Abstract
The essential toxin in Clostridium perfringens-mediated gas gangrene or clostridial myonecrosis is alpha-toxin, although other toxins and extracellular enzymes may also be involved. In many bacterial pathogens extracellular sialidases are important virulence factors, and it has been suggested that sialidases may play a role in gas gangrene. C. perfringens strains have combinations of three different sialidase genes, two of which, nanI and nanJ, encode secreted sialidases. The nanI and nanJ genes were insertionally inactivated by homologous recombination in derivatives of sequenced strain 13 and were shown to encode two functional secreted sialidases, NanI and NanJ. Analysis of these derivatives showed that NanI was the major sialidase in this organism. Mutation of nanI resulted in loss of most of the secreted sialidase activity, and the residual activity was eliminated by subsequent mutation of the nanJ gene. Only a slight reduction in the total sialidase activity was observed in a nanJ mutant. Cytotoxicity assays using the B16 melanoma cell line showed that supernatants containing NanI or overexpressing NanJ enhanced alpha-toxin-mediated cytotoxicity. Finally, the ability of nanI, nanJ, and nanIJ mutants to cause disease was assessed in a mouse myonecrosis model. No attenuation of virulence was observed for any of these strains, providing evidence that neither the NanI sialidase nor the NanJ sialidase is essential for virulence.
Clostridium perfringens type A is the causative agent of human gas gangrene, or clostridial myonecrosis, and human food poisoning (25, 27). It produces many secreted hydrolytic enzymes and toxins, including alpha-toxin and perfringolysin O. C. perfringens strains can also encode up to three sialidases, but the three sialidase genes, nanH, nanI, and nanJ, are not present in all of the strains that have been completely sequenced. Strain ATCC 13124 encodes all three sialidases (18), while strain 13 encodes both of the large sialidases, NanI and NanJ, but not the smaller NanH enzyme (32). The food poisoning isolate SM101 encodes NanH but not NanI or NanJ (18).
Sialidases have been implicated in the virulence of several bacterial pathogens. They have been shown to enhance the pathogenesis of disease through synergistic effects with other bacterial factors. For example, Vibrio cholerae sialidase enhances the activity of cholera toxin (10), Pseudomonas aeruginosa sialidase increases the binding of this organism to the cells of susceptible patients (6), and the two sialidases of Streptococcus pneumoniae contribute to the progression of infection in several animal models (16, 23, 37). More recently, a surface-exposed sialidase was shown to be required for persistence of the canine pathogen Capnophagia canimorsus (15).
Alpha-toxin is an essential virulence factor in gas gangrene (2), and perfringolysin O, although not essential, has been found to have a synergistic role with alpha-toxin, enhancing the disease process (3). Synergy between alpha-toxin and the NanI sialidase was also observed in experiments that showed that purified alpha-toxin had greater pathological effects on cultured cell lines that had been pretreated with NanI (8). Inoculation of mice with both purified alpha-toxin and NanI resulted in increased levels of plasma creatine kinase, a marker for muscle necrosis, compared to the levels after inoculation of alpha-toxin alone (8).
The sialidases of C. perfringens have different cellular locations. NanH (43 kDa) lacks a signal peptide and is located in the cytoplasm (12, 24). It has been proposed that NanH is involved in the cleavage of short oligosaccharides that enter the cell and are subsequently broken down for nutritional purposes (41). By contrast, NanI (77 kDa) contains a signal peptide, is secreted from the cell, and is readily isolated from cell-free supernatants (19, 38). A high-resolution structure of the catalytic domain of NanI in a complex with its sialic acid substrate has recently been described (20). NanI may also play a role in nutrition, releasing sialic acid from higher-order gangliosides for subsequent transport into the cell (41). As a result of its location, NanI may also interact with the extracellular environment of the host tissue during infection. In addition to its synergy with alpha-toxin (8), NanI has also been shown to have synergistic effects with ɛ-toxin (31), which is required for C. perfringens type B- and D-mediated diseases (34, 39, 40). Very limited information is available for the 129-kDa NanJ enzyme, but recent studies have shown that in addition to sialidase motifs this enzyme contains an additional galactose binding domain (5).
The objective of our study was to determine the role of NanI and NanJ in the pathogenesis of gas gangrene. Mutagenesis of the genes (nanI and nanJ) encoding each of the secreted sialidases was carried out using the strain 13 derivative JIR325. Then the relative contribution of each sialidase to total sialidase production was determined, and virulence was assessed using the mouse myonecrosis model. The results showed that NanI is the major sialidase in this strain but that neither enzyme is essential for virulence.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All C. perfringens strains were derivatives of JIR325, a rifampin (rifampicin)-resistant and nalidixic acid-resistant derivative of strain 13 (13, 14). C. perfringens strains were grown in brain heart infusion broth (Oxoid), fluid thioglycolate broth (Difco Laboratories), Trypticase-peptone-glucose broth (28), or Todd-Hewitt (TH) broth (Oxoid) supplemented with 0.02% glucose (Amresco) and 0.1% sodium thioglycolate (Sigma). All clostridial broth media were boiled prior to inoculation, and cultures were grown at 37°C. For growth on solid media, C. perfringens was grown at 37°C on nutrient agar (26) in anaerobic jars (Oxoid) with an atmosphere containing 10% H2, 10% CO2, and 80% N2. When an antibiotic was required, C. perfringens cultures were supplemented with erythromycin (50 μg/ml), tetracycline (10 μg/ml), rifampin (10 μg/ml), nalidixic acid (10 μg/ml), chloramphenicol (30 μg/ml), or thiamphenicol (10 μg/ml). The Escherichia coli cultures were derivatives of DH5α (Life Technologies) or NovaBlue (Novagen) and were propagated in 2× YT medium (17) supplemented with ampicillin (100 μg/ml), chloramphenicol (30 μg/ml), erythromycin (150 μg/ml), or tetracycline (10 μg/ml).
Molecular and genetic techniques.
Plasmid DNA was isolated using QIAprep spin miniprep kits (Qiagen) by following the manufacturer's instructions. C. perfringens genomic DNA was prepared as described previously (22). Other molecular procedures were carried out essentially as previously described (29). C. perfringens transformation was performed by electroporation (30) at 1,800 V, 25 μF, and 200 Ω.
Construction of recombinant plasmids and mutants.
The characteristics of plasmids utilized in this study are shown in Table 1, and the oligonucleotide primers used are shown in Table 2. The nanI suicide vector pJIR2822 was constructed as follows. The downstream region of the nanI gene was PCR amplified using oligonucleotide primers JRP2016 and JRP2015, and the product was digested with XbaI and HindIII and cloned into pJIR2783 (11) to construct pJIR2812. The upstream region of nanI was PCR amplified with JRP2049 and JRP2050, digested with PstI and BamHI, and cloned into the corresponding sites in pJIR2812. The resultant plasmid, pJIR2822, which also carried an erm(B) erythromycin resistance gene, was used to transform C. perfringens strain JIR325, and transformants that were resistant to chloramphenicol but susceptible to erythromycin were isolated.
