Abstract
In Saccharomyces cerevisiae, the simultaneous absence of Slt2 and Rim101 prevents growth in nonosmotically stabilized media (F. Castrejon et al., Eukaryot. Cell 5:507-517, 2006). The double mutant slt2Δ rim101Δ displays altered chitin rings, together with a significant reduction in the overall levels of chitin. Cultures of this mutant lyse upon transfer to nonosmotically stabilized media, mostly through the bud, and such lysis is partially prevented by deletion of the chitinase gene (CTS1). Growth of the slt2Δ rim101Δ double mutant was restored by the overexpression of the GFA1 or CCT7 genes, which code for two biologically unrelated proteins. Further characterization of the mutant and its suppressors indicated that both Slt2 and Rim101 were independently required for the correct assembly of the septum machinery and that their concomitant absence reduced Chs3 accumulation at the neck, leading to lower levels of chitin. GFA1 overexpression, as well as the addition of glucosamine to the growth medium, specifically suppressed the growth defects by activating chitin synthesis at the neck and restoring the normal assembly of the chitin ring. In contrast, overexpression of CCT7, a Cct chaperonin subunit, alleviated the defect in the septum machinery without affecting chitin synthesis. Both suppressors thus act by reducing neck fragility through different mechanisms and allow growth in nonstabilized media. This work reports new roles for Slt2 and Rim101 in septum formation in budding yeast and confirms the homeostatic role of the chitin ring in the maintenance of neck integrity during cell division.
The Saccharomyces cerevisiae cell responds to changing environmental conditions by means of a broad array of signal transduction pathways that contribute to the remodeling of cell physiology to adapt to such events (12). The external part of the fungal cell is the cell wall, which constitutes the major interface with the environment and is also responsible for the maintenance of cellular shape. It is therefore not surprising that yeasts have a signal transduction pathway involved in cell wall remodeling in response to environmental aggression, the PKC or cell wall integrity (CWI) pathway (33). This pathway depends on a signal transmission mechanism based on a kinase cascade that, in turn, activates a transcription factor, Rlm1, which then triggers a specific transcriptional response directly involved in cell wall remodeling (17). Although this response is the major event triggered by activation of the pathway, there is increasing evidence to suggest that several components of the cascade participate in the control of other cellular processes. The overall working model for the route has recently been reviewed extensively (33), and hence, herein we shall focus on the specific role of Slt2 (Mpk1), the mitogen-activated protein kinase (MAPK) of this route. In addition to its role as a direct activator of Rlm1, this kinase has been proposed to regulate the SBF transcription factor in response to cell wall stress, although some evidence suggests that Slt2 may also regulate SBF along the cell cycle (2, 34). Slt2 cycles between the nucleus and the cytoplasm (24), accumulating at the site of polarized growth (46). Based on these localizations, it has been proposed that Slt2 would act on multiple cytoplasmic targets, including the Cch1/Mid1 Ca2+ channel and several protein phosphatases, although it is generally believed that most of the cytoplasmic targets of Slt2 remain to be described (12, 33).
For several years, the CWI response was thought to be the only signaling response involved in cell wall control. However, increasing evidence has suggested that almost all yeast signaling pathways contribute to cell wall remodeling during both vegetative growth and specialized developmental stages (reviewed in reference 33). In this respect, it has recently been shown that the PKC, high osmotic glycerol (HOG), and Ca2+/calcineurin pathways regulate chitin synthesis in Candida albicans (35). More surprisingly, the cell wall damage produced by zymolyase is translated into the CWI transcriptional response through the HOG signaling pathway (4). These new results complement those of previous studies reporting the role of the HOG (19, 20) and calcineurin (28) signaling pathways in cell wall construction.
Contrary to what occurs in most signal transduction routes, the RIM101 signaling pathway does not rely on a cascade of protein kinases for signaling but instead relies on the proteolytic processing of a transcription factor, Rim101 (36). This proteolytic processing activates a transcriptional response that allows yeast cells to thrive in alkaline pH media in combination with activation of the calcineurin pathway (44). However, Rim101 also acts as a transcriptional repressor under noninducing conditions. Accordingly, several genes are overexpressed in the rim101Δ mutant, including some cell wall-related genes (30). In addition, it has been shown that several mutants in the PKC cascade are alkali sensitive, suggesting that cell wall remodeling occurs in response to alkalinization of the medium (43). In light of this evidence, it is not surprising to find that rim101Δ mutants have cell wall-associated defects and, more importantly, that the concomitant absence of Slt2 and Rim101 leads to cell lysis (10).
Beyond the general importance of the fungal cell wall in the maintenance of cellular shape, remodeling of the cell wall plays a critical role during the process of cell division in yeasts (32). Separation of mother and daughter cells is achieved by the synthesis of a primary septum formed by chitin that is later covered with other cell wall layers to form the secondary septum (7). The primary septum is surrounded by a chitin ring made by chitin synthase III (CSIII). This ring is not essential for cell division but apparently reinforces the neck region, favoring the correct assembly of the different layers of the cell wall in this region (32). The regulation of CSIII is complex and depends on the correct intracellular trafficking of Chs3, its catalytic subunit (38). Chs3 is delivered to the neck in a polarized mode, where it interacts with Chs4. This interaction mediates the anchorage of Chs4 to septins through Bni4 (16, 26). In addition, Chs4 promotes CSIII activation (37).
Correct assembly of the septum machinery and the septum formation depend directly on septins. The absence of the septin ring prevents the formation of primary and secondary septa, and the cells die, although the synthesis of cell wall materials persists (42). Incorrect assembly of the septin ring produces viable cells with altered primary septa, whose defects are severely aggravated in the absence of the chitin ring. These results have led to the proposal that both septins and the chitin ring are required for neck integrity in budding yeast (42).
After septum formation, the mother and daughter cells are physically separated by coordinated degradation of the yeast cell walls at the neck. This degradation is mediated by the chitinase (27) and glucanase (3) activities encoded by the CTS1 and ENG1 genes, respectively, but also by other as yet not fully characterized activities. This degradation is cell cycle regulated through the Ace2 transcription factor, which induces the expression of several genes, including CTS1 and ENG1, specifically in the daughter cell (13). An imbalance between the chitin synthase I (CSI) and chitinase activities has been shown to trigger cell lysis (9).
Previous work at our lab led to the identification of the synthetic lethality of the slt2Δ rim101Δ mutant. This was explained in terms of a general defect in the assembly of the yeast cell wall (10), based on the deregulation of the expression of several cell wall-associated genes caused by the absence of a functional RIM101 pathway (30). In order to explore the molecular basis for this lethality in greater detail, herein we carried out a screening for multicopy suppressors of this lethality. Our screening highlighted the defects of this mutant, showing that it dies because of an unbalanced formation of the septum produced by the accumulation of the independent defects caused by the slt2Δ and rim101Δ mutations. Thus, in the present work, we explored the roles of Slt2 and Rim101 in the septum formation.
MATERIALS AND METHODS
Strains, plasmids, and yeast genetic methods.
