Abstract
Enteropathogenic Escherichia coli (EPEC) strains cause watery diarrhea almost exclusively in young children. The basis for this age discrimination has never been determined, but it may be related to host cell receptors. During infection, EPEC strains express type IV bundle-forming pili composed of repeating subunits of the protein called bundlin. The very first interaction between EPEC and in vitro-cultured epithelial cells is mediated by the binding of α-bundlin to a carbohydrate receptor that contains, at a minimum, the N-acetyllactosamine (LacNAc) glycan sequence. However, bundlins expressed from the β-bundlin allele do not bind LacNAc glycan sequences. Herein, we investigated whether EPEC strains use α-bundlin to mediate early adherence to human intestinal biopsy specimens cultured in vitro by assessing the ability of isogenic EPEC mutants expressing either the α1- or β1-bundlin allele or a bundlin-deficient EPEC strain to bind to these specimens. Furthermore, we directly compared the abilities of a wild-type EPEC strain to bind to the epithelial surfaces of both human adult and pediatric biopsy specimens. Our results demonstrate that β-bundlin does not act as an adhesin during early EPEC adherence to adult duodenal biopsy specimens. The results also indicate that EPEC binds equally well to adult and pediatric biopsy specimens in an early adherence assay. This result is supported by the finding that the early adherence of EPEC to both adult and pediatric biopsy specimens was inhibited by LacNAc neoglycoconjugates, suggesting that organisms expressing α-bundlin-type bundle-forming pili initially bind to related glycan receptors in both age groups.
Enteropathogenic Escherichia coli (EPEC) strains cause watery diarrhea in young children, an illness associated with the EPEC-mediated disruption of the small-intestinal epithelium (21, 22, 24). While EPEC strains cause clinical disease predominantly in children under the age of 2 years, disease can also be elicited in adult volunteers given a very high infectious dose of bacteria (1, 7, 9, 11). The basis for this age discrimination by EPEC has never been determined but may be related to either differences in susceptibility to EPEC colonization between adults and infants or to acquired protective immunity from repeated infections in adults.
EPEC pathogenesis is dependent on a two-stage mechanism of adherence of the bacteria to host enterocytes. In the first step, EPEC cells bind to the host cell via their type IV bundle-forming pili (BFP) in a process known as localized adherence (LA). Following LA, EPEC strains inject effector proteins and their own receptor, a protein called the translocated intimin receptor (Tir), into the host cell via a type III secretion system. Tir is inserted into the host cell plasma membrane, where it acts as the receptor for the EPEC adhesin intimin and recruits cytoskeletal components, the net effect of which is the effacement of the microvilli and the formation of actin-rich pedestals at the site of EPEC adherence. This is known as the attaching-and-effacing process.
BFP are homopolymers of a protein called bundlin. EPEC strains express one of two bundlin types, α or β, based on sequence homology (5). There are currently three α-bundlin alleles and seven β-bundlin alleles that have been described among diverse EPEC strains (4, 5). The α-bundlin proteins are N-acetyllactosamine (LacNAc) lectins, whereas the β-bundlins are not, since they lack the carbohydrate binding domain found in the α-bundlins (13, 16). The divergence between bundlins may be the result of selective immunological pressure, since the β-bundlins are less immunogenic in rabbits and mice than are the α-bundlins (8). It appears that α1-bundlin is the only adhesin mediating early (45-min) LA of EPEC strain E2348/69 to HEp-2 cells, since replacing the α1-bundlin allele in this strain with β-bundlin alleles 1 to 3 abolishes early adherence despite the expression of BFP (16). The early LA of this strain, and of all the other α-bundlin allele-expressing EPEC strains tested to date, can also be inhibited by LacNAc conjugated to bovine serum albumin (BSA) or gold nanoparticles (LacNAc-Au) (14-16). We previously reported (14) that LacNAc-based neoglycoconjugates inhibit α1-bundlin-expressing EPEC LA as a result of their ability to (i) competitively inhibit the binding of the organisms to host cell glycan receptor sequences and (ii) induce BFP retraction, resulting in the dispersal of EPEC autoaggregates. Those strains that harbor the native β-bundlin allele are either nonadherent after a 45-min incubation with HEp-2 cells or not inhibited by LacNAc neoglycoconjugates (16). The inhibition of the α-bundlin allele-carrying strains is also lost when the bacteria are incubated on HEp-2 tissue culture cells for extended periods of time (3 h) (16). These data are consistent with evidence presented previously by Cleary and colleagues (6) that demonstrates a requirement for BFP during EPEC adherence to Caco-2 cells during the first 60 min of infection. After the first 60 min, other adhesins such as intimin and EspA then contribute to EPEC adherence.
