gp10044-59. (C) Human TRP-1277-297 is a potent auto-antigen. DR4 Tg mice were immunized twice with mTRP-1 protein and extracted LN cells were stimulated with hTRP-1277-297 and expanded with IL-2. 14 days after restimulation cells were assayed for specific IFN-γ secretion by ELISA. Immune responses were superior to hTRP-1 peptide (pulsed onto DR4+ 1088 EBV-B at 50 μM) and hTRP-1 protein (pulsed onto DR4+ 1088 EBV-B at 25 μg/ml) when compared with reactivities observed to mTRP-1 peptide and protein. No significant immune response was observed to the control peptide HA306-318 or control protein OVA. All experiments were performed 2-3 times with similar results.
Given the relative low binding affinity, it was not clear if the murine variant was presented to specific circulating T cells. To resolve this question, we immunized DR4 Tg mice twice with mTRP-1 protein then stimulated extracted LN cells two weeks after the second vaccination with hTRP-1277-297. Nine days later, cells were restimulated with peptide pulsed splenocytes and expanded with IL-2. Fourteen days after restimulation, cells were assayed for specific IFN-γ secretion by ELISA. Immunization with murine TRP-1 successfully primed immune responses to both human and murine TRP-1 (Fig. 1C). More importantly, immune responses were superior to both hTRP-1 peptide (pulsed onto DR4+ 1088 EBV-B at 50 μM) and hTRP-1 protein (pulsed onto DR4+ 1088 EBV-B at 25 μg/ml) when compared with reactivities observed to the weaker binding mTRP-1 peptide and protein. No significant immune response was observed to control peptide HA306-318 or control protein OVA (both pulsed at similar concentrations). Given these findings, it appeared that both species-specific forms of the 277-297 auto-epitope were presented by HLA-DR4 and recognized by host CD4+ T cells. Moreover, these results suggested that hTRP-1 would serve as the stronger immunogen for subsequent experimental work.
Immune responses to TRP1277-297 are significantly greater in TRP1-/-//DR4+ Tg mice
Having established that the 277-297 DR4-restricted epitope is an experimental self-antigen, we sought to evaluate the effect of germline antigen loss on self-tolerance. TRP-1B-w mice were generated at Oak Ridge laboratories by exposure to gamma irradiation and found to express a unique “cappuccino” phenotype secondary to a large chromosomal rearrangement juxtaposing the promoter sequence from the coding region of the TRP-1 gene, confirming its pivotal role in normal melanin (black) production. Previous studies had demonstrated that RNA extracted from neonatal TRP-1B-w mice skin samples and retinal pigment epithelium (both sources of peripheral melanocytes) were fully deficient in RNA-level expression of the TRP-1 gene (29). TRP-1B-w mice were then bred with DR4 Tg (also previously backcrossed onto a C57BL/6 background) mice to generate TRP1-/-//DR4+ (KO) and control TRP1+/-//DR4+ (WT) littermates (Fig. 2A).
We next sought to determine if functional differences in the TRP-1-specific, DR4-restricted CD4+ T cell repertoire were observable in mice germline-deficient for TRP-1. To evaluate differential immune responses, TRP1-/-//DR4+ (KO) and control TRP1+/-//DR4+ (WT) littermates were each immunized with recombinant hTRP-1 protein. Two weeks after the second immunization, LN cells were analyzed by multicolor flow cytometry for phenotypic features. Both populations had largely equivalent and non-significant differences in the percentage of total CD4+ T cells (74% WT vs. 83% KO, panels 1 and 4) and Tregs (CD4+/CD25+/Foxp3+: 2.2% WT vs. 3.6% KO of all CD4's, Fig. 2B, panels 2-3 and 5-6, respectively). LN cells were then stimulated for 24 hours with peptides (100 μM), protein (50 μg/ml), or lysate (105 cell equivalents) pulsed onto DR4+ EBV-B cells (1088 EBV-B). T cell responses were then assessed for ex vivo reactivity using a standard ELISPOT assay for IFN-γ production. Strong and equivalent ex vivo reactivity was detected in both groups to hTRP-1 peptide and control anti-CD3 stimulation, however reactivity to mTRP-1 peptide, human and murine TRP-1 protein, and lysate (SK23 and B16) was significantly greater with CD4+ T cells derived from TRP1-/-//DR4+ in comparison with control TRP1+/-//DR4+ littermates. Reactivity was also shown to be fully inhibited with mAb HB55 (anti-HLA-DR), but not with control mAb W6/32 (anti-MHC-class I) (Fig. 2C). Importantly, no IL-4 or IL-17 secretion was detected from either group, excluding the possibility of an ex vivo Th2 or Th17 response (data not shown).
Although lower T cell frequencies were observed by ex vivo ELISPOT in immunized TRP1+/-//DR4+ (WT) mice to murine TRP-1277-297 peptide, recombinant TRP-1 protein, and melanoma lysates, no significant difference in frequency was seen in response to hTRP-1277-297 peptide (mean = 174 vs. 154 per 4×105 LN cells) at 100 μM. We hypothesized, therefore, that T cell frequencies between TRP1-/-//DR4+ and TRP1+/-//DR4+ mice could be contrasted at titrating peptide concentrations, particularly in light of the differences in reactivity already observed to the less-effective binding peptide (mTRP-1277-297), recombinant protein and lysates. Using the same immunization protocol, LN cells were assessed by ex vivo ELISPOT against hTRP-1277-297 pulsed onto DR4+ EBV-B (1088 EBV-B) cells. A 1.5-log difference in T cell frequency (mean = 84 vs. 3) was observed at half-maximal (1 μM) stimulation for the TRP1-/-//DR4+ group, despite a similar number of total CD4+ T cells and Tregs. Again no difference in frequency was observed at 100 μM (mean = 163 vs. 158), suggesting that the presence or absence of self-antigen has little effect on the prevalence of the lowest avidity T cells (Fig. 2D).
In vitro expanded CD4+ T cells derived from TRP-1 germline deficient mice recognize tumor and retain superior avidity compared with littermate controls
Given the potent ex vivo reactivity that was observed with CD4+ T cells derived from TRP1-/-//DR4+ mice, we sought to determine if further expansion and enrichment of those cells would result in similar differential potency. Moreover, we investigated whether or not T cells from both groups would recognize intact tumor and exhibit a cytokine secretion profile consistent with that of a polarized T-helper phenotype. This latter issue has become increasingly relevant as recent reports have documented the importance of individual Th-subsets in inflammation, autoimmunity and anti-tumor responses (38). We felt this was of particular relevance given that many of the interpretations and functional studies of these populations have been performed following differential skewing with cytokine environments that promote selective subset development.
