Abstract
The C-type lectin dendritic cell (DC)-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN) is the major receptor on DCs for mycobacteria of the Mycobacterium tuberculosis complex. Recently, we have shown that although the mannose caps of the mycobacterial surface glycolipid lipoarabinomannan (ManLAM) are essential for the binding to DC-SIGN, genetic removal of these caps did not diminish the interaction of whole mycobacteria with DC-SIGN and DCs. Here we investigated the role of the structurally related glycolipids phosphatidylinositol mannosides (PIMs) as possible ligands for DC-SIGN. In a binding assay with both synthetic and natural PIMs, DC-SIGN exhibited a high affinity for hexamannosylated PIM6, which contains terminal α(1→2)-linked mannosyl residues identical to the mannose cap on ManLAM, but not for di- and tetramannosylated PIM2 and PIM4, respectively. To determine the role of PIM6 in the binding of whole mycobacteria to DC-SIGN, a mutant strain of M. bovis bacillus Calmette-Guérin deficient in the production of PIM6 (ΔpimE) was created, as well as a double knockout deficient in the production of both PIM6 and the mannose caps on LAM (ΔpimE ΔcapA). Compared to the wild-type strain, both mutant strains bound similarly well to DC-SIGN and DCs. Furthermore, the wild-type and mutant strains induced comparable levels of interleukin-10 and interleukin-12p40 when used to stimulate DCs. Hence, we conclude that, like ManLAM, PIM6 represents a bona fide DC-SIGN ligand but that other, as-yet-unknown, ligands dominate in the interaction between mycobacteria and DCs.
Tuberculosis (TB), caused by the bacterium Mycobacterium tuberculosis, is a major cause of death world wide and claims approximately 1.7 million lives each year (74). Currently, it is estimated that about one-third of the total world population is latently infected and this number continues to rise. Besides macrophages, which are the primary target for infection by mycobacteria (21), dendritic cells (DCs) are pivotal in determining the nature of the antimycobacterial response (15, 35, 38). Importantly, mycobacteria, including the vaccine strain M. bovis bacillus Calmette-Guérin (BCG), are able to suppress the immune response by interfering with the normal function of DCs (23, 73). Induction of the immunosuppressive cytokine interleukin-10 (IL-10) has a negative effect on the production of the proinflammatory cytokine IL-12 and inhibits a Th1-biased response which is necessary for mycobacterial clearance (14, 45). Responsible for at least part of this immune modulation is the binding of mycobacteria to the C-type lectin DC-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN [CD209]) (26, 69). This lectin has been shown to be the major mycobacterial receptor expressed by DCs (6, 66). DC-SIGN signaling enhances the transcription of the IL-10 gene upon Toll-like receptor (TLR) signaling (30). In addition, the level of DC-SIGN expression on host cells has been related to susceptibility to and the pathogenicity of mycobacterial infections (7, 51, 70).
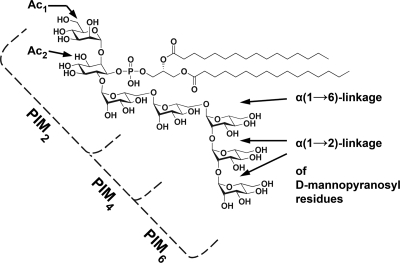
The cell wall components on mycobacteria that dominate the interaction of mycobacteria with DC-SIGN are currently unknown. Several ligands have been studied so far: the mannosylated 19-kDa and 45-kDa antigens, mannose-capped lipoarabinomannan (ManLAM), arabinomannan (AM), lipomannan (LM), and phosphatidylinositol mannosides (PIMs) (27, 46, 59, 67). The 19- and 45-kDa glycoproteins were shown to have an inhibitory effect on mycobacterial binding to DC-SIGN (59). However, M. tuberculosis mutants deficient in these glycoproteins bound DC-SIGN as well as the wild-type strain did (59). For a long time, mycobacterial binding to DC-SIGN was thought to be mediated by ManLAM. Several studies showed that ManLAM, in contrast to AraLAM, which is ManLAM devoid of mannose caps, strongly binds to DC-SIGN and induces the production of IL-10 (27, 46). Yet, these studies were all performed with purified compounds. Strikingly, mutation of capA (Rv1635c), i.e., the gene responsible for the mannose capping of LAM (16), did not attenuate M. bovis BCG in in vitro and in vivo infection models (5). In addition, there was no significant reduction in binding of the mutant strain to DC-SIGN, compared to that of the wild-type strain (5). Therefore, additional DC-SIGN ligands must be present. The third group of ligands, the PIMs, were also shown to be able to bind to DC-SIGN (67). However, as for ManLAM, functional studies with live bacteria assessing the role of PIMs in the Mycobacterium-DC-SIGN interaction have yet to be conducted. PIMs are structurally related to LAM (for a review, see references 13 and 52). Like LAM, PIMs contain a mannosylated glycosylphosphatidylinositol (GPI) anchor, which can be further acylated at two positions (Fig. 1). The number of mannosyl residues can range from one to six, as is denoted by the subscript to PIM (PIM1 to PIM6), with tri- and tetra-acylated PIM2 and PIM6 as the predominant species (48). LAM and PIMs share the initial steps of their biosynthesis (40), with PIM4 as the assumed branching point (11, 43). It is noteworthy that the α(1→2)-linkage, which is only present between the terminal mannosyl residues of the polar PIMs, i.e., PIM5 and PIM6, is identical to the linkage between the terminal mannosyl residues in the mannose caps of LAM (Fig. 1). Previous studies have shown that DC-SIGN recognizes α(1→2)-linked dimannosyl and trimannosyl structures, with the affinity increasing with length (20, 42, 61). Furthermore, PIMs are present in the outermost layer of the mycobacterial cell envelope (54), where they function as adhesins for mycobacterial binding to both macrophages and nonphagocytic cells (36, 47, 71). These observations suggest that PIMs may be involved in the interaction between mycobacteria and DC-SIGN.
FIG. 1.
Structure of synPIM6. PIM6 consist of a GPI anchor mannosylated with six mannosyl residues. natPIMs can be further acylated at the mannosyl group attached to the C-2 position of the inositol ring (Ac1 PIMs) and subsequently at the C-3 position of inositol itself (Ac2 PIMs). PIM4 consists of a GPI anchor with four mannosyl residues in which the three mannosyl residues extending from the C-6 position of the inositol ring are linked in an α(1→6) configuration. The terminal mannosyl residue in PIM4 is substituted with two α(1→2)-linked mannosyl residues to form PIM6. This part of PIM6 is identical to the α(1→2)-linked mannosyl residues in the mannose cap on ManLAM.
In this study, we investigated the role of PIMs in the interaction between mycobacteria and DC-SIGN. We first determined the ability of DC-SIGN to interact with distinct species of PIMs by using both natural and synthetic PIMs (natPIMs and synPIMs, respectively). We found that DC-SIGN exhibited a high affinity for polar PIM6, yet binding to the apolar, less mannosylated PIMs, i.e., PIM2 and PIM4, could not be observed. The next step was to further clarify the role of PIMs in the binding of mycobacteria to DC-SIGN. The enzyme catalyzing the transfer of the fifth mannose to PIM4 to produce PIM5 is PimE (Rv1159) (49); thus, a mutant strain of M. bovis BCG (ΔpimE) that expresses PIM2 and PIM4 but not PIM6 was created. To overcome the possibility of redundancy between ligands for DC-SIGN, we also constructed a double mutant devoid of both PIM6 and the mannose caps on LAM (ΔpimE ΔcapA). Unexpectedly, both mutant strains bound as well to both DC-SIGN and DCs as did the wild-type strain. Furthermore, when the wild-type and mutant strains were used to stimulate DCs, we did not find indications of a different skewing (IL-12p40/IL-10 ratio) of the immune response between the strains. Hence, we conclude that although PIM6 is a ligand for DC-SIGN, its role in the interaction between mycobacteria and DC-SIGN, like that of the mannose caps on LAM, is limited. Binding of the mutant strains to DC-SIGN and DCs could still be blocked by mannan and antibodies against DC-SIGN, suggesting that other, as-yet-unknown, ligands for DC-SIGN must be present on the mycobacterial cell surface.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. bovis BCG strain Copenhagen (8) was grown in Middlebrook 7H9 broth (Difco) with 10% Middlebrook albumin-dextrose-catalase enrichment (BBL) and 0.05% Tween 80 or on Middlebrook 7H10 agar (Difco) with 10% Middlebrook oleic acid-albumin-dextrose-catalase enrichment (BBL) at 37°C. Escherichia coli strain DH5α (32) was grown on Luria-Bertani agar at 37°C. The concentrations of antibiotics used were 25 μg ml−1 kanamycin and 50 and 100 μg ml−1 hygromycin for M. bovis BCG and E. coli, respectively.
Construction of M. bovis BCG mutant strains.