Recently, significant differences were observed in the mechanism by which EPEC induces attaching and effacing lesions in tissue culture cells compared to ex vivo human intestinal biopsy specimens (3, 20). As such, we sought to determine if the roles that we have demonstrated for bundlin and LacNAc in E2348/69 early LA to intestinal cells grown in tissue culture also apply to early EPEC adherence to human intestinal explants. Of particular interest was whether the β-bundlins, represented in these studies by the β1-bundlin allele, had completely lost the ability to act as adhesins as a result of selective pressure or whether tissue culture cells simply lack the appropriate receptor for β-bundlin. We also wished to determine if the predilection of EPEC for causing disease in the pediatric population might be related to BFP-mediated early adherence.
MATERIALS AND METHODS
Bacterial strains, plasmids, and reagents.
Wild-type EPEC strain E2348/69 (serotype O127:H6), harboring the α1-bundlin allele, was initially isolated from an infant with diarrhea and was provided by M. Finlayson (Provincial Laboratory of Alberta, Edmonton, Alberta, Canada). UMD901 is an isogenic E2348/69 mutant strain that contains a missense mutation in bfpA that encodes C129S and leads to an unstable bundlin product that is rapidly degraded. UMD901 and plasmids pRPA100 (α1 bfpA complement) and pXLW13 (β1 bfpA complement) were kind gifts from Michael Donnenberg, University of Maryland, Baltimore, MD. Both plasmids contain the bfpA allele cloned behind the native BFP promoter from E2348/69 in low-copy-number vector pWKS30 (2, 8). These bacteria were routinely cultured on conventional tryptic soy agar (TSA) plates with ampicillin (100 μg/ml), as required, at 37°C to obtain single colonies. All strains in this study express equivalent amounts of BFP, which was assessed by electron microscopy, immunoblotting, and enzyme-linked immunosorbent assay against the bundlin protein reported in our previous studies (13, 16).
The chemically synthesized 8-methoxycarbonyloctyl glycoside of LacNAc was covalently coupled to BSA and was provided by Om Srivastava, Optimer Pharmaceuticals, San Diego, CA. The neoglycoconjugate was dissolved in sterile distilled water to a final concentration of 5 mg/ml.
HEp-2 cell EPEC adherence inhibition assay.
HEp-2 cells (ATCC CCL-23) were obtained from the American Type Culture Collection (Manassas, VA) and propagated according to their recommendations. Cell monolayers were prepared in 96-well microtiter plates containing polystyrene disks, as described previously by Vanmaele and colleagues (25). Prior to each experiment, BFP expression was induced by inoculating 10 μl of a tryptic soy broth bacterial culture, grown statically overnight at 37°C from a single colony picked from a TSA plate, into 1 ml of Dulbecco modified Eagle medium (DMEM; Invitrogen, Burlington, Ontario, Canada), which was preequilibrated overnight in a CO2 incubator. After 45 min, 0.8 mg/ml of either LacNAc-BSA or negative control Pk-BSA [BSA containing covalently coupled 8-methoxycarbonyloctyl glycosides of the αGal(1,4)βGal(1,4)βGlc trisaccharide sequence] was added to the cultures, and the mixtures were incubated for an additional 30 min in the CO2 incubator. These samples were then transferred into the wells of 96-well microtiter plates containing HEp-2 cells, and the samples were further incubated in the CO2 incubator for 45 min. The tissue culture cells were washed three times with phosphate-buffered (pH 7.2) physiological saline (PBS). Cells were either methanol fixed and Giemsa stained for light microscopy or solubilized using 0.01% (vol/vol) Triton X-100, serially diluted in sterile PBS, and spread onto TSA plates for determining the number of CFU as described previously (14). Whole genomic DNA was extracted from a third subset of EPEC-infected HEp-2 cells using the QIAmp DNA microkit (Qiagen, Mississauga, Ontario, Canada) for quantitative PCR (qPCR) determinations of the number of adherent bacteria as described below.
Intestinal explants.
The method and process used for obtaining the intestinal biopsy specimens were reviewed and approved by the Conjoint Ethics Review Panel of the University of Calgary and Alberta Children's Hospital. Following informed parental or patient consent, duodenal (DD) tissue samples were obtained during upper endoscopy procedures using standard biopsy forceps. Biopsy specimens from adjacent regions were used for pathological assessment by an expert pathologist as a part of routine clinical care. Only samples with no microscopic evidence of abnormality were included in this study. The age and sex of the subjects from whom biopsy specimens were obtained are shown in Table 1. The mean age of the nine pediatric subjects was 32.1 months (range, 45 months). The mean age for the 16 adult subjects was 41.5 years (range, 50 years). Once removed, the biopsy specimens were placed into ice-chilled PBS and used within 1 h.
TABLE 1.