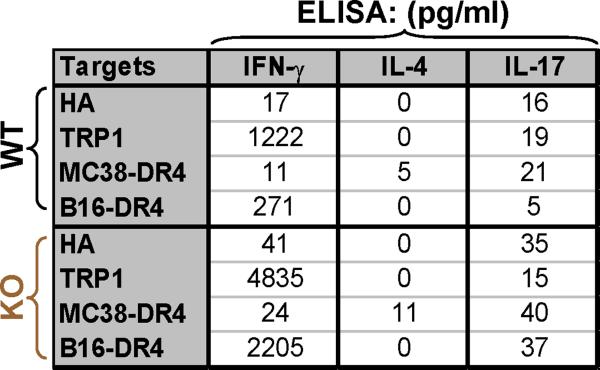
To address these questions, LN cells from immunized mice (using the standard regimen described in Fig. 2) were subsequently expanded in vitro with two rounds of peptide stimulation and IL-2. Following expansion, cells from both groups were more than 90% CD4+/CD25+ but negative for Foxp3, suggesting the absence of Tregs (Fig. 3A, panels 1-4). Despite equivalent activated phenotypic features, T cells continued to exhibit a pronounced differential titration effect to peptide hTRP-1277-297 with T cells derived from TRP1-/-//DR4+ mice recognizing peptide at a ratio of 15 times (KO IFN-γ/WT IFN-γ) greater than the control population at half-maximal stimulation (100 nM), and 25 times greater than the control population at the lowest level stimulation (1 nM) (Fig. 3B). In addition, CD4+ T cells from both groups were found to have similar differential reactivity to both the TRP-1 peptide and the recombinant protein pulsed onto DR4+ targets (DR4 Tg splenocytes) and no reactivity when pulsed onto DR4- APCs (C57BL/6 splenocytes, H-2 Kb/Db and I-Ab) (Fig. 3C). More importantly, although CD4+ T cells from both animal groups were capable of recognizing intact human tumor expressing both the appropriate restriction-element (DR4) and antigen (hTRP-1), T cells from TRP1-/-//DR4+ mice exhibited a more than 20-fold greater degree of cytokine release compared with the control population (Fig. 3C). T cells derived from the KO mice were also capable of recognizing the endogenously expressed self-antigen (mTRP-1) presented by HLA-DR4 (on B16 stably transduced with the DR4 transgene, but not native B16) and specific reactivity was abrogated with mAbs against MHC class II (HB55), but not class I (W6/32) (Fig. 3B). Although immediate ex vivo stimulation revealed a polarized Th1 phenotype, it was not clear if artificial in vitro culturing techniques used to expand CD4+ T cells would alter the cytokine profile. When the two Th populations were tested against exogenously loaded peptide and endogenously presented antigen (B16-DR4), we identified a fully polarized Th1 cytokine profile characterized by predominant IFN-γ secretion as detected by ELISA (and some IL-2, GM-CSF and TNF-α, data not shown), but virtually no IL-4 or IL-17A (Fig. 3D).
Figure 3.
CD4+ T cells derived from TRP-1 KO mice recognize tumor in vitro. TRP1-/-//DR4+and TRP1+/-//DR4+ Tg littermates were immunized with hTRP-1 protein and expanded in vitro. (A) Flow cytometric analysis of experimental T cell populations. Both groups of in vitro IL-2 expanded WT and KO T cells are equivalently CD4high/CD8dim (panels 1 and 3) and Treg negative CD4high/CD25high/Foxp3dim (panel 2 and 4). The panels are representative of three distinct experiments. (B) Significant differences in in vitro reactivity to titering concentrations of hTRP-1277-297 pulsed onto DR4+ EBV-B cells (1088 EBV-B) were observed between groups. (C) CD4+ T cells derived from germline-deficient hosts strongly recognize intact melanoma. CD4+ T cells from both groups differentially react to both the TRP-1 peptide and the recombinant protein when pulsed on DR4+ targets (DR4 Tg splenocytes), but not when pulsed onto DR4- APCs (C57BL/6 splenocytes, I-Ab+). No reactivity was observed to control protein OVA. Specific reactivity was also observed to both HLA-DR4+ and TRP-1+ human melanomas (526 and 624 Mel pretreated with IFN-γ and 1088 Mel stably transduced with CIITA) and to murine melanoma B16-DR4 (B16 stably transduced with HLA-DRB1*0401). No reactivity was observed against control tumor B16 (DR4-). Recognition of B16-DR4 was blocked with mAb HB55 (anti-class II), but not with W6/32 (anti-class I). (D) CD4+ T cells from both groups exhibit a Th1 cytokine profile (IFN-γ production, but none to IL-4 or IL-17A by ELISA) in response to specific peptide and tumor (B16-DR4, but not MC-38-DR4). All experiments were performed 2-3 times with similar results.
High avidity autoreactive CD4+ T cells derived from antigen-deficient hosts indirectly mediate tumor destruction through elicitation of host CD8+ T cells
Given the potent and superior reactivity of CD4+ T cells derived from TRP1-/-//DR4+ mice to both exogenously loaded and endogenously presented self-antigen, we sought to determine if those cells were more effective at eradicating tumor than those derived from WT controls. Moreover, we sought to determine if the treatment effect was not only antigen-specific and DR4-restricted, but mediated by direct or indirect recognition of tumor. To test these questions, we took advantage of the unique genetic differences in MHC expression on both the tumor cells (DR4-) and host APCs (DR4+), as well as the restriction elements that mediate the reactivity of both host CD8+ T cells (Kb/Db+) and the adoptively transferred CD4+ T cell treatment population (DR4+). A general schematic for all tumor treatment experiments (Figs. 4 and 5) is shown in Fig. 4A.
Figure 4.
