Abstract
African trypanosomes are the causative agents of human trypanosomiasis (sleeping sickness). The pathogenic stage of the parasite has unique adaptations to life in the bloodstream of the mammalian host, including upregulation of endocytic and lysosomal activities. We investigated stage-specific requirements for cytoplasmic adaptor/clathrin machinery in post-Golgi apparatus biosynthetic sorting to the lysosome using RNA interference silencing of the Tbμ1 subunit of adaptor complex 1 (AP-1), in conjunction with immunolocalization, kinetic analyses of reporter transport, and quantitative endocytosis assays. Tbμ1 silencing was lethal in both stages, indicating a critical function(s) for the AP-1 machinery. Transport of soluble and membrane-bound secretory cargoes was Tbμ1 independent in both stages. In procyclic parasites, trafficking of the lysosomal membrane protein, p67, was disrupted, leading to cell surface mislocalization. The lysosomal protease trypanopain was also secreted, suggesting a transmembrane-sorting receptor for this soluble hydrolase. In bloodstream trypanosomes, both p67 and trypanopain trafficking were unaffected by Tbμ1 silencing, suggesting that AP-1 is not necessary for biosynthetic lysosomal trafficking. Endocytosis in bloodstream cells was also unaffected, indicating that AP-1 does not function at the flagellar pocket. These results indicate that post-Golgi apparatus sorting to the lysosome is critically dependent on the AP-1/clathrin machinery in procyclic trypanosomes but that this machinery is not necessary in bloodstream parasites. We propose a simple model for stage-specific default secretory trafficking in trypanosomes that is consistent with the behavior of other soluble and glycosylphosphatidylinositol-anchored cargos and which is influenced by upregulation of endocytosis in bloodstream parasites as an adaptation to life in the mammalian bloodstream.
African trypanosomes (Trypanosoma brucei subspecies), the agents of African sleeping sickness, are alone among the kinetoplastid parasites (including Trypanosoma cruzi and Leishmania spp.) in having a pathogenic bloodstream stage that exists and replicates extracellularly in the mammalian host. This places unique constraints on the parasite in terms of dealing with host immune responses and on acquisition of essential nutrients. The parasite has evolved many strategies to deal with these constraints, the best known of which is the process of antigenic variation (9). Another is the lysosome, which impacts the host-pathogen balance in multiple ways. Trypanosomes have a single terminal lysosome that is the final repository of endocytic cargo acquired from the host serum for nutritional purposes (30), as well as for potentially lytic immune complexes removed from the cell surface (4, 8). Both endocytosis and lysosomal hydrolytic activities are differentially regulated through the trypanosome life cycle (11, 30), and there are stage-specific differences in the biosynthetic trafficking of essential lysosomal components (discussed below). The release of lysosomal proteases is a factor in the signature event of human infection, penetration of the central nervous system (36). Finally, lysosomal physiology is critical to the activity of an innate human serum resistance trait, trypanolytic factor, which limits the host range of Trypanosoma species (38).
Clearly, given its multiple roles in pathogenesis, biogenesis of the lysosome is critical to the success of trypanosomes as human parasites. As in all eukaryotes, lysosomal biogenesis is a balance between the proper sorting of newly synthesized membranes and proteins and recycling of established membranes and proteins internalized from the cell surface. In each case, protein sorting involves recognition of specific signals in cargo molecules by cellular machinery for inclusion in nascent transport vesicles destined for downstream delivery. Unique sets of cytoplasmic coat complexes at discrete intracellular locations serve the dual purpose of simultaneously mediating vesicle formation and selective cargo loading. The best characterized of these machineries is the clathrin/adaptin system for formation of coated vesicles at the Golgi apparatus and the plasma membrane (10, 41). Adaptor complexes (APs) are cytosolic heterotetramers that interact with specific signals in the cytoplasmic domains of membrane cargo proteins, such as dileucine motifs ([E/D]XXXL[L/I]) and tyrosine motifs (YXXØ, where Ø is a bulky hydrophobic residue). The prototypic AP complexes are AP-1 and AP-2, which function at the trans-Golgi network and plasma membrane, respectively. Both are composed of two large subunits (γ/β1 in AP-1; α/β2 in AP-2) and two smaller subunits (σ1/μ1 in AP-1; σ2/μ2 in AP-2). YXXØ motifs interact with μ adaptins, and dileucine motifs interact with combinations of adaptin subunits in both AP-1 and AP-2 (26, 40, 42). It is the large subunits, particularly β adaptin, that mediate clathrin recruitment (19, 44). Other APs, AP-3 and AP-4, with discrete subunit compositions, also exist. AP-3 functions in trafficking to lysosome-related organelles, such as melanosomes, and AP-4 may be involved in basolateral trafficking in polarized epithelial cells (10). The genome of the African trypanosome, T. brucei, encodes a complete complement of orthologous subunits for AP-1, AP-3, and AP-4 but has no genes for AP-2, the major adaptor complex mediating endocytosis in vertebrate cells (16). This is likely due to evolutionary loss, since the closely related T. cruzi has orthologues of all four APs.
Two major lysosomal cargo proteins have been studied in T. brucei, the LAMP (lysosome-associated membrane protein)-like protein p67 and the cathepsin L orthologue trypanopain. p67 is a type I membrane protein with a large glycosylated lumenal domain and a short cytoplasmic domain (1, 27). In procyclic insect stage (PCF) trypanosomes, the cytoplasmic domain is both necessary and sufficient for lysosomal targeting of a heterologous reporter, and its deletion results in mistargeting of p67 to the cell surface (1). The cytoplasmic domain contains two canonical dileucine motifs, mutation of which also results in delivery to the cell surface (47). These findings strongly indicate the existence of cognate cytoplasmic machinery for lysosomal delivery of p67 in PCF trypanosomes. Strikingly, however, the cytoplasmic domain, and its motifs, are totally dispensable for lysosomal targeting in bloodstream stage (BSF) trypanosomes (1). Deletion of the cytoplasmic domain results in minor mislocalization to the cell surface, but p67 is still overwhelmingly delivered to the lysosome. Ongoing lysosomal targeting cannot easily be attributed to misfolding of the lumenal domain, as suggested by others (3), since the normal transport-associated patterns of p67 glycosylation and cleavage prevail in these deletion constructs.
Less is known about targeting of soluble trypanopain. In mammalian cells, soluble hydrolases are targeted to the lysosome by the addition of mannose-6-phosphate (M6P) moieties in the Golgi apparatus, which serve as ligands for recognition and lysosomal targeting by downstream M6P receptors (28). Soluble hydrolases can also be sorted by receptors that recognize polypeptide motifs, such as sortilins in mammalian cells (12) and Vps10 in yeast (13, 32). These receptors have lumenal cargo recognition domains and cytoplasmic domains containing signals for late endosomal targeting and recycling. M6P-modified N-linked glycans are not found in trypanosomes, and genes encoding the necessary enzymatic activities are absent from the genome (16), ruling out this possibility for trypanopain sorting. However, the T. cruzi orthologue, cruzipain, has been shown to rely on peptide motifs in the N-terminal prodomain for targeting (24), raising the possibility of a sortilin/Vps10p-like sorting receptor. Although there are no obvious orthologues of these proteins in the T. brucei genome, overexpression of trypanopain in PCF trypanosomes leads to secretion, an observation that is consistent with saturation of a specific sorting receptor (S. S. Sutterwala and J. D. Bangs, unpublished observations).
Having previously studied the innate signals involved in p67 targeting (1, 47), we now turned our attention to the cognate machinery for post-Golgi apparatus sorting. Specifically, we investigate the role of trypanosomal AP-1 in stage-specific biosynthetic trafficking to the lysosome using RNA interference (RNAi)-mediated silencing of the Tbμ1 (geneDB no. Tb927.7.3180 [www.genedb.org]) subunit as our primary strategy. Our results demonstrate that AP-1 and clathrin are critical for lysosomal targeting of p67 and trypanopain in PCF trypanosomes but that they are essentially dispensable in BSF parasites. These data, in conjunction with the behavior of p67-targeting mutants (1) and other trypanosomal secretory reporters, lead us to propose a simple model for stage-specific default trafficking in African trypanosomes. Although in some respects our results are similar to those of a recent publication using RNAi silencing of the Tbγ1 subunit of AP-1 (3), they differ in key aspects, leading us to significantly different conclusions.