TABLE 1.
Plasmids
| Plasmid | Relevant characteristicsa | Reference or origin |
|---|---|---|
| pUC18 | Cloning vector, Apr | 42 |
| pT7Blue | Commercial cloning vector, Apr | Novagen |
| pJIR750 | E. coli-C. perfringens shuttle vector, carries pIP404 replication region, Cmr | 4 |
| pJIR751 | E. coli-C. perfringens shuttle vector, carries pIP404 replication region, Emr | 4 |
| pJIR2783 | Mobilizable base suicide vector for clostridia, Cmr Emr | 11 |
| pJIR2812 | pJIR2783 (HindIII/XbaI)ΩJRP2015/JRP2016 (HindIII/XbaI, 2.4 kb) PCR product, Cmr Emr | This study |
| pJIR2822 | pJIR2212 (PstI/BamHI)ΩJRP2049/JRP2050 (PstI/BamHI, 2.4 kb) nanI suicide vector, Cmr Emr | This study |
| pJIR2958 | pT7BlueΩJRP2152/JRP2153 (3.5 kb) nanJ+, Apr | This study |
| pJIR3057 | pJIR751 (PstI/XbaI)ΩpJIR3046 (PstI/XbaI 2.6 kb) nanI+ complementation plasmid, Emr | This study |
| pJIR3109 | pMOD-3 R6Kγori/MCS (Epicentre)Ωerm(B), Apr Emr | This study |
| pJIR3181 | pJIR2958 EZ-Tn5 mutant at nanJ nucleotide 2153, nanJ suicide vector, Apr Emr | This study |
| pJIR3270 | pJIR750 (BamHI), pJIR2593 (BamHI, 4 kb) tet(M), Tcr Cmr | This study |
| pJIR3308 | pJIR2958 (SmaI)ΩpJIR3270 (SmaI, 8.2 kb) tet(M), catP, C. perfringens rep and ori, nanJ+ complementation vector, Tcr Cmr Apr | This study |
| pJIR3309 | pJIR3248 (SmaI)ΩpJIR3270 (SmaI, 8.2 kb) tet(M), catP, C. perfringens rep and ori, Tcr Cmr Apr | This study |
Apr, Cmr, Emr, Tcr, Rifr, and Nalr, resistance to ampicillin, chloramphenicol (thiamphenicol), erythromycin, tetracycline, rifampin, and nalidixic acid, respectively.
TABLE 2.
Oligonucleotide primers
| Primer | Sequence (5′-3′) | Usea |
|---|---|---|
| JRP1877 | CTCAGTACTGAGAGGGAACTTAGATGGTAT | catP |
| JRP1878 | CCGGGATCCTTAGGGTAACAAAAAACACC | catP |
| JRP2015 | ACAACGGCCGACAAATGAAAAAGGTAAACAAT | nanI RHS |
| JRP2016 | GTTTCCTCATTTGGGTCATTAC | nanI RHS |
| JRP2048 | GTAAAGTAAGAAAAGTCGACGTAT | nanI LHS |
| JRP2049 | AACTGCAGTGTTGAAAGTAGTGATGAA | nanI LHS |
| JRP2050 | TAGGATCCATTAAATCTTACAACAATA | nanI LHS |
| JRP2085 | GATCTAAACCTAATAATGAATC | nanI LHS |
| JRP2115 | GGTCTAGATAAAGAGTAGTAAGAAAGAATA | nanI |
| JRP2118 | GCAGGCTTATTTCCACATCCA | nanI |
| JRP2148 | TGGAGAAGAAGTTGGAAATGC | nanJ |
| JRP2149 | CTTTACCATTTATTTTTCCAC | nanJ |
| JRP2152 | GATCTAGATGATGTTTTTTAGCTTTTTGT | nanJ |
| JRP2153 | GACTGCAGTAACATACCTAATAATTTTTT | nanJ |
| JRP2154 | GCTATTATTGAAACTGCTATT | nanJ |
| JRP2258 | ATCTTCATCTAAAACTTCAAT | nanJ |
| #2980 | ATAAAGTAAACAGGTAACGTCT | erm(B) |
| #2981 | GCTCCTTGGAAGCTGTCAGTAG | erm(B) |
RHS and LHS refer to upstream and downstream regions, respectively.
To construct the nanJ suicide vector pJIR3181, the nanJ gene was PCR amplified using primers JRP2152 and JRP2153 and cloned into pT7Blue to construct pJIR2958. Then a suicide vector was constructed by performing transposon mutagenesis using a modification of the commercially available EZ-Tn5 pMOD-3 <RK6γori/MCS> transposon mutagenesis system (Epicentre). The transposon was modified so that it contained an erm(B) antibiotic resistance cassette that could be used for selection in C. perfringens. Using pJIR3109 as the delivery vector (Table 1), pJIR3181, which had an insertion in nanJ, was isolated. Sequence analysis showed that in pJIR3181 the transposon was inserted into nanJ codon 715. Transformation of both wild-type strain JIR325 and its nanI mutant JIR4915 with pJIR3181 was used to isolate nanJ mutants. Erythromycin-resistant colonies were screened by PCR using primers JRP2148 and JRP2258.
To construct a nanI complementation vector, the nanI gene was PCR amplified and cloned into pUC18 before it was subcloned into the C. perfringens-E. coli shuttle vector pJIR751 to construct pJIR3057. Similarly, a nanJ complementation vector was constructed by initially cloning the tet(M) tetracycline resistance gene into pJIR750 to construct pJIR3270. The tet(M) gene and the C. perfringens rep and ori region were then cloned into pJIR2958 to construct the nanJ complementation vector pJIR3308 and into pT7Blue to construct the vector control plasmid pJIR3309.
Southern hybridization analysis.
Genomic DNA was digested with an appropriate restriction enzyme, and the DNA fragments were separated by electrophoresis on a 0.8% agarose gel and then transferred to a nylon membrane (Hybond N; Amersham Pharmacia Biotech) (29, 35). The blots were hybridized with 40 ng of either an internal 2-kb nanJ probe (amplified using primers JRP2154 and JRP2149), a 1-kb erm(B) probe (amplified using primers #2980 and #2981), a 0.7-kb catP probe (amplified using primers JRP1877 and JRP1878), or a 1.1-kb probe amplified from genomic DNA upstream of nanI using primers JRP2048 and JRP2085. Probe DNA was labeled using digoxigenin by following the manufacturer's instructions (Roche). Hybridization was detected by chemiluminescent detection using CDP-Star (Roche) by following the manufacturer's instructions.
Toxin assays.