An unmarked deletion mutation in pimE (BCG_1220, homolog of Rv1159) was constructed in both wild-type M. bovis BCG and the M. bovis BCG ΔcapA (BCG_1673c, homolog of Rv1635c) mutant, which produces LAM devoid of mannose caps (5), by using a two-step strategy involving vectors pGOAL19 and p2NIL (57). First, two fragments harboring the up- and downstream regions of pimE, respectively, were amplified by PCR from M. bovis BCG genomic DNA with primer set BCG_1220-LF (5′-CTGGGCAAACTATTGGTGGT-3′) and BCG_1220-LR (5′-CAGGTGATGATCCCGTCTTT-3′), primer set BCG_1220-RF (5′-CGATCGAGGGGTACATGAAG-3′) and BCG_1220-RR (5′-CAGATAGGTCCAGGCGAGTC-3′), and Pfu DNA polymerase (Fermentas). The temperature program was as follows: 94°C for 5 min; 30 cycles of 30 s at 96°C, 30 s at 56.5°C, and 2 min at 72°C; followed by 7 min at 72°C and subsequent cooling to 4°C. PCR products were cloned separately into pCRII-TOPO according to the manufacturer's instructions (Invitrogen). They were cut out by using the EcoRI sites of pCRII-TOPO and ligated to each other in order to obtain the knockout pimE construct in which 771 bp of the gene were deleted. Constructs were checked for the correct orientation of the two fragments with primers BCG_1220-LR and BCG_1220-RF and recloned into pCRII-TOPO. A HinDIII/PstI digest from pCRII-TOPO-ΔpimE was ligated into HinDIII/PstI-digested p2NIL. A hyg-PAg85-lacZ-Phsp60-sacB marker was cassette cut out from the pGOAL19 plasmid with PacI and subsequently cloned into the PacI site of p2NIL-ΔpimE to obtain the final plasmid, which was then electroporated into wild-type and ΔcapA mutant M. bovis BCG (56). Selection of mutants was performed as described previously (57). In short, after single-crossover events, we selected Kanr Hygr Sucs blue colonies which harbor both wild-type pimE and a pimE gene with a deletion mutation, the hygromycin and kanamycin resistance genes (hyg and kan, respectively), lacZ, and sacB. Double-crossover events were induced by selection on plates containing 2% (wt/vol) sucrose. The colonies obtained (Kans Hygs Sucr) either contained a wild-type or a mutant copy of pimE. Potential mutants were screened for the presence or absence of the deletion fragment by PCR with various primer sets.
Complementation of M. bovis BCG mutant strains.
The pimE gene was amplified from genomic BCG DNA with primers BCG_1220-F-BamHI (5′-GGATCCGATGGTTGGCATGTCTCT-3′) and BCG_1220-R (5′-AGGTGGTATCACGGAAAACG-3′) and Pfu DNA polymerase (Fermentas). The following PCR program was used: 95°C for 3 min; 35 cycles of 30 s at 94°C, 30 s at 55°C, and 3 min at 72°C; and 7 min at 72°C. The PCR product obtained was cloned into pCRII-TOPO. This plasmid was digested with BamHI and Eco32I, and the fragment was ligated into BamHI/Eco32I-digested pSMT3-eGFP (1, 33). In the resulting plasmid, pSMT3-pimE, the pimE gene was located behind the heat shock promoter 60 gene. pSMT3-pimE was isolated from E. coli DH5α cells with a QIAprep Miniprep kit (Qiagen) and electroporated into M. bovis BCG ΔpimE as described previously (55). Transformants were selected on hygromycin. For complementation of the M. bovis BCG ΔpimE ΔcapA mutant, pimE was amplified with primers BCG_1220-F-BamHI and BCG_1220-R-BcuI (5′-ACTAGTAGGTGGTATCACGGAAAA-3′) with the following PCR program: 95°C for 5 min; 35 cycles of 30 s at 94°C, 30 s at 58°C, and 3 min at 72°C; and 7 min at 72°C. The PCR product was cloned into pCRII-TOPO. The BamHI/BcuI digest of this plasmid was cloned into the BamHI/BcuI site of plasmid pSMT3-capA (5) to obtain pSMT3-pimE-capA.
MAbs and DC-SIGN-Fc.
Murine monoclonal antibodies (MAbs) of the immunoglobulin M (IgM) class were used. MAb F30-5 recognizes the carbohydrate structure (Ara)6 (for its structure, see reference 42) and binds both ManLAM and AraLAM (5, 41). MAb 55.92.1A1 binds the mannose cap on LAM (5). F183-24 recognizes α(1→2)-linked mannosyl residues as present in the mannose cap on ManLAM and PIM6 (41). The DC-SIGN-Fc construct consists of the extracellular portion of DC-SIGN C terminally fused to a human IgG1-Fc fragment (25). In all experiments involving DC-SIGN-Fc, Tris-buffered saline containing 20 mM Tris, 150 mM NaCl, 1 mM MgCl2, 2 mM CaCl2 (TSM), and 0.5% bovine serum albumin (Fluka) was used instead of phosphate-buffered saline (PBS).
PIMs.
Synthetic 2,6-(di-O-α-d-mannopyranosyl)-1-O-(1,2-di-O-palmitoyl-sn-glycero-3-phosphoryl)-d-myo-inositol (synPIM2) and synthetic 1-O-(1,2-di-O-hexadecanoyl-sn-glycero-3-phosphoryl)-2-O-(α-d-mannopyranosyl)-6-O-[(α-d-mannopyranosyl)-(1→6)-(α-d-mannopyranosyl)-(1→6)-(α-d-mannopyranosyl)]-d-myo-inositol (synPIM4) were prepared by following methods published elsewhere (3). A synthetic sample of 1-O-(1,2-di-O-hexadecanoyl-sn-glycero-3-phosphoryl)-2-O-(α-d-mannopyranosyl)-6-O-[(α-d-mannopyranosyl)-(1→2)-(α-d-mannopyranosyl)-(1→2)-(α-d-mannopyranosyl)-(1→6)-(α-d-mannopyranosyl)-(1→6)-(α-d-mannopyranosyl)]-d-myo-inositol (synPIM6) was synthesized from the starting material 1-O-allyl-3,4,5-tri-O-benzyl-d-myo-inositol. Key synthetic steps included the thioglycosylation of 1-O-allyl-2-O-(2-O-benzoyl-3,4,6-tri-O-benzyl-α-d-mannopyranosyl)-6-O-(2-O-benzoyl-3,4-di-O-benzyl-α-d-mannopyranosyl)-3,4,5-tri-O-benzyl-d-myo-inositol with phenyl (2-O-benzoyl-3,4,6-tri-O-benzyl-α-d-mannopyranosyl)-(1→2)-(3,4,6-tri-O-benzyl-α-d-mannopyranosyl)-(1→2)-(3,4,6-tri-O-benzyl-α-d-mannopyranosyl)-(1→6)-2-O-benzoyl-3,4-di-O-benzyl-1-thio-α-d-mannopyranoside in 76% yield and introduction of the diacylglycerol moiety by phosphoramidite coupling methodology. synPIM6 analyzed for C77H138O43P; high-resolution mass spectrometry-electrospray ionization [M-H]− found, 1,781.8358. synPIM6 was further characterized by treatment with hydrazine to afford dPIM6; 1H NMR (500 MHz, D2O) δ 5.30 (br s, 1H), 5.20 (br s, 1H), 5.13 (br s, 1H), 5.12 (br s, 1H), 5.06 (br s, 1H), 4.91 (br s, 1H), 4.35 (br s, 1H), 4.23 to 3.59 (m, 45H), 3.39 (t, J = 9.1 Hz, 1H). 13C NMR (125 MHz, D2O, selected data) δ 103.1, 102.6, 102.2, 101.6, 100.5, 99.2. High-resolution mass spectrometry-electrospray ionization [M-H]−; calculated for C45H78O41P, 1,305.3756; found, 1,305.3738.
The natPIMs (natPIM1,2 and natPIM6) were purified from M. tuberculosis H37Rv and contained a mixture of differently acylated PIMs. The natPIMs were a gift from Colorado State University.
SDS-PAGE and immunoblotting.
M. bovis BCG was grown until mid to late log phase and disrupted with a Beadbeater (BioSpec). Supernatants were equalized for protein content, as quantified by the bicinchoninic acid protein assay kit according to the manufacturer's instructions (Pierce). Cell lysates were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in glycine buffer for immunostaining with MAb F30-5 or 55.92.1A1 or to 12% SDS-PAGE in Tricine buffer for incubation with MAb F183-24 or DC-SIGN-Fc (44). After transfer onto a polyvinylidene difluoride membrane (Millipore) (68), the membrane was incubated in blocking reagents (Boehringer), followed by incubation with the MAbs. After washing, incubation with peroxidase-labeled goat-anti-mouse IgM (American Qualex) and 0.5% normal goat serum followed. Membranes probed with DC-SIGN-Fc were blocked with 1% bovine serum albumin (Fluka), and goat anti-human IgG-peroxidase (Jackson Laboratories) in 0.5% normal goat serum was used as the secondary antibody (5, 6). Membranes were developed with a mixture of 4-chloro-1-naphthol (Bio-Rad) and 3,3′-diaminobenzidine.4HCl (Sigma-Aldrich).