Characteristics of pediatric and adult subjects from whom DD biopsy specimens were obtained
| Patient | Age | Sexa |
|---|---|---|
| Pediatric | ||
| 774 | 15 mo | M |
| 349 | 16 mo | M |
| 108 | 17 mo | M |
| 930 | 19 mo | F |
| 039 | 20 mo | M |
| 376 | 24 mo | F |
| 189 | 58 mo | M |
| 635 | 60 mo | M |
| 604 | 60 mo | M |
| Adult | ||
| 622 | 21 yr | F |
| 911 | 27 yr | F |
| 699 | 28 yr | F |
| 310 | 33 yr | M |
| 570 | 35 yr | F |
| 109 | 46 yr | F |
| 358 | 64 yr | M |
| 425 | 67 yr | F |
| 976 | 71 yr | F |
| 644 | 71 yr | M |
| 772 | 44 yr | M |
| 123 | 73 yr | F |
| 692 | 62 yr | F |
| 607 | 59 yr | F |
| 540 | 27 yr | F |
| 694 | 21 yr | F |
M, male; F, female.
Polystyrene biopsy specimen holders, described in our previous article (15) (Fig. 1), were used to restrict EPEC binding to the epithelial surface of the biopsy specimens. The holders also served to restrict the surface area to which EPEC is presented, since the exposed biopsy specimen surface area is the same in all holders. Biopsy specimens were oriented with the aid of a dissecting microscope and mounted luminal side up. Immediately following mounting, the chambers of the biopsy specimen holder were filled with ice-cold PBS, and the samples were kept on ice in a 12-well plate until infected with bacteria (typically 10 min).
FIG. 1.
Biopsy specimen holder utilized in this study.
Prior to each experiment, static (optical density at 600 nm of 0.6) EPEC tryptic soy broth cultures grown overnight were inoculated at a 1:100 dilution into DMEM that had been preequilibrated overnight in a CO2 incubator, as described previously (25). Following a 45-min incubation at 37°C in the CO2 incubator to induce BFP expression, EPEC bacteria were added to the epithelial surface of the biopsy specimens in duplicate, and the samples were incubated in the CO2 incubator for an additional 45 min. Biopsy specimens were then washed thoroughly with PBS, removed from the biopsy specimen holders, and placed directly into ATL buffer (Qiagen, Mississauga, Ontario, Canada) for overnight digestion and further processing. One uninfected biopsy specimen per subject was kept in reserve as a negative control to test for endogenous reactivity in the EPEC-specific qPCR assay described below. In preliminary experiments, aliquots of DMEM from the bottom biopsy specimen holder chamber were cultured on TSA to confirm that EPEC bacteria were restricted to the upper chamber; no CFU were found in any of the samples tested (data not shown).
For inhibition assays, either LacNAc-BSA or Pk-BSA (14) was added, to a final concentration of 0.8 mg/ml, to EPEC DMEM cultures following the initial 45-min incubation in the 37°C CO2 incubator. These cultures were incubated for an additional 30 min prior to adding them to the biopsy samples, as described above. E2348/69-DMEM-glycoconjugate mixtures were incubated on the biopsy specimens for 45 min in the 37°C CO2 incubator and processed exactly as described above for the HEp-2 cell adherence assays.
Quantitative real-time PCR assay.
Whole genomic DNA was extracted from infected HEp-2 cells or intestinal biopsy specimens using the QIAamp DNA microkit (Qiagen, Mississauga, Ontario, Canada). Bacteria were quantified by qPCR of the intimin (eae) gene using the oligonucleotide primers described elsewhere previously (18). qPCR was performed using a Bio-Rad (Hercules, CA) IQ5 instrument with the QuantiTect SYBR green PCR kit (Qiagen, Mississauga, Ontario, Canada) according to the manufacturer's instructions. Five 10-fold dilutions of whole genomic DNA, isolated from EPEC E2348/69 using a standard protocol (15), were used as an internal control for the qPCR. qPCR conditions were described in our previous article (15).
Statistical analyses.
GraphPad (La Jolla, CA) Prism 5.0 was used for calculations. Statistical significance was defined as a P value of <0.5.
RESULTS
Assessment of qPCR as a method to enumerate adherent EPEC bacteria.
