Abstract
The supermucoid Pseudomonas aeruginosa strain PDO300Δalg8(pBBR1MCS-5:alg8) showed strongly impaired attachment compared with the respective mucoid or nonmucoid strains and formed a thicker biofilm with large extended mushroom-like microcolonies. Alginate lyase treatment dissolved microcolonies. The data suggested that alginate overproduction impairs attachment but plays a structural role in microcolony formation.
Alginate is an important virulence factor for Pseudomonas aeruginosa, and the conversion of nonmucoid strains to alginate-overproducing mucoid strains early after the infection of cystic fibrosis patients is associated with a decline of pulmonary function and survival rate (11, 13). Alginate functions as extracellular matrix material, enabling the formation of differentiated biofilms in which the diffusion of clinical antibiotics is decreased and the embedded cells are protected against human antibacterial defense mechanisms (9, 12). Although alginate is not required for P. aeruginosa biofilm formation (15), previous studies have provided evidence that it plays a role in the formation of thick and three-dimensional biofilms (5, 9). To further investigate the impact of alginate on attachment and biofilm architecture, we used a recently generated supermucoid strain, PDO300Δalg8(pBBR1MCS-5:alg8) (14). This strain showed about 15-fold alginate overproduction compared to alginate-producing mucoid P. aeruginosa. The gene alg8 encodes the proposed catalytic subunit of alginate polymerase and is essential for alginate biosynthesis (14).
Quantitative analysis of attachment and biofilm formation.
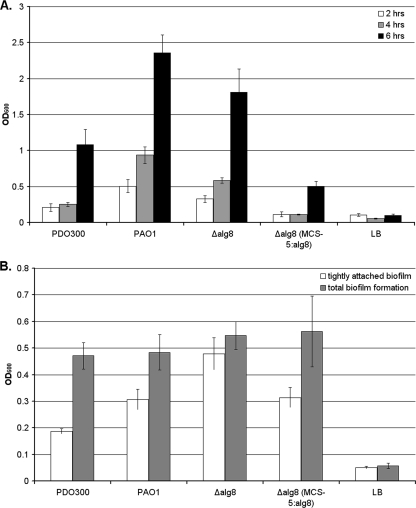
The attachment characteristics of the supermucoid strain PDO300Δalg8(pBBR1MCS-5:alg8) were compared with those of the wild-type strain PAO1 and the mucoid strain PDO300 (an isogenic mucA22 mutant of PAO1) (8) and its alginate-negative isogenic alg8 deletion mutant (14). A modification of the solid-surface assay (SSA) (10) was used to assess attachment in microtiter plates after incubation for 2, 4, and 6 h. Stationary cultures at 37°C in Luria-Bertani medium (containing gentamicin at 300 μg/ml when appropriate) were adjusted to an optical density at 600 nm of 0.05, and 100-μl aliquots were added to one column (8 wells) of each of five replicate 96-well tissue culture plates. After incubation at 37°C for the respective times, nonadherent bacteria were washed off by filling the wells three times with sterile water and then removing the well contents with gentle suction. Plates were then air dried, and adherent bacteria were stained with 100 μl of 0.1% (wt/vol) crystal violet for 20 min at room temperature. The crystal violet was removed by washing as described above and dissolved in 100 μl dimethyl sulfoxide. After 20 min, the absorbance at 595 nm was measured. The data presented here are the averages of results from three independent experiments with eight replicates each. The results showed excellent intra-assay and interassay reproducibility, with minimal background. During the early attachment phase (2 to 4 h), the nonmucoid strains (wild-type strain PAO1 and PDO300Δalg8) showed significantly more attachment than any of the other strains (Fig. 1A). The supermucoid strain PDO300Δalg8(pBBR1MCS-5:alg8) showed the weakest attachment at 2, 4, and 6 h but wild-type levels of attachment and biofilm formation after 4 days (Fig. 1B). Biofilm analysis after stringent (3) or gentle (see description above) washing was used to evaluate the strength of the attachment of the grown biofilm. The difference between the effects of stringent and gentle washing was minimal for the PDO300Δalg8 mutant biofilm, where the extracellular matrix lacked alginate, while the greatest differences were observed in the alginate-rich biofilms of PDO300 and the supermucoid strain (Fig. 1B). These results suggest that alginate interferes with early attachment but subsequently promotes the formation of a thick, although loosely attached, biofilm.
