Abstract
Yersinia enterocolitica and other Yersinia species, such as Y. pseudotuberculosis, Y. bercovieri, and Y. intermedia, were differentiated using Fourier transform infrared spectroscopy (FT-IR) combined with artificial neural network analysis. A set of well defined Yersinia strains from Switzerland and Germany was used to create a method for FT-IR-based differentiation of Yersinia isolates at the species level. The isolates of Y. enterocolitica were also differentiated by FT-IR into the main biotypes (biotypes 1A, 2, and 4) and serotypes (serotypes O:3, O:5, O:9, and “non-O:3, O:5, and O:9”). For external validation of the constructed methods, independently obtained isolates of different Yersinia species were used. A total of 79.9% of Y. enterocolitica sensu stricto isolates were identified correctly at the species level. The FT-IR analysis allowed the separation of all Y. bercovieri, Y. intermedia, and Y. rohdei strains from Y. enterocolitica, which could not be differentiated by the API 20E test system. The probability for correct biotype identification of Y. enterocolitica isolates was 98.3% (41 externally validated strains). For correct serotype identification, the probability was 92.5% (42 externally validated strains). In addition, the presence or absence of the ail gene, one of the main pathogenicity markers, was demonstrated using FT-IR. The probability for correct identification of isolates concerning the ail gene was 98.5% (51 externally validated strains). This indicates that it is possible to obtain information about genus, species, and in the case of Y. enterocolitica also subspecies type with a single measurement. Furthermore, this is the first example of the identification of specific pathogenicity using FT-IR.
The genus Yersinia belongs to the bacterial family Enterobacteriaceae and encompasses three well-known human pathogens: Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica. Pathogenic strains of Y. enterocolitica cause yersiniosis, an acute enteric disease. In Germany and Switzerland, strains of Y. enterocolitica belong to the most frequently isolated pathogens connected with bacterial gastroenteritis (27, 31). Y. enterocolitica also causes other clinical syndromes, such as enterocolitis, acute mesenteric lymphadenitis, mimicking appendicitis, postinfectious arthritis, and systemic infections (7, 21). It is assumed that the main contamination source is food of animal origin, especially pork meat or raw milk (8, 21, 27). Therefore, the focus of diagnosis for these bacteria as food-borne pathogens includes the examination of food samples in food inspection and veterinary controls of livestock.
The species Y. enterocolitica sensu lato as described by Frederiksen (9) was recently subdivided into several species: Y. enterocolitica sensu stricto, Y. intermedia, Y. frederiksenii, Y. kristensenii, Y. aldovae, Y. mollaretii, Y. rohdei, and Y. bercovieri (20). The identification of Y. enterocolitica sensu stricto by traditional agar plate techniques (ISO standard 10273:2003) is complicated by the fact that on the commonly used selective agar plates, especially the cefsulodin-irgasan-novobiocin (CIN) agar, several unrelated bacteria also grow (1, 20). In addition, some Yersinia strains are inhibited by CIN agar (10). The differentiation of putative Yersinia strains isolated from the CIN agar is additionally impeded because the commonly used commercial identification systems (for example, API 20E or API Rapid 32IDE) do not include all Yersinia strains in their databases and usually misidentify them as Y. enterocolitica (12). Nevertheless, the biochemical test system API 20E is still used as an affordable tool for the identification of Y. enterocolitica. This probably results in a constant misidentification of certain Yersinia species, particularly Y. bercovieri, Y. rohdei, and Y. intermedia, as Y. enterocolitica (1, 12, 15).
Y. enterocolitica sensu stricto comprises pathogenic and nonpathogenic members. The species can be grouped into various biotypes by biochemical tests and independently into different serotypes by immunological tests. Both types are connected with different pathogenic potential. The most common biotype-serotype combinations associated with human diseases were biotype 1B/serotype O:8, 2/O:5,27, 2/O:9, 3/O:3, and 4/O:3 (7). Biotype 1A is deemed to be non- or less pathogenic for humans. Biotype 1B is widespread in the United States and only rarely detected in Europe and Japan (11, 14, 26, 28). Based on different DNA-DNA hybridization values and 16S rRNA gene sequences, it was proposed to name the “American” strains Y. enterocolitica subsp. enterocolitica (19). Biotypes 2 and 4 are often isolated from yersiniosis patients, and biotype 3 seems to be pathogenic but rare (6, 21).