Cultures were grown to the mid-exponential to late exponential growth phase (4 h) in TH broth, and 15-ml samples of culture supernatant were harvested by centrifugation at 5,800 × g for 10 min. The strains all had similar growth rates. Culture supernatants were used directly in the perfringolysin O assay or concentrated 20-fold using Amicon Ultra centrifugal filter devices (Millipore) with a nominal 30,000-molecular-weight cutoff for use in the sialidase or alpha-toxin assays. Perfringolysin O activity was measured by a double-dilution assay as described previously (36), except that 5 mM dithiothreitol was included. The titer was expressed as the reciprocal of the value for the last well that showed complete hemolysis. Alpha-toxin activity was assayed as previously described (33). Total protein content was determined with a bicinchoninic acid protein assay kit (Pierce).
Sialidase assays.
For each sialidase assay, 20 μl of concentrated culture supernatant was added to 60 μl of 100 mM sodium acetate buffer (pH 5.5 or pH 7.5) in a microtiter tray. Then 20 μl of 4 mM 5-bromo-4-chloro-3-indolyl-α-d-N-acetylneuraminic acid (Sigma) was added, and the tray was incubated at 37°C. The absorbance at 620 nm was determined with a Multiskan microplate reader (Labsystems) and was analyzed using Genesis ThermoLabsystem software (GenLite, version 3.05). The initial rate of enzyme activity was determined by measuring the absorbance every 10 min for NanI and every 30 min for NanJ. One unit of sialidase activity was defined as a 1-U change in absorbance at 620 nm/min, standardized using the turbidity at 600 nm of the bacterial culture.
Cytotoxicity assays.
Each strain was grown to a turbidity at 650 nm of 0.7 in TH broth, and the culture supernatants were ultrafiltered through 10-kDa membranes (Millipore) and sterilized by filtration through 0.22-μm Millex filters (Millipore). The amounts of total protein and alpha-toxin in the supernatants were determined as described above. Mouse melanoma cell line B16 and a derivative of this cell line, the ganglioside-deficient mutant cell line GM95 (8), were cultured in 96-well plates in Eagle's minimal essential medium supplemented with 10% fetal bovine serum, l-glutamine (5 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml) in a humid atmosphere containing 5% CO2 at 37°C. Monolayers grown to 80 to 90% confluence were exposed to twofold serial dilutions of the ultrafiltrates diluted in supplemented medium for 24 h at 37°C. Cell viability was assessed 24 h later using a neutral red assay (9). Briefly, the cells were incubated for 2 h with 200 μl/well of neutral red (50 μg/ml) dissolved in supplemented culture medium, and the incorporated dye was extracted with 100 μl of acetic acid-ethanol-water (1:50:49) before the absorbance at 540 nm was recorded. The results were expressed as the percentage of cell survival; 100% survival was defined as the value for parallel cultures incubated with medium. The data are presented below as averages of at least three independent experiments, each with three replicate samples.
Virulence studies.
Virulence studies were performed as previously reported (3, 7). Briefly, cultures were grown in TH broth for 4 h, until they reached mid-exponential to late exponential growth phase. Cells were harvested by centrifugation at 5,800 × g for 10 min, washed once in phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 1.4 mM KH2PO4, 4.3 mM Na2HPO4), and then resuspended in a volume of PBS equivalent to three times the packed cell volume to obtain a concentration of approximately 1 × 1010 viable cells/ml. For each strain, 50 μl or 100 μl of the cell suspension was injected into the thighs of 10 6- to 8-week-old BALB/c mice. The mice were scored hourly for malaise, limping, swelling of the thigh and footpad, and blackening of the thigh and footpad. Each mouse was scored for each pathological parameter as follows: 0, little or no pathology; 0.5, moderate pathology; and 1, severe pathology (3, 7). The data for all animals for each strain were pooled and used to calculate a quantitative score for each pathological parameter (maximum score, 10). For mice that had to be euthanized for ethical reasons prior to the end of the experiment, a score of 1 was used for each parameter at all subsequent time points.
In separate experiments designed to measure myotoxicity quantitatively, exponential-phase cultures were harvested by centrifugation at 6,000 × g for 5 min, washed twice with PBS, and then resuspended in PBS to obtain 5 × 109 washed cells/ml. Myotoxicity was estimated by measuring the creatine kinase activity in the plasma after intramuscular injection of 5 × 108 bacteria into the right gastrocnemius muscle for groups of CD-1 mice (body weight, 16 to 18 g). The creatine kinase activity in plasma was determined 5 h later as described previously (1), using a kinetic assay (Biocon Diagnostik). The data presented below are averages and standard deviations for at least two independent experiments, each performed with a group of eight mice.
RESULTS
Construction of nanI, nanJ, and nanIJ mutants.
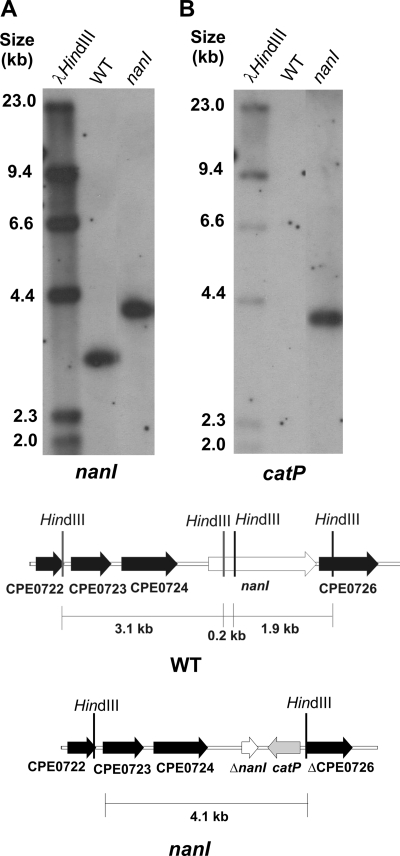
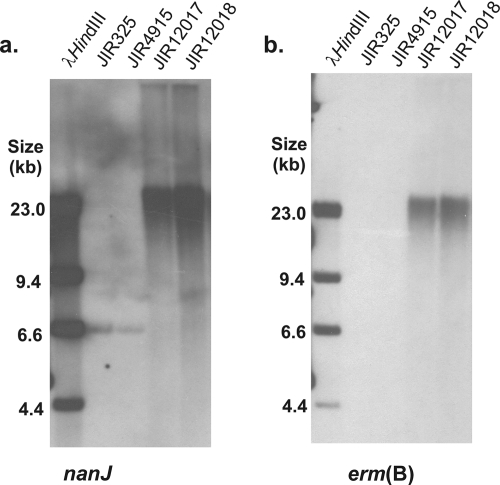
To determine the relative importance of each of the two sialidases encoded by derivatives of strain 13, chromosomal nanI, nanJ, and nanIJ mutants were constructed by insertional inactivation. The nanIJ mutant was derived by transformation of the nanI mutant with the nanJ suicide vector. Southern blotting confirmed that the nanI mutant (JIR4915) was the result of a double-crossover event (Fig. 1). Mutagenesis of nanI also disrupted the downstream gene CPE0726, which encodes a putative conserved hypothetical protein with a low level of similarity to N-acetylneuraminate mutarotases and kelch repeat domain proteins. As expected, we amplified a 0.9-kb product from the wild-type nanJ gene and a 2.6-kb product in the nanJ (JIR12018) and nanIJ (JIR12017) mutants (data not shown), and in Southern blots a 6.6-kb NsiI fragment from the parent strains, JIR325 and JIR4915, hybridized with the nanJ probe. Unexpectedly, both the nanJ and erm(B) probes hybridized to a >23-kb fragment in the nanJ and nanIJ mutants (Fig. 2). This fragment was much larger than the 8.4-kb fragment predicted for a simple double-crossover event. These results can be explained by postulating that the suicide vector disrupted nanJ by inserting into the chromosome in a concatamerized manner following an initial double-crossover event, as indicated by the absence of a wild-type nanJ PCR product in the mutants. Nonetheless, the mutants clearly had a stable, insertionally inactivated nanJ gene and were therefore suitable for functional analysis.
