Abstract
PURPOSE
To determine the incidence of new chorioretinal lesions in children with toxoplasmosis diagnosed after, and therefore not treated during, their first year.
DESIGN
Prospective longitudinal cohort study.
METHODS
Thirty-eight children were evaluated in Chicago between 1981 and 2005 for new chorioretinal lesions. Thirty-eight children and mothers had serum IgG antibody to Toxoplasma gondii.
RESULTS
Twenty-eight of 38 children had one of the following: diagnosis with serum antibody to T. gondii indicative of chronic infection at age 24 months, central nervous system calcifications, hydrocephalus, illness compatible with congenital toxoplasmosis perinatally but not diagnosed at that time. Twenty-five returned for follow-up during 1981 to 2005. Their mean (range) age at last exam was 10.9 ± 5.7 (range, 3.5 to 27.2) years and mean follow-up was 5.7 ± 2.9 years. Eighteen (72%) children developed at least one new lesion. Thirteen (52%) had new central lesions, 11 (44%) had new peripheral lesions, and six (24%) had both. Thirteen (52%) had new lesions diagnosed at age ≥ 10 years. New lesions were found at more than one visit in four (22%), and bilateral new lesions developed in seven (39%) of 18 children who developed new lesions. Of 10 additional children with eye findings and serologic tests indicative of chronic infection, six returned for follow-up, four (67%) developing new lesions at ≥ 10 years of age.
CONCLUSIONS
More than 70% developed new chorioretinal lesions. New lesions were commonly diagnosed after the first decade of life.
CONGENITAL TOXOPLASMOSIS THAT WAS UNTREATED or treated only for one month has been described in small series of patients as a relapsing, recrudescent disease causing significant visual impairment.1,2 However, the prospective follow-up into adolescence of chorioretinal lesions in children with congenital toxoplasmosis, other than a small group of those with congenital toxoplasmosis diagnosed at birth and treated during the first year of life, has not been rigorously defined. Herein we describe a cohort of 38 children with toxoplasmosis presenting after one year of age, who were followed prospectively in a single center according to a standardized protocol.
METHODS
DESIGN OF STUDY
The children in this study and their mothers were referred by their physicians and evaluated at the University of Chicago by a group of specialists at the onset of enrollment into the study and later at prespecified times: 1, 3.5, 5, 7.5, 10, 15, 20, 25, 30, 35, 40, and 45 years of age.3,4 Written informed consent was obtained from all parents or legal guardians of participating minor-age children and/or directly from the patient (if of legal adult age).
PATIENT COHORT
This cohort consists of 38 persons who were children at the time of diagnosis of their ocular toxoplasmosis, who, with their mothers, had nonacute serologic tests for Toxoplasma gondii infection at the time their contact with the Chicago Toxoplasmosis Center was initiated. They were not diagnosed until after their first year of life. Patients in this cohort were not treated during their first year of life. All children with active chorioretinitis were treated when this occurred and was detected. It appears that children in the present cohort seem to have much more frequent new lesions than we report in a cohort of children who were detected at birth and treated throughout the first year of life, even though the children detected at birth often initially had more severe and significant systemic, neurologic, and eye disease. The fetus in utero and infants during the first year of life have less mature immune systems including immune mechanisms important in containing T. gondii. When medicines are discontinued at one year of age, most infants treated throughout the first year of life had rebound in their antibody titers, suggesting that parasite growth had occurred but no new disease was detected in conjunction with this serologic rebound. This suggested to us that after one year of age the child had an immune system capable of containing the parasite; and therefore, since no medicines eliminate the latent parasites, if there was no activity children were not treated after that time. Available medicines do not eliminate the latent bradyzoite. We do not have data to address whether children treated after the first year of life have less eye disease in adolescence. Thirty-seven children had brain computed tomography (CT) scans and one had magnetic resonance imaging (MRI) at the time of enrollment; 22 presented with brain calcifications, four presented with or developed hydrocephalus, one had mild ventricular dilatation and four had both calcifications and hydrocephalus. All patients and their mothers (other than the mothers of three adopted children) had serologic tests performed in the United States (US) reference serology laboratory in Palo Alto, California (http://www.pamf.org/serology).
