Abstract
Listeriolysin O (LLO) is an essential virulence factor for the gram-positive bacterium Listeria monocytogenes. Our goal was to determine if altering the topology of LLO would alter the virulence and toxicity of L. monocytogenes in vivo. A recombinant strain was generated that expressed a surface-associated LLO (sLLO) variant secreted at 40-fold-lower levels than the wild type. In culture, the sLLO strain grew in macrophages, translocated to the cytosol, and induced cell death. However, the sLLO strain showed decreased infectivity, reduced lymphocyte apoptosis, and decreased virulence despite a normal in vitro phenotype. Thus, the topology of LLO in L. monocytogenes was a factor in the pathogenesis of the infection and points to a role of LLO secretion during in vivo infection. The sLLO strain was cleared by severe combined immunodeficient (SCID) mice. Despite the attenuation of virulence, the sLLO strain was immunogenic and capable of eliciting protective T-cell responses.
Listeria monocytogenes is a gram-positive facultative intracellular pathogen extensively used to understand host-pathogen interactions (44, 51, 53). It expresses the highly conserved pore-forming toxin listeriolysin O (LLO), a member of a large family of cholesterol-dependent cytolysins found in many important pathogens (11, 33, 50). LLO is required for L. monocytogenes virulence both in vivo and vitro. L. monocytogenes genetically deficient in LLO (Δhly) is incapable of any growth in vivo or inducing protective immunity except at extremely high infectious doses (27). This finding supports the relationship between virulence and development of strong protective immunity.
The effect of LLO on cells is multifaceted. It induces calcium fluxes at the cell surface (16, 46), cytokine production by splenocytes (40), apoptosis of dendritic cells and T lymphocytes (8, 26), and Toll-like receptor 4 signaling (43). LLO is also a source of T-cell epitopes of L. monocytogenes in multiple major histocompatibility complex (MHC) haplotypes (22, 52). Most studies on LLO have focused on its ability to perforate the phagolysosome at acidic pH, which allows L. monocytogenes to enter the cytosol of the infected phagocytic cell (24, 45). The Δhly strain does not cross from the phagosome to the cytosol and is effectively killed (20, 45). L. monocytogenes constructed to express variable levels of LLO protein in vitro has shown a dose threshold that must be reached for entry into the cytosol (14). There is recent work that suggests that low levels of LLO secretion can lead to maintenance of L. monocytogenes in vacuoles, suggesting a possible mechanism for granuloma formation and persistent infection in immunocompromised strains of mice (6).
During infection with L. monocytogenes, there is a phase of lymphocyte apoptosis in the spleen that peaks at the second day of the infection (30, 34). Apoptosis is dependent on the level of infection with L. monocytogenes. High doses of LLO-deficient or heat-killed L. monocytogenes do not induce the lesions, demonstrating that live, virulent infection is required for their development. Induction of lymphocyte apoptosis is immunomodulatory and a major determinant in the virulence of L. monocytogenes infection (11). For example, mice deficient in type I interferon (IFN) receptor signaling are more resistant to listeriosis and have decreased levels of lymphocyte and macrophage apoptosis (2, 10, 41). Tumor necrosis factor (TNF)-related apoptosis-inducing ligand−/− (TRAIL) mice are also more resistant to infection and have less apoptosis (54), while liver X receptor−/− (LXR) mice have increased macrophage apoptosis and are more susceptible to listeriosis (32). And pointedly, mice deficient in lymphocytes have no detectable splenic apoptosis and are more resistant early after infection than conventional mice (3, 9).
Our interpretation on the pathogenesis of the lesions is that LLO released extracellularly during the strong exponential phase of growth is central for their development. Treatment of cultured T cells at neutral pH with sublytic nanomolar and subnanomolar doses of LLO can induce apoptosis (8). The apoptosis induced by LLO on activated T cells is highly dependent on granzyme expression within the affected cell (12). Nonactivated lymphocytes can be sensitized to the apoptotic effect of LLO by treatment with type I IFN (10).
To examine the possible role of LLO as an extracellular protein, we engineered a recombinant strain of L. monocytogenes in which the LLO protein is covalently linked to the bacterial cell wall (surface-associated LLO [sLLO]). The sLLO strain expresses wild-type levels of bacterially associated LLO but secretes 40-fold less total protein. The growth, pathogenicity, and inflammatory properties of the sLLO strain are severely attenuated in vivo. Importantly, there is reduced apoptosis in the infectious foci following infection with the sLLO mutant. This strain is also capable of eliciting memory T-cell responses and protective immunity.
MATERIALS AND METHODS
Generation of sLLO-expressing L. monocytogenes.
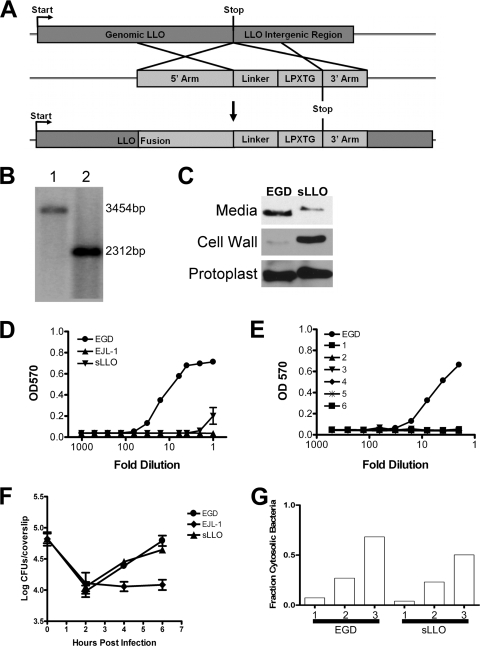
Figure 1A shows a schematic of the strategy employed to generate sLLO (17, 48). A fragment of the LLO gene (hly) was isolated from the EGD strain genome by PCR with primers 5′-GTCGACCTAAATCAAACGTTAACAACGCAG-3′ and 5′-GGATCCTTCGATTGGATTATCTAC-3′. Primers were designed to amplify a region spanning the last 1,067 bp of the LLO coding region and incorporate a 5′ SalI site and a 3′ BamHI site for subsequent cloning. The fragment of LLO was cloned into the gram-positive shuttle vector pKSV7 by directional cloning using SalI and BamHI digests of the vector and insert (48). This fragment of LLO served as the “5′ Arm” for homologous recombination and removed the stop codon of the LLO gene. Next, the region of internalin A (inlA) known to encode the cell wall association motif (LPXTG) was amplified by PCR with primers 5′-TTAAGGATCCCACCACCACCACCACCACCTCCCTACAACTGGCGATAGCG-3′ and 5′-AATTGGATCCGCGGCCGCTTATTATTTACTAGCACGTGCTTTTTTAGTAAGAGCC-3′ from the EGD genome. Both primers contained a BamHI site, the 3′ primer contained a NotI site, and the 5′ primer contained a hexahistidine linker that was included in the recombinant LLO construct previously described (21, 24). This fragment was cloned into pKSV7 containing the 5′ Arm fragment to yield LLO followed by a hexahistidine linker and finally the internalin A sorting sequence with a stop codon, 5′ Arm/Linker/LPXTG. The final step was to PCR amplify the 500 bp immediately 3′ of the LLO stop codon (3′ Arm) with primers 5′-AATTGCGGCCGCTTGTAAAAGTAATAAAAAATTAAG-3′ and 5′-AATTGCGGCCGCTTGTAAAAGTAATAAAAAATTAAG-3′. This fragment was cloned after the LPXTG sequence. The final construct was grown up in TOP10 cells, and the plasmid was isolated. Transformation and homologous recombination were carried out into the L. monocytogenes strain EGD using the penicillin G transformation protocol described previously (17).
