Abstract
The cellular response to hydrogen peroxide (H2O2) is characterized by a repression of growth-related processes and an enhanced expression of genes important for cell defense. In budding yeast, this response requires the activation of a set of transcriptional effectors. Some of them, such as the transcriptional activator Yap1, are specific to oxidative stress, and others, such as the transcriptional activators Msn2/4 and the negative regulator Maf1, are activated by a wide spectrum of stress conditions. How these general effectors are activated in response to oxidative stress remains an open question. In this study, we demonstrate that the two cytoplasmic thioredoxins, Trx1 and Trx2, are essential to trigger the nuclear accumulation of Msn2/4 and Maf1, specifically under H2O2 treatment. Contrary to the case with many stress conditions previously described for yeast, the H2O2-induced nuclear accumulation of Msn2 and Maf1 does not correlate with the downregulation of PKA kinase activity. Nevertheless, we show that PP2A phosphatase activity is essential for driving Maf1 dephosphorylation and its subsequent nuclear accumulation in response to H2O2 treatment. Interestingly, under this condition, the lack of PP2A activity has no impact on the subcellular localization of Msn2, demonstrating that the H2O2 signaling pathways share a common route through the thioredoxin system and then diverge to activate Msn2 and Maf1, the final integrators of these pathways.
The Saccharomyces cerevisiae response to stress conditions involves a genome-wide reprogramming of gene expression that leads to the repression of growth-related processes and to the rapid induction of cellular protection mechanisms (5, 17). Stress-protective genes have been classified into those that are specifically induced in response to a given stress (e.g., heat, osmotic or acidic shocks, oxidative stress, DNA damage, or nutrient starvation) and those that are induced under all stress conditions. The latter ones are part of the so-called environmental stress response (ESR) cluster (5, 17). The mechanisms of these transcriptional modifications are complex and remain poorly understood, although several effectors have been identified. In particular, the transcription factors Msn2 and Msn4 (Msn2/4) are key players of the response to stress since they regulate many genes of the ESR cluster (5, 17). The conserved Maf1 protein also has an important role under adverse growth conditions (8, 34, 35, 47) since it mediates the transcriptional repression of RNA polymerase III (essentially dedicated to the transcription of the 5S rRNA and tRNA genes).
Despite the fact that the Msn2/4 transcriptional activators and the Maf1 negative regulator do not share any significant sequence homology, their behaviors display many similarities during the stress response, leading to the proposal that observations on Msn2/4 could provide a paradigm for Maf1 (30). Indeed, both are localized in the cytoplasm under optimal growth conditions and redistribute into the nucleus in response to stress conditions (1, 19, 30, 33, 37), and their nuclear export depends upon the Msn5 nuclear export factor (10, 45). Both Maf1 and Msn2 are direct substrates of the cAMP-dependent protein kinase A (PKA) (4), and it has been shown that their regulated nucleocytoplasmic trafficking and transcriptional activities are governed by PKA-mediated phosphorylation (3, 19, 30, 42). In particular, under optimal growth conditions, high PKA activity correlates with the cytoplasmic retention and negative regulation of both Msn2/4 and Maf1. The implication of other protein kinase activities in the general response to stress remains poorly documented except for the Hog1 MAP kinase pathway, which has been shown to be required for Msn2/4 activation in response to osmotic shock (19, 36, 40).
In yeast, the hydrogen peroxide (H2O2) stress response involves the activation of the general ESR cluster genes (5, 17) and the specific H2O2 stimulon, containing most of the antioxidant enzymes, such as thioredoxins, thioredoxin reductase, thioredoxin peroxidase (peroxiredoxin), superoxide dismutase, and cytochrome c peroxidase (18, 21). Thioredoxins are highly conserved redox enzymes that reduce disulfide bonds by a thiol-disulfide exchange mechanism with protons donated by NADPH through the flavoenzyme thioredoxin reductase. S. cerevisiae carries two cytoplasmic thioredoxins, Trx1 and Trx2, which are important for DNA synthesis, sulfate assimilation, and H2O2 tolerance due to their role of reducing ribonucleotide reductase, 3′-phosphoadenosine 5′-phosphosulfate reductase, and thiol-peroxidases, respectively (for a review, see reference 44). Trx1 and Trx2 have redundant activities, as shown by the phenotypes of null mutations of their respective genes. Indeed, strains lacking either TRX1 or TRX2 do not display any remarkable phenotype, whereas a strain lacking both of these genes displays a cell cycle S phase elongation (31), methionine auxotrophy (31), and marked hypersensitivity to H2O2 (16).
How the general effectors Msn2/4 and Maf1 are activated in response to oxidative stress remains an open question. Here we examined the protein neosynthesis in cells lacking both cytoplasmic thioredoxins, Trx1 and Trx2, under H2O2 treatment. We showed that the induction of typical proteins, which are encoded by genes belonging to the ESR cluster and regulated by the Msn2/4 transcription factors, is specifically impaired under H2O2 treatment. Microscopy observations reveal that the cytoplasmic thioredoxins are essential to trigger the nuclear accumulation of Msn2/4 and Maf1 under H2O2 treatment. Interestingly, the H2O2-induced nuclear accumulation of both transcriptional effectors does not require the downregulation of PKA kinase activity. However, the H2O2 signaling pathways are different for each transcriptional effector, since protein phosphatase 2A (PP2A) activity is required only for Maf1 nuclear accumulation.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
Strains used in this study are listed in Table 1. Strains expressing either the Msn2-green fluorescent protein (GFP) or Msn4-GFP fusion protein were transformed, respectively, either with the pAMG plasmid (CEN, LEU2) (19), which encodes the fusion protein Msn2-GFP (GFP is fused at the C-terminal part of Msn2) under the control of the ADH1 promoter, or with the pGR247 plasmid (23), which encodes the fusion protein Msn4-GFP. pGR247 derives from pAMG, from which the MSN2 open reading frame has been replaced by the MSN4 open reading frame.
TABLE 1.
Saccharomyces cerevisiae strains
| Strain or description | Genotype | Reference |
|---|---|---|
| YPH98 | MATaura3-52 ade2-101 lys2-801 trp1Δ1 leu2Δ1 | 41 |
| trx1Δ trx2Δ | MATα ura3-52 ade2-101 lys2-801 trp1Δ1 leu2Δ1 his3Δ200 trx1::TRP1 trx2::kanMX4 | 27 |
| trr1Δ | MATα ura3-52 ade2-101 lys2-801 trp1Δ1 leu2Δ1 his3Δ200 trr1::kanMX4 | 27 |
| trr1Δ trx1Δ trx2Δ | MATα ura3-52 ade2-101 lys2-801 trp1Δ1 leu2Δ1 his3Δ200 trx1::URA3 trx2::kanMX4 trr1::kanMX4 | 27 |
| tsa1Δ | MATaura3-52 ade2-101 lys2-801 trp1Δ1 leu2Δ1 tsa1::TRP1 | 26 |
| pde2Δ | MATα ura3-52 ade2-101 lys2-801 trp1Δ1 leu2Δ1 his3Δ200 pde2::kanMX4 | 3 |
| MW671-Cmyc | MATα ura3-52 ade2-101 lys2-801 trp1Δ63 leu2-Δ1 his3Δ200 rpc160::HIS3 MAF1-13myc::kanMX6 pC160-240 [TRP1 3HA-RPC160] | 34 |
| AY925 | MATaura3-1 ade2-1 trp1-1 leu2-3,112 his3-11 can1-100 ssdl-d2 | 12 |
| DEY217 (pp2aΔ) | MATaura3-1 ade2-1 trp1-1 leu2-3,112 his3-11 can1-100 ssdl-d2 pph22-172::URA3 pph21Δ1::HIS3 pph3Δ1::LYS2 lys2-953 | 12 |
For two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) and mRNA Northern blot analyses, cells were grown in SD (synthetic defined) minimal medium (6.7 g·liter−1 yeast nitrogen base, 20 g·liter−1 glucose) supplemented with amino acids plus adenine, uracil, and glutathione (0.5 mM). For microscopy experiments, cells were transformed with the nonessential pAMG (LEU2) or pGR247 (LEU2) plasmid and grown in complete supplement mixture (CSM) lacking leucine (6.7 g·liter−1 yeast nitrogen base, 0.85 g·liter−1 CSM-LEU [Bio 101], 20 g·liter−1 glucose) to select for the presence of the plasmids. For pre-tRNA Northern blot and Maf1 Western blot analyses, cells were grown in CASA medium (6.7 g·liter−1 yeast nitrogen base, 1 g·liter−1 Casamino Acid, 20 g·liter−1 glucose). All strains were grown at 30°C except for AY925 and DEY217, which were grown at 24°C.