OPHTHALMOLOGIC EXAMINATIONS AND DEFINITIONS
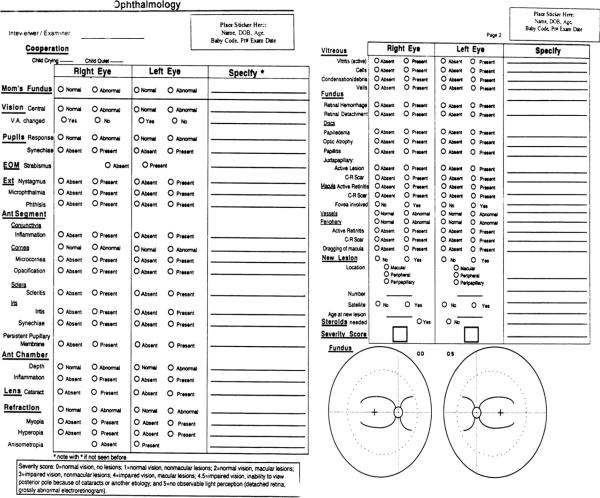
Children were examined in a single center according to a standardized protocol as described.3,4 The ophthalmology examination proceeded as indicated on the data form (Figure 1). New chorioretinal lesions are defined as lesions that were not identified in the initial evaluation or occurred in previously uninvolved retina. Any lesion not seen previously, including those in uninvolved retina, geographically new lesions, satellite lesions, enlarged lesions, and active lesions all were considered new lesions and these are included in the incidence figures presented. Recurrences are considered additional new lesions. New lesions refer to both newly detected and newly active lesions and both occur, the latter in the absence of routine periodic screening visits. Furthermore, satellite lesions were defined as those contiguous to or in close proximity to an older lesion. New lesions are further described as central or peripheral, where central lesions are those in the macula, which means within the vascular arcades and/or in peripapillary areas, which are limited to within 1 disc diameter of the optic nerve, and peripheral lesions are those outside of the major temporal arcades and not within the peripapillary areas. Juxtapapillary means touching the optic disc. If a lesion is both peripapillary and macular we have referred to that lesion as peripapillary. After eye examinations, patients were assigned an eye severity score for each eye, which was used to uniformly characterize the impact of the infection on the patient's vision. The score was defined as follows: (0) normal vision, no lesion; (1) normal vision, nonmacular lesions; (2) normal vision, macular lesions; (3) impaired vision, nonmacular lesions; (4) impaired vision, macular lesions; (4.5) impaired vision, inability to view posterior because of cataracts or another etiology; and (5) no observable light perception (retinal detachment and grossly abnormal electroretinogram).
FIGURE 1.
Data form for ophthalmology examination for toxoplasmosis study.
DATA EVALUATION AND STATISTICS
A data form was completed during each ophthalmologic evaluation (Figure 1). Full fundus photographs in all gaze positions, and especially of the chorioretinal lesions, were obtained from children depending on their ability to cooperate with photography. Children less than 3.5 years old and severely cognitively impaired children did not have photographs unless those were taken when they were sedated or anesthetized for other reasons, or they had an examination under anesthesia specifically for their ophthalmologic disease. We did not routinely perform examinations under anesthesia for the purpose of the study, to obtain fundus photographs, or to examine the periphery of the fundus as thoroughly as would be performed with an examination under anesthesia. Examinations of the retina were performed while children were awake, without lid speculum, and without scleral depression. As soon as a child could cooperate with obtaining photographs, photographs were obtained. For young children under the age of 3.5 years, we usually were unable to obtain a photograph unless anesthesia was given for another purpose or an examination under anesthesia for clinical care was performed, which was infrequent. For those children 3.5 years old or older, we did obtain photographs of the central retina; and for children over 7.5 years old we generally were able to photograph the periphery as well. Results of examinations and photographs were compared. In almost all instances, the examining ophthalmologist had already detected lesions seen in the photographs. Reliability of results based on ophthalmologists' observations of central lesions is similar to observations of the periphery, except for very young children where examination beyond the vascular arcades or equator were more limited by cooperation. Data collected on the data form and photographs taken during these evaluations, as well as all the medical records and scans obtained from the patient's physicians, were used to complete the data analysis of this cohort.
Incidence rates of new lesions were calculated at each visit. The number of new lesions seen at each age-specific visit was divided by the total number of person-years at risk to obtain the incidence rates. The number of person-years a person contributed at a particular visit was the amount of time (in years) elapsed since their last examination. A 95% confidence interval (CI) for the incidence rates was calculated assuming a Poisson distribution using exact methods. In this work, we define age-specific incidence rates for a cohort of US patients diagnosed after their first year of life with toxoplasmosis. We note that our sample size is limited and that we do not know that those in our study represent all those throughout the US.