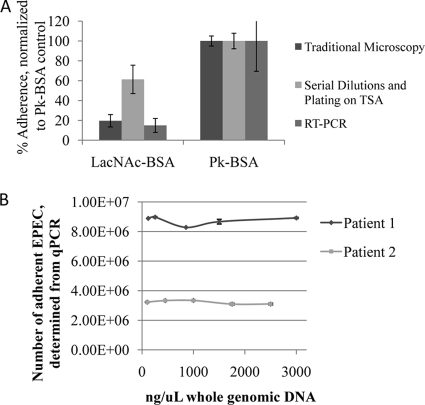
In our previous articles, we employed the accepted light microscopy method to assess EPEC LA to tissue culture cells (25). However, light microscopic observation of EPEC bacteria bound to intestinal biopsy specimens could not be performed for technical reasons, and the biopsy specimens are also exceedingly difficult to homogenize for determining CFU. Accordingly, we employed a qPCR method to determine the number of adherent EPEC bacteria on each biopsy specimen. With this method, whole genomic DNA was extracted from the biopsy specimens, and qPCR was performed on the intimin (eaeA) gene, which was then compared to a standard curve created using known concentrations of E2348/69 DNA. The equivalency of the qPCR, light microscopy, and CFU counting methods for enumerating adherent EPEC was assessed by comparing the methods using HEp-2 cells infected with E2348/69 in the presence of 0.8 mg/ml LacNAc-BSA or Pk-BSA (Fig. 2A). We found no significant difference among the three methods of assessing EPEC LA to HEp-2 cell monolayers (P = 0.45 by a Student's t test). We also tested the adherence of a mutant EPEC strain, JPN15, with our biopsy adherence assay. This strain lacks plasmid pEAF, which carries the BFP operon and PerC, a positive regulator for intimin and Tir (19). JPN15 was nonadherent in our tissue culture early-LA assay, and we could not detect an adherence of this strain to biopsy specimens obtained from the duodenum of four adult patients by the intestinal biopsy adherence assay (data not shown).
FIG. 2.
Validation of the qPCR method for enumerating EPEC strain E2348/69 LA to intestinal biopsy specimens. (A) Comparison of microscopic evaluation, serial dilutions and plating, and qPCR enumeration of E2348/69 cells bound to HEp-2 cells in the presence of 0.8 mg/ml LacNAc-BSA or Pk-BSA. Data are normalized to the Pk-BSA negative control. Each bar represents the mean of three experiments, and error bars indicate the standard deviations from the means. (B) Effect of biopsy specimen size on qPCR enumeration of E2348/69 cells bound to adult DD biopsy specimens. Each point represents the average of data obtained from two specimens, with standard errors. Numbers of EPEC E2348/69 bacteria were determined by qPCR of the eaeA gene, whereas the whole genomic DNA concentration of EPEC E2348/69-infected biopsy specimens was determined by spectrophotometry, as outlined in Materials and Methods. RT, reverse transcription.
In the intestinal explant infection model, the surface area of the biopsy specimen to which EPEC cells were exposed is restricted by the size of the pore in the upper well of the biopsy specimen holders (Fig. 1). Regardless, we wondered if biopsy specimen size might affect the outcome, since larger amounts of tissue might act as a carrier for the relatively small amounts of EPEC DNA extracted by the QIAmp DNA microkit. To test this, we obtained three biopsy specimens from the duodenum of two individuals. The biopsy specimens were trimmed into five pieces of increasing size and infected with E2348/69 as described in Materials and Methods. One biopsy specimen was left uninfected as a negative control. Genomic DNA was extracted, quantified using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE), and assessed for E2348/69 adherence using our qPCR assay. In these experiments, biopsy specimen size, gauged by the final DNA concentration, did not have any effect on the amount of E2348/69 DNA detected by qPCR (Fig. 2B), and no E2348/69 DNA was detected in the uninfected specimens (data not shown).
β-Bundlin-expressing EPEC strains adhere poorly to adult DD biopsy specimens.
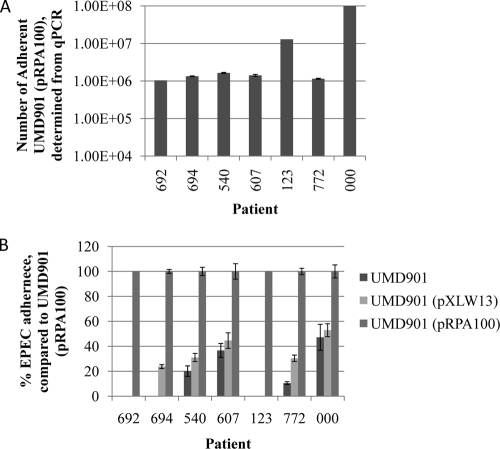
We previously demonstrated that UMD901, a bundlin-null isogenic E2348/69 EPEC mutant strain, binds in the 45-min LA assay to neither HEp-2 cells (16) nor Caco-2 cells (our unpublished observations) grown in tissue culture. Furthermore, if UMD901 is complemented with a β-bundlin allele, adherence is not restored, whereas BFP expression is. We now wished to determine if α-bundlin is also necessary for early adherence to human intestinal explants. Two adult DD biopsy specimens from seven adult subjects were infected with either UMD901; UMD901(pRPA100), which expresses α1-bundlin; or UMD901(pXLW13), which expresses β1-bundlin, and adherence was assessed after 45 min using the qPCR method. On average, 1.79 × 107 UMD901(pRPA100) EPEC bacteria (range, 1.05 × 108) adhered to the DD samples (Fig. 3A), which represents 1% of the inoculum (data not shown). In contrast, both UMD901 and UMD901(pXLW13) were significantly (P < 0.001 for all samples; n = 4) attenuated in their abilities to bind the DD biopsy specimens (Fig. 3B). For two subjects, no UMD901 or UMD901(pXLW13) cells adhered to the biopsy specimens despite the biopsy specimens from individual 123 harboring the second highest number of UMD901(pRPA100) bacteria (compare Fig. 3A and B). In the remaining five subjects, more UMD901(pXLW13) cells adhered to the DD biopsy specimens than UMD901 cells. However, this difference was significant for only three of the samples (Fig. 3B). These data suggest that early adherence to the native intestinal epithelium is mediated by α-bundlin.