FIG. 1.
Genotype and in vitro phenotype of the sLLO mutant of L. monocytogenes. (A) Genomic construct of LLO bearing a hexahistidine linker and the LPXTG cell wall targeting motif of internalin A. (B) Southern analysis of the wild-type (1) and sLLO (2) strains on BamHI-digested genomic DNA with a probe directed against LLO. The wild-type sequence migrated at 3,454 bp, and the mutant variant migrated at 2,312 bp. (C) Immunoblot analysis of bacterially associated LLO. Mid-log culture supernatants (media), cell wall fraction (cell wall), or protoplasts were isolated as described in Materials and Methods. LLO was detected by immunoblotting with an LLO-specific monoclonal antibody (A4-8). (D) Culture supernatants of the mid-log-cultured sLLO, EJL-1, and EGD strains were tested for hemolytic activity on human RBC. (E) Mice were infected with 106 EGD or sLLO strain cells, and spleens were homogenized and plated on BHI. Single colonies were picked and grown overnight in standing BHI culture at 37°C. Numbers 1 to 6 in panel E represent individual colonies of the sLLO strain. Supernatants were serially diluted, and hemolytic activity was determined by RBC lysis assay. (F) PEC were infected with equivalent doses of the EGD, EJL-1, or sLLO strain, and at the indicated times, cells were lysed and total bacterial number was determined by titration on BHI agar plates. Points represent the mean ± standard error for triplicate wells. (G) PEC (2.5 × 105) were infected with equivalent numbers of EGD or sLLO strain cells (MOI of 1), and at the indicated times (hours), the cells were fixed and stained for L. monocytogenes and phalloidin. The percentage of cytosolic bacteria was determined by counting phalloidin-positive L. monocytogenes cells and dividing it by the total number of bacteria. Each bar represents the percentage determined from counting four to six microscopic fields from two independent experiments. The total number of bacteria counted was between 350 and 800 per bar. There was no statistical difference between the EGD and sLLO groups (Mann-Whitney U test).
Genotypic characterization of the L. monocytogenes sLLO mutant.
Transformants were first screened by PCR using a primer specific for the 5′ end of the LLO gene (5′-GTCCTCTCGTAAAAGCGAATTCG-3′) and a 3′ primer specific for the LPXTG sequence of inlA (5′-AATTGCGGCCGCTTGTAAAAGTAATAAAAAATTAAG-3′). Putative recombinants were further screened by Southern analysis. Bacteria were subcloned by streaking out on brain heart infusion (BHI)-agar plates until the Southern blot and PCR were both positive for 100% of the bacteria after subcloning. For Southern analysis, genomic bacterial DNA was cut with BamHI, resolved on a 1% agarose gel, transferred to a nylon membrane, and probed against the 5′ end of the coding region of LLO. The wild-type band was expected to migrate at 3,454 bp and the properly targeted mutant at 2,312 bp. The Southern blot was also performed with BsaAI for further confirmation. BamHI and BsaAI were selected because novel sites of each were introduced into the LLO locus by the manipulations used to generate the recombinant sLLO strain. Individual clones were screened and subcloned until homogeneous by both Southern analysis and PCR. Stability of the mutation was verified after infection in both wild-type and SCID mice followed by screening of 6 to 10 colonies per infected mouse.
Immunoblot analysis.
Bacterial strain EGD and the Δhly (LLO-deficient) and sLLO strains were grown in standing BHI culture overnight at 37°C. Overnight cultures were used to inoculate shaking cultures of BHI. Bacteria were grown at 37°C until mid-log phase (optical density at 560 nm [OD560] of 0.8 to 1.0) and centrifuged at a relative centrifugal force (RCF) of 8,000, and the supernatants were collected. Culture supernatant, cell wall, and protoplasts were fractionated and precipitated as described previously with lysozyme treatment for 1 h instead of endolysin treatment (49). Protein was transferred onto polyvinylidene difluoride (PVDF) membranes, and LLO was detected with the anti-LLO monoclonal antibody A4-8 (37). A Coomassie blue gel was run in parallel to confirm equivalent loading of lanes.
RBC lysis assays.
Bacteria were grown as described in the previous section. Bacteria were spun down at an RCF of 8,000 and washed twice in a buffer containing phosphate-buffered saline (PBS [pH 6.0]), 0.1% bovine serum albumin (BSA), and 0.01 M dithiothreitol. Twofold dilutions of the supernatants were combined with human red blood cells (RBC) and incubated for 1 h at 37°C. Human RBC were isolated from various normal patients and washed three times in PBS (pH 6.0)-0.1% BSA. The final assay conditions were 1× PBS (pH 6.0), 0.1% BSA, 0.005 M dithiothreitol, and 3% RBC and dilution of culture supernatant. After incubation, cells were spun out at an RCF of 600 to remove unlysed RBC, supernatants were collected, and OD570 was determined for the supernatant of each sample.
Mice and L. monocytogenes infection.
C.B-17 and C.B-17 SCID mice were bred and maintained at Washington University School of Medicine under specific-pathogen-free conditions. All protocols were performed in accordance with the guidelines of the Division of Comparative Medicine of Washington University School of Medicine. Mice of both genders were infected at 8 to 12 weeks of age. Bacteria were stored as frozen glycerol stocks, thawed, and diluted into pyrogen-free saline prior to infection. All mice were infected intraperitoneally with a final volume of 0.5 ml. All antibodies used in this study were generated, purified, and used as described previously (18). Hematoxylin and eosin staining and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling (TUNEL) staining were performed as described previously (34). L. monocytogenes was detected in tissue sections by immunofluorescence using a 1:200 dilution of rabbit polyclonal anti-Listeria antibody (BD Pharmingen, Valencia, CA) followed by detection with a 1:200 dilution of goat anti-rabbit Atto 488 (Sigma, St. Louis, MO). Mann-Whitney U test was used for all statistical analyses.
In vitro assays.
Coverslip assays for growth and for determination of escape into the cytosol were performed as described previously (19). Plaque assays were performed as described previously with slight modifications (4, 31). Plaque assays were carried out in six-well plates and overlaid with methylcellulose instead of agarose. L929 cells were used to form the monolayers for plaque assays. Enzyme-linked immunospot (ELISPOT) assays for gamma IFN (IFN-γ) and interleukin-2 (IL-2) were performed with matched ELISPOT antibody sets (BD Pharmingen) and used according to the manufacturer's instructions. Cytokines from culture supernatants or sera were determined by cytometric bead array assay (BD Pharmingen) using the manufacturer's instructions. Type I IFN in the spleen was determined by enzyme-linked immunosorbent assay (PBL Laboratories). Relative IFN-β induction was determined by quantitative reverse transcription-PCR (qRT-PCR) using SYBR green (Bio-Rad iCycler) and cycle threshold (ΔΔCt) analysis using hypoxanthine phosphoribosyltransferase (HPRT) as the housekeeping control.
RESULTS
Generation and properties of a strain of L. monocytogenes that expressed LLO associated with its cell wall.
We hypothesized that LLO released into the extracellular milieu was responsible for the extensive lymphocyte apoptosis during infection. By limiting LLO secretion, we would reduce apoptosis and attenuate L. monocytogenes virulence. Our goal was to limit the amount of LLO released into the cytosol and extracellular space while still allowing the bacteria to escape the phagosome of infected macrophages. To this end, a mutant (sLLO) L. monocytogenes strain was generated that expressed LLO covalently associated with the cell wall via the bacterial sortase. One major mechanism gram-positive bacteria use to associate their proteins with the cell wall is sortase-mediated covalent binding to the peptidoglycan (38, 49). The minimal sequence required for sortase-mediated association of the L. monocytogenes protein internalin A has been well studied, so our strategy involved adding the inlA gene sorting sequence to the 3′ end of LLO. The final DNA construct (Fig. 1A) was generated and transformed into the wild-type EGD strain of L. monocytogenes. Bacterial clones were screened by PCR and Southern blotting to identify successful recombinants (Fig. 1B).