MATERIALS AND METHODS
Cell culture, metabolic labeling, and immunoprecipitation.
Standard propagation of the cultured PCF and BSF Lister 427 strain T. brucei brucei in Cunningham's and HMI9 media (15, 23) is described elsewhere (38, 47). The tetracycline-responsive 29-13 PCF and 13-90 BSF derivatives of Lister 427 were used for all experiments (50). Certified tetracycline-free fetal bovine serum (FBS) (Clonetech, Mountain View, CA) was used for all in vitro cultures. Pulse-chase radiolabeling and immunoprecipitation of cultured trypanosomes were performed as described previously (38, 47), as was analysis of variant surface glycoprotein (VSG) trafficking (45). For PCF cultures, pulse times were 15 min (p67) or 7.5 min (trypanopain, BiPN, and VSG). For BSF cultures, pulse times were 2 min (VSG), 10 min (trypanopain and BiPN), and 15 min (p67). All immunoprecipitates were fractionated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the gels were analyzed by phosphorimaging using a Molecular Dynamics Typhoon Storm 860 system with native ImageQuant Software (Amersham Biosciences Inc., Piscataway, NJ). For quantification of specific band intensities, signals from equivalent unlabeled areas of each lane were subtracted as background. All data analyses were performed with Prism4 software (GraphPad Software, Inc., San Diego, CA).
Construction of vectors and cell lines.
A stem-loop construct was generated for RNAi silencing of Tbμ1. Briefly, the tetracycline-responsive vector pLew100 (50) was modified by insertion of XhoI and XbaI linkers into the HindIII and BamHI sites, respectively. A 477-bp “stuffer” fragment was generated by PCR from pJM326 (49) (a gift from Paul Englund, Johns Hopkins University) and inserted into the XhoI/BamHI sites to generate the pLew100X:Pex11 stem-loop vector. Using trypanosome genomic DNA, an 800-bp region of the Tbμ1 open reading frame (ORF) (geneDB no. Tb927.7.3180) was amplified with flanking XhoI/AscI sites and inserted upstream of the stuffer fragment. The same region was amplified again with flanking BamHI/XbaI sites and inserted in the opposite orientation downstream of the stuffer fragment. The resultant Tbμ1 RNAi stem-loop double-stranded RNA (dsRNA) vector was linearized with NotI for electroporation into 29-13 PCF and 13-90 BSF trypanosomes. Tbμ1 RNAi cell lines were selected with phleomycin as described previously (38, 47) and cloned by limiting dilution. Expression of dsRNA was induced with 1 μg/ml tetracycline. The clathrin heavy-chain dsRNA vector, p2T7ti:TbCHC (2) (a gift from Mark Field, University of Cambridge), was introduced into 29-13 PCF trypanosomes and induced in a similar manner. The transgenic BiPN and VSG117 secretory reporters in the constitutive expression vector pXS5pac (48) were linearized with XhoI and introduced into the PCF Tbμ1 dsRNA cell line, and transformants were selected with puromycin. Likewise, pXS5pac:BiPN was introduced into the BSF Tbμ1 dsRNA cell line. The trypanopain ORF was amplified from genomic DNA with a hemagglutinin (HA) tag fused to the C terminus, inserted into pXS5neo with flanking HindIII (5′) and EcoRI (3′) sites, and introduced into PCF trypanosomes as described above. There are 11 tandemly arrayed ORFs in the trypanopain locus (geneDB no. Tb927.6.960 to -1060); therefore, the precise amplicon is not clear.
Northern analysis.
Total RNA was extracted from mid-log-phase parasites using Trizol reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Probes were labeled with [32P]dCTP with a random-priming kit (Stratagene, San Diego, CA) and desalted on ProbeQuant 96 G-50 columns (GE Healthcare, Piscataway, NJ). RNA (5 μg/lane) was fractionated on 1% agarose/formaldehyde gels and transferred to Zeta Probe nylon membranes (Bio-Rad Laboratories Inc., Hercules, CA) by capillary action. Membrane-bound RNA was photo cross-linked at 1,200 μJ (1 min). The membranes were prehybridized (4 h; 42°C; 5× SSPE [1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA {pH 7.7}], 5× Denhardt's solution, 50% formamide, 0.5% SDS, 200 μg/ml denatured salmon testis DNA). Denatured probes were added in fresh prewarmed hybridization buffer. Following hybridization (42°C overnight), the membranes were washed once in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.1% SDS (15 min at room temperature); twice in 0.2× SSC, 0.1% SDS (15 min at 42°C); and once in 2× SSC (5 min at room temperature). To normalize loading, the blots were stripped (three times; 80°C; 30 min; 2× SSC, 0.5% SDS) and reprobed with a tubulin probe. An identical procedure was used to access levels of Tbμ3 and Tbμ4 messages. In all cases, hybridization was analyzed by phosphorimaging using a Molecular Dynamics Typhoon Storm 860 system with native ImageQuant software.
Antibodies.
Rabbit anti-VSG117, anti-VSG221, anti-BiP, and anti-trypanopain and monoclonal anti-p67 were described previously (34, 38). Monoclonal anti-Leishmania paraflagellar rod was a kind gift from Diane McMahon-Pratt (Yale University). Rabbit anti-HA was from Invitrogen (Carlsbad, CA). Alexa 488 and Alexa 633 secondary reagents were from Molecular Probes Inc. (Eugene, OR).
Immunofluorescence imaging.
Staining of formaldehyde-fixed permeabilized and nonpermeabilized PCF cells was described previously (47). Staining of methanol/acetone-fixed BSF cells was described previously (38). All samples were stained with DAPI (4′,6′-diamidino-2-phenylindole) (0.5 μg/ml) before being mounted in 50% (vol/vol) glycerol in phosphate-buffered saline. Acquisition and deconvolution of serial three-channel Z stacks was described previously (38). Single Z-plane images are presented for fixed permeabilized cells, and nondeconvolved images are presented for surface labeling of nonpermeabilized cells.
TL binding/uptake studies.
Tomato lectin (TL), TL-biotin, and TL-fluorescein isothiocyanate (FITC) conjugate were purchased from Vector Laboratories (Burlingame, CA). Alexa 488 carboxylic acid, tetrafluorophenyl ester (Molecular Probes), was coupled to TL according to the manufacturer's instructions. For localization of bound and endocytosed TL, cultured BSF cells (107) were washed with HEPES-buffered saline (HBS) (50 mM HEPES, 50 mM NaCl, 5 mM KCl, 70 mM glucose, pH 7.5) and resuspended in 1 ml of HMI9-bovine serum albumin (serum-free HMI9 medium supplemented with 0.5 mg/ml bovine serum albumin) containing TL-biotin at 5 μg/ml. The cells were incubated at 5°C for 30 min, followed by extensive washing with HBS, 1% FBS. The cells were then processed for epifluorescence microscopy as described above, using Alexa 488-strepavidin (Molecular Probes) to detect TL-biotin localization. For quantitative determination of binding and uptake, cells were incubated with TL-Alexa 488 (5 μg/ml) at 5°C (binding) or 37°C (uptake) for 30 min, followed by extensive washing with HBS, 1% FBS. The cells were then resuspended in phosphate-buffered saline containing DAPI (5 μg/ml) to stain dead cells and analyzed by flow cytometry on a BD LSR instrument (BD Biosciences, Mountain View, CA) using native FACSDiva 5.0.2 acquisition software; 10,000 Alexa 488-positive/DAPI-negative events were collected for each data point. To measure the rate of endocytosis, cells were exposed to TL-FITC (5 μg/ml) at 5°C to allow binding in the flagellar pocket, washed, shifted to 37°C to allow endocytosis, and then analyzed by flow cytometry as a function of time. The FITC fluorescence yield is pH dependent (pKa, ∼6.4), and delivery to endosomal/lysosomal compartments results in a decreased fluorescent signal. All flow cytometry data were analyzed using FlowJo 8.7 (Tree Star, Ashland, OR).
RESULTS
Tbμ1 localization.