FIG. 3.
(A) Adherence of UMD901(pRPA100), which expresses α1-bundlin, to human adult DD specimens. The number of adherent EPEC cells was determined by qPCR. (B) Adherence of UMD901 and UMD901(pXLW13), which expresses β1-bundlin, to human adult DD specimens relative to the adherence of UMD901(pRPA100). In both panels, bars represent the averages of data from four samples, and the error bars are the standard deviations from the means.
Effect of LacNAc-BSA on E2348/69 adherence to adult and pediatric biopsy specimens.
In a previous article, we demonstrated that LacNAc conjugated to gold nanoparticles (LacNAc-Au) inhibited the LA of E2348/69 to adult biopsy specimens obtained from the DD, terminal ileum, and transverse colon. Herein, we sought to extend these results to biopsy specimens obtained from pediatric subjects. However, our access to these samples was limited. Accordingly, we tested only samples obtained from the DD since EPEC naturally infects the small intestine. Furthermore, while we endeavored to obtain biopsy specimens only from children under the age of 2 years, we extended the age limit to 5 years, similarly to others (12), also because of the limited availability of samples.
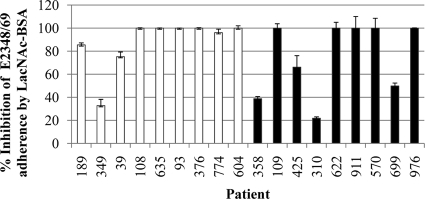
These experiments revealed that LacNAc-BSA inhibited E2348/69 adherence to all of the DD specimens (Fig. 4) in both the adult and pediatric specimens (P < 0.0001). For six of the pediatric specimens and five of the adult specimens, LacNAc-BSA reduced EPEC adherence below the detection limit (100 bacteria, 100% inhibition) (Fig. 4) of the assay. Overall, no correlation between the number of adherent EPEC cells and the inhibition of EPEC binding using LacNAc-BSA was observed, even when the subjects over the age of 2 years were removed from the analysis (data not shown).
FIG. 4.
LacNAc-BSA inhibition (percent relative to Pk-BSA glycan specificity controls) of EPEC E2348/69 cell adherence to DD biopsy specimens obtained from adult and pediatric subjects. White bars represent pediatric specimens (n = 2), and black bars represent adult specimens (n = 4). Error bars represent standard errors (pediatric specimens) or deviations from the means (adult specimens).
Comparison of early E2348/69 adherence to pediatric and adult biopsy specimens.
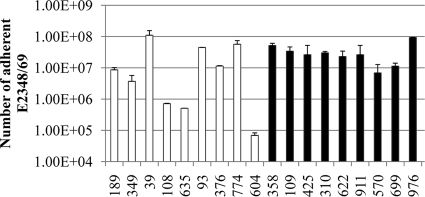
In our previous article, we observed significant (5-log range) subject-to-subject variability in the number of E2348/69 cells attached to the biopsy specimens collected from the terminal ileum and transverse colon of adults (15). In contrast, we previously observed little variability in E2348/69 binding to adult DD biopsy specimens (with the exception of one outlier). The data presented in Fig. 4 confirm these results. However, in contrast to adult specimens, the pediatric DD biopsy specimens displayed significant variability (P = 0.0306 by a Student's t test; range, 3.2 logs) in the number of adherent E2348/69 EPEC cells. Moreover, biopsy specimen preparations from adults generally bound more bacteria than did biopsy specimens from pediatric subjects, with eight of nine adult specimens displaying bacterial binding over 1 × 107 organisms per biopsy specimen, compared to only five of nine pediatric biopsy specimens displaying EPEC binding of over 1 × 107 bacteria per preparation. However, this difference failed to achieve statistical significance (P = 0.06 by Fisher exact test). Moreover, no correlation between the number of adherent EPEC bacteria and donor age or sex, or with breastfeeding relative to formula feeding among the pediatric subjects, was observed (P > 0.05 by a Student's t test).