Standard procedures were employed for yeast genetic manipulations (39) and DNA manipulations (40). The S. cerevisiae strains used in this study and their sources are listed in Table S1 in the supplemental material. Single mutants were made using the one-step gene replacement technique, while multiple mutants were always obtained after tetrad dissection of the heterozygous mutants obtained by conjugation of the appropriate mutant strains.
The plasmids used throughout this work are listed in Table S1 in the supplemental material and have been described previously, with the exception of pRS314-BNI4::YFP. In this case, the yellow fluorescent protein (YFP) tag was introduced as a NotI fragment in a NotI site created specifically at the last codon of BNI4, following a strategy previously used in our laboratory (14). The tagged protein was confirmed to be fully functional in the Δbni4 mutant.
Culture conditions.
YEPD (1% yeast extract, 2% peptone, and 2% glucose) or synthetic minimal medium (SC) (0. 7% yeast nitrogen base without amino acids and 2% glucose) supplemented with 1 M sorbitol were used as routine media. SC was supplemented with appropriate amino acids and nucleic acid bases. Typically, growth of the different mutants was assessed in YEPD-sorbitol media, but SC-sorbitol was used in all experiments involving plasmid-containing cells. Alternatively, plain YEPD or SC with or without 15 mM glucosamine was used in some of the experiments, as indicated.
Isolation of suppressors.
The FCM328 (slt2Δ rim101Δ) strain was transformed with a multicopy library made in plasmid YEp13 (ATCC 37323), and leucine prototrophs were grown on selective SC-sorbitol medium. In two independent transformations, we obtained approximately 10,000 transformants, which were individually picked and tested for growth on SC-sorbitol and YEPD plates at different temperatures (28°C, 35°C, and 37°C). Direct selection of suppressors was not possible in SC, since this medium allowed for partial growth of the double mutant (not shown), making the identification of bona fide suppressors extremely difficult. All transformants that grew at least at 28°C and 35°C on YEPD were reconfirmed and eventually selected for further analysis. The screening provided 15 bona fide suppressors that were able to restore partial growth to the slt2Δ rim101Δ mutant in nonosmotically stabilized medium. We were able to rescue the plasmids from 14 of these transformants, which in all cases restored growth in the mutant after retransformation, and hence, the plasmids contained the expected suppressors. Most of the plasmids contained several open reading frames, some of which were subcloned, allowing the identification of some suppressor genes (see Results). Some of these plasmids are still under characterization, and the results will be published elsewhere.
Chitin determinations.
Chitin measurements were performed using chitinase from Serratia marcescens (Sigma-Aldrich) and colorimetric determination of N-acetyl-d-glucosamine (GlcNAc), as described previously (41). Chitin levels are expressed as millimoles of GlcNAc released per 100 mg of cells (wet weight).
Northern analyses.
Total RNA was obtained, as described previously (40), and the poly(A) fractions were purified using the mRNA purification kit (GE Healthcare). A total of 10 μg of each mRNA was loaded per lane, and the RNAs were finally transferred to Hybond-N membranes. Blots were hybridized with internal probes from the different genes labeled using the Rediprime II system (GE Healthcare) and were visualized using a PhosphorImager system (BAS-1500; Fujifilm). The intensities of the bands were quantified, and the reported values are always referred to actin, used as a loading control.
Immunoblot analyses.
Proteins were analyzed by Western blotting after sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting, as described previously (37), but using 2 mM phenylmethylsulfonyl fluoride in the protease inhibitor cocktail. Commercial mouse monoclonal antibodies against the green fluorescent protein (GFP) epitope (JL-8; Clontech Laboratories, Inc.) or β-tubulin (Sigma-Aldrich) were used as primary antibodies. Western blots were developed using the ECL kit (GE Healthcare). All Western blots reported were repeated at least three times, and a single representative experiment is shown. When required, quantification was performed using Quantity One software (Bio-Rad), and values represent band intensities relative to the weight, using tubulin as a loading control for each sample.
Microscopic techniques.
Calcofluor vital staining was observed in cells grown in YEPD-sorbitol in the presence of 50 μg ml−1 calcofluor for 2 h at 28°C. Alternatively, the chitin ring was observed in fixed cells (3.2% formaldehyde, 30 min) stained with calcofluor for 5 min. For GFP visualization, yeast cells containing the corresponding centromeric plasmid were grown to early logarithmic phase in SC-sorbitol medium supplemented with 0.2% adenine. All microscopic observations were performed using a Leica RX150 epifluorescence microscope with a 100 W Hg lamp, using the following appropriate filters: UV for calcofluor, GFPblue for GFP, and C3 for YFP observations. Images were obtained with a Sensys digital camera and were processed with Adobe Photoshop CS software. All images shown in each figure were acquired under identical conditions and processed in parallel to preserve the relative intensities of fluorescence for comparative purposes. Cell measurements were performed using the tools included in the ImageJ 1.38x package (NIH).
Electron microscopy.
Transmission electron microscopy (TEM) was performed essentially as described previously (10). Briefly, S. cerevisiae cultures were grown to an optical density at 600 nm of 0.5 to 1.0 in 1 M sorbitol-supplemented YEPD, transferred to YEPD, and grown for an additional 1.5 h. Then, 12.5 optical-density-at-600-nm units of cells was collected by centrifugation, rapidly washed, and fixed in 2% potassium permanganate for 1 h at room temperature. Excess potassium permanganate was removed by exhaustive washing, and the stained cells were finally dehydrated by incubation in increasing concentrations of ethanol. Samples were processed in the embedding medium (Spurr's resin embedding kit; TAAB) by successive 2-hour incubations in 1:1 and 1:3 ethanol/embedding medium mixtures and, finally, in fresh embedding medium. Cells were concentrated in 500 μl of resin, and samples were allowed to polymerize overnight at 70°C. Ultrathin sections were obtained using an LKB Ultratome III microtome, and samples were visualized under a Zeiss EM 900 transmission electron microscope. Images were processed with Adobe Photoshop software, preserving relative magnifications.
Statistical analysis.
One-way analysis of variance was used to test for differences in chitin synthesis values or the inner diameter of chitin rings among yeast strains. Tukey's honestly significant difference method (equal sample sizes) and the GT2 method (unequal sample sizes) were used as a posteriori tests to perform multiple comparisons. Planned comparisons were performed with the least significant difference procedure. Dunnett's method was used to compare several strains to the controls. The chi-square test was used to test differences in proportions. In all tests, P values of <0.05 were considered statistically significant, and significant values are indicated in the text.
RESULTS
The slt2Δ rim101Δ mutant shows altered chitin rings at the cell division sites.
Previous work carried out at our lab identified the synthetic lethality between the rim101Δ and slt2Δ mutations, which was bypassed by osmotic stabilization of the growth media (10). This lethality was explained in terms of the complementary roles of Rim101 and Slt2 in cell wall assembly. This explanation was not very precise in molecular terms; therefore, we further characterized the phenotypes of the double mutant.