Before investigating Tbμ1 function, we first attempted to localize the gene product by introducing an HA epitope tag into the C terminus of Tbμ1 by in situ chromosomal recombination (43) in both PCF and BSF trypanosomes, a strategy we have used before with success (46). However, we were unable to confirm expression of the epitope-tagged proteins by either immunoprecipitation or immunofluorescence, despite the fact that correct in situ tagging was confirmed by genomic PCR and sequencing of both the PCF and BSF cell lines. We attribute this to low expression levels or to masking of the epitope within the AP-1 heterotetramer. Similar difficulties have been encountered by others attempting to localize AP-1 with antibodies to recombinant subunits (3).
Tbμ1 is essential in trypanosomes.
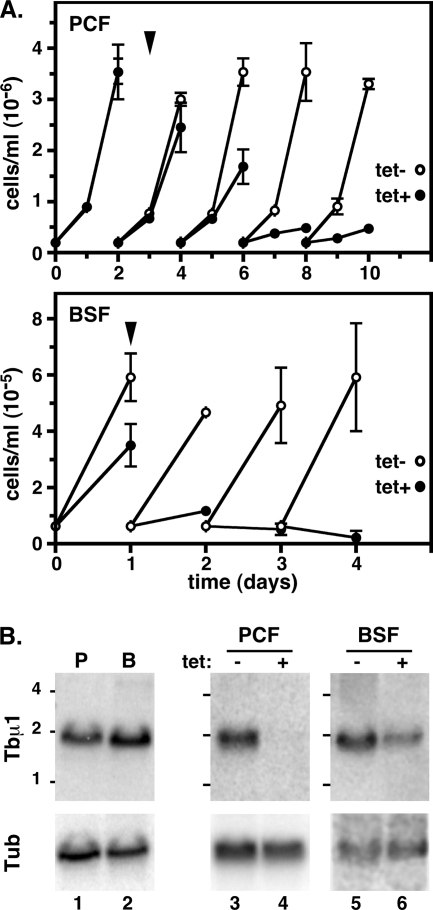
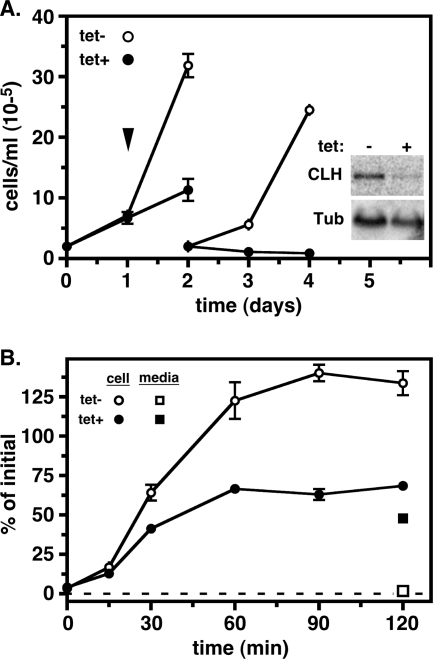
To investigate the role of AP-1 complexes in lysosomal trafficking in trypanosomes, we created an inducible dsRNA construct targeting the Tbμ1 subunit and introduced it into both cultured PCF and BSF parasites. Induction of specific dsRNA expression in each case led to a cessation of cell growth (Fig. 1A). PCF trypanosomes required 6 days to fully arrest, while in BSF parasites, growth arrest occurred within 24 h and significant death was evident after 48 h. Northern analyses indicated that normal Tbμ1 message levels were elevated 1.6 ± 0.3-fold (mean ± standard error of the mean [SEM]; n = 3) in BSF relative to PCF trypanosomes (Fig. 1B, lane 1 versus 2), consistent with stage-specific elevations seen for other clathrin and adaptin subunits (35), as well as the higher rates of biosynthetic and endocytic lysosomal trafficking in this stage of the life cycle (1, 14, 17, 30). Induction of specific dsRNA led to dramatic reductions in Tbμ1 expression: 94.0% ± 2.0% in PCF trypanosomes on day 3 (Fig. 1B, lane 3 versus 4) and 58.8% ± 1.2% in BSF trypanosomes on day 1 (Fig. 1B, lane 5 versus 6) (means ± SEM; n = 3). No effect was seen on the levels of Tbμ3 or Tbμ4 messages, confirming the specificity of silencing (data not shown). These results confirm that growth arrest associated with dsRNA induction is specifically related to ablation of Tbμ1 expression. All further phenotypic analyses were performed on day 3 and day 1 of RNAi induction for PCF and BSF trypanosomes, respectively.
FIG. 1.
Tbμ1 silencing in trypanosomes. Transgenic PCF and BSF cell lines containing the Tbμ1 RNAi construct were cultured without (tet−) or with (tet+; 1 μg/ml) tetracycline to initiate specific dsRNA synthesis. (A) Growth. Cell density was monitored by microscopic counting, and cultures were adjusted to starting densities every 2 days (PCF) or 1 day (BSF). Data from triplicate cultures are presented as means ± SEM. The arrowheads indicate time points (PCF, day 3; BSF, day 1) for all subsequent RNAi phenotypic analyses. (B) Northern analyses. As indicated, Tbμ1 mRNA levels were determined in control PCF (P) and BSF (B) cells and in PCF and BSF cell lines containing the Tbμ1 RNAi construct after culture without (−) or with (+) tetracycline to induce dsRNA synthesis. The blots were first probed for Tbμ1 (top blots) and then stripped and reprobed for tubulin (bottom blots) as a loading control. All lanes contained 5 μg total RNA. Phosphorimages are presented. Size is indicated in kilobases.
Tbμ1 mediates lysosomal trafficking in procyclic trypanosomes.
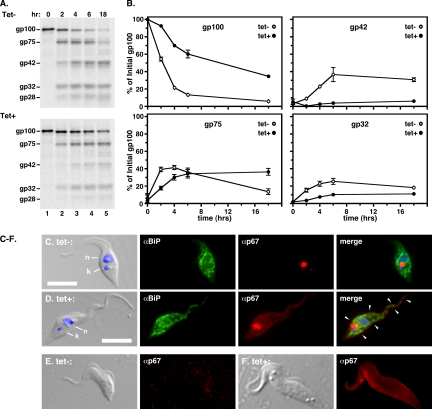
Our previous work indicated that lysosomal targeting of the membrane glycoprotein p67 in PCF trypanosomes is mediated by saturable interactions of two canonical C-terminal dileucine motifs with trafficking machinery (1, 47), presumably cytosolic components of transport vesicles, such as the AP-1 heterotetramer. Therefore, we tested the effect of Tbμ1 silencing on p67 trafficking in PCF trypanosomes by pulse-chase radiolabeling (Fig. 2A). In control cells (top gel), the normal pattern of posttranslational processing associated with lysosomal delivery was seen (1). Initially, p67 was detected as the full-length endoplasmic reticulum (ER) gp100 glycoform, and this was processed to the gp75 intermediate and subsequently to the quasi-stable end products, gp42, gp32, and gp28. A similar pattern of processing was observed in Tbμ1-silenced cells (bottom gel); however, quantification (Fig. 2B) revealed a dramatic reduction in the kinetics of processing. The turnover rate of the gp100 precursor was reduced fivefold (half-life, ∼2.2 versus 11 h), the appearance of the gp75 intermediate was delayed, and it subsequently failed to decline, and the appearance of the gp42 and gp32 end products was nearly eliminated. These data indicate a clear impact of Tbμ1 silencing on the efficiency of p67 trafficking.
FIG. 2.
Effect of Tbμ1 silencing on p67 trafficking in PCF trypanosomes. (A) p67 turnover. After culture without (Tet−) or with (Tet+) induction of dsRNAi synthesis, PCF Tbμ1 cells were subjected to pulse-chase radiolabeling as described in Materials and Methods. p67 polypeptides were specifically immunoprecipitated from cell extracts at the indicated times and analyzed by SDS-PAGE/phosphorimaging. The mobilities of intact p67 (gp100) and its derivative fragmentary glycoforms (gp75 to gp28) are indicated. All lanes contained 107 cell equivalents. (B) p67 quantitation. The relative amounts of specific p67 fragments were quantified by phosphorimaging. The data are presented as percentages of the original gp100 glycoform and represent means ± SEM for triplicate experiments. (C and D) Immunofluorescence assays were performed on fixed/permeabilized PCF cells without (tet−) (C) or with (tet+) (D) specific silencing of Tbμ1. The cells were stained with anti-BiP (green) and anti-p67 (red) as indicated (middle images). Merged DAPI/DIC images are presented on the left, and the positions of nuclei (n) and kinetoplasts (k) are indicated. Three-channel fluorescent merges are presented on the right. The bars indicate 10 μm. The arrowheads indicate regions of p67 mislocalization to the cell surface. (E and F) Nonpermeabilized control (tet−) (E) and Tbμ1-silenced (tet+) (F) cells were stained with anti-p67 (red) and imaged at identical exposure times. Matched DIC and immunofluorescence images are presented.