RESULTS
PATIENT COHORT
This cohort, consisting of 38 patients who were children at the time of diagnosis of their ocular toxoplasmosis, was composed of 25 females and 13 males. There were 29 White patients, seven persons of Hispanic background, and two of Asian and White descent. The current mean ± standard deviation (SD) [range] age for patients in this cohort is 18.9 ± 9.0 [two to 43] years. Of the 38 children enrolled into this study, 31 returned for standardized follow-up visits (see Supplemental Figure 1 available at AJO.com) or came with new active lesions and were evaluated for new chorioretinal lesions (Figures 2 through 6). Twenty-eight children had one of the following: perinatal signs and symptoms consistent with congenital toxoplasmosis, diagnosis at ≤2 years of age, and/or cerebral calcifications or hydrocephalus (see Supplemental Figure 2 available at AJO.com). Of the 28, 16 were female and 12 male. There were 19 White patients, seven Hispanics, and two of Asian and White descent. Of these 28 children, 25 returned for standardized follow-up visits or came with new active lesions and were evaluated for new chorioretinal lesions (Figures 2 through 6). Of these 25 children, mean age at last exam was 10.9 ± 5.7 (range, 3.5 to 27.2) years. Mean follow-up was 5.7 ± 2.9 years.
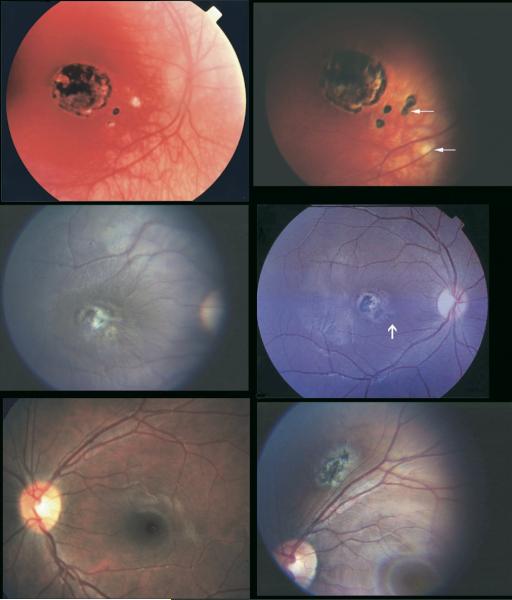
FIGURE 2.
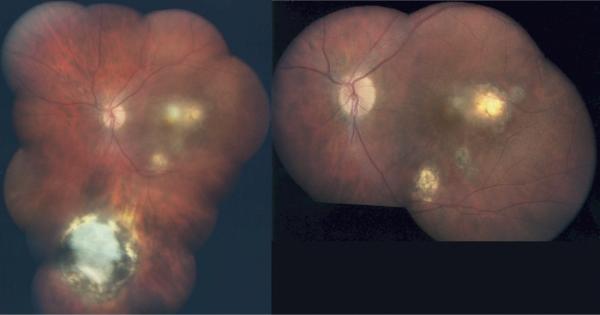
Representative example of new toxoplasmic chorioretinal lesions in patients no. 159 (top), 118 (middle), and 89 (bottom). (Top left) Patient at age 4½ years before new lesion. (Top right) Patient pictured at top left at age 7 years with active disease. (Middle left) Patient at age 8 years before new lesion. (Middle right) Patient pictured at middle left with active disease at age 10 years. (Bottom left) Twelve-year-old patient before new lesion. (Bottom right) Patient pictured at bottom left at age 20 with active disease.
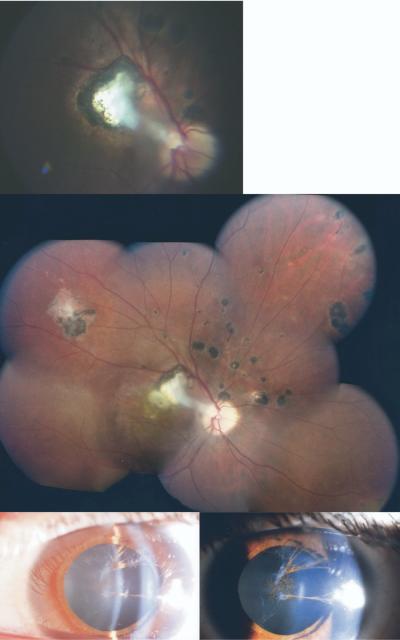
FIGURE 6.