Pathogenic strains of Y. enterocolitica harbor certain virulence factors, such as the plasmid-encoded yadA gene and the chromosomally encoded ail gene (17, 32). In contrast, apathogenic strains of Y. enterocolitica do not contain these two genes. However, the plasmid harboring the yadA gene can be lost under certain cultivation conditions in the laboratory (4). This may lead to false-negative results in any test system based on the presence of this plasmid. Therefore, the ail gene appears to be the best-suited marker for the detection of pathogenic Y. enterocolitica strains. The product of the ail gene is an adhesion and invasion factor (17). Therefore, the detection of the ail gene by PCR is used as an indication of the presence of pathogenic strains of Y. enterocolitica in selective enrichments or isolated pure cultures (33).
Recently, Fourier transform infrared spectroscopy (FT-IR) has been established as a new method for identification of bacteria, yeasts, and other microorganisms (3, 16, 22, 24, 38). This method analyzes the total composition of all components of the cell using infrared spectroscopy (13, 18). The FT-IR method is rapid and reliable and therefore can be easily adapted to routine analysis. Furthermore, there accrue almost no costs for consumables during sample preparation and measurements. The technique offers a wide range of applications for differentiation at the species and subspecies levels. It has already been used for the differentiation of several food-borne pathogens, like Listeria monocytogenes (25), Escherichia coli (13), and Bacillus cereus (23, 29). Recently, promising results were obtained by combination of FT-IR and multivariate methods for data processing, in particular artificial neural networks (ANN) (25, 35).
In the present work, FT-IR combined with ANN analysis was applied for classification of Yersinia strains at the species level and of Y. enterocolitica at the subspecies level. Furthermore, differentiation between pathogenic and apathogenic strains of Y. enterocolitica by FT-IR was attempted.
MATERIALS AND METHODS
Bacterial strains.
A total of 123 Yersinia strains were obtained from human patients, raw retail pork, and pig feces in Switzerland. These strains were previously characterized by API 20E, extended biochemical differentiation as described by Bockemühl and Wong (5) and Wauters et al. (37), and bio- and serotyping (2, 15). In addition, 50 strains from food and veterinary samples which had been isolated at the Chemisches und Veterinäruntersuchungsamt Stuttgart (CVUAS, Germany) were analyzed. A total of eight Yersinia strains were provided by the Chemisches und Veterinäruntersuchungsamt Freiburg, Germany, and Y. enterocolitica SZ5108/01 was allocated by the Institut für Hygiene und Umwelt, Hamburg, Germany. Y. enterocolitica DSM 4780T (DSMZ, Braunschweig, Germany) was used for comparison. Bacterial strains used in this study are listed in Table 1. In addition, 674 well-defined non-Yersinia gram-negative strains belonging to 56 species from 26 genera were involved, including strains of the presumed background flora growing under the same temperature conditions as those indicated in Fig. 1. The Betaproteobacteria group contained 33 isolates (nine species from five genera). The Salmonella group included 359 isolates, and the third group encompassed Gammaproteobacteria (Enterobacteriales [249 isolates/35 species/16 genera], Aeromonadales [3/1/1], Pasteurellales [40/3/3], and Vibrionales [15/5/1]).
TABLE 1.
Strains used in the present study
| Speciesa | No. of isolates from:
|
Total | |||
|---|---|---|---|---|---|
| Pork meat | Pork feces | Human | Other | ||
| Y. enterocoliticad | 12 | 12 | 24 | ||
| Y. enterocolitica | 20 | 21 | 47 | 1 | 89 |
| Y. bercovieri | 11 | 11 | |||
| Y. intermedia group I | 20 | 20 | |||
| Y. rohdei | 2 | 2 | |||
| Y. kristensenii/frederiksenii/intermedia group IId | 8 | 6 | 14 | ||
| Y. pseudotuberculosis | 11b | 11 | |||
| Y. ruckeri | 12c | 12 | |||
Y. bercovieri, Y. intermedia group I, and Y. rohdei were identified as Y. enterocolitica by API 20E and identified earlier by amplified fragment length polymorphism analysis and subsequently by phenotypic analysis (15). The isolates of Y. frederiksenii/intermedia group II showed an API profile distinguished from that of isolates of Y. enterocolitica. These strains were grouped together with Y. kristensenii.
Isolated from several mammalian species.
Isolated from fish.
As determined by API 20E.