We next asked if silencing affects the steady-state localization of p67 (Fig. 2C to F). Immunofluorescent staining in permeabilized control cells revealed the typical pattern for endogenous p67, a single discrete vacuole in the postnuclear region (Fig. 2C). Silenced cells, however, in addition to typical lysosomal localization, displayed diffuse staining with considerable signal at the perimeter of the cell, consistent with mistargeting to the cell surface. This was confirmed by staining of nonpermeabilized cells; Tbμ1 silencing led to strong surface labeling (Fig. 2F) relative to uninduced controls (Fig. 2E), indicating that disruption of lysosomal targeting leads to mislocalization of p67 to the plasma membrane.
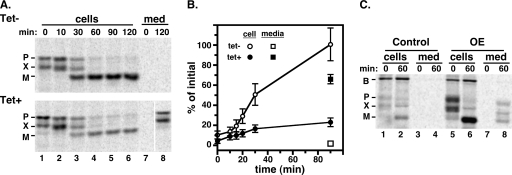
These data strongly suggest a direct role for AP-1 in post-Golgi apparatus trafficking of lysosome membrane proteins. To investigate its role in trafficking of lumenal proteins, we followed the posttranslational processing of trypanopain, a cathepsin L orthologue (31), in control and Tbμ1-silenced PCF trypanosomes (Fig. 3). As was seen previously (38), in controls (Fig. 3A, top gel, lanes 1 to 6) trypanopain was synthesized as a cell-associated 53-kDa precursor that was subsequently converted to the mature 44-kDa lysosomal form by proteolytic removal of an N-terminal prodomain. In addition, an ∼50-kDa “precursor X” form (Fig. 3A) was also seen initially, and it, too, was converted to the mature form. No trypanopain was detected in the medium fractions, indicating that lysosomal trafficking was efficient in the control cells (Fig. 3A, lanes 7 and 8). The exact nature of the precursor X is not clear, and its presence is variable (38). Trypanopain is known to have two distinct transcripts (31), and our own observations (Sutterwala and Bangs, unpublished) indicate precursor X is derived from the trypanopain ORF, suggesting that it may result from alternate trans-splicing of pre-mRNA and/or initiation of translation. A similar pattern of processing was seen in Tbμ1-silenced cells, in which the two precursors were converted to cell-associated mature trypanopain (Fig. 3A, bottom gel, lanes 1 to 6), albeit at greatly reduced levels. In contrast to control cells, however, significant amounts of both precursor forms were detected in the final medium fraction (Fig. 3A, compare lanes 7 and 8). Careful quantification (Fig. 3B) indicated that Tbμ1 silencing reduced lysosomal delivery 78% with a compensatory 66% increase in total secreted forms. These results indicate that normal trafficking of trypanopain is also dependent on Tbμ1 and, furthermore, suggest the existence of transmembrane receptors for sorting this soluble hydrolase to the lysosome. To test this possibility, we constitutively overexpressed trypanopain in PCF trypanosomes and assayed its fate by pulse-chase labeling (Fig. 3C). Trypanopain was dramatically overexpressed (lanes 5 and 6) relative to control parental cells (lanes 1 and 2) and was overwhelmingly converted to the mature form within cells, indicating proper targeting to the lysosome. In addition, overexpression led to secretion of considerable amounts of all forms of trypanopain (lanes 7 and 8), whereas no secretion was detected in control cells (lanes 3 and 4). These results are consistent with the existence of a saturable transmembrane receptor with a lumenal domain for recognition of trypanopain precursors and a cytoplasmic domain for interaction with the AP-1 complex.
FIG. 3.
Effect of Tbμ1 silencing on trypanopain trafficking in PCF trypanosomes. (A) After culture without (Tet−) or with (Tet+) induction of dsRNAi synthesis, procyclic Tbμ1 cells were subjected to pulse-chase radiolabeling as described in Materials and Methods. Trypanopain polypeptides were specifically immunoprecipitated from equivalent cell extract and medium (med) fractions at the indicated chase times and analyzed by SDS-PAGE/phosphorimaging. The mobilities of precursor (P) and mature (M) trypanopain and the uncharacterized trypanopain-related polypeptide (X) are indicated. All lanes contained 107 cell equivalents. The vertical white line in the lower blot indicates intervening lanes that were digitally excised for the sake of presentation (all remaining lanes are from the same exposure). (B) Relative amounts of cell-associated and secreted trypanopain in control (tet−) and silenced (tet+) cells were quantified by phosphorimaging. For cell-associated trypanopain (circles), the mature species was quantified as a percentage of all initial species (P + X + M). For secreted trypanopain (squares), all species (P + X + M) were quantified as a percentage of all initial species (P + X + M). The data represent means ± SEM for triplicate experiments. (C) Control and trypanopain-overexpressing (OE) cell lines were pulse-chase radiolabeled, and trypanopain (P, X, and M) and BiP (B) polypeptides were simultaneously immunoprecipitated from equivalent cell extracts and medium fractions at the indicated chase times. The BiP polypeptide served as an internal control for equal loading of cell samples and as a negative control for secretion. Samples were analyzed as for panel A; all lanes contained 107 cell equivalents.
Tbμ1 silencing does not affect secretory trafficking in PCF trypanosomes.
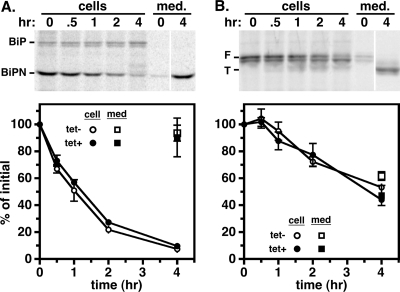
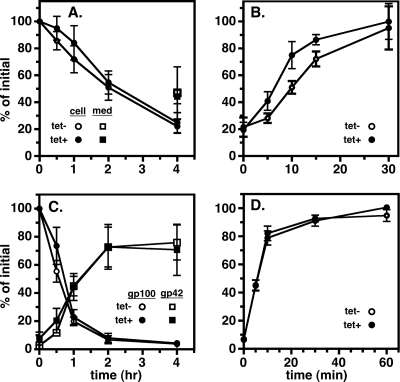
Our results collectively indicate that both membrane-bound and soluble lysosomal cargoes are critically dependent on AP-1 for proper trafficking and that disruption of this process results in misdirection into the secretory pathway. This suggests that normal secretory trafficking is independent of AP-1. To test this, we introduced two secretory reporters, BiPN and VSG117, into the Tbμ1 RNAi cell line and performed standard pulse-chase analyses (Fig. 4). BiPN is the soluble globular ATPase domain of the ER molecular chaperone BiP that we engineered previously to study secretion (6); VSG117 is a BSF-specific VSG that we had previously used to study glycosylphosphatidylinositol (GPI)-dependent trafficking (34, 48). BiPN was efficiently and quantitatively exported from control cells, and Tbμ1 silencing had no effect on the kinetics or extent of secretion (Fig. 4A). As previously established (7, 22, 29), GPI-anchored VSG117 is delivered to the surface of control cells, from where it is released in truncated form by the action of MSP-B, an endogenous surface metalloprotease. Again, silencing of Tbμ1 had no effect on the kinetics or extent of secretion (Fig. 4B). These results confirm that normal post-Golgi apparatus trafficking of soluble and membrane secretory cargoes in PCF trypanosomes is independent of the AP-1 machinery.
FIG. 4.