2D polyacrylamide gel analysis.
Cells were grown to mid-log phase (optical density at 600 nm [OD600] = 0.2 to 0.3) prior to being treated or not with H2O2 (0.6 mM) for 15 min. Cells were subsequently labeled with 100 to 200 μCi [35S]methionine for 15 min. Cell harvesting, protein extraction, and 2D-PAGE were performed as described previously (28).
Northern blot analyses.
Cells were grown to mid-log phase (OD600 = 0.2 to 0.3) prior to being treated with H2O2 (0.6 mM for mRNA analyses and 0.8 mM for pre-tRNA analyses) for different periods of time. Total RNAs were phenol extracted as described previously (25). For mRNA analyses, 20 μg of total RNAs were separated on a 1.5% agarose gel, and DNA probes were generated by PCR on genomic DNA with the following oligonucleotides: FW-ACT1, 5′-CAAGACACCAAGGTATCATGG; RV-ACT1, 5′-TCTTTCAGCAGTGGTGGAGA; FW-CTT1, 5′-GTACTCTCATCACCCATACG; RV-CTT1, 5′-GGAGTACCTCTATCACCAAAC; FW-HSP12, 5′-GTCTGACGCAGGTAGAAAAGG; RV-HSP12, 5′-CTTCTTGGTTGGGTCTTCTTC; FW-PGM2, 5′-CTAAAGGTGCCACTCTTGTTG; and RV-PGM2, 5′-ATAGCCTTACCGTATGGTCC.
PCR products were labeled with [α-32P]dCTP by random priming (Amersham Megaprime DNA labeling systems).
Pre-tRNAs were analyzed as described previously (32) from 20 μg of total RNAs and using the following oligonucleotides: U4, 5′-CCATGAGGAGACGGTCTGG; pre-tRNA Leu, 5′-CCAAACAACCACTTATTTGTTGA; and pre-tRNA Ileu, 5′-GGCCTGTTTGAAAGGTCTTTGGCAC.
Oligonucleotides were labeled with [γ-32P]ATP and T4 polynucleotide kinase.
Microscopy experiments.
Cells were grown to mid-log phase (OD600 = 0.2 to 0.3) before being treated or not with H2O2 (0.1 to 0.8 mM), NaCl (0.5 M), and rapamycin (0.5 μg·ml−1) for 10 min. For Msn2-GFP observations with a pde2Δ strain, cells were pretreated or not with 3 mM of cAMP for 30 min at 30°C prior to being treated as described above.
For Msn2-GFP and Msn4-GFP fluorescence microscopy, cells were harvested and fixed by adding 1 volume of 7.4% formaldehyde in phosphate-buffered saline (PBS) (1×). After a 2-min incubation at room temperature (RT), cells were washed with cold PBS (1×), kept on ice for 5 min, and washed once more with cold PBS (1×). Nuclei were stained by addition of 2 μg·ml−1 of 4′,6′-diamidino-2-phenylindole (DAPI) before microscopy observations using a Leica Dmrxa upright fluorescence microscope (100×, NA1.3 PL-fluotar objective) and a charge-coupled-device camera (Micromax 1300Y/HS; Roper Scientific). Quantitative data were obtained by analysis of at least 400 individual cells during three independent experiments.
For Maf1 immunofluorescence microscopy, cells were harvested and formaldehyde (3.7% final) was added for 15 min at RT. Cells were collected by centrifugation and washed twice with KP-sorbitol buffer (1.2 M sorbitol, 50 mM KPO4 pH 6.5, 0.5 mM MgCl2). Spheroplasts were obtained by incubating the cells for 30 min at 37°C in KP-sorbitol buffer containing 0.2 mg·ml−1 of Zymolyase 100T and 28 mM of 2-mercaptoethanol. Spheroplasts were collected by gentle centrifugation and washed once with PBS (1×)-1% fish gelatin, twice with PBS (1×)-1% fish gelatin-0.1% Triton X-100, and once with PBS (1×)-1% fish gelatin. Spheroplasts were incubated for 1 h at RT with anti-Maf1 antibodies (dilution 1/100) (33) in PBS (1×)-1% fish gelatin. After washes (as described above), spheroplasts were incubated for 1 h at RT with anti-rabbit immunoglobulin G Alexa 594 (dilution 1/100) in PBS (1×)-1% fish gelatin. After washes (as above), nuclei were stained by addition of 0.5 μg·ml−1 of DAPI before observations using a Leica Dmrxa upright fluorescence microscope (100×; NA1.3 PL-fluotar objective) and a charge-coupled-device camera (Micromax 1300Y/HS; Roper Scientific).
Protein analyses.
Analyses of Maf1 phosphorylations were performed by sodium dodecyl sulfate (SDS)-PAGE using a specific ratio of acrylamide/bisacrylamide (33.5/0.3) and by Western blotting using polyclonal anti-Maf1 antibodies as described previously (33).
For Maf1-Myc immunoprecipitation, MW671-Cmyc cells were grown in CASA medium to mid-log phase (OD600 = 0.2 to 0.3) prior to being treated or not with H2O2 (0.8 mM), rapamycin (0.5 μg·ml−1), or chlorpromazine (250 μM). Cells were harvested by centrifugation after a 30-min incubation (see Fig. 4B) or at the indicated time for the H2O2 time course experiment (see Fig. 4C) and stored at −80°C. Cells were resuspended in 500 μl of immunoprecipitation buffer (50 mM HEPES [pH 7.5], 1 mM EDTA, 1 mM dithiothreitol, 10% glycerol, 100 mM NaCl, 0.05% NP-40, protease inhibitors [Complete; Roche], and Phosphatase inhibitors [Cocktail 2; Sigma]) and disrupted by vigorous vortexing (30 min, 4°C) in the presence of 200 μl of glass beads (425- to 600μm; Sigma). After centrifugation (14,000 rpm, 15 min, 4°C), the supernatants were incubated for 2 h at 4°C with monoclonal anti-Myc antibodies (9E10; Santa Cruz) coupled to Dynal PanMouse immunoglobulin G magnetic beads (Dynal Biotech). The beads were extensively washed with immunoprecipitation buffer, resuspended in 60 μl of Laemmli buffer (1×), and incubated at 90°C for 10 min. Fifteen μl of the samples were analyzed by SDS-PAGE using a standard ratio of acrylamide/bisacrylamide (20/0.53), and the Maf1 protein was revealed by Western blot analyses using monoclonal anti-phospho-PKA substrate antibodies (Cell Signaling). The amount of immunoprecipitated Maf1 was monitored with anti-Maf1 antibodies (33). The same membrane was used for both measurements, since the signal arising from anti-Maf1 antibodies is far stronger than the signal arising from anti-phospho-PKA substrate antibodies. As a control, the amount of immunoprecipitated Maf1 was also monitored with anti-Maf1 antibodies incubated with independent membranes.