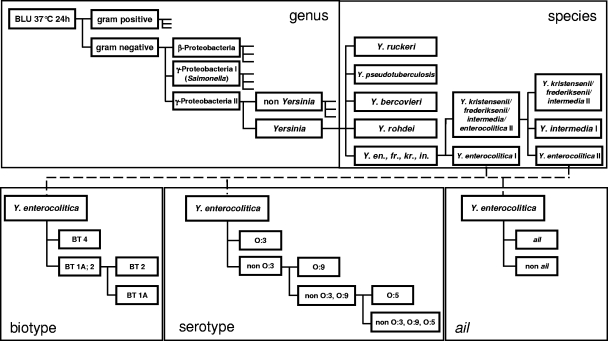
FIG. 1.
Hierarchical ANN classification scheme for the differentiation of Yersinia strains at the genus and species levels and of the bio-, sero-, and ail groups of Y. enterocolitica. “Y. en., fr., kr., in.” indicates Y. enterocolitica/frederiksenii/kristensenii/intermedia.
Biotyping and serotyping.
Biotypes were characterized previously by discriminatory tests (15) and by reference methods at the Swiss National Center for Enteropathogenic Bacteria (NENT, Lucerne, Switzerland). Serotyping was done by seroagglutination with anti-O:3, -O:5, and -O:9 rabbit antisera (SIFIN, Berlin, Germany) (Table 2) .
TABLE 2.
Distribution of the ail gene among various biotype/serotype combinations of Y. enterocolitica and several Y. enterocolitica-like speciesa
| Species | Biotype | Serotype | No. of strains tested | No. of ail-positive strains | % ail-positive strains |
|---|---|---|---|---|---|
| Y. enterocolitica | 1A | Otherb | 34 | 0 | 0 |
| 1A | O:5 | 7 | 0 | 0 | |
| 2 | O:5 | 10 | 10 | 100 | |
| 2 | O:9 | 18 | 18 | 100 | |
| 3 | ND | 1 | 0 | 0 | |
| 4 | O:3 | 19 | 19 | 100 | |
| ND | ND | 24 | 10 | ||
| Y. bercovieri | 11 | 0 | 0 | ||
| Y. intermedia group I | 20 | 0 | 0 | ||
| Y. rohdei | 2 | 0 | 0 | ||
| Y. kristensenii/frederiksenii/intermedia group IIc | 6 | 0 | 0 | ||
| Total | 160 | 57 |
ND, not determined.
Serotypes other than O:3, O:5, or O:9.
As determined by API 20E.
PCR.
Molecular detection of the ail gene was performed using the modified protocol of Thisted Lambertz et al. (33). DNA was extracted from bacterial colonies by thermal lysis. The PCR mixtures (total volume of 25 μl) consisted of 2 μl cell lysate containing bacterial DNA; 0.5 U Taq DNA polymerase in 1× polymerase buffer (Roche, Mannheim, Germany) and 1.5 mM MgCl2; 1 mg/ml bovine serum albumin (Promega, Madison, WI); 100 μM each of dATP, dCTP, dGTP, and dTTP (Eppendorf, Hamburg, Germany); and 0.3 μM each of primers 9A and 10A (Roth, Karlsruhe, Germany) (33). Amplification conditions were as follows: one initial cycle at 92°C for 30 s, 54°C for 1 min, and 72°C for 1 min, followed by three consecutive cycles with a decreasing annealing temperature of 53°C, 52°C, and 51°C, respectively, followed by 30 cycles with a constant annealing temperature of 50°C and a final synthesis step at 72°C for 7 min.
FT-IR.
All isolates were cultivated independently in six to nine replicas for 24 h at 37°C on different blood agar plates (Oxoid, Basingstoke, United Kingdom). Cells of each isolate were harvested with a platinum loop and suspended in 80 μl of deionized water. An aliquot of 35 μl was placed in the sample zone of a zinc selenide optical plate (BrukerOptics GmbH, Ettlingen, Germany) and dried under reduced pressure for 30 min to a homogeneous solid film, which was used directly for FT-IR spectroscopy. One position on the optical plate was free, to provide a reference reading.
The infrared spectra were recorded for each sample in transmission mode between 4,000 and 500 cm−1 with an FT-IR spectrometer (IFS 28/B, BrukerOptics). The FT-IR spectrometer was equipped with a deuterated triglycine sulfate detector coadding 64 scans. The acquisition and first analysis of data were carried out using OPUS software (version 4.2; BrukerOptics).