Tbμ1 silencing does not affect secretory trafficking in PCF trypanosomes. Control (tet−) and Tbμ1-silenced (tet+) PCF cell lines expressing either the soluble secretory reporter BiPN (A) or the GPI-anchored reporter VSG117 (B) were pulse-chase radiolabeled as for Fig. 3. Reporter polypeptides were specifically immunoprecipitated from cell and medium (med.) fractions at the indicated chase times and analyzed by SDS-PAGE/phosphorimaging (107 cell equivalents per lane). Representative gels from control cells are presented (top). The mobilities of endogenous BiP, recombinant BiPN reporter, and the full-length (F) and truncated (T) forms of the recombinant VSG117 reporter are indicated. Cell-associated (circles) and secreted (squares) reporters for triplicate experiments were quantified by phosphorimaging and are presented as percentages of initial reporters (means ± SEM). Release of truncated VSG is due to cleavage by a resident metalloprotease following arrival at the cell surface (7).
TbCLH silencing in PCF trypanosomes.
Our data demonstrate a role for AP-1 in post-Golgi apparatus trafficking to the lysosome and, by inference, for clathrin-coated vesicles. Two studies have looked at the role of clathrin in PCF trypanosomes, both using RNAi silencing of clathrin heavy chain (TbCLH) and immunolocalization of p67, but no kinetic analyses were performed in either case. One study concluded that biosynthetic lysosomal trafficking was dependent on TbCLH (2), while the other concluded it was not (25). To clarify this issue, we performed our own TbCLH RNAi analyses (Fig. 5) in PCF trypanosomes using endogenous trypanopain as a lysosomal reporter. Specific silencing of TbCLH resulted in complete growth arrest by day 2 (Fig. 5A), consistent with the previous studies, and as expected, TbCLH message was almost completely eliminated (Fig. 5A, inset). Processing of trypanopain, as an index of lysosomal trafficking, was reduced ∼50% with a compensatory rise in secreted forms (Fig. 5B). These findings are fully consistent with the effects of Tbμ1 silencing and confirm that biosynthetic vesicular trafficking to the lysosome in PCF trypanosomes is mediated primarily by the AP-1/clathrin machinery.
FIG. 5.
Silencing of clathrin heavy chain in PCF trypanosomes. A PCF cell line containing the clathrin heavy-chain RNAi construct was cultured without (tet−) and with (tet+) tetracycline to induce synthesis of specific dsRNA. (A) Growth was monitored by microscopy for triplicate experiments (means ± SEM), and the arrowhead indicates the time point for all subsequent RNAi phenotypic analyses. Northern analyses (inset) were performed as for Fig. 1B. (B) Transport and processing of trypanopain were monitored as described in the legend to Fig. 3. Cell-associated (circles) and secreted (squares) trypanopain was quantified for triplicate experiments and is presented as a percentage of initial trypanopain (means ± SEM).
Role of Tbμ1 in protein trafficking in BSF trypanosomes.
We next investigated the role of AP-1 adaptin in secretory and lysosomal trafficking in BSF trypanosomes. Standard pulse-chase analyses were performed on control and Tbμ1-silenced BSF cells to assess the kinetics of p67 and trypanopain trafficking as endogenous lysosomal markers and to assess the kinetics of BiPN and native VSG as secretory markers (Fig. 6). The VSG assay in BSF trypanosomes relies on the temporal coincidence of susceptibility to release by endogenous GPI-specific phospholipase C with arrival at the cell surface and is well documented in many of our publications (5, 46, 48). As expected, transport of secretory cargo was unaffected by Tbμ1 ablation, and transport of both BiPN (Fig. 6A) and VSG (Fig. 6B) was essentially unimpaired. Strikingly, but not unexpectedly (see Discussion), the transport of the lysosomal cargos p67 and trypanopain also was not affected by Tbμ1 silencing. p67, which in BSF cells is converted in the Golgi apparatus to a gp150 isoform by addition of poly-N-acetyllactosamine (pNAL) to N-glycans (1) and then to similar quasi-stable lysosomal degradation products, as seen in PCF cells, had exactly the same profile of processing fragments (Fig. 6C). The rate of gp100 disappearance, which reflects transport from the ER to the Golgi apparatus, was identical, as was the rate of appearance of the gp42 fragment, which represents arrival in the lysosome from the Golgi apparatus. Similar to p67, trypanopain was processed to the mature form with exactly the same kinetics in control and Tbμ1-ablated cells (Fig. 6D) and in each case remained >90% cell associated, indicating little or no secretion. These results indicate, as is the case in PCF trypanosomes, that transport of bona fide secretory cargo to the cell surface, and beyond, is independent of the AP-1 machinery. In contrast to PCF trypanosomes, however, delivery of lysosomal cargo was unaffected, suggesting that the AP-1 machinery is not necessary in BSF trypanosomes or that there is an alternate route to the lysosome that is cryptic until normal trafficking is disrupted.
FIG. 6.
Effect of Tbμ1 silencing on protein trafficking in BSF trypanosomes. BSF cell lines containing the Tbμ1 dsRNA construct were assayed for transport of the transgenic reporter BiPN (A) and for endogenous VSG221 (B), p67 (C), and trypanopain (D) (means ± SEM). Control (tet−) and silenced (tet+; 24 h) cells were analyzed by standard pulse-chase analysis for the various reporters. Note that the maximum secreted BiPN seen in BSF cells was always ∼50% (48) and that the endogenous VSG transport assay used in BSF trypanosomes was different from that used in transgenic PCF cells (see the text).
Tbμ1 and endocytosis in BSF trypanosomes.
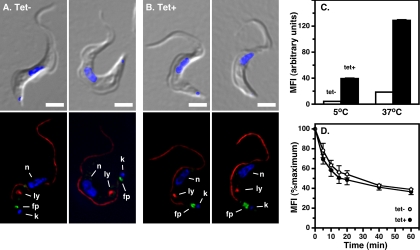
Endocytosis in BSF trypanosomes is critically dependent on clathrin (2), yet the AP-2 adaptor that mediates clathrin-dependent endocytosis in other eukaryotes is completely absent. This raises the possibility that one of the other APs, AP-1, AP-3, or AP-4, fulfills this critical role. We first determined the abilities of control and Tbμ1-silenced BSF cells to bind TL as a surrogate for receptor-mediated endocytic cargo. This ligand binds to pNAL epitopes in the flagellar pocket and is endocytosed and delivered to the lysosome (1, 37, 38). Binding occurs at 5°C, but endocytosis occurs only at higher temperatures. At low temperature, both live control and Tbμ1-silenced BSF cells bound TL-biotin conjugate in a region between the posterior kinetoplast and the base of the paraflagellar rod, consistent with flagellar-pocket localization (Fig. 7A and B). At elevated temperature, bound TL was delivered with fidelity to the lysosome (not shown). The lysosomal morphology appeared unaltered, as judged by immunofluorescence with anti-p67, nor was cell surface p67 detected by this methodology. However, at a qualitative level, Tbμ1-silenced cells displayed more intensive staining with TL-biotin, suggesting an enlargement of the flagellar pocket and/or an elevation in pNAL density, both of which could result from impairment of endocytosis. No evidence for flagellar-pocket enlargement, the so-called big-eye phenotype associated with CLH RNAi in BSF cells (2), was seen in transmitted-light images (compare Fig. 7A and B, top), nor did electron microscopy reveal any disruption of flagellar-pocket architecture (not shown). Therefore, we looked at pNAL density more quantitatively. Control and silenced cells were incubated with TL-Alexa 488 conjugate at 5°C and 37°C, and cell-associated fluorescence was measured by flow cytometry (Fig. 7C). Typical levels of binding and uptake were seen in control cells, but the levels of each were seven- to ninefold higher in silenced cells. We next measured the rates of endocytosis in control and ablated cells. TL-FITC conjugate was allowed to bind at 5°C, the cells were then shifted to 37°C to initiate endocytosis, and cell-associated fluorescence was determined as a function of time (Fig. 7D). FITC fluorescence is pH sensitive and is rapidly quenched as the probe is internalized into acidified endosomes and delivered to lysosomes (39), which in trypanosomes have an internal pH of ∼4.8 (33). When normalized to the initial signal, cell-associated fluorescence decayed with essentially identical kinetics and to the same endpoint in control and silenced cells, consistent with equivalent internalization and delivery to the lysosome. These results indicate that while AP-1 does not significantly influence endocytosis in BSF trypanosomes, its ablation leads to a dramatic increase in pNAL epitopes at the cell surface.
FIG. 7.