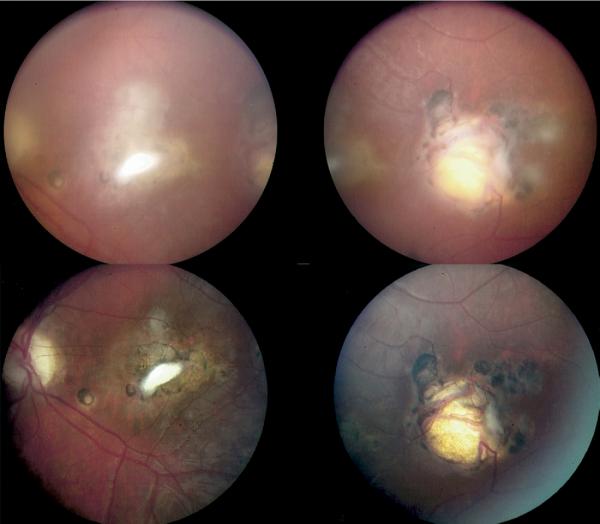
Representative example of new toxoplasmic chorioretinal lesions in patient no. 27. Right eye of patient at age 15 years (Top) and at age 18 years (Middle). (Bottom) Cataract in eye of same patient at age 18 years.
CHARACTERIZATION OF NEW CHORIORETINAL LESIONS
Almost all the patients (36/38 = 95%) in this cohort of children, who missed being treated in their first year of life, had eye disease at presentation. New lesions are those of any type that were not present in the prior evaluation. These were newly seen and not present on an earlier routine evaluation, that is, geographically new, new documented activity, or larger than the earlier lesion in the same place. Two patients never had ocular lesions: one a child with congenital toxoplasmosis with cognitive problems at presentation and one twin of a child with ocular toxoplasmosis who was also seropositive. Both twins were premature and their mother was also seropositive. All other children had ocular lesions when first examined. These lesions were not considered to be new when first detected unless they were active. They were only described as new if they had not been seen before. For these children in our series, most of whom had quiescent eye lesions when first diagnosed, frequent new lesions also developed (Supplemental Figure 1). Children who had one active recurrence tended to seek medical care sooner with a second recurrence. The size of the new lesion often reflected whether there was a delay in seeking treatment. Figures 2 through 6 show the development and evolution of new lesions over time in patients who had recurrences. More than half of the cohort (22/31 = 71%, 95% CI [52%, 86%]) developed at least one new chorioretinal lesion that was previously not detected (Table). Sixteen (52%) developed central chorioretinal lesions, and 14 children (45%) had newly seen peripheral lesions. New lesions were diagnosed at age 10 or later in 17 children (55%). Of the 22 children with new eye lesions, lesions were found at multiple visits in five patients (23%), and bilateral involvement of the retina occurred in nine patients (41%). In three of the 38 patients (four recurrences), new lesions were also found to be active (Supplemental Figure 1). All of these three patients had symptoms of active ocular toxoplasmosis in each of the four recurrences rather than new lesions detected at routine follow-up examinations. These symptoms prompted evaluations by their primary ophthalmologists. Supplemental Figure 1 shows the time the new lesions occurred or were detected. If the vertical bar indicating they were detected is at the same time for each eye, then they were observed at that same time in each eye. Of the 30 times new lesions were detected, bilaterial lesions were detected simultaneously nine times and new lesions were detected unilaterally 21 times. A vertical bar indicates when new lesions were detected, but this does not necessarily mean that this is when new lesions developed. For example, they could be peripheral lesions that were newly seen because of increased patient cooperation, or they could have developed in the period between visits to Chicago.
TABLE.
Numbers and Locations of New Toxoplasmic Chorioretinal Lesions and Eyes in Which They Occur in Each Patient
| Number (%) With Findings |
||
|---|---|---|
| All Ptsa |
Pts Prob CTb |
|
| Findings | N=38 (n=31) | N=28 (n=25) |
| Chriorentinal lesion of any kind | 22 (71 %) | 18 (72%) |
| Central chorioretinal lesion | 16 (52%) | 13 (52%) |
| OD eye | 10 | 8 |
| OS eye | 6 | 5 |
| OU eyes | 4 | 3 |
| Peripheral chorioretinal lesion | 14 (45%) | 11 (44%) |
| OD eye | 8 | 7 |
| OS eye | 8 | 6 |
| OU eyes | 2 | 2 |
CT = congenital toxoplasmosis; N = total number of patients in each group; n = number of patients who had return visits and were evaluated for new lesions; OD = right eye; OS = left eye; OU= both eyes.
Central lesions are lesions in the macula and peripapillary area; peripapillary lesion is defined as a lesion within 1 disc diameter of the optic nerve; peripheral lesions are lesions outside the macula and peripapillary area.
Note that patients were counted multiple times when tabulating the numbers in the OD, OS, and OU rows of the table if they had lesions at multiple visits.
Patients (Pts): There were 38 persons who were children at the time of diagnosis who were not treated during the first year of life.
Patients with probable congenital toxoplasmosis (Pts Prob CT): There were 28 patients in whom congenital infection was likely attributable to presence of serum antibody to T. gondii indicative of chronic infection at age ≤24 months, and who had central nervous system calcifications or hydrocephalus, or illness compatible with congenital toxoplasmosis perinatally but not diagnosed as congenital toxoplasmosis at that time.