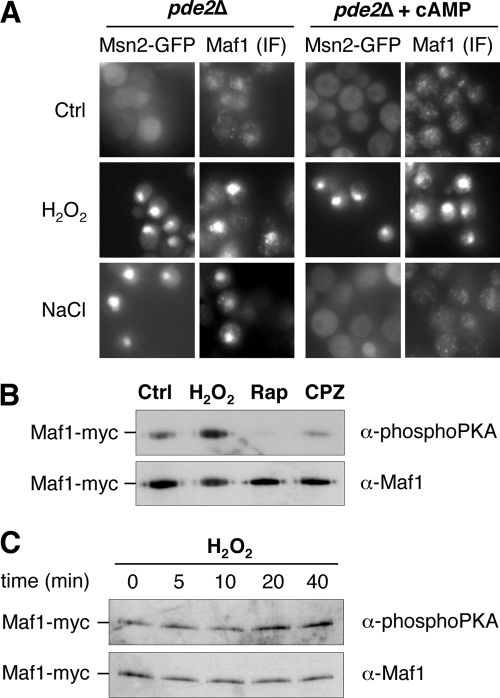
FIG. 4.
The level of PKA phosphorylation does not decrease under H2O2 conditions. (A) pde2Δ mutant expressing an Msn2-GFP fusion protein was grown to exponential phase, and cAMP (3 mM) was added or not to the culture for 30 min. Cells were either harvested without supplemental treatment (Ctrl) or incubated for 10 min with H2O2 (0.8 mM) or NaCl (0.5 M), as indicated. Msn2-GFP subcellular localization was assessed by fluorescence microscopy, and Maf1 subcellular localization was assessed by immunofluorescence (IF) microscopy using a polyclonal anti-Maf1 antibody. (B) Cells carrying Myc-tagged Maf1 were grown to exponential phase in the absence of stress before being incubated, or not (Ctrl), for 30 min with H2O2 (0.8 mM), rapamycin (0.5 μg·ml−1) (Rap), or chlorpromazine (0.25 mM) (CPZ). Maf1-Myc was immunoprecipitated, and the level of phosphorylation of the PKA consensus sites was revealed by Western blotting using a monoclonal anti-phosphoPKA antibody (α-phosphoPKA). As a control, the amount of immunoprecipitated Maf1-Myc was monitored on the same membrane using polyclonal anti-Maf1 antibodies (α-Maf1). (C) Cells carrying Myc-tagged Maf1 were grown to exponential phase in the absence of stress before being submitted to H2O2 treatment (0.8 mM) and harvested after different periods of time, as indicated. The phosphorylation level of the PKA consensus sites was monitored as for panel B.
RESULTS
Cytoplasmic thioredoxins are required for Msn2/4 response to H2O2.
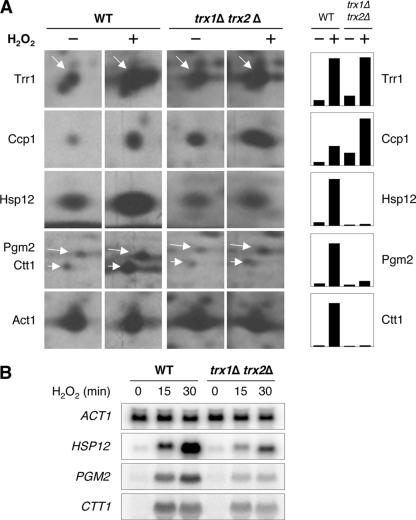
Using [35S]methionine pulse-labeling and quantitative two-dimensional gel electrophoresis, we monitored the protein neosynthesis in a wild-type (WT) strain and in a strain lacking both cytoplasmic thioredoxins (trx1Δ trx2Δ) during the response to H2O2 treatment. WT cells treated with H2O2 displayed an increase in the synthesis rate of proteins required for the oxidative and general stress responses, as seen in previous studies (18, 21, 26). In particular, typical targets of the Yap1 transcription factor, such as thioredoxin reductase (Trr1) and cytochrome c peroxidase (Ccp1), were highly induced (8- and 6-fold, respectively) (Fig. 1A). Targets of the Msn2/4 transcription factor, such as cytosolic catalase (Ctt1), Pgm2, and Hsp12, were also highly induced (34-, 14-, and 13-fold, respectively) (Fig. 1A). Proteome analysis of trx1Δ trx2Δ cells revealed two main differences. First, Yap1-regulated proteins had a higher basal synthesis rate but were still further induced by H2O2 (fivefold for Trr1 and fourfold for Ccp1), as described previously and in keeping with the Yap1 constitutive activation resulting from inactivation of the thioredoxin pathway (7, 22). Second, and more importantly, trx1Δ trx2Δ cells failed to increase the synthesis rate of Msn2/4-regulated proteins (no induction for Ctt1 and three- and twofold for Pgm2 and Hsp12, respectively), suggesting that thioredoxins are required for proper activation of Msn2/4 by H2O2. Northern blot experiments confirmed these observations at the transcriptional level. Whereas WT cells displayed a dramatic increase in the mRNA level of HSP12 and to a lesser extent of PGM2 and CTT1 during the response to H2O2 treatment, trx1Δ trx2Δ cells were severely impaired for the transcriptional induction of these genes (Fig. 1B).
FIG. 1.
Induction of Msn2/4-dependent proteins requires cytoplasmic thioredoxins under H2O2 treatment. (A) Exponentially growing WT (YPH98) and trx1Δ trx2Δ mutant cells were treated (+) or not (−) with 0.6 mM H2O2 for 15 min before being labeled with [35S]methionine and analyzed by quantitative two-dimensional gel electrophoresis. The local areas of gel autoradiograms are displayed, and a summary of the quantification is presented as histograms (count of radioactivity measured for each spot [arbitrary units] normalized with Act1 signals). Arrows indicate Trr1, Pgm2, and Ctt1 protein spots. (B) Total RNAs were extracted from WT (YPH98) and trx1Δ trx2Δ mutant cells treated with H2O2 (0.6 mM) for different periods of time, as indicated. HSP12, PGM2, and CTT1 transcript levels were monitored in a Northern blot experiment (ACT1 mRNA was used as the standard).
H2O2-induced Msn2/4 nuclear localization is impaired in thioredoxin mutants.
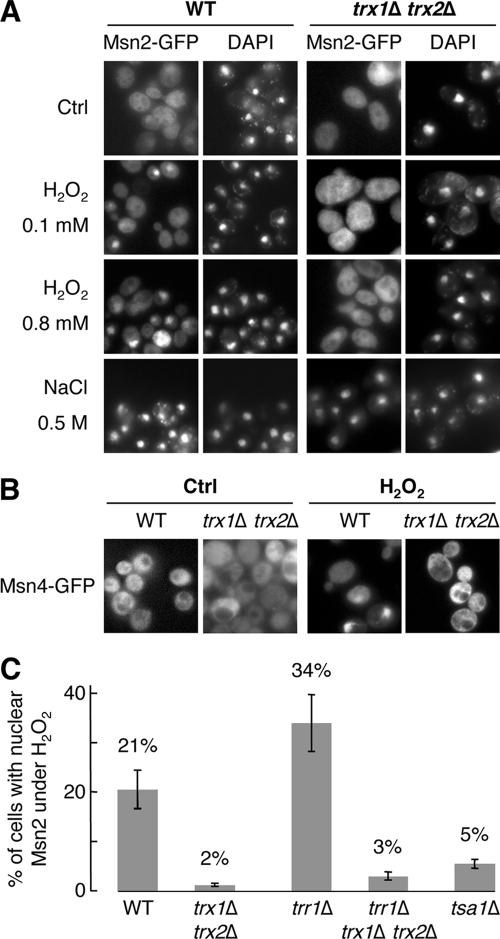
Although the nuclear accumulation of Msn2/4 is not sufficient to alone explain the regulation of its activity (10), this process represents a crucial control level of Msn2/4 activation. We thus examined the subcellular localization of Msn2 using a GFP fusion protein. In both the WT and trx1Δ trx2Δ strains, GFP staining had a diffuse cellular pattern in the absence of stress (Fig. 2A). Exposure of WT cells to H2O2 led to a dose-dependent nuclear GFP staining. At a low concentration of H2O2 (0.1 mM), GFP nuclear staining was observed in 21% of the cells (Fig. 2A). This proportion increased gradually with higher H2O2 concentrations and reached a plateau at 0.8 mM H2O2, where about 50% of the cells displayed GFP nuclear staining (Fig. 2A). In contrast, exposure of trx1Δ trx2Δ cells to H2O2 did not modify GFP staining, which remained diffuse, even at elevated H2O2 concentrations (Fig. 2A). We also tested the Msn2-GFP response to other stressors known to trigger the nuclear accumulation of Msn2 and the activation of the ESR gene cluster. In both WT and trx1Δ trx2Δ strains, Msn2 accumulated in the nucleus under different stress conditions, such as NaCl (0.5 M) (Fig. 2A), ethanol (7%), and tBOOH (0.5 mM) (data not shown), suggesting that the defective Msn2 response in the trx1Δ trx2Δ strain is specific to H2O2.