FIG. 1.
Southern hybridization analysis of nanI mutant. HindIII-digested genomic DNA from wild-type strain JIR325 (WT) and the nanI mutant JIR4915 were probed with a fragment upstream of nanI (A) and with a catP probe (B). The HindIII fragments used for the Southern hybridization analysis are indicated in the diagrams. The genomic organization of the nanI region in the wild-type and mutant (nanI) strains is also shown.
FIG. 2.
Southern hybridization analysis of nanJ and nanIJ mutants. NsiI-digested genomic DNA from wild-type strain JIR325, the nanI mutant JIR4915, the nanJ mutant JIR12018, and the nanIJ mutant JIR12017 were probed with nanJ-specific (a) and erm(B)-specific (b) probes.
To assess the impact of the nanI and nanJ mutations, the wild-type genes were complemented in trans so that molecular Koch's postulates could be fulfilled. The nanI+ complementation plasmid pJIR3057 or the vector control plasmid pJIR751 was used to transform the nanI mutant, and the nanJ+ complementation plasmid pJIR3308 or the vector control plasmid pJIR3309 was used to transform the nanJ and nanIJ mutants. The nanIJ mutant was not complemented with the wild-type nanI gene since the role of NanI was examined using the nanI mutant and its derivatives.
NanI is the major sialidase in strain 13.
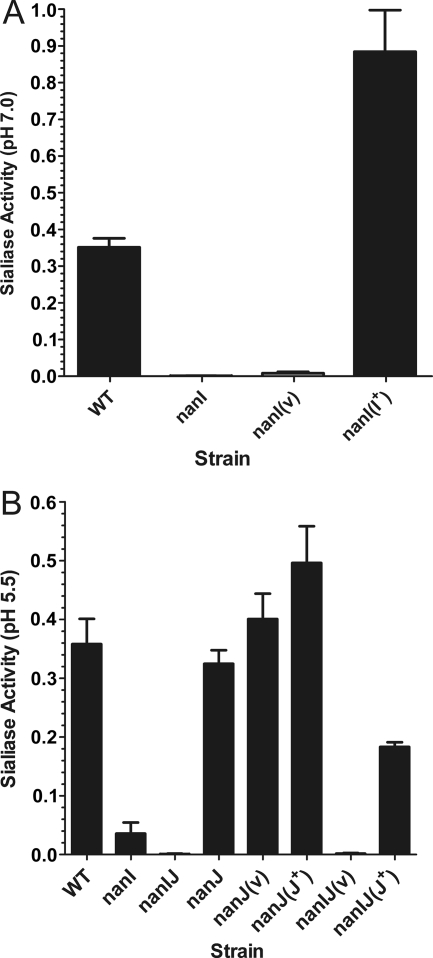
To determine the effects of the mutation of each sialidase gene on the total sialidase activity, sialidase assays were carried out using concentrated supernatants of the mutant strains and their complemented derivatives. Compared to the high level of sialidase activity observed in supernatants from the wild-type strain at pH 7.0, almost negligible activity was present in supernatants from the nanI mutant (Fig. 3A). Complementation with the wild-type nanI gene in trans resulted in levels of activity that were higher than the wild-type levels, most likely due to the gene dosage effect of complementation with a shuttle plasmid. No complementation was observed with the vector plasmid. These data indicate that NanI is the major extracellular sialidase produced by derivatives of C. perfringens strain 13.
FIG. 3.
Sialidase activity in culture supernatants derived from nanI and nanJ mutants. Samples of each strain were harvested from 4-h cultures in TH broth. The culture supernatants were concentrated 20-fold, and sialidase activity at pH 7.0 (A) (n = 6) or pH 5.5 (B) (n = 4) was assayed. Genotypes are indicated on the x axis (WT, wild type). nanI(v), nanJ(v), and nanIJ(v), nanI, nanJ, and nanIJ mutants carrying the vector plasmid, respectively; nanI(I+), nanI mutant carrying the nanI+ complementation plasmid; nanI(J+) and nanIJ(J+), nanI and nanIJ mutants carrying the nanJ+ complementation plasmids, respectively. The error bars indicate standard errors of the means.
When preparations were assayed over a longer period of time and at pH 5.5, low levels of sialidase activity were detected in concentrated supernatants from the nanI mutant (Fig. 3B). When both nanI and nanJ were disrupted, virtually no sialidase activity was detected, indicating that NanJ was responsible for the residual sialidase activity. Complementation of the nanJ mutation with the wild-type nanJ gene, but not complementation with the vector control, resulted in levels of sialidase activity that were greater than the levels detected for the nanI mutant, again presumably due to gene dosage effects. These results provide the first demonstration that the C. perfringens nanJ gene encodes a functional sialidase, even though two modules of NanJ, including a module that binds sialic acid, have been crystallized and their structures have been determined (5).
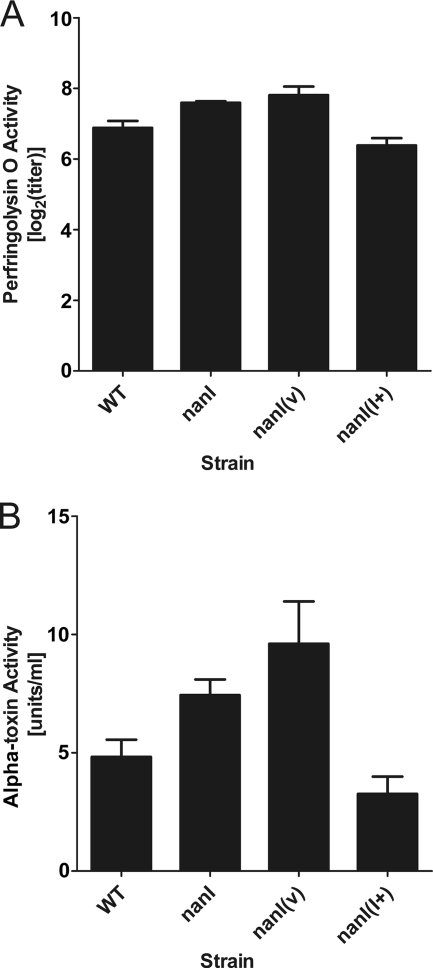
Disruption of nanI affects perfringolysin O and alpha-toxin activities in a culture supernatant.