DC-SIGN-Fc adhesion assay.
synPIMs and natPIMs were used to coat enzyme-linked immunosorbent assay (ELISA) plates (Nunc 96-well PolySorp) by adding methanol-dissolved PIMs (1 or 10 μg per well) and overnight evaporation to dryness at room temperature. Titration of DC-SIGN-Fc (starting from 1.25 μg ml−1) was performed with the protocol as described for immunoblotting with o-phenylenediamine dihydrochloride (Sigma-Aldrich) as the coloring reagent (27). To verify proper coating, ELISA plates were coated with various concentrations of PIMs and probed with MAb HA-1A, which recognizes hydrophobic structures (34). HA-1A bound to the less mannosylated PIMs as well as to PIM6. The median staining ratios (measured by absorption at 490 nm) in nine data sets derived from three independent experiments were 0.9 for synPIM2/synPIM6, 1.1 for synPIM4/synPIM6, and 0.9 for natPIM1,2/natPIM6.
Two-dimensional (2D) thin-layer chromatography (TLC) for polar lipid analysis.
Cells were harvested by centrifugation and washed once with PBS, and small-scale lipid extraction was performed to afford polar lipids as described previously (5, 10). The extracts were resuspended in CHCl3-CH3OH (2:1), and crude lipids were applied to the corners of aluminum-backed TLC plates (Merck). The plates were developed in solvent system E for polar lipid extracts (10). TLC was run once in each direction with CHCl3-CH3OH-H2O (60:30:6) in the first direction and CHCl3-acetic acid-CH3OH-H2O (40:25:3:6) in the second. Lipids were visualized by staining with α-naphthol and brief charring at 100°C until lipids appeared, which could then be compared to known standards (10).
Cells.
Human peripheral blood mononuclear cells were isolated from buffy coats (Sanquin Blood Bank, Amsterdam, The Netherlands) by centrifugation on a Ficoll-Paque gradient (GE Healthcare), followed by selection for CD14+ monocytes with anti-CD14 MAb-coupled magnetic beads and the Midi-MACS system (Miltenyi Biotech) (30). Fresh monocytes were cultured in the presence of IL-4 (500 U ml−1) and granulocyte-macrophage colony-stimulating factor (800 U ml−1; PeproTech) to produce immature monocyte-derived DCs (MoDCs) (62), which were used for further experiments on day 6 after isolation. MoDCs, Raji cells, and transfected Raji cells expressing DC-SIGN (Raji + DC-SIGN cells) (24, 75) were cultured in RPMI 1640 medium (Gibco) supplemented with 10% fetal calf serum (Gibco), 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin at 37°C in a 5% CO2 atmosphere.
Binding of DC-SIGN to PIMs and M. bovis BCG as determined by flow cytometry.
synPIMs and natPIMs were used to coat 1.0-μm fluorescent beads (Polysciences no. 17154) by adding 50 μg PIMs to 1 ml 0.1 M carbonate-bicarbonate buffer (pH 9.6) containing 1.4 × 109 beads in presiliconized tubes (Sigma-Aldrich). After 1 h of rotating at 37°C, beads were collected by centrifugation and incubated for another hour in 1 ml 5% human serum albumin (HSA; Sigma-Aldrich) in TSM and washed in 0.5% HSA in TSM (65).
M. bovis BCG cultures were grown until mid-log phase, concentrated, and incubated with 1 mg ml−1 fluorescein isothiocyanate (Sigma-Aldrich) for 15 min at room temperature, after which the suspensions were washed and declumped by passage through a 5-μm filter (Millipore).
Fluorescence of the different bead and mycobacterial suspensions was quantified with a FLUOstar-Galaxy microplate reader (BMG). Beads were added to human cells at a ratio of 50:1, and mycobacteria were added at a multiplicity of infection (MOI) of 0.5, 2, or 8. After incubation for 45 min at 37°C in the absence or presence of 2 mg ml−1 mannan (Sigma-Aldrich), the percentage of fluorescent cells was determined with a FACScan analytic flow cytometer (Becton Dickinson) and analyzed with the manufacturer's software (CellQuest version 3.1f).
Phagocytosis.
MoDCs and mycobacteria were cocultured at a MOI of 1 to 2 (in triplicate in each independent experiment) for 3 h at 37°C, after which amikacin (Sigma) was added to a concentration of 200 μg ml−1 for 2 h of incubation at 37°C. The MoDCs were then washed three times in PBS and finally lysed with 1% Triton X-100 (Sigma) in PBS for 20 min. Serial dilutions of the inoculum and the released mycobacteria were made in PBS-0.05% Tween 80 and plated on 7H10 agar. CFU were counted after 4 weeks (1).
Cytokine induction.
MoDCs and mycobacteria were cocultured for 24 h at 37°C in the absence or presence of 10 ng ml−1 lipopolysaccharide (LPS) from Salmonella enterica serotype Abortusequi (Sigma-Aldrich). IL-10 and IL-12p40 concentrations in supernatants were analyzed with an ELISA against human IL-10 and IL-12p40 according to the manufacturer's instructions (BioSource).
Statistical analysis.
All values were expressed as means of triplicates ± standard deviation. The unpaired Student t test was used to identify differences between groups. A P value of <0.05 was considered statistically significant.
RESULTS
DC-SIGN has a higher affinity for PIM6 than for less mannosylated PIMs.
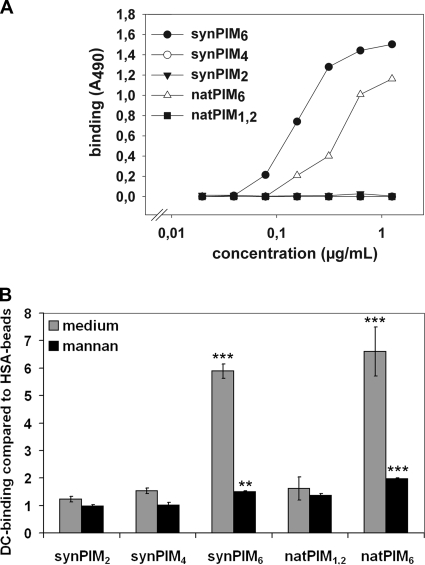
DC-SIGN exhibits a high affinity for α(1→2)-linked mannosyl residues (42). As α(1→2)-linked mannosyl residues are present in polar PIMs, i.e., PIM6, but absent in apolar PIMs, i.e., PIM4, PIM2, and PIM1 (Fig. 1), we set out to investigate the affinity of DC-SIGN for different species of PIMs with two independent assays. First, individual PIM compounds (purified natPIMs and synPIMs) were used to coat ELISA plates and probed with a DC-SIGN-Fc construct. As shown in Fig. 2A, DC-SIGN-Fc strongly bound to both synPIM6 and natPIM6 but not to synPIM2, synPIM4, and natPIM1,2 (a mixture of PIM1 and PIM2), suggesting that DC-SIGN-Fc specifically recognized PIM6. To determine whether native, membrane-localized DC-SIGN exhibited a similar specificity, fluorescent polystyrene beads were coated with the different species of PIMs and assayed for the capacity to bind to MoDCs (Fig. 2B) and Raji cells expressing DC-SIGN (see Fig. S1 in the supplemental material). Consistent with the results for DC-SIGN-Fc, only beads coated with PIM6 (both natural and synthetic), compared to control beads coated with HSA, showed significantly increased binding. The interaction could be blocked by the addition of mannan (Fig. 2B) and by DC-SIGN-blocking antibody AZN-D1 (see Fig. S2 in the supplemental material), confirming that binding was dependent on DC-SIGN.
FIG. 2.
Affinity of DC-SIGN(-Fc) for different species of PIMs (the degree of mannosylation is indicated by subscripts). (A) PIMs were used to coat ELISA plates; this was followed by titration with DC-SIGN-Fc. One representative experiment out of five (synPIMs) or three (natPIMs) is shown. (B) PIMs were used to coat fluorescent beads and analyzed for binding to MoDCs by flow cytometry. Indicated are the means of triplicates and the standard deviations. One representative experiment out of three (synPIMs) or two (natPIMs) is shown. This experiment was also repeated five and two times for the synPIMs and natPIMs, respectively, with Raji + DC-SIGN cells (see Fig. S1 in the supplemental material). ***, P < 0.0005; **, P < 0.005; *, P < 0.05 (compared to control HSA-beads).
Construction of an M. bovis BCG pimE mutant deficient in polar PIM production.