The data set for strains used for the construction of the differentiation method (six to nine spectra) was divided into two equal parts. The first one was used to create the method (creation set). The second one was used to verify the created method to gain the recovery rates (internal recovery set). An internal validation was performed when fewer than 20 strains of a respective class were available. If there were more strains available, as is necessary for method construction, the residual strains were used for an external validation (24). Results were given as probability for repeated determination, based on the results of the respective internal and external recovery sets.
The differentiation methods were developed with NeuroDeveloper software (Synthon, Heidelberg, Germany), which is based on an ANN. Generally, the second derivatives of the vector-normalized, five-point smoothed spectra of the creation sets in the wave number range of 1,800 to 500 cm−1 were used for analysis of data in the covar mode by use of a significance of 95%. The 80% randomly selected spectra of the creation set were used as the intrinsic “training set” of the developer module. The internal method optimization of wavelength combinations was carried out routinely, with the remaining 20% of the spectra put in the “validation set” (30, 35).
The hierarchical classification at the genus and species levels was integrated in a database of 674 non-Yersinia gram-negative isolates as indicated in Fig. 1. The methods for the subspecies analysis of Y. enterocolitica were performed independently for serotype, biotype, and the ail marker.
RESULTS AND DISCUSSION
Construction of a hierarchically structured method for the identification of members of the genus Yersinia using an artificial neural net.
A collection of 183 Yersinia strains, which had been isolated from different food and animal sources in Germany and Switzerland, was analyzed. These strains had been identified previously by various phenotypic tests (Table 1) and also partially further differentiated by bio- and serotyping. The FT-IR spectra obtained were separated into classes, and an ANN (NeuroDeveloper software) was used to construct a hierarchically structured modulated method (35, 36). The hierarchical model obtained consisted of a top level and various subsequent subclassification levels. If an FT-IR spectrum of an unknown isolate was predicted to belong to one of the nets in the higher levels, this spectrum was projected to the next level to distinguish between the respective classes available in the accordant subnet.
The strains were initially divided into three main classes, which were used as a preliminary filter. The strains from the genus Yersinia belonged to the third group, which was differentiated in a second step down to the genus level (Fig. 1).
For validation, 4,500 FT-IR spectra were obtained from 674 different gram-negative isolates which did not belong to the genus Yersinia and classified by the newly created method. The probability for an incorrect identification of these strains as Yersinia was less than 0.2% at the genus level (data not shown).
The probability for correct identification of Yersinia strains at the genus level was 91.5% (n = 151, external validation), with an error of only 1.5% (Table 3). The difference to 100% was caused by the additional existence of uncertain results.
TABLE 3.
Validation of genus and species classification by FT-IR, given as probability for correct identification of strains (repeated determinations)a
| Organism | nref | All strains
|
External strains
|
||||
|---|---|---|---|---|---|---|---|
| nval | Identification (%)
|
nval | Identification (%)b
|
||||
| Correct | Incorrect | Correct | Incorrect | ||||
| Yersinia (genus level) | 32 | 183 | 92.1 | 1.3 | 151 | 91.5 | 1.5 |
| Yersinia (species level) | 101 | 183 | 77.9 | 2.0 | |||
| Y. enterocolitica | 48 | 113 | 79.9 | 2.6 | 65 | 78.7 | 3.9 |
| Y. ruckeri | 6 | 12 | 73.7 | 1.6 | |||
| Y. pseudotuberculosis | 5 | 11 | 84.3 | 1.4 | |||
| Y. bercovieri | 11 | 11 | 96.7 | 0 | |||
| Y. rohdei | 2 | 2 | 100 | 0 | |||
| Y. intermedia I | 19 | 19 | 45.0 | 1.3 | |||
| Y. kristensenii/frederiksenii/intermedia IIc | 12 | 14 | 89.0 | 1.3 | |||
nref, number of isolates used for reference; nval, number of isolates used for validation. For internal validation, all spectra which had not been included in the reference data set were used. The probability of obtaining uncertain results during repeated determinations is given by the residual to 100%.
For genus-level determination and for identification of Y. enterocolitica, an external validation was applied using all isolates not included in the reference.
As determined by API 20E.
Identification of different Yersinia species by FT-IR plus ANN analysis.
