Abstract
Lactobacillus acidophilus NCFM derivatives containing deletion mutations in the transporter genes LBA0552, LBA1429, LBA1446, and LBA1679 exhibited increased sensitivity to bile. These strains showed unique patterns of sensitivity to a variety of inhibitory compounds, as well as differential accumulations of ciprofloxacin and taurocholate.
Lactobacillus acidophilus NCFM is a probiotic strain that is widely used in yogurt formulations and dietary supplements (17). Analysis of its genomic sequence has facilitated functional characterization of many of its probiotic features (1, 3-6, 10). The global transcriptional response of this strain to bile was previously characterized and showed the induction of 78 genes (12). Among these were two transporters of the major facilitator superfamily (MFS) (LBA1429 and LBA1446) and the permease and ATPase subunits of an ABC transporter (LBA1679 and LBA1680). Each of these genes was annotated as a member of the family of multidrug resistance (MDR) transporters, a class of transporters that can act as a defense mechanism against inhibitory compounds by extruding a wide variety of structurally dissimilar substrates from the cytoplasm, including antibiotics, bile salts, and peptides. MDR transporters can belong to different classes of transporters, including those of the MFS and ABC transporter families (13, 15).
Of the 10 genes most highly induced by bile in L. acidophilus NCFM, two encode MDR transporters (LBA1446 and LBA1679), which suggests that MDR transport systems may be important in achieving bile tolerance in this species. Additionally, these transporters have been shown to play a role in bile tolerance in other species, notably, Listeria monocytogenes and Lactobacillus reuteri (18, 21). This study investigated the role of transporter genes in bile tolerance in L. acidophilus NCFM. It also examined the role of these versatile transporters in tolerance to other compounds whose presence is detrimental to the cell.
Previous microarray analysis of L. acidophilus NCFM (12) indicated the induction of three transporter genes, LBA1429 (MFS transporter), LBA1446 (MFS transporter), and LBA1679 (ABC transporter [permease component]), in the presence of 0.5% oxgall, as well as the slight repression of LBA0552, an MFS transporter also annotated as MDR. BLAST analyses (2) of these proteins indicated that all are widespread among members of the Firmicutes, with similar proteins being found in high-GC gram-positive bacteria. LBA1429, however, was more widespread, with similar proteins present in members of the Bacteroides, the Betaproteobacteria, and the Deltaproteobacteria. LBA0552 and LBA1446 showed sequence similarity to EmrB/QacA family drug resistance transporters in Enterococcus faecalis and L. monocytogenes. This type of transporter was previously shown to be involved in bile efflux and was induced in E. faecalis in the presence of bile (19, 20). Additionally, TBLASTN analysis showed similarity (57% identity) between LBA1446 and lr1265, the L. reuteri protein which was implicated in bile shock survival in this species (2, 21). LBA1429 shows similarity to the quinolone resistance protein GlpT in Bacillus cereus (50% positive results). LBA1679 does not show similarity to any protein encoded by any named gene but shows similarity to other ABC transporter permeases.
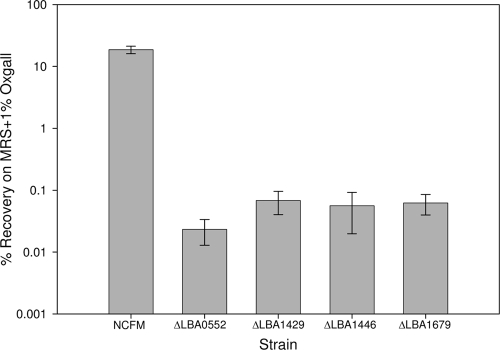
Because LBA1429, LBA1446, and LBA1679 were induced in the presence of bile in L. acidophilus NCFM, in-frame deletion mutant strains were created as described previously (12, 16); the method used included excising internal fragments from each of these genes in order to examine their role in bile tolerance. Lists of the strains used in this study and of the primers used to generate them can be found in Tables S1 and S2 in the supplemental material. Although LBA0552 was not induced by the presence of bile, a deletion mutation was created in this gene because of its strong annotation as an MFS transporter. Survival of early-log-phase cells (optical density at 600 nm [OD600], 0.2 to 0.3) was assayed by plating cells on MRS agar and MRS agar plus 1% (wt/vol) oxgall. While there was no difference in the results with respect to recovery of the strains on MRS plates, all mutant strains, including ΔLBA0552, were more sensitive to oxgall than the wild-type strain (Fig. 1).
FIG. 1.
Recovery of early-log-phase L. acidophilus NCFM strains on MRS agar plates containing 1% (wt/vol) oxgall. Error bars represent the standard deviations of the results of three replicate experiments.
Since transporter proteins of this type typically interact with more than one substrate (13), the mutant strains were examined for growth in a number of compounds, including individual bile salts, detergents, and antibiotics. Early-log-phase cells (OD600, 0.2 to 0.3) were inoculated into 200 μl of MRS broth containing dilutions of the inhibitory compound in 96-well plates. Plates were held anaerobically for 24 h at 37°C, after which the OD600 of each strain was measured. These assays were performed in triplicate, and the results showed the concentration of compound required to inhibit growth of the strain in MRS broth by 50%, as indicated by a 50% decrease in final OD of the cultures (Table 1).
TABLE 1.
Concentration of compound added to MRS broth needed to reduce the OD600 of the culture by 50% compared to the results seen with MRS broth alonea
| Compound | Compound concn range | Inhibitory concnb
|
||||
|---|---|---|---|---|---|---|
| NCFM | ΔLBA0552 | ΔLBA1429 | ΔLBA1446 | ΔLBA1679 | ||
| Antibiotics | ||||||
| Ampicillin | 0.1-0.5 μg/ml | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Chloramphenicol | 1-5 μg/ml | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Ciprofloxacin | 50-90 μg/ml | 70 | 50 | 70 | 60 | 70 |
| Erythromycin | 0.1-0.5 μg/ml | 0.3 | 0.2 | 0.3 | 0.3 | 0.3 |
| Detergents | ||||||
| Sodium dodecyl sulfate | 2-6% | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Triton X-100 | 0.2-0.6% | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 |
| Bile salts | ||||||
| Glycocholate | 0.4-1.2% | 0.8 | 0.6 | 0.4 | 0.4 | 0.4 |
| Taurocholate | 1.0-1.8% | 1.2 | 1.0 | 1.0 | 1.0 | 1.0 |
| Glycodeoxycholate | 0.1-0.5% | 0.4 | 0.4 | 0.4 | 0.3 | 0.4 |
| Taurodeoxycholate | 0.6-1.0% | 0.9 | 1.0 | 0.9 | 0.6 | 0.6 |
Values are representative of the results of three replicate experiments.
Units for antibiotic concentrations are μg/ml; inhibitory concentrations for detergents and bile salts are given as percentages.
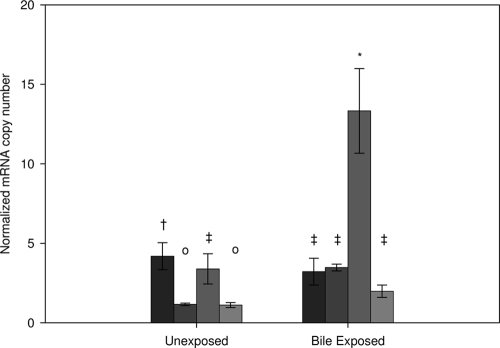
Expression of LBA0552, LBA1429, LBA1446, and LBA1679 in the presence and absence of 0.5% oxgall was analyzed by reverse transcriptase quantitative PCR (RT-QPCR). Expression of LBA1429, LBA1446, and LBA1679 was induced in the presence of bile, and the results with respect to expression of LBA0552 were similar under the two sets of conditions, although the expression level was slightly reduced in bile (Fig. 2). A list of the primers utilized in these experiments can be found in Table S3 in the supplemental material.
FIG. 2.
RTQ-PCR analysis of the expression of (from left to right) LBA0552, LBA1429, LBA1446, and LBA1679 in the presence and absence of 0.5% oxgall. Copy numbers were normalized to the number of mRNA copies of LBA383 (DNA polymerase III [delta subunit]). Error bars represent the standard deviations of the results of four biological replicate experiments. Bars with different symbols represent significant differences, as determined by analysis of variance (P < 0.05).
Sequence analysis, expression data, and mutant phenotype analysis suggest that these four proteins act to transport bile salts and/or antibiotics from the cellular cytoplasm. In order to confirm this activity, assays were conducted to examine the accumulation of the fluoroquinolone antibiotic ciprofloxacin and the bile salt taurocholate in the wild-type and mutant strains (Table 2).
TABLE 2.
Accumulation of ciprofloxacin and taurocholate in L. acidophilus cellsa
| L. acidophilus strain | Compound accumulation
|
|
|---|---|---|
| Ciprofloxacin (ng/OD600 unit) | Taurocholate (pmol/mg total protein) | |
| NCFM | 79.0 ± 8.6 | 3.9 ± 0.17 |
| ΔLBA0552 | 97.0 ± 1.8* | 4.4 ± 0.50 |
| ΔLBA1429 | 74.0 ± 2.0 | 4.4 ± 0.38* |
| ΔLBA1446 | 73.5 ± 10.9 | 4.6 ± 0.70* |
| ΔLBA1679 | 77.3 ± 10.9 | 4.3 ± 0.52 |
Mean values ± standard deviations of the results of three replicate experiments are shown. Values marked with an asterisk are significantly different from L. acidophilus NCFM values, as determined by Student's t test (P < 0.05).
Because of the sensitivity of the ΔLBA0552 mutant to ciprofloxacin relative to the other strains, the accumulation of this antibiotic was assayed by the method of Chapman and Georgopapadakou (7). ΔLBA0552 was shown to accumulate more ciprofloxacin per OD600 unit than the other strains, which correlates with the inhibition of growth that was observed for ΔLBA0552 in the presence of ciprofloxacin. ΔLBA0552 was considerably more sensitive to ciprofloxacin, with sensitivity occurring at a concentration of 50 μg/ml, whereas the rest of the strains did not exhibit sensitivity until a higher concentration (60 to 70 μg/ml) of ciprofloxacin was used. This increased accumulation of ciprofloxacin in ΔLBA0552 correlates with its increased sensitivity to this compound.
Tritium-labeled taurocholate was used to assay accumulation of this bile salt by the use of the method of Sleator et al. (18) with slight modifications. Cells were exposed to 200 pmol of [3H]taurocholate (Perkin Elmer) for 15 min, after which they were collected by centrifugation through silicon oil (70% fluid 550 and 30% fluid 510; Dow Corning). In these assays, the mutant strains accumulated more taurocholate than wild-type NCFM, with strains ΔLBA1429 and ΔLBA1446 accumulating significantly more taurocholate than NCFM, albeit all strains accumulated relatively low amounts of this compound. Compared to other bile salts, taurocholate has a low pKa (1.5) and the ionized form predominates at the pH of the assay (7.0). Since the ionized form of the bile salt is less likely to passively diffuse into the cell, a relatively small amount of this compound was accumulated in all strains. Other studies that have examined the transport of bile salts used deconjugated bile salts with higher pKas (11, 14, 18). Taurocholate was selected for this study because all of the mutant strains were sensitive to it.
Each of the genes examined in this study is annotated as an MDR transporter, and each gene makes a contribution to bile tolerance in L. acidophilus NCFM, while at the same time each exhibits differences in expression and contributes uniquely to resistance to various compounds. Initially, the dramatic bile sensitivity of ΔLBA0552 was surprising, since expression of this gene was previously shown to be slightly repressed in the presence of bile. However, RT-QPCR analysis indicated that this gene is expressed at a level equal to that of the induced expression of LBA1429 and LBA1679. The ΔLBA0552 mutant showed the most sensitivity to antibiotics due to its reduced growth in the presence of erythromycin and ciprofloxacin. This mutant strain also retained significantly more ciprofloxacin than the other strains when exposed to this compound, correlating with its increased sensitivity and demonstrating a role in ciprofloxacin extrusion for this transporter. This strain was also sensitive to the bile salts glycocholate and taurocholate, contributing to its overall bile sensitivity. Taking these data together, the constitutive expression of this gene, along with the wide range of sensitivities exhibited by the mutant strain, suggests that LBA0552 acts as a true MDR transporter, protecting the cell from a number of toxic substrates that could be encountered in its environment.
The considerable induction of LBA1446 in the presence of bile was confirmed by RT-QPCR, and the sensitivity of ΔLBA1446 to bile was demonstrated by loss of viability on bile plates. This strain was sensitive to all bile salts tested and accumulated significantly more taurocholate than the wild-type strain, indicating a role in the extrusion of bile salts for this species. The high level of gene induction, along with the mutant phenotypes, suggests that LBA1446 may be the most critical transporter contributing to bile tolerance in L. acidophilus NCFM.
The ΔLBA1429 and ΔLBA1679 mutants were sensitive to growth on oxgall plates and sensitive to some of the individual bile salts tested. Overall, however, these strains showed sensitivity to the fewest compounds. The ΔLBA1429 mutant showed a slight increase in resistance to the detergent Triton X-100, although the reason for this phenotype is unclear. Despite their being sensitive to fewer compounds than ΔLBA0552 and ΔLBA1446, it is apparent that LBA1429 and LBA1679 also play a critical role in bile tolerance in L. acidophilus NCFM. The redundancy of transporters necessary for bile tolerance in the NCFM genome indicates the importance of this trait in this intestinal species.
There is some concern over the potential for exchange of antibiotic resistance genes between microbes in the gastrointestinal environment. Although the genes examined in this study contributed at minor levels to increased resistance to erythromycin and ampicillin, the levels were not physiologically relevant. The level of resistance of ΔLBA0552 to ciprofloxacin was generally similar to levels previously reported for L. acidophilus strains (8, 9).
Transcriptional analysis has suggested the importance of transporters in the bile tolerance phenotype of L. acidophilus NCFM. The subsequent generation of transporter mutants and the associated phenotypic analyses confirmed the role of these proteins in bile tolerance and indicated that they may transport a wide range of substrates that can be detrimental to the cell.
Supplementary Material
Acknowledgments
This study was supported by the North Carolina Dairy Foundation, Dairy Management, Inc., and Danisco USA, Inc. E.A.P. was supported by a Department of Education Graduate Assistance in Areas of National Need Biotechnology Fellowship and a George H. Hitchings New Investigator Award in Health Sciences.
We gratefully acknowledge helpful discussions and technical advice from E. Durmaz, J. Goh, and S. O'Flaherty.
Footnotes
Published ahead of print on 24 July 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Altermann, E., W. M. Russell, M. A. Azcarate-Peril, R. Barrangou, B. L. Buck, O. McAuliffe, N. Souther, A. Dobson, T. Duong, M. Callanan, S. Lick, A. Hamrick, R. Cano, and T. R. Klaenhammer. 2005. Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc. Natl. Acad. Sci. USA 102:3906-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azcarate-Peril, M. A., E. Altermann, R. L. Hoover-Fitzula, R. J. Cano, and T. R. Klaenhammer. 2004. Identification and inactivation of genetic loci involved with Lactobacillus acidophilus acid tolerance. Appl. Environ. Microbiol. 70:5315-5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azcarate-Peril, M. A., J. M. Bruno-Barcena, H. M. Hassan, and T. R. Klaenhammer. 2006. Transcriptional and functional analysis of oxalyl-coenzyme A (CoA) decarboxylase and formyl-CoA transferase genes from Lactobacillus acidophilus. Appl. Environ. Microbiol. 72:1891-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azcarate-Peril, M. A., O. McAuliffe, E. Altermann, S. Lick, W. M. Russell, and T. R. Klaenhammer. 2005. Microarray analysis of a two-component regulatory system involved in acid resistance and proteolytic activity in Lactobacillus acidophilus. Appl. Environ. Microbiol. 71:5794-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buck, B. L., E. Altermann, T. Svingerud, and T. R. Klaenhammer. 2005. Functional analysis of putative adhesion factors in Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 71:8344-8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman, J. S., and N. H. Georgopapadakou. 1989. Fluorometric assay for fleroxacin uptake by bacterial cells. Antimicrob. Agents Chemother. 33:27-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danielsen, M., and A. Wind. 2003. Susceptibiltiy of Lactobacillus spp. to antimicrobial agents. Int. J. Food Microbiol. 82:1-11. [DOI] [PubMed] [Google Scholar]
- 9.Elkins, C. A., and L. B. Mullis. 2004. Bile-mediated aminoglycoside sensitivity in Lactobacillus species likely results from increased membrane permeability attributable to cholic acid. Appl. Environ. Microbiol. 70:7200-7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klaenhammer, T. R., R. Barrangou, B. L. Buck, M. A. Azcarate-Peril, and E. Altermann. 2005. Genomic features of lactic acid bacteria effecting bioprocessing and health. FEMS Microbiol. Rev. 29:393-409. [DOI] [PubMed] [Google Scholar]
- 11.Mallonee, D., and P. Hylemon. 1996. Sequencing and expression of a gene encoding a bile acid transporter from Eubacterium sp. strain VPI 12708. J. Bacteriol. 178:7053-7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeiler, E. A., M. A. Azcarate-Peril, and T. R. Klaenhammer. 2007. Characterization of a novel bile-inducible operon encoding a two-component regulatory system in Lactobacillus acidophilus. J. Bacteriol. 189:4624-4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piddock, L. J. V. 2006. Multidrug-resistance efflux pumps—not just for resistance. Nat. Rev. Microbiol. 4:629-636. [DOI] [PubMed] [Google Scholar]
- 14.Price, C. E., S. J. Reid, A. J. M. Driessen, and V. R. Abratt. 2006. The Bifidobacterium longum NCIMB 702259T ctr gene codes for a novel cholate transporter. Appl. Environ. Microbiol. 72:923-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Putman, M., H. W. van Veen, and W. N. Konings. 2000. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64:672-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell, W. M., and T. R. Klaenhammer. 2001. Efficient system for directed integration into the Lactobacillus acidophilus and Lactobacillus gasseri chromosomes via homologous recombination. Appl. Environ. Microbiol. 67:4361-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanders, M. E., and T. R. Klaenhammer. 2001. The scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. J. Dairy Sci. 84:319-331. [DOI] [PubMed] [Google Scholar]
- 18.Sleator, R. D., H. H. Wemekamp-Kamphuis, C. G. Gahan, T. Abee, and C. Hill. 2005. A PrfA-regulated bile exclusion system (BilE) is a novel virulence factor in Listeria monocytogenes. Mol. Microbiol. 55:1183-1195. [DOI] [PubMed] [Google Scholar]
- 19.Solheim, M., A. Aakra, H. Vebo, L. Snipen, and I. F. Nes. 2007. Transcriptional responses of Enterococcus faecalis V583 to bovine bile and sodium dodecyl sulfate. Appl. Environ. Microbiol. 73:5767-5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thanassi, D. G., L. W. Cheng, and H. Nikaido. 1997. Active efflux of bile salts by Escherichia coli. J. Bacteriol. 179:2512-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitehead, K., J. Versalovic, S. Roos, and R. A. Britton. 2008. Genomic and genetic characterization of the bile stress response of probiotic Lactobacillus reuteri ATCC 55730. Appl. Environ. Microbiol. 74:1812-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.