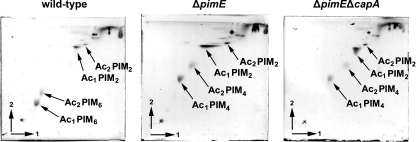
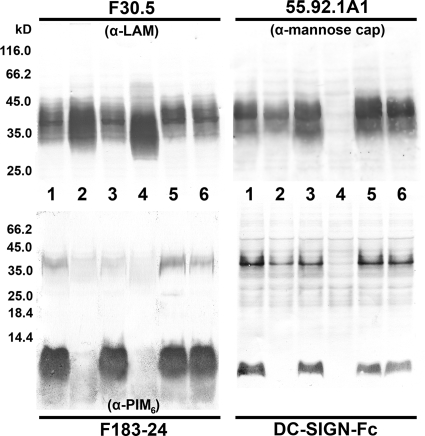
As demonstrated above, DC-SIGN specifically bound to PIM6. To study the role of PIM6 in the interaction between DC-SIGN and whole mycobacteria, our next goal was to create a mutant strain deficient in the production of PIM6. Recently, it was demonstrated that pimE encodes the mannosyltransferase responsible for the elongation of PIM4 to PIM5 and that mutation of the gene abolished the production of polar PIMs (49). Hence, we created a pimE knockout of M. bovis BCG. As PIM6 may show redundancy with ManLAM in binding to DC-SIGN, we also constructed a double knockout deficient in both PIM6 and the mannose caps on LAM (ΔpimE ΔcapA). First, the composition of PIMs in the cell wall of the wild-type and mutant strains was analyzed by 2D TLC. As shown in Fig. 3, all of the strains produced PIM2, yet PIM6 was only present in the wild-type strain. In contrast, the mutant strains accumulated PIM4, which is consistent with the report on the mutation of the homologous gene in M. smegmatis mc2155 (49). Next, whole-cell lysates were probed by SDS-PAGE and immunoblotting with MAb F183-24, which recognizes PIM6 (A. H. J. Kolk, unpublished data) (Fig. 4, lower left panel). In the low-molecular-weight part of the blot (<14 kDa), which is the region harboring the PIMs (39, 59), F183-24 bound to PIMs from wild-type M. bovis BCG and the complemented strains (Fig. 4, lanes 1 and 6 and lanes 3 and 5, respectively) but not to PIMs from the ΔpimE and ΔpimE ΔcapA mutant strains (lanes 2 and 4, respectively). As expected, since the LAM/PIM biosynthesis pathway bifurcates after PIM4 (11, 43), the mutant strains still produced LAM, as indicated by the retained interaction with anti-LAM MAb F30.5 (Fig. 4, upper left panel). M. bovis BCG ΔpimE ΔcapA only produced capless AraLAM, as shown by the lack of staining with cap-specific MAb 55.92.1A1 (Fig. 4, upper right panel, lane 4). Compared to wild-type M. bovis BCG, no additional alterations of the lipid composition in the mutant strains were observed (see Fig. S7 in the supplemental material).
FIG. 3.
2D TLC lipid profile of wild-type and ΔpimE and ΔpimE ΔcapA mutant M. bovis BCG with solvent system E for polar lipid extracts (10). Ac1 PIM2, Ac1 PIM4, and Ac1 PIM6 are triacylated PIM2, PIM4, and PIM6, respectively; Ac2 PIM2, Ac2 PIM4, and Ac2 PIM6 are tetra-acylated PIM2, PIM4, and PIM6, respectively.
FIG. 4.
SDS-PAGE and immunoblotting. M. bovis BCG cell lysates were subjected to glycine-SDS-PAGE (upper panels) or Tricine-SDS-PAGE (lower panels), transferred to polyvinylidene difluoride membrane, and probed with MAbs F30-5 (anti-AraLAM), 55.92.1A1 (anti-ManLAM), and F183-24 (anti-PIM6) and DC-SIGN-Fc. The position of LAM on the blot is between 35 and 45 kDa, and that of the PIMs is below 14 kDa. Lanes 1 and 6, wild-type M. bovis BCG; lane 2, M. bovis BCG ΔpimE; lane 3, M. bovis BCG ΔpimE complemented with pSMT3-pimE; lane 4, M. bovis BCG ΔpimE ΔcapA; lane 5, M. bovis BCG ΔpimE ΔcapA complemented with pSMT3-pimE-capA.
Binding of DC-SIGN-Fc to LAM and PIMs from wild-type M. bovis BCG and the ΔpimE and ΔpimE ΔcapA mutant strains.
To evaluate the consequences of the pimE and capA mutations for the recognition of LAM and PIMs by DC-SIGN-Fc, whole-cell lysates from the wild-type and mutant strains were analyzed by Tricine-SDS-PAGE, followed by immunoblotting with DC-SIGN-Fc. As shown in Fig. 4 (lower right panel), DC-SIGN-Fc did not show binding at the position of PIMs for both the ΔpimE and ΔpimE ΔcapA mutant strains (lanes 2 and 4, respectively). This binding pattern is similar to the immunostaining with F183-24 (Fig. 4, lower left panel, lanes 2 and 4, respectively), which confirms the selective affinity of DC-SIGN-Fc for α(1→2)-interlinked mannosyl residues present in PIM6. Loss of DC-SIGN-Fc binding to LAM without the mannose cap (ΔcapA) has been demonstrated in previous studies (5, 27) and was also observed at the position of LAM in the cell lysate from M. bovis BCG ΔpimE ΔcapA (Fig. 4, lane 4). Hence, in the M. bovis BCG ΔpimE ΔcapA mutant, two ligands for DC-SIGN were absent. Complementation of the mutant strains restored the binding of DC-SIGN-Fc to both LAM and the PIMs (Fig. 4, lanes 3 and 5, respectively).
Binding to DC-SIGN and phagocytic uptake of wild-type M. bovis BCG and the ΔpimE and ΔpimE ΔcapA mutant strains.
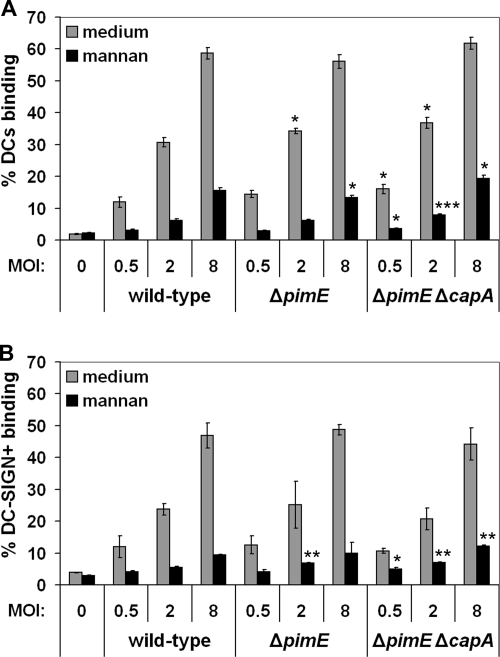
To determine the effect of the pimE and capA mutations on the binding of whole mycobacteria to DC-SIGN-expressing cells, the wild-type and mutant strains were tested for the ability to bind MoDCs by flow cytometry. As shown in Fig. 5A, all three strains bound similarly well at all three of the MOIs tested. To test if a reduction in binding to DC-SIGN was possibly compensated for by other receptors on the MoDCs, the same assay was performed with Raji cells expressing DC-SIGN. As indicated in Fig. 5B, the M. bovis BCG ΔpimE and ΔpimE ΔcapA mutants showed a cell-binding pattern similar to that of the wild-type strain. Furthermore, the binding to MoDCs could be blocked by the addition of mannan (Fig. 5A) and by DC-SIGN-blocking antibody AZN-D1 (see Fig. S4 in the supplemental material), confirming that the interaction of the mutant strains with MoDCs was still dependent on DC-SIGN. Although variation in binding was observed in some experiments, binding of the mutant strains was never significantly lower. In contrast, in three out of six experiments, a 1.5-fold increase in the binding of the mutant strains could be observed (data not shown). These data, together with the observation that the interactions could still be blocked by mannan, suggest that even in the ΔpimE ΔcapA double knockout, additional ligands for DC-SIGN were present. Next, the wild-type and mutant strains were assayed for phagocytic uptake by MoDCs after 3 h of coculturing (see Fig. S5 in the supplemental material). A high variation in uptake efficiency was observed between the different donors. Nevertheless, the M. bovis BCG ΔpimE ΔcapA mutant strain showed a consistent increase in phagocytic uptake compared to that of the wild-type strain in five of the six experiments (see Fig. S5 in the supplemental material). The median of the ratios of the uptake of M. bovis BCG ΔpimE ΔcapA to the uptake of wild-type M. bovis BCG was 2.2, which is in line with the increased binding of the mutant strain that was observed in the earlier binding studies. The data from the CFU count of the M. bovis BCG ΔpimE mutant strain were less consistent. For two donors, the bacterial uptake was increased, whereas for the third donor it was lower (see Fig. S5 in the supplemental material). Hence, drawing conclusions for this mutant strain was not possible.
FIG. 5.
Binding assay with (A) MoDCs and (B) Raji cells expressing DC-SIGN. Cells were incubated with wild-type M. bovis BCG or with the ΔpimE and ΔpimE ΔcapA mutants at a MOI of 0.5, 2, or 8 for 45 min in the presence or absence of mannan. The percentage of the cell population binding M. bovis BCG was determined by flow cytometry. Shown are means of triplicates and the standard deviations. For binding to both MoDCs and Raji + DC-SIGN cells, one representative experiment out of three is shown. See Fig. S3 in the supplemental material for the control binding assay with wild-type Raji cells. ***, P < 0.0005; **, P < 0.005; *, P < 0.05 (compared to wild-type M. bovis BCG at equal MOIs).
Wild-type M. bovis BCG and the ΔpimE and ΔpimE ΔcapA mutant strains induce similar cytokine profiles in MoDCs.