The identification of Yersinia strains at a species level was developed in several steps. First, Y. ruckeri, Y. pseudotuberculosis, Y. bercovieri, and Y. rohdei isolates were separated clearly by the ANN analysis of their FT-IR spectra from the group containing Y. enterocolitica, Y. frederiksenii, Y. kristensenii, and Y. intermedia. For further classification it was helpful to divide Y. enterocolitica isolates into two IR groups, because it facilitates the separation of the remaining subgroup in the following step. Strains of Y. enterocolitica group I (comprises Y. enterocolitica biotype 4) were separated in the second level of the species identification, and finally in the next level, Y. enterocolitica group II could be differentiated from strains of Y. intermedia group I (Fig. 1). These strains of Y. intermedia were identified as Y. enterocolitica by API 20E but identified as Y. intermedia by biochemical analyses (5, 37). In addition to Y. intermedia I, in this step a heterogeneous group of strains was separated from Y. enterocolitica group II. This group was not further differentiated and consisted of isolates of Y. kristensenii, Y. frederiksenii, and Y. intermedia II. These strains could also be differentiated from Y. enterocolitica by biochemical profiles. So finally, the FT-IR/ANN analysis resulted in the differentiation of eight classes at the species level (Fig. 1).
In an external validation, 78.7% (n = 65) of Y. enterocolitica sensu stricto isolates were identified correctly, with an error of only 3.9% (Table 3). All other Yersinia species comprised less than 20 isolates. Therefore, an internal validation was performed, and 75% of these strains were classified correctly.
This demonstrated that the differentiation of Yersinia strains at the species level using FT-IR in combination with ANN analysis has great potential. Thus, for some Yersinia strains, which were misidentified as Yersinia enterocolitica by API 20E, the probability of being correctly classified as Y. intermedia, Y. bercovieri, or Y. rohdei was 65.6%.
Differentiation of Y. enterocolitica isolates at the subspecies level.
A main problem in the identification of enteropathogenic strains of Y. enterocolitica is that only certain groups of Y. enterocolitica sensu stricto appear to be associated with human pathogenicity. Therefore, for practical purposes it is not sufficient to identify Y. enterocolitica sensu stricto; in addition, it is necessary to identify the enteropathogenic strains within this species. This has traditionally been attempted by the classification of biotypes and serotypes, and it has been found that enteropathogenic isolates are found mainly in certain biotype-serotype combinations, although recently some doubts have been raised about the significance of this classification (34).
It has previously been shown that FT-IR provides sufficient resolution power to distinguish microbes even at a subspecies level (24, 25). Therefore, two independent methods were established by use of ANN analysis for classification of Y. enterocolitica isolates at a subspecies level. One method was created to represent biotypes 1A, 2, and 4 of Y. enterocolitica (Fig. 1) using FT-IR-spectra from 47 isolates with well-defined biotypes (Table 4). An external validation used spectra of Y. enterocolitica strains belonging to the same biotypes, which were obtained independently from those taken for method development. In the experiments, 98.3% of the strains (n = 41) were allocated to the correct biotype.
TABLE 4.
External validation of the IR spectral database for subspecies classificationsa
| Biotype
|
Serotype
|
ail
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | nref | nval | Identification (%)
|
Type | nref | nval | Identification (%)
|
ail status | nref | nval | Identification (%)
|
|||
| Correct | Incorrect | Correct | Incorrect | Correct | Incorrect | |||||||||
| 1A | 26 | 15 | 100 | 0 | O:3 | 9 | 10 | 99.7 | 0 | Positive | 31 | 26 | 99.9 | 0 |
| 2 | 12 | 16 | 99.2 | 0 | O:5 | 12 | 7 | 78.3 | 1.8 | Negative | 31 | 25 | 91.8 | 1.3 |
| 4 | 9 | 10 | 94.2 | 0 | O:9 | 6 | 12 | 99.5 | 0 | |||||
| Otherb | 21 | 13 | 88.0 | 8.5 | ||||||||||
| Total | 47 | 41 | 98.3 | 0 | Total | 48 | 42 | 92.5 | 2.9 | Total | 62 | 51 | 98.5 | 0.7 |
nref, number of isolates used for reference; nval, number of isolates used for validation.
Serotypes other than O:3, O:5, or O:9.
For differentiation of serotypes O:3, O:5, and O:9, a second method was created by use of ANN analysis based on 48 serotyped isolates (Table 4). The classification of the serotypes by FT-IR was correct in 92.5% of the cases (n = 42, external validation). Most misidentifications were due to difficulties in IR separation of serotype O:5 from the other class excluding serotypes O:3, O:5, and O:9.