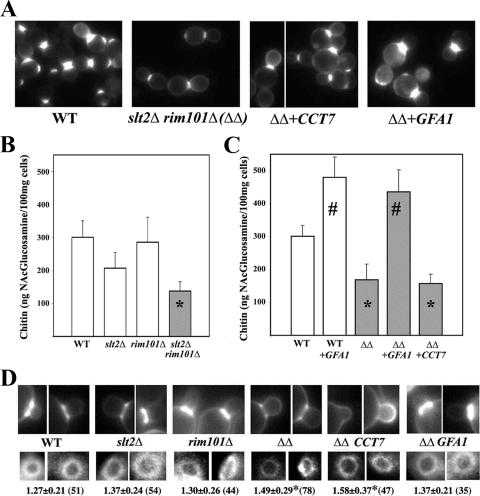
The rim101Δ slt2Δ double mutant stained poorly with calcofluor (Fig. 1A) and, more importantly, showed a significant (P < 0.05) reduction in chitin synthesis, displaying approximately half of the chitin displayed by the wild type (wt) (Fig. 1B). This reduction in chitin can be explained only in terms of the combination of both mutations, since the individual mutants did not show significant reductions in their chitin levels compared to that of the wt (Fig. 1B). In S. cerevisiae, most chitin forms the chitin ring that surrounds the neck during cell division, so we addressed the formation of the chitin ring by calcofluor staining of fixed cells. The single slt2Δ and rim101Δ mutants showed levels of staining similar to those of wt cells (Fig. 1D). However, the slt2Δ rim101Δ double mutant showed reduced staining, in agreement with the observed chitin levels (Fig. 1B). Moreover, the inner (Fig. 1D) and the outer (not shown) diameters of the chitin rings increased significantly (P < 0.001) in the double mutant. Apparently, the double mutant fails to assemble a normal chitin ring.
FIG. 1.
Chitin synthesis in selected strains. (A) Calcofluor staining of the double mutant (ΔΔ) transformed with the indicated suppressors. The wt is shown as a control. (B, C) Chitin levels in the indicated strains. Values are the means from three independent experiments, and standard deviation bars are indicated. Data marked with asterisks indicate values that are significantly (P < 0.05) different from those of the wt, and data marked with number signs indicate values that are significantly (P < 0.05) different from those of the wt or the double mutant. (D) Staining of the chitin ring after 5 min of calcofluor treatment in the indicated strains. All images were processed identically to preserve relative intensities (top row). The numbers indicate the inner diameter of the ring expressed in micrometers (bottom row). Standard deviations, as well as the number of rings measured for each strain (in parentheses), are indicated. Values that are significantly different (P < 0.05) from those of the wt are indicated with an asterisk.
The slt2Δ rim101Δ mutant lyses through the neck because of the unbalanced synthesis/degradation of the chitin ring.
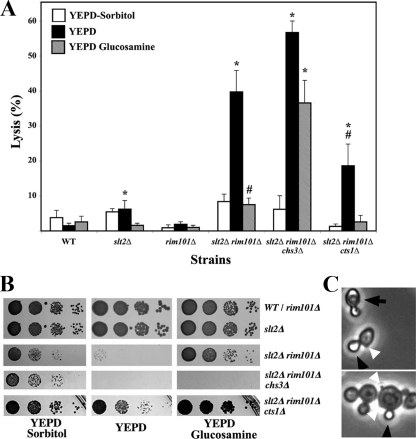
The rim101Δ slt2Δ mutant was unable to grow in a nonosmotically stabilized media. Accordingly, we addressed the behavior of these cells upon transfer to plain YEPD media. All strains tested showed marginal levels of lysis in osmotically supplemented media. However, the transfer of the slt2Δ rim101Δ double mutant to plain YEPD produced a high degree of lysis (Fig. 2A), in accordance with the growth defect observed in solid media (Fig. 2B). Interestingly, the lysed cultures of the slt2Δ rim101Δ double mutant showed many refringent daughter cells (Fig. 2C). Approximately 40% of the lysis occurred only in the daughter cells, suggesting that lysis had occurred very late in the cell cycle, i.e., after the physical separation between mother and daughter cells. This phenotype is very similar to that described for the chs1Δ mutant in SC (acidic), which has been shown to be produced by an unbalanced function of CSI and chitinase activities (8). It thus became clear that we had to test whether overexpression of CHS1 or deletion of the chitinase gene would suppress the lethality of the double mutant. Expression of CHS1 in a multicopy plasmid, which has been shown to increase CSI by severalfold (5), did not improve the growth of the double mutant (not shown). However, deletion of the CTS1 gene, which encodes the major chitinase in S. cerevisiae (9), clearly improved the growth of the double mutant (Fig. 2B), significantly (P < 0.05) reducing the lysis of the culture in plain YEPD (Fig. 2A). This suppression was only partially based on the lysis (18.5% ± 5.6%) still observed in the slt2Δ rim101Δ cts1Δ mutant in YEPD, significantly (P < 0.05) higher than that in the wt. Interestingly, the slt2Δ rim101Δ double mutant showed a higher expression level of CTS1 (see Table S2 in the supplemental material), in accordance with the described role of Rim101 as a transcriptional repressor (30). This result therefore reinforces the idea that an imbalance between the synthesis and degradation of chitin at the neck could be responsible for the lethality of the slt2Δ rim101Δ double mutant.
FIG. 2.
Cell lysis in nonosmotically stabilized media. (A) Percentage of lysis after transferring logarithmically growing cells of the indicated strains from YEPD-sorbitol medium to the indicated media. Results were scored 3 h after the transfer, and lysis was determined by counting refringent cells using phase-contrast microscopy. The results presented are the means from two independent experiments, and standard deviations are indicated as bars. The degree of lysis observed in each medium was compared statistically with the values obtained for the wt in the corresponding media. Values significantly different (P < 0.05) from those of the corresponding wt are marked with an asterisk. A number sign indicates a significant (P < 0.05) reduction in lysis compared with the degree of lysis for the double mutant grown in YEPD. (B) Growth of the indicated mutants in different media. Logarithmically growing cells in YEPD-sorbitol were spotted onto the indicated media at a 1/10 serial dilution. Plates were incubated at 28°C for 48 h. (C) Overall aspect of the slt2Δ rim101Δ mutant grown in YEPD. Note the higher proportion of small daughter cells lysed (black arrowheads) than paired cells lysed (arrows). Note also the prominence of some bud scars in the double mutant (white arrowheads).
In order to test this hypothesis, we attempted to suppress this synthetic lethality by supplementing the growth media with glucosamine, a well-known inducer of chitin synthesis (6). This treatment increased chitin synthesis in the mutant (not shown, see below) and reduced lysis to a significant extent (Fig. 2A), allowing for growth of the double mutant on plain YEPD medium supplemented with 15 mM glucosamine (Fig. 2B). However, the triple mutant slt2Δ rim101Δ chs3Δ, which completely lacks CSIII activity, was able to grow in osmotically stabilized media, but its growth defect could not be suppressed by glucosamine (Fig. 2B). Lysis remained significantly (P < 0.05) high (Fig. 2A and B) in this medium, thus indicating that the upregulation of the CSIII induced by glucosamine (6) should be responsible for the suppression.