FIG. 2.
In response to H2O2, Msn2-GFP and Msn4-GFP nuclear accumulations require cytoplasmic thioredoxins. (A) WT (YPH98) and trx1Δ trx2Δ mutant cells expressing an Msn2-GFP fusion protein were grown to exponential phase before being incubated, or not (Ctrl), under stress conditions for 10 min (0.1 mM or 0.8 mM H2O2 or 0.5 M NaCl, as indicated). Msn2-GFP subcellular localization (Msn2-GFP) and the position of the cell nucleus (DAPI) were assessed by fluorescence microscopy. (B) WT (YPH98) and trx1Δ trx2Δ mutant cells expressing an Msn4-GFP fusion protein were grown to exponential phase before being incubated, or not (Ctrl), under H2O2 conditions for 10 min (0.1 mM). Msn4-GFP subcellular localization was assessed by fluorescence microscopy. (C) WT (YPH98), trx1Δ trx2Δ, trr1Δ, trx1Δ trx2Δ trr1Δ, and tsa1Δ cells expressing an Msn2-GFP fusion protein were grown to exponential phase before being incubated with 0.1 mM H2O2 for 10 min. Proportions of cells displaying a nuclear Msn2-GFP accumulation were monitored by fluorescence microscopy. The given percentages result from an observation of around 400 individual cells per mutant and from three independent experiments. Error bars represent standard deviations.
We examined the localization of Msn4, which is structurally related to Msn2 (32% identity and 46% similarity) but differentially regulated in response to various stress conditions (15). As seen with Msn2, upon exposure to a low dose of H2O2 (0.1 mM), Msn4 went from a diffuse cellular localization in unstressed cells to a nuclear localization in 22% of the WT cells but not in trx1Δ trx2Δ cells, in which it remained in the cytoplasm (Fig. 2B).
In summary, the nuclear accumulation of Msn2 and Msn4 triggered by H2O2 but not by other environmental stressors is defective in cells lacking cytoplasmic thioredoxins.
Inactivation of thioredoxin reductase improves Msn2 response to H2O2.
To further understand the thioredoxin requirement in Msn2/4 activation, we explored the other components of this pathway. Thioredoxins are themselves reduced by thioredoxin reductase with protons donated by NADPH. Moreover, thioredoxins use as one of their main substrates the peroxiredoxins, which are enzymes that catalytically reduce intracellular peroxides (13). We thus examined the Msn2 response to H2O2 in strains lacking either cytoplasmic thioredoxin reductase (trr1Δ) or Tsa1 (tsa1Δ), one of the most abundant of the five peroxiredoxins and one of the three peroxiredoxins having a cytoplasmic subcellular localization. Interestingly, in the trr1Δ strain, Msn2 still accumulated in the nucleus in response to low doses of H2O2. Furthermore, this response was even more potent than that of the WT strain, with a higher proportion (34%) of H2O2-treated cells carrying GFP nuclear staining (Fig. 2C). This potent Msn2 response of the trr1Δ strain was almost totally abolished (3% of cells with GFP nuclear staining) upon further removal of TRX1 and TRX2 (trr1Δ trx1Δ trx2Δ) (Fig. 2C). Finally, lack of Tsa1 also abolished the Msn2 response to H2O2 (5% of GFP-stained cells) (Fig. 2C).
These results reveal a dedicated role of the thioredoxin pathway in relaying the H2O2 signal to Msn2. Furthermore, the opposite effects of the deletion of each of the components of this pathway in the subcellular redistribution of Msn2 by H2O2 suggest that the primary effector in the activation of Msn2 nuclear accumulation is the oxidized form of the thioredoxins (see Discussion). Therefore, one can picture a simple mechanism in which oxidized thioredoxins trigger Msn2 nuclear localization through a direct redox interaction. This hypothesis requires the presence of at least one redox cysteine residue within the sequence of the transcription factor. Interestingly, the sequence of a truncated Msn2 mutant (amino acids 257 to 642) which still resumes the behavior of the full-length protein in response of a wide variety of stresses (14), including H2O2 (our observations), contains a unique cysteine. We mutated this cysteine residue into an alanine (C395A) and assessed the subcellular localization of the Msn2-GFP mutant. Like the full-length Msn2 protein, this mutated form of truncated Msn2 displayed a thioredoxin-dependent nuclear accumulation in response to H2O2 (data not shown), making the possibility of a direct modification of Msn2 by thioredoxins in their oxidized form unlikely.
Maf1 response to H2O2 requires thioredoxins.
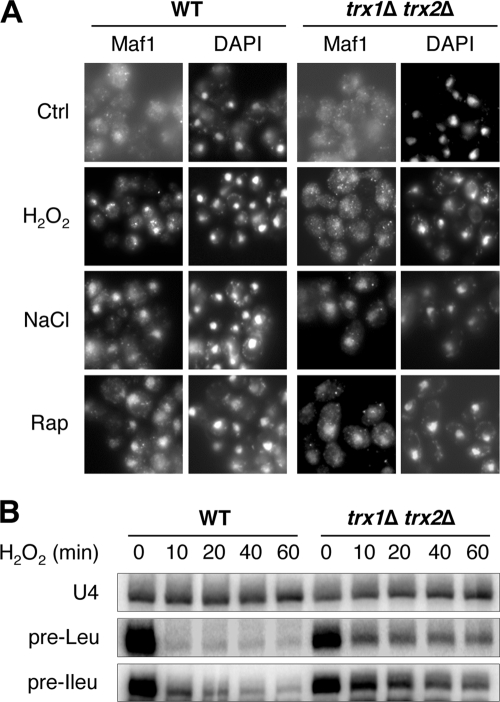
The transcriptional repressor Maf1 is required to achieve the downregulation of RNA polymerase III activity under H2O2 treatment (8). Because Maf1 appears to share common regulatory and physiological features with Msn2/4 (see the introduction), we asked whether the lack of the two cytoplasmic thioredoxins could also impair Maf1 nuclear accumulation under H2O2 treatment. Using Maf1-specific immunofluorescence staining (Fig. 3A), we found that Maf1 displays a diffuse cellular localization in exponentially growing WT cells, with a few cells exhibiting a very weak nuclear accumulation, as previously reported (33, 45). Maf1 redistributed from a diffuse cellular localization to a nuclear accumulation upon treatment with rapamycin (0.5 μg·ml−1), as previously reported (33), or NaCl (0.5 M). Similarly, Maf1 redistributed in the nucleus upon treatment with H2O2 (0.8 mM) in about 40% of WT cells (Fig. 3A). However, Maf1 nuclear accumulation was totally impaired in trx1Δ trx2Δ cells upon exposure to H2O2 but not to other stress conditions. Thus, as for Msn2/4, cytoplasmic thioredoxins are required for Maf1-specific nuclear accumulation under H2O2 treatment.