Tbμ1 does not function in endocytosis in BSF trypanosomes. (A and B) Control and Tbμ1-silenced BSF cells were loaded (30 min) with TL-biotin (5 μg/ml) at 5°C. After extensive washing, the cells were fixed and stained with anti-p67 (red), anti-paraflagellar rod (red), and streptavidin-Alexa 488 (TL; green). Merged DAPI/DIC (top) and three-channel epifluorescence (bottom) images of two representative control (Tet−) (A) and Tbμ1-silenced (Tet+) (B) cells are presented. Lysosomes (ly) and flagellar pockets (fp) are indicated. Nuclei (n) and kinetoplasts (k) were stained with DAPI. The bars indicate 5 μm. (C) Cells were loaded (30 min) with TL-Alexa 488 (5 μg/ml) at 5°C (binding) or at 37°C (uptake). In each case, after the cells were washed, cell-associated fluorescence was determined by flow cytometry. Mean fluorescence intensities (MFI) are presented for triplicate experiments (mean + standard deviation). (D) Kinetics of endocytosis. Cells were loaded with the pH-sensitive conjugate TL-FITC (5 μg/ml) at 5°C. After being washed, the cells were shifted to 37°C, and the mean fluorescence intensity was determined by flow cytometry as a function of time. Means ± SEM are presented for three independent experiments. Viability (C and D), as determined by DAPI exclusion, was excellent in all experiments (>90%).
DISCUSSION
Our current work focuses on the machinery of post-Golgi apparatus trafficking and specifically on the role of the AP-1 adaptor complex in lysosomal targeting, using RNAi silencing to ablate expression of the Tbμ1 subunit. While Tbμ1 expression is essential in both PCF and BSF stages of the life cycle, it apparently plays no role in post-Golgi apparatus transport of bona fide secretory cargo (VSG and BiPN) in either. However, it has very distinct stage-specific effects on lysosomal targeting. In PCF cells, Tbμ1 ablation results in dramatically impaired targeting of endogenous p67, with compensatory delivery to the cell surface. The simplest interpretation of these data is a direct physical interaction of the p67 dileucine motifs with one or more of the AP-1 subunits, as is the case in mammalian systems, where such motifs have been shown to bind to both β and μ adaptin subunits (10). Targeting of endogenous trypanopain was also severely disrupted, leading to secretion of immature proenzyme, and similar results were observed with silencing of clathrin heavy chain, resolving a longstanding discrepancy in PCF trypanosomes concerning the role of clathrin in post-Golgi apparatus sorting (2, 25). Mistargeting of a soluble lumenal hydrolase by ablation of the cytosolic AP-1/clathrin machinery provides compelling, albeit circumstantial, evidence for a specific trans-membrane targeting receptor with a lumenal cargo recognition domain and a cytoplasmic domain capable of interaction with the AP-1 heterotetramer. This interpretation is supported by the observation that overexpression of trypanopain in PCF trypanosomes results in secretion, consistent with saturation of a specific targeting receptor. Overall, these results establish for the first time that the AP-1/clathrin machinery mediates post-Golgi apparatus sorting of lysosomal cargo from secretory cargo in PCF trypanosomes.
In stark contrast, when Tbμ1 was ablated in BSF trypanosomes, trafficking of all reporters, lysosomal and secretory, was completely unaffected. A trivial explanation for this result could be that the knockdown is partial and that residual Tbμ1 provides sufficient AP-1 for normal sorting. We feel this is unlikely for several reasons. First, silencing is effective enough to achieve complete arrest of cell growth, as well as alterations of cell surface phenotypes. Second, ablation of the AP-1 γ subunit (geneDB no. Tb927.4.760) also has no effect on p67 trafficking (3) (discussed below). Finally, these results are completely consistent with the behavior of p67-targeting mutants (1, 47). The finding that lysosomal targeting of p67 can be independent of the AP-1 machinery in BSF trypanosomes may seem remarkable but is in fact not surprising. If lysosomal targeting of p67 continues in the absence of the normal dileucine motifs that operate in PCF trypanosomes, it can be reasonably expected that it will also continue in the absence of the normal machinery for motif recognition. This does not preclude p67 dileucine motifs and the AP-1 machinery from functioning normally in BSF parasites; thus, the dileucine motifs may be sufficient but not necessary. Rather, we hypothesize that in their absence a “cryptic” default route becomes apparent. Alternatively, it is possible that other cognate machinery mediates Golgi apparatus-to-lysosome trafficking of p67, either supplemental to normal sorting or in place of AP-1. Such alternate machinery could be any of the other adaptors, but AP-3 can be excluded as the sole agent, since RNAi silencing of Tbμ3 does not affect p67 trafficking in our hands (unpublished data). However, we currently prefer the default model because of its simplicity, because it functions equally well for soluble (trypanopain) and membrane-bound lysosomal (p67) cargoes, and, finally, because it is consistent with the behavior of a totally independent reporter protein, GPI-anchored VSG. Native VSG is delivered rapidly and efficiently to the cell surface in BSF trypanosomes and likewise when expressed transgenically in PCF trypanosomes (5, 7, 18). However, when expressed in soluble GPI-minus form, it is quantitatively secreted from PCF trypanosomes (6, 7, 34) but is overwhelmingly delivered to the lysosome in BSF cells (48). Thus, in BSF trypanosomes, the GPI anchor is a positive targeting signal for delivery to the cell surface, and its loss leads to capture into the default pathway to the lysosome, much like the p67 deletion mutants.
All of this begs the questions of exactly what the default pathway is and why it differs between PCF and BSF trypanosomes. We feel that the answers to these questions lie in the relative endocytic activities of the two stages, very low in PCF and very high in BSF trypanosomes (14, 17, 30). Thus, when targeting signals are deleted in PCF trypanosomes, the resultant neutral reporters are carried along by bulk secretory flow to the cell surface and beyond (Fig. 8). In BSF parasites, the very same reporters are carried by bulk endocytic flow back to the lysosome. The same phenomena are expected for ablation of cognate targeting machinery. This scenario imposes requirements for specific targeting signals to be diverted to other locations, for instance, dileucine motifs for lysosomal targeting in PCF trypanosomes and GPI anchors for plasma membrane targeting in BSF forms. This scenario also dictates an obligate physical intersection of the secretory and endosomal pathways in BSF trypanosomes. A likely point for this intersection is the recycling endosome, where VSG is sorted from endocytic cargo and recycled to the flagellar pocket (20, 21), although other crossroads can be envisioned.
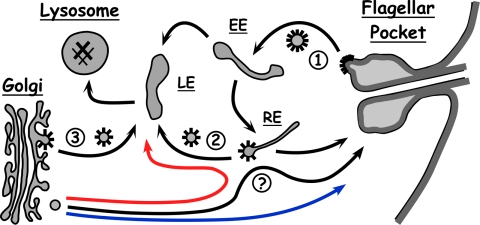
FIG. 8.
Hypothetical default pathways in trypanosomes. Known pathways between endomembrane compartments, including the early endosome (EE), recycling endosome (RE), and late endosome (LE), are indicated by black arrows. The numbers indicate sites where clathrin-coated vesicles are known to operate, including the flagellar pocket (no. 1) (2), the recycling endosome (no. 2) (21), and the Golgi apparatus (no. 3) (this work). Our work indicates that AP-1 functions at site no. 3 of clathrin-mediated trafficking in PCF cells, and possibly also in BSF cells, but not at site no. 1. No information is available for site no. 2. The question mark in the Golgi apparatus-to-flagellar pocket pathway indicates uncertainty as to whether this is a direct route or indirect via the endocytic pathway. The proposed BSF default pathway to the lysosome is indicated in red. The proposed default PCF pathway to the cell surface is in blue and essentially follows the normal pathway to the flagellar pocket.