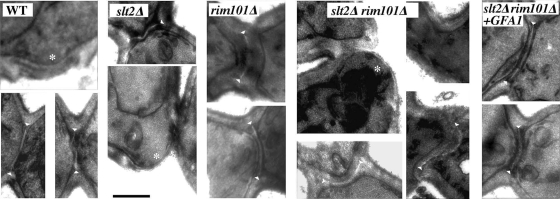
Together, these results suggest that lysis could be due to the altered assembly of the septum at the neck region during cell division, where CSIII is involved in the construction of the chitin ring and chitinase acts to promote cell separation. TEM analysis of the double mutant indicated that bud scars were more prominent (Fig. 2C and 3), and the neck region seemed to be more cylindrical, similar to that observed in the chs3Δ mutant (42). The double mutant was able to form primary septa, although these appeared displaced into the daughter cells and were more irregular than in control strains. None of these defects was apparent in the single mutants. Deletion of CTS1 in the double mutant did not improve these phenotypes (data not shown), indicating that these characteristics are not directly related to the overproduction of chitinase.
FIG. 3.
Ultrastructure of the yeast septum. TEM images of cells showing the septum structure in different strains. Note the more prominent scars (*) in the slt2Δ rim101Δ double mutant compared to those in the wt or slt2Δ strains and the irregular shape of the primary septum, observed as a white region between septal walls (arrowheads). Also note the more tubular aspect of the neck in the double mutant. The bar corresponds to 0.5 μm.
Identification of multicopy suppressors: overproduction of chitin restores cell growth in the slt2Δ rim101Δ double mutant.
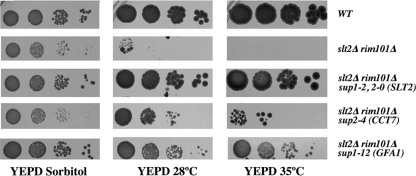
To further investigate the molecular basis of this lethality, we selected multicopy suppressors that might improve the growth of the rim101Δ slt2Δ double mutant. For this purpose, the double mutant was transformed with a gene library constructed in the multicopy vector YEp13. More than 10,000 individual transformants were selected in SC-sorbitol media and tested for growth at different temperatures on YEPD solid media (see Materials and Methods). We identified 14 bona fide transformants able to grow on YEPD at 28°C or 35°C (Fig. 4). Plasmids were rescued, sequenced, and retransformed into the double mutant. A preliminary characterization indicated that only four plasmids (1-2, 2-0, 2-4, and 1-12) restored, at least partially, calcofluor staining in the double mutant (Fig. 1A and data not shown) and promoted good growth on plates (Fig. 4). Plasmids 1-2 and 2-0 contained the SLT2 gene, explaining the normal behavior of these transformants (Fig. 4). Thus, we focused on plasmids 2-4 and 1-12, which were subcloned and tested for suppression. We found that the GFA1 and CCT7 genes were responsible for the observed suppression, although they were unable to restore full growth compared to the wt or single mutants (Fig. 4). A comprehensive description of the other suppressors will be published elsewhere. Surprisingly, no plasmid containing the RIM101 gene was identified in our screening, although overexpression of this gene was able to suppress the synthetic lethality of the double mutant (not shown). Therefore, it may be assumed that the screening was not yet saturated and/or that the RIM101 gene may have been underrepresented in our gene library.
FIG. 4.
Suppression of the synthetic lethality of the slt2Δ rim101Δ mutant. (A) Growth of the slt2Δ rim101Δ mutant transformed with the different multicopy suppressors. Logarithmically growing cells in SC-sorbitol were spotted onto the indicated media at a 1/10 serial dilution. Plates were incubated at the indicated temperatures, and growth was scored 48 h after inoculation.
Gfa1 is required for the synthesis of the metabolic precursors of chitin, acting as a bottleneck in the regulation of the synthesis of this polymer. Increased or decreased levels of Gfa1 are directly reflected in higher or lower levels of chitin synthesis, respectively (29). In addition, GFA1 expression is induced after cell wall damage in a PKC-dependent mode (17, 28). CCT7 encodes a Cct chaperonin subunit (45), and the CCT complex is involved in multiple cellular processes. However, Cct7 has not been previously related to chitin synthesis, suggesting that there are two independent suppression mechanisms.
In order to check this hypothesis, we tested for chitin synthesis in the different strains (Fig. 1C). Overexpression of GFA1 dramatically increased chitin synthesis in the slt2Δ rim101Δ double mutant as well as in the wt, as described previously (29). This result is in complete agreement with the suppression observed after glucosamine addition, since both GFA1 overexpression and glucosamine have been shown to increase chitin synthesis through an upregulation of CSIII activity (6, 29). On the contrary, CCT7 overexpression did not significantly increase chitin synthesis in the double mutant (Fig. 1C). Thus, GFA1 suppression increased chitin deposition at the neck, restoring the normal diameter of the chitin ring (Fig. 1D) and the normal architecture of the septa (Fig. 3). In contrast, CCT7 suppression did not alter chitin synthesis nor significantly reduce the diameter of the chitin ring (Fig. 1D). Apparently, the GFA1 and CCT7 genes suppress by different mechanisms.
The slt2Δ rim101Δ mutant is impaired in the assembly of functional CSIII at the neck.
The defects described above are linked, at least partially, to a defect in CSIII, which may be due to a decrease in the precursors of chitin, as suggested by the suppression caused by GFA1. However, the levels of GFA1 expression in all the mutant strains were similar to those of the wt (see Table S2 in the supplemental material). Thus, reduced levels of GFA1 cannot explain the observed reduction in chitin synthesis, and we therefore concentrated on the molecular machinery of CSIII activity.
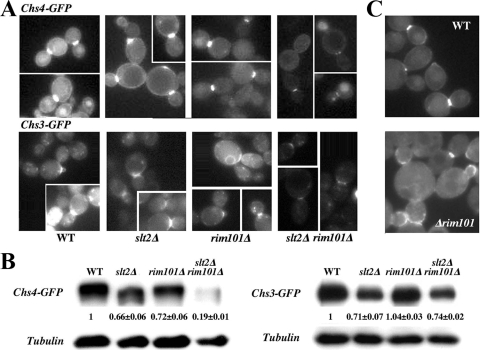
CSIII depends on the correct delivery of Chs3 to the plasma membrane (PM) around the neck constriction, a process that depends at several levels on the function of Chs4 (16, 37, 41). Accordingly, we determined the intracellular distribution of Chs4 and Chs3 (Fig. 5A). Both proteins localized normally at the neck in all strains tested, but the signal for Chs3-GFP and Chs4-GFP in the slt2Δ rim101Δ double mutant was reduced, showing a lower accumulation at the neck than in the wt or single mutants. Such a defect could be due to lower protein levels or to an improper anchoring of CSIII to the septum machinery. In light of this, we tested both possibilities.