Our initial examination of the sLLO strain involved growth in shaking culture at 37°C to determine rates of growth. Wild-type EGD and the sLLO mutant grew at the same rate in multiple experiments, both reaching an OD600 reading of mid-log growth (0.8 to 1.2) 3 to 4 h after the beginning of culture in BHI. Both strains also reached the same level of absorbance (OD600 of 1.2 to 1.5) following an overnight standing culture in BHI. Therefore, the introduction of a mutation into the LLO gene did not adversely affect the growth of the sLLO mutant compared to its wild-type counterpart.
Next, we wanted to determine if the recombinant LLO was covalently associated with the cell wall. Bacterial culture media, cell wall fractions, and protoplast fractions of EGD and the sLLO mutant were prepared, and LLO in each fraction was detected by immunoblot analysis. Figure 1C shows an immunoblot of the three fractions. EGD secreted more LLO into culture media than the sLLO strain. In contrast, the sLLO strain contained more cell wall-associated LLO than EGD. Both bacteria contained comparable levels of protoplast-associated LLO. We also performed all three isolations with the LLO-deficient strain EJL-1 and did not detect any protein by immunoblotting (data not shown). We conclude from this that the sLLO strain expresses covalently attached LLO on its cell wall and secretes qualitatively less LLO into culture media than EGD.
Since it is difficult to quantify the amount of LLO being secreted by either EGD or the sLLO strain by immunoblotting, we used RBC hemolysis to determine the amount of functional LLO that was secreted. Human RBC were treated with culture supernatant from mid-log-phase cultures of the EGD or sLLO strain. The sLLO strain supernatant showed an ∼40-fold reduced lytic activity compared to the EGD supernatant (Fig. 1D), a finding consistent with the immunoblot analysis shown in Fig. 1C. Additionally, the hemolytic activity could be neutralized by addition of 1 mg/ml anti-LLO A4-8 (data not shown).
To demonstrate that the sLLO genotype was stable, mice were infected with 104 sLLO mutant cells, and bacterial colonies were isolated from spleens by standard techniques. Consistent with the results shown above, six out of six sLLO isolates had diminished or no lytic activity in culture supernatants (Fig. 1E). The presence of the sLLO mutation was also confirmed by PCR and Southern blotting (data not shown). Thus, the sLLO mutant is a stable strain of L. monocytogenes that sheds ∼40-fold less than the wild-type strain.
LLO allows egress of L. monocytogenes from the phagosome to the cytosol and cell-to-cell migration of bacteria. The handling of the EGD and sLLO strains by macrophages was tested to determine if there was impairment in the in vitro growth of the sLLO strain (4, 19). Figure 1F shows a time course of infection of peritoneal exudate cells (PEC) with the sLLO, EGD, and Δhly strains. Both the EGD and sLLO strains showed the typical growth pattern, with an initial reduction followed by progressive growth, the latter an indication that the bacteria had reached the cytosol. The Δhly strain did not grow in macrophages. We determined that the EGD and sLLO strains entered into the cytosol at the same rate and in comparable numbers by staining infected cells with phalloidin and anti-L. monocytogenes antibody (Fig. 1G). Colocalization of phalloidin and bacteria is an indication of the actin polymerization that occurs only in the cytosol. Combined, these results show that the sLLO mutant strain is capable of normal growth and phagosomal escape in infected macrophages.
An additional function of LLO is to enhance bacterial spread from one infected cell to an adjacent cell (21). In the cell-to-cell spread assay, L. monocytogenes either transits from one cell to the next or is killed by gentamicin present in the culture. Both the EGD and the sLLO mutant strains formed equivalent numbers of plaques of approximately the same size. It was previously shown that LLO-deficient strains form small plaques, which was not the case for the sLLO mutant (4). The sLLO or the small amount of LLO shed by the sLLO mutant was sufficient to permit cell-to-cell spread. The terminal stage of a productive infection with L. monocytogenes is the death of the infected cell. EGD and sLLO mutant cells that gained access to the cytosol were toxic to the infected cell. We detected nuclear condensation, cell detachment, and propidium iodide staining in macrophages and PEC cells infected with sLLO mutant and EGD cells for more than 16 h (data not shown). Therefore, sLLO mutant cells can gain access to the cytosol and grow well, eventually compromising the integrity of the infected phagocytic cell.
Comparison of EGD versus sLLO mutant infection in C.B-17 mice.
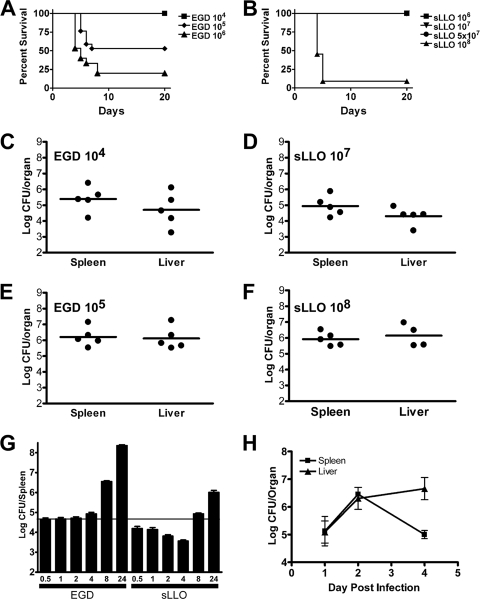
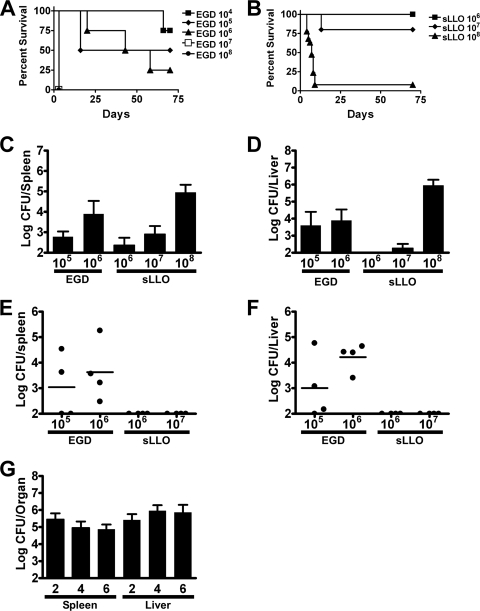
The infectivity and virulence of EGD and sLLO in vivo were tested in wild-type C.B-17 mice. The 50% lethal dose (LD50) for EGD in C.B-17 mice was 105 CFU, and that for the sLLO mutant was >5 × 107 for sLLO (Fig. 2A and B). Figure 2C to F show a comparison of EGD against the sLLO strain 2 days after infection. C.B-17 mice infected with 104 CFU of EGD had the same colony counts as mice infected with 107 CFU of the sLLO strain (Fig. 2C and D). Infection with 105 CFU of EGD gave the same colony counts as infection with 108 CFU of the sLLO strain (Fig. 2E and F). We concluded that the sLLO strain had a 1,000-fold virulence attenuation compared to EGD. Similar differences in colony counts were seen at day 4 postinfection (data not shown).
FIG. 2.