For the 28 children (25 with follow-up) for whom congenital infection was likely attributable to presence of serum antibody to T. gondii indicative of chronic infection at age ≤24 months, who had central nervous system (CNS) calcifications or hydrocephalus, or who had illness compatible with congenital toxoplasmosis perinatally but not diagnosed as congenital toxoplasmosis at that time, 18 children (18/25 = 72%, 95% CI [51%, 88%]) developed at least a single new lesion (Table; Supplemental Figure 1 (Top); Figures 2 through 6). Thirteen children (52%) had new central lesions, 11 (44%) had new peripheral lesions, and six (24%) had both. Thirteen children (52%) had new lesions diagnosed at age ≥10 years. Of the 18 children with new lesions, four (22%) had lesions at more than one visit and seven (39%) had bilateral involvement.
For the remaining 10 children (six with follow-up), four (67%) of six developed at least a single new lesion (Supplemental Figure 1, Bottom). Three children (50%) of six had new central lesions, three (50%) had new peripheral lesions, and two (33%) of six had both. Four children (67%) of six had new lesions diagnosed at age ≥10 years. Among the four children who developed at least one new lesion, new lesions were found during multiple visits in one child (25%), and bilateral new lesions developed in two children (50%).
INCIDENCE AND TIMES OF OCCURRENCE OF NEW RETINAL LESIONS
The incidence of new lesions occurring in the cohort of 28 persons with highly likely congenital toxoplasmosis increased in the second half of adolescence (15 to 20 years of age) with a small increase also at the age of 5 years (Figure 7). Incidence is based on the number of patients with new lesions per patient-year. However, as indicated by the wide confidence intervals, more follow-up into adolescence is needed. Of the 22 children in this entire cohort of 38 who developed new chorioretinal lesions, 17 of them (77%) had a lesion(s) at age 10 years or later, as demonstrated in Supplemental Figure 1. There was a total amount of follow-up (ie, time at risk of developing the first new lesion) of 296 person-years, and the incidence of first new lesions was 0.061 per person-year with an approximate 95% CI of 0.032, 0.090.
FIGURE 7.
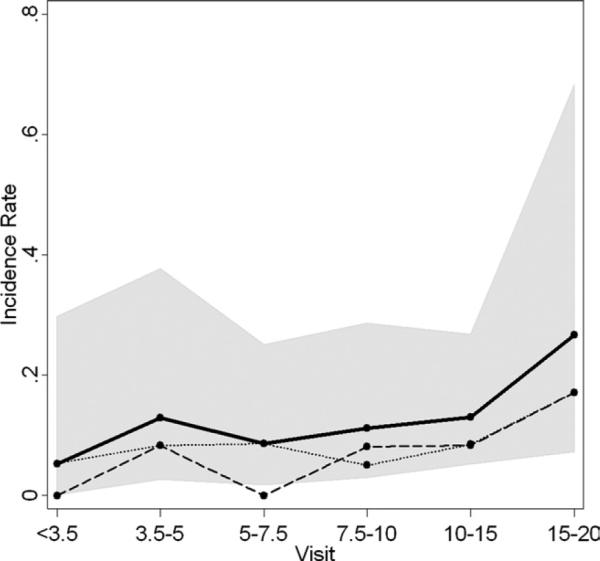
Incidence of new toxoplasmic chorioretinal lesions by age of visit. Incidence rates of new lesions were calculated at each visit by dividing the number of patients seen with new lesions by the total number of person-years at risk. The dashed line is for peripheral lesions, the dotted line for central lesions, and the solid line is for all lesions (peripheral and central). The shaded region indicates the 95% confidence bounds for all lesions incidence rates.