FIG. 5.
Intracellular behavior of CSIII proteins. (A) Localization of the indicated proteins in the different strains. The images of each protein were acquired under identical conditions, and pictures were processed in parallel for comparative purposes. The intensity of the label should therefore roughly reflect the relative amount of a protein in the different strains. Note the reduced intensity of both proteins in the double mutant. (B) Total protein levels, as determined by Western blotting. Numbers indicate the relative levels of Chs4-GFP or Chs3-GFP, using tubulin as a loading control. The data presented are the averages from two independent experiments. (C) Localization of Chs4-GFP integrated into the chromosome. Cells in the experiments shown in panels A and B were grown in sorbitol-supplemented SC, while the cells shown here were grown in plain SC. Note the absence of Chs4-GFP along the PM in all rim101Δ strains and the significant delocalization (see the text) of the integrated Chs4-GFP to the bud shown here.
Chs4-GFP levels were reduced in the double mutant in comparison with the wt or single mutants (Fig. 5B), thereby explaining the reduced fluorescence observed for this protein. However, Chs3-GFP levels were similar in the wt and the double mutant (Fig. 5B). Careful analysis of Chs4 distribution indicated that both rim101Δ and the double mutant cells lacked the peripheral PM staining observed in the wt and the slt2Δ single mutant. This distribution is fairly similar to that of nonprenylated forms of Chs4 (37), suggesting that the absence of Rim101 could alter the intracellular localization of Chs4. We confirmed the effect of the rim101Δ mutation in Chs4 localization using an integrated version of Chs4-GFP. As shown in Fig. 5C, Chs4-GFP delocalized to the bud in the rim101Δ mutant, with the number of buds showing staining in the rim101Δ mutant increasing to 38.5 ± 0.7 (n = 102) compared to the number of buds being 12.2 ± 3.4 (n = 196) in the wt (P < 0.05). These observations indicated a limited functionality of Chs4 that would account for the reduced accumulation of Chs3 at the neck and the reduced levels of chitin in the double mutant. However, they do not explain the molecular basis for the reduced accumulation of Chs4 at the neck.
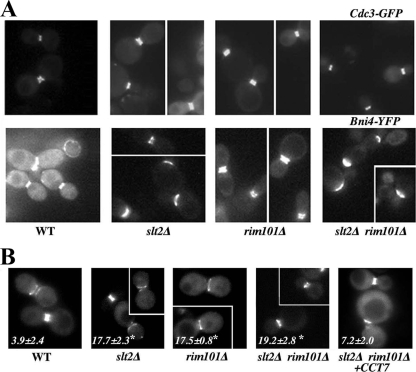
The accumulation of CSIII at the neck depends on the septum machinery by anchoring Chs4 to septins through the intermediate protein Bni4 (16, 41). Thus, we tested Bni4, Cdc3, and Myo1 localization in the mutant strains. Myo1 is part of the contractile ring that promotes cell separation and is directly involved in the formation of primary septa. Myo1 localization was similar in all strains tested, including in the double mutant (see Table S3 in the supplemental material), which allowed the formation of primary septum, as observed in TEM images (Fig. 3). Cdc3 is an essential part of the septin ring, and its localization was similar in the single mutants and in the wt, with approximately 50% of the cells showing a clear double ring (Fig. 6A; Table 1) (P > 0.05). However, the double mutant displayed a significantly (P < 0.05) reduced number of double rings (Table 1), as highlighted in the images presented. Closer scrutiny of the cultures indicated that the single mutants had indeed undergone some alterations in septin assembly. The wt cultures showed a low number of cells with asymmetric double rings, but this number increased significantly in the single mutants and in the double mutant (P < 0.05) (Fig. 6B), strongly suggesting a role for Slt2 and Rim101 in the control of septin assembly. Interestingly, the septin ring controls the asymmetric distribution of some neck proteins during cell division (25), and among them is Bni4, a key protein for Chs4 localization (26, 41). Bni4 appeared to be asymmetrically accumulated at the mother cell side in the slt2Δ mutant and the double mutant, whose cultures showed a significantly (P < 0.05) reduced number of Bni4 double rings in comparison with those of the wt (Fig. 6A and Table 1), together with a significantly wider distribution of the protein (Fig. 6A; see also Table S3 in the supplemental material). The delocalization of Bni4 is intrinsically due to the absence of Slt2, since the slt2Δ single mutant showed similar defects, regardless of the growth conditions used (not shown). In light of this, it came as no surprise to observe the reduced accumulation of Chs4/Chs3 at the neck in the slt2Δ rim101Δ double mutant, which accumulated the defects caused by the slt2Δ and rim101Δ mutations.
FIG. 6.
Intracellular distribution of septum proteins. (A) Localization of the indicated proteins in the different strains. The images of each protein were acquired under identical conditions, and pictures were processed in parallel for comparative purposes. The intensity of the label should therefore roughly reflect the relative amount of a protein in the different strains. The reduced number of septin double rings in the slt2Δ and slt2Δ rim101Δ strains is appreciable in the images, but a quantitative analysis is shown in Table 1. (B) Asymmetric distribution of Cdc3 in the indicated strains. Values indicate the numbers of cells showing double rings in which either the diameter or the intensity of one of the rings is significantly different from the other, as highlighted in the images. The results are the averages from two independent experiments (n > 123), and values significantly different (P < 0.05) from those of the wt are marked with asterisks. Cells were grown in SC-sorbitol medium.
TABLE 1.
Localization of Bni4 and Cdc3 in the indicated strains
| Strain | % Localization of Bni4 and Cdc3 tested in the indicated mediaa
|
|||
|---|---|---|---|---|
| Bni4-YFP
|
Cdc3-GFP
|
|||
| Sorbitol | Glucosamine | Sorbitol | Glucosamine | |
| wt | 44.0 ± 6.4 (89) | 56.5 ± 7.7 (75) | 52.8 ± 8.9 (78) | 46.2 ± 3.8 (78) |
| rim101Δ | 60.6 ± 2.8 (112) | ND | 41.9 ± 12.7 (120) | ND |
| slt2Δ | 29.5 ± 1.1 (132)b | ND | 50.0 ± 3.1 (95) | ND |
| slt2Δ rim101Δ | 27.1 ± 7.4 (193)b | 27.3 ± 2.3 (55)b | 28.4 ± 5.3 (191)b | 15.2 ± 4.2 (46)b |
| slt2Δ rim101Δ plus CCT7 | ND | ND | 45.5 ± 0.05 (101)c | ND |
Values indicate the number of double rings observed in reference to the total number of cells showing fluorescence at the neck. The results are the means from three independent experiments, and standard deviations are indicated. Numbers in parentheses indicate the total number of cells counted. Typically, cells were grown on SC-sorbitol, but data on Bni4 and Cdc3 localization in some strains were also determined in SC-glucosamine medium. ND, not determined.
Values are significantly (P < 0.05) lower than those of the wt.
CCT7 overexpression increased significantly (P < 0.05) the number of double rings compared to the double mutant values, which were not significantly different from those of the wt.