Infection of wild-type mice with either the sLLO or EGD strain. (A and B) C.B-17 mice were infected with the indicated doses of the EGD or sLLO strain and monitored for survival. (B) We saw no lethality in the sLLO strain at 106, 107, or 5 × 107, so the lines overlap. (C to F) C.B-17 mice were infected with the indicated doses of either the EGD (C and E) or sLLO (D and F) strain, and bacterial burden in the spleen and liver was determined at day 2 postinfection. (G) C.B-17 mice were infected with 107 EGD or sLLO strain cells, and colony counts per spleen were determined at the indicated hours postinfection. Each bar represents the mean ± the standard error of the mean of at least eight mice per group. (H) C.B-17 mice were infected with 5 × 107 CFU of the sLLO strain, and bacterial titers were determined at days 1, 2, and 4 postinfection. Points represent the mean ± standard error of the mean of at least four mice per group.
In order to determine when the sLLO growth defect occurred, mice were infected and colony counts determined during the first few hours following infection (Fig. 2G). C.B-17 mice were infected intravenously (i.v.), and colony counts were determined at 0.5, 1, 2, 4, 6, and 24 h postinfection. The initial level of infectivity with the sLLO strain was reduced compared with that of the EGD strain: a statistically significant difference was found within the first 30 min following infection (P = 0.0037 at 0.5 h by Mann-Whitney U test). During the first hour, both bacteria showed no change in colony counts. However, after 4 h, the sLLO strain decreased in number. Other studies have shown that L. monocytogenes is initially trapped in the marginal zone of the spleen, where it resides during the first 4 to 6 h. At the 4- to 6-h time period, L. monocytogenes is transported to the periarteriolar lymphoid sheaths (PALS), where it grows exponentially (1, 29, 36). This migration is dependent on LLO expression, because the Δhly strain does not enter into PALS. Both the sLLO mutant and EGD grew exponentially after 4 to 6 h of infection, but the sLLO strain never reached the same rate of growth as EGD: compare the 4-, 8-, and 24-h time points in Fig. 2G. Figure 2H shows that the sLLO strain can grow progressively in both the spleens and livers, reaching peak levels at the second day of infection. The sLLO strain was rapidly cleared from the spleen after the 4th day of infection. In additional experiments, both the sLLO and EGD strains were cleared from both livers and spleens by day 8 postinfection (data not shown).
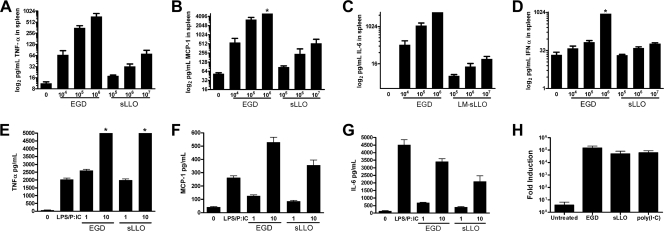
LLO is an important component of the inflammation induced by L. monocytogenes infection. We infected C.B-17 mice with EGD and sLLO mutant cells and determined the level of serum cytokines at day 3 of the infection. Infection of mice with L. monocytogenes EGD induced high levels of TNF-α, monocyte chemoattractant protein 1 (MCP-1), and IL-6 and modest levels of IFN-α that were compatible with levels seen by our laboratory and others (Fig. 3A to D). Infection with the sLLO strain did not lead to robust inflammatory cytokine production at lower doses of infection. Consistent with colony count information, we detected comparable levels of inflammatory cytokine induction in vivo at a 3-log-higher input infection of the sLLO strain (Fig. 3A to D).
FIG. 3.
Inflammatory cytokine responses and T-cell responses following infection with the sLLO or EGD strain. (A to D) Mice were either uninfected or infected with the indicated doses of the EGD or sLLO strain for 3 days. Spleens were isolated from mice and tested by cytometric bead array for TNF-α (A), MCP-1 (B), IL-6 (C), or IFN-α (D). (E to G) C.B-17 PEC were left untreated (0), infected with an MOI of 1 or 10 of either the sLLO or EGD strain for 24 h, or treated with 10 ng/ml lipopolysaccharide and 100 ng/ml poly(I · C), and then culture supernatants were assayed for either TNF-α (E), MCP-1 (F), or IL-6 (G) content by CBA. Bars represent the mean ± standard error for either four to six mice or three culture supernatants per group. Asterisks indicate assays in which the maximal limit of detection was reached. The Mann-Whitney U test did not find a statistical difference between any of the MOI 1 or 10 cytokine responses between the EGD and sLLO strains. (H) A total of 106 C.B-17 PEC were infected with the EGD or sLLO strain at an MOI of 10 or 100 ng/ml poly(I·C), and after 6 h, total RNA was isolated, treated with DNase I, and reverse transcribed, and qRT-PCR was performed using SYBR green with an internal fluorescein loading control (iCycler, IQ master mix; Bio-Rad). Induction (fold) of IFN-β mRNA was determined by ΔΔCt calculation with HPRT as the housekeeping control. Bars represent the mean of independent samples assayed in duplicate.
The reduction in cytokine production in vivo was not due to an inability of the sLLO strain to induce macrophage cytokine production. Peritoneal macrophages were infected with the sLLO strain or EGD, and then TNF-α, MCP-1, and IL-6 were measured at 24 h postinfection (Fig. 3E to G). We found similar levels of all three cytokines for the sLLO and EGD strains. This reinforces the results seen earlier, where the phenotype of the sLLO strain in vitro is the same as that of EGD despite a dramatic difference in vivo. We also measured the induction of IFN-β mRNA by qRT-PCR. Peritoneal macrophages induced IFN-β >10,000-fold 6 h after infection with either EGD or the sLLO strain (multiplicity of infection [MOI] of 10) or treatment with poly(I·C) (100 ng/ml) (Fig. 3H). We conclude that sLLO has reduced inflammatory capacity in vivo which correlates with infectious dose, despite normal induction of cytokines in infected cultured cells.
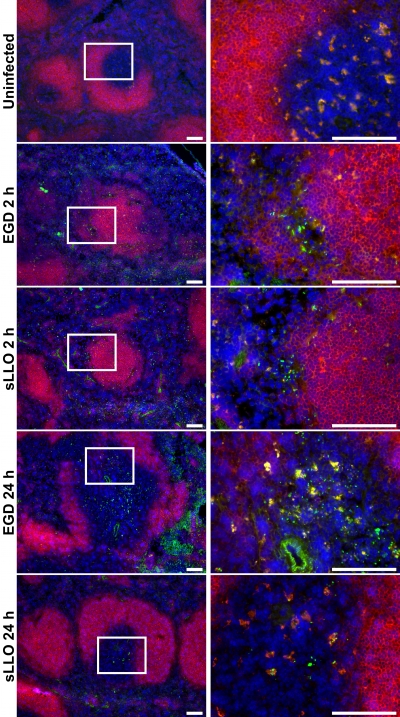
In light of the suggested role of LLO in bacterial migration into the PALS, the localization of EGD and sLLO mutant cells in the spleens of infected C.B-17 mice was compared by immunofluorescence. As expected, 2 h postinfection, both EGD and sLLO mutant cells localized to the marginal zone (Fig. 4). By 24 h postinfection, both strains were found in the PALS, although fewer sLLO strain than EGD bacteria were detected. Therefore, the sLLO strain was able to access PALS.
FIG. 4.
Localization of the sLLO and EGD strain cells in infected spleens. Mice were either left uninfected or were infected with the EGD or sLLO strain for the times indicated. For the 2-h infections, mice received 108 CFU of either the EGD or sLLO strain. For the 24-h infections, mice received 106 CFU of the EGD strain or 107 CFU of the sLLO strain. Spleens were isolated from all mice, flash frozen, sectioned, and stained against L. monocytogenes (green), CD19 (red), or DAPI (4′,6-diamidino-2-phenylindole; blue). The white insert box in the left panels indicates the region shown on the corresponding right panels. White bars represent 100 μm. Fields are representative of at least three mice per group.