DISCUSSION
In our previous studies, we demonstrated that EPEC employs its BFP to adhere to host cells during the early stages of infection (16). Bundlin, the primary structural subunit of the BFP filament, is a lectin-like adhesin that demonstrates specificity for LacNAc or LacNAc-related glycan receptors on intestinal epithelial cells in adults when expressed from the α-bundlin allele (15, 16). Accordingly, synthetic LacNAc conjugated to BSA or gold nanoparticles represents an effective means of inhibiting this initial interaction between α-bundlin-expressing EPEC strains and the host cell (14-16). However, those strains expressing β-bundlin alleles apparently produce alternate adhesins that mediate early adherence since the β-bundlins do not bind tissue culture cells. We have now demonstrated that this is also true for intestinal explants, since bundlin-null EPEC strain UMD901 was significantly attenuated in its ability to bind DD biopsy samples in vitro (Fig. 3). However, unlike the results with HEp-2 and Caco-2 cells, EPEC strain UMD901 displays some residual adherence, indicating that other adhesins also play a role in the early adherence of E2348/69 to the human intestine. Cleary and colleagues previously demonstrated a clear role for EspA in early E2348/69 adherence, and this adhesin may also be playing a role in our studies. Interestingly, we observed significant LacNAc-BSA inhibition of E2348/69 adherence to both pediatric and adult biopsy specimens. In some cases, LacNAc-BSA completely abolished E2348/69 adherence (Fig. 4). No lectin-like activity has been described for EspA, nor has a receptor been identified for this protein. However, the ability of LacNAc-BSA to inhibit E2348/69 adherence could be explained by our recent observations indicating that LacNAc-BSA, which induces BFP retraction and degradation, also induces a downregulation of the E2348/69 locus-of-enterocyte-effacement operons, including LEE4, on which espA is carried (our unpublished data).
While strain UMD901(pXLW13), which expresses β1-bundlin, adhered to the biopsy specimens obtained from three of the seven subjects significantly better than UMD901, it was still significantly attenuated in adherence compared to strain UM901(pRPA100). In our tissue culture assays, UMD901(pXLW13) was completely nonadherent in the 45-min assay, but adherence was evident after extended periods of incubation (3 h). However, we were concerned about the viability of the biopsy specimens for extended incubation times and therefore did not test the ability of EPEC cells to bind to biopsy specimens at later times. Since β-bundlin-expressing EPEC strains have been isolated from infants with diarrhea (5), it is likely that these EPEC strains have acquired adhesins, other than BFP, by which to colonize the host during infection. These additional adhesins might include flagella (10) or other pili. Moreover, under the physiochemical conditions that EPEC encounters in the small intestine, additional adhesins may be expressed that are not expressed when the organisms are growing in cell culture medium.
Bacterial lectins often impart host tropism, a phenomenon that was first described for E. coli K99 (17), which infects newborn but not adult pigs or humans. The K99 fimbrial lectin is specific for glycolipids containing N-glycolylneuraminic acid but not those containing N-acetylneuraminic acid. N-Glycolylneuraminic acid is expressed on the intestinal cells of piglets but is replaced by N-acetylneuraminic acid as the animals mature. Humans express N-acetylneuraminic acid from birth, which may explain why E. coli strains expressing K99 pili infect neonatal piglets but not mature pigs (17). In humans, EPEC strains cause illness almost exclusively in children less than 2 years of age, and in the present study, we sought to determine if the basis for this age-related discrimination might be related to the expression of alternate intestinal BFP receptors for EPEC in infants. Herein we report that E2348/69 apparently binds to related receptors in both pediatric and adult intestines, since adherence was inhibited in both cases by LacNAc-BSA (Fig. 4). Moreover, E2348/69 apparently does not adhere to pediatric DD biopsy specimens in higher numbers. In fact, the adherence potential of E2348/69 appears to be greater for the adult intestine than for the pediatric intestine (Fig. 5). Together, these results suggest that E2348/69 is possibly binding LacNAc-related receptors in both pediatric and adult intestines, and the predilection of EPEC to cause illness in infants may therefore be unrelated to preferential BFP-mediated adherence to the pediatric intestinal epithelium. In contrast, it would appear that BFP are responsible for the host species tropism displayed by EPEC based on data presented previously by Tobe and Sasakawa demonstrating that BFP bind tissue culture cells of human but not animal origin (23).
FIG. 5.
Numbers of E2348/69 cells adherent to DD biopsy specimens determined by qPCR of the eae gene. White bars represent pediatric specimens (n = 2), and black bars represent adult specimens (n = 4). Error bars represent standard errors (pediatric specimens) or deviations from the means (adult specimens).