We reported above that glucosamine or GFA1 and CCT7 overexpression suppressed the growth defect of the double mutant; accordingly, we addressed their effects on septin assembly. The increase in chitin synthesis mediated by glucosamine (Table 1) or GFA1 overexpression (not shown) did not significantly increase the number of Cdc3 or Bni4 double rings above those observed in the double mutant (Table 1), and hence, they did not have any effect on the assembly of the septum machinery. However, CCT7 overexpression increased significantly (P < 0.05) the percentage of double rings observed in the double mutant (Table 1) and alleviated the asymmetric accumulation of Cdc3 (Fig. 6B). These results confirm the initial hypothesis that the suppression promoted by GFA1 or CCT7 will act through independent mechanisms.
DISCUSSION
Suppression of lethality in the slt2Δ rim101Δ mutant highlights its defects in chitin ring assembly.
The results reported here identified specific defects of the slt2Δ rim101Δ mutant in the assembly of the septum that are directly linked to its lethality in nonosmotically stabilized media. Our data clearly indicate that the slt2Δ rim101Δ mutant has reduced levels of chitin, which are translated into a deficient formation of the chitin ring. However, this defect alone did not suffice to explain the lethality of the slt2Δ rim101Δ mutant, since the chs3Δ cells, which lack the chitin ring, were perfectly viable under all conditions tested. The absence of Rim101 also led to increased levels of CTS1 expression (30), and overexpression of CTS1 had no effect on chs3Δ but reduced growth in the slt2Δ mutant (data not shown). Accordingly, the chs3Δ rim101Δ mutant is fully viable (10), pointing to the absence of Slt2 as a major culprit in the described lethality. It therefore seems clear that the synthetic lethality of the slt2Δ rim101Δ double mutant is caused by a combination of several factors, including reduced levels of chitin together with chitinase overexpression and the absence of Slt2. Accordingly, the increase in chitin synthesis or the deletion of CTS1 alleviates the synthetic lethality of the double mutant (Fig. 1 and 2).
Although a specific role for Slt2 in septum formation remains to be clearly established (see below), chitinase and the chitin ring are major players in the assembly of the septum, and hence, the apparent fragility of the neck region in the double mutant, as determined by TEM or by the abnormally wide bud scars, was not surprising (Fig. 1D). Such fragility was not apparent in any of the single mutants, in agreement with their higher resistance to chitinase overexpression. Chitin overproduction, but not cts1Δ, restored neck architecture in the double mutant, indicating that chitinase overproduction contributes only to the lysis of an already structurally defective neck region.
Slt2 and Rim101 contribute independently to the correct assembly of the septum machinery.
Our work also highlights the defective assembly of the septin ring in the rim101Δ and slt2Δ single mutants, explaining some of the small alterations associated with them, such as calcofluor resistance or hypersensitivity to chitinase, respectively. These individual defects were exacerbated in the double mutant, leading to reduced levels of chitin, which together severely affected the assembly of the septum, promoting cell lysis. This is in agreement with previous reports (42). A similar argument could explain the synthetic lethality of the slt2Δ bni4Δ mutant, which would also accumulate the slt2Δ defects as well as a defective chitin ring formation due to the absence of Bni4 (31). This dramatic phenotype is not observed in the slt2Δ or rim101Δ single mutant because neither showed an accumulation of these defects, and hence, they exhibited fairly normal bud scars. This hypothesis is fully compatible with the viability of chs3Δ rim101Δ and rlm1Δ rim101Δ (10), with neither of which showing the septin defects associated with the slt2Δ mutation.
The septin defects observed are marginal compared to others previously reported (i.e., cla4Δ) (42), since cell lysis depends mostly on chitinase action. In agreement with this, the actomyosin ring appeared to be fully functional in the synthesis of the primary septum, contrary to what occurs in the cla4Δ mutant (42). Interestingly, the main defect observed in septin organization was the asymmetric distribution of Cdc3 in the mutants, rarely observed in the wt. The nature of Cdc3 asymmetry seemed to be different in the slt2Δ and rim101Δ single mutants, causing different biological effects, such as the asymmetric accumulation of Bni4, which is observed only in the slt2Δ strain. These differences would explain the cumulative defects observed in the double mutant.
The defects in septin assembly are also supported by the nature of one of the suppressors isolated, CCT7. This is a member of the CCT chaperonin complex that has multiple functions in yeast, including the assembly of actin and microtubules (23). It has recently been shown to interact physically with septins, being required for correct assembly of the septin ring (15). In our case, CCT7 overexpression alleviated the defect in the septin assembly of the double mutant without affecting chitin synthesis or the assembly of the chitin ring (Fig. 2). In light of this, it appears that CCT7 overexpression provides an alternative basis for septin assembly that, to some extent, prevents septum collapse. This hypothesis is in complete agreement with the recently proposed regulatory role of the CCT complex in septin assembly (15). The reasons for the increased calcofluor staining promoted by CCT7 overexpression are still unclear but could be attributed to the altered assembly of the septum rather than to the upregulation of chitin synthesis (Fig. 1).
It is much more difficult to pinpoint the specific role of Slt2 or Rim101 in the assembly of septins. Slt2 is a MAPK that has been shown to localize to the septum during part of the cell cycle (33); accordingly, it may be suspected that it would carry out some function at this place. Slt2 has been shown to interact genetically with Glc7 (1, 18), which is physically and biologically linked to Bni4 at the neck (26, 31). Therefore, it is possible to link the alterations in Bni4 localization observed in the slt2Δ mutant to Glc7 function (Fig. 6; Table 1). The Bni4 localization defects can be translated directly into an altered septin assembly, in agreement with previous reports (21). Alternatively, Slt2 could phosphorylate additional substrates at the neck, since most of the cytosolic substrates of this kinase remain unknown (33). Our work clearly demonstrates a function for Slt2 at the neck and provides the basis for searching for its substrates. More surprising is the hypothetical role of a transcription factor such as Rim101 in the assembly of the septum. Very recently, it has been shown that RSB1, which is downregulated in the rim101Δ mutant (30), is required for maintaining the lipid asymmetry of yeast membranes (22). The role of lipid asymmetry in membranes is not well understood, but it could affect the association of multiple proteins with membranes. Interestingly, in the rim101Δ mutant, Chs4 localized to the neck but was absent along the PM of the mother cells and accumulated abnormally at the buds (Fig. 5C). This localization of Chs4 resembles that of the nonprenylated forms of the protein, which associate poorly with membranes (37). This phenotype is thus perfectly compatible with the lipid asymmetry alteration caused by the downregulation of RSB1. Interestingly, both rim101Δ and rsb1Δ cells showed partially delocalized chitin deposition, and more importantly, the slt2Δ rim101Δ double mutant, although not lethal, showed a significant degree of lysis (data not shown), unequivocally linking some of the defects of the rim101Δ mutant to the downregulation of RSB1. In light of these results, changes would be expected in the localization of other membrane-associated proteins, some of which could contribute to the defects observed.
Slt2 and Rim101 are required for proper Chs4 localization.