Comparison of EGD versus sLLO strain infection in SCID mice.
We initially hypothesized that secreted LLO was responsible for lymphocyte apoptosis seen after infection of wild-type mice (8). If the decreased virulence of the sLLO strain was entirely caused by reduction of lymphocyte apoptosis following infection, we would expect that SCID mice would not show as profound a difference in control of the sLLO strain versus EGD as the wild-type mice.
C.B-17 SCID mice were infected with both strains over a range of doses. SCID mice were quite resistant to both bacterial strains (Fig. 5A and B), consistent with previous observations from our laboratory (3, 9). Despite the absence of lymphocytes, the sLLO strain was still highly attenuated. For example, at 70 days postinfection, a 106-CFU inoculum of EGD resulted in 75% lethality compared to 0% lethality when mice were infected with the sLLO strain. At the 106 infectious dose, ∼104 colony counts for the EGD strain were found 4 days later (Fig. 5C and D) but bacteria were not recovered from the sLLO strain-infected SCID mice. Similar differences were also observed at higher infectious doses.
FIG. 5.
Infection of SCID mice with either the sLLO or EGD strain. (A and B) SCID mice were infected intraperitoneally with the indicated doses and monitored for survival. (C and D) SCID mice were infected with the indicated doses of EGD or sLLO strain cells, and bacterial burden was determined at day 4 postinfection. (E and F) SCID mice were infected with the indicated doses of EGD or sLLO strain cells, and bacterial burden was determined at day 14 postinfection. (G) SCID mice were infected with 108 CFU of the sLLO strain intraperitoneally, and colony counts were measured at the indicated days postinfection. Each point represents the CFU per organ for one mouse. Bars represent the mean for each group.
Due to the absence of T cells, wild-type L. monocytogenes establishes a chronic infection in SCID mice (5). We were able to recover EGD but not sLLO strain cells from mouse spleens and livers 14 days after infection (Fig. 5E and F). We were unable to find a dose of the sLLO strain that established a chronic infection: even though the sLLO strain was infectious and virulent, it did not persist in SCID mice. At the very high 108-CFU dose, both strains killed the mice within the first 4 to 8 days of infection. The time course of bacterial burden in SCID mice following infection with 108 CFU of the sLLO strain is shown in Fig. 5G: there were high titers in both the spleen and liver from days 2 to 6 postinfection. The sLLO strain was difficult to detect by the 4th day of infection in SCID mice, and in several experiments, we found no bacterial burden at day 14 postinfection, a time at which the EGD strain is found chronically infecting SCID mice.
Together, our data suggest that the sLLO strain is poorly virulent in SCID mice at low doses, but once a threshold dose is reached, it can grow vigorously and kill mice. At sublethal doses, the sLLO strain was unable to establish the chronic infection typically seen with sublethal infection with EGD.
Other features of the infection with the sLLO strain of L. monocytogenes.
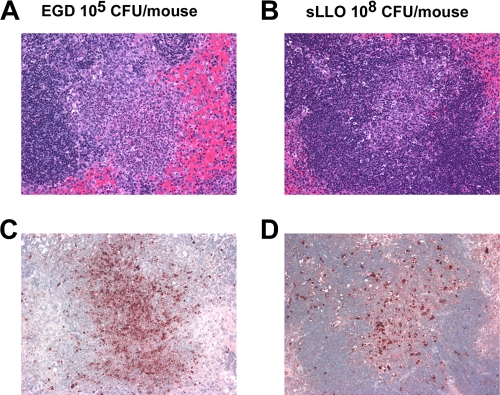
Histological analysis of C.B-17 mice that had equivalent EGD and sLLO strain burdens at day 2 postinfection showed infective foci in the PALS of the white pulp (Fig. 6A and B). When adjacent sections were stained by TUNEL, lymphocyte apoptosis was detected with both strains (Fig. 6C and D). However, the extent of the lesions was markedly reduced in sLLO strain-infected mice despite an equivalent bacterial burden. At sLLO strain infectious doses of ≤107 CFU, few TUNEL-positive cells were found despite a measurable bacterial burden in the spleen (Fig. 2D). In contrast, EGD-infected mice with the same bacterial burden showed significant TUNEL-positive lesions. The livers of mice infected with either bacterial strain had comparable pathology and at 48 h showed the typical microabscesses.
FIG. 6.
Histological analysis of sLLO strain-infected mice. C.B-17 mice were infected with 105 CFU of EGD (A and C) or 108 CFU of the sLLO strain (B and D). At day 2 postinfection, mice were sacrificed and the spleens were removed, fixed in formalin, sectioned, and stained by hematoxylin and eosin (A and B) or TUNEL (C and D) as described in Materials and Methods. All panels are at a magnification of ×400.
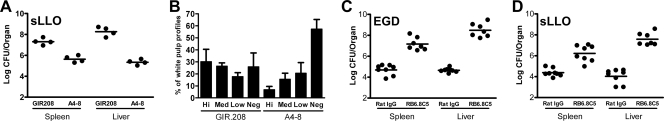
Neutralization of LLO using the monoclonal antibody A4-8 renders wild-type L. monocytogenes avirulent (18, 19). Figure 7A shows that A4-8 inhibits the in vivo growth of the sLLO strain, indicating that the LLO produced by both strains contributed to their infectivity. Histological sections of spleens from the same mice in Fig. 7A were analyzed for the extent of apoptosis found in their white pulp profiles. Mice that received control GIR.208 antibodies had 30% and 26% of their white pulp profiles containing either large or medium apoptotic lesions. In contrast, after A4-8 treatment, 6% and 15% of white pulp profiles contained large and medium apoptotic lesions, respectively. In the GIR.208 group, 25% of the white pulp profiles contained no apoptotic lesions versus 57% for the A4-8-treated group. Therefore, treatment with A4-8 reduced the number and extent of apoptotic lesions following sLLO strain infection by over 50%.
FIG. 7.
Characterization of sLLO strain infection in C.B-17 mice. (A and B) C.B-17 mice were treated with 1 mg of either the LLO-neutralizing antibody A4-8 or the GIR.208 control antibody, and 24 h later, they were infected with 108 CFU of the sLLO strain. (A) Mice were sacrificed at day 2 postinfection, and colony counts were determined. (B) Histology from the mice was scored for extent of apoptosis. Lesions were classified by size from negative (neg; negative/no apoptosis) through low (small apoptotic lesions; <25% white pulp profile apoptotic), medium (med; medium apoptotic lesions, 25 to50% white pulp profile apoptotic), and high (hi; large apoptotic lesions; >75% white pulp profile apoptotic). The data show the percentage of white pulp profiles from each mouse that fit each of the four categories. Bars are the mean ± standard error of the mean from four mice per group. (C and D) C.B-17 mice were treated with 250 μg of RB6-8C5 or rat immunoglobulin G (IgG) at days −3 and −1. At day 0, mice were infected with either 104 CFU of EGD or 107 CFU of the sLLO strain. At day 3, mice were sacrificed and colony counts were determined. Points represent individual titers per mouse organ. Bars are the mean ± standard error for two independent experiments with three or four mice per group.