FIG. 1.
SSA analysis of various P. aeruginosa strains. PDO300, P. aeruginosa PDO300; PAO1, P. aeruginosa PAO1; Δalg8, P. aeruginosa PDO300Δalg8; Δalg8(MCS-5:alg8), supermucoid strain; LB, uninoculated Luria-Bertani medium control; OD600, optical density at 600 nm. (A) Differences during early attachment phase; (B) differences between loosely and tightly attached 4-day-old biofilms (adherent biofilms after gentle and stringent washing, respectively). Values and error bars represent the averages and standard deviations, respectively, for eight independent replicates.
Biofilm analysis.
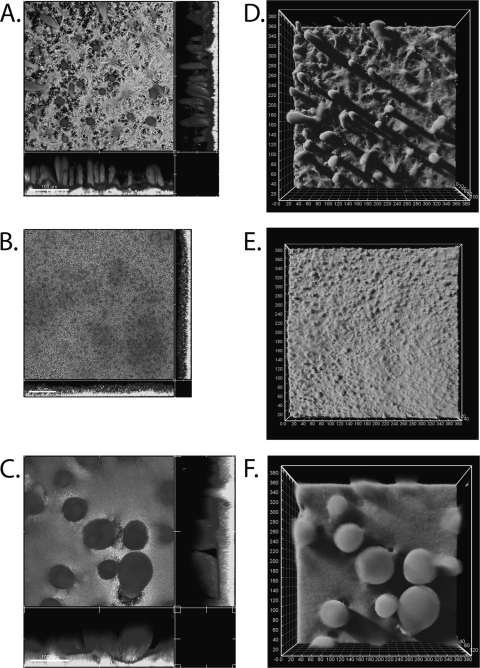
The morphology and architecture of biofilms of strains PDO300(pBBR1MCS-5) and PDO300Δalg8(pBBR1MCS-5) and the supermucoid strain PDO300Δalg8(pBBR1MCS-5:alg8) were analyzed in a continuous-culture flow chamber as described previously (2). After 4 days of growth in Pseudomonas isolation medium (14), biofilms were stained with dye from the LIVE/DEAD BacLight bacterial viability kit (Molecular Probes Inc.) as described previously (2) and observed under a confocal laser scanning microscope (Leica TCS 4D) using the appropriate filters. Image analysis was performed and measurements were compiled using Imaris software (Bitplane Inc.). Strain PDO300(pBBR1MCS-5) showed a mature, fully differentiated biofilm consisting of well-defined finger-like microcolonies ranging in height from 75 to 145 μm with a diameter of 15 to 35 μm. The base layer of the biofilm ranged in thickness from 10 to 35 μm. The volume of this biofilm was 24.30 ± 3.78 μm3/μm2 (Fig. 2A and D). Strain PDO300Δalg8(pBBR1MCS-5) showed a thinner, more uniform biofilm with no apparent microcolonies and a thickness ranging from 15 to 35 μm. The volume of this biofilm was 21.90 ± 5.76 μm3/μm2. Interestingly, the surface of this biofilm was visibly rougher and small clusters of cells were distinguishable, while both PDO300 and the supermucoid strain cells were embedded in the matrix and indistinguishable (Fig. 2A and C). The supermucoid strain PDO300Δalg8(pBBR1MCS-5:alg8) showed the thickest biofilm, ranging from 90 to 145 μm in height and consisting of large (up to 100-μm-diameter) spherical microcolonies. The concentrations of cells appeared to be highest at the bases of the microcolonies and decreased toward the tops of the microcolonies, where the edges became loosely defined and individual cells could be detected. Presumably this region contained high concentrations of alginate, keeping the cells associated with the microcolony. The base layer of the biofilm was also significantly thicker than those of the other biofilms, ranging from 35 to 55 μm. The volume of this supermucoid strain biofilm was 2.4 times that of the mucoid PDO300 strain biofilm, at 57.37 ± 4.34 μm3/μm2 (Fig. 2C and F). Proportions of dead and live cells did not differ among the tested strains (data not shown).