FIG. 3.
Absence of cytoplasmic thioredoxins impairs Maf1 activation in response to H2O2. (A) WT (YPH98) and trx1Δ trx2Δ mutant cells were grown to exponential phase before being incubated, or not (Ctrl), with H2O2 (0.8 mM), NaCl (0.5 M), or rapamycin (0.5 μg·ml−1) (Rap) for 10 min. Maf1 subcellular localization was assessed by immunofluorescence microscopy using a polyclonal anti-Maf1 antibody. (B) Total RNAs were extracted from WT (YPH98) or trx1Δ trx2Δ mutant cells treated with H2O2 (0.8 mM) for different periods of time, as indicated. The levels of precursor tRNA Leu and tRNA Ileu were monitored by a Northern blot experiment (U4 snRNA was used as the standard).
Yeast cells devoid of functional Maf1 are characterized by a defect in the downregulation of RNA polymerase III transcription under adverse growth conditions (for a review, see reference 48). Therefore, we compared the regulation of RNA polymerase III transcriptional activity in WT and trx1Δ trx2Δ cells as illustrated by the level of the neosynthesized precursor tRNAs (45, 47). Northern blot experiments revealed that under H2O2 treatment, the decrease in the precursor tRNA Leu and tRNA Ileu levels is significantly affected in trx1Δ trx2Δ cells compared to WT cells (Fig. 3B). This result is consistent with the abnormal localization of Maf1 in trx1Δ trx2Δ cells upon exposure to H2O2 and confirms that cytoplasmic thioredoxins are required for a proper Maf1 activation under H2O2 treatment.
The PKA pathway does not mediate Msn2/4 and Maf1 responses to H2O2.
Several reports have established a link between the decrease in PKA activity under stress conditions and the nuclear accumulation of Msn2/4 (3, 14, 19, 23, 42) and Maf1 (30, 48). We thus sought to determine whether H2O2 activation of these regulators involved the PKA pathway. To this purpose, we used a strain lacking the high-affinity cAMP phosphodiesterase gene PDE2. Exposure of this mutant strain to exogenous cAMP allows maintenance of a high PKA activity. As in the WT strain, in the pde2Δ strain in the absence of added cAMP, Msn2-GFP, and Maf1 redistributed into the nucleus in response to both H2O2 (0.8 mM) and NaCl (0.5 M) (Fig. 4A). Of note for the pde2Δ strain, the Msn2 response to H2O2 was even more potent than the one seen in the WT, since more than 95% of the cells displayed a GFP nuclear staining after H2O2 treatment. In the presence of cAMP (3 mM), the NaCl-induced Msn2-GFP nuclear redistribution was totally impaired, as previously shown (20), and a similar result was obtained with Maf1 (Fig. 4A). Surprisingly, in the presence of cAMP, H2O2-induced nuclear redistribution of both Msn2-GFP and Maf1 was maintained. This last result strongly suggests that an elevated PKA activity does not predominantly impact the subcellular localization of both Msn2 and Maf1 under H2O2 treatment.
We also examined the phosphorylation status of Maf1 by Western blotting using an anti-PKA phospho-substrate antibody that specifically recognizes the phosphorylated PKA consensus sites RRXpS/pT of Maf1 sequence (30). Here we used WT cells carrying a Myc-tagged version of Maf1 and monitored Maf1 phosphorylation after immunoprecipitation of the protein with an anti-Myc antibody. In untreated cells, Maf1 appeared PKA phosphorylated, whereas in cells treated with rapamycin (0.5 μg·ml−1) or with chlorpromazine (0.25 mM), phosphorylation significantly decreased (Fig. 4B), as previously reported (30). However, H2O2 treatment (0.8 mM) did not alter Maf1 phosphorylation, since the signal even increased after 20 min of H2O2 treatment (Fig. 4B and C). Under these conditions, the level of immunoprecipitated Maf1 remained constant, as shown by Western blot analysis using a specific anti-Maf1 antibody. This result indicates that the PKA phosphorylation status of Maf1 does not have a predominant impact on the control of Maf1 nuclear accumulation in the response to H2O2.
Altogether, we conclude that in response to H2O2, the regulation of cAMP-PKA system activity is not a key player in the control of Msn2 and Maf1 nuclear accumulation. This reinforces the idea that the response to H2O2 has regulatory features that are unique from those for other stress conditions.
Thioredoxins are essential for Maf1 dephosphorylation under H2O2 conditions.
The regulation of Maf1 and Msn2/4 activation is a complex process resulting from an antagonism between kinase and phosphatase activities. In addition to that of PKA, other, undefined kinase activities are likely to be involved in the process of Maf1 nuclear accumulation, depending of the nature of the stress (6, 45). It was thus important to explore the impact of H2O2 on the Maf1 and Msn2 phosphorylation pattern. Since we did not observe any difference in Msn2 electrophoretic mobility using different experimental procedures, we took advantage of the fact that Maf1 has been described to resolve in three main bands under particular SDS-PAGE conditions (Fig. 5A, time zero), with the two upper bands corresponding to hyperphosphorylated forms of the protein and the lower one to the hypophosphorylated form (30, 33, 37). Using these experimental procedures, we observed that addition of H2O2 (0.8 mM) and NaCl (0.5 M) led to a rapid accumulation of the Maf1 hypophosphorylated form in WT cells (Fig. 5A). These results are consistent with previous observations reported for other stress conditions leading to Maf1 nuclear accumulation (6, 30, 33, 37). We believe that the difference between the Maf1 phosphorylation patterns under H2O2 or NaCl treatment reflects the variation of the cell proportion exhibiting a clear Maf1 nuclear accumulation (i.e., 40% under H2O2 treatment and more than 80% under NaCl treatment).
FIG. 5.
PP2A-dependent dephosphorylation of Maf1 is specifically impaired in trx1Δ trx2Δ cells under H2O2 treatment. (A) WT (YPH98) cells were grown to exponential phase and were submitted to H2O2 (0.8 mM) treatment for different periods of time or to NaCl (0.5 M) treatment for 10 min. The Maf1 phosphorylation state was analyzed by Western blotting using a polyclonal anti-Maf1 antibody. The positions of the two upper bands, corresponding to hyperphosphorylated Maf1, and the lower band, corresponding to hypophosphorylated Maf1, are indicated. (B) pph21Δ pph3Δ pph22-172 (DEY217, pp2aΔ) and isogenic WT (AY925, WT) cells expressing an Msn2-GFP fusion protein were grown to exponential phase at 24°C before being harvested in the absence of stress (Ctrl) or after a 10-min H2O2 (0.8 mM) treatment. Msn2-GFP subcellular localization (Msn2-GFP, upper panel) was assessed by fluorescence microscopy, whereas Maf1 subcellular localization (lower panel) was assessed by immunofluorescence microscopy (IF) using a polyclonal anti-Maf1 antibody. The cell nuclei were localized by DAPI staining. (C) pph21Δ pph3Δ pph22-172 (DEY217, pp2aΔ) cells were grown to exponential phase at 24°C and submitted, or not (Ctrl), to H2O2 (0.8 mM) or NaCl (0.5 M) treatment for 10 min before being harvested. Maf1 phosphorylation states were monitored as for panel A. (D) The Maf1 phosphorylation state was monitored in trx1Δ trx2Δ mutant cells as described for panel A.
It has been proposed that PP2A activity is essential for Maf1 dephosphorylation and nuclear accumulation in response to rapamycin treatment (33). We used a triple mutant strain (pph21Δ pph3Δ pph22-172), previously shown to be defective for PP2A catalytic activity (11), to test the role of this phosphatase in Msn2 and Maf1 nuclear accumulation during the response to H2O2 (0.8 mM). As shown in Fig. 5B, Maf1 nuclear accumulation was totally abolished under H2O2 treatment in the triple mutant strain. This observation correlated with Maf1 remaining fully phosphorylated in the pph21Δ pph3Δ pph22-172 strain during both H2O2 and NaCl treatment (Fig. 5C). Interestingly, the presence of the triple mutation pph21Δ pph3Δ pph22-172 did not affect Msn2-GFP nuclear accumulation during the H2O2 treatment (Fig. 5B). This shows that in response to H2O2, nuclear localization of Msn2 and that of Maf1 are mediated by different phosphatases.