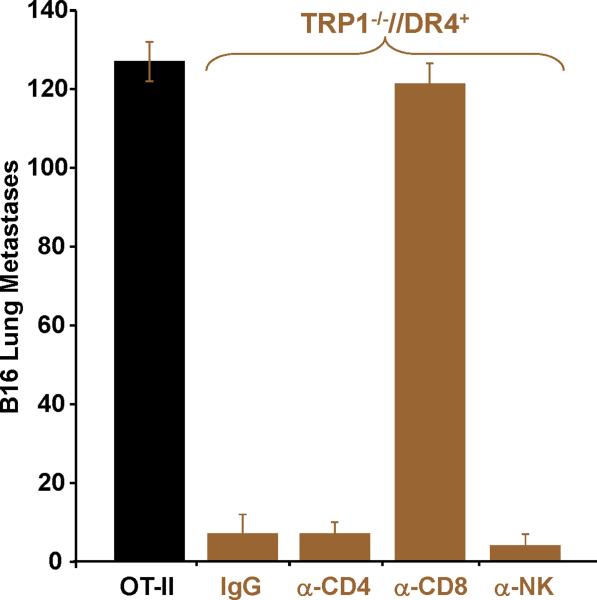
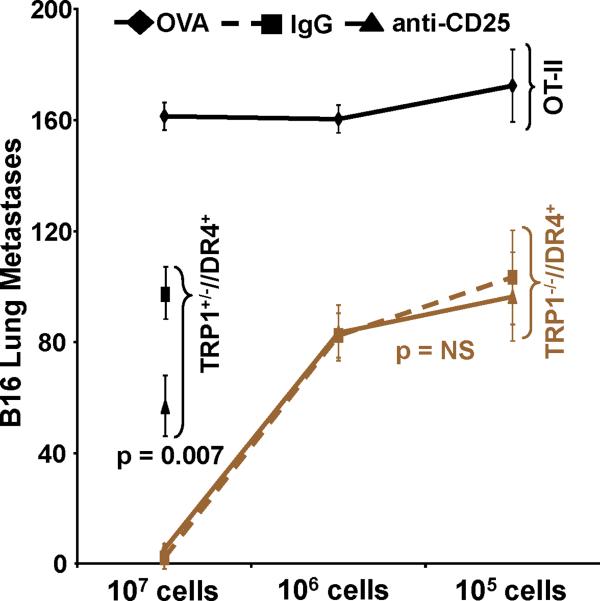
Adoptively transferred CD4+ T cells derived from TRP-1 KO mice eradicate established tumor by priming host CD8+ T cells. To prepare cells for adoptive transfer, TRP1-/-//DR4+ and TRP1+/-//DR4+ Tg littermates were immunized with hTRP-1 protein and expanded in vitro. Control OT-II mice were similarly immunized and expanded. (A) A general schematic of all tumor treatment experiments. (B) Treatment is antigen-specific and DR4-restricted. Expanded T cells were adoptively transferred (107 per mouse) into three different tumor-bearing hosts at 5 mice per group: 1) B16 (TRP-1+) lung metastases in DR4 transgenics (DR4+); 2) B16 lung metastases in C57BL/6 mice (DR4-); 3) MC38 (TRP-1-) lung metastases in DR4 transgenics. Each group had been previously IV tail vein inoculated with tumor. CD4+ T cells derived from the TRP-1-/-//DR4+ mice nearly fully eradicate the 4-day lung metastases (mean = 5; p = 0.0006 using a two-tailed Student T-test) when compared to littermate (WT) controls (mean = 68) and control OVA-specific CD4+ T cells (mean: 156). No treatment effect was observed (mean = >150 lung metastases for all treatment groups) in mice bearing the control tumor cell line (MC38) or in recipient mice lacking the specific restriction element (C57BL/6 mice; DR4-). (C) Treatment of B16 lung metastases is dependent upon host CD8+ T cells. Tumor bearing (B16 only) DR4 Tg mice were depleted of individual effector-cell subsets (CD4, CD8, NK and NKT) following IP injection of mAb's prior to adoptive transfer of CD4+ T cells derived from TRP1-/-//DR4+ mice. Control OVA-specific CD4+ T cells were used to measure the non-specific effects of adoptive transfer. Each experimental arm involved 5 mice per group and all experiments were performed 2-3 times with similar results.
Figure 5.
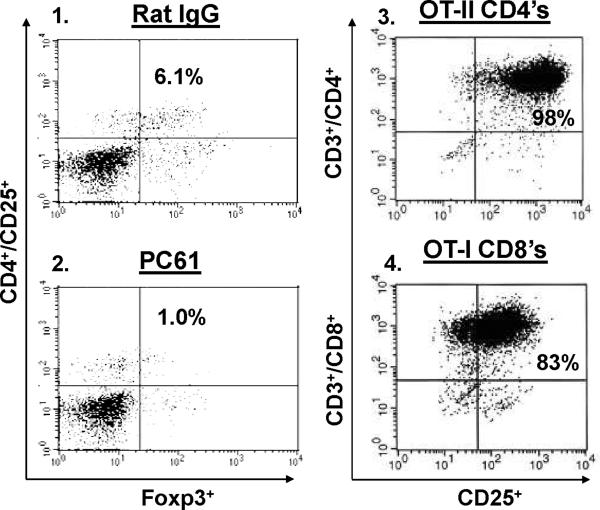
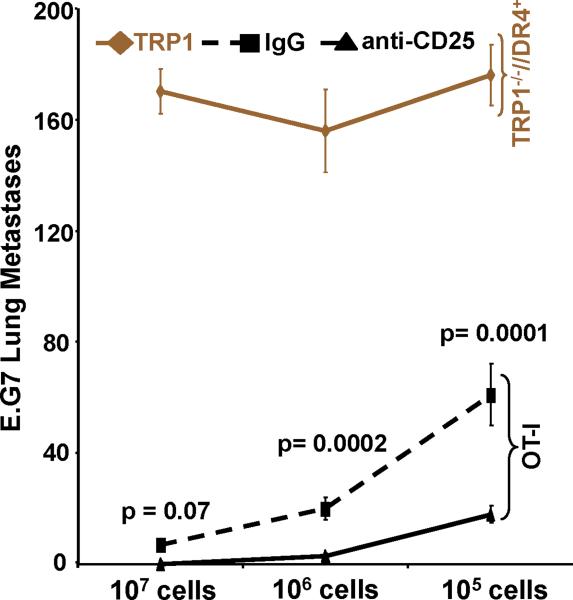
Adoptively transferred CD4+ T cells derived from TRP-1 KO's are maximally effective against tumor regardless of Treg status. (A) Flow cytometric analysis obtained from peripheral blood samples on the day of adoptive transfer of host Treg status (CD4+/CD25+/Foxp3+) following depletion with control rat IgG (panel 1) or with PC61 (panel 2), respectively. Flow cytometric analysis of control in vitro expanded OT-II and OT-I cells are CD4high/CD25high (panel 3) and CD8high/CD25high (panel 4), respectively; the panels are representative of three distinct experiments. (B) Treatment of B16 lung metastases is unaffected by Treg depletion. Tumor bearing (B16 only) DR4 Tg mice were depleted of Tregs following IP injection of mAb PC61 prior to adoptive transfer. CD4+ T cells derived from KO mice and control OT-II mice were transferred at titering concentrations (107, 106, and 105). CD4+ T cells derived from TRP-1 WT mice were transferred at 107 cells only. Pre-treatment with PC61 has no impact on treatment when compared with control rat IgG administration using KO cells when compared with WT cells. No significant treatment effect was observed using the control OVA-specific CD4+ T cell transfer. (C) OVA-specific CD8+/CD25+ T cells more effectively eradicate established tumor in hosts depleted of Tregs. Tumor bearing (E.G7 only) C57BL/6 mice were similarly depleted of Tregs prior to adoptive transfer. CD8+ T cells derived from OT-I mice and control TRP-1-specific CD4's were adoptively transferred at titering concentrations. Pre-treatment with PC61 significantly improved the overall treatment effect at both 106 and 105 cells infused when compared with control rat IgG. No significant treatment effect was observed using control TRP-1-specific CD4+ T cells. Each experimental arm involved 5 mice per group, and all experiments were performed at least 2-3 times with similar results.
