Abstract
Human brucellosis is caused mainly by Brucella melitensis, which is often acquired by ingesting contaminated goat or sheep milk and cheese. Bacterial factors required for food-borne infection of humans by B. melitensis are poorly understood. In this study, a mouse model of oral infection was characterized to assess the roles of urease, the VirB type IV secretion system, and lipopolysaccharide for establishing infection through the digestive tract. B. melitensis strain 16M was consistently recovered from the mesenteric lymph node (MLN), spleen, and liver beginning at 3 or 7 day postinfection (dpi). In the gut, persistence of the inoculum was observed up to 21 dpi. No inflammatory lesions were observed in the ileum or colon during infection. Mutant strains lacking the ureABC genes of the ure1 operon, virB2, or pmm encoding phosphomannomutase were constructed and compared to the wild-type strain for infectivity through the digestive tract. Mutants lacking the virB2 and pmm genes were attenuated in the spleen (P < 0.05) and MLN (P < 0.001), respectively. The wild-type and mutant strains had similar levels of resistance to low pH and 5 or 10% bile, suggesting that the reduced colonization of mutants was not the result of reduced resistance to acid pH or bile salts. In an in vitro lymphoepithelial cell (M-cell) model, B. melitensis transited rapidly through polarized enterocyte monolayers containing M-like cells; however, transit through monolayers containing only enterocytes was reduced or absent. These results indicate that B. melitensis is able to spread systemically from the digestive tract after infection, most likely through M cells of the mucosa-associated lymphoid tissue.
Brucella spp. are facultative intracellular gram-negative bacteria that cause chronic infections in mammalian hosts. Brucella melitensis infects mainly goats and sheep, causing abortion, placentitis, and infertility, and it is considered the most pathogenic species of Brucella to humans (15). Human brucellosis is an important worldwide zoonosis that causes social and economic impacts on public health and animal production. The disease is prevalent mostly in the Middle East, Mediterranean, Central Asia, Latin America, and North Africa, with over 500,000 new cases of human brucellosis registered annually (41).
Human brucellosis usually does not present with specific clinical symptoms. It is frequently associated with intermittent fever, anorexia, weakness, soreness, and inflammation affecting several organs. Spondylitis, arthritis, osteomyelitis, and epididymo-orchitis are common manifestations of the infection. However, infection of the heart and brain may also occur and can result in death (34). Human brucellosis caused by B. melitensis results mostly from ingestion of unpasteurized milk and dairy products from infected goats, sheep, camels, and cattle. The importance of food-borne infection is underlined by a recent study in Mexico, which reported that in 98% of cases, the major risk factor for brucellosis was ingestion of contaminated dairy products (32). Human infections may also be acquired via breaks in the skin, mucosal contact, or inhalation of aerosols containing bacteria that may occur during occupational contact with infected animals or during laboratory manipulation of the agent (15, 22). The epidemiological evidence showing the important role of food-borne infection (consumption of raw milk or unpasteurized cheese) in human zoonosis suggests that the digestive tract is an important site of B. melitensis infection (22). However, the mechanisms of entry to the host after food-borne infection, which is most likely to occur somewhere in the digestive tract, are poorly known.
The pathogenicity of Brucella species is associated with its capability to survive and replicate within phagocytic and nonphagocytic cells which results in its ability to cause chronic infection in the host (23). These aspects of Brucella infection have been intensively investigated in cell culture or animal models. The mouse is the animal model most frequently used to study the chronic infection caused by pathogenic Brucella species (20). The type IV secretion system (T4SS) and lipopolysaccharide (LPS) are among the most important virulence factors already identified in Brucella. While the T4SS is required for intracellular survival and replication, defects in LPS structure that eliminate O antigen result in a number of phenotypes, including sensitivity to complement-mediated killing, increased sensitivity to killing by antimicrobial peptides, increased maturation of dendritic cells, and attenuation in tissue culture and animal infection models (9, 25, 31). Recent studies have demonstrated a potential role of urease and bile salt hydrolase in oral infection by Brucella abortus and Brucella suis in mice (4, 18, 52). However, the roles of T4SS and LPS during B. melitensis infection via the digestive tract are not known.
A better understanding of the pathogenesis of human brucellosis requires an expansion of our knowledge about the mechanisms involved in invasion and dissemination of the organism as well as the host immune response to infection. Although the digestive tract is thought to be an important route of B. melitensis infection in humans, the kinetics of infection and innate immune response in the digestive tract are not well characterized. Furthermore, there is little information available regarding the requirement of virulence factors during establishment of systemic infection through the digestive tract. Therefore, in this study, we characterized a mouse model of oral infection and investigated the requirement of B. melitensis urease, T4SS, and LPS O antigen for infection via this route.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
Brucella melitensis virulent strain 16M and isogenic mutant strains were used in this study. Bacterial inocula for infection of mice were cultured on tryptic soy agar plates (TSA) (Difco/Becton Dickinson, Sparks, MD) with 5% sheep blood at 37°C in a 5% CO2 atmosphere for 4 days and resuspended in sterile phosphate-buffered saline (PBS). Strains isolated from mice were cultured on TSA with or without appropriate antibiotics or in Farrell's medium (Brucella selective supplement SR83; Oxoid, United Kingdom) to count the number of CFU. Potato infusion agar plates with appropriate antibiotics were used to select candidate colonies of mutant strains. Strains were also grown on tryptic soy broth (TSB) (Difco/Becton Dickinson) at 37°C with agitation. Carbenicillin (100 mg/liter) or kanamycin (100 mg/liter) was added when necessary. All experiments with live Brucella strains were performed under biosafety level 3 conditions.
Generation of mutant strains and recombinant DNA techniques.
B. melitensis strains carrying deletion of ureA, ureB, and ureC of the ure1 operon, a polar deletion of virB2, or disruption of pmm (phosphomannomutase), named TAP4 (ureABC1), TAP1 (virB2), and TAP5 (pmm), respectively, were constructed as detailed below.
For generating the ureABC1 mutant, a plasmid that generates a marked deletion of ureC1, ureB1, and ureA1 genes of the ure1 operon, encoding the αβγ subunits of urease (BMEI1652 to BMEI1654, GenBank accession number NC003317), was constructed. Up- and downstream fragments of ure1 were amplified using primers BM1652upF-BM1652upR and BM1654dnF-BM1654dnR, respectively (Table 1). These PCR products were cloned into the pCR2.1 TOPO vector using a TOPO-TA cloning kit (Invitrogen, Carlsbad, CA). The upstream fragment was excised by double digestion with XbaI and EcoRI and cloned into pBluescript KS (Stratagene, CA). The downstream fragment was excised by digesting with EcoRI and SalI and cloned into the same plasmid. Then, the kanamycin resistance cassette from pUC4-KSAC (Amersham Pharmacia Biotech, Freiburg, Germany) was digested by EcoRI and cloned between up- and downstream fragments, generating pUR. The correct sequence and direction of cloned fragments were verified by sequencing. This pUR plasmid was introduced by electroporation into B. melitensis 16M, and clones with recombinant resistance to kanamycin and sensitivity to carbenicillin were selected. The deletion of ureA1, ureB1, and ureC1 from chromosome I of B. melitensis was confirmed by Southern blotting (not shown). To confirm the mutant phenotype, loss of urease activity for strain TAP4 (ΔureABC1) was confirmed using urease test broth (Difco).
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Bacterial strain, plasmid, or primer | Description, relevant genotype, or sequencea |
|---|---|
| Bacterial strains | |
| B. melitensis | |
| 16M | Wild type |
| TAP1 (ΔvirB2) | ΔvirB2::kan |
| TAP4 (Δure1) | ΔureABC1::kan |
| TAP5 (Δpmm) | pmm::pNH1.3 |
| E. coli DH5α | Used for cloning |
| Plasmids | |
| pCR2.1-TOPO | TA cloning vector |
| pBluescript KS | Cloning vector |
| pUC4-KSAC | Plasmid with KSAC kanamycin resistance cassette |
| pUR | PCR products of ure fragments from nucleotides 1703818 to 1704817 and nucleotides 1707273 to 1708272 separated by KSAC in pBluescript KS |
| pAV2.2 | PCR products of virB fragments from nucleotides 988 to 1562 and nucleotides 1796 to 2458 separated by KSAC in pBluescript KS |
| pNH1.3 | PCR product of pmm nucleotides 355 to 610 in pBluescript KS |
| Primers | |
| BM1652upF | TCTAGAGGCTTGCAGGAGATTGAT (XbaI) |
| BM1652upR | GAATTCGCCGGAGTATGAGATATG (EcoRI) |
| BM1654dnF | GAATTCGGCGGCCATCGCGATCAA (EcoRI) |
| BM1654dnR | GTCGACCGTCAACGGGACCGGTGA (SalI) |
| PMM355F | GCTCCACCGAAACCGATGC |
| PMM610R | TCGCTTTTGCCCCATTGG |
The restriction sites underlined in the primer sequences are shown in parentheses after the sequences.