FIG. 2.
P. aeruginosa biofilm formation in a continuous-culture flow cell 4 days postinoculation as analyzed using a confocal laser scanning microscope. (A and D) P. aeruginosa PDO300(pBBR1MCS-5); (B and E) P. aeruginosa PDO300Δalg8(pBBR1MCS-5); (C and F) P. aeruginosa PDO300Δalg8(pBBR1MCS-5:alg8). Panels A, B, and C are sectional views; panels D, E, and F are three-dimensional projections. Images are representative of results from three independent experiments. The magnification is ×400.
Mature biofilms were treated with a 100-μg/ml (1-U/ml) solution of alginate lyase from Flavobacterium sp. by exposure to a laminar flow (0.3 ml min−1). This treatment showed no apparent effect on the PDO300(pBBR1MCS-5) and PDO300Δalg8(pBBR1MCS-5) biofilms, whereas the supermucoid PDO300Δalg8(pBBR1MCS-5:alg8) biofilm showed the large microcolonies dissolving from their tops and cells being released (see Movies S1 to S3 in the supplemental material). The same treatment with DNase or buffer alone did not cause any apparent dissolving or cell release (data not shown).
Discussion.
In this study, the role of alginate in attachment and biofilm formation was studied using a recently genetically engineered supermucoid strain, PDO300Δalg8(pBBR1MCS-5:alg8), which produces 15 times more alginate, when grown as a biofilm, than the already alginate-overproducing mucoid strain PDO300 (14). Exopolysaccharides produced by P. aeruginosa have been shown previously to be involved in attachment and biofilm formation (2, 6, 9, 10). Alginate has been shown to play a role in attachment to surfaces (7). However, the SSA analysis in this study clearly suggested that alginate overproduction impairs attachment to solid surfaces (Fig. 1A), as the mucoid strains showed the weakest attachment whereas the alginate-negative strain and the nonmucoid wild type showed the strongest attachment when assayed 2 to 6 h after inoculation (Fig. 1). Although PAO1 and PDO300Δalg8 are both nonmucoid, it should be noted that they showed some differences in the SSA which may be due to the PDO300 strain's defective mucA22 allele, allowing the unrestricted activity of the AlgU sigma factor and thus promoting the transcription of not only the alginate biosynthesis operon but also other genes (4). Nivens and colleagues (9) provided evidence that alginate contributes to the biofilm architecture. Our results support this hypothesis; the finger-like microcolonies present in the mucoid strain biofilm were completely absent in the alginate-negative mutant biofilm (Fig. 2A, B, D, and E), indicating that alginate is essential for the formation of these biofilm structures. Additionally, it was shown that further increasing the levels of alginate production leads to the formation of extremely large microcolonies, demonstrating the important role this exopolysaccharide has in microcolony formation (Fig. 2C and F).
Treatment of the supermucoid biofilm with alginate lyase resulted in the breakdown of the microcolony structure, but this treatment had little effect on the mucoid and nonmucoid biofilms. This finding confirms that the supermucoid microcolonies were composed of high proportions of alginate. It is unclear why the presumably alginate-containing mucoid biofilm was not dissolved by the alginate lyase treatment in the same way. This outcome may be due to the lower proportion of alginate and, thus, the higher proportion of other biofilm matrix components present in the microcolonies, maintaining the biofilm structure.