Despite the finding that pediatric patients display an increased susceptibility to EPEC infection, our data suggest that at least the early BFP-mediated adherence of α-bundlin-expressing EPEC occurs by similar mechanisms involving LacNAc recognition. In this study, we also present data suggesting that β1-bundlin does not possess lectin-like properties, since strain UMD901(pXLW13) is significantly attenuated in its ability to bind adult DD biopsy specimens. To our knowledge, this study represents the first direct comparison of EPEC adherence to adult and pediatric intestinal biopsy specimens and reveals that BFP receptor usage during the initial interaction between EPEC and the host intestinal epithelium may not play a role in the age-related specificity of these organisms.
Finally, LacNAc is present as the common core glycan sequence for the A, B, H, Lewis X, Lewis Y, Lewis X-related, and Lewis Y-related blood group antigens, which are present on human intestinal epithelial cells in various amounts. It is also well established that the biosynthetic pathway of complex glycan sequences is not 100% efficient, further adding to the diversity of these sequences on the surface of eukaryotic cells. The incomplete substitution of fucose at the number 3 position of GlcNAc in the Lewis X antigen, for example, would result in a complex glycan sequence terminating in unsubstituted LacNAc. Moreover, although we reported that α-bundlin generally demonstrates a preference for LacNAc glycoconjugates in vitro, LA of EPEC strains expressing BFP assembled from α3-bundlin is inhibited by Lewis X-BSA derivatives (13). In reality, we should emphasize that α-bundlin probably does not show a strict specificity for any one unique glycan sequence, perhaps binding simultaneously to multiple LacNAc-related sequences in vivo.
Acknowledgments
Material support was provided by the Alberta Ingenuity Centre for Carbohydrate Science (G.D.A.). Financial support was provided by the Canadian Institutes for Health Research (P.B.) and the Alberta Heritage Foundation for Medical Research (P.B.). R.M.H. was the recipient of a studentship from NSERC.
Intestinal biopsy specimens were obtained from the University of Calgary Intestinal Inflammation Tissue Bank. We thank Michael Donnenberg for providing enteropathogenic Escherichia coli mutant strains and Om Srivastava for providing LacNAc-BSA.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 27 July 2009.
REFERENCES
- 1.Albert, M. J., A. S. Faruque, S. M. Faruque, R. B. Sack, and D. Mahalanabis. 1999. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J. Clin. Microbiol. 37:3458-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anantha, R. P., K. D. Stone, and M. S. Donnenberg. 2000. Effects of bfp mutations on biogenesis of functional enteropathogenic Escherichia coli type IV pili. J. Bacteriol. 182:2498-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai, L., S. Schuller, A. Whale, A. Mousnier, O. Marches, L. Wang, T. Ooka, R. Heuschkel, F. Torrente, J. B. Kaper, T. A. Gomes, J. Xu, A. D. Phillips, and G. Frankel. 2008. Enteropathogenic Escherichia coli O125:H6 triggers attaching and effacing lesions on human intestinal biopsy specimens independently of Nck and TccP/TccP2. Infect. Immun. 76:361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blank, T. E., D. W. Lacher, I. C. Scaletsky, H. Zhong, T. S. Whittam, and M. S. Donnenberg. 2003. Enteropathogenic Escherichia coli O157 strains from Brazil. Emerg. Infect. Dis. 9:113-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blank, T. E., H. Zhong, A. L. Bell, T. S. Whittam, and M. S. Donnenberg. 2000. Molecular variation among type IV pilin (bfpA) genes from diverse enteropathogenic Escherichia coli strains. Infect. Immun. 68:7028-7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleary, J., L. C. Lai, R. K. Shaw, A. Straatman-Iwanowska, M. S. Donnenberg, G. Frankel, and S. Knutton. 2004. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology 150:527-538. [DOI] [PubMed] [Google Scholar]
- 7.Cravioto, A., R. E. Reyes, R. Ortega, G. Fernandez, R. Hernandez, and D. Lopez. 1988. Prospective study of diarrhoeal disease in a cohort of rural Mexican children: incidence and isolated pathogens during the first two years of life. Epidemiol. Infect. 101:123-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandes, P. J., Q. Guo, and M. S. Donnenberg. 2007. Functional consequences of sequence variation in bundlin, the enteropathogenic Escherichia coli type IV pilin protein. Infect. Immun. 75:4687-4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Germani, Y., E. Begaud, P. Duval, and C. Le Bouguenec. 1996. Prevalence of enteropathogenic, enteroaggregative, and diffusely adherent Escherichia coli among isolates from children with diarrhea in new Caledonia. J. Infect. Dis. 174:1124-1126. [DOI] [PubMed] [Google Scholar]
- 10.Giron, J. A., A. G. Torres, E. Freer, and J. B. Kaper. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44:361-379. [DOI] [PubMed] [Google Scholar]
- 11.Gomes, T. A., V. Rassi, K. L. MacDonald, S. R. Ramos, L. R. Trabulsi, M. A. Vieira, B. E. Guth, J. A. Candeias, C. Ivey, M. R. Toledo, et al. 1991. Enteropathogens associated with acute diarrheal disease in urban infants in Sao Paulo, Brazil. J. Infect. Dis. 164:331-337. [DOI] [PubMed] [Google Scholar]
- 12.Hicks, S., G. Frankel, J. B. Kaper, G. Dougan, and A. D. Phillips. 1998. Role of intimin and bundle-forming pili in enteropathogenic Escherichia coli adhesion to pediatric intestinal tissue in vitro. Infect. Immun. 66:1570-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphries, R. M., M. S. Donnenberg, J. Strecker, E. Kitova, J. S. Klassen, L. Cui, T. P. Griener, G. L. Mulvey, and G. D. Armstrong. 2009. From alpha to beta: identification of amino acids required for the N-acetyllactosamine-specific lectin-like activity of bundlin. Mol. Microbiol. 72:859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyland, R., T. P. Griener, G. L. Mulvey, P. I. Kitov, O. P. Srivastava, P. Marcato, and G. D. Armstrong. 2006. Basis for N-acetyllactosamine-mediated inhibition of enteropathogenic Escherichia coli localized adherence. J. Med. Microbiol. 55:669-675. [DOI] [PubMed] [Google Scholar]
- 15.Hyland, R. M., P. Beck, G. L. Mulvey, P. I. Kitov, and G. D. Armstrong. 2006. N-Acetyllactosamine conjugated to gold nanoparticles inhibits enteropathogenic Escherichia coli colonization of the epithelium in human intestinal biopsy specimens. Infect. Immun. 74:5419-5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyland, R. M., J. Sun, T. P. Griener, G. L. Mulvey, J. S. Klassen, M. S. Donnenberg, and G. D. Armstrong. 2008. The bundlin pilin protein of enteropathogenic Escherichia coli is an N-acetyllactosamine-specific lectin. Cell. Microbiol. 10:177-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyogashima, M., V. Ginsburg, and H. C. Krivan. 1989. Escherichia coli K99 binds to N-glycolylsialoparagloboside and N-glycolyl-GM3 found in piglet small intestine. Arch. Biochem. Biophys. 270:391-397. [DOI] [PubMed] [Google Scholar]
- 18.Leverton, L. Q., and J. B. Kaper. 2005. Temporal expression of enteropathogenic Escherichia coli virulence genes in an in vitro model of infection. Infect. Immun. 73:1034-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter, M. E., P. Mitchell, A. J. Roe, A. Free, D. G. Smith, and D. L. Gally. 2004. Direct and indirect transcriptional activation of virulence genes by an AraC-like protein, PerA from enteropathogenic Escherichia coli. Mol. Microbiol. 54:1117-1133. [DOI] [PubMed] [Google Scholar]
- 20.Schuller, S., Y. Chong, J. Lewin, B. Kenny, G. Frankel, and A. D. Phillips. 2007. Tir phosphorylation and Nck/N-WASP recruitment by enteropathogenic and enterohaemorrhagic Escherichia coli during ex vivo colonization of human intestinal mucosa is different to cell culture models. Cell. Microbiol. 9:1352-1364. [DOI] [PubMed] [Google Scholar]
- 21.Staley, T. E., L. D. Corley, and E. W. Jones. 1970. Early pathogenesis of colitis in neonatal pigs monocontaminated with Escherichia coli. Fine structural changes in the colonic epithelium. Am. J. Dig. Dis. 15:923-935. [DOI] [PubMed] [Google Scholar]
- 22.Staley, T. E., L. D. Corley, and E. W. Jones. 1972. Malabsorption in neonatal pigs monocontaminated with Escherichia coli (055B5). Am. J. Dig. Dis. 17:239-247. [DOI] [PubMed] [Google Scholar]
- 23.Tobe, T., and C. Sasakawa. 2002. Species-specific cell adhesion of enteropathogenic Escherichia coli is mediated by type IV bundle-forming pili. Cell. Microbiol. 4:29-42. [DOI] [PubMed] [Google Scholar]
- 24.Ulshen, M. H., and J. L. Rollo. 1980. Pathogenesis of Escherichia coli gastroenteritis in man—another mechanism. N. Engl. J. Med. 302:99-101. [DOI] [PubMed] [Google Scholar]
- 25.Vanmaele, R. P., L. D. Heerze, and G. D. Armstrong. 1999. Role of lactosyl glycan sequences in inhibiting enteropathogenic Escherichia coli attachment. Infect. Immun. 67:3302-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]