Abstract
The fungal pathogen Cryptococcus neoformans causes approximately one million cases of cryptococcosis per year in people with AIDS. In contrast, the related species C. gattii is responsible for a much smaller number of cases, but these often occur in immunocompetent people. In fact, C. gattii has emerged in the last decade as the frequent cause of cryptococcosis in otherwise healthy people in British Columbia. We analyzed the immune responses elicited by three C. gattii strains and one C. neoformans strain in mice as a first step toward understanding why C. gattii is able to cause disease in immunocompetent hosts. The C. gattii strains all induced a less protective inflammatory response in C57BL/6 mice by inhibiting or failing to provoke the migration of neutrophils to sites of infection. The C. gattii strains also failed to elicit the production of protective cytokines, such as tumor necrosis factor alpha, compared to the ability of the C. neoformans strain. Despite these differences, the strain representing the major outbreak genotype from British Columbia showed a virulence equivalent to that of the C. neoformans strain, while two other C. gattii strains had reduced virulence. Taken together, our results indicate that C. gattii strains thrive in immunocompetent hosts by evading or suppressing the protective immune responses that normally limit the progression of disease caused by C. neoformans.
Cryptococcus neoformans is an opportunistic fungal pathogen that can cross the blood-brain barrier to cause a life-threatening disease of the central nervous system (5). In the environment, the fungus is found in trees, soil, and avian excreta, where it exists as spores or desiccated yeast cells (9, 16, 26, 50). Infection is initiated primarily by the inhalation of these cells, and the fungus can spread from the lungs to cause systemic cryptococcosis and meningoencephalitis. C. neoformans var. grubii (serotype A) typically infects people with compromised immune systems, and this serotype causes the majority of cryptococcosis cases in AIDS patients (33). In contrast, a related species, C. gattii (serotype B), does not have the same impact on AIDS patients and tends to infect people regardless of their immune status (27, 43). C. gattii infections originally were found primarily in tropical and subtropical regions of the world, such as South America and Australia (34). However, since 1999, an outbreak of C. gattii infections has been occurring in the temperate climate of Vancouver Island in British Columbia. This outbreak has resulted in at least 200 human cases and eight deaths (3, 21). More recently, cases of cryptococcosis caused by C. gattii have occurred on the mainland in British Columbia as well as in Washington and Oregon in the United States. (8, 45).
The mechanisms of the immune response to cryptococcal species have been best established for infections caused by C. neoformans (using strains of both the A and D serotypes). Normally, most of the fungal spores or yeast cells that are inhaled do not even reach the lungs and are cleared due to air turbulence and ciliary action (42). The small numbers of Cryptococcus spores or yeast cells that do reach the lung parenchyma are cleared by a protective inflammatory immune response involving the production of cytokines such as tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ), and a leukocyte infiltrate consisting of neutrophils, Th1-associated classically activated macrophages, Th1-lymphocytes, and dendritic cells (DCs) (12, 24, 31, 32). In contrast, a nonprotective response involves the production of high levels of the Th2 cytokine interleukin-4 (IL-4) as well as a diffuse pulmonary infiltrate consisting of immature DCs and alternatively activated macrophages (12, 24, 31, 32, 47). Protective inflammation involving neutrophil recruitment is crucial for the induction of adaptive Th1 immune responses against cryptococcal infections and for an effective response to other respiratory pathogens (1, 10, 15). Furthermore, the timing of the cellular infiltration is important, and early neutrophilia is associated with protective immune responses against C. neoformans (24). Additionally, mice deficient in myeloperoxidase, an enzyme preferentially expressed in neutrophils, produce weak Th1 immune responses and have a significantly reduced survival time upon C. neoformans infection compared to that of wild-type mice (1).