Depletion of neutrophils with the anti-GR1 antibody has a profound effect on murine listeriosis (13, 47). Strains of L. monocytogenes that express hypervirulent mutants of LLO are presumed to be more sensitive to neutrophils and antibiotics because they gain access to the extracellular milieu too quickly (23). In order to rule out the possibility that the sLLO strain is a hyperactive mutant in vivo, we depleted treated mice with the neutrophil-depleting antibody RB6.8C5 prior to infection. Figure 7C and D show the effect of neutrophil depletion on infection with the sLLO and EGD strains. Both strains of L. monocytogenes were responsive to the effect of neutrophil depletion, with about a 100-fold increase in colony counts in the spleen and a 1,000-fold increase in colony counts in the liver. Thus, the sLLO strain was as sensitive to neutrophil deletion as the EGD strain. Based on this data, we concluded that the LLO expressed by the sLLO strain is not attenuating infection due to increased accessibility to the extracellular space.
T-cell response to the sLLO strain.
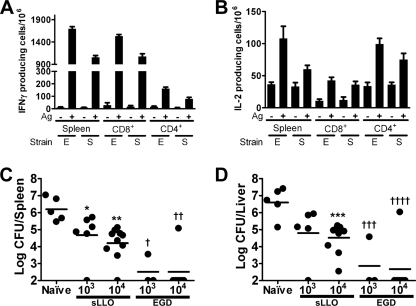
We wanted to determine if the sLLO strain, despite its dramatically reduced in vivo virulence, could elicit T-cell responses. C.B-17 mice were infected with 104 CFU of either the sLLO or EGD strain, and the number of antigen-specific T cells was determined by ELISPOT for IFN-γ or IL-2 (Fig. 8A and B). Three different proteins were used as recall antigens: (i) whole LLO protein with the W492A mutation to decrease bystander cell lysis (35); (ii) the LLO peptide 188-201, which binds the MHC class II molecule I-Ad (22); or (iii) the LLO 91-99 peptide, which binds the MHC class I molecule H-2Kd (52). Both strong CD4 and CD8 T-cell responses were found from both the sLLO and EGD strains after 1 week of infection. Against LLO protein (the W492A mutant), there were ∼1,700 IFN-γ and ∼110 IL-2-producing T cells per million total spleen cells for EGD and ∼1,000 IFN-γ and ∼60 IL-2-producing T cells per million total spleen cells for the sLLO strain. Thus, the sLLO strain elicited a strong T-cell response of comparable magnitude to the wild-type EGD strain at infectious doses 104-fold lower than its LD50. LLO alone did not cause proliferation of CD4+ or CD8+ cells isolated from immunized lymph nodes. In assays involving purified CD4+ or CD8+ cells, exogenous irradiated syngeneic spleen cells were used as the antigen-presenting cells.
FIG. 8.
T-cell responses and protective immunity following infection with the sLLO or EGD strain. (A and B) Mice were infected with 104 CFU of either the sLLO or EGD strain for 7 days. Spleen cells were isolated, and the number of IFN-γ (A)- or IL-2 (B)-producing cells was determined by ELISPOT analysis. Bars represent the mean ± standard error of the mean from three mice per group. For total spleen responses, LLOW492A mutant protein was used as the recall antigen. For CD8+ responses, the LLO(91-99) peptide was used as the recall antigen. For CD4+ responses, the LLO(188-201) peptide was used as the recall antigen. (C and D) Mice were either mock infected or were infected with the indicated doses of the sLLO or EGD strain. After 21 days, mice were rechallenged with 105 CFU of EGD. After 3 days, colony counts were determined. Bars represent the mean of two independent experiments. In panels A and B, E refers to EGD and S refers to the sLLO strain. *, P = 0.0357; **, P = 0.0007; ***, P = 0.0087; †, P = 0.0303; ††, P = 0.0007; †††, P = 0.0357; and ††††, P = 0.0013 by Mann-Whitney U test. Statistical analysis was done by comparing the immunized samples to the unimmunized control.
In order to demonstrate protective immunity, C.B-17 mice were infected with either the sLLO or EGD strain at two different doses (103 or 104 CFU/mouse). After 21 days, both groups were challenged with 105 CFU of EGD (LD50), and colony counts were determined at the 3rd day of infection. Immunization with both strains led to decreased colony counts in the spleen and liver compared to those in the unimmunized control (Fig. 8C and D). The sLLO strain at the 104-CFU dose conferred an ∼100-fold protection, while the EGD strain was more effective, about 1,000-fold. Even though the protection afforded by the sLLO strain is not a robust as that of EGD, it is far better than that of the Δhly strain, where i.v. 109-CFU infection is required to detect any protective immunity (27). Additionally, we infected C.B-17 mice with 106 sLLO strain CFU and then 28 days later rechallenged them with a 10× LD50 dose of EGD (106 CFU/mouse) and found that 7/8 mice were protected from lethality. The mock-treated controls all died following the infection (0/8 survival). Therefore, the sLLO mutant can confer a survival advantage against a lethal challenge with the wild-type strain. Even though there are many variants of L. monocytogenes that have been used as good vaccines (ΔActA and killed but metabolically active), this approach represents a new method for generating recombinant bacteria that can be used as an immunogen (7, 25).
DISCUSSION
A genomic mutant of L. monocytogenes, the sLLO strain, was generated that expressed LLO as a fusion protein with the LPXTG cell wall-targeting motif of internalin A. The novel strain of L. monocytogenes had LLO anchored to its cell wall and secreted about 40-fold less LLO into culture supernatants. In vitro examination demonstrated that the sLLO mutant behaved like the wild-type strain with respects to all known in vitro functions of LLO, including hemolysis, phagosomal escape, cell-to-cell spread, and cytotoxicity. The in vitro behavior of the sLLO strain was confounding, because it was markedly attenuated in virulence (about a 3-log difference) compared to the wild type. One thousand-fold more bacteria were required to get equivalent colony counts over a 3-log range of infective doses, and this translated into increased survival after infection with the sLLO strain.
At face value, the differences between the behavior of the sLLO and EGD strains suggest a role for high levels of secretions of LLO in virulence that cannot be ascribed to its known biology within the infected cell. LLO may be released from L. monocytogenes during its short extracellular stage before uptake by macrophages or after its uptake, when inflammation sets in and cell death takes place. Structural studies during this stage show occasional profiles of free L. monocytogenes cells within cellular debris (34). Once LLO is released from the phagocytes that first capture the bacteria, it may drive inflammation and cytotoxicity that is detrimental to the host. One possible hypothesis is that more LLO is being shed in vacuoles than in broth culture, thereby allowing the sLLO strain to grow in vitro. Even if this is occurring, it cannot account for the profound difference in virulence observed in vivo. We know that within the first few hours L. monocytogenes is localized to the marginal zone, where it resides in phagocytic macrophages (Fig. 4D) (1, 36). Even at these early time points, where the sLLO strain resides in macrophages in vivo, it is incapable of growth, and in fact decays over the first 4 h following infection (Fig. 2G).
The sLLO strain allows us to speculate on the biology of this important experimental model of bacterial infection. In particular, three findings merit discussion. The first finding relates to the pronounced lymphocyte apoptosis which translates into a brief period of susceptibility to the infection. At infective doses that yielded the same bacterial burden with the sLLO and EGD strains, we found both strains localized in the PALS. However, the extent of lymphocyte apoptosis was diminished in the sLLO strain. This occurred both within individual PALS and in the percentage of affected PALS profiles per infected spleen. We infer that EGD was more apoptogenic because it released LLO, most likely at a time when there were few profiles of extracellular LLO in the infective foci (34). This result supports our original hypothesis that LLO was one causative factor of lymphocyte apoptosis and subsequent immunosuppression after infection of wild-type mice (8, 9). One should note that monoclonal antibodies to LLO decreased lymphocyte apoptosis, which can now be explained by neutralization of the extracellular cytotoxic LLO (18).