DISCUSSION
HEREIN, WE DESCRIBE THE NATURAL HISTORY OF CHORIO retinal lesions in a cohort of children with toxoplasmosis who were not treated during their first year of life. New and recurrent chorioretinal lesions occurred commonly, especially during middle to late adolescence, 22 (71%) of 31 overall and in 18 (72%) of the 25 children who had sufficient follow-up and likely had congenital infection because of other findings or age at diagnosis. In this work, we define age-specific incidence rates for US patients diagnosed after their first year of life with toxoplasmosis. We note that new lesions develop in those with and without older lesions in the same location in the eye but are more frequent in locations where scars are present. Specifically, out of 38 patients (76 eyes), 12 patients (14 eyes) had progression of eye disease in the same eye where scars were present while three patients (three eyes) developed eye disease where no lesions were initially present. Twenty-eight (45 eyes) of 38 patients (76 eyes) had stable eye disease. Seven patients (14 eyes) did not have sufficient follow-up to determine this. We note that our sample size is limited and that we do not know that those in our study represent all those throughout the US. The reasons for these frequent occurrences of new chorioretinal lesions in this cohort are not known. It may be attributable to selection bias, with parents and physicians of children with more severe eye disease seeking out our study and returning for follow-up visits once they are in the study (leading to an overestimation of the true incidence rates). Most of these children, however, had no obvious findings at birth that would have led to a diagnosis of congenital toxoplasmosis with usual neonatal evaluations, making them less severely involved at birth than >80% of the children in our cohort of treated infants and children for whom new retinal lesions were substantially less frequent.3-8 Alternatively, this finding of new, frequently recurrent ocular disease is consistent with earlier reports of children who were untreated or treated for only one month perina-tally,1-5 in whom subsequent chorioretinal disease occurred frequently. Eighty-two percent of such children had new eye lesions by their teenage years.1
At the time we began this work, the incidence of ocular disease following acute infection acquired postnatally was thought to be very small;7 thus we initially considered every person in this cohort to be congenitally infected since the mothers were seropositive and retinal disease in young children and teenagers was generally considered to be congenitally acquired.9-11 Perinatal signs and symptoms consistent with congenital toxoplasmosis, diagnosis at ≤2 years of age, and/or cerebral calcifications or hydrocephalus occurred in 28 of the children in this cohort, which makes it highly likely that these 28 children have congenital infection. Of the remaining 10 children, four had ocular signs/symptoms noted when they were less than 10-years-old, four had ocular signs/symptoms noted between 10 and 15 years of age, two were more than 15-years-old when ocular signs/symptoms were noted, and none of these children had serologic tests indicative of acute T. gondii infection. In areas of low seroprevalence such as the US, it is less likely that both the mother and the child at a young age would be chronically infected with T. gondii secondary to acute acquired infection in early childhood, although there are occurrences of family clusters of acute acquired infection because of shared risk factors; therefore, for these 10 children it seems less likely, but not impossible, that infection might have been acquired postnatally.
Some of the peripheral lesions we detected may actually have been present at earlier ages but not recognized. Because of the limitations in examination of the peripheral retina in infants and young children without anesthesia or sedation, these peripheral lesions may not have been appreciated at those earlier ages. However, examinations of the retina are usually accurate in their detection of new central chorioretinal lesions.
Koppe and associates1 and Wilson and associates2 addressed the occurrence of new eye disease in children who had no eye disease detected at birth who received one month or less of treatment in the first year of life. In these children, between 82% and 92% had eye lesions by adolescence. Almost all (36 out of 38) of the children in our cohort, who missed being treated in their first year of life, had eye disease at presentation. For these children in our series, most of whom had quiescent eye lesions when first diagnosed, they also had new lesions develop. The frequency of new chorioretinal lesions in adolescence in this cohort indicates that ophthalmologic examinations for children with toxoplasmosis through at least the second decade are essential before outcomes of treatment studies such as that described in the companion article8 can be considered definitive. Follow-up into later decades of life is also of importance.
The rates of occurrence of new lesions are high in our cohort of children who were not treated during the first year of life. This rate is consistent with the rates described by Koppe and associates and Wilson and associates for infants without lesions at birth with subclinical infection. These rates of new lesions provide a striking comparison with the rates of new lesions in our cohort of children who were treated during their first year of life. These treated children are younger and had much more significant disease at birth. The children with more severe involvement at birth but who were treated have an incidence of new eye lesions of ~10% while the children in the present cohort who were not treated during their first year of life had a much higher incidence: 22 of 38 (71%) of new lesions. Although it is possible that the untreated children in the cohort described herein had either host or parasite genetics that predisposed them to developing new eye lesions, it seems less likely, since they had fewer symptoms and signs in the perinatal period than children in the treated cohort for whom the severity of disease at birth also correlated with later development of new eye lesions.8 Another possible explanation is that treatment in the first year of life reduces the incidence of later eye disease. However, since the cohorts are not directly comparable, these data suggest but do not prove that treatment in the first year of life may reduce subsequent eye disease. Longer follow-up is needed for both the treated and historical cohorts.