Although only a few studies have examined the immune response during C. gattii infection, there is some evidence that C. gattii induces less protective immune responses than C. neoformans. For example, Dong and Murphy showed that culture filtrate antigens from C. gattii strains inhibit neutrophil migration in vitro and in vivo, whereas culture filtrate antigens from C. neoformans stimulate neutrophil migration (17). They proposed that C. gattii strains are able to cause disease in immunocompetent people because they inhibit neutrophil migration into the lungs during the initial stage of infection. A more recent study found that even though a strain of C. gattii inhibited neutrophil migration in vitro, neutrophil infiltration into the lungs of infected rats was similar between C. neoformans and C. gattii infections (52). Thus, it was suggested that C. gattii infections are not cleared efficiently by immunocompetent hosts because they do not induce protective inflammation during infection, and as a result they are not able to elicit the adaptive Th1-type immune responses that are elicited with infection with C. neoformans (52). However, this idea has not been explored beyond the study of differential C. neoformans and C. gattii effects on neutrophil function.
In light of these studies, we examined the cytokine profiles and pulmonary infiltrates in the lungs of infected mice to determine whether there were differences in the immune responses for a commonly studied C. neoformans isolate (serotype A) and selected C. gattii strains representing the genotypes causing the current outbreak in British Columbia. Our study revealed lower levels of neutrophil infiltration and reduced inflammatory cytokine production in the lungs of mice infected with C. gattii compared to those of mice infected with C. neoformans. This analysis is an important first step toward understanding the ability of C. gattii strains to cause disease in immunocompetent hosts.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice were obtained from Charles River Laboratories (Montreal, Quebec, Canada) and were infected at 12 to 14 weeks of age. Female A/JCr mice were obtained from the NIH animal program (Frederick, MD) and infected at 7 to 8 weeks of age. Mice were housed under specific-pathogen-free conditions using sterilized cages with a microisolator cage top. Clean food and water were given ad libitum. The mice were maintained by the Wesbrook Animal Unit at the University of British Columbia in accordance with the methods and regulations approved by the University of British Columbia's Animal Care Committee.
Strains.
The C. neoformans H99 strain and the C. gattii strains R265, R272, and WM276 were used in this study. H99 and WM276 were obtained from Joseph Heitman (Duke University Medical Center). R265 and R272 both were isolated in 2001 from the bronchial washings of infected patients from the Vancouver Island outbreak (29). WM276 is an environmental isolate from Australia. The lac1 lac2 double mutant and the ure1 mutant both were derived from the H99 strain and obtained from the American Type Culture Collection (Manassas, VA).
Murine infection.
Cryptococcus strains were grown for 24 h in Sabouraud dextrose broth (SDB), washed three times with phosphate-buffered saline (PBS), counted in a hemocytometer, and resuspended in PBS at a concentration of 1.0 × 106 yeast cells/ml. The intranasal inoculation method has been described elsewhere (25, 28). In brief, mice were anesthetized with 82.25 mg/kg of body weight ketamine and 5.5 mg/kg xylazine by intraperitoneal injection. Anesthetized mice then were suspended by their incisors on a thread to fully extend their necks, and 50 μl of the yeast suspension (5 × 104 cells) was slowly pipetted into the nares of each mouse. The mice were suspended for an additional 10 min and then placed on a heated blanket for recovery. For the virulence assay, mice were euthanized using CO2 inhalation if they showed signs of illness or had weight loss of greater than 20%. For all other assays, mice were euthanized on the indicated days by CO2 inhalation.
Lung leukocyte isolation.
The procedure for leukocyte isolation has been described elsewhere (36). Briefly, whole lungs were excised, minced, and enzymatically digested in 10 ml of digestion buffer (RPMI medium, 5% fetal bovine serum [FBS], 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mg/ml collagenase IV, 30 μg/ml DNase). Cell suspensions were homogenized further by drawing them through the bore of an 18-guage needle attached to a 3-ml syringe. The total cell suspensions then were pelleted and washed with PBS before resuspension in 3 ml of red blood cell lysis buffer (0.9% NH4Cl in H2O) for 5 min. Subsequently, 10 ml of complete medium (RPMI medium, 10% FBS, 2 mM l-glutamine, 1 mM sodium pyruvate, and antibiotics) was added to the suspension to return the solution to isotonicity. The cell suspension then was strained through a 70-μm filter before being pelleted and resuspended in complete medium. Total lung leukocytes were enumerated in the presence of trypan blue using a hemocytometer.