In trx1Δ trx2Δ cells, the Maf1 phosphorylation pattern did not change significantly with the presence of H2O2 (Fig. 5D), demonstrating that the absence of the two cytoplasmic thioredoxins impairs the control of the Maf1 phosphorylation state. Under saline stress (NaCl, 0.5 M), trx1Δ trx2Δ cells still accumulated the hypophosphorylated form of Maf1 (Fig. 5D), which indicates that at the molecular level, the defect of Maf1 dephosphorylation in trx1Δ trx2Δ cells is also restricted to H2O2 stress conditions.
Altogether, these results demonstrate that the presence of H2O2 impacts Maf1 phosphorylation in a PP2A-dependent manner, which is not the case for Msn2. Thus, if H2O2 sensing requires the presence of the cytoplasmic thioredoxins for both Maf1 and Msn2, the pathways leading to their nuclear accumulation appear to be different.
DISCUSSION
In this study, we showed that the lack of the two cytoplasmic thioredoxins impairs the nuclear accumulation of two transcriptional regulators, Msn2/4 and Maf1, specifically under H2O2 treatment. The data indicate that during the response to H2O2, the modulation of PKA activity does not have a prevailing role in controlling the nuclear redistribution of both transcriptional effectors. We also show that the presence of the cytoplasmic thioredoxins is required for Maf1 PP2A-dependent dephosphorylation, leading to its nuclear accumulation, whereas a different mechanism seems to apply to Msn2, which still responds to H2O2 in the absence of PP2A activity.
Under oxidative stress conditions, cell viability requires the transcriptional activation of many genes that have protective roles. This includes both specific oxidative stress genes and a common set of genes involved in the general response to stress. The latter group is part of the ESR cluster and is mainly regulated by the Msn2/4 transcription factors. Our observations provide several lines of evidence highlighting the role of the yeast cytoplasmic thioredoxins in the activation of the general response to H2O2. We found that in a yeast strain lacking the two genes encoding the cytoplasmic thioredoxins (trx1Δ and trx2Δ), the transcriptional induction of Msn2/4 target genes and the increased neosynthesis of the corresponding proteins were nearly abolished (Fig. 1). The specific requirement of cytoplasmic thioredoxins for signaling the presence of H2O2 was confirmed by monitoring the subcellular localization of Msn2/4 (Fig. 2), expressed as GFP fusion proteins under the control of the strong ADH1 promoter, and Maf1 (Fig. 3), expressed at its native chromosomal locus.
It is noticeable that in contrast to other stress responses, the nuclear localization of Msn2 and Maf1 occurred in only a fraction of the population. The limited proportion of apparently responsive cells could reflect either that the H2O2 signaling pathway is less efficient than others in the general response to stress or that the cells display different sensitivities to this environmental condition. It is also possible that this heterogeneity reflects the dynamic behavior of the transcription factors, as recently reported for Msn2 (14, 23, 29). Indeed, upon particular stress conditions, Msn2 oscillates between the cytoplasm and the nucleus. Thus, when the population is fixed by formaldehyde for observation, it is expected that Msn2 will accumulate in the nucleus in only a portion of the cells.
One of the main questions raised by the thioredoxin-dependent H2O2 signaling is how the H2O2 signal goes to and from thioredoxins. Our Msn2-GFP experiments with different thioredoxin pathway mutants provide some interesting clues about the initial mechanisms. The amount of cells displaying a nuclear accumulation of Msn2-GFP in response to H2O2 treatment is higher for a trr1Δ strain than for a WT strain (Fig. 2C). On the contrary, we observed a very low proportion of cells exhibiting a nuclear GFP staining in a tsa1Δ strain. In accordance with the role of peroxidase reduction through the thioredoxin pathway (Fig. 6), a trr1Δ strain accumulates oxidized thioredoxins in the presence of H2O2 (46), whereas thioredoxins are likely to be less oxidized in a tsa1Δ strain under the same conditions. Based on these results, we suggest a model in which the thioredoxins, in their oxidized form, are essential for signaling the presence of H2O2 to the effectors of the general stress response (Fig. 6). In this working model, cytoplasmic thioredoxins, which are involved in H2O2 detoxification, are also required for driving the general response to stress in the presence of H2O2. Thus, the global yeast response to stress induced by H2O2 is essentially activated by two highly sensitive peroxidases, Orp1/Gpx3 and Tsa1, directly oxidized by H2O2. Orp1 activates the Yap1 transcription factor through a direct redox interaction, leading to the transcription of genes specifically dedicated to the H2O2 stress response (43). Similarly, through a direct redox interaction, Tsa1 activates the cytoplasmic thioredoxins (Fig. 6), which are required to set up the general response to stress. This response is characterized by the repression of growth-related processes and the induction of cellular protection mechanisms, as illustrated respectively by the nuclear accumulation of Maf1 and Msn2/4. How the H2O2 signal is transmitted from the oxidized thioredoxins to the transcriptional effectors remains to be determined.
FIG. 6.
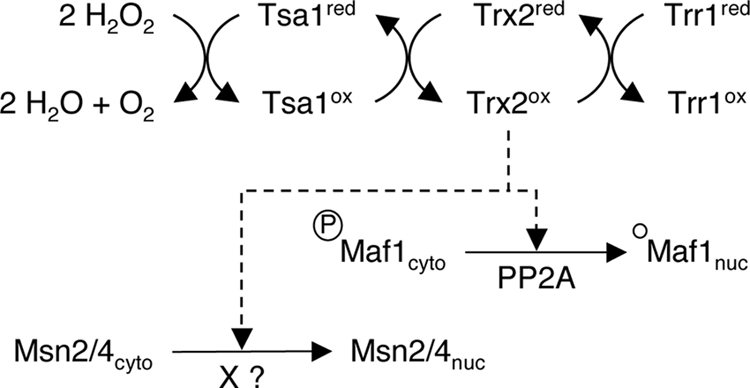
A working model of the thioredoxin signaling pathway. Accordingly to a model proposed by Ross and colleagues (38), in the course of H2O2 detoxification by the thioredoxin peroxidase Tsa1, the cytoplasmic thioredoxin Trx2 is oxidized. Our results demonstrate that the cytoplasmic thioredoxins are specifically required to trigger the nuclear localization of Msn2/4 and Maf1, effectors of the general response to stress, in the presence of H2O2. The signaling pathways, which appear to be different for each transcription factor, do not require a downregulation of the PKA activity. In addition, Maf1 nuclear accumulation correlates with the PP2A-depend accumulation of the hypophosphorylated form of the protein. “X” is an unknown phosphatase, which is required for Msn2/4 dephosphorylation and nuclear accumulation.
Under optimal growth conditions, Msn2 and Maf1 are phosphorylated in vivo, and it is now well established that their nuclear accumulation correlates tightly with modifications of their phosphorylation states. Our observations strongly suggest that at least in the case of Maf1, the H2O2 redox signal leads to the modification of the phosphorylation/dephosphorylation ratio. It could be speculated that thioredoxins would impact the cAMP/PKA pathway, inducing Msn2/4 and Maf1 nuclear accumulation through a decrease in PKA activity. Indeed, independent studies clearly established a link between the inhibition of PKA activity and the nuclear localization of Msn2/4 (2, 15, 19, 20, 42) and Maf1 (for a review, see reference 48). Furthermore, Maf1 and Msn2 are substrates of PKA (4). In this framework, our results on PKA activity during the H2O2 response are striking. We observed that addition of cAMP in the culture medium of pde2Δ cells had no impact on the nuclear accumulation of Msn2-GFP and Maf1 during H2O2 treatment (Fig. 4A). In addition, the phosphorylation level of the PKA-consensus sites increased significantly when monitored on immunoprecipitated Maf1 after 30 min of H2O2 treatment (Fig. 4C). From these results, we conclude that the ESR response to H2O2 is not mediated by an inhibition of PKA activity, highlighting the particular regulatory features of the response to H2O2 compared to other stress conditions.