The delocalization of Chs4 in the rim101Δ mutants led to partial delocalization of chitin to the daughter cells, which could explain the partial resistance of this strain to calcofluor despite its normal levels of chitin (10). In the slt2Δ rim101Δ double mutant, the function of Chs4 is compromised even further due to the altered localization of Bni4, the natural link of Chs4 to septins. Under these conditions, the accumulation of Chs4, and concomitantly Chs3, at the neck is severely reduced, compromising the formation of the chitin ring (Fig. 1D). Interestingly, Bni4 distribution in this mutant is significantly wider (see Table S3 in the supplemental material), also promoting the formation of a wider chitin ring (Fig. 1D). The limited anchorage of Chs4 to membranes favors its abnormal accumulation to the vacuole (Fig. 5A) and its eventual degradation in the rim101Δ slt2Δ mutant (Fig. 5B), in agreement with previous data (37). In addition, the deficient function of Chs4 in the double mutant could alter the endocytic turnover of Chs3, which would contribute to chitin delocalization (37). This delocalization would also explain the dramatic defect in the formation of the chitin ring observed in the double mutant compared to the much more modest reduction in total chitin levels. Overexpression of GFA1 or glucosamine addition activates the residual amounts of Chs3 at the neck, restoring the formation of the chitin ring and cell growth. The mechanisms for this activation are unclear, and they have been extensively discussed in previous reports (6, 29). However, these conditions do not correct the septin asymmetry significantly (Table 1 and data not shown), indicating that such defects are intrinsically associated with the absence of Slt2 and Rim101 independent of growth conditions. These defects are physiologically compensated by the increase in chitin synthesis.
Characterization of the synthetic lethality of the slt2Δ rim101Δ double mutant through the selection of multicopy suppressors has thus provided the opportunity to uncover additional roles of Slt2 and Rim101 in septum assembly, which have been previously underestimated owing to the role of these proteins as members of the corresponding signal transduction pathways. In addition, the homeostatic role of the chitin ring in the maintenance of septum assembly is highlighted, explaining the evolutionary conservation of the CSIII complex machinery (38), despite its limited biological relevance in favorable environments.
Supplementary Material
Acknowledgments
We thank N. Skinner for language revision and R. Valle for her technical support with this work. Special thanks are due to C. R. Vazquez for providing strains and to the staff from the TEM facilities of the University of Salamanca for their help.
A.G. and A.R. are supported by predoctoral fellowships from the MEC and JCyL, respectively. J.P. is supported by a postdoctoral fellowship from the BSCH through the University of Salamanca. This research was supported by the Spanish CICYT grants BIO2004-00280 and BIO2007-60779 and the JCyL grants SA127A08 and GR231.
Footnotes
Published ahead of print on 24 July 2009.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Andrews, P. D., and M. J. Stark. 2000. Type 1 protein phosphatase is required for maintenance of cell wall integrity, morphogenesis and cell cycle progression in Saccharomyces cerevisiae. J. Cell Sci. 113:507-520. [DOI] [PubMed] [Google Scholar]
- 2.Baetz, K., J. Moffat, J. Haynes, M. Chang, and B. Andrews. 2001. Transcriptional coregulation by the cell integrity mitogen-activated protein kinase Slt2 and the cell cycle regulator Swi4. Mol. Cell. Biol. 21:6515-6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baladron, V., S. Ufano, E. Duenas, A. B. Martin-Cuadrado, F. del Rey, and C. R. Vazquez de Aldana. 2002. Eng1p, an endo-1,3-beta-glucanase localized at the daughter side of the septum, is involved in cell separation in Saccharomyces cerevisiae. Eukaryot. Cell 1:774-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermejo, C., E. Rodríguez, R. García, J. M. Rodríguez-Peña, M. L. Rodríguez de la Concepción, C. Rivas, P. Arias, C. Nombela, F. Posas, and J. Arroyo. 2008. The sequential activation of the yeast HOG and SLT2 pathways is required for cell survival to cell wall stress. Mol. Biol. Cell 19:1113-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulawa, C. E., M. Slater, E. Cabib, J. Au-Young, A. Sburlati, W. L. J. Adair, and P. W. Robbins. 1986. The S. cerevisiae structural gene for chitin synthase is not required for chitin synthesis in vivo. Cell 46:213-225. [DOI] [PubMed] [Google Scholar]
- 6.Bulik, D. A., M. Olczak, H. A. Lucero, B. C. Osmond, P. W. Robbins, and C. A. Specht. 2003. Chitin synthesis in Saccharomyces cerevisiae in response to supplementation of growth medium with glucosamine and cell wall stress. Eukaryot. Cell 2:886-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabib, E., D. H. Roh, M. Schmidt, L. B. Crotti, and A. Varma. 2001. The yeast cell wall and septum as paradigms of cell growth and morphogenesis. J. Biol. Chem. 276:19679-19682. [DOI] [PubMed] [Google Scholar]
- 8.Cabib, E., A. Sburlati, B. Bowers, and S. J. Silverman. 1989. Chitin synthase 1, an auxiliary enzyme for chitin synthesis in Saccharomyces cerevisiae. J. Cell Biol. 108:1665-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabib, E., S. J. Silverman, and J. A. Shaw. 1992. Chitinase and chitin synthase 1: counterbalancing activities in cell separation of Saccharomyces cerevisiae. J. Gen. Microbiol. 138:97-102. [DOI] [PubMed] [Google Scholar]
- 10.Castrejon, F., A. Gomez, M. Sanz, A. Duran, and C. Roncero. 2006. The RIM101 pathway contributes to yeast cell wall assembly and its function becomes essential in the absence of mitogen-activated protein kinase Slt2p. Eukaryot. Cell 5:507-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reference deleted.