Recently, using RNAi silencing of the Tbγ1 subunit, Allen et al. (3) reached very different conclusions about the role of AP-1 in post-Golgi apparatus trafficking. In agreement with our work, they found that AP-1 was essential in both life cycle stages and that knockdown did not affect p67 trafficking in BSF cells. They interpreted this as indicating that normal delivery of p67 to the lysosome is largely independent of AP-1, but as we argue for our own work with Tbμ1, such data do not preclude the AP-1 machinery from functioning in normal lysosomal trafficking in BSF cells. They also concluded, based solely on ultrastructural images, that AP-1 does not mediate a major trafficking route in PCF trypanosomes, whereas our kinetic analyses with two native lysosomal reporters, p67 and trypanopain, clearly indicate that post-Golgi apparatus sorting to the lysosome in PCF trypanosomes is critically dependent on AP-1. Finally, Allen et al. concluded that surface expression is the default pathway for transmembrane proteins in BSF trypanosomes, based on a fusion of the p67 transmembrane and cytoplasmic domains to our BiPN reporter. Judged by imaging alone, the chimera trafficked normally to the lysosome, and mutation of one of the two canonical dileucine motifs resulted in surface expression. However, substantial lysosomal localization for the mutant was also apparent, and no quantification of the percentage of total reporter at the cell surface was provided. Nevertheless, Allen et al. concluded that the p67 cytoplasmic domain is both necessary and sufficient for lysosomal trafficking in BSF cells and that in its absence the plasma membrane is the default destination. In contrast, we feel that the results with the chimeric reporter, although qualitative in nature, are in general agreement with our quantitative results with p67 deletion mutants, which also accessed the cell surface in small amounts but are largely delivered to the lysosome.
A final conclusion of our work is that AP-1 is not involved in endocytosis in BSF trypanosomes; ablation of Tbμ1 had no effect on the rate of uptake of a solid-phase endocytic cargo, TL. We did note a significant increase in binding of TL in the flagellar pocket, which we interpret as an increase in available pNAL targets, but despite considerable effort, we have been unable to assign this to a specific protein. In particular, all efforts to detect p67 on the cell surface by immunofluorescence or biotinylation failed. Tentatively, we conclude that ablation of Tbμ1 leads to dysregulation of endosomal recycling. Whether this is a direct effect of Tbμ1 silencing or a secondary effect of dying cells is not clear, but it is worth noting that no disruption of the flagellar pocket or other internal structures were seen at the time point (24 h) at which our analyses were carried out. Resolution of this issue will require additional studies.
In summary, we find that the AP-1/clathrin machinery is critical for post-Golgi apparatus targeting of membrane and soluble lysosomal cargoes in PCF trypanosomes but that AP-1 is dispensable for lysosomal targeting in BSF parasites. Contained in these results is compelling evidence for an as-yet-unidentified sorting receptor for trypanopain targeting. Overall, these findings are consistent with abundant published data from our laboratory concerning the roles of p67 targeting signals and VSG GPI anchors in post-Golgi apparatus trafficking, and they lead us to propose stage-specific default pathways for soluble and membrane proteins in trypanosomes: the cell surface and extracellular milieu in PCF trypanosomes and the lysosome in pathogenic BSF trypanosomes. Furthermore, we propose that it is the innate endocytic activities of the two stages—low in PCF trypanosomes and high in BSF trypanosomes—that influence these pathways. This simple model sets the framework for future efforts to define the subtleties of interplay between secretion and endocytosis, processes critical to the pathogenesis associated with this important human parasite.
Acknowledgments
We are grateful to Laura Knoll, UW—Madison, for helpful discussions and critical comments.
This work was supported by United States Public Health Service Grant R01 AI056866 to J.D.B. E. S. Sevova was supported by an NIH Microbial Pathogenesis and Host Responses Training Grant (T32 AI055397) to UW—Madison.
Footnotes
Published ahead of print on 6 July 2009.
REFERENCES
- 1.Alexander, D. L., K. J. Schwartz, A. E. Balber, and J. D. Bangs. 2002. Developmentally regulated trafficking of the lysosomal membrane protein p67 in Trypanosoma brucei. J. Cell Sci. 115:3255-3263. [DOI] [PubMed] [Google Scholar]
- 2.Allen, C. L., D. Goulding, and M. C. Field. 2003. Clathrin-mediated endocytosis is essential in Trypanosoma brucei. EMBO J. 22:4991-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, C. L., D. Liao, W.-L. Chung, and M. C. Field. 2007. Dileucine signal-dependent and AP-1-independent targeting of a lysosomal glycoprotein in Trypanosoma brucei. Mol. Biochem. Parasitol. 156:175-190. [DOI] [PubMed] [Google Scholar]
- 4.Balber, A. E., J. D. Bangs, S. M. Jones, and R. L. Proia. 1979. Inactivation or elimination of potentially trypanolytic, complement-activating immune complexes by pathogenic trypanosomes. Infect. Immun. 24:617-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bangs, J. D., N. Andrews, G. W. Hart, and P. T. Englund. 1986. Posttranslational modification and intracellular transport of a trypanosome variant surface glycoprotein. J. Cell Biol. 103:255-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bangs, J. D., E. M. Brouch, D. M. Ransom, and J. L. Roggy. 1996. A soluble secretory reporter system in Trypanosoma brucei: studies on endoplasmic reticulum targeting. J. Biol. Chem. 271:18387-18393. [DOI] [PubMed] [Google Scholar]
- 7.Bangs, J. D., D. M. Ransom, M. A. McDowell, and E. M. Brouch. 1997. Expression of bloodstream variant surface glycoproteins in procyclic stage Trypanosoma brucei: role of GPI anchors in secretion. EMBO J. 16:4285-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barry, J. D. 1979. Capping of variable antigen on Trypanosoma brucei, and its immunological and biological significance. J. Cell Sci. 37:287-302. [DOI] [PubMed] [Google Scholar]
- 9.Barry, J. D., L. Marcello, L. J. Morrison, A. F. Read, K. Lythgoe, N. Jones, M. Carrington, G. Blandin, U. Böhme, E. C. Hertz-Fowler, H. Renauld, N. El-Sayed, and M. Berriman. 2005. What the genome sequence is revealing about trypansome antigenic variation. Biochem. Soc. Trans. 33:986-989. [DOI] [PubMed] [Google Scholar]
- 10.Bonifacino, J. S., and L. M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395-447. [DOI] [PubMed] [Google Scholar]
- 11.Caffrey, C. R., E. Hansell, K. D. Lucas, L. S. Brinen, A. A. Hernandez, J. Cheng, S. L. Gwalteny, W. R. Roush, Y.-D. Stierhof, M. Bogyo, D. Steverding, and J. H. McKerrow. 2001. Active site mapping, biochemical properties and subcellular localization of rhodesain, the major cysteine protease of Trypanosoma brucei rhodesiense. Mol. Biochem. Parasitol. 118:61-73. [DOI] [PubMed] [Google Scholar]
- 12.Canuel, M., A. Korkidakis, K. Konnyu, and C. R. Morales. 2008. Sortilin mediates the lysosomal targeting of cathepsins D and H. Biochem. Biophys. Res. Commun. 373:292-297. [DOI] [PubMed] [Google Scholar]
- 13.Cooper, A. A., and T. H. Stevens. 1996. Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J. Cell Biol. 133:529-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coppens, I., F. R. Opperdoes, P. J. Courtoy, and P. Baudhin. 1987. Receptor-mediated endocytosis in the bloodstream form of Trypanosoma brucei. J. Protozool. 34:344-349. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham, I. 1977. New culture medium for maintenance of tsetse tissues and growth of trypanosomatids. J. Protozool. 24:325-329. [DOI] [PubMed] [Google Scholar]
- 16.Engstler, M., J. D. Bangs, and M. C. Field. 2006. Intracellular transport systems in trypanosomes: function, evolution and virulence, p. 281-317. In J. D. Barry, J. C. Mottram, R. McCulloch, and A. Acosta-Serrano (ed.), Trypanosomes—after the genome. Horizon Scientific Press, Wymondham, United Kingdom.