Since it was previously reported that NanI enhances the activity of alpha-toxin with cultured cell lines and when it is injected into mice in vivo (8), the effect of the nanI mutation on perfringolysin O and alpha-toxin activities was determined. The results showed that culture supernatants of the nanI mutant derivatives JIR4915 and JIR4915(pJIR751) exhibited small but significant increases in alpha-toxin (P < 0.04) and perfringolysin O activities (P < 0.03) compared to culture supernatants of the wild-type strain (Fig. 4). When the ability to produce NanI was restored by complementation, the toxin levels were statistically the same as the wild-type levels (P > 0.15). When the nanJ and nanIJ mutants and their derivatives were assayed, each derivative showed toxin levels that were not significantly different from those of its parent strain; that is, the nanJ mutant had toxin levels that were indistinguishable from those of the wild-type strain JIR325, and the nanIJ mutant had slightly higher levels that were not significantly different from those of its nanI parent JIR4915 (data not shown).
FIG. 4.
Perfringolysin O and alpha-toxin production by the nanI mutant and its derivatives. Culture supernatants (n = 4) and 20-fold-concentrated supernatants (n = 6) were assayed to determine perfringolysin O (A) and alpha-toxin (B) activities, respectively. Genotypes are indicated on the x axis (WT, wild type). nanI(v), nanI mutant carrying the vector plasmid; nanI(I+), nanI mutant carrying the nanI+ complementation plasmid. The error bars indicate standard errors of the means.
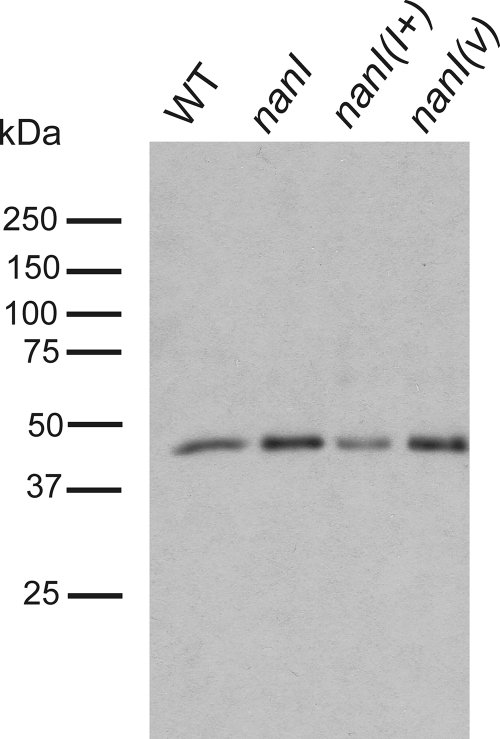
The apparent increase in toxin activity in the supernatant could have been the result of either NanI-mediated inhibition of alpha-toxin and perfringolysin O activities or increased toxin production. Western blotting of culture supernatants with an alpha-toxin antibody revealed that there was a higher level of alpha-toxin in the strains that lacked NanI than in the wild-type strain and in the complemented mutant (Fig. 5). SYPRO ruby staining confirmed that equivalent levels of total protein were present in the samples (data not shown). These results indicate that the unexpected activity profile observed in the in vitro alpha-toxin assays was the result of increased extracellular alpha-toxin production rather than any inhibitory effect of the sialidase on enzyme activity.
FIG. 5.
Western blots of nanI mutants and their derivatives. Identical volumes of culture supernatants from cultures previously examined using the in vitro alpha-toxin assay were examined by Western blotting using anti-alpha-toxin antibodies. WT, wild type; nanI(I+), nanI mutant carrying the nanI+ complementation plasmid; nanI(v), nanI mutant carrying the vector plasmid.
Both NanI and NanJ enhance alpha-toxin-mediated cytotoxic effects on cultured cell lines.
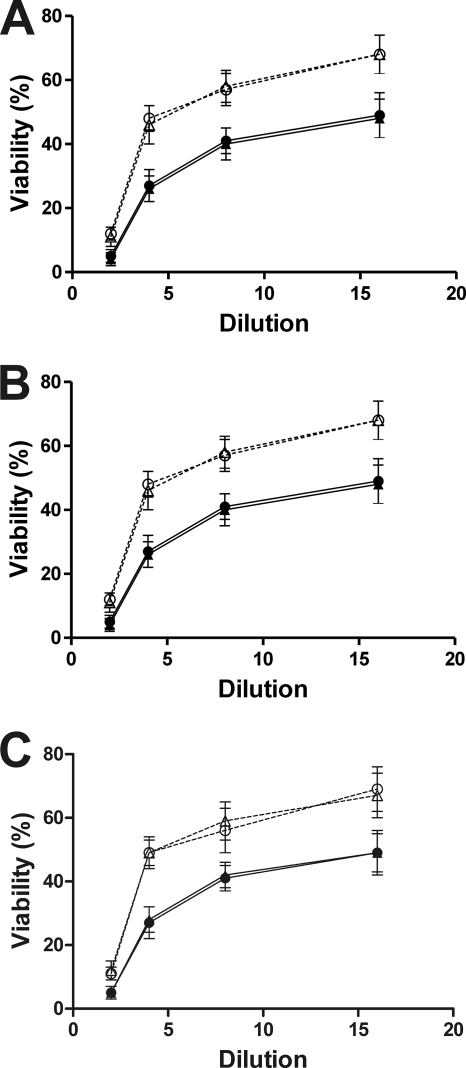
Previous results obtained with purified toxins indicated that pretreatment with the NanI sialidase predisposed host cells to the cytotoxic effects of alpha-toxin (8). To see if similar effects were observed when culture supernatants from the isogenic wild-type and mutant strains were used, ultrafiltered supernatants of the wild-type strain and the nanI, nanJ, and nanIJ mutants and their complemented derivatives were added to B16 mouse melanoma cells, and their cytotoxicities were determined. These preparations exhibited equivalent levels of alpha-toxin activity and had similar cytotoxic activities with ganglioside-deficient GM95 (sialidase-resistant) cells (data not shown), indicating that equivalent amounts of alpha-toxin were present in the samples. However, the cytotoxic activity with B16 cells was significantly lower (P < 0.02) for supernatants of the nanI mutant than for supernatants of the wild-type strain (Fig. 6A). Transformation with the control vector plasmid had no effect on cytotoxicity, but complementation with the wild-type nanI gene restored wild-type activity. These results indicate that the sensitivity of B16 cells to the cytotoxic effect of alpha-toxin is enhanced in the presence of NanI. By contrast, mutation of the nanJ gene had no effect on cytotoxicity (Fig. 6B). Complementation of the nanIJ double mutant with the wild-type nanJ gene did restore wild-type cytotoxicity (P < 0.025), suggesting that it is the level of sialidase in the culture supernatant that is important, not the specific sialidase enzyme.