Since the virB operon is 100% conserved between B. abortus and B. melitensis, a previously constructed plasmid, pAV2.2 (18a), designed based on the B. abortus genome sequence was used for construction of a B. melitensis strain carrying a virB2 deletion. Plasmid pAV2.2 is pBluescript KS with PCR products of virB nucleotides 988 to 1562 and virB nucleotides 1796 to 2458 (GenBank accession number AF226278) separated by a kanamycin resistance cassette. This plasmid was introduced by electroporation into B. melitensis 16M, and clones with recombinant resistance to kanamycin and sensibility to carbenicillin were selected. The deletion of virB2 from chromosome II of B. melitensis in strain TAP1 (ΔvirB2) was confirmed by Southern blotting (not shown).
For construction of a LPS rough strain of B. melitensis, plasmid pNH1.3, which is a pBluescript KS derivative containing an internal fragment of pmm amplified using primers PMM355F and PMM610R (Table 1), was used. This plasmid was introduced by electroporation into B. melitensis 16M, and clones with resistance to carbenicillin were selected. Integration of the plasmid into the genome results in two truncated copies of pmm (pmm [GenBank accession number BAB2_0855] of B. abortus or BMEII0899 of B. melitensis). The LPS rough phenotype of strain TAP5 (Δpmm) was confirmed by crystal violet staining. All bacterial strains used or generated in this study as well as all plasmids and primers are listed in Table 1.
Infection of mice.
Six- to 8-week-old female BALB/c ByJ mice were obtained from Jackson Laboratory (Bar Harbor, ME). Groups of 5 to 10 mice were inoculated intragastrically by gavage with 0.1 ml PBS containing approximately 1 × 1010 CFU of wild-type (wt) B. melitensis or mutant strains. Control mice were given PBS intragastrically with PBS. For mixed infection experiments, groups of five mice were inoculated intragastrically with 0.1 ml PBS containing approximately 2 × 1010 CFU of 1:1 mixture of the wt B. melitensis strain and one mutant strain. Infected mice were kept in cages within a biosafety level 3 facility. At the indicated time points, mice were euthanized using inhalation anesthesia followed by cervical dislocation. At necropsy, the organs were collected aseptically for bacteriologic analyses. Fragments of the spleen, liver, mesenteric lymph node (MLN), Peyer's patches, ileum, colon, and cecum were collected in sterile PBS, weighed, homogenized, and plated for CFU counting. Tissue samples were also snap-frozen in liquid nitrogen and stored at −80°C for RNA isolation. Additional fragments were fixed by immersion in 10% buffered formalin for histopathology.
RNA isolation, cDNA, and quantitative reverse transcription-PCR (RT-PCR).
Total RNA from mouse tissues was isolated using the Tri reagent (Molecular Research Center, Cincinnati, OH) according to the manufacturer's instructions. The cDNA was prepared with 500 ng of total RNA from each sample using TaqMan reverse transcription reagents (Applied Biosystems, Branchburg, NJ). Briefly, purified RNA was mixed 5 μl of 10× TaqMan buffer, 11 μl of 25 mM MgCl2, 10 μl of deoxynucleoside triphosphates, 2.5 μl of random hexamer primers, 1 μl of RNase inhibitor, 1.25 μl of reverse transcriptase, and water to a final volume of 50 μl. Reverse transcription was performed at 25°C for 10 min followed by 48°C for 30 min, and inactivation was performed at 95°C for 5 min. Real-time PCR was performed using 4 μl of cDNA, 300 nM of each pair of primers, and SYBR green PCR master mix (Applied Biosystems). Reactions were run in an ABI PRISM 7900HT fast real-time pCR system (Applied Biosystems) according to the manufacturer's instructions. The data were analyzed using the comparative threshold cycle method (31a). The change in gene expression in infected mice was normalized against glyceraldehyde-3-phosphate dehydrogenase expression and calculated relative to the gene expression level in noninfected mice. The genes analyzed in this study and the primers used for real-time RT-PCR analysis are listed in Table 2.
TABLE 2.
Primer pairs used for real-time RT-PCR analysis of genes
| Genea | Primer sequence |
|---|---|
| GAPDH | 5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
| 5′-AGGTCGGTGTGAACGGATTTG-3′ | |
| IFN-γ | 5′-TCAAGTGGCATAGATGTGGAAGAA-3′ |
| 5′-TGGCTCTGCAGGATTTTCATG-3′ | |
| IL-6 | 5′-GAGGATACCACTCCCAACAGACC-3′ |
| 5′-AAGTGCATCATCGTTGTTCATACA-3′ | |
| IL-10 | 5′-GGTTGCCAAGCCTTATCGGA-3′ |
| 5′-ACCTGCTCCACTGCCTTGCT-3′ | |
| IL-12 | 5′-GGAAGCACGGCAGCAGAATA-3′ |
| 5′-AACTTGAGGGAGAAGTAGGAATGG-3′ | |
| iNOS | 5′-TTGGGTCTTGTTCACTCCACGG-3′ |
| 5′-CCTCTTTCAGGTCACTTTGGTAGG-3′ | |
| KC | 5′-TGCACCCAAACCGAAGTCAT-3′ |
| 5′-TTGTCAGAAGCCAGCGTTCAC-3′ | |
| MIP-3α | 5′-CCAGGCAGAAGCAAGCAACT-3′ |
| 5′-TCGGCCATCTGTCTTGTGAA-3′ | |
| MIP-2 | 5′-AGTGAACTGCGCTGTCAATGC-3′ |
| 5′-AGGCAAACTTTTTGACCGCC-3′ | |
| TNF-α | 5′-CATCTTCTCAAAATTCGAGTGACAA-3′ |
| 5′-TGGGAGTAGACAAGGTACAACCC-3′ |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IFN-γ, gamma interferon; TNF-α, tumor necrosis factor alpha.
Histopathology.
Fragments of the spleen, liver, ileum with Peyer's patches, and colon were collected from mice at selected time points. Tissue fragments were fixed for 24 h in 10% neutral-buffered formalin, processed for paraffin embedding, sectioned in 5-μm-thick sections, and stained with hematoxylin and eosin. Inflammatory lesions were scored by a veterinary pathologist in a blinded analysis.
In vitro susceptibility to low pH.
The pH of the PBS was adjusted to pH 2, 4, or 7 by adding 1 N HCl. PBS solutions with different pHs were inoculated with 1 × 109 CFU/ml of wild-type or mutant strains and incubated at 37°C. At the end of 30 min of incubation, serial dilutions of each culture were plated on TSA for CFU counting.
In vitro susceptibility to bile salt.
The susceptibility of B. melitensis strains to bile salts was tested by the method of Delpino et al. (18). The wt and mutant strains were grown in TSB to exponential phase and were suspended at 108 CFU/ml in TSB alone or in TSB containing 5% or 10% bovine bile (oxgall; U.S. Biological, MA). Cultures were incubated at 37°C with agitation for 18 h, and aliquots were plated on TSA for CFU counting.
Urease enzyme activity qualitative analyses.
B. suis 1330, B. melitensis 16M and TAP4 (ureABC1) were grown for 72 h in TSB at 37°C with agitation. Fifty microliters of culture was used to inoculate 5-ml volumes of urease test broth (Difco). The cultures were incubated at 37°C with agitation. At 8, 24, 48, 72, and 96 h after incubation, the color change in urease test broth was checked. The presence of a pink color was indicative of urease activity. The noninoculated urease test broth was used as a negative control.
Infection of polarized intestinal epithelial cell monolayers.
The in vitro M-cell coculture model was essentially as described by Gullberg et al. (24). The Caco-2 cell line, an enterocyte cell line derived from colorectal adenocarcinoma, was cultured in Dulbecco modified Eagle medium (Sigma) supplemented with 20% fetal calf serum, 1% nonessential amino acids, and antibiotics/antimycotics (Sigma) at 37°C in 5% CO2. After trypsinization, 5 × 105 Caco-2 cells were seeded onto 1.2-cm-diameter Transwell (Corning) polycarbonate inserts (3-μm pore) and incubated at 37°C in 5% CO2 for 14 days to allow differentiation. Raji B cells were grown in RPMI 1640 medium (Gibco-BRL) supplemented with 10% fetal calf serum and antibiotics/antimycotics at 37°C in 5% CO2 and were added to the basal compartment. A total of 5 × 105 Raji B cells were added to the basolateral chamber of insert. The cocultures were maintained for 6 days. Monocultures of Caco-2 cells cultivated as described above (except that Raji B cells were replaced with Raji B-cell medium) were used as controls.