- 17.Engstler, M., L. Thilo, F. Weise, C. G. Grünfelder, H. Schwarz, M. Boshart, and P. Overath. 2004. Kinetics of endocytosis and recycling of the GPI-anchored variant surface glycoprotein in Trypanosoma brucei. J. Cell Sci. 117:1105-1115. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson, M. A. J. 1999. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J. Cell Sci. 112:2799-2809. [DOI] [PubMed] [Google Scholar]
- 19.Goodman, O. B., and J. H. Keen. 1995. The α chain of the AP-2 adaptor is a clathrin binding subunit. J. Biol. Chem. 270:23768-23773. [DOI] [PubMed] [Google Scholar]
- 20.Grünfelder, C. G., M. Engstler, F. Wieise, H. Schwarz, Y.-D. Stierhof, M. Boshart, and P. Overath. 2002. Accumulation of a GPI-anchored protein at the cell surface requires sorting at multiple intracellular levels. Traffic 3:547-559. [DOI] [PubMed] [Google Scholar]
- 21.Grünfelder, C. G., M. Engstler, F. Wieise, H. Schwarz, Y.-D. Stierhof, G. W. Morgan, M. C. Field, and P. Overath. 2003. Endocytosis of a glycosylphosphatidylinositol-anchored protein via clatherin-coated vesicles, sorting by default in endosomes and exocytosis via RAB11-positive carriers. Mol. Biol. Cell 14:2029-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruszynski, A. E., A. DeMaster, N. M. Hooper, and J. D. Bangs. 2003. Surface coat remodeling during differentiation of Trypanosoma brucei. J. Biol. Chem. 278:24665-24672. [DOI] [PubMed] [Google Scholar]
- 23.Hirumi, H., and K. Hirumi. 1989. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 75:985-989. [PubMed] [Google Scholar]
- 24.Huete-Perez, J. A., J. C. Engel, J. C. Brinen, J. C. Mottram, and J. H. McKerrow. 1999. Protease trafficking in two primitive eukaryotes is mediated by a prodomain protein motif. J. Biol. Chem. 274:16249-16256. [DOI] [PubMed] [Google Scholar]
- 25.Hung, C.-H., Q. Xugang, P.-T. Lee, and M. G.-S. Lee. 2004. Clathrin-dependent targeting of receptors to the flagellar pocket of procyclic-form Trypanosoma brucei. Eukaryot. Cell 3:1004-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janvier, K., Y. Kato, M. Boehm, J. R. Rose, J. A. Martina, B.-Y. Kim, S. Venkatesan, and J. S. Bonifacino. 2003. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 γ-σ1 and AP-3 δ-σ3 hemicomplexes. J. Cell Biol. 163:1281-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelley, R., D. Alexander, C. Cowan, A. Balber, and J. Bangs. 1999. Molecular cloning of p67, a lysosomal membrane glycoprotein from Trypanosoma brucei. Mol. Biochem. Parasitol. 98:17-28. [DOI] [PubMed] [Google Scholar]
- 28.Kornfeld, S., and I. Mellman. 1989. The biogenesis of lysosomes. Annu. Rev. Cell Biol. 5:483-525. [DOI] [PubMed] [Google Scholar]
- 29.LaCount, D. J., A. E. Gruszynski, P. M. Grandgenett, J. D. Bangs, and J. E. Donelson. 2003. Expression and function of the Trypanosoma brucei major surface protease (GP63) genes. J. Biol. Chem. 278:24658-24664. [DOI] [PubMed] [Google Scholar]
- 30.Langreth, S. G., and A. E. Balber. 1975. Protein uptake and digestion in bloodstream and culture forms of Trypanosoma brucei. J. Protozool. 22:40-53. [DOI] [PubMed] [Google Scholar]
- 31.Mackey, Z. B., T. C. O'Brien, D. C. Greenbaum, R. B. Blank, and J. H. McKerrow. 2004. A cathespin B-like protease is required for host protein degradation in Trypanosoma brucei. J. Biol. Chem. 279:48426-48433. [DOI] [PubMed] [Google Scholar]
- 32.Marcusson, E. G., B. F. Horazdovsky, J. L. Cereghino, E. Gharakhanian, and S. D. Emr. 1994. The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell 77:579-586. [DOI] [PubMed] [Google Scholar]
- 33.McCann, A. K., K. J. Schwartz, and J. D. Bangs. 2008. A determination of the steady state lysosomal pH of bloodstream stage African trypanosomes. Mol. Biochem. Parasitol. 159:146-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDowell, M. A., D. A. Ransom, and J. D. Bangs. 1998. Glycosyl phosphatidylinositol-dependent secretory transport in Trypanosoma brucei. Biochem. J. 335:681-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan, G. W., C. L. Allen, T. R. Jeffries, M. Hollinshead, and M. C. Field. 2001. Developmental and morphologicl regulation of clathrin-mediated endocytosis in Trypanosoma brucei. J. Cell Sci. 114:2605-2615. [DOI] [PubMed] [Google Scholar]
- 36.Nikolskaia, O. V., A. P. de A Lima, Y. V. Kim, J. D. Lonsdale-Eccles, T. Fukuma, J. Scharfstein, and D. J. Grab. 2006. Blood-brain barrier traversal by African trypanosomes requires calcium signaling induced by parasite cysteine protease. J. Clin. Investig. 116:2739-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nolan, D. P., G. Geuskens, and E. Pays. 1999. N-Linked glycans containing linear poly-N-acetyllactosamine as sorting signals in endocytosis in Trypanosoma brucei. Curr. Biol. 9:1169-1172. [DOI] [PubMed] [Google Scholar]
- 38.Peck, R. F., A. M. Shiflett, K. J. Schwartz, A. McCann, S. L. Hajduk, and J. D. Bangs. 2008. The LAMP-like protein p67 plays an essential role in the lysosome of African trypanosomes. Mol. Microbiol. 68:933-946. [DOI] [PubMed] [Google Scholar]
- 39.Poole, B., and S. Ohkuma. 1981. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J. Cell Biol. 90:665-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rapoport, I., Y. C. Chen, P. Cupers, S. E. Shoelson, and T. Kirchhausen. 1998. Dileucine-based sorting signals bind to the β chain of AP-1 at a site distinct and regulated differently frorm the tyrosine-based motif binding site. EMBO J. 17:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson, M. R., and J. S. Bonifacino. 2001. Adaptor-related proteins. Curr. Opin. Cell Biol. 13:444-453. [DOI] [PubMed] [Google Scholar]
- 42.Rodionov, D. G., and O. Bakke. 1998. Medium chains of adaptor complexes AP-1 and AP-2 recognize leucine-sorting signals from the invariant chain. J. Biol. Chem. 273:6005-6008. [DOI] [PubMed] [Google Scholar]
- 43.Shen, S., G. K. Arhin, E. Ullu, and C. Tschudi. 2001. In vivo epitope tagging of Trypanosoma brucei genes using a one step PCR strategy. Mol. Biochem. Parasitol. 113:171-173. [DOI] [PubMed] [Google Scholar]
- 44.Shih, W., A. Gallusser, and T. Kirchhausen. 1995. A clathrin binding site in the hinge region of the β2 chain of mammalian AP-2 complexes. J. Biol. Chem. 270:31083-31090. [DOI] [PubMed] [Google Scholar]
- 45.Sutterwala, S. S., C. H. Creswell, S. Sanyal, A. K. Menon, and J. D. Bangs. 2007. De novo sphingolipid synthesis is essential for viability, but not transport of glycosylphosphatidylinositol-anchored proteins in African trypanosomes. Eukaryot. Cell 6:454-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutterwala, S. S., F.-F. Hsu, E. S. Sevova, K. J. Schwartz, K. Zhang, P. Key, J. Turk, S. M. Beverley, and J. D. Bangs. 2008. Developmentally regulated sphingolipid synthesis in African trypanosomes. Mol. Microbiol. 70:281-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tazeh, N. N., and J. D. Bangs. 2007. Multiple signals regulate trafficking of the lysosomal membrane protein p67 in African trypanosomes. Traffic 8:1007-1017. [DOI] [PubMed] [Google Scholar]
- 48.Triggs, V. P., and J. D. Bangs. 2003. Glycosylphosphatidylinositol-dependent protein trafficking in bloodstream stage Trypanosoma brucei. Eukaryot. Cell 2:76-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, Z., J. C. Morris, M. E. Drew, and P. T. Englund. 2000. Inhibition of Trypanosoma brucei gene expression by RNA interference using an integratable vector with opposing T7 promotors. J. Biol. Chem. 275:40174-40179. [DOI] [PubMed] [Google Scholar]
- 50.Wirtz, E., S. Leal, C. Ochatt, and G. Cross. 1999. A tightly regulated inducible expression system for conditional gene knockouts and dominant-negative genetics in Trypanosoma brucei. Mol. Biochem. Parasitol. 99:89-101. [DOI] [PubMed] [Google Scholar]