Differentiation of Y. enterocolitica strains containing the ail gene by FT-IR.
Pathogenic strains of Y. enterocolitica harbor the virulence factor which is encoded by the ail gene. Therefore, the presence of the ail gene was analyzed with various Yersinia species by PCR. The gene was detected in 57 strains of Y. enterocolitica which represented the biotype-serotype combinations 2/O:5, 2/O:9, and 4/O:3. These combinations of bio- and serotypes were already shown to encompass the majority of pathogenic Y. enterocolitica strains (7). In contrast, all strains of Y. enterocolitica biotype 1A, Y. bercovieri, Y. intermedia, and Y. rohdei were ail negative. Therefore it was attempted to differentiate ail-positive and ail-negative strains using FT-IR spectra. Thus, a database of 62 isolates of Y. enterocolitica was established, and another method was created by use of ANN analysis for separation of ail-positive and ail-negative strains (Fig. 1). The external validation with 51 Y. enterocolitica isolates resulted in a 98.5% probability for correct classification, with only 0.7% of identifications incorrect (Table 4). All 33 strains of Y. bercovieri, Y. intermedia, or Y. rohdei which could not be separated from Y. enterocolitica by using the API 20E test system were allocated to the non-ail class using this method.
The present study demonstrated that it is possible to identify Yersinia strains at the species level as well as at different subspecies levels using FT-IR in combination with various ANN subnets. The promising external validation of the three different strategies for subspecies differentiation, especially for the differentiation of the pathogenicity marker ail, reflects the usefulness of FT-IR for the risk evaluation of contaminations in food and veterinary samples. The integration of the various classification systems allows, in the case of Y. enterocolitica strains, the simultaneous classification at the species level and at the serotype or biotype level in a single analysis. In addition to the great accuracy, the FT-IR analysis is also significantly faster than the conventional test systems. Thus, it is possible to differentiate Yersinia species by FT-IR in combination with ANN-based methods in 1 day with better results than those from the commonly used API 20E system for determination of presumptive pathogenic strains of Y. enterocolitica.
FT-IR methods are very flexible concerning separation of various bacterial strains at the genus, species, or subspecies level. It is very likely that it is possible to extend the methods established here for other rarely detected Yersinia species or Y. enterocolitica biotypes.
Acknowledgments
We thank M. Contzen for performing the ail PCR.
Footnotes
Published ahead of print on 17 July 2009.
REFERENCES
- 1.Arnold, T., H. Neubauer, K. Nikolaou, U. Roesler, and A. Hensel. 2004. Identification of Yersinia enterocolitica in minced meat: a comparative analysis of API 20E, Yersinia identification kit and a 16S rRNA-based PCR method. J. Vet. Med. B 51:23-27. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner, A., M. Küffer, D. Suter, T. Jemmi, and P. Rohner. 2007. Antimicrobial resistance of Yersinia enterocolitica strains from human patients, pigs and retail pork in Switzerland. Int. J. Food Microbiol. 115:110-114. [DOI] [PubMed] [Google Scholar]
- 3.Beekes, M., P. Lasch, and D. Naumann. 2007. Analytical applications of Fourier transform-infrared (FT-IR) spectroscopy in microbiology and prion research. Vet. Microbiol. 123:305-319. [DOI] [PubMed] [Google Scholar]
- 4.Blais, B. W., and L. M. Phillippe. 1995. Comparative analysis of yadA and ail polymerase chain reaction methods for virulent Yersinia enterocolitica. Food Control 6:211-214. [Google Scholar]
- 5.Bockemuhl, J., and J. D. Wong. 2003. Yersinia, p. 672-683. In P. R. Murray, E. J. Baron, M. A. Pfaller, J. H. Jorgensen, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 6.Bottone, E. J. 1997. Yersinia enterocolitica: the charisma continues. Clin. Microbiol. Rev. 10:257-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottone, E. J. 1999. Yersinia enterocolitica: overview and epidemiologic correlates. Microbes Infect. 1:323-333. [DOI] [PubMed] [Google Scholar]
- 8.Bucher, M., C. Meyer, B. Grötzbach, S. Wacheck, A. Stolle, and M. Fredriksson-Ahomaa. 2008. Epidemiological data on pathogenic Yersinia enterocolitica in southern Germany during 2000-2006. Foodborne Pathog. Dis. 5:273-280. [DOI] [PubMed] [Google Scholar]
- 9.Frederiksen, W. 1964. A study of some Yersinia pseudotuberculosis-like bacteria (Bacterium enterocoliticum and Pasteurella X), p. 103-104. In Proceedings of the XIV Scandinavian Congress of Pathology and Microbiology, Oslo 1964. Norwegian Universities Press, Oslo, Norway.