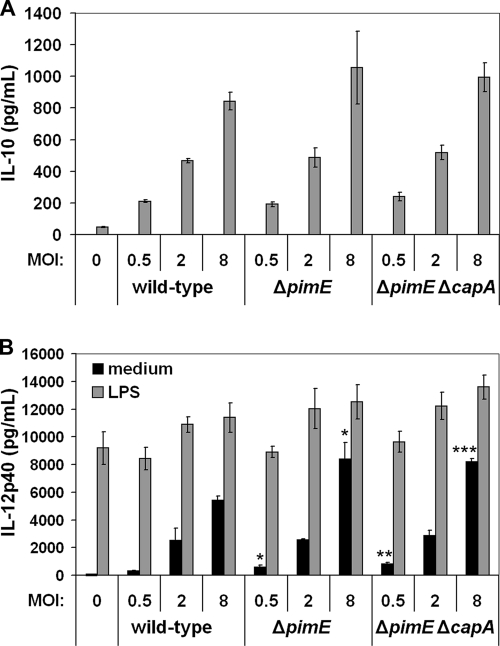
DC-SIGN ligation interferes with TLR signaling and enhances transcription of IL-10 (27, 30). In addition, it has been shown that purified ManLAM inhibits the production of IL-12 by human DCs (53). Hence, removal of DC-SIGN ligands may induce an altered cytokine secretion profile in Mycobacterium-stimulated DCs. To determine the effect of the pimE and capA mutations on the ability to induce cytokine secretion in MoDCs, MoDCs (both immature and LPS primed) were stimulated with the wild-type and mutant bacteria, after which the amounts of secreted IL-10 and IL-12p40 were determined. Although the Mycobacterium-induced cytokine secretion varied among the five different donors tested, no consistent differences within a single donor were observed. Nevertheless, in some experiments, such as the one shown in Fig. 6, significant differences at certain MOIs could be observed. In general, the mutant strains induced more IL-12p40 and IL-10 (see Fig. S9 in the supplemental material). Of note, in all five of the donors tested, both immature and LPS-primed MoDCs produced IL-12p40 following stimulation. In contrast, IL-10 secretion was only observed in LPS-primed MoDCs. Since bioactive IL-12 consists of two subunits, i.e., IL-12p40 and IL-12p35, with the p40 subunit also being present in other IL-12 family members, we also determined the amount of secreted IL-12p70. Although a difference in absolute cytokine production was observed, with the amount of secreted IL-12p40 being higher than that of IL-12p70 by a factor 10 to 100, depending on the donor, their relative levels of induction were similar (see Fig. S8 in the supplemental material). The ratios of IL-12p40/IL-10 induced by M. bovis BCG ΔpimE to IL-12p40/IL-10 induced by wild-type M. bovis BCG and of IL-12p40/IL-10 induced by M. bovis BCG ΔpimE ΔcapA to IL-12p40/IL-10 induced by wild-type M. bovis BCG were calculated for LPS-primed MoDCs from four different donors (one donor did not produce IL-10). These ratios ranged from 0.66 to 1.33 (22 triplicate data sets), with a median of 1.01. Hence, we concluded that the IL-12p40/IL-10 ratios of the two mutant strains were identical to that of the wild-type strain, meaning that the absence of PIM6, either alone or in combination with missing mannose caps on LAM, did not alter the IL-12p40/IL-10 balance.
FIG. 6.
Induction of IL-10 (A) and IL-12p40 (B) secretion in MoDCs by M. bovis BCG. MoDCs were cocultured with mycobacteria for 24 h in the absence or presence of LPS, after which cytokine secretion was measured by ELISA. Shown are means of triplicates and the standard deviations of one representative experiment (five were conducted). IL-10 secretion by non-LPS-activated MoDCs upon infection with BCG was below the limit of detection (<20 pg ml−1). ***, P < 0.0005; **, P < 0.005; *, P < 0.05 (compared to wild-type M. bovis BCG at equal MOIs).
DISCUSSION
In this study, we demonstrated that the degree of mannosylation, in particular, the presence of α(1→2)-linked mannosyl residues in polar PIM6 (Fig. 1), determines the interaction of PIMs with DC-SIGN. DC-SIGN showed a high affinity for PIM6 but not for less mannosylated PIMs, including PIM4 (Fig. 2). Yet, a mutant strain deficient in the production of polar PIMs bound as well to DC-SIGN as did wild-type M. bovis BCG (Fig. 5). Even a double mutant which was also devoid of mannose caps on LAM did not bind less to DC-SIGN and DCs. Binding of the mutant strains could still be blocked by mannan, indicating that additional DC-SIGN ligands were present.
It has been suggested that mycobacteria target DC-SIGN to escape immune surveillance (26, 69). However, studies investigating single nucleotide polymorphisms in the promoter region of DC-SIGN have shown a reduced risk of TB associated with both downregulation (70) and upregulation (7) of DC-SIGN expression or even no association with susceptibility to TB at all (9, 29). A study with mice lacking SIGNR1—one of the murine homologs of DC-SIGN—showed that these mice had stronger T-helper 1 responses to M. tuberculosis and a reduced level of IL-10 (72). However, no differences in bacterial load and mouse survival rates could be detected (72). In a recent publication on human DC-SIGN transgenic mice, a decreased pathology and prolonged survival during mycobacterial infection have been reported. In this case, no differences in IL-10 secretion could be detected (64). It should be taken into account that the murine DC-SIGN system is different from the human one in both expression and specificity and it is not known yet which homolog is functionally most similar to the human DC-SIGN version (58, 60). Hence, many questions regarding the functional consequences of the interaction between mycobacteria and DC-SIGN still exist (51).
Mycobacterial strains that show a reduced binding to DC-SIGN will help to elucidate the role of DC-SIGN in mycobac-terial pathogenesis. In principle, such strains can be developed by deleting the major DC-SIGN ligand(s) present. In contrast to what has long been thought, deletion of the mannose cap on LAM did not abrogate the DC-SIGN-mycobacterial interaction (5). Polar PIMs, i.e., PIM5 and PIM6, have been proposed before as possible ligands for DC-SIGN (67). Although it was shown that the degree of PIM mannosylation did not determine its binding to DC-SIGN (67), we decided to reconsider the case for two separate reasons. First, DC-SIGN shows an unambiguous high affinity of for α(1→2)-interlinked mannosyl residues and this affinity increases with the number of mannosyl residues present, suggesting that DC-SIGN, in theory, should display an increased affinity toward higher-order PIMs (20, 42), and second, previous studies on PIMs were only performed with purified or synthetic compounds and not with whole mycobacteria.
In two independent assay systems with (i) ELISA plates coated with different species of PIMs and probing with DC-SIGN-Fc and (ii) PIM-coated beads used in binding studies with DC-SIGN expressing cells, we observed a clear difference in the affinity of DC-SIGN for the individual species of PIMs (Fig. 2); only PIM6 was recognized by DC-SIGN. Even at high concentrations (10 μg per well with ELISA) or at high bead-to-cell ratios (100:1; data not shown), we did not detect significant binding of DC-SIGN to synPIM2, synPIM4, or natPIM1,2. Whether the degree and nature of acylation of the PIMs played a role in their binding to DC-SIGN was not directly assessed. However, these data suggest that this is not important because the synPIMs, which were all diacylated with identical fatty acids and were single discrete species, displayed binding properties similar to those of the analogous natPIMs, which contained mixtures of mono- to tetra-acylated PIMs. Moreover, natPIM6 and synPIM6 both showed a high affinity for DC-SIGN, indicating that differences in affinity are directly related to the degree of mannosylation. Strikingly, our results are different from those reported by Torrelles et al. (67). In that study, it was demonstrated that all PIMs, irrespective of their degree of mannosylation, showed similar binding to DC-SIGN. We have no ready explanation for the difference in outcome; however, we do note that the magnitude of the binding of PIMs to DC-SIGN reported in the study by Torrelles et al. was very small for all of the species tested and thus lacked specificity. Our data are more in agreement with a recent study by Boonyarattanakalin and coworkers (12). This study on the chemical synthesis of PIMs showed a high affinity of DC-SIGN for the polar PIMs, i.e., PIM5 and PIM6, and low or no affinity for the less mannosylated PIMs, i.e., PIM2 and PIM4, respectively, in a fluorescence scanning assay of a PIM microarray.
The M. bovis BCG ΔpimE and ΔpimE ΔcapA mutant strains were created to investigate the role of polar PIMs in the interaction between DC-SIGN and whole mycobacteria. Although polar PIMs have been reported to be essential for cell wall integrity and mycobacterial growth (31), no differences in growth between the mutant and parent strains were observed (see Fig. S6 in the supplemental material), except for a higher tendency of the mutant strains to clump in older cultures (>1.5 weeks) at high optical densities at 600 nm (data not shown). Interestingly, the M. bovis BCG ΔpimE and ΔpimE ΔcapA mutant strains accumulated PIM4, a feature which was not observed in wild-type M. bovis BCG. This confirms that in wild-type M. bovis BCG, PIM4 is an intermediate in polar PIM biosynthesis (28).