Second, despite the findings in vitro, tissue phagocytes controlled the growth of the sLLO strain very effectively. This was particularly evident in the SCID mouse, where only very high doses were infective. The results following infection of SCID mice with 108 sLLO strain cells suggest that there was an initial infectious threshold that must be crossed but that required large amounts of secreted LLO. Once this threshold was crossed, the bacteria could grow well in tissue and caused the death of mice. The infection with SCID mice also suggests that lymphocyte apoptosis alone could not explain all of the limited virulence of the sLLO strain. There was no detectable lymphocyte apoptosis in the SCID mice: in fact, we saw no increase in TUNEL-positive cells in the spleen following infection of SCID mice with EGD (9).
We hypothesize that the control of sLLO strain growth by tissue phagocytes is also exerted in normal mice by resident macrophages, represented by the marginal zone macrophages in the spleen. What this apparent control means—whether it results from a microbicidal effect of the marginal zone macrophages or differences in the dynamics of cells entering and leaving—is not clear. What was evident was the lack of growth of EGD and a clear decrease in the sLLO strain during the early colonization of the marginal zone. As others have indicated, L. monocytogenes must be translocated to the PALS, where it can grow exponentially (1, 36, 39). We have attempted to isolate the marginal zone macrophages to determine if they are more bactericidal than other phagocytic populations, but have been unable to isolate a splenic population consistent with the phenotype detected by tissue staining.
A third and important finding is that the sLLO mutant elicited a memory response that allowed for protection against infection from wild-type L. monocytogenes. These findings make the sLLO strain a good vector for immunization against L. monocytogenes or heterologous antigens. L. monocytogenes has been an effective vector for vaccine development requiring the expression of LLO (15, 27, 28, 42). We do not know another immunogenic strain of L. monocytogenes that is cleared by SCID/RAG−/− mice. Our own experiments have shown that that the ActA-deficient strain chronically infects RAG−/− mice; therefore, it is not as safe as the sLLO strain in immunocompromised settings (J. Carrero and E. Unanue, unpublished observations). Given the importance of LLO and other cholesterol-dependent cytolysins in the virulence of a wide range of gram-positive bacteria, these findings merit careful scrutiny, not only for the development of better vaccines, but also to better understand the role of pore-forming bacterial toxins in virulence and immunogenicity during live infection.
Acknowledgments
We thank Kathy Frederick for help with animal husbandry and Roger Belizaire for critical reading of the manuscript.
This work was funded by grant no. AI062832 from the National Institutes of Health (E.R.U.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
The authors have no financial conflicts of interest.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 10 August 2009.
REFERENCES
- 1.Aoshi, T., B. H. Zinselmeyer, V. Konjufca, J. N. Lynch, X. Zhang, Y. Koide, and M. J. Miller. 2008. Bacterial entry to the splenic white pulp initiates antigen presentation to CD8+ T cells. Immunity 29:476-486. [DOI] [PubMed] [Google Scholar]
- 2.Auerbuch, V., D. G. Brockstedt, N. Meyer-Morse, M. O'Riordan, and D. A. Portnoy. 2004. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 200:527-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bancroft, G. J., M. J. Bosma, G. C. Bosma, and E. R. Unanue. 1986. Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: induction of Ia expression by a T cell-independent mechanism. J. Immunol. 137:4-9. [PubMed] [Google Scholar]
- 4.Barry, R. A., H. G. A. Bouwer, D. A. Portnoy, and D. J. Hinrichs. 1992. Pathogenicity and immunogenicity of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect. Immun. 60:1625-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhardwaj, V., O. Kanagawa, P. E. Swanson, and E. R. Unanue. 1998. Chronic Listeria infection in SCID mice: requirements for the carrier state and the dual role of T cells in transferring protection or suppression. J. Immunol. 160:376-384. [PubMed] [Google Scholar]
- 6.Birmingham, C. L., V. Canadien, N. A. Kaniuk, B. E. Steinberg, D. E. Higgins, and J. H. Brumell. 2008. Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature 451:350-354. [DOI] [PubMed] [Google Scholar]
- 7.Brockstedt, D. G., K. S. Bahjat, M. A. Giedlin, W. Liu, M. Leong, W. Luckett, Y. Gao, P. Schnupf, D. Kapadia, G. Castro, J. Y. Lim, A. Sampson-Johannes, A. A. Herskovits, A. Stassinopoulos, H. G. Bouwer, J. E. Hearst, D. A. Portnoy, D. N. Cook, and T. W. Dubensky, Jr. 2005. Killed but metabolically active microbes: a new vaccine paradigm for eliciting effector T-cell responses and protective immunity. Nat. Med. 11:853-860. [DOI] [PubMed] [Google Scholar]
- 8.Carrero, J. A., B. Calderon, and E. R. Unanue. 2004. Listeriolysin O from Listeria monocytogenes is a lymphocyte apoptogenic molecule. J. Immunol. 172:4866-4874. [DOI] [PubMed] [Google Scholar]
- 9.Carrero, J. A., B. Calderon, and E. R. Unanue. 2006. Lymphocytes are detrimental during the early innate immune response against Listeria monocytogenes. J. Exp. Med. 203:933-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrero, J. A., B. Calderon, and E. R. Unanue. 2004. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J. Exp. Med. 200:535-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrero, J. A., and E. R. Unanue. 2006. Lymphocyte apoptosis as an immune subversion strategy of microbial pathogens. Trends Immunol. 27:497-503. [DOI] [PubMed] [Google Scholar]
- 12.Carrero, J. A., H. Vivanco-Cid, and E. R. Unanue. 2008. Granzymes drive a rapid listeriolysin O-induced T cell apoptosis. J. Immunol. 181:1365-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czuprynski, C. J., J. F. Brown, N. Maroushek, R. D. Wagner, and H. Steinberg. 1994. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J. Immunol. 152:1836-1846. [PubMed] [Google Scholar]
- 14.Dancz, C. E., A. Haraga, D. A. Portnoy, and D. E. Higgins. 2002. Inducible control of virulence gene expression in Listeria monocytogenes: temporal requirement of listeriolysin O during intracellular infection. J. Bacteriol. 184:5935-5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietrich, G., J. Hess, I. Gentschev, B. Knapp, S. H. Kaufmann, and W. Goebel. 2001. From evil to good: a cytolysin in vaccine development. Trends Microbiol. 9:23-28. [DOI] [PubMed] [Google Scholar]
- 16.Dramsi, S., and P. Cossart. 2003. Listeriolysin O-mediated calcium influx potentiates entry of Listeria monocytogenes into the human Hep-2 epithelial cell line. Infect. Immun. 71:3614-3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dramsi, S., P. Dehoux, M. Lebrun, P. L. Goossens, and P. Cossart. 1997. Identification of four new members of the internalin multigene family of Listeria monocytogenes EGD. Infect. Immun. 65:1615-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edelson, B. T., P. Cossart, and E. R. Unanue. 1999. Cutting edge: paradigm revisited. Antibody provides resistance to Listeria infection. J. Immunol. 163:4087-4090. [PubMed] [Google Scholar]
- 19.Edelson, B. T., and E. R. Unanue. 2001. Intracellular antibody neutralizes Listeria growth. Immunity 14:503-512. [DOI] [PubMed] [Google Scholar]