To investigate these issues, we initially performed a 4-day lung metastasis treatment experiment using three different tumor-bearing hosts: 1) B16 (TRP-1+) lung metastases in DR4 Tg (DR4+); 2) B16 lung metastases in C57BL/6 (DR4-); 3) MC38 (TRP-1-) lung metastases in DR4 Tg. T cells (from both WT and KO mice) were again obtained from a LN harvest following a similar immunization and in vitro expansion and adoptively transferred (107 cells per mouse) into tumor-bearing recipients. Importantly, an equal number of both total cells and CD4+ T cells, including an equal but small number of Tregs (CD4+/CD25+/Foxp3+: Fig. 3A, panels 1-4) were used for adoptive transfer. Control OVA-specific CD4+ T cells were obtained from OT-II mice, after a similar immunization and in vitro expansion with ovalbumin323-339 (34). We observed a significant difference in treatment effect using T cells obtained from TRP1-/-//DR4+ mice (mean = 5 lung mets; p = 0.0006 using a two-tailed Student T-test) vs. WT littermates (mean = 68 lung metastases) vs. control OVA CD4's (mean = 156 lung metastases) in DR4 Tg recipients bearing B16 lung metastases (Fig. 4B). This result was contrasted with the complete loss of any treatment effect (mean = >150 lung metastases for all treatment groups) in mice bearing our control antigen-deficient tumor cell line (MC38) or in recipient mice lacking the specific restriction element (C57BL/6 mice; DR4-). Unlike other models of adoptive transfer (17, 39), no exogenous IL-2 was administered, nor were recipient animals vaccinated or irradiated for these experiments. These results confirmed that the treatment effect was both melanoma-specific and restricted by host DR4 expression.
To more specifically determine if the treatment effect was mediated by individual T cell subset populations (from either the host or those transferred), we performed a similar adoptive transfer experiment using CD4+ T cells derived only from TRP1-/-//DR4+ mice. Tumor bearing (B16 only) recipient mice were first depleted of individual effector-cell subsets (CD4, CD8, NK and NKT) prior to adoptive transfer (elimination of >95% of each population was confirmed by flow cytometry, data not shown). The treatment effect was entirely abrogated following host CD8+ T cell depletion with no significant difference between the CD8 depletion group and the OVA control group (mean = 121 vs. 127 lung mets; p = 0.106). Moreover, we excluded the contribution of other host components by observing a nearly complete and equivalent treatment effect following host CD4 and NK/NKT depletion (mean = 7 vs. 4 lung mets; p = 0.128) or with control rat IgG administration (Fig. 4C).
High avidity autoreactive CD4+ T cells overcome host Tregs and mediate tumor destruction
A preponderance of animal and clinical studies have demonstrated that elimination of Tregs can break self-tolerance, enhance anti-tumor immunity, and improve the anti-tumor effects of both cancer vaccines and adoptively transferred cells (40-42). We hypothesized, therefore, that depletion of host Tregs would improve the treatment effect of adoptively transferred CD4+ T cells. To address this question, we employed an established protocol (43) using an anti-CD25 mAb (PC61) capable of depleting CD4+/CD25+/Foxp3+ Tregs (as determined by flow cytometry on the day of adoptive transfer from peripheral blood samples, Fig. 5A, panels 1-2) while simultaneously having no effect on host CD25- cells (both CD8+ and NK+), nor any suppressive effect on members of the IL-2 cytokine family including IL-21 (44). To more precisely measure the depletion effect on treatment, we adoptively transferred titering numbers of CD4+ T cells derived from TRP1-/-//DR4+ mice in comparison with those from control OVA-specific cells (CD4+/CD25+, as determined by flow cytometry, Fig. 5A, panel 3). Although we found a decreasing treatment effect with titering numbers (107, 106, and 105) of infused specific cells, we also found that pre-treatment with PC61 had no demonstrable impact on the overall efficacy when compared with the control IgG administration. This result was contrasted by a differential treatment effect (mean = 96 vs. 47 lung metastases; p = 0.007) that favored Treg elimination prior to adoptive transfer of the lower avidity WT TRP-1 specific T cells (107 cells only). As before, we found no treatment effect with the control OVA-specific CD4+/CD25+ T cell transfer (Fig. 5B).
Although neutralizing levels of PC61 (administered 8 days prior to adoptive transfer) appeared to have no functional inhibitory effect on the potentially susceptible infused CD4+/CD25+ T cell population, a finding noted by other investigators (43, 45, 46), that issue still remained unclear. Therefore, to control for the confounding effects of residual circulating CD25 antibodies on the adoptive transfer cell population, and gauge the relative impact of Treg depletion on anti-tumor responses, we infused titering numbers of CD8+/CD25+ (Fig. 5A, panel 4) Kb-restricted OVA specific T cells (generated with 3 rounds of in vitro stimulation using peptide and IL-2) into C57BL/6 mice implanted with the OVA-expressing thymoma cell line E.G7. Unlike high avidity CD4+ T cells, OVA-specific CD8+ T cells had greater and statistically significant (p = 0.0002 at 106 and p = 0.0001 at 105 cells infused) therapeutic efficacy when host animals were depleted of Tregs prior to adoptive transfer when compared to control depletion (rat IgG) or control cells (TRP-1 specific CD4's) (Fig. 5C). These unexpected results strongly suggested that high avidity TRP-1 specific CD4+ T cells were maximally effective against established tumor regardless of host Treg status.