Neutrophil counts.
Leukocyte suspensions were normalized for cell concentration, cytospun onto glass slides, and stained using the Hemacolour stain set (Harelco, EMD Chemicals, Gibbstown, NJ). A total of 200 neutrophils from randomly chosen micrographs were visually counted, and frequencies were expressed as percentages of the total number of leukocytes present in the sample.
Cytokine assays.
Lung tissue was excised, weighed, and homogenized in 5 ml of PBS using a mixer mill (MM200; Retsch, Haan, Germany). The tissue homogenates then were clarified by centrifugation, and aliquots of the supernatant were stored at −80°C. The cytokine analysis of the undiluted supernatant was performed on a BD FACSCalibur flow cytometer using the BD cytometric bead array (CBA) mouse inflammation kit (Becton Dickinson, San Jose, CA) according to the manufacturer's instructions. The data were analyzed using BD CellQuest, BD CBA software (Becton Dickinson, San Jose, CA), and FlowJo software (Tree Star Inc., San Carlos, CA).
Flow-cytometric analysis.
For flow cytometry, 1.0 × 106 cells from the lung leukocyte suspension were stained in 30 μl of buffer (Hanks balanced salt solution [HBSS], 20 mM HEPES, 0.2% sodium azide, 2% FBS) and mixed with the following antibodies: fluorescein isothiocyanate (FITC)-labeled anti-Gr.1, peridinin chlorophyll protein-labeled anti-CD11b, and allophycocyanin (APC)- labeled anti-CD11c. Staining was performed on ice for 20 min, and then cells were washed with the buffer, pelleted, resuspended in 0.5% paraformaldehyde in PBS, and analyzed on a BD FACSCalibur flow cytometer. A total of 100,000 events were collected per sample. Initial gates were set based on light scatter characteristics to exclude debris, red blood cells, and clusters of cells (see Fig. S1A in the supplemental material). Neutrophils were gated based on side scatter as well as the expression of Gr.1 and CD11b. Pulmonary macrophages are highly autofluorescent, express very low levels of F4/80 and high levels of CD11c, and have various levels of expression of CD11b depending on their activation status (23, 48). DCs express high levels of CD11b and CD11c and can be differentiated from macrophages by their low autofluorescence (23, 48). Therefore, to differentiate between macrophages and DCs, all cells that were CD11c+ with various levels of expression of CD11b were gated from the total leukocyte population (see Fig. S1B in the supplemental material). These CD11c+ cells then were used to draw a histogram showing the autofluorescent populations so that the threshold between autofluorescence-negative and autofluorescence-positive cells could be determined (see Fig. S1C in the supplemental material). This threshold was used to draw a gate to differentiate between the population of CD11c+ cells that were macrophages and DCs (see Fig. S1D in the supplemental material). All fluorescence-activated cell sorting (FACS) data were analyzed using BD Cellquest Pro software (Becton Dickinson, San Jose, CA). The anti-Gr.1 antibody was purchased from Caltag (Cedarlane Laboratories, Burlington, Ontario, Canada) and all other antibodies were purchased from Biolegend (Biolegend Inc., San Diego, CA).
MPO measurement.
The method for the measurement of myeloperoxidase (MPO) has been described elsewhere (49). Briefly, lung tissue was excised, weighed, snap-frozen in liquid nitrogen, and stored at −80°C. Frozen tissue then was homogenized in hexadecyltrimethylammonium bromide (HTAB) buffer (10 ml 50 mM KH2PO4, pH 6.0, 5 mM EDTA, 0.5% HTAB) at 1 ml per 50-mg tissue sample using a mixer mill (MM200; Retsch, Haan, Germany). The tissue homogenates then were clarified by centrifugation, and 50 μl of the supernatant was mixed with 1.45 ml of freshly prepared assay buffer (100 mM KH2PO4, pH 6.0, 0.005% H2O2, 0.005 g O-dianisideine dihydroschloride). The change in absorbance at 450 nm was measured every minute for 4 min in a spectrophotometer. The results are expressed in units of MPO per gram of tissue (wet weight), where 1 U of MPO activity is defined as that degrading 1 μmol peroxide per min at 25°C (49).