- 10.Fukushima, H., and M. Gomyoda. 1986. Growth of Yersinia pseudotuberculosis and Yersinia enterocolitica biotype 3B serotype O3 inhibited on Cefsulodin-Irgasan-Novobiocin agar. J. Clin. Microbiol. 24:116-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gierczynski, R., J. Szych, W. Rastawicki, S. Wardak, and M. Jagielski. 2009. Molecular characterization of human clinical isolates of Yersinia enterocolitica bioserotype 1B/O8 in Poland: emergence and dissemination of three highly related clones. J. Clin. Microbiol. 47:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallanvuo, S., J. Peltola, T. Heiskanen, and A. Siitonen. 2006. Simplified phenotypic scheme evaluated by 16S rRNA sequencing for differentiation between Yersinia enterocolitica and Y. enterocolitica-like species. J. Clin. Microbiol. 44:1077-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helm, D., H. Labischinski, G. Schallhehn, and D. Naumann. 1991. Classification and identification of bacteria by Fourier-transformed infrared spectroscopy. J. Gen. Microbiol. 177:69-79. [DOI] [PubMed] [Google Scholar]
- 14.Ichinohe, H., M. Yoshioka, H. Fukushima, S. Kaneko, and T. Maruyama. 1991. First isolation of Yersinia enterocolitica serotype O:8 in Japan. J. Clin. Microbiol. 29:846-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuehni-Boghenbor, K., S. L. W. On, B. Kokotovic, A. Baumgartner, T. M. Wassenaar, M. Wittwer, B. Bissig-Choisat, and J. Frey. 2006. Genotyping of human and porcine Yersinia enterocolitica, Yersinia intermedia, and Yersinia bercovieri strains from Switzerland by amplified fragment length polymorphism analysis. Appl. Environ. Microbiol. 72:4061-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kümmerle, M., S. Scherer, and H. Seiler. 1998. Rapid and reliable identification of food-borne yeasts by Fourier-transform infrared spectroscopy. Appl. Environ. Microbiol. 64:2207-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller, V. L., J. J. Farmer III, W. E. Hill, and S. Falkow. 1989. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect. Immun. 57:121-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naumann, D., D. Helm, and H. Labischinski. 1990. Einsatzmöglichkeiten der FT-IR Spektroskopie in Diagnostik und Epidemiologie: ein neuartiges Verfahren zur Charakterisierung pathogener Bakterien. Bundesgesundheitsblatt 33:387-393. [Google Scholar]
- 19.Neubauer, H., S. Aleksic, A. Hensel, E.-J. Finke, and H. Meyer. 2000. Yersinia enterocolitica 16S rRNA gene types belong to the same genospecies but form three homology groups. Int. J. Med. Microbiol. 290:61-64. [DOI] [PubMed] [Google Scholar]
- 20.Neubauer, H., L. D. Sprague, H. Scholz, and A. Hensel. 2001. The diagnostic of Yersinia enterocolitica infections: a review on classical identification techniques and new molecular methods. Berl. Münch. Tierärztl. Wschr. 114:1-7. [PubMed] [Google Scholar]
- 21.Neubauer, H., L. D. Sprague, H. Scholz, and A. Hensel. 2001. Yersinia enterocolitica infections. 2. Impact on human health. Berl. Münch. Tierärztl. Wochenschr. 114:81-87. [PubMed] [Google Scholar]
- 22.Oberreuter, H., H. Seiler, and S. Scherer. 2002. Identification of coryneform bacteria and related taxa by Fourier transform infrared spectroscopy. Int. J. Syst. Evol. Microbiol. 52:91-100. [DOI] [PubMed] [Google Scholar]
- 23.Rau, J., R. Perz, G. Klittich, and M. Contzen. 2009. Cereulidbildende präsumtive Bacillus cereus aus Lebensmitteln: differenzierende Untersuchungen mittels kultureller Methoden, LC-MS/MS, PCR und Infrarotspektroskopie unter Berücksichtigung thermotoleranter Vertreter. Berl. Munch. Tierarztl. Wochenschr. 122:25-36. [PubMed] [Google Scholar]