Discussion
In this report, we establish a new model for studying immune tolerance and the antitumor effects of high avidity autoreactive CD4+ T cells. Having previously identified a HLADR4-restricted epitope from the melanocyte antigen TRP-1, we proposed a series of experiments to better understand the potential immunotherapeutic effects of CD4+ T cells. Using this epitope in combination with a TRP-1 germline knockout, we demonstrate that systemic expression of TRP-1 alters the functionality of the endogenous T cell repertoire. These conclusions were initially supported by ex vivo experiments in which reactivity to exogenously loaded antigen was demonstrably superior by T cells derived from the antigen-deficient host. By utilizing titering concentrations of antigen, a differential frequency between WT and KO groups was unmasked. We observed that at the highest concentration of peptide, no difference in T cell frequency was observed. These results suggested that tolerance to TRP-1 does not result in the complete loss of autoreactive T cells, but instead leaves behind lower avidity clones still capable of recognizing exogenously loaded antigen.
Having established basic functional differences in T cell avidity between WT and KO hosts, we next tested the potential anti-tumor effects of these cells and their interaction with individual cellular popopulations. Using genetically MHC-mismatched combinations of tumors and tumor-bearing hosts, we demonstrate that fully polarized high IFN-γ producing Th1 CD4+ T cells eradicate established lung metastases in a manner that is both melanoma-specific and DR4-restricted. More importantly, we show that this anti-tumor effect is superior when mediated by CD4+ T cells derived from antigen-deficient donors. These results were therefore consistent with the aforementioned differences in T cell avidity. Although cells from WT and KO mice were infused into TRP-1 “self”-expressing DR4 Tg mice, systemic expression of TRP-1 appeared to have no apparent functional ill effect on the KO cells' ability to perform superiorly in comparison with their WT counterparts.
Using mAbs to deplete individual lymphocyte populations, we then demonstrate that the anti-tumor effect is entirely dependent on host CD8+ T cells. Although a Kb-restricted epitope from TRP-1 has been previously described (23), it is still unknown what specific antigen or restriction element is utilized by the CTL's. We had found that MCA-205 stably transfected with mTRP-1 was rejected following adoptive transfer (but not the control GFP transfectant) (data not shown) confirming that anti-tumor responses were indeed TRP-1 specific. The results, however, still do not exclude the contribution of other antigens to the overall effector CD8 response. Regardless of the specific CD8+ T cell in question, it is clear that host conditions require the expression of HLA-DR4, presumably on host APCs. The ability of activated, adoptively transferred CD4+ T cells to treat established tumor suggest that endogenous APCs are unable on their own to activate CD8+ T cells, but become de novo induced following adoptive transfer. It is also important to point out that specific CD4+ T cells were incapable of recognizing the TRP-1277-297 epitope when presented on murine class I or II (present on C57BL/6 splenocytes), thus excluding a false positive interaction between the effector population and tumor.
We conclude our study by demonstrating, rather unexpectedly, that the tumor treatment effect was unimproved (at titering concentrations of infused cells) following the depletion of host Tregs. WT TRP-1 specific CD4's were more effective against tumor when host Tregs were depleted, while TRP-1 specific CD4's derived from the KO mice were maximally active against tumor and thus capable of overcoming the inhibitory effects of Tregs. Despite their high avidity and action against a highly immunogenic foreign antigen, OT-I CD8+ T cells were relatively less effective in Treg replete hosts than the auto-reactive KO-derived T cell population. There also appears to be no evidence that the host tumor bearing state or the relatively naïve unprimed state of the endogenous APC pool has any negative effects on the function of activated, high avidity CD4+ T cells to cross-prime anti-tumor CD8+ T cells (47).
Over the past 20 years, the majority of human trials have focused on the unilateral activation or delivery of CD8+ T cells. Although many of these studies have opened doors into understanding basic principles of clinical cancer immunotherapy, the results have been mostly disappointing. To a large degree the focus on CD8+ T cells has appeared secondary to a number of technical and epidemiologic factors including: the availability of known immunogenic MHC class I epitopes; the high frequency of one particular class I allele (HLA-A*0201) in melanoma patients (50%); the straightforward assays used to measure class I-restricted immune responses; the relative ease in cultivating CD8+ T cells compared with their CD4+ T cell counterparts; the widespread MHC class I expression on the tumor surface; and the counter-beneficial actions of regulatory T cell activation (48). Despite this emphasis on CD8+ T cell responses, there has been mounting evidence to support the critical role for CD4+ T-helper cells in autoimmunity, antitumor immunity and long-term immunity (49, 50). There is also accumulating evidence that the combined application of class I and class II epitopes derived from the same tumor antigen can potentiate durable antitumor effector function (51-53).
Although other investigators have utilized either ubiquitous or foreign antigen models or employed TCR transgenics T cells, our study formally demonstrates for the first time that the adoptive transfer of non-transgenic CD4+ T cells specific for a normally expressed endogenous self/tumor antigen can directly cross-prime host-derived endogenous CD8+ T cells. Reports in the literature indicate that CD4+ T cells recognize Ag/MHC complexes either on classic APCs or on class II expressing endothelial cells (54). CD4+ T cells in turn activate or condition DC's, principally through the interaction of CD40 with its ligand, resulting in the upregulation of B7-1, B7-2 and OX40 costimulatory molecules (55-57). Conditioned DC's can then cross-present antigen to cognate CD8+ T cells at the tumor site or in remote locations (58). We hypothesized that the anti-tumor effects of the transferred CD4+ T cells could occur by several different mechanisms-of-action, including: the release of cytokines that directly kill tumor or the supporting stroma; the activation of APCs that in turn activate NK/NKT cells or macrophages that in turn kill stroma; or through the activation of APCs which in turn cross-prime and activate CD8+ T cells on the same APC. It is also plausible that IFN-γ production by CD4+ T cells potentiates (or enables) the action of CTL's either by upregulating tumor MHC class I and/or by enhancing antigen processing. In the end, only host CD8+ T cells were required for tumor elimination.