FIG. 6.
Comparative cytotoxicities of supernatants from C. perfringens mutants. The cytotoxicities of supernatants from the strains indicated below were determined using the B16 melanoma cell line as described in Materials and Methods. The averages and standard deviations for at least three independent experiments are indicated. (A) Symbols: •, wild type; ▴, nanI mutant carrying the nanI+ complementation plasmid; ○, nanI mutant; ▵, nanI mutant carrying the vector plasmid. (B) Symbols: •, wild type; ▴, nanJ mutant carrying the nanJ+ complementation plasmid; ○, nanJ mutant; ▵, nanJ mutant carrying the vector plasmid. (C) Symbols: •, wild type; ▴, nanIJ mutant carrying the nanIJ+ complementation plasmid; ○, nanIJ mutant; ▵, nanIJ mutant carrying the vector plasmid. Twofold dilutions from 1/2 to 1/16 are indicated on the x axes.
NanI and NanJ are not essential for virulence in the mouse myonecrosis model.
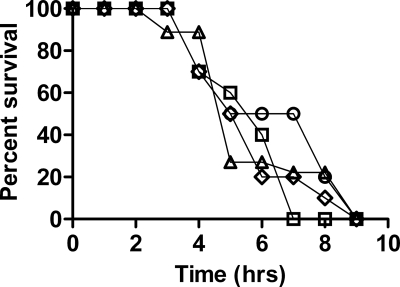
The virulence of the isogenic nanI, nanJ, and nanIJ mutants was assessed using the mouse myonecrosis model. Mice were monitored hourly, several gross pathological features were scored, and the overall levels of survival were determined (Fig. 7). The nanI and nanJ mutants were not significantly different from the wild-type strain for any facet of disease examined, and they were fully virulent. The onset of limping in the nanJ mutant appeared to be slightly delayed, but this result was not consistently observed in different experimental replicates. The sialidase null mutant, JIR12017, which has mutations in both nanI and nanJ, also showed no alteration in virulence; the disease caused by this strain was indistinguishable from the disease caused by the wild type (Fig. 7). Because no differences in virulence were observed, for ethical reasons it was deemed unnecessary to test the virulence of the complemented derivatives.
FIG. 7.
Survival of mice infected with sialidase mutants. Groups of 10 BALB/c mice were inoculated with the strains indicated, and the overall survival curves are shown. Symbols: ⋄, wild-type strain JIR325; □, JIR4915 (nanI); ▵, JIR12017 (nanIJ); ○, JIR12018 (nanJ).
To determine if the characteristic leukocyte accumulation in the blood vessels and the paucity of leukocytes at the site of infection that are observed in C. perfringens-mediated myonecrosis (7) were affected by the sialidase mutations, muscle tissue from infected mice was stained with hematoxylin and eosin and examined microscopically. The tissues from mice infected with the wild-type and mutant strains were indistinguishable, and necrosis in the muscle tissue was evident with all of the strains examined. There was also no significant difference between the strains in the levels of leukocyte accumulation in the blood vessels (data not shown).
In a separate series of experiments it was shown that 5 h after intramuscular injection of 5 × 109 cells of strain JIR325, damage to the muscle tissue could be detected biochemically by the presence of a significant increase in plasma creatine kinase activity (Table 3). Consistent with the results of the other virulence experiments, the increase in creatine kinase activity induced by the mutants was similar to the increase induced by parent strain JIR325. These results confirmed that absence of the nanI and nanJ genes, either singly or in tandem, did not result in a decrease in mouse myotoxicity.
TABLE 3.
Plasma creatine kinase activity after infection with C. perfringens mutants
| Strain | Properties | Creatine kinase activity (IU/liter)a |
|---|---|---|
| JIR325 | Wild-type strain 13 Rifr Nalr | 4,970 ± 840 |
| JIR325 | Wild type (heat-killed cells) | 201 ± 45 |
| JIR4915 | JIR325 ΔnanI::catP, nanI mutant (Rifr Nalr Cmr) | 5,080 ± 750 |
| JIR12017 | JIR4915 nanJ::erm(B), nanIJ double mutant (Rifr Nalr Cmr Emr) | 5,020 ± 680 |
| JIR12018 | JIR325 nanJ::erm(B), nanJ mutant (Rifr Nalr Emr) | 5,370 ± 810 |
Myotoxicity was evaluated after intramuscular injection (n = 8), and the creatine kinase activity in plasma was determined 5 h later. The values are the averages and standard deviations for at least three independent experiments. One international unit is the amount of enzyme that transforms 1 μmol of substrate per min under standard conditions.
DISCUSSION
The use of molecular genetics for determination of the role of C. perfringens sialidases in gas gangrene was somewhat complicated by the presence of two secreted sialidases in this organism. NanI has previously been characterized and shown to be functional (19, 20, 38). The NanJ sialidase was identified by genome sequencing projects (18, 32); however, although its binding domains have recently been analyzed (5), the biological activity of this enzyme has not been characterized. By using mutants with mutations in both the nanI and nanJ sialidase genes we assessed the relative contribution of each sialidase to the total sialidase activity and we showed that the nanJ gene encodes a functional extracellular sialidase. NanI is the major sialidase produced in vitro, and when assessed under optimum conditions for this enzyme, the nanI mutation resulted in an almost complete loss of sialidase activity. This phenotype was restored by complementation with the wild-type nanI gene. This result also showed that disruption of the downstream gene CPE0726 did not affect sialidase production in the mutant since the phenotype was restored by addition of nanI alone.
When the sialidase activity of the nanI mutant was assayed at pH 5.5, residual sialidase activity was observed. This activity represented only 9% of the sialidase activity of the wild-type strain under the same conditions. This loss of sialidase activity is similar to that observed for the sialidase mutants of S. pneumoniae. In this species, a nanA mutant retains a small amount of sialidase activity (∼10%), attributed to the second sialidase, NanB, when it is grown in vitro (16). The residual sialidase activity of the nanI mutant was abolished following subsequent mutation of nanJ, clearly demonstrating that this activity is mediated by NanJ. Complementation of the nanIJ double mutant with the wild-type nanJ gene resulted in levels of activity greater than the wild-type levels, which definitively demonstrates the functional nature of the second secreted sialidase.
Previous studies showed that the enhancement of in vivo alpha-toxin activity by NanI is dependent on the presence of gangliosides on the surface of the cell (8). Cleavage of the sialic acid units of these gangliosides, which protrude from the cell surface, most likely changes the electrostatic potential of the microenvironment and allows better interaction of alpha-toxin with its substrates at the cellular interface (8). The results presented here are consistent with these findings. No differences in cytotoxicity were observed between NanI-deficient and wild-type culture supernatants when they were assessed using the GM95 cell line. Since this cell line lacks surface gangliosides, the protective effect of sialic acid on alpha-toxin-mediated cytotoxicity was not observed, and, as expected, the presence of NanI did not enhance the activity of the alpha-toxin, as the sialidase lacked a target. However, when assessed using the ganglioside-proficient cell line B16, both NanI and NanJ enhanced cytotoxicity. C. perfringens supernatants that contained both alpha-toxin and NanI or NanJ exhibited higher levels of cytotoxicity with B16 cells than supernatants that contained alpha-toxin but lacked NanI. This result confirmed previous data that showed that there was alpha-toxin and NanI synergy when purified proteins were used (8).