The high rate of recurrence in the present cohort suggests that suppressive therapy for such persons with lesions near the optic disc or macula, especially when bilateral, may be indicated. There are no data about suppressive therapy for children who missed being treated during the first year of life, especially as they enter their teenage years. Disease close to the optic disc or fovea, especially for a child who has severe disease with impairment of vision in one or both eyes, presents a particular threat to visual acuity (VA) and may warrant suppression. The only suppressive regimen that has documented efficacy is trimethoprim sulfamethoxazole, and this was only documented for Brazilian patients.12 We have not felt that the use of this suppressive regimen is warranted because of the high incidence of hypersensitivity to sulfonamides (25% of older children and adults), which can result in loss of the sulfonamides for subsequent treatment. Since 2005, we have treated such children, especially those with more than one recurrence, with prolonged pyrimethamine plus clindamycin or clarithromycin and then clindamycin or clarithromycin or azithromycin alone. Prolonged clarithromycin therapy has been associated with yeast vaginitis. When such suppressive therapy was given to four children, who had multiple antecedent sequential sight-threatening recurrences, no further recurrences have occurred. An alternative approach has been to rigorously utilize prompt self-reporting of symptoms and daily monitoring of VA using an amsler grid, a home vision test, print-outs of newspaper, or Allen card pictures, with prompt attention by the patient's ophthalmologist if any new visual symptoms occur. This is so that treatment can be initiated immediately for any recrudescence. Younger children should be, and are in this cohort, followed using home vision testing suitable for young children and age-appropriate office eye examinations. Additional data, including data about parasite genetics and host genetics, also will contribute to determining best approaches for this difficult problem.
It is of interest that six of 38 children had a worsening in severity score with the development of new central lesions (data not shown). Those children who had symptomatic lesions and were treated promptly seemed to have less diminution of VA than those whose lesions were detected at routine visits (Kuo and associates, data not shown; manuscript in preparation).
Results from the study of this cohort suggest that children with toxoplasmosis diagnosed after, and therefore not treated in, the first year of life commonly develop new eye lesions with time and that the new lesions are often diagnosed after the first decade of life.
Supplementary Material
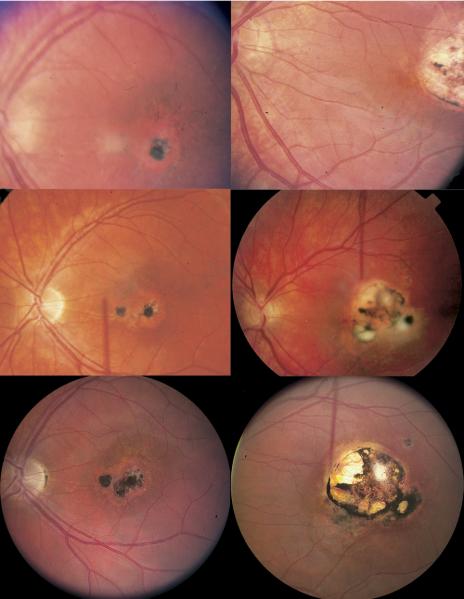
FIGURE 3.
Representative example of new toxoplasmic chorioretinal lesions in patient no. 42. Right eye of patient at age 4 years (Top left), age 11 years (Middle left), and age 19 years (Bottom left). Left eye of patient at age 4 (Top right), at age 11 years (Middle right), and at age 19 years (Bottom right).
FIGURE 4.
Representative example of new toxoplasmic chorioretinal lesions in patient no. 25. Left eye of patient at age 23 years two months (Left) and at age 23 years three months (Right).
FIGURE 5.
Representative example of new toxoplasmic chorioretinal lesions in patient no. 84. Left eye of patient from two different angles at age 12 years (Top) and at age 13 years (Bottom).
Acknowledgments
THIS STUDY WAS SUPPORTED IN PART BY THE NIH (RO1 NIAID AI 1-27530-15), BETHESDA, MARYLAND AND THE RESEARCH TO Prevent Blindness Foundation, New York, New York; the Hyatt Hotel Foundation, Chicago, Illinois; United Airlines Friendly Skies, Braniff International Airways, Southwest Airlines, American Airlines, Air Canada, and Angel Flight America; and the Langel, Morel, Kiewit, Rooney-Alden, Blackman, Rosenstein, Kapnick, Cussen, Samuel, Taub, and Singer families. The authors indicate no financial conflict of interest. Involved in design of study (S.M., K.B., C.S., M.M., N.R., P.M., R.M.); conduct of study (W.M., P.R., K.B., S.C., J.R., P.M., M.S., R.M.); collection, management, analysis, and interpretation of the data (L.P., K.K., J.J., A.G.N., P.L., A.K., W.M., S.M., P.R., K.B., C.S., M.M., N.R., S.C., J.R., P.M., M.S., R.M.); and preparation, review, or approval of manuscript (L.P., K.K., J.J., A.G.N., P.L., W.M., S.M., P.R., C.S., M.M., N.R., S.C., M.S., R.M.). Institutional Review Board (IRB) approval was obtained at the University of Chicago for this study. Written informed consent was obtained from all parents or legal guardians of participating minor-age children and/or directly from the patient (if of legal adult age). This study also is in compliance with all Health Insurance Portability and Accountability Act of 1996 (HIPAA) regulations. Because this cohort study deals with only historical patients, those who were not treated during the first year of life, this study does not qualify as a clinical trial.