Despite substantial evidence that Tregs are crucial in maintaining self-tolerance and blunting the onset of autoimmunity and tumor immunity, the most surprising finding of our present study was the demonstration that the suppressive actions of Tregs is not absolute. In particular, we found that the actions of high avidity autoreactive CD4+ T cells can overcome Tregs and mediate tumor immunity. High avidity CD8+ T cells have been shown to be susceptible to regulatory suppression in similar adoptive transfer and tumor treatment models (59, 60), including suppression generated within the immediate tumor microenvironment (61). In our hands, high avidity OT-I CD8+ T cells were in fact susceptible to suppression. Tregs are thought to oppose the sustained activation and expansion of CD4+ and CD8+ T cells through the secretion of suppressive cytokines (IL-10, TGF-β), the engagement of CTLA-4 or PD-1, the down-regulation of DC antigen-presentation function, or through the direct elimination of autoreactive CD4+ T cells (62-65). But the adoptive transfer of high avidity CD4+ TRP-1 specific T cells may operate outside the constraints of regulatory suppression by either counterbalancing the actions of CTLA-4 or PD-1 through an overwhelmingly strong avidity interaction between TCR and the Ag/MHC complex as demonstrated by the differential Treg effects between WT and KO TRP-1 specific CD4+ T-cells (Fig. 5B). This interaction may also be further advantaged by the release of cytokines (perhaps the high level release of IFN-γ alone) that counterbalance the suppressor cytokines released by Tregs.
The experimental cornerstone of our studies is the potent reactivity generated against TRP-1 in TRP-1 deficient mice. Our results indicate that systemic self-antigen expression can diminish the avidity and functional activity of autoreactive CD4+ T cells. Immune tolerance mechanisms have evolved to protect the host against autoimmunity and broadly include deletion, anergy, regulation, activated-induced cell death (AICD) and ignorance (41, 66, 67). In each case, immune responses to self-antigens are qualitatively and quantitatively diminished or absent. Promiscuous expression of tissue-specific genes within the medullary thymic epithelium (mTEC) has emerged as a powerful mechanism of central tolerance (68, 69). Although MDA's gp100 and tyrosinase have been detected by PCR, no causal relationship to thymic selection has been demonstrated (70). Similarly, it is unclear if TRP-1 is expressed within mTEC's. What is known, however, is that TRP-1 is the most abundant glycoprotein in melanocytic cells and a secreted fraction of the protein has been found to contain the 277-297 epitope (19). Given these circumstantial findings, it is plausible that negative selection mediated by ectopically presented antigen from BM-derived DC's or by mTEC's alters the composition of the T cell repertoire (71, 72). It is also plausible that peripheral mechanisms mediated by the secreted, but inappropriately presented antigen could have a similar functional outcome. These issues are currently under investigation.
Finally, our results highlight the importance of isolating high avidity T helper cells. The anti-tumor potency of CD4+ T cells is still largely untested, and the exploitation of T cells derived from antigen-deficient hosts, as demonstrated in this study, magnifies their potential experimental and clinical utility. A potently tumor reactive CD8+ p53-specific TCR has been isolated from p53 KO mice and shown to instigate T cell help (not the reverse), but its clinical efficacy still remains in question (73, 74). As demonstrated in our studies, high avidity CD4+ T cells have the capacity to disrupt homeostatic balance and overcome the inhibitory effects of Tregs. A further advantage of this model system is that it relies on a normally expressed self-antigen, presented by the most common class II allele (DR4) in patients with metastatic melanoma (75, 76). The binding of the hTRP1 peptide to HLA-DR1, DR7, and DR11 has also been found to be very high (data not shown), thus expanding the potential scope of its clinical application to more than 50% of patients with melanoma (77). Unlike other models, our system also did not involve the exogenous administration of IL-2 or vaccination or require myelodepletion to facilitate the anti-tumor effects. These results further emphasize the global self-sufficient impact that high avidity T cells have on the host immune system. We believe these overall findings have tremendous therapeutic implications if TCR genes from CD4+ T cells can be isolated from antigen-deficient hosts and transferred either to peripheral T cells or hematopoietic stem cells. It is potentially at that point that the full therapeutic capacity of CD8+ T cells can be achieved.
Acknowledgements
The authors would like to thank Steven A. Rosenberg, Paul Robbins, Hal Broxmeyer, and Keith Lillemoe for all their advice and support.
Funding for this research project was supported by the American Surgical Association Fellowship Prize
Andrew Brandmaier was supported by an NIH T32 AI 060519 Training Grant through NIAID
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this article.
The authors have not conflicting financial interests.
Reference List
- 1.Boon T, Coulie PG, Van Den Eynde BJ, van der BP. Human T cell responses against melanoma. Annu.Rev.Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat.Rev.Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peggs KS, Segal NH, Allison JP. Targeting immunosupportive cancer therapies: accentuate the positive, eliminate the negative. Cancer Cell. 2007;12:192–199. doi: 10.1016/j.ccr.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat.Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lizee G, Cantu MA, Hwu P. Less yin, more yang: confronting the barriers to cancer immunotherapy. Clin.Cancer Res. 2007;13:5250–5255. doi: 10.1158/1078-0432.CCR-07-1722. [DOI] [PubMed] [Google Scholar]
- 6.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv.Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 7.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat.Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams MA, Holmes BJ, Sun JC, Bevan MJ. Developing and maintaining protective CD8+ memory T cells. Immunol.Rev. 2006;211:146–153. doi: 10.1111/j.0105-2896.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 9.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J.Exp.Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv.Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- 11.Dighe AS, Richards E, OLD LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 12.Williamson BD, Carswell EA, Rubin BY, Prendergast JS, OLD LJ. Human tumor necrosis factor produced by human B-cell lines: synergistic cytotoxic interaction with human interferon. Proc.Natl.Acad.Sci.U.S.A. 1983;80:5397–5401. doi: 10.1073/pnas.80.17.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coughlin CM, Salhany KE, Gee MS, LaTemple DC, Kotenko S, Ma X, Gri G, Wysocka M, Kim JE, Liu L, Liao F, Farber JM, Pestka S, Trinchieri G, Lee WM. Tumor cell responses to IFNgamma affect tumorigenicity and response to IL-12 therapy and antiangiogenesis. Immunity. 1998;9:25–34. doi: 10.1016/s1074-7613(00)80585-3. [DOI] [PubMed] [Google Scholar]