Transepithelial electrical resistance (TER) was measured before and after each experiment, using the voltohmmeter Millicell-ERS (Millipore). To infect the monolayers, B. melitensis strains were grown overnight in TSB at 37°C. A bacterial suspension of 50 μl in supplemented Dulbecco modified Eagle medium containing approximately 5 × 107 bacteria was added to the epithelial monolayer from the lumenal side. The basolateral medium was sampled over time, serially diluted, and plated on TSA with the appropriate antibiotic to estimate the number of CFU.
Scanning electron microscopy.
After infection, monolayers were washed three times with PBS and then fixed in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 16 h at 4°C. The samples were rinsed with 0.1 M sodium phosphate buffer to remove fixative and then dehydrated in graduating increments of ethanol for 15 min for each step with three changes of 95% ethanol and three changes of 100% ethanol (200 proof). Following dehydration through graded ethanol solutions (30 min each in 25%, 50%, 75%, and 100% absolute alcohol), hexamethyldisilazane (Electron Microscopy Sciences, Hatfield, PA) was added to each insert and allowed to evaporate overnight in a fume hood. The samples were then mounted on aluminum stubs using double sticky carbon tape and then sputter coated with gold using a Pelco SC-7 automatic sputter coater (Ted Pella, Inc., Redding, CA). The specimens were examined using a FEI XL-30 TMP scanning electron microscope (FEI Company, Hillsboro, The Netherlands), and images were acquired using iTEM software from Olympus Soft Imaging Systems (Lakewood, CO).
Immunofluorescence microscopy.
After infection, monolayers were washed three times with PBS, fixed in a solution of 4% paraformaldehyde for 1 h at room temperature, and washed three times in PBS. The cells were permeabilized with 0.1% (vol/vol) Triton X-100 in PBS for 10 min. Following three washes (each wash performed for 5 min on a shaker), the samples were blocked in PBS with 1% goat serum for 60 min. The samples were washed three times in PBS with agitation and incubated for 60 min with rabbit anti-ZO-1 (ZO-1 is zona occludens 1) (1:40, Invitrogen) and 2% goat serum in PBS. Monolayers were washed five times with PBS with agitation and incubated with goat anti-rabbit immunoglobulin G labeled with Alexa Fluor 594 (1:200; Invitrogen) and 2% goat serum in PBS for 60 min with shaking. After additional washes, the membranes were removed with a scalpel and the monolayers were placed with their side up on glass slides, before a coverslip (no. 1.5) with Slow Fade Gold antifade mounting medium (Invitrogen) supplemented with Hoechst dye (0.5 μg/ml; Molecular Probes, Inc.) was placed over the monolayer. The slides were examined by scanning microscopy (Zeiss LSM5 Pascal), and image analysis was performed by using LSM5 Image Browser.
Statistical analyses.
The CFU data were logarithmically transformed and expressed as means and standard deviations. The change in gene expression data were also logarithmically transformed and expressed as geometric means and standard deviations. Data were analyzed by analysis of variance, and means were compared by unpaired Student's t test. For mixed infection experiments, data were logarithmically transformed and means were compared by paired Student's t test. The competitive index was calculated as log (CFU of mutant/CFU of wild type) and adjusted in each case to the ratio of mutant to wild type in the inoculum. A P value of ≤0.05 was considered significant.
RESULTS
Kinetics of Brucella melitensis infection through the digestive tract in mice.
In order to study the kinetics of B. melitensis infection via the gastrointestinal tract, female BALB/c mice were inoculated with 1 × 1010 CFU of B. melitensis virulent strain 16M by gavage. The bacterial loads in the ileum, colon, cecum, Peyer's patches, MLN, spleen, and liver were assessed at 8 h and 1, 3, 7, 14, and 21 dpi. Although 1 × 1010 CFU per animal may be considered a high inoculum, it was chosen in order to obtain consistent colonization of tissues, particularly during early stages of infection, which is crucial to characterizing the dissemination of B. melitensis from the digestive tract to systemic sites of infection, and for comparison of colonization by wild-type and mutant B. melitensis strains. As shown in Fig. 1, higher numbers of B. melitensis CFU were recovered from the colon or cecum at an early time point during the course of infection, i.e., 8 h postinfection (0.3 dpi). These numbers quickly decreased at subsequent time points during the course of infection, but a low number of bacteria was still detectable in the large intestine (colon and cecum) up to 21 dpi. In the ileum, a slight, but not statistically significant, decline in the numbers of CFU was observed throughout the time course of infection. Lack of statistical significance in this case reflected a high variation among experimental animals in the number of bacteria recovered from the ileum. Conversely, small numbers of B. melitensis CFU were cultured from the MLN, spleen, and liver at 8 h and 1 dpi. The bacterial load in these organs significantly increased at 3 and 7 dpi and stabilized at 14 and 21 dpi. In the Peyer's patches, bacterial load was very low during the whole period, with significant increases in the number of CFU at 7 and 14 dpi, which returned to undetectable levels at 21 dpi, indicating that colonization of Peyer's patches is transient. Bacteria were also detected by bacteriology in the blood and cervical lymph node transiently beginning at 1 dpi (data not shown).
FIG. 1.
Kinetics of Brucella melitensis infection through the digestive tract in BALB/c mice. Fragments of ileum (A), cecum (B), colon (C), Peyer's patch (PP) (D), MLN (E), spleen (F), and liver (G) were homogenized in PBS and plated for CFU counting at 0.3, 1, 3, 7, 14, and 21 dpi. Values for different time points that are statistically significantly different are indicated by brackets and asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Data points represent arithmetic means plus standard errors (error bars) of 5 or 10 mice at each time point. N.S., not significantly different.
Brucella melitensis does not elicit an inflammatory response or histopathological lesions in the gut.
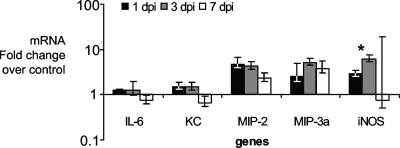
Inflammatory responses during Brucella infection have been characterized previously in the spleens of mice inoculated via the intraperitoneal route. Gamma interferon, tumor necrosis factor alpha, and interleukin 12 (IL-12) were upregulated in the spleen during Brucella infection in mice (42a, 50a). Importantly, the spleens from orally infected mice had similar profiles of mRNA expression for these inflammatory genes (data not shown). To investigate whether B. melitensis elicits an inflammatory response in the gut during oral infection, mRNA expression of selected proinflammatory chemokines and cytokines was assessed by real-time RT-PCR in the ilea of mice sampled at 1, 3, and 7 dpi as shown in Fig. 2. IL-6 and the chemokines macrophage inflammatory protein 2 (MIP-2/Cxcl2 [Cxcl2 is chemokine {C-X-C motif} ligand 2]), macrophage inflammatory protein 3 alpha (MIP-3α/Ccl20 [CCl20 is chemokine {C-C motif} ligand 2]), and neutrophil chemoattractant (keratinocyte-derived chemokine [KC]/Cxcl1) mRNA expression did not significantly change over the time course of infection. While IL-6 and KC did not have any significant increase in expression, MIP-2 and MIP-3α had approximately fourfold increases in mRNA levels at all time points. The inducible nitric oxide synthase (iNOS) mRNA expression increased significantly (P = 0.05) at 3 dpi, but showed only a mild (approximately sixfold) increase in mRNA levels compared to uninfected controls.
FIG. 2.
Expression of proinflammatory genes in the ilea of BALB/c mice infected with Brucella melitensis at 1, 3, and 7 dpi in comparison to uninfected controls as measured by quantitative real-time RT-PCR. The expression of IL-6, KC, MIP-2, MIP-3α (MIP-3a), and iNOS genes is shown. The change in expression for iNOS is statistically significantly different (P = 0.05) between 1 and 3 dpi (*). Data points represent geometric means ± standard errors (error bars) of five mice per data point.