Even though we observed enhancement of alpha-toxin-mediated cytotoxicity by NanI and NanJ in the cell culture model, this result did not translate to the mouse model of myonecrosis. Infection with the wild type or with a nanI, nanJ, or nanIJ mutant did not result in any differences in the abilities of the organisms to cause disease, in the rates at which the mice displayed the gross pathological features of disease, or in the rates at which the mice had to be euthanized for ethical reasons. The overall levels of muscle necrosis were also the same, as were the levels of leukocyte accumulation in the vasculature. Since all the mice displayed similar levels of disease, these results clearly demonstrate that the NanI sialidase and the NanJ sialidase, either singly or in tandem, are not essential for the pathogenesis of gas gangrene.
The inability of the sialidase mutants to mediate an effect in vivo does not, however, eliminate the possibility that sialidases are involved in the pathogenesis of gas gangrene. In other pathogens, sialidase is not essential for virulence, but it enhances the virulence of the pathogens (6, 10, 37). It is possible that subtle effects that are mediated by the sialidases are masked in this model, which of necessity involves infection with a large number of cells to ensure that anaerobic conditions are created. This model is the only way in which virulent disease can be reproduced. Inoculation with a lower infectious dose of C. perfringens does not lead to fulminant gangrene (21; L. Young, J. Emmins, and J. Rood, unpublished observations).
In conclusion, the data presented in this paper clearly demonstrate that C. perfringens strain JIR325 encodes two functional secreted sialidases. The majority of sialidase activity is mediated by NanI, although under certain conditions NanJ contributes to the total sialidase activity of the organism. The alpha-toxin-mediated cytotoxicity for cultured cell lines was enhanced by the presence of NanI or NanJ, but neither sialidase was found to be essential for the pathogenesis of gas gangrene.
Acknowledgments
This work was supported by a program grant from the Australian National Health and Medical Research Council, by the Australian Research Council (ARC) through the ARC Centre of Excellence in Structural and Functional Microbial Genomics, and by grant AI056177-03 from the United States National Institute of Allergy and Infectious Diseases.
We thank P. Hauer, Center for Veterinary Biologics, Ames, IA, for kindly providing alpha-toxin antibodies.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 3 August 2009.
REFERENCES
- 1.Alape-Girón, A., M. Flores-Díaz, I. Guillouard, C. E. Naylor, R. W. Titball, A. Rucavado, B. Lomonte, A. K. Basak, J. M. Gutiérrez, S. T. Cole, and M. Thelestam. 2000. Identification of residues critical for toxicity in Clostridium perfringens phospholipase C, the key toxin in gas gangrene. Eur. J. Biochem. 267:5191-5197. [DOI] [PubMed] [Google Scholar]
- 2.Awad, M. M., A. E. Bryant, D. L. Stevens, and J. I. Rood. 1995. Virulence studies on chromosomal α-toxin and θ-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of α-toxin in Clostridium perfringens-mediated gas gangrene. Mol. Microbiol. 15:191-202. [DOI] [PubMed] [Google Scholar]
- 3.Awad, M. M., D. M. Ellemor, R. L. Boyd, J. J. Emmins, and J. I. Rood. 2001. Synergistic effects of α-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect. Immun. 69:7904-7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannam, T. L., and J. I. Rood. 1993. Clostridium perfringens-Escherichia coli shuttle vectors that carry single antibiotic resistance determinants. Plasmid 29:223-235. [DOI] [PubMed] [Google Scholar]
- 5.Boraston, A. B., E. Ficko-Blean, and M. Healey. 2007. Carbohydrate recognition by a large sialidase toxin from Clostridium perfringens. Biochemistry 46:11352-11360. [DOI] [PubMed] [Google Scholar]
- 6.Davies, J., A. Dewar, A. Bush, T. Pitt, D. Gruenert, D. M. Geddes, and E. W. Alton. 1999. Reduction in the adherence of Pseudomonas aeruginosa to native cystic fibrosis epithelium with anti-asialoGM1 antibody and neuraminidase inhibition. Eur. Respir. J. 13:565-570. [DOI] [PubMed] [Google Scholar]
- 7.Ellemor, D. M., R. N. Baird, M. M. Awad, R. L. Boyd, J. I. Rood, and J. J. Emmins. 1999. Use of genetically manipulated strains of Clostridium perfringens reveals that both alpha-toxin and theta-toxin are required for vascular leukostasis to occur in experimental gas gangrene. Infect. Immun. 67:4902-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flores-Díaz, M., A. Alape-Girón, G. Clark, B. Catimel, Y. Hirabayashi, E. Nice, J. M. Gutierrez, R. Titball, and M. Thelestam. 2005. A cellular deficiency of gangliosides causes hypersensitivity to Clostridium perfringens phospholipase C. J. Biol. Chem. 280:26680-26689. [DOI] [PubMed] [Google Scholar]
- 9.Flores-Díaz, M., A. Alape-Girón, R. Titball, M. Moos, I. Guillouard, S. Cole, A. Howells, C. von Eichel-Streiber, I. Florin, and M. Thelestam. 1998. UDP-glucose deficiency causes hypersensitivity to the cytotoxic effect of Clostridium perfringens phospholipase C. J. Biol. Chem. 273:24433-24438. [DOI] [PubMed] [Google Scholar]
- 10.Galen, J. E., J. M. Ketley, A. Fasano, S. H. Richardson, S. S. Wasserman, and J. B. Kaper. 1992. Role of Vibrio cholerae neuraminidase in the function of cholera toxin. Infect. Immun. 60:406-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes, M. L., R. Poon, V. Adams, S. Sayeed, S. Saputo, F. A. Uzal, B. A. McClane, and J. I. Rood. 2007. Epsilon toxin plasmids of Clostridium perfringens type D are conjugative. J. Bacteriol. 189:7531-7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruse, S., R. G. Kleineidam, P. Roggentin, and R. Schauer. 1996. Expression and purification of a recombinant small sialidase from Clostridium perfringens A99. Protein Expr. Purif. 7:415-422. [DOI] [PubMed] [Google Scholar]
- 13.Lyristis, M., A. E. Bryant, J. Sloan, M. M. Awad, I. T. Nisbet, D. L. Stevens, and J. I. Rood. 1994. Identification and molecular analysis of a locus that regulates extracellular toxin production in Clostridium perfringens. Mol. Microbiol. 12:761-777. [DOI] [PubMed] [Google Scholar]