- 14.Kurts C, Carbone FR, Barnden M, Blanas E, Allison J, Heath WR, Miller JF. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J.Exp.Med. 1997;186:2057–2062. doi: 10.1084/jem.186.12.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Surman DR, Dudley ME, Overwijk WW, Restifo NP. Cutting edge: CD4+ T cell control of CD8+ T cell reactivity to a model tumor antigen. J.Immunol. 2000;164:562–565. doi: 10.4049/jimmunol.164.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong CA, Botella R, Galloway TH, Murray N, Kramp JM, Song IS, Ansel JC. Antitumor effects of granulocyte-macrophage colony-stimulating factor production by melanoma cells. Cancer Res. 1996;56:2191–2198. [PubMed] [Google Scholar]
- 19.Xu Y, Setaluri V, Takechi Y, Houghton AN. Sorting and secretion of a melanosome membrane protein, gp75/TRP1. J.Invest Dermatol. 1997;109:788–795. doi: 10.1111/1523-1747.ep12340971. [DOI] [PubMed] [Google Scholar]
- 20.Murisier F, Beermann F. Genetics of pigment cells: lessons from the tyrosinase gene family. Histol.Histopathol. 2006;21:567–578. doi: 10.14670/HH-21.567. [DOI] [PubMed] [Google Scholar]
- 21.Touloukian CE, Leitner WW, Robbins PF, Li YF, Kang X, Lapointe R, Hwu P, Rosenberg SA, Restifo NP. Expression of a "self-"antigen by human tumor cells enhances tumor antigen-specific CD4(+) T-cell function. Cancer Res. 2002;62:5144–5147. [PMC free article] [PubMed] [Google Scholar]
- 22.Overwijk WW, Lee DS, Surman DR, Irvine KR, Touloukian CE, Chan CC, Carroll MW, Moss B, Rosenberg SA, Restifo NP. Vaccination with a recombinant vaccinia virus encoding a "self" antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc.Natl.Acad.Sci.U.S.A. 1999;96:2982–2987. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowne WB, Srinivasan R, Wolchok JD, Hawkins WG, Blachere NE, Dyall R, Lewis JJ, Houghton AN. Coupling and uncoupling of tumor immunity and autoimmunity. J.Exp.Med. 1999;190:1717–1722. doi: 10.1084/jem.190.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leitner WW, Hwang LN, deVeer MJ, Zhou A, Silverman RH, Williams BR, Dubensky TW, Ying H, Restifo NP. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat.Med. 2003;9:33–39. doi: 10.1038/nmxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Javerzat S, Jackson IJ. White-based brown (Tyrp1B-w) is a dominant mutation causing reduced hair pigmentation owing to a chromosomal inversion. Mamm.Genome. 1998;9:469–471. doi: 10.1007/s003359900798. [DOI] [PubMed] [Google Scholar]
- 26.Yu Z, Theoret MR, Touloukian CE, Surman DR, Garman SC, Feigenbaum L, Baxter TK, Baker BM, Restifo NP. Poor immunogenicity of a self/tumor antigen derives from peptide-MHC-I instability and is independent of tolerance. J.Clin.Invest. 2004;114:551–559. doi: 10.1172/JCI21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colella TA, Bullock TN, Russell LB, Mullins DW, Overwijk WW, Luckey CJ, Pierce RA, Restifo NP, Engelhard VH. Self-tolerance to the murine homologue of a tyrosinase-derived melanoma antigen: implications for tumor immunotherapy. J.Exp.Med. 2000 Apr. 3191:1221–1232. doi: 10.1084/jem.191.7.1221. 191.(7.):1221.-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Touloukian CE, Leitner WW, Schnur RE, Robbins PF, Li Y, Southwood S, Sette A, Rosenberg SA, Restifo NP. Normal tissue depresses while tumor tissue enhances human T cell responses in vivo to a novel self/tumor melanoma antigen, OA1. J.Immunol. 2003;170:1579–1585. doi: 10.4049/jimmunol.170.3.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson IJ, Chambers D, Rinchik EM, Bennett DC. Characterization of TRP-1 mRNA levels in dominant and recessive mutations at the mouse brown (b) locus. Genetics. 1990;126:451–459. doi: 10.1093/genetics/126.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smyth IM, Wilming L, Lee AW, Taylor MS, Gautier P, Barlow K, Wallis J, Martin S, Glithero R, Phillimore B, Pelan S, Andrew R, Holt K, Taylor R, McLaren S, Burton J, Bailey J, Sims S, Squares J, Plumb B, Joy A, Gibson R, Gilbert J, Hart E, Laird G, Loveland J, Mudge J, Steward C, Swarbreck D, Harrow J, North P, Leaves N, Greystrong J, Coppola M, Manjunath S, Campbell M, Smith M, Strachan G, Tofts C, Boal E, Cobley V, Hunter G, Kimberley C, Thomas D, Cave-Berry L, Weston P, Botcherby MR, White S, Edgar R, Cross SH, Irvani M, Hummerich H, Simpson EH, Johnson D, Hunsicker PR, Little PF, Hubbard T, Campbell RD, Rogers J, Jackson IJ. Genomic anatomy of the Tyrp1 (brown) deletion complex. Proc.Natl.Acad.Sci.U.S.A. 2006;103:3704–3709. doi: 10.1073/pnas.0600199103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakeland E, Morel L, Achey K, Yui M, Longmate J. Speed congenics: a classic technique in the fast lane (relatively speaking) Immunol.Today. 1997;18:472–477. doi: 10.1016/s0167-5699(97)01126-2. [DOI] [PubMed] [Google Scholar]
- 32.Ito K, Bian HJ, Molina M, Han J, Magram J, Saar E, Belunis C, Bolin DR, Arceo R, Campbell R, Falcioni F, Vidovic D, Hammer J, Nagy ZA. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J.Exp.Med. 1996;183:2635–2644. doi: 10.1084/jem.183.6.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J.Exp.Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol.Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 35.Touloukian CE, Leitner WW, Topalian SL, Li YF, Robbins PF, Rosenberg SA, Restifo NP. Identification of a MHC class II-restricted human gp100 epitope using DR4-IE transgenic mice. J.Immunol. 2000 Apr. 1164:3535–3542. doi: 10.4049/jimmunol.164.7.3535. 164.(7.):3535.-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Touloukian CE, Leitner WW, Robbins PF, Rosenberg SA, Restifo NP. Mining the melanosome for tumor vaccine targets: P.polypeptide is a novel tumor-associated antigen. Cancer Res. 2001;61:8100–8104. [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson IJ. Homologous pigmentation mutations in human, mouse and other model organisms. Hum.Mol.Genet. 1997;6:1613–1624. doi: 10.1093/hmg/6.10.1613. [DOI] [PubMed] [Google Scholar]