To characterize the inflammatory lesions caused by B. melitensis in intestinal tissues of orally infected mice, sections of the ileum with Peyer's patches and sections of the colon, liver, and spleen were histologically evaluated, and in situ localization of B. melitensis was performed by immunohistochemistry (data not shown). No significant inflammation was observed in the ileum and cecum during the time course of infection. In a few mice, there was rare exocytosis of neutrophils in the ileal mucosa. A variable degree of lymphoid hyperplasia was observed in the Peyer's patches. The absence of B. melitensis-induced inflammatory lesions in the gut is in good agreement with the profile of chemokine mRNA expression, which was characterized by extremely modest increases in expression. Immunolabeled B. melitensis was rarely observed in the cytoplasm of mononuclear inflammatory cells in the lamina propria. Inflammatory changes in the spleen were characterized by mild foci of neutrophils and mild to moderate histiocytosis in the marginal zone. Intracellular immunolabeled B. melitensis were rarely detected within macrophages in the red pulp. In the liver, multiple granulomas composed of macrophages, lymphocytes, and rare neutrophils were observed. Although immunohistochemistry is a tool to localize Brucella in tissues, in this study there was poor detection of immunolabeled B. melitensis due to the low sensitivity of this method in tissues of mice as previously described (35a).
Urease, T4SS, and LPS O antigen are required for wt levels of B. melitensis infection via the digestive tract.
Previous reports have indicated that urease of B. suis and B. abortus may play an important role in survival and invasion through the digestive tract (4, 52), whereas the T4SS and LPS have been recognized as important virulence factors for intracellular survival, but their roles during the initial steps of infection through the oral route are not known (9, 31). In order to investigate the requirement of urease, T4SS, and LPS for establishing B. melitensis infection through the digestive tract in mice, mutant strains carrying deletion of the ureABC1 of the ure1 operon (TAP4), deletion of virB2 (TAP1), or insertional inactivation of pmm (TAP5) were constructed. Deletion of ureABC1 in the ureABC1 strain TAP4 was confirmed by Southern blotting, and lack of urease activity was assessed by using urease test broth. The wt strain turned the color of the medium at 48 h, while strain TAP4 (ureABC1) required 96 h at 37°C with agitation. This residual activity may be from the second urease, encoded by the ure2 locus, which is predicted to be functional in B. melitensis (52). Deletion of virB2 in strain TAP1 was also confirmed by Southern blotting. Deletion of pmm in strain TAP5 was confirmed by the mutant LPS phenotype as demonstrated by positive crystal violet staining.
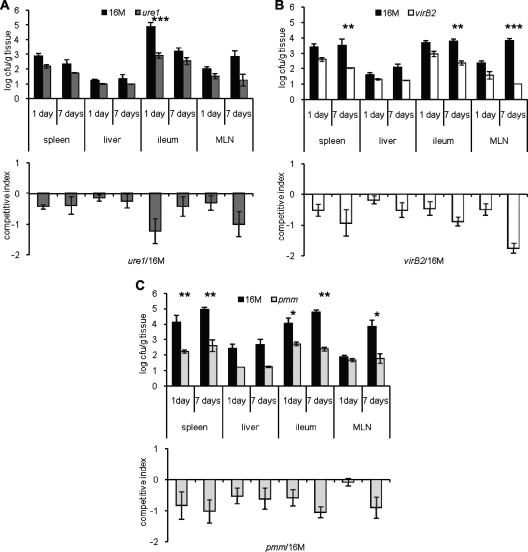
BALB/c mice were intragastrically inoculated with the wt strain or one of the mutant strains, and local and systemic bacterial dissemination and multiplication were established by CFU counts. Bacterial loads were determined in the ileum, MLN, spleen, and liver at 1 and 7 dpi. At 1 dpi, bacteria were recovered from the MLN or spleen in higher numbers in mice infected with the wt strain compared to mice infected with the virB2 (TAP1) and pmm (TAP5) mutant strains, but the differences were not statistically significant (Fig. 3A). At 7 dpi, there were significantly lower numbers of CFU recovered from the ileum, MLN, spleen, and liver of mice infected with the virB2 (TAP1) and pmm (TAP5) mutant strains compared to the tissues of mice infected with the wt strain. Lower numbers of CFU of the ure1 mutant (TAP4) were also recovered in the spleen and liver (Fig. 3B). Mixed infections were performed to confirm attenuation of mutant strains by the intragastric route, and similar results were observed for virB2 (TAP1; Fig. 4A and B) and pmm (TAP5; Fig. 4C and D). Lower numbers of CFU of the ureABC1 (TAP4) mutant strain were recovered only in the ileum at 1 dpi and in the MLN at 7 dpi (Fig. 4E and F). Taken together, these results indicate that the virB2 and pmm mutants were attenuated in the spleen, liver, and MLN at 7 dpi.
FIG. 3.
Number of CFU of bacteria in tissues of BALB/c mice inoculated intragastrically with wild-type or mutant Brucella melitensis strains. The wt strain 16M and mutant strains TAP4 (ure1), TAP1 (virB2), and TAP5 (pmm) were used. Fragments of the ileum, MLN, spleen, and liver were homogenized in PBS and plated for CFU counting at 1 dpi (A) and 7 dpi (B). There are significant differences between wt and mutant strains as indicated by asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Black circles represent the values for individual animals, and black lines represent arithmetic means of 5 or 10 mice at each time point.
FIG. 4.
Number of CFU of bacteria from tissues of BALB/c mice intragastrically inoculated with a mixed suspension (1:1) of wild-type Brucella melitensis 16M and one of the three mutant strains, strain TAP4 (ure1), TAP1 (virB2), and TAP5 (pmm), as indicated. Fragments of ileum, MLN, spleen, and liver were homogenized in PBS and plated for CFU counting at 1 and 7 dpi. The top graphs in panels A to C show the means and errors (error bars) of the number of CFU of wt and mutant strains. The bottom graphs in panels A to C show the competitive index of mixed infection, calculated as log (CFU of mutant/CFU of wild type) adjusted for the same ratio in the inoculum. Differences between the CFU values for wt and mutant strains were analyzed using a repeated-measure analysis of variance for paired data (GraphPad Instat). Values that are statistically significantly different are indicated by asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
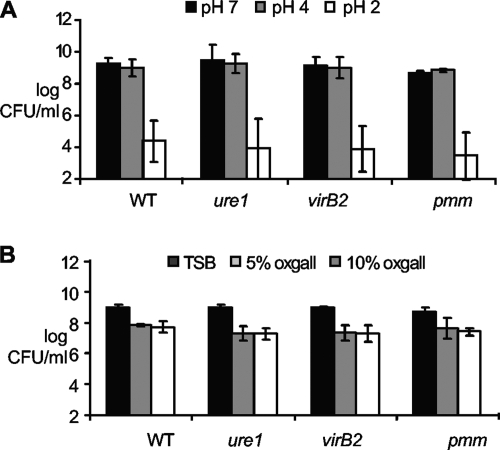
Reduced colonization of the pmm, virB2, and ure1 mutant strains is not associated with increased susceptibility to acid pH or bile salts in vitro.
Both gastric acidity and bile salts are major barriers to bacterial survival in the digestive tract (8). In order to test whether mutants lacking pmm (TAP5), virB2 (TAP1), or ureABC1 (TAP4) displayed increased susceptibility to gastric acidity, a low-pH susceptibility assay was performed. No significant differences were observed in the numbers of CFU of the wt or mutant strains recovered from pH 7 and pH 4 medium after 30 min. All B. melitensis strains were killed at pH 2, with no significant differences between the wt and mutant strains (Fig. 5A). Concurrently, the susceptibility of wt and mutant strains to bile salts was assessed using TSB with 0, 5, or 10% bovine bile. There was a decrease of 1.2 orders of magnitude for B. melitensis 16M and the pmm mutant at 5 or 10% of bovine bile, whereas virB2 and ureABC1 mutants were decreased by 1.6 orders of magnitude. However, there was no significant difference between the wt and mutant strains (Fig. 5B). These results indicate that wt and mutant strains have similar susceptibilities to low pH and bile salts, supporting the notion that reduced colonization by the mutant strains was not the result of reduced resistance to acid pH or bile salts during passage through the digestive tract.
FIG. 5.
Survival of wild-type and mutant Brucella melitensis strains at low pH (A) and bile salts (B). (A) The bacteria were incubated in PBS at different pHs for 30 min at 37°C. (B) The bacteria were incubated in TSB alone or supplemented with bile salts for 18 h with agitation at 37°C. Bacteria were diluted and plated for CFU counting. Data points represent arithmetic means ± standard deviations (error bars) from two or three different independent experiments. The values for the strains were not statistically significantly different by unpaired t test.