The M. bovis BCG ΔpimE and ΔpimE ΔcapA mutant strains did not show apparent phenotypic changes other than the altered production of PIM6 and the mannose caps on LAM. Since DC-SIGN specifically bound PIM6 in vitro (Fig. 2), we hypothesized that deletion of PIM6 may reduce mycobacterial binding to cells expressing DC-SIGN. To test this, we first compared the wild-type and mutant bacteria for binding to MoDCs. The results indicated that both the M. bovis BCG ΔpimE and ΔpimE ΔcapA mutant strains bound as well to MoDCs as did the wild-type strain (Fig. 5A). To exclude the possibility that other receptors on MoDCs masked a reduced affinity of DC-SIGN for the mutant strains, binding of the wild-type and mutant strains to Raji cells expressing DC-SIGN was also assessed. Also in this case, no reduction and in some cases even a small increase in the binding of the mutant strains compared to that of the wild-type strain was observed (Fig. 5B). This latter finding could be due to a less hydrophobic cell surface of the mutant strains, as was reported for an M. smegmatis ΔpimE mutant (49), since the increased binding was also observed for wild-type Raji cells, which do not express DC-SIGN (see Fig. S3 in the supplemental material). Binding of the mutant strains to MoDCs could still be blocked by mannan (Fig. 5), as well as by DC-SIGN-blocking antibody AZN-D1 (see Fig. S4 in the supplemental material). Therefore, we conclude that the absence of PIM5 and PIM6 in the mutant strains did not alter receptor usage for MoDCs: DC-SIGN was still the major receptor.
Finally, wild-type M. bovis BCG and the ΔpimE and ΔpimE ΔcapA mutant strains were assayed for the ability to induce IL-12p40 and IL-10 secretion by MoDCs. The balance between IL-10 and IL-12 is important for shaping the host immune response, and alterations in it may thus influence the outcome of mycobacterial infections (4, 37, 50). Binding to DC-SIGN enhances the transcription of the IL-10 gene upon TLR signaling (30). Furthermore, PIMs have been reported to suppress allergic airway disease, which is associated with increased production of IL-10 (2, 63). However, PIM1 and PIM2 were used in the latter two studies (2, 63) and IL-10 induction in this case likely involves a different pathway (22). In our experiments, in which LPS-primed MoDCs were cocultured with wild-type M. bovis BCG or the ΔpimE and ΔpimE ΔcapA mutant strains, no decrease in IL-10 secretion by MoDCs incubated with the mutant strains compared to wild-type M. bovis BCG was found (Fig. 6A). Instead, there was a tendency toward higher cytokine (IL-10, IL-12p40, and IL-12p70) induction by the mutant strains compared to that by wild-type M. bovis BCG (see Fig. S8 and S9 in the supplemental material). However, because the mutant strains induced higher production of both anti-inflammatory IL-10 and proinflammatory IL-12p40, the increased cytokine production is probably not biologically significant. Importantly, it should be noted that IL-10 induction by mycobacteria alone could not be detected. Only after costimulation with LPS did M. bovis BCG induce the secretion of IL-10. Since mycobacteria do not contain LPS, although other TLR4 ligands may be present (18), it will be important to investigate DC-SIGN-dependent IL-10 induction also with other classes of TLR ligands (particularly TLR2).
In summary, we have shown that although DC-SIGN has a high affinity for PIM6, this glycolipid does not dictate the Mycobacterium-DC-SIGN interaction. This finding demonstrates, like our previous study (5), that the interaction of mycobacteria with DC-SIGN is much more complex than initially anticipated. Furthermore, it demonstrates that studies with purified compounds should be interpreted with great care. In addition, our study shows that mycobacteria do not express one or two but probably many more ligands for DC-SIGN. As shown in Fig. 4 (lower right panel), DC-SIGN-Fc recognized, besides ManLAM and PIM6, many additional bands (lane 4). These bands most likely represent mannosylated proteins, which are known to be expressed by mycobacteria (19). For at least the 45-kDa glycoprotein, it has been reported that the mannobiose and mannotriose units present are all α(1→2) linked and thus reminiscent of the mannose cap on ManLAM (17). If these proteins are indeed involved in the binding of mycobacteria to DC-SIGN, creation of mutant strains displaying reduced binding will be a huge task to perform. It will therefore be of great importance to evaluate the role of mannosylated proteins in the binding of mycobacteria to DC-SIGN.
Supplementary Material
Acknowledgments
B.J.A. acknowledges support for R.U. and J.G. from the EU (FP6 grant ImmunovacTB 37388). The mycobacterial glycolipids (natPIMs) were a gift from J. Belisle, Colorado State University, Fort Collins, and the National Institutes of Health, Bethesda, MD (contract NO1 AI-75320).
We are grateful to Arend Kolk (Royal Tropical Institute, Amsterdam, The Netherlands) for providing MAbs F30-5 and F183-24. Without the generosity of Theo Geijtenbeek, Yvette van Kooyk, and Jeroen den Dunnen (Molecular Cell Biology and Immunology, VU University Medical Center, Amsterdam, The Netherlands) in providing the DC-SIGN-Fc construct, Raji (+ DC-SIGN) cells, and technical assistance, this study would not have been possible. We appreciate very much the cooperation of Peter van der Ley (Netherlands Vaccine Institute, Bilthoven, The Netherlands) on this project. We thank Arun Mishra (School of Biosciences, University of Birmingham) and Marion Sparrius (Medical Microbiology, VU University Medical Center, Amsterdam, The Netherlands) for technical assistance and Wilbert Bitter (Medical Microbiology, VU University Medical Center, Amsterdam, The Netherlands) for helpful advice.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 3 August 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Abdallah, A. M., T. Verboom, F. Hannes, M. Safi, M. Strong, D. Eisenberg, R. J. Musters, C. M. Vandenbroucke-Grauls, B. J. Appelmelk, J. Luirink, and W. Bitter. 2006. A specific secretion system mediates PPE41 transport in pathogenic mycobacteria. Mol. Microbiol. 62:667-679. [DOI] [PubMed] [Google Scholar]
- 2.Ainge, G. D., J. Hudson, D. S. Larsen, G. F. Painter, G. S. Gill, and J. L. Harper. 2006. Phosphatidylinositol mannosides: synthesis and suppression of allergic airway disease. Bioorg. Med. Chem. 14:5632-5642. [DOI] [PubMed] [Google Scholar]
- 3.Ainge, G. D., N. A. Parlane, M. Denis, C. M. Hayman, D. S. Larsen, and G. F. Painter. 2006. Phosphatidylinositol mannosides: synthesis and adjuvant properties of phosphatidylinositol di- and tetramannosides. Bioorg. Med. Chem. 14:7615-7624. [DOI] [PubMed] [Google Scholar]
- 4.Altare, F., A. Durandy, D. Lammas, J. F. Emile, S. Lamhamedi, F. Le Deist, P. Drysdale, E. Jouanguy, R. Doffinger, F. Bernaudin, O. Jeppsson, J. A. Gollob, E. Meinl, A. W. Segal, A. Fischer, D. Kumararatne, and J. L. Casanova. 1998. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280:1432-1435. [DOI] [PubMed] [Google Scholar]
- 5.Appelmelk, B. J., J. den Dunnen, N. N. Driessen, R. Ummels, M. Pak, J. Nigou, G. Larrouy-Maumus, S. S. Gurcha, F. Movahedzadeh, J. Geurtsen, E. J. Brown, M. M. Eysink Smeets, G. S. Besra, P. T. Willemsen, T. L. Lowary, Y. van Kooyk, J. J. Maaskant, N. G. Stoker, P. van der Ley, G. Puzo, C. M. Vandenbroucke-Grauls, C. W. Wieland, T. van der Poll, T. B. Geijtenbeek, A. M. van der Sar, and W. Bitter. 2008. The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium-host interaction. Cell. Microbiol. 10:930-944. [DOI] [PubMed] [Google Scholar]
- 6.Appelmelk, B. J., I. van Die, S. J. van Vliet, C. M. J. E. Vandenbroucke-Grauls, T. B. H. Geijtenbeek, and Y. van Kooyk. 2003. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 170:1635-1639. [DOI] [PubMed] [Google Scholar]