Phenotypic assays.
To examine the production of the main cryptococcal virulence factors, fungal cells were grown in SDB medium for 24 h at 30°C in a shaker, washed twice with PBS, and adjusted to a concentration of 2.0 × 108 cells/ml. The cell suspensions then were diluted 10-fold serially, and 5 μl of each dilution was spotted onto Sabouraud dextrose agar plates, l-DOPA plates (0.5 mM 3,4-hydroxyl-l-phenylalanine [L-DOPA], 1 mM MgSO4·7H2O, 22 mM KH2PO4, 3 μM thiamine-HCl, 0.1% glucose, 0.1% L-asparagine, pH 5.6), or urease plates (333 mM urea, 86 mM NaCl, 15 mM KH2PO4, 0.1% peptone, 0.1% glucose, 0.0012% phenol red) to assess growth at 37°C, melanin production, and urease production, respectively. All incubations were performed at 30°C unless otherwise specified. To examine capsule formation, strains were grown in low-iron medium for 48 h at 30°C in a shaker, washed with low-iron water, stained with India ink, and examined by differential interference microscopy.
Histopathology.
Both A/JCr and C57BL/6 mice were used for histology experiments. At 7 days postinfection, lungs were fixed in 10% neutral buffered formalin. The tissue then was embedded in paraffin and cut into 5-μm-thick sections, stained with hematoxylin and eosin (H&E) or Mayer's mucicarmine (MM) to visualize the cryptococcal capsule, and then fixed on slides. Slides were examined by light microscopy. Capsule sizes were measured for at least 275 fungal cells from randomly chosen micrographs for each sample.
Statistics.
For the virulence assays, the time to mortality was evaluated for statistical significance with Kaplan-Meier survival curves, and P values were obtained from a log-rank test. An unpaired two-tailed t test was used to analyze capsule size data. For all other assays, statistical significance was calculated using one-way analysis of variance and using the Student-Newman-Keuls postanalysis to obtain P values. All statistical analyses were done using Graphpad Prism 4.0 software (GraphPad Software, Inc.). All values are reported as means ± standard errors of the means (SEM).
RESULTS
Phenotypic comparison of C. neoformans and C. gattii strains for virulence factor expression.
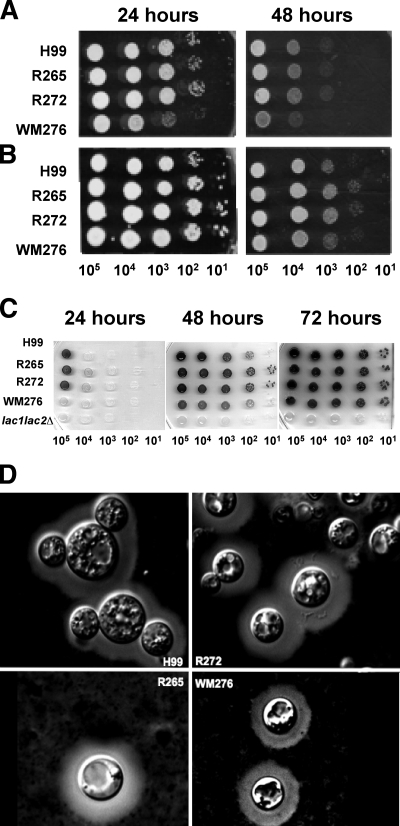
C. neoformans and C. gattii are known to share the major virulence factors needed to cause disease in mammalian hosts, and these include capsule and melanin formation and growth at 37°C. To set the stage for comparative studies of the host response, we compared the virulence-associated phenotypes of three strains of C. gattii to those of the commonly studied C. neoformans strain H99. Two of the C. gattii strains, R265 and R272, represent the most common molecular subtypes (VGIIa and VGIIb, respectively) from the outbreak in British Columbia. The third C. gattii strain, WM276, was included as a representative of the other subtype (VGI) that is associated with the outbreak. However, this specific strain is an environmental isolate from Australia, and it was selected as the VGI representative because, like R265, its genome has been sequenced. Our analysis of the virulence factors revealed that the clinical C. gattii strains R265 and R272, as well as the clinical C. neoformans strain H99, grew well at 37°C and were similarly able to produce melanin and the polysaccharide capsule (Fig. 1). In contrast, the C. gattii strain WM276 grew more slowly at 37°C and exhibited a delay in melanin production. We also noted that all four strains were similar in their production of another virulence trait, the enzyme urease (data not shown). Taken together, these results indicate that all four strains possess the major virulence factors, but WM276 appears to be less robust with regard to growth at 37°C and melanin production.