Our observations showing the rapid accumulation of nuclear Maf1 (Fig. 3) and a hypophosphorylated form of Maf1 (Fig. 5A) while PKA activity remained elevated suggest the involvement of another kinase. We thus propose that through thioredoxins, the presence of H2O2 leads to the inhibition of at least one other, still-unknown protein kinase activity. Interestingly, upon the transition from glucose to a nonfermentable carbon source, Ciesla and coworkers have also implicated another uncharacterized protein kinase activity, since an altered level of PKA activity affects neither the pattern of Maf1 phosphorylation nor its nuclear accumulation (6).
An alternative, and nonexclusive, hypothesis would be that H2O2 leads to the activation of a phosphatase activity. Indeed, the dephosphorylation step is the crucial event for the nuclear accumulation of these factors. In the case of Msn2, it has been shown that different phosphatase activities control its nuclear accumulation (24, 39). In particular, upon glucose depletion, the protein phosphatase 1 (PP1) is the direct antagonist of PKA-dependent phosphorylation (9), whereas the PP2A activity was also implicated in the light-induced oscillation of Msn2 (14).
In this report we focus on the Maf1 negative regulator, for which nuclear accumulation depends upon PP2A phosphatase activity under rapamycin treatment (33). We monitored a clear correlation between Maf1 nuclear accumulation and its dephosphorylation: in the absence of cytoplasmic thioredoxins, Maf1 remains cytoplasmic and its dephosphorylation is impaired. Furthermore, we identify PP2A as the phosphatase responsible for Maf1 dephosphorylation in the presence of H2O2. Thus, the PP2A phosphatase appears as a key player in the control of Maf1 nuclear import, since its activity is required in response to rapamycin (33), NaCl, and H2O2 (Fig. 5C). In this respect, our study describes a major difference in the regulation of Msn2 and Maf1 nuclear accumulation, since the H2O2 response of Msn2 is not affected by the lack of the PP2A phosphatase. Since Msn2 nuclear accumulation very likely requires a dephosphorylation step, this means that an additional phosphatase activity remains to be identified. Thus, the scheme in Fig. 6 indicates that parts of the H2O2 signaling pathway share a common route through the thioredoxin system and then diverge to activate Msn2 and Maf1, which are transcription factors of the general response to stress and the final integrators of these pathways.
Acknowledgments
We thank Joel Acker, Peggy Baudouin-Cornu, Christine Conesa, and Olivier Lefebvre for helpful discussions and critical reading of the manuscript. We are grateful to Olivier Lefebvre for the gift of polyclonal anti-Maf1 antibodies and to the members of the LBI for support and technical assistance.
S.B. was a recipient of a grant from the “Programme de Toxicologie Nucléaire Environnementale.”
Footnotes
Published ahead of print on 6 July 2009.
REFERENCES
- 1.Beck, T., and M. N. Hall. 1999. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 402:689-692. [DOI] [PubMed] [Google Scholar]
- 2.Boy-Marcotte, E., M. Perrot, F. Bussereau, H. Boucherie, and M. Jacquet. 1998. Msn2p and Msn4p control a large number of genes induced at the diauxic transition which are repressed by cyclic AMP in Saccharomyces cerevisiae. J. Bacteriol. 180:1044-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boy-Marcotte, E., D. Tadi, M. Perrot, H. Boucherie, and M. Jacquet. 1996. High cAMP levels antagonize the reprogramming of gene expression that occurs at the diauxic shift in Saccharomyces cerevisiae. Microbiology (Reading) 142:459-467. [DOI] [PubMed] [Google Scholar]
- 4.Budovskaya, Y. V., J. S. Stephan, S. J. Deminoff, and P. K. Herman. 2005. An evolutionary proteomics approach identifies substrates of the cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 102:13933-13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Causton, H. C., B. Ren, S. S. Koh, C. T. Harbison, E. Kanin, E. G. Jennings, T. I. Lee, H. L. True, E. S. Lander, and R. A. Young. 2001. Remodeling of yeast genome expression in response to environmental changes. Mol. Biol. Cell 12:323-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciesla, M., J. Towpik, D. Graczyk, D. Oficjalska-Pham, O. Harismendy, A. Suleau, K. Balicki, C. Conesa, O. Lefebvre, and M. Boguta. 2007. Maf1 is involved in coupling carbon metabolism to RNA polymerase III transcription. Mol. Cell. Biol. 27:7693-7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delaunay, A., A. D. Isnard, and M. B. Toledano. 2000. H2O2 sensing through oxidation of the Yap1 transcription factor. EMBO J. 19:5157-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai, N., J. Lee, R. Upadhya, Y. Chu, R. D. Moir, and I. M. Willis. 2005. Two steps in Maf1-dependent repression of transcription by RNA polymerase III. J. Biol. Chem. 280:6455-6462. [DOI] [PubMed] [Google Scholar]
- 9.De Wever, V., W. Reiter, A. Ballarini, G. Ammerer, and C. Brocard. 2005. A dual role for PP1 in shaping the Msn2-dependent transcriptional response to glucose starvation. EMBO J. 24:4115-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durchschlag, E., W. Reiter, G. Ammerer, and C. Schüller. 2004. Nuclear localization destabilizes the stress-regulated transcription factor Msn2. J. Biol. Chem. 279:55425-55432. [DOI] [PubMed] [Google Scholar]
- 11.Düvel, K., A. Santhanam, S. Garrett, L. Schneper, and J. R. Broach. 2003. Multiple roles of Tap42 in mediating rapamycin-induced transcriptional changes in yeast. Mol. Cell 11:1467-1478. [DOI] [PubMed] [Google Scholar]
- 12.Evans, D. R., and M. J. Stark. 1997. Mutations in the Saccharomyces cerevisiae type 2A protein phosphatase catalytic subunit reveal roles in cell wall integrity, actin cytoskeleton organization and mitosis. Genetics 145:227-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fourquet, S., M. Huang, B. D'autreaux, and M. B. Toledano. 2008. The dual functions of thiol-based peroxidases in H2O2 scavenging and signaling. Antioxid. Redox Signal. 10:1565-1576. [DOI] [PubMed] [Google Scholar]
- 14.Garmendia-Torres, C., A. Goldbeter, and M. Jacquet. 2007. Nucleocytoplasmic oscillations of the yeast transcription factor Msn2: evidence for periodic PKA activation. Curr. Biol. 17:1044-1049. [DOI] [PubMed] [Google Scholar]
- 15.Garreau, H., R. N. Hasan, G. Renault, F. Estruch, E. Boy-Marcotte, and M. Jacquet. 2000. Hyperphosphorylation of Msn2p and Msn4p in response to heat shock and the diauxic shift is inhibited by cAMP in Saccharomyces cerevisiae. Microbiology (Reading) 146:2113-2120. [DOI] [PubMed] [Google Scholar]
- 16.Garrido, E. O., and C. M. Grant. 2002. Role of thioredoxins in the response of Saccharomyces cerevisiae to oxidative stress induced by hydroperoxides. Mol. Microbiol. 43:993-1003. [DOI] [PubMed] [Google Scholar]
- 17.Gasch, A. P., P. T. Spellman, C. M. Kao, O. Carmel-Harel, M. B. Eisen, G. Storz, D. Botstein, and P. O. Brown. 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11:4241-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godon, C., G. Lagniel, J. Lee, J. M. Buhler, S. Kieffer, M. Perrot, H. Boucherie, M. B. Toledano, and J. Labarre. 1998. The H2O2 stimulon in Saccharomyces cerevisiae. J. Biol. Chem. 273:22480-22489. [DOI] [PubMed] [Google Scholar]