- 14.Mahony, D. E., and T. J. Moore. 1976. Stable L-forms of Clostridium perfringens and their growth on glass surfaces. Can. J. Microbiol. 22:953-959. [DOI] [PubMed] [Google Scholar]
- 15.Mally, M., H. Shin, C. Paroz, R. Landmann, and G. R. Cornelis. 2008. Capnocytophaga canimorsus: a human pathogen feeding at the surface of epithelial cells and phagocytes. PLoS Pathog. 4:e1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manco, S., F. Hernon, H. Yesilkaya, J. C. Paton, P. W. Andrew, and A. Kadioglu. 2006. Pneumococcal neuraminidases A and B both have essential roles during infection of the respiratory tract and sepsis. Infect. Immun. 74:4014-4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 18.Myers, G. S., D. A. Rasko, J. K. Cheung, J. Ravel, R. Seshadri, R. T. DeBoy, Q. Ren, J. Varga, M. M. Awad, L. M. Brinkac, S. C. Daugherty, D. H. Haft, R. J. Dodson, R. Madupu, W. C. Nelson, M. J. Rosovitz, S. A. Sullivan, H. Khouri, G. I. Dimitrov, K. L. Watkins, S. Mulligan, J. Benton, D. Radune, D. J. Fisher, H. S. Atkins, T. Hiscox, B. H. Jost, S. J. Billington, J. G. Songer, B. A. McClane, R. W. Titball, J. I. Rood, S. B. Melville, and I. T. Paulsen. 2006. Skewed genomic variability in strains of the toxigenic bacterial pathogen, Clostridium perfringens. Genome Res. 16:1031-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nees, S., and R. Schauer. 1974. Induction of neuraminidase from Clostridium perfringens and the correlation of this enzyme with acylneuraminate pyruvate-lyase. Behring Inst. Mitt. 55:68-78. [Google Scholar]
- 20.Newstead, S. L., J. A. Potter, J. C. Wilson, G. Xu, C. H. Chien, A. G. Watts, S. G. Withers, and G. L. Taylor. 2008. The structure of Clostridium perfringens NanI sialidase and its catalytic intermediates. J. Biol. Chem. 283:9080-9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Brien, D. K., and S. B. Melville. 2004. Effects of Clostridium perfringens alpha-toxin (PLC) and perfringolysin O (PFO) on cytotoxicity to macrophages, on escape from the phagosomes of macrophages, and on persistence of C. perfringens in host tissues. Infect. Immun. 72:5204-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Connor, J. R., D. Lyras, K. A. Farrow, V. Adams, D. R. Powell, J. Hinds, J. K. Cheung, and J. I. Rood. 2006. Construction and analysis of chromosomal Clostridium difficile mutants. Mol. Microbiol. 61:1335-1351. [DOI] [PubMed] [Google Scholar]
- 23.Orihuela, C. J., G. Gao, K. P. Francis, J. Yu, and E. I. Tuomanen. 2004. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J. Infect. Dis. 190:1661-1669. [DOI] [PubMed] [Google Scholar]
- 24.Roggentin, P., B. Rothe, F. Lottspeich, and R. Schauer. 1988. Cloning and sequencing of a Clostridium perfringens sialidase gene. FEBS Lett. 238:31-34. [DOI] [PubMed] [Google Scholar]
- 25.Rood, J. I. 2007. Clostridium perfringens and histotoxic disease, p. 753-770. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes: a handbook on the biology of bacteria, 3rd ed., vol. 4. Springer, New York, NY. [Google Scholar]
- 26.Rood, J. I. 1983. Transferable tetracycline resistance in Clostridium perfringens strains of porcine origin. Can. J. Microbiol. 29:1241-1246. [DOI] [PubMed] [Google Scholar]
- 27.Rood, J. I., and B. A. McClane. 2002. Clostridium perfringens: enterotoxaemic diseases, p. 1117-1139. In M. Sussman (ed.), Molecular medical microbiology, vol. 1. Academic Press, London, United Kingdom. [Google Scholar]
- 28.Rood, J. I., V. N. Scott, and C. L. Duncan. 1978. Identification of a transferable resistance plasmid (pCW3) from Clostridium perfringens. Plasmid 1:563-570. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.Scott, P. T., and J. I. Rood. 1989. Electroporation-mediated transformation of lysostaphin-treated Clostridium perfringens. Gene 82:327-333. [DOI] [PubMed] [Google Scholar]
- 31.Shimamoto, S., E. Tamai, O. Matsushita, J. Minami, A. Okabe, and S. Miyata. 2005. Changes in ganglioside content affect the binding of Clostridium perfringens epsilon-toxin to detergent-resistant membranes of Madin-Darby canine kidney cells. Microbiol. Immunol. 49:245-253. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu, T., K. Ohtani, H. Hirakawa, K. Ohshima, A. Yamashita, T. Shiba, N. Ogasawara, M. Hattori, S. Kuhara, and H. Hayashi. 2002. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. USA 99:996-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sloan, J., T. A. Warner, P. T. Scott, T. L. Bannam, D. I. Berryman, and J. I. Rood. 1992. Construction of a sequenced Clostridium perfringens-Escherichia coli shuttle plasmid. Plasmid 27:207-219. [DOI] [PubMed] [Google Scholar]
- 34.Songer, J. G. 2005. Clostridial diseases in domestic animals, p. 527-542. In P. Durre (ed.), Handbook on clostridia. Taylor and Francis, Boca Raton, FL.
- 35.Southern, E. M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503-517. [DOI] [PubMed] [Google Scholar]
- 36.Stevens, D. L., J. Mitten, and C. Henry. 1987. Effects of α and θ toxins from Clostridium perfringens on human polymorphonuclear leukocytes. J. Infect. Dis. 156:324-333. [DOI] [PubMed] [Google Scholar]
- 37.Tong, H. H., L. E. Blue, M. A. James, and T. F. DeMaria. 2000. Evaluation of the virulence of a Streptococcus pneumoniae neuraminidase-deficient mutant in nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect. Immun. 68:921-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Traving, C., R. Schauer, and P. Roggentin. 1994. Gene structure of the ‘large’ sialidase isoenzyme from Clostridium perfringens A99 and its relationship with other clostridial nanH proteins. Glycoconj. J. 11:141-151. [DOI] [PubMed] [Google Scholar]
- 39.Uzal, F. A., and W. R. Kelly. 1997. Effects of the intravenous administration of Clostridium perfringens type D epsilon toxin on young goats and lambs. J. Comp. Pathol. 116:63-71. [DOI] [PubMed] [Google Scholar]
- 40.Uzal, F. A., and W. R. Kelly. 1998. Experimental Clostridium perfringens type D enterotoxemia in goats. Vet. Pathol. 35:132-140. [DOI] [PubMed] [Google Scholar]
- 41.Walters, D. M., V. L. Stirewalt, and S. B. Melville. 1999. Cloning, sequence, and transcriptional regulation of the operon encoding a putative N-acetylmannosamine-6-phosphate epimerase (nanE) and sialic acid lyase (nanA) in Clostridium perfringens. J. Bacteriol. 181:4526-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]