FIG. 1.
Virulence-associated phenotypes of the C. gattii strains R265, R272, and WM276 and the C. neoformans strain H99. The strains were grown at 30°C overnight and spotted onto different types of media as described in Materials and Methods. The photographs show growth at 30°C (A), growth at 37°C (B), melanin production (C), and capsule size (D). The results are representative of three independent experiments.
C. gattii isolates vary in their virulence in a mouse model of cryptococcosis.
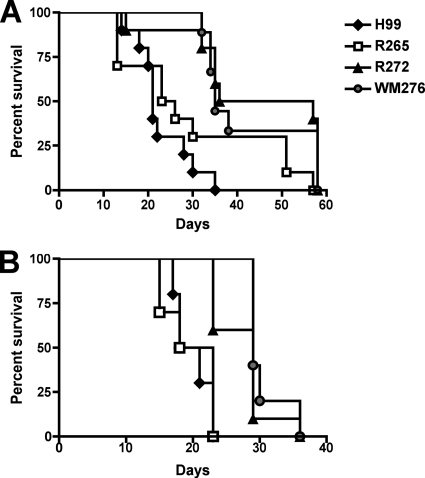
We next tested the virulence of our selected isolates of C. neoformans and C. gattii by infecting two different strains of mice using the intranasal inhalation method and assessing survival during a 60-day period. We employed C57BL/6 mice in this analysis, because this inbred strain is used commonly in studies of the immune response to C. neoformans. In parallel, we also used A/JCr mice, because this strain commonly is used to examine the virulence of C. neoformans and C. gattii isolates (14, 19, 51). Our virulence assays revealed that the C. neoformans strain H99 and the C. gattii strain R265 both were significantly more virulent than the C. gattii strains R272 and WM276 (P < 0.001) in both C57BL/6 and A/JCr mice (Fig. 2). Furthermore, there was no significant difference in the virulence of H99 compared to that of R265, nor was there a difference in the virulence of R272 compared to that of WM276 in either mouse strain. In addition to revealing the equivalent virulence of H99 and R265, these results indicate that the differences in growth at 37°C and melanin production between WM276 and R272 were not reflected in their virulence. Furthermore, given that the two clinical isolates from the Vancouver Island outbreak (R265 and R272) have similar genotypes (19, 29) and phenotypes (Fig. 1), these results indicate that R272 must have differences in other traits that reduce its virulence relative to that of R265. The results shown in Fig. 2 revealed that A/JCr mice were significantly more susceptible to infection with the two C. gattii strains with lower virulence (R272 and WM276) (P < 0.01) than were C57BL/6 mice. C57BL/6 mice infected with R272 and WM276 reached the endpoint at 47 and 35 days postinfection, respectively. In contrast, A/JCr mice infected with either of these strains reached the endpoint at 29 days postinfection.
FIG. 2.
Virulence of the C. