Abstract
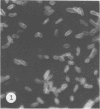
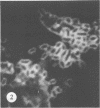
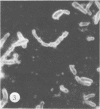
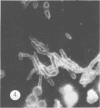
Antisera against seven strains of Bacteroides fragilis subspecies fragilis were produced from dense suspensions of whole cells. These sera exhibited high agglutination titers with homologous antigens. Reciprocal cross-reactions in agglutination tests with each immunizing strain yielded lower titers. Both the indirect and direct fluorescent-antibody techniques were used to evaluate these reagents in the serological identification of 24 defined strains of B. fragilis subspecies fragilis. Subspecies and even strain specificities were noted with particular antisera. A pooled antiserum and conjugate were prepared and studied. Study results showed that specific and high-titered antisera against strains within this subspecies can be produced by the methods described herein and that possibly more than one serotype exists within the seven strains studied. The development of more antibody pools will be necessary to encompass a wider antigenic coverage before the fluorescent-antibody technique can be relied upon altogether for serologically identifying isolates of B. fragilis subspecies fragilis. Test data showed that the indirect method of fluorescent-antibody staining with whole antiserum is an excellent means of identifying strains of this organism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell W. J., Stulberg C. S., Peterson W. D., Jr Somatic and flagellar immunofluorescence of Salmonella. J Bacteriol. 1966 Oct;92(4):1177–1187. doi: 10.1128/jb.92.4.1177-1187.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dack G. M. NON-SPOREFORMING ANAEROBIC BACTERIA OF MEDICAL IMPORTANCE. Bacteriol Rev. 1940 Dec;4(4):227–259. doi: 10.1128/br.4.4.227-259.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELDERING G., EVELAND W. C., KENDRICK P. L. Fluorescent antibody staining and agglutination reactions in Bordetella pertussis cultures. J Bacteriol. 1962 Apr;83:745–749. doi: 10.1128/jb.83.4.745-749.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felner J. M., Dowell V. R., Jr Anaerobic bacterial endocarditis. N Engl J Med. 1970 Nov 26;283(22):1188–1192. doi: 10.1056/NEJM197011262832203. [DOI] [PubMed] [Google Scholar]
- Garcia M. M., Neil D. H., McKay K. A. Application of immunofluorescence to studies on the ecology of Sphaerophorus necrophorus. Appl Microbiol. 1971 May;21(5):809–814. doi: 10.1128/am.21.5.809-814.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin M. H. Fluorescent antibody techniques in the identification of the gram-negative nonsporeforming anaerobes. Health Lab Sci. 1970 Apr;7(2):78–83. [PubMed] [Google Scholar]
- Kasper D. L. The polysaccharide capsule of Bacteroides fragilis subspecies fragilis: immunochemical and morphologic definition. J Infect Dis. 1976 Jan;133(1):79–87. doi: 10.1093/infdis/133.1.79. [DOI] [PubMed] [Google Scholar]
- Kislak J. W. The susceptibility of Bacteroides fragilis to 24 antibiotics. J Infect Dis. 1972 Mar;125(3):295–299. doi: 10.1093/infdis/125.3.295. [DOI] [PubMed] [Google Scholar]
- Lambe D. W., Jr Determination of Bacteroides melaninogenicus serogroups by fluorescent antibody staining. Appl Microbiol. 1974 Oct;28(4):561–567. doi: 10.1128/am.28.4.561-567.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe D. W., Jr, Moroz D. A. Serogrouping of Bacteroides fragilis subsp. fragilis by the agglutination test. J Clin Microbiol. 1976 Jun;3(6):586–592. doi: 10.1128/jcm.3.6.586-592.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., Gardner M., Washington J. A., 2nd In vitro antimicrobial susceptibility of anaerobic bacteria isolated from clinical specimens. Antimicrob Agents Chemother. 1972 Feb;1(2):148–158. doi: 10.1128/aac.1.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOKES E. J. Anaerobes in routine diagnostic cultures. Lancet. 1958 Mar 29;1(7022):668–670. doi: 10.1016/s0140-6736(58)91087-0. [DOI] [PubMed] [Google Scholar]
- Spendlove R. S. Optimal labeling of antibody with fluorescein isothiocyanate. Proc Soc Exp Biol Med. 1966 Jun;122(2):580–583. doi: 10.3181/00379727-122-31196. [DOI] [PubMed] [Google Scholar]
- Stauffer L. R., Hill E. O., Holland J. W., Altemeier W. A. Indirect fluorescent antibody procedure for the rapid detection and identification of Bacteroides and Fusobacterium in clinical specimens. J Clin Microbiol. 1975 Oct;2(4):337–344. doi: 10.1128/jcm.2.4.337-344.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd Evaluation of two commercially available media for detection of bacteremia. Appl Microbiol. 1972 May;23(5):956–959. doi: 10.1128/am.23.5.956-959.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W. R., Martin W. J., Wilkowske C. J., Washington J. A., 2nd Anaerobic bacteremia. Mayo Clin Proc. 1972 Sep;47(9):639–646. [PubMed] [Google Scholar]