B. melitensis can penetrate enterocyte monolayers via M-like cells.
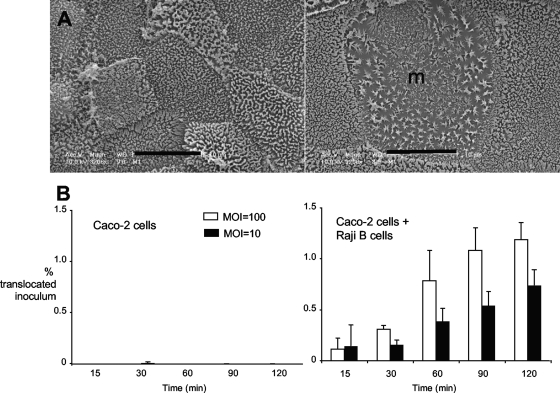
To gain insight into how B. melitensis disseminates from the gastrointestinal tract, we used an in vitro model to study penetration of polarized epithelial monolayers containing only enterocytes or containing enterocytes and lymphoepithelial cells (M cells) (24). In this model, differentiation of polarized Caco-2 cells to M-like cells is induced by coculture with Raji B cells, which is a modification of the procedure previously reported by Kerneis et al. (30). To determine whether breach of the intestinal mucosa by B. melitensis occurs via invasion of enterocytes or via M cells, we determined whether B. melitensis can translocate through either enterocyte monolayers or monolayers containing M-like cells. Apical infection of these cell monolayers with B. melitensis at ratios of 10 or 100 bacteria per cell resulted in recovery of B. melitensis 16M from the basolateral side of monolayers containing M-like cells within 15 min of infection (Fig. 6B, right panel). The numbers of translocated bacteria increased until 2 h after inoculation, after which they remained constant. The total number of translocated bacteria was around 1% of the inoculum, which is of low efficiency compared to the 20% value that has been reported for Salmonella enterica serotype Typhimurium using the same model and range of inocula (35). In contrast to the cocultures, monolayers containing only Caco-2 enterocytes had very rare translocation (10- to 1,000-fold-fewer bacteria than in cocultures) of B. melitensis up to 2 h in some experiments, and no recovery of B. melitensis from the basolateral compartment in other experiments, suggesting that translocation through enterocytes is much less efficient than translocation via M-like cells.
FIG. 6.
Transcytosis of wild-type and mutant B. melitensis strains through Caco-2 enterocyte monolayers and monolayers containing M-like cells. (A) Scanning electron microscopy of representative Transwell membranes containing Caco-2 cells only (left) or Caco-2 cells cocultured with Raji B cells (right). m, M cell. (B) Transcytosis of B. melitensis mutants through monolayers containing Caco-2 enterocytes only (left) or Caco-2 cells cocultured with Raji B cells (right). (Left) The Caco-2 cells were infected at a multiplicity of infection (MOI) of 10 and 100. Data shown are from individual experiments that are representative of at least two experimental replicates.
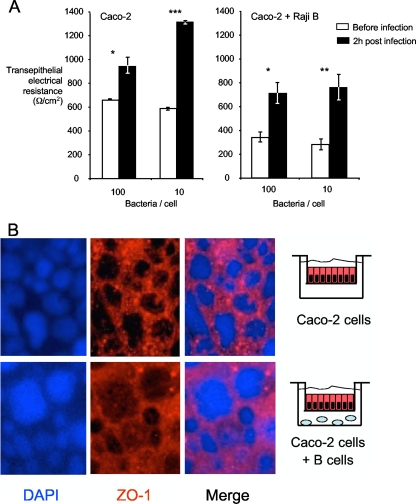
Enteric pathogens such as S. Typhimurium and enteropathogenic Escherichia coli have been shown to induce loss of transepithelial resistance by disruption of tight junctions in epithelial monolayers (17, 48). To exclude the possibility that penetration of enterocyte monolayers containing M-like cells by B. melitensis is a result of damage to the monolayer, we determined whether infection of monolayers with B. melitensis affected their integrity by measuring transepithelial electrical resistance. The TER of monolayers was measured before and 2 h after treatment with B. melitensis 16M (Fig. 7). A value of above 175 Ω/cm2 in this model is considered indicative of an intact monolayer (49). B. melitensis infection did not lead to damage to the monolayer or loss of epithelial integrity (Fig. 7A), as the transepithelial resistance actually increased after infection, suggesting that the monolayer was still intact. The Caco-2 cells cocultured with Raji B cells did not achieve a TER value as high as that of Caco-2 monolayers, consistent with a previous report (24). Staining of monolayers with antibodies specific for the tight-junction protein ZO-1 confirmed the presence of larger cells with M-cell-like morphology in the Caco-2 cell/Raji B-cell cocultures (Fig. 7B). Further, no loss of ZO-1 from cell-cell junctions was observed after infection, providing further evidence that B. melitensis did not disrupt the monolayer. These data suggest that transit of the monolayer containing M-like cells by B. melitensis occurs by transcytosis, rather than via disruption of cell-cell junctions.
FIG. 7.
Interaction of B. melitensis with polarized Caco-2 enterocyte monolayers and Caco-2 cells with M-like cells. (A) Transepithelial electrical resistance was measured before infection of monolayers and 2 h after infection. Data shown are from individual experiments that are representative of at least three experimental replicates. The statistical significance of differences in the values for the experimental groups was assessed using Student's t test. Values that are statistically significantly different are indicated by asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) Distribution of the tight-junction marker ZO-1 in monolayers after infection with B. melitensis. The large cells in the bottom panels have the characteristic large size of M cells. DAPI, 4′,6′-diamidino-2-phenylindole.
DISCUSSION
Although the predominance of food-borne infection in human brucellosis suggests that the digestive tract is an important site of bacterial invasion, there are a limited number of studies investigating Brucella infection by this route (4, 18, 26, 42, 52). Early studies documented that B. melitensis is more efficient at causing infection by the oral route than B. abortus. Fleischner and colleagues showed that monkeys could be readily infected with B. melitensis given with food, but that repeated inoculation with B. abortus was required to establish infection (21). Similarly, Morales Otero reported that human volunteers could be infected with a single dose of B. melitensis given orally, but that repeated inoculation was required to transmit B. abortus by this route (38). On the basis of the higher oral infectivity of B. melitensis, we characterized an animal model of B. melitensis oral infection, demonstrating the kinetics of infection up to 21 days after intragastric inoculation in mice. Furthermore, for the first time, we experimentally demonstrated some of the requirements for B. melitensis infection through the digestive tract, which include a functional T4SS and intact LPS.
The digestive tract is a restrictive environment to colonization and multiplication of pathogenic microorganisms. It has limiting factors such as defensins, lactoferrin, and lysozyme in saliva, gastric acid pH, bile salts, antimicrobial peptides, mucosal antibody, epithelial barrier, commensal microbiota, and local immune response (8, 33). Therefore, B. melitensis must resist at least a subset of these defenses to reach its intracellular niche and establish a systemic infection through the digestive tract. In contrast to findings with enteroinvasive pathogens such as nontyphoidal Salmonella enterica serotypes (37), disruption of the intestinal microbiota with streptomycin did not increase oral infectivity of B. melitensis for mice, suggesting that B. melitensis does not compete with the streptomycin-sensitive intestinal microbiota to establish infection (data not shown).
By characterizing the kinetics of B. melitensis infection through the digestive tract, we demonstrated that Brucella can resist this harsh environment and survive in low but significant numbers (around 100 CFU bacteria/g tissue) at 7 days after infection in the gut, allowing enough time for the organism to reach cells and vessels in intestinal submucosa and spread systemically. In addition, B. melitensis was detected in the gut until 21 dpi in some mice. Though we did not determine whether these bacteria were in the lumen or in tissue, this result suggests colonization of cells, possibly macrophages and dendritic cells in the lamina propria and intestinal lymphoid follicles.
A previous study by Ackermann et al., using bovine ligated ileal loops, showed that B. abortus vaccine strain 19 is internalized by lymphoepithelial cells (M cells) in the follicle-associated epithelium (FAE) and taken up by phagocytes in the intestinal lamina propria (1). Further, Salcedo et al. used a murine ileal loop model to demonstrate localization of B. abortus to dendritic cells of the FAE (51). These studies suggest that one potential site of bacterial invasion in food-borne brucellosis may be the ileal Peyer's patches; however, they do not exclude the possibility that FAE at other sites in the digestive tract may also provide a port of entry for B. melitensis. From the FAE, it is likely that bacteria reach regional lymph nodes, from whence they multiply and spread to other tissues. Here we have shown that B. melitensis consistently and rapidly reach mesenteric lymph nodes and systemic sites at similar time points during the course of infection, suggesting that systemic colonization may occur through blood vessels and that it may be independent of MLN colonization. Colonization of systemic organs independently of MLN colonization has also been demonstrated in infection with enteropathogenic bacteria such as Yersinia pseudotuberculosis in mice (5). Interestingly, in this study, bacteria were detected in the blood at different dpi, suggesting a consistent bacteremia in mice. In humans, B. melitensis may cause a transient and initial bacteremia, followed by invasion of phagocytes, and reappearance in the blood in low numbers, either continuously or intermittently (40).
B. melitensis is not considered a pathogen able to cause intestinal inflammation. However, there are some clinical reports of gastrointestinal inflammation caused by Brucella spp. in humans (29, 44, 45, 54). Here we demonstrated that B. melitensis is able to invade via the gut and disseminate to systemic sites without causing any significant inflammation in the digestive tract. We observed no expression of KC and only very mild induction of expression of MIP-2 and MIP-3 in the ileum (Fig. 2). KC and MIP-2 are chemotactic for polymorphonuclear cells, while MIP-3α is a T-lymphocyte chemotactic factor. All these chemokines may be produced by macrophages and dendritic cells in response to infection by several pathogens (27). Other inflammatory genes such as IL-6, IL-12, and IL-10 genes were not significantly expressed (data not shown). In addition, in this study, neither neutrophil influx nor significant histopathological changes were observed in the ileum or colon. Conversely, histopathological lesions observed in the spleen and liver, including neutrophilic infiltrate and granulomas were similar to the lesions described in mice inoculated by other routes of infection (19, 36).
The lack of Brucella-elicited inflammatory responses is consistent with the view that Brucella spp. are stealth pathogens that avoid activation of the innate immune response during establishment of infection (6, 10, 12, 51). In mice, a lack of proinflammatory response during infection with B. abortus has been observed (6). In addition, B. abortus is able to impair proinflammatory responses by bovine trophoblastic cells (10). Therefore, in good agreement with these previous studies, we demonstrated here that B. melitensis can invade through the digestive tract without inducing a strong local inflammatory response. Thus, although human brucellosis is a food-borne disease, it is expected that little or no intestinal inflammation takes place during oral infection with B. melitensis. Since inflammatory responses can result in containment of bacterial spread, eliciting a weak local inflammatory response at the site of B. melitensis invasion may be an essential virulence strategy of Brucella spp., as this would allow bacteria to disseminate unchecked from the mucosa to systemic sites, a strategy described previously for typhoid fever caused by S. enterica serotype Typhi (46, 47, 55, 56).
Recent reports have identified Brucella genes that may be important for bacterial survival in the digestive tract and, consequently, to establish systemic infection in mice (4, 18, 52). The genes that encode urease are required for establishing B. suis and B. abortus infection through the oral route in mice (4, 52). Urease is a multisubunit enzyme involved in nitrogen metabolism, which causes an increase in pH due to production of ammonia as a result of urea hydrolysis (13). B. abortus and B. suis have two nonadjacent urease operons (11, 43). The ure1 operon is responsible for urease activity, and its inactivation causes attenuation in mice inoculated through the digestive tract (4, 14, 52). B. melitensis also has two nonadjacent urease operons, and it usually has lower urease activity than B. suis and B. abortus. However, some B. melitensis isolates have urease activity comparable to that of B. suis and B. abortus (3, 16). In this study, a B. melitensis mutant strain carrying a deletion of genes encoding urease subunits αβγ of the ure1 operon was generated to determine the role of urease as a virulence factor after intragastric infection of B. melitensis. Interestingly, the ureABC1 mutant (TAP4) was attenuated after 7 dpi in the spleen and liver of mice, which is similar to the phenotype reported for the B. suis mutant (4), but the B. melitensis ureABC1 mutant was not attenuated in the MLN. In contrast, during mixed infection with the wt B. melitensis strain, the ureABC1 mutant was attenuated in the ileum or MLN, but not in the spleen and liver. These results suggest that independent or mixed infections have distinct outcomes for ureABC1 mutant attenuation, which may be due to compensatory effects of coinfection with the wt. It is noteworthy that lower bacterial loads in the spleens of mice infected with the B. melitensis ureABC1 mutant are not necessarily due to a reduced ability of the mutant strain to survive in the digestive tract and consequently reach systemic sites. This notion is supported by our results indicating that the ureABC1 mutant had a survival rate in low pH that was similar to that of the wt strain. These results suggest that the gastric acid pH can significantly affect the survival of B. melitensis through the digestive tract without impeding systemic infection.
The T4SS and LPS O antigen are two important virulence factors of Brucella. The T4SS is essential for survival and multiplication in macrophages and persistent infection in mice (25, 39, 53). The LPS O-antigen side chain is implicated in extracellular and early intracellular survival, apoptosis inhibition, modulation of host immune response, and persistence in mice (2, 28, 31). However, the requirement of these virulence factors has not been investigated in infections via mucosal routes. We demonstrated that virB2 (TAP1) and pmm (TAP5) mutants were attenuated in intragastrically infected mice. Our results could not distinguish between impaired invasion of intestinal tissue or reduced dissemination, since differences in bacterial loads detected in the tissues of mice infected with virB2 and wt B. melitensis strains were not statistically significant at 1 dpi and because we had difficulty detecting the low number of bacteria in intestinal tissues by immunohistochemistry. Since significant differences were detected only at 7 dpi, we conclude that the T4SS is required for wt B. melitensis levels of infection through the digestive tract after 7 days, which is similar to experimental infections via the intraperitoneal route in mice (50). This attenuation observed in the T4SS-deficient strain may reflect a lack of ability to survive intracellularly regardless of the route of infection, and therefore, our results do not support an invasion or dissemination defect that is specific to the oral route of infection. In contrast, the pmm mutant (TAP5) was attenuated in mice at 1 and 7 dpi. A hypothesis to explain this phenotype is that the rough LPS strain TAP5 (pmm) may be more easily killed in the extracellular environment by complement or antimicrobial peptides, such as intestinal defensins, as well as impaired intracellular survival, thus accounting for its initial and late attenuation in mice (31).
To gain insight into how B. melitensis penetrates the gastrointestinal epithelium, we examined transcytosis of B. melitensis in polarized enterocyte monolayers. Our results show for the first time that B. melitensis has a poor ability to transit through polarized enterocyte monolayers unless they contain M-like cells (Fig. 7). Compared to nontyphoidal S. enterica, B. melitensis is not efficient at transcytosing via M-like cells, and it is possible that, since humans are only incidental hosts, B. melitensis may lack adhesins or other virulence factors that increase the efficiency of this process.
Compared to intranasal inoculation of mice, in which a dose of 1 × 105 bacteria resulted in reproducible infection, intragastric infection of mice was relatively inefficient, requiring an inoculum of 1 × 1010 CFU. A requirement for a high oral infection dose in the mouse has also been reported for B. abortus (42, 52). Since infection via the respiratory tract is more efficient than via the digestive tract in the mouse, the epidemiologic importance of the food-borne infection route for human brucellosis may reflect the larger numbers of individuals exposed to B. melitensis via unpasteurized milk and cheese than via procedures likely to result in exposure to aerosolized bacteria (36). Alternatively, in humans, mucosa-associated lymphoid tissue at other sites of the digestive tract, such as the pharyngeal tonsils, may be more efficient sites for invasion of Brucella than the ileal Peyer's patches. However, since mice lack pharyngeal tonsils, we were unable to test this possibility in our model.
Salcedo et al. showed that in murine Peyer's patches, B. abortus could be localized to dendritic cells of the FAE (51). Since both virB mutants and mutants defective in LPS are unable to survive in human dendritic cells (7), one possible explanation for the attenuation of these mutants after oral inoculation is that after uptake by Peyer's patch M cells, they are taken up and killed by dendritic cells. For the virB mutant, this pathway appears to be different from infection by the intraperitoneal route, since the numbers of the B. abortus virB mutant remain at wt levels for several days after infection by this route (50), while after oral infection, there is a more rapid decrease in virB2 mutant numbers, with only one mouse having detectable virB2 mutant in the spleen by 7 dpi in the individual infection experiment (Fig. 3).
In conclusion, our results with an oral infection model show that B. melitensis is able to disseminate rapidly from the intestine to systemic sites without triggering overt intestinal inflammation. The penetration of the intestinal barrier likely occurs via M cells overlying mucosa-associated lymphoid tissue. Further experiments will be needed to identify additional B. melitensis and host factors that mediate invasion of the digestive tract and systemic dissemination to sites of persistent infection.
Acknowledgments
This work was supported by Public Health Service grant AI050553 to R.M.T. T.A.P. and R.L.S. are recipients of fellowships from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brasília, Brazil).
We thank N. Heuvelmans for generating pNH1.3 and S. Barthold and E. Hodzic for helpful discussions on this work.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 3 August 2009.
REFERENCES
- 1.Ackermann, M. R., N. F. Cheville, and B. L. Deyoe. 1988. Bovine ileal dome lymphoepithelial cells: endocytosis and transport of Brucella abortus strain 19. Vet. Pathol. 25:28-35. [DOI] [PubMed] [Google Scholar]
- 2.Allen, C. A., L. G. Adams, and T. A. Ficht. 1998. Transposon-derived Brucella abortus rough mutants are attenuated and exhibit reduced intracellular survival. Infect. Immun. 66:1008-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alton, G. G., L. M. Jones, R. D. Angus, and J. M. Verger. 1988. Techniques for the brucellosis laboratory. INRA Publications, Paris, France.
- 4.Bandara, A. B., A. Contreras, A. Contreras-Rodriguez, A. M. Martins, V. Dobrean, S. Poff-Reichow, P. Rajasekaran, N. Sriranganathan, G. G. Schurig, and S. M. Boyle. 2007. Brucella suis urease encoded by ure1 but not ure2 is necessary for intestinal infection of BALB/c mice. BMC Microbiol. 7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes, P. D., M. A. Bergman, J. Mecsas, and R. R. Isberg. 2006. Yersinia pseudotuberculosis disseminates directly from a replicating bacterial pool in the intestine. J. Exp. Med. 203:1591-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barquero-Calvo, E., E. Chaves-Olarte, D. S. Weiss, C. Guzman-Verri, C. Chacon-Diaz, A. Rucavado, I. Moriyon, and E. Moreno. 2007. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS One 2:e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billard, E., C. Cazevieille, J. Dornand, and A. Gross. 2005. High susceptibility of human dendritic cells to invasion by the intracellular pathogens Brucella suis, B. abortus, and B. melitensis. Infect. Immun. 73:8418-8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borriello, S. P. 1984. Bacteria and gastrointestinal secretion and motility. Scand. J. Gastroenterol. Suppl. 93:115-121. [PubMed] [Google Scholar]
- 9.Boschiroli, M. L., S. Ouahrani-Bettache, V. Foulongne, S. Michaux-Charachon, G. Bourg, A. Allardet-Servent, C. Cazevieille, J. P. Lavigne, J. P. Liautard, M. Ramuz, and D. O'Callaghan. 2002. Type IV secretion and Brucella virulence. Vet. Microbiol. 90:341-348. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho Neta, A. V., A. P. Stynen, T. A. Paixão, K. L. Miranda, F. L. Silva, C. M. Roux, R. M. Tsolis, R. E. Everts, H. A. Lewin, L. G. Adams, A. F. Carvalho, A. P. Lage, and R. L. Santos. 2008. Modulation of the bovine trophoblastic innate immune response by Brucella abortus. Infect. Immun. 76:1897-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chain, P. S., D. J. Comerci, M. E. Tolmasky, F. W. Larimer, S. A. Malfatti, L. M. Vergez, F. Aguero, M. L. Land, R. A. Ugalde, and E. Garcia. 2005. Whole-genome analyses of speciation events in pathogenic brucellae. Infect. Immun. 73:8353-8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirl, C., A. Wieser, M. Yadav, S. Duerr, S. Schubert, H. Fischer, D. Stappert, N. Wantia, N. Rodriguez, H. Wagner, C. Svanborg, and T. Miethke. 2008. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat. Med. 14:399-406. [DOI] [PubMed] [Google Scholar]
- 13.Collins, C. M., and S. E. D'Orazio. 1993. Bacterial ureases: structure, regulation of expression and role in pathogenesis. Mol. Microbiol. 9:907-913. [DOI] [PubMed] [Google Scholar]
- 14.Contreras-Rodriguez, A., J. Quiroz-Limon, A. M. Martins, H. Peralta, E. Avila-Calderon, N. Sriranganathan, S. M. Boyle, and A. Lopez-Merino. 2008. Enzymatic, immunological and phylogenetic characterization of Brucella suis urease. BMC Microbiol. 8:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbel, M. J. 1997. Brucellosis: an overview. Emerg. Infect. Dis. 3:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbel, M. J., and D. M. Hendry. 1985. Urease activity of Brucella species. Res. Vet. Sci. 38:252-253. [PubMed] [Google Scholar]
- 17.Dean, P., and B. Kenny. 2004. Intestinal barrier dysfunction by enteropathogenic Escherichia coli is mediated by two effector molecules and a bacterial surface protein. Mol. Microbiol. 54:665-675. [DOI] [PubMed] [Google Scholar]
- 18.Delpino, M. V., M. I. Marchesini, S. M. Estein, D. J. Comerci, J. Cassataro, C. A. Fossati, and P. C. Baldi. 2007. A bile salt hydrolase of Brucella abortus contributes to the establishment of a successful infection through the oral route in mice. Infect. Immun. 75:299-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.den Hartigh, A. B., Y. H. Sun, D. Sondervan, N. Heuvelmans, M. O. Reinders, T. A. Ficht, and R. M. Tsolis. 2004. Differential requirements for VirB1 and VirB2 during Brucella abortus infection. Infect. Immun. 72:5143-5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enright, F. M., L. N. Araya, P. H. Elzer, G. E. Rowe, and A. J. Winter. 1990. Comparative histopathology in BALB/c mice infected with virulent and attenuated strains of Brucella abortus. Vet. Immunol. Immunopathol. 26:171-182. [DOI] [PubMed] [Google Scholar]
- 20.Ficht, T. A. 2003. Intracellular survival of Brucella: defining the link with persistence. Vet. Microbiol. 2516:1-11. [DOI] [PubMed] [Google Scholar]
- 21.Fleischner, E. C., M. Vecki, E. B. Shaw, and K. F. Meyer. 1921. The pathogenicity of B. abortus and B. melitensis for monkeys. Studies on the genus Brucella nov. gen. III. J. Infect. Dis. 29:663-705. [Google Scholar]
- 22.Godfroid, J., A. Cloeckaert, J. P. Liautard, S. Kohler, D. Fretin, K. Walravens, B. Garin-Bastuji, and J. J. Letesson. 2005. From the discovery of the Malta fever's agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. 36:313-326. [DOI] [PubMed] [Google Scholar]
- 23.Gorvel, J. P., and E. Moreno. 2002. Brucella intracellular life: from invasion to intracellular replication. Vet. Microbiol. 90:281-297. [DOI] [PubMed] [Google Scholar]
- 24.Gullberg, E., M. Leonard, J. Karlsson, A. M. Hopkins, D. Brayden, A. W. Baird, and P. Artursson. 2000. Expression of specific markers and particle transport in a new human intestinal M-cell model. Biochem. Biophys. Res. Commun. 279:808-813. [DOI] [PubMed] [Google Scholar]
- 25.Hong, P. C., R. M. Tsolis, and T. A. Ficht. 2000. Identification of genes required for chronic persistence of Brucella abortus in mice. Infect. Immun. 68:4102-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izadjoo, M. J., M. G. Mense, A. K. Bhattacharjee, T. L. Hadfield, R. M. Crawford, and D. L. Hoover. 2008. A study on the use of male animal models for developing a live vaccine for brucellosis. Transbound. Emerg. Dis. 55:145-151. [DOI] [PubMed] [Google Scholar]
- 27.Jenner, R. G., and R. A. Young. 2005. Insights into host responses against pathogens from transcriptional profiling. Nat. Rev. Microbiol. 3:281-294. [DOI] [PubMed] [Google Scholar]
- 28.Jiménez De Bagüés, M. P., A. Terraza, A. Gross, and J. Dornand. 2004. Different responses of macrophages to smooth and rough Brucella spp.: relationship to virulence. Infect. Immun. 72:2429-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jorens, P. G., P. P. Michielsen, E. J. Van den Enden, N. H. Bourgeois, E. A. Van Marck, G. R. Krueger, A. M. Ramon, and Y. M. Van Maercke. 1991. A rare cause of colitis-Brucella melitensis. Report of a case. Dis. Colon Rectum 34:194-196. [DOI] [PubMed] [Google Scholar]
- 30.Kerneis, S., A. Bogdanova, J. P. Kraehenbuhl, and E. Pringault. 1997. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science 277:949-952. [DOI] [PubMed] [Google Scholar]
- 31.Lapaque, N., I. Moriyon, E. Moreno, and J. P. Gorvel. 2005. Brucella lipopolysaccharide acts as a virulence factor. Curr. Opin. Microbiol. 8:60-66. [DOI] [PubMed] [Google Scholar]
-
31a.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the
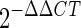 method 25:402-408. [DOI] [PubMed] [Google Scholar]
method 25:402-408. [DOI] [PubMed] [Google Scholar] - 32.Luna-Martinez, J. E., and C. Mejia-Teran. 2002. Brucellosis in Mexico: current status and trends. Vet. Microbiol. 90:19-30. [DOI] [PubMed] [Google Scholar]
- 33.Magalhaes, J. G., I. Tattoli, and S. E. Girardin. 2007. The intestinal epithelial barrier: how to distinguish between the microbial flora and pathogens. Semin. Immunol. 19:106-115. [DOI] [PubMed] [Google Scholar]
- 34.Mantur, B. G., S. K. Amarnath, and R. S. Shinde. 2007. Review of clinical and laboratory features of human brucellosis. Indian J. Med. Microbiol. 25:188-202. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Argudo, I., and M. A. Jepson. 2008. Salmonella translocates across an in vitro M cell model independently of SPI-1 and SPI-2. Microbiology 154:3887-3894. [DOI] [PubMed] [Google Scholar]
- 35a.Meador, V. P., L. B. Tabatabai, W. A. Hagemoser, and B. L. Deyoe. 1986. Identification of Brucella abortus in formalin-fixed, paraffin-embedded tissues of cows, goats, and mice with an avidin-biotin-peroxidase complex immunoenzymatic staining technique. Am. J. Vet. Res. 47:2147-2150. [PubMed] [Google Scholar]
- 36.Mense, M. G., L. L. Van De Verg, A. K. Bhattacharjee, J. L. Garrett, J. A. Hart, L. E. Lindler, T. L. Hadfield, and D. L. Hoover. 2001. Bacteriologic and histologic features in mice after intranasal inoculation of Brucella melitensis. Am. J. Vet. Res. 62:398-405. [DOI] [PubMed] [Google Scholar]
- 37.Miller, C. P., and M. Bohnhoff. 1963. Changes in the mouse's enteric microflora associated with enhanced susceptibility to Salmonella infection following streptomycin treatment. J. Infect. Dis. 113:59-66. [DOI] [PubMed] [Google Scholar]
- 38.Morales Otero, P. 1948. Studies of Brucella infection in Puerto Rico. University of Puerto Rico, San Juan.
- 39.O'Callaghan, D., C. Cazevieille, A. Allardet-Servent, M. L. Boschiroli, G. Bourg, V. Foulongne, P. Frutos, Y. Kulakov, and M. Ramuz. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 33:1210-1220. [DOI] [PubMed] [Google Scholar]
- 40.Pappas, G., and P. Papadimitriou. 2007. Challenges in Brucella bacteraemia. Int. J. Antimicrob. Agents 30(Suppl. 1):S29-S31. [DOI] [PubMed] [Google Scholar]
- 41.Pappas, G., P. Papadimitriou, N. Akritidis, L. Christou, and E. V. Tsianos. 2006. The new global map of human brucellosis. Lancet Infect. Dis. 6:91-99. [DOI] [PubMed] [Google Scholar]
- 42.Pasquali, P., A. Rosanna, C. Pistoia, P. Petrucci, and F. Ciuchini. 2003. Brucella abortus RB51 induces protection in mice orally infected with the virulent strain B. abortus 2308. Infect. Immun. 71:2326-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42a.Pasquali, P., R. Adone, L. C. Gasbarre, C. Pistoia, and F. Ciuchini. 2001. Mouse cytokine profiles associated with Brucella abortus RB51 vaccination or B. abortus 2308 infection. Infect. Immun. 69:6541-6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paulsen, I. T., R. Seshadri, K. E. Nelson, J. A. Eisen, J. F. Heidelberg, T. D. Read, R. J. Dodson, L. Umayam, L. M. Brinkac, M. J. Beanan, S. C. Daugherty, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, W. C. Nelson, B. Ayodeji, M. Kraul, J. Shetty, J. Malek, S. E. Van Aken, S. Riedmuller, H. Tettelin, S. R. Gill, O. White, S. L. Salzberg, D. L. Hoover, L. E. Lindler, S. M. Halling, S. M. Boyle, and C. M. Fraser. 2002. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc. Natl. Acad. Sci. USA 99:13148-13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrella, R., and E. J. Young. 1988. Acute brucella ileitis. Am. J. Gastroenterol. 83:80-82. [PubMed] [Google Scholar]
- 45.Potasman, I., L. Even, M. Banai, E. Cohen, D. Angel, and M. Jaffe. 1991. Brucellosis: an unusual diagnosis for a seronegative patient with abscesses, osteomyelitis, and ulcerative colitis. Rev. Infect. Dis. 13:1039-1042. [DOI] [PubMed] [Google Scholar]
- 46.Raffatellu, M., D. Chessa, R. P. Wilson, R. Dusold, S. Rubino, and A. J. Bäumler. 2005. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect. Immun. 73:3367-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raffatellu, M., R. L. Santos, D. Chessa, R. P. Wilson, S. E. Winter, C. A. Rossetti, S. D. Lawhon, H. Chu, T. Lau, C. L. Bevins, L. G. Adams, and A. J. Bäumler. 2007. The capsule encoding the viaB locus reduces interleukin-17 expression and mucosal innate responses in the bovine intestinal mucosa during infection with Salmonella enterica serotype Typhi. Infect. Immun. 75:4342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raffatellu, M., R. P. Wilson, D. Chessa, H. Andrews-Polymenis, Q. T. Tran, S. Lawhon, S. Khare, L. G. Adams, and A. J. Bäumler. 2005. SipA, SopA, SopB, SopD, and SopE2 contribute to Salmonella enterica serotype Typhimurium invasion of epithelial cells. Infect. Immun. 73:146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ragnarsson, E. G., I. Schoultz, E. Gullberg, A. H. Carlsson, F. Tafazoli, M. Lerm, K. E. Magnusson, J. D. Soderholm, and P. Artursson. 2008. Yersinia pseudotuberculosis induces transcytosis of nanoparticles across human intestinal villus epithelium via invasin-dependent macropinocytosis. Lab. Investig. 88:1215-1226. [DOI] [PubMed] [Google Scholar]
- 50.Rolán, H. G., and R. M. Tsolis. 2007. Mice lacking components of adaptive immunity show increased Brucella abortus virB mutant colonization. Infect. Immun. 75:2965-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50a.Roux, C. M., H. G. Rolan, R. L. Santos, P. D. Beremand, T. L. Thomas, L. G. Adams, and R. M. Tsolis. 2007. Brucella requires a functional type IV secretion system to elicit innate immune responses of mice. Cell. Microbiol. 9:1851-1869. [DOI] [PubMed] [Google Scholar]
- 51.Salcedo, S. P., M. I. Marchesini, H. Lelouard, E. Fugier, G. Jolly, S. Balor, A. Muller, N. Lapaque, O. Demaria, L. Alexopoulou, D. J. Comerci, R. A. Ugalde, P. Pierre, and J. P. Gorvel. 2008. Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp1. PLoS Pathog. 4:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sangari, F. J., A. Seoane, M. C. Rodriguez, J. Aguero, and J. M. Garcia Lobo. 2007. Characterization of the urease operon of Brucella abortus and assessment of its role in virulence of the bacterium. Infect. Immun. 75:774-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sieira, R., D. J. Comerci, D. O. Sanchez, and R. A. Ugalde. 2000. A homologue of an operon required for DNA transfer in Agrobacterium is required in Brucella abortus for virulence and intracellular multiplication. J. Bacteriol. 182:4849-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stermer, E., N. Levy, I. Potasman, M. Jaffe, and J. Boss. 1991. Brucellosis as a cause of severe colitis. Am. J. Gastroenterol. 86:917-919. [PubMed] [Google Scholar]
- 55.Tsolis, R. M., G. M. Young, J. V. Solnick, and A. J. Bäumler. 2008. From bench to bedside: stealth of enteroinvasive pathogens. Nat. Rev. Microbiol. 6:883-892. [DOI] [PubMed] [Google Scholar]
- 56.Winter, S. E., M. Raffatellu, R. P. Wilson, H. Russmann, and A. J. Bäumler. 2008. The Salmonella enterica serotype Typhi regulator TviA reduces interleukin-8 production in intestinal epithelial cells by repressing flagellin secretion. Cell. Microbiol. 10:247-261. [DOI] [PubMed] [Google Scholar]