- 20.Gaillard, J. L., P. Berche, and P. Sansonetti. 1986. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect. Immun. 52:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gedde, M. M., D. E. Higgins, L. G. Tilney, and D. A. Portnoy. 2000. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect. Immun. 68:999-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geginat, G., S. Schenk, M. Skoberne, W. Goebel, and H. Hof. 2001. A novel approach of direct ex vivo epitope mapping identifies dominant and subdominant CD4 and CD8 T cell epitopes from Listeria monocytogenes. J. Immunol. 166:1877-1884. [DOI] [PubMed] [Google Scholar]
- 23.Glomski, I. J., A. L. Decatur, and D. A. Portnoy. 2003. Listeria monocytogenes mutants that fail to compartmentalize listerolysin O activity are cytotoxic, avirulent, and unable to evade host extracellular defenses. Infect. Immun. 71:6754-6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glomski, I. J., M. M. Gedde, A. W. Tsang, J. A. Swanson, and D. A. Portnoy. 2002. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J. Cell Biol. 156:1029-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goossens, P. L., and G. Milon. 1992. Induction of protective CD8+ T lymphocytes by an attenuated Listeria monocytogenes actA mutant. Int. Immunol. 4:1413-1418. [DOI] [PubMed] [Google Scholar]
- 26.Guzman, C. A., E. Domann, M. Rohde, D. Bruder, A. Darji, S. Weiss, J. Wehland, T. Chakraborty, and K. N. Timmis. 1996. Apoptosis of mouse dendritic cells is triggered by listeriolysin, the major virulence determinant of Listeria monocytogenes. Mol. Microbiol. 20:119-126. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton, S. E., V. P. Badovinac, A. Khanolkar, and J. T. Harty. 2006. Listeriolysin O-deficient Listeria monocytogenes as a vaccine delivery vehicle: antigen-specific CD8 T cell priming and protective immunity. J. Immunol. 177:4012-4020. [DOI] [PubMed] [Google Scholar]
- 28.Ikonomidis, G., Y. Paterson, F. J. Kos, and D. A. Portnoy. 1994. Delivery of a viral antigen to the class I processing and presentation pathway by Listeria monocytogenes. J. Exp. Med. 180:2209-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jablonska, J., K. E. Dittmar, T. Kleinke, J. Buer, and S. Weiss. 2007. Essential role of CCL2 in clustering of splenic ERTR-9+ macrophages during infection of BALB/c mice by Listeria monocytogenes. Infect. Immun. 75:462-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang, J., L. L. Lau, and H. Shen. 2003. Selective depletion of nonspecific T cells during the early stage of immune responses to infection. J. Immunol. 171:4352-4358. [DOI] [PubMed] [Google Scholar]
- 31.Jones, S., and D. A. Portnoy. 1994. Small plaque mutants. Methods Enzymol. 236:526-531. [DOI] [PubMed] [Google Scholar]
- 32.Joseph, S. B., M. N. Bradley, A. Castrillo, K. W. Bruhn, P. A. Mak, L. Pei, J. Hogenesch, M. O'Connell, R., G. Cheng, E. Saez, J. F. Miller, and P. Tontonoz. 2004. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell 119:299-309. [DOI] [PubMed] [Google Scholar]
- 33.Kayal, S., and A. Charbit. 2006. Listeriolysin O: a key protein of Listeria monocytogenes with multiple functions. FEMS Microbiol. Rev. 30:514-529. [DOI] [PubMed] [Google Scholar]
- 34.Merrick, J. C., B. T. Edelson, V. Bhardwaj, P. E. Swanson, and E. R. Unanue. 1997. Lymphocyte apoptosis during early phase of Listeria infection in mice. Am. J. Pathol. 151:785-792. [PMC free article] [PubMed] [Google Scholar]
- 35.Michel, E., K. A. Reich, R. Favier, P. Berche, and P. Cossart. 1990. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol. Microbiol. 4:2167-2178. [DOI] [PubMed] [Google Scholar]
- 36.Muraille, E., R. Giannino, P. Guirnalda, I. Leiner, S. Jung, E. G. Pamer, and G. Lauvau. 2005. Distinct in vivo dendritic cell activation by live versus killed Listeria monocytogenes. Eur. J. Immunol. 35:1463-1471. [DOI] [PubMed] [Google Scholar]
- 37.Nato, F., K. Reich, S. Lhopital, S. Rouyre, C. Geoffroy, J. C. Mazie, and P. Cossart. 1991. Production and characterization of neutralizing and nonneutralizing monoclonal antibodies against listeriolysin O. Infect. Immun. 59:4641-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarre, W. W., and O. Schneewind. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63:174-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neuenhahn, M., K. M. Kerksiek, M. Nauerth, M. H. Suhre, M. Schiemann, F. E. Gebhardt, C. Stemberger, K. Panthel, S. Schroder, T. Chakraborty, S. Jung, H. Hochrein, H. Russmann, T. Brocker, and D. H. Busch. 2006. CD8alpha+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity 25:619-630. [DOI] [PubMed] [Google Scholar]
- 40.Nomura, T., I. Kawamura, K. Tsuchiya, C. Kohda, H. Baba, Y. Ito, T. Kimoto, I. Watanabe, and M. Mitsuyama. 2002. Essential role of interleukin-12 (IL-12) and IL-18 for gamma interferon production induced by listeriolysin O in mouse spleen cells. Infect. Immun. 70:1049-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Connell, R. M., S. K. Saha, S. A. Vaidya, K. W. Bruhn, G. A. Miranda, B. Zarnegar, A. K. Perry, B. O. Nguyen, T. F. Lane, T. Taniguchi, J. F. Miller, and G. Cheng. 2004. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J. Exp. Med. 200:437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan, Z. K., G. Ikonomidis, A. Lazenby, D. Pardoll, and Y. Paterson. 1995. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat. Med. 1:471-477. [DOI] [PubMed] [Google Scholar]
- 43.Park, J. M., V. H. Ng, S. Maeda, R. F. Rest, and M. Karin. 2004. Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists. J. Exp. Med. 200:1647-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Portnoy, D. A., V. Auerbuch, and I. J. Glomski. 2002. The cell biology of Listeria monocytogenes infection: the intersection of bacterial pathogenesis and cell-mediated immunity. J. Cell Biol. 158:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Repp, H., Z. Pamukci, A. Koschinski, E. Domann, A. Darji, J. Birringer, D. Brockmeier, T. Chakraborty, and F. Dreyer. 2002. Listeriolysin of Listeria monocytogenes forms Ca2+-permeable pores leading to intracellular Ca2+ oscillations. Cell. Microbiol. 4:483-491. [DOI] [PubMed] [Google Scholar]
- 47.Rogers, H. W., and E. R. Unanue. 1993. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect. Immun. 61:5090-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith, G. A., H. Marquis, S. Jones, N. C. Johnston, D. A. Portnoy, and H. Goldfine. 1995. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 63:4231-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ton-That, H., L. A. Marraffini, and O. Schneewind. 2004. Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochim. Biophys. Acta 1694:269-278. [DOI] [PubMed] [Google Scholar]
- 50.Tweten, R. K. 2005. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 73:6199-6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Unanue, E. R. 1997. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol. Rev. 158:11-25. [DOI] [PubMed] [Google Scholar]
- 52.Villanueva, M. S., A. J. Sijts, and E. G. Pamer. 1995. Listeriolysin is processed efficiently into an MHC class I-associated epitope in Listeria monocytogenes-infected cells. J. Immunol. 155:5227-5233. [PubMed] [Google Scholar]
- 53.Zenewicz, L. A., and H. Shen. 2007. Innate and adaptive immune responses to Listeria monocytogenes: a short overview. Microbes Infect. 9:1208-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng, S. J., J. Jiang, H. Shen, and Y. H. Chen. 2004. Reduced apoptosis and ameliorated listeriosis in TRAIL-null mice. J. Immunol. 173:5652-5658. [DOI] [PubMed] [Google Scholar]