- 24.Rebuffo, C. A., J. Schmitt, M. Wenning, F. von Stetten, and S. Scherer. 2006. Reliable and rapid identification of Listeria monocytogenes and Listeria species by artificial neural network-based Fourier transform infrared spectroscopy. Appl. Environ. Microbiol. 72:994-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebuffo-Scheer, C. A., J. Schmitt, and S. Scherer. 2007. Differentiation of Listeria monocytogenes serovars by using artificial neural network analysis of Fourier-transformed infrared spectra. Appl. Environ. Microbiol. 73:1036-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.RKI (Robert Koch Institut). 2002. Fallbericht: Enteritis durch Yersinia enterocolitica serogruppe O:8, biovar 1B. Epidemiol. Bull. 27:221-222. [Google Scholar]
- 27.RKI (Robert Koch Institut). 2006. Ausgewählte Zoonosen im Jahr 2005: durch Lebensmittel übertragbare bakterielle gastrointestinale Infektionen. Epidemiol. Bull. 41:351-356. [Google Scholar]
- 28.Sakai, T., A. Nakayama, M. Hashida, Y. Yamamoto, H. Takebe, and S. Imai. 2005. Outbreak of food poisoning by Yersinia enterocolitica serotype O8 in Nara prefecture: the first case report in Japan. Jpn. J. Infect. Dis. 58:257-258. [PubMed] [Google Scholar]
- 29.Schleif, J., and H. Mietke. 2003. Charakterisierung und Identifizierung von Bacillus cereus-Isolaten aus Futtermitteln und Lebensmitteln mittels Fourier-transform-infrarot (FT-IR) Spektroskopie. Schriftenreihe der Sächsischen Landesanstalt für Landwirtschaft. Heft 3-8. Jahrgang, Dresden, Germany.
- 30.Schmitt, J., M. Beekes, A. Brauer, T. Udelhoven, P. Lasch, and P. Naumann. 2002. Identification of scrapie infection from blood serum by Fourier transform infrared spectroscopy. Anal. Chem. 74:3865-3868. [DOI] [PubMed] [Google Scholar]
- 31.SFOPH (Swiss Federal Office of Public Health). 1998. Infectious diseases—reports of physicians and laboratories. Wkly. Bull. 52:16. [Google Scholar]
- 32.Skurnik, M., and H. Wolf-Watz. 1989. Analysis of the yopA gene encoding the Yop1 virulence determinants of Yersinia spp. Mol. Microbiol. 3:517-529. [DOI] [PubMed] [Google Scholar]
- 33.Thisted Lambertz, S., A. Ballagi-Pordany, A. Nilsson, P. Nordberg, and M.-L. Danielsson-Tham. 1996. A comparison between a PCR method and a conventional culture method for detecting pathogenic Yersinia enterocolitica in food. J. Appl. Bacteriol. 81:303-308. [DOI] [PubMed] [Google Scholar]
- 34.Thoerner, P., C. I. B. Kingombe, K. Bögli-Stuber, B. Bissig-Choisat, T. M. Wassenaar, J. Frey, and T. Jemmi. 2003. PCR detection of virulence genes in Yersinia enterocolitica and Yersinia pseudotuberculosis and investigation of virulence gene distribution. Appl. Environ. Microbiol. 69:1810-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Udelhoven, T., D. Naumann, and J. Schmitt. 2000. Development of a hierarchical classification system with artificial neural networks and FT-IR spectra for the identification of bacteria. Appl. Spectrosc. 54:1471-1479. [Google Scholar]
- 36.Udelhoven, T., M. Novozhilov, and J. Schmitt. 2003. The NeuroDeveloper: a tool for modular neural classification of spectroscopic data. Chemometr. Intell. Lab. 66:219-226. [Google Scholar]
- 37.Wauters, G., M. Janssens, A. G. Steigerwalt, and D. J. Brenner. 1988. Yersinia mollaretii sp. nov. and Yersinia bercovieri sp. nov., formerly called Yersinia enterocolitica biogroups 3A and 3B. Int. J. Syst. Bacteriol. 38:424-429. [Google Scholar]
- 38.Wenning, M., H. Seiler, and S. Scherer. 2002. Fourier-transform infrared microspectroscopy, a novel and rapid tool for identification of yeasts. Appl. Environ. Microbiol. 68:4717-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]