- 12.Chen, R. E., and J. Thorner. 2007. Function and regulation of MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1773:1311-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colman-Lerner, A., T. E. Chin, and R. Brent. 2001. Yeast Cbk1 and Mob2 activate daughter-specific genetic programs to induce asymmetric cell fates. Cell 107:739-750. [DOI] [PubMed] [Google Scholar]
- 14.Cos, T., R. A. Ford, J. A. Trilla, A. Duran, E. Cabib, and C. Roncero. 1998. Molecular analysis of Chs3p participation in chitin synthase III activity. Eur. J. Biochem. 256:419-426. [DOI] [PubMed] [Google Scholar]
- 15.Dekker, C., P. C. Stirling, E. A. McCormack, H. Filmore, A. Paul, R. L. Brost, M. Costanzo, C. Boone, M. R. Leroux, and K. R. Willison. 2008. The interaction network of the chaperonin CCT. EMBO J. 27:1827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeMarini, D. J., A. E. M. Adams, H. Fares, C. De Virgilio, G. Valle, J. S. Chuang, and J. R. Pringle. 1997. A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J. Cell Biol. 139:75-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia, R., C. Bermejo, C. Grau, R. Perez, J. M. Rodriguez-Pena, J. Francois, C. Nombela, and J. Arroyo. 2004. The global transcriptional response to transient cell wall damage in Saccharomyces cerevisiae and its regulation by the cell integrity signaling pathway. J. Biol. Chem. 279:15183-15195. [DOI] [PubMed] [Google Scholar]
- 18.García-Gimeno, M. A., I. Muñoz, J. Ariño, and P. Sanz. 2003. Molecular characterization of Ypi1, a novel Saccharomyces cerevisiae type 1 protein phosphatase inhibitor. J. Biol. Chem. 278:47744-47752. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Rodriguez, L. J., A. Duran, and C. Roncero. 2000. Calcofluor antifungal action depends on chitin and a functional high-osmolarity glycerol response (HOG) pathway: evidence for a physiological role of the Saccharomyces cerevisiae HOG pathway under noninducing conditions. J. Bacteriol. 182:2428-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García-Rodríguez, L. J., R. Valle, A. Durán, and C. Roncero. 2005. Cell integrity signaling activation in response to hyperosmotic shock in yeast. FEBS Lett. 579:6186-6190. [DOI] [PubMed] [Google Scholar]
- 21.Gladfelter, A. S., L. Kozubowski, T. R. Zyla, and D. J. Lew. 2005. Interplay between septin organization, cell cycle and cell shape in yeast. J. Cell Sci. 118:1617-1628. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda, M., A. Kihara, A. Denpoh, and Y. Igarashi. 2008. The rim101 pathway is involved in rsb1 expression induced by altered lipid asymmetry. Mol. Biol. Cell 19:1922-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabir, M. A., J. Kaminska, G. B. Segel, G. Bethlendy, P. Lin, F. Della Seta, C. Blegen, K. M. Swiderek, T. Zoładek, K. T. Arndt, and F. Sherman. 2005. Physiological effects of unassembled chaperonin Cct subunits in the yeast Saccharomyces cerevisiae. Yeast 22:219-239. [DOI] [PubMed] [Google Scholar]
- 24.Kamada, Y., U. S. Jung, J. Piotrowski, and D. E. Levin. 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9:1559-1771. [DOI] [PubMed] [Google Scholar]
- 25.Kozubowski, L., J. R. Larson, and K. Tatchell. 2005. Role of the septin ring in the asymmetric localization of proteins at the mother-bud neck in Saccharomyces cerevisiae. Mol. Biol. Cell 16:3455-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozubowski, L., H. Panek, A. Rosenthal, A. Bloecher, D. J. DeMarini, and K. Tatchell. 2003. A Bni4-Glc7 phospatase complex that recruits chitin synthase to the site of bud emergence. Mol. Biol. Cell 14:26-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuranda, M. J., and P. W. Robbins. 1991. Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J. Biol. Chem. 266:19758-19767. [PubMed] [Google Scholar]
- 28.Lagorce, A., N. C. Hauser, D. Labourdette, C. Rodriguez, H. Martin-Yken, J. Arroyo, J. D. Hoheisel, and J. Francois. 2003. Genome-wide analysis of the response to cell wall mutations in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 278:20345-20357. [DOI] [PubMed] [Google Scholar]
- 29.Lagorce, A., V. Le Berre-Anton, B. Aguilar-Uscanga, H. Martin-Yken, A. Dagkessamanskaia, and J. Francois. 2002. Involvement of GFA1, which encodes glutamine-fructose-6-phosphate amidotransferase, in the activation of the chitin synthesis pathway in response to cell-wall defects in Saccharomyces cerevisiae. Eur. J. Biochem. 269:1697-1707. [DOI] [PubMed] [Google Scholar]
- 30.Lamb, T. M., and A. P. Mitchell. 2003. The transcription factor Rim101p governs ion tolerance and cell differentiation by direct repression of the regulatory genes NRG1 and SMP1 in Saccharomyces cerevisiae. Mol. Cell. Biol. 23:677-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larson, J. R., J. P. Bharucha, S. Ceaser, J. Salamon, C. J. Richardson, S. M. Rivera, and K. Tatchell. 2008. Protein phosphatase type 1 directs chitin synthesis at the bud neck in Saccharomyces cerevisiae. Mol. Biol. Cell 19:3040-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lesage, G., and H. Bussey. 2006. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70:317-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin, D. E. 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69:262-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madden, K., Y. J. Sheu, K. Baetz, B. Andrews, and M. Snyder. 1997. SBF cell cycle regulator as a target of the yeast PKC-MAP kinase pathway. Science 275:1781-1784. [DOI] [PubMed] [Google Scholar]
- 35.Munro, C. A., S. Selvaggini, I. de Bruijn, L. Walker, M. D. Lenardon, B. Gerssen, S. Milne, A. J. Brown, and N. A. Gow. 2007. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol. Microbiol. 63:1399-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peñalva, M. A., J. Tilburn, E. Bignell, and H. N. J. Arst. 2008. Ambient pH gene regulation in fungi: making connections. Trends Microbiol. 16:291-300. [DOI] [PubMed] [Google Scholar]
- 37.Reyes, A., M. Sanz, A. Duran, and C. Roncero. 2007. Chitin synthase III requires Chs4p-dependent translocation of Chs3p into the plasma membrane. J. Cell Sci. 120:1998-2009. [DOI] [PubMed] [Google Scholar]
- 38.Roncero, C. 2002. The genetic complexity of chitin synthesis in fungi. Curr. Genet. 41:367-378. [DOI] [PubMed] [Google Scholar]
- 39.Rose, M. D., F. Wisnton, and P. Hieter. 1990. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 41.Sanz, M., F. Castrejon, A. Duran, and C. Roncero. 2004. Saccharomyces cerevisiae Bni4p directs the formation of the chitin ring and also participates in the correct assembly of the septum structure. Microbiology 150:3229-3241. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt, M., A. Varma, T. Drgon, B. Bowers, and E. Cabib. 2003. Septins, under Cla4p regulation, and the chitin ring are required for neck integrity in budding yeast. Mol. Biol. Cell 14:2128-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serrano, R., H. Martín, A. Casamayor, and J. Ariño. 2006. Signaling alkaline pH stress in the yeast Saccharomyces cerevisiae through the Wsc1 cell surface sensor and the Slt2 MAPK pathway. J. Biol. Chem. 281:39785-39795. [DOI] [PubMed] [Google Scholar]
- 44.Serrano, R., A. Ruiz, D. Bernal, J. R. Chambers, and J. Arino. 2002. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol. Microbiol. 46:1319-1333. [DOI] [PubMed] [Google Scholar]
- 45.Stoldt, V., F. Rademacher, V. Kehren, J. F. Ernst, D. A. Pearce, and F. Sherman. 1996. Review: the Cct eukaryotic chaperonin subunits of Saccharomyces cerevisiae and other yeasts. Yeast 12:523-529. [DOI] [PubMed] [Google Scholar]
- 46.van Drogen, F., and M. Peter. 2002. Spa2p functions as a scaffold-like protein to recruit the Mpk1p MAP kinase module to sites of polarized growth. Curr. Biol. 12:1698-1703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.