The most striking difference found in the biofilm architecture of the supermucoid strain was the extensive formation of large, alginate-dense (as demonstrated by the alginate lyase treatment) microcolonies, at the tops of which individual cells could be detected, suggesting a function for alginate in microcolony structure with an impact on the dispersal of cells from the biofilm as discussed elsewhere (1).
Supplementary Material
Acknowledgments
We are grateful to Palmerston North Medical Research Foundation for funding the capillary flow cell biofilm equipment and to Massey University for the research fund and postdoctoral fellowship. I.D.H. was supported by a Massey University doctoral scholarship.
Footnotes
Published ahead of print on 31 July 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Boyd, A., and A. M. Chakrabarty. 1994. Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 60:2355-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campisano, A., C. Schroeder, M. Schemionek, J. Overhage, and B. H. A. Rehm. 2006. PslD is a secreted protein required for biofilm formation by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 72:3066-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finelli, A., C. V. Gallant, K. Jarvi, and L. L. Burrows. 2003. Use of in-biofilm expression technology to identify genes involved in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 185:2700-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Firoved, A. M., and V. Deretic. 2003. Microarray analysis of global gene expression in mucoid Pseudomonas aeruginosa. J. Bacteriol. 185:1071-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hay, I. D., U. Remminghorst, and B. H. A. Rehm. 2009. MucR, a novel membrane-associated regulator of alginate biosynthesis in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 75:1110-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson, K. D., M. Starkey, S. Kremer, M. R. Parsek, and D. J. Wozniak. 2004. Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J. Bacteriol. 186:4466-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mai, G. T., J. G. McCormack, W. K. Seow, G. B. Pier, L. A. Jackson, and Y. H. Thong. 1993. Inhibition of adherence of mucoid Pseudomonas aeruginosa by alginase, specific monoclonal antibodies, and antibiotics. Infect. Immun. 61:4338-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathee, K., C. Sternberg, O. Ciofu, P. Jensen, J. Campbell, M. Givskov, D. E. Ohman, N. Hoiby, S. Molin, and A. Kharazmi. 1999. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology 145:1349-1357. [DOI] [PubMed] [Google Scholar]
- 9.Nivens, D. E., D. E. Ohman, J. Williams, and M. J. Franklin. 2001. Role of alginate and its O acetylation in formation of Pseudomonas aeruginosa microcolonies and biofilms. J. Bacteriol. 183:1047-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overhage, J., M. Schemionek, J. S. Webb, and B. H. A. Rehm. 2005. Expression of the psl operon in Pseudomonas aeruginosa PAO1 biofilms: PslA performs an essential function in biofilm formation. Appl. Environ. Microbiol. 71:4407-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedersen, S. S., N. Hoiby, F. Espersen, and C. Koch. 1992. Role of alginate in infection with mucoid Pseudomonas aeruginosa in cystic-fibrosis. Thorax 47:6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pier, G. B., F. Coleman, M. Grout, M. Franklin, and D. E. Ohman. 2001. Role of alginate O acetylation in resistance of mucoid Pseudomonas aeruginosa to opsonic phagocytosis. Infect. Immun. 69:1895-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rehm, B. H. A., E. Grabert, J. Hein, and U. K. Winkler. 1994. Antibody response of rabbits and cystic fibrosis patients to an alginate-specific outer membrane protein of a mucoid strain of Pseudomonas aeruginosa. Microb. Pathog. 16:43-51. [DOI] [PubMed] [Google Scholar]
- 14.Remminghorst, U., and B. H. A. Rehm. 2006. In vitro alginate polymerization and the functional role of Alg8 in alginate production by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 72:298-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wozniak, D. J., T. J. Wyckoff, M. Starkey, R. Keyser, P. Azadi, G. A. O'Toole, and M. R. Parsek. 2003. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. USA 100:7907-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.