- 38.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr.Opin.Immunol. 2006;18:349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J.Exp.Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 41.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat.Rev.Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 42.Baecher-Allan C, Anderson DE. Immune regulation in tumor-bearing hosts. Curr.Opin.Immunol. 2006;18:214–219. doi: 10.1016/j.coi.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Sutmuller RP, van Duivenvoorde LM, van EA, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J.Exp.Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Comes A, Rosso O, Orengo AM, Di CE, Sorrentino C, Meazza R, Piazza T, Valzasina B, Nanni P, Colombo MP, Ferrini S. CD25+ regulatory T cell depletion augments immunotherapy of micrometastases by an IL-21-secreting cellular vaccine. J.Immunol. 2006;176:1750–1758. doi: 10.4049/jimmunol.176.3.1750. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J.Exp.Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsushita N, Pilon-Thomas SA, Martin LM, Riker AI. Comparative methodologies of regulatory T cell depletion in a murine melanoma model. J.Immunol.Methods. 2008;333:167–179. doi: 10.1016/j.jim.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleindienst P, Wiethe C, Lutz MB, Brocker T. Simultaneous induction of CD4 T cell tolerance and CD8 T cell immunity by semimature dendritic cells. J.Immunol. 2005;174:3941–3947. doi: 10.4049/jimmunol.174.7.3941. [DOI] [PubMed] [Google Scholar]
- 48.Rosenberg SA. Development of effective immunotherapy for the treatment of patients with cancer. J.Am.Coll.Surg. 2004;198:685–696. doi: 10.1016/j.jamcollsurg.2004.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr.Opin.Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 50.Gerloni M, Zanetti M. CD4 T cells in tumor immunity. Springer Semin.Immunopathol. 2005;27:37–48. doi: 10.1007/s00281-004-0193-z. [DOI] [PubMed] [Google Scholar]
- 51.Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J.Exp.Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knutson KL, Schiffman K, Disis ML. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J.Clin.Invest. 2001;107:477–484. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langlade-Demoyen P, Garcia-Pons F, Castiglioni P, Garcia Z, Cardinaud S, Xiong S, Gerloni M, Zanetti M. Role of T cell help and endoplasmic reticulum targeting in protective CTL response against influenza virus. Eur.J.Immunol. 2003;33:720–728. doi: 10.1002/eji.200323287. [DOI] [PubMed] [Google Scholar]
- 54.Marelli-Berg FM, Frasca L, Weng L, Lombardi G, Lechler RI. Antigen recognition influences transendothelial migration of CD4+ T cells. J.Immunol. 1999;162:696–703. [PubMed] [Google Scholar]
- 55.Schoenberger SP, Toes RE, van,d. V, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 56.Ndejembi MP, Teijaro JR, Patke DS, Bingaman AW, Chandok MR, Azimzadeh A, Nadler SG, Farber DL. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J.Immunol. 2006;177:7698–7706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 57.Soroosh P, Ine S, Sugamura K, Ishii N. OX40-OX40 ligand interaction through T cell-T cell contact contributes to CD4 T cell longevity. J.Immunol. 2006;176:5975–5987. doi: 10.4049/jimmunol.176.10.5975. [DOI] [PubMed] [Google Scholar]
- 58.Ridge JP, Di RF, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 59.Imai H, Saio M, Nonaka K, Suwa T, Umemura N, Ouyang GF, Nakagawa J, Tomita H, Osada S, Sugiyama Y, Adachi Y, Takami T. Depletion of CD4+CD25+ regulatory T cells enhances interleukin-2-induced antitumor immunity in a mouse model of colon adenocarcinoma. Cancer Sci. 2007;98:416–423. doi: 10.1111/j.1349-7006.2006.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J.Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bai A, Higham E, Eisen HN, Wittrup KD, Chen J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc.Natl.Acad.Sci.U.S.A. 2008;105:13003–13008. doi: 10.1073/pnas.0805599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+ J.Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 63.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate costimulatory molecules on antigen-presenting cells. Eur.J.Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 64.Hori S, Takahashi T, Sakaguchi S. Control of autoimmunity by naturally arising regulatory CD4+ T cells. Adv.Immunol. 2003;81:331–371. doi: 10.1016/s0065-2776(03)81008-8. [DOI] [PubMed] [Google Scholar]
- 65.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 66.Hildeman DA, Zhu Y, Mitchell TC, Kappler J, Marrack P. Molecular mechanisms of activated T cell death in vivo. Curr.Opin.Immunol. 2002;14:354–359. doi: 10.1016/s0952-7915(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 67.Spiotto MT, Fu YX, Schreiber H. Tumor immunity meets autoimmunity: antigen levels and dendritic cell maturation. Curr.Opin.Immunol. 2003;15:725–730. doi: 10.1016/j.coi.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 68.Magalhaes DA, Silveira EL, Junta CM, Sandrin-Garcia P, Fachin AL, Donadi EA, Sakamoto-Hojo ET, Passos GA. Promiscuous gene expression in the thymus: the root of central tolerance. Clin.Dev.Immunol. 2006;13:81–99. doi: 10.1080/17402520600877091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kyewski B, Derbinski J. Self-representation in the thymus: an extended view. Nat.Rev.Immunol. 2004;4:688–698. doi: 10.1038/nri1436. [DOI] [PubMed] [Google Scholar]
- 70.Sospedra M, Ferrer-Francesch X, Dominguez O, Juan M, Foz-Sala M, Pujol-Borrell R. Transcription of a broad range of self-antigens in human thymus suggests a role for central mechanisms in tolerance toward peripheral antigens. J.Immunol. 1998;161:5918–5929. [PubMed] [Google Scholar]
- 71.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J.Exp.Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 73.Kuball J, Schmitz FW, Voss RH, Ferreira EA, Engel R, Guillaume P, Strand S, Romero P, Huber C, Sherman LA, Theobald M. Cooperation of human tumor-reactive CD4+ and CD8+ T cells after redirection of their specificity by a high-affinity p53A2.1-specific TCR. Immunity. 2005;22:117–129. doi: 10.1016/j.immuni.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 74.Cohen CJ, Zheng Z, Bray R, Zhao Y, Sherman LA, Rosenberg SA, Morgan RA. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J.Immunol. 2005;175:5799–5808. doi: 10.4049/jimmunol.175.9.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barger BO, Acton RT, Soong SJ, Roseman J, Balch C. Increase of HLA-DR4 in melanoma patients from Alabama. Cancer Res. 1982;42:4276–4279. [PubMed] [Google Scholar]
- 76.Marincola FM, Shamamian P, Rivoltini L, Salgaller M, Cormier J, Restifo NP, Simonis TB, Venzon D, White DE, Parkinson DR. HLA associations in the antitumor response against malignant melanoma. J.Immunother.Emphasis.Tumor Immunol. 1995;18:242–252. doi: 10.1097/00002371-199511000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Southwood S, Sidney J, Kondo A, del Guercio MF, Appella E, Hoffman S, Kubo RT, Chesnut RW, Grey HM, Sette A. Several common HLA-DR types share largely overlapping peptide binding repertoires. J.Immunol. 1998;160:3363–3373. [PubMed] [Google Scholar]