We would like to acknowledge Theodore Karrison, Department of Health Studies, University of Chicago, Chicago, Illinois for his statistical consultation and assistance.
Biographies

Laura Phan, MD, MPH, is currently a resident in Ophthalmology at Virginia Commonwealth University-Medical College of Virginia, Richmond, Virginia, where she also received her medical degree. Dr Phan developed her interest in research while obtaining her Master's in Public Health at Yale University School of Public Health, New Haven, Connecticut as the Wilbur G. Downs' Research Fellow.

Dr A. Gwendolyn Noble earned her PhD in 1980 from the University of Florida, Gainesville, Florida studying herpes simplex virus, which she continued as a postdoctoral fellow at the University of Chicago, Chicago, Ilinois then completed her MD from Chicago Medical School in 1987. After ophthalmology residency, Dr Noble obtained fellowship training in pediatric ophthalmology at Children's National Medical Center, Washington, DC. She practices pediatric ophthalmology in Chicago's western suburbs at Children's Memorial Hospital and in the Congenital Toxoplasmosis Study (2001).
REFERENCES
- 1.Koppe JG, Loewer-Sieger DH, de Roever-Bonnet H. Results of 20-year follow-up of congenital toxoplasmosis. Lancet. 1986;1:254–256. doi: 10.1016/s0140-6736(86)90785-3. [DOI] [PubMed] [Google Scholar]
- 2.Wilson CB, Remington JS, Stagno S, Reynolds DW. Development of adverse sequelae in children born with subclinical congenital Toxoplasma infection. Pediatrics. 1980;66:767–774. [PubMed] [Google Scholar]
- 3.Mets MB, Holfels E, Boyer KM, et al. Eye manifestations of congenital toxoplasmosis. Am J Ophthalmol. 1996;122:309–324. doi: 10.1016/s0002-9394(14)72057-4. [DOI] [PubMed] [Google Scholar]
- 4.McAuley J, Boyer KM, Patel D, et al. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial. Clin Infect Dis. 1994;18:38–72. doi: 10.1093/clinids/18.1.38. [DOI] [PubMed] [Google Scholar]
- 5.Roizen N, Swisher CN, Stein MA, et al. Neurologic and developmental outcome in treated congenital toxoplasmosis. Pediatrics. 1995;95:11–20. [PubMed] [Google Scholar]
- 6.McLeod R, Boyer K, Karrison T, et al. Outcome of treatment for congenital toxoplasmosis, 1981-2004: the National Collaborative Chicago-Based Congenital Toxoplasmosis Study. Clin Infect Dis. 2006;42:1383–1394. doi: 10.1086/501360. [DOI] [PubMed] [Google Scholar]
- 7.Hogan MJ. Ocular toxoplasmosis. Am J Ophthalmol. 1958;46:467–494. doi: 10.1016/0002-9394(58)91127-9. [DOI] [PubMed] [Google Scholar]
- 8.Phan L, Kasza K, Jalbrzikowski J, et al. Longitudinal study of new eye lesions in treated congenital toxoplasmosis. Ophthalmology. 2008;115:553–559. doi: 10.1016/j.ophtha.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 9.Binquet C, Wallon M, Quantin C, et al. Prognostic factors for the long-term development of ocular lesions in 327 children with congenital toxoplasmosis. Epidemiol Infect. 2003;131:1157–1168. doi: 10.1017/s0950268803001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couvreur J, Desmonts G, Tournier G, Szusterkac M. A homogeneous series of 210 cases of congenital toxoplasmosis in 0 to 11-month-old infants detected prospectively [in French] Ann Pediatr (Paris) 1984;31:815–819. [PubMed] [Google Scholar]
- 11.Desmonts G, Couvreur J. Congenital toxoplasmosis. Prospective study of the outcome of pregnancy in 542 women with toxoplasmosis acquired during pregnancy [in French] Ann Pediatr (Paris) 1984;31:805–809. [PubMed] [Google Scholar]
- 12.Silveira C, Belfort R, Jr, Muccioli C, et al. The effect of long-term intermittent trimethoprim/sulfamethoxazole treatment on recurrences of toxoplasmic retinochoroiditis. Am J Ophthalmol. 2002;134:41–46. doi: 10.1016/s0002-9394(02)01527-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.