- 7.Barreiro, L. B., O. Neyrolles, C. L. Babb, L. Tailleux, H. Quach, K. McElreavey, P. D. van Helden, E. G. Hoal, B. Gicquel, and L. Quintana-Murci. 2006. Promoter variation in the DC-SIGN-encoding gene CD209 is associated with tuberculosis. PLoS Med. 3:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Ali, M., L. B. Barreiro, A. Chabbou, R. Haltiti, E. Braham, O. Neyrolles, K. Dellagi, B. Gicquel, L. Quintana-Murci, and M. R. Barbouche. 2007. Promoter and neck region length variation of DC-SIGN is not associated with susceptibility to tuberculosis in Tunisian patients. Hum. Immunol. 68:908-912. [DOI] [PubMed] [Google Scholar]
- 10.Besra, G. S. 1998. Preparation of cell-wall fractions from mycobacteria, p. 91-107. In T. Parish and N. G. Stoker (ed.), Methods in molecular biology: mycobacteria protocols. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 11.Besra, G. S., C. B. Morehouse, C. M. Rittner, C. J. Waechter, and P. J. Brennan. 1997. Biosynthesis of mycobacterial lipoarabinomannan. J. Biol. Chem. 272:18460-18466. [DOI] [PubMed] [Google Scholar]
- 12.Boonyarattanakalin, S., X. Liu, M. Michieletti, B. Lepenies, and P. H. Seeberger. 2008. Chemical synthesis of all phosphatidylinositol mannoside (PIM) glycans from Mycobacterium tuberculosis. J. Am. Chem. Soc. 130:16791-16799. [DOI] [PubMed] [Google Scholar]
- 13.Briken, V., S. A. Porcelli, G. S. Besra, and L. Kremer. 2004. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol. Microbiol. 53:391-403. [DOI] [PubMed] [Google Scholar]
- 14.Demangel, C., P. Bertolino, and W. J. Britton. 2002. Autocrine IL-10 impairs dendritic cell (DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur. J. Immunol. 32:994-1002. [DOI] [PubMed] [Google Scholar]
- 15.Demangel, C., and W. J. Britton. 2000. Interaction of dendritic cells with mycobacteria: where the action starts. Immunol. Cell Biol. 78:318-324. [DOI] [PubMed] [Google Scholar]
- 16.Dinadayala, P., D. Kaur, S. Berg, A. G. Amin, V. D. Vissa, D. Chatterjee, P. J. Brennan, and D. C. Crick. 2006. Genetic basis for the synthesis of the immunomodulatory mannose caps of lipoarabinomannan in Mycobacterium tuberculosis. J. Biol. Chem. 281:20027-20035. [DOI] [PubMed] [Google Scholar]
- 17.Dobos, K. M., K. H. Khoo, K. M. Swiderek, P. J. Brennan, and J. T. Belisle. 1996. Definition of the full extent of glycosylation of the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. J. Bacteriol. 178:2498-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doz, E., S. Rose, J. Nigou, M. Gilleron, G. Puzo, F. Erard, B. Ryffel, and V. F. J. Quesniaux. 2007. Acylation determines the Toll-like receptor (TLR)-dependent positive versus TLR2-, mannose receptor-, and SIGNR1-independent negative regulation of pro-inflammatory cytokines by mycobacterial lipomannan. J. Biol. Chem. 282:26014-26025. [DOI] [PubMed] [Google Scholar]
- 19.Espitia, C., and R. Mancilla. 1989. Identification, isolation and partial characterization of Mycobacterium tuberculosis glycoprotein antigens. Clin. Exp. Immunol. 77:378-383. [PMC free article] [PubMed] [Google Scholar]
- 20.Feinberg, H., R. Castelli, K. Drickamer, P. H. Seeberger, and W. I. Weis. 2007. Multiple modes of binding enhance the affinity of DC-SIGN for high mannose N-linked glycans found on viral glycoproteins. J. Biol. Chem. 282:4202-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenton, M. J., and M. W. Vermeulen. 1996. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect. Immun. 64:683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagliardi, M. C., R. Teloni, F. Giannoni, M. Pardini, V. Sargentini, L. Brunori, L. Fattorini, and R. Nisini. 2005. Mycobacterium bovis bacillus Calmette-Guérin infects DC-SIGN− dendritic cell and causes the inhibition of IL-12 and the enhancement of IL-10 production. J. Leukoc. Biol. 78:106-113. [DOI] [PubMed] [Google Scholar]
- 23.Gagliardi, M. C., R. Teloni, S. Mariotti, E. Iona, M. Pardini, L. Fattorini, G. Orefici, and R. Nisini. 2004. Bacillus Calmette-Guérin shares with virulent Mycobacterium tuberculosis the capacity to subvert monocyte differentiation into dendritic cell: implication for its efficacy as a vaccine preventing tuberculosis. Vaccine 22:3848-3857. [DOI] [PubMed] [Google Scholar]
- 24.Geijtenbeek, T. B. H., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. F. van Duijnhoven, J. Middel, I. L. M. H. Cornelissen, H. S. L. M. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 25.Geijtenbeek, T. B. H., G. C. F. van Duijnhoven, S. J. van Vliet, E. Krieger, G. Vriend, C. G. Figdor, and Y. van Kooyk. 2002. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J. Biol. Chem. 277:11314-11320. [DOI] [PubMed] [Google Scholar]
- 26.Geijtenbeek, T. B. H., and Y. van Kooyk. 2003. Pathogens target DC-SIGN to influence their fate—DC-SIGN functions as a pathogen receptor with broad specificity. APMIS 111:698-714. [DOI] [PubMed] [Google Scholar]
- 27.Geijtenbeek, T. B. H., S. J. van Vliet, E. A. Koppel, M. Sanchez-Hernandez, C. M. J. E. Vandenbroucke-Grauls, B. Appelmelk, and Y. van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilleron, M., C. Ronet, M. Mempel, B. Monsarrat, G. Gachelin, and G. Puzo. 2001. Acylation state of the phosphatidylinositol mannosides from Mycobacterium bovis bacillus Calmette-Guérin and ability to induce granuloma and recruit natural killer T cells. J. Biol. Chem. 276:34896-34904. [DOI] [PubMed] [Google Scholar]
- 29.Gómez, L. M., J. M. Anaya, E. Sierra-Filardi, J. Cadena, A. Corbi, and J. Martin. 2006. Analysis of DC-SIGN (CD209) functional variants in patients with tuberculosis. Hum. Immunol. 67:808-811. [DOI] [PubMed] [Google Scholar]
- 30.Gringhuis, S. I., J. den Dunnen, M. Litjens, B. V. Hof, Y. van Kooyk, and T. B. H. Geijtenbeek. 2007. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-κB. Immunity 26:605-616. [DOI] [PubMed] [Google Scholar]
- 31.Haites, R. E., Y. S. Morita, M. J. McConville, and H. B. Jacobe. 2005. Function of phosphatidylinositol in mycobacteria. J. Biol. Chem. 280:10981-10987. [DOI] [PubMed] [Google Scholar]
- 32.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 33.Hayward, C. M., P. O'Gaora, D. B. Young, G. E. Griffin, J. Thole, T. R. Hirst, L. R. Castello-Branco, and D. J. Lewis. 1999. Construction and murine immunogenicity of recombinant bacille Calmette-Guérin vaccines expressing the B subunit of Escherichia coli heat labile enterotoxin. Vaccine 17:1272-1281. [DOI] [PubMed] [Google Scholar]
- 34.Helmerhorst, E. J., J. J. Maaskant, and B. J. Appelmelk. 1998. Anti-lipid A monoclonal antibody Centoxin (HA-1A) binds to a wide variety of hydrophobic ligands. Infect. Immun. 66:870-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henderson, R. A., S. C. Watkins, and J. A. L. Flynn. 1997. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J. Immunol. 159:635-643. [PubMed] [Google Scholar]
- 36.Hoppe, H. C., B. J. M. Dewet, C. Cywes, M. Daffé, and M. R. W. Ehlers. 1997. Identification of phosphatidylinositol mannoside as a mycobacterial adhesin mediating both direct and opsonic binding to nonphagocytic mammalian cells. Infect. Immun. 65:3896-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobs, M., N. Brown, N. Allie, R. Gulert, and B. Ryffel. 2000. Increased resistance to mycobacterial infection in the absence of interleukin-10. Immunology 100:494-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiao, X. N., R. Lo-Man, P. Guermonprez, L. Fiette, E. Deriaud, S. Burgaud, B. Gicquel, N. Winter, and C. Leclerc. 2002. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J. Immunol. 168:1294-1301. [DOI] [PubMed] [Google Scholar]
- 39.Kaur, D., S. Berg, P. Dinadayala, B. Gicquel, D. Chatterjee, M. R. Mcneil, V. D. Vissa, D. C. Crick, M. Jackson, and P. J. Brennan. 2006. Biosynthesis of mycobacterial lipoarabinomannan: role of a branching mannosyltransferase. Proc. Natl. Acad. Sci. USA 103:13664-13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khoo, K. H., A. Dell, H. R. Morris, P. J. Brennan, and D. Chatterjee. 1995. Structural definition of acylated phosphatidylinositol mannosides from Mycobacterium tuberculosis—definition of a common anchor for lipomannan and lipoarabinomannan. Glycobiology 5:117-127. [DOI] [PubMed] [Google Scholar]
- 41.Kolk, A. H. J., M. L. Ho, P. R. Klatser, T. A. Eggelte, S. Kuijper, S. Dejonge, and J. Vanleeuwen. 1984. Production and characterization of monoclonal antibodies to Mycobacterium tuberculosis, M. bovis (BCG) and M. leprae. Clin. Exp. Immunol. 58:511-521. [PMC free article] [PubMed] [Google Scholar]