- 19.Görner, W., E. Durchschlag, M. T. Martínez-Pastor, F. Estruch, G. Ammerer, B. Hamilton, H. Ruis, and C. Schüller. 1998. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 12:586-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Görner, W., E. Durchschlag, J. Wolf, E. L. Brown, G. Ammerer, H. Ruis, and C. Schüller. 2002. Acute glucose starvation activates the nuclear localization signal of a stress-specific yeast transcription factor. EMBO J. 21:135-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasan, R., C. Leroy, A. D. Isnard, J. Labarre, E. Boy-Marcotte, and M. B. Toledano. 2002. The control of the yeast H2O2 response by the Msn2/4 transcription factors. Mol. Microbiol. 45:233-241. [DOI] [PubMed] [Google Scholar]
- 22.Izawa, S., K. Maeda, K. Sugiyama, J. Mano, Y. Inoue, and A. Kimura. 1999. Thioredoxin deficiency causes the constitutive activation of Yap1, an AP-1-like transcription factor in Saccharomyces cerevisiae. J. Biol. Chem. 274:28459-28465. [DOI] [PubMed] [Google Scholar]
- 23.Jacquet, M., G. Renault, S. Lallet, J. De Mey, and A. Goldbeter. 2003. Oscillatory nucleocytoplasmic shuttling of the general stress response transcriptional activators Msn2 and Msn4 in Saccharomyces cerevisiae. J. Cell Biol. 161:497-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaida, D., H. Yashiroda, A. Toh-e, and Y. Kikuchi. 2002. Yeast Whi2 and Psr1-phosphatase form a complex and regulate STRE-mediated gene expression. Genes Cells 7:543-552. [DOI] [PubMed] [Google Scholar]
- 25.Laferté, A., E. Favry, A. Sentenac, M. Riva, C. Carles, and S. Chédin. 2006. The transcriptional activity of RNA polymerase I is a key determinant for the level of all ribosome components. Genes Dev. 20:2030-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, J., D. Spector, C. Godon, J. Labarre, and M. B. Toledano. 1999. A new antioxidant with alkyl hydroperoxide defense properties in yeast. J. Biol. Chem. 274:4537-4544. [DOI] [PubMed] [Google Scholar]
- 27.Le Moan, N., G. Clement, S. Le Maout, F. Tacnet, and M. B. Toledano. 2006. The Saccharomyces cerevisiae proteome of oxidized protein thiols: contrasted functions for the thioredoxin and glutathione pathways. J. Biol. Chem. 281:10420-10430. [DOI] [PubMed] [Google Scholar]
- 28.Maillet, I., G. Lagniel, M. Perrot, H. Boucherie, and J. Labarre. 1996. Rapid identification of yeast proteins on two-dimensional gels. J. Biol. Chem. 271:10263-10270. [DOI] [PubMed] [Google Scholar]
- 29.Medvedik, O., D. W. Lamming, K. D. Kim, and D. A. Sinclair. 2007. MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae. PLoS Biol. 5:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moir, R. D., J. Lee, R. A. Haeusler, N. Desai, D. R. Engelke, and I. M. Willis. 2006. Protein kinase A regulates RNA polymerase III transcription through the nuclear localization of Maf1. Proc. Natl. Acad. Sci. USA 103:15044-15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller, E. G. 1991. Thioredoxin deficiency in yeast prolongs S phase and shortens the G1 interval of the cell cycle. J. Biol. Chem. 266:9194-9202. [PubMed] [Google Scholar]
- 32.O'Connor, J. P., and C. L. Peebles. 1991. In vivo pre-tRNA processing in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:425-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oficjalska-Pham, D., O. Harismendy, W. J. Smagowicz, A. Gonzalez de Peredo, M. Boguta, A. Sentenac, and O. Lefebvre. 2006. General repression of RNA polymerase III transcription is triggered by protein phosphatase type 2A-mediated dephosphorylation of Maf1. Mol. Cell 22:623-632. [DOI] [PubMed] [Google Scholar]
- 34.Pluta, K., O. Lefebvre, N. C. Martin, W. J. Smagowicz, D. R. Stanford, S. R. Ellis, A. K. Hopper, A. Sentenac, and M. Boguta. 2001. Maf1p, a negative effector of RNA polymerase III in Saccharomyces cerevisiae. Mol. Cell. Biol. 21:5031-5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reina, J. H., T. N. Azzouz, and N. Hernandez. 2006. Maf1, a new player in the regulation of human RNA polymerase III transcription. PLoS ONE 1:e134.17205138 [Google Scholar]
- 36.Rep., M., M. Krantz, J. M. Thevelein, and S. Hohmann. 2000. The transcriptional response of Saccharomyces cerevisiae to osmotic shock. Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J. Biol. Chem. 275:8290-8300. [DOI] [PubMed] [Google Scholar]
- 37.Roberts, D. N., B. Wilson, J. T. Huff, A. J. Stewart, and B. R. Cairns. 2006. Dephosphorylation and genome-wide association of Maf1 with Pol III-transcribed genes during repression. Mol. Cell 22:633-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross, S. J., V. J. Findlay, P. Malakasi, and B. A. Morgan. 2000. Thioredoxin peroxidase is required for the transcriptional response to oxidative stress in budding yeast. Mol. Biol. Cell 11:2631-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santhanam, A., A. Hartley, K. Düvel, J. R. Broach, and S. Garrett. 2004. PP2A phosphatase activity is required for stress and Tor kinase regulation of yeast stress response factor Msn2p. Eukaryot. Cell 3:1261-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schüller, C., J. L. Brewster, M. R. Alexander, M. C. Gustin, and H. Ruis. 1994. The HOG pathway controls osmotic regulation of transcription via the stress response element (STRE) of the Saccharomyces cerevisiae CTT1 gene. EMBO J. 13:4382-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith, A., M. P. Ward, and S. Garrett. 1998. Yeast PKA represses Msn2p/Msn4p-dependent gene expression to regulate growth, stress response and glycogen accumulation. EMBO J. 17:3556-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toledano, M. B., A. Delaunay, L. Monceau, and F. Tacnet. 2004. Microbial H2O2 sensors as archetypical redox signaling modules. Trends Biochem. Sci. 29:351-357. [DOI] [PubMed] [Google Scholar]
- 44.Toledano, M. B., C. Kumar, N. Le Moan, D. Spector, and F. Tacnet. 2007. The system biology of thiol redox system in Escherichia coli and yeast: differential functions in oxidative stress, iron metabolism and DNA synthesis. FEBS Lett. 581:3598-3607. [DOI] [PubMed] [Google Scholar]
- 45.Towpik, J., D. Graczyk, A. Gajda, O. Lefebvre, and M. Boguta. 2008. Derepression of RNA polymerase III transcription by phosphorylation and nuclear export of its negative regulator, Maf1. J. Biol. Chem. 283:17168-17174. [DOI] [PubMed] [Google Scholar]
- 46.Trotter, E. W., and C. M. Grant. 2003. Non-reciprocal regulation of the redox state of the glutathione-glutaredoxin and thioredoxin systems. EMBO Rep. 4:184-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Upadhya, R., J. Lee, and I. M. Willis. 2002. Maf1 is an essential mediator of diverse signals that repress RNA polymerase III transcription. Mol. Cell 10:1489-1494. [DOI] [PubMed] [Google Scholar]
- 48.Willis, I. M., and R. D. Moir. 2007. Integration of nutritional and stress signaling pathways by Maf1. Trends Biochem. Sci. 32:51-53. [DOI] [PubMed] [Google Scholar]