- 42.Koppel, E. A., I. S. Ludwig, M. S. Hernandez, T. L. Lowary, R. R. Gadikota, A. B. Tuzikov, C. M. J. E. Vandenbroucke-Grauls, Y. van Kooyk, B. J. Appelmelk, and T. B. H. Geijtenbeek. 2004. Identification of the mycobacterial carbohydrate structure that binds the C-type lectins DC-SIGN, L-SIGN and SIGNR1. Immunobiology 209:117-127. [DOI] [PubMed] [Google Scholar]
- 43.Kovacevic, S., D. Anderson, Y. S. Morita, J. Patterson, R. Haites, B. N. I. McMillan, R. Coppel, M. J. McConville, and H. Billman-Jacobe. 2006. Identification of a novel protein with a role in lipoarabinomannan biosynthesis in mycobacteria. J. Biol. Chem. 281:9011-9017. [DOI] [PubMed] [Google Scholar]
- 44.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 45.Madura Larsen, J., C. S. Benn, Y. Fillie, D. van der Kleij, P. Aaby, and M. Yazdanbakhsh. 2007. BCG stimulated dendritic cells induce an interleukin-10 producing T-cell population with no T helper 1 or T helper 2 bias in vitro. Immunology 121:276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maeda, N., J. Nigou, J. L. Herrmann, M. Jackson, A. Amara, P. H. Lagrange, G. Puzo, B. Gicquel, and O. Neyrolles. 2003. The cell surface receptor DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J. Biol. Chem. 278:5513-5516. [DOI] [PubMed] [Google Scholar]
- 47.McCarthy, T. R., J. B. Torrelles, A. S. MacFarlane, M. Katawczik, B. Kutzbach, L. E. DesJardin, S. Clegg, J. B. Goldberg, and L. S. Schlesinger. 2005. Overexpression of Mycobacterium tuberculosis manB, a phosphomannomutase that increases phosphatidylinositol mannoside biosynthesis in Mycobacterium smegmatis and mycobacterial association with human macrophages. Mol. Microbiol. 58:774-790. [DOI] [PubMed] [Google Scholar]
- 48.Morita, Y. S., J. H. Patterson, H. Billman-Jacobe, and M. J. McConville. 2004. Biosynthesis of mycobacterial phosphatidylinositol mannosides. Biochem. J. 378:589-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morita, Y. S., C. B. C. Sena, R. F. Waller, K. Kurokawa, M. F. Sernee, F. Nakatani, R. E. Haites, H. Billman-Jacobe, M. J. McConville, Y. Maeda, and T. Kinoshita. 2006. PimE is a polyprenol-phosphate-mannose-dependent mannosyltransferase that transfers the fifth mannose of phosphatidylinositol mannoside in mycobacteria. J. Biol. Chem. 281:25143-25155. [DOI] [PubMed] [Google Scholar]
- 50.Murray, P. J., and R. A. Young. 1999. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect. Immun. 67:3087-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neyrolles, O., B. Gicquel, and L. Quintana-Murci. 2006. Towards a crucial role for DC-SIGN in tuberculosis and beyond. Trends Microbiol. 14:383-387. [DOI] [PubMed] [Google Scholar]
- 52.Nigou, J., M. Gilleron, and G. Puzo. 2003. Lipoarabinomannans: from structure to biosynthesis. Biochimie 85:153-166. [DOI] [PubMed] [Google Scholar]
- 53.Nigou, J., C. Zelle-Rieser, M. Gilleron, M. Thurnher, and G. Puzo. 2001. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 166:7477-7485. [DOI] [PubMed] [Google Scholar]
- 54.Ortalo-Magné, A., A. Lemassu, M. A. Laneelle, F. Bardou, G. Silve, P. Gounon, G. Marchal, and M. Daffé. 1996. Identification of the surface-exposed lipids on the cell envelopes of Mycobacterium tuberculosis and other mycobacterial species. J. Bacteriol. 178:456-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parish, T., B. G. Gordhan, R. A. McAdam, K. Duncan, V. Mizrahi, and N. G. Stoker. 1999. Production of mutants in amino acid biosynthesis genes of Mycobacterium tuberculosis by homologous recombination. Microbiology 145(Pt. 12):3497-3503. [DOI] [PubMed] [Google Scholar]
- 56.Parish, T., and N. G. Stoker. 1998. Electroporation of mycobacteria. Methods Mol. Biol. 101:129-144. [DOI] [PubMed] [Google Scholar]
- 57.Parish, T., and N. G. Stoker. 2000. Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiology 146:1969-1975. [DOI] [PubMed] [Google Scholar]
- 58.Park, C. G., K. Takahara, E. Umemoto, Y. Yashima, K. Matsubara, Y. Matsuda, B. E. Clausen, K. Inaba, and R. M. Steinman. 2001. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int. Immunol. 13:1283-1290. [DOI] [PubMed] [Google Scholar]
- 59.Pitarque, S., J. L. Herrmann, J. L. Duteyrat, M. Jackson, G. R. Stewart, F. Lecointe, B. Payre, O. Schwartz, D. B. Young, G. Marchal, P. H. Lagrange, G. Puzo, B. Gicquel, J. Nigou, and O. Neyrolles. 2005. Deciphering the molecular bases of Mycobacterium tuberculosis binding to the lectin DC-SIGN reveals an underestimated complexity. Biochem. J. 392:615-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Powlesland, A. S., E. M. Ward, S. K. Sadhu, Y. Guo, M. E. Taylor, and K. Drickamer. 2006. Widely divergent biochemical properties of the complete set of mouse DC-SIGN-related proteins. J. Biol. Chem. 281:20440-20449. [DOI] [PubMed] [Google Scholar]
- 61.Reina, J. J., I. Diaz, P. M. Nieto, N. E. Campillo, J. A. Paez, G. Tabarani, F. Fieschi, and J. Rojo. 2008. Docking, synthesis, and NMR studies of mannosyl trisaccharide ligands for DC-SIGN lectin. Org. Biomol. Chem. 6:2743-2754. [DOI] [PubMed] [Google Scholar]
- 62.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and down-regulated by tumor necrosis factor α. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sayers, I., W. Severn, C. B. Scanga, J. Hudson, G. Le Gros, and J. L. Harper. 2004. Suppression of allergic airway disease using mycobacterial lipoglycans. J. Allergy Clin. Immunol. 114:302-309. [DOI] [PubMed] [Google Scholar]
- 64.Schaefer, M., N. Reiling, C. Fessler, J. Stephani, I. Taniuchi, F. Hatam, A. O. Yildirim, H. Fehrenbach, K. Walter, J. Ruland, H. Wagner, S. Ehlers, and T. Sparwasser. 2008. Decreased pathology and prolonged survival of human DC-SIGN transgenic mice during mycobacterial infection. J. Immunol. 180:6836-6845. [DOI] [PubMed] [Google Scholar]
- 65.Schlesinger, L. S., S. R. Hull, and T. M. Kaufman. 1994. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J. Immunol. 152:4070-4079. [PubMed] [Google Scholar]
- 66.Tailleux, L., O. Schwartz, J. L. Herrmann, E. Pivert, M. Jackson, A. Amara, L. Legres, D. Dreher, L. P. Nicod, J. C. Gluckman, P. H. Lagrange, B. Gicquel, and O. Neyrolles. 2003. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torrelles, J. B., A. K. Azad, and L. S. Schlesinger. 2006. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J. Immunol. 177:1805-1816. [DOI] [PubMed] [Google Scholar]
- 68.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Kooyk, Y., B. Appelmelk, and T. B. Geijtenbeek. 2003. A fatal attraction: Mycobacterium tuberculosis and HIV-1 target DC-SIGN to escape immune surveillance. Trends Mol. Med. 9:153-159. [DOI] [PubMed] [Google Scholar]
- 70.Vannberg, F. O., S. J. Chapman, C. C. Khor, K. Tosh, S. Floyd, D. Jackson-Sillah, A. Crampin, L. Sichali, B. Bah, P. Gustafson, P. Aaby, K. P. McAdam, O. Bah-Sow, C. Lienhardt, G. Sirugo, P. Fine, and A. V. Hill. 2008. CD209 genetic polymorphism and tuberculosis disease. PLoS ONE. 3:e1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Villeneuve, C., M. Gilleron, I. Maridonneau-Parini, M. Daffé, C. Astarie-Dequeker, and G. Etienne. 2005. Mycobacteria use their surface-exposed glycolipids to infect human macrophages through a receptor-dependent process. J. Lipid Res. 46:475-483. [DOI] [PubMed] [Google Scholar]
- 72.Wieland, C. W., E. A. Koppel, J. den Dunnen, S. Florquin, A. N. McKenzie, Y. van Kooyk, T. van der Poll, and T. B. Geijtenbeek. 2007. Mice lacking SIGNR1 have stronger T helper 1 responses to Mycobacterium tuberculosis. Microbes Infect. 9:134-141. [DOI] [PubMed] [Google Scholar]
- 73.Wolf, A. J., B. Linas, G. J. Trevejo-Nunez, E. Kincaid, T. Tamura, K. Takatsu, and J. D. Ernst. 2007. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J. Immunol. 179:2509-2519. [DOI] [PubMed] [Google Scholar]
- 74.World Health Organization. 2008. Global tuberculosis control: surveillance, planning, financing. WHO/HTM/TB/2008.393. World Health Organization, Geneva, Switzerland.
- 75.Wu, L., T. D. Martin, M. Carrington, and V. N. KewalRamani. 2004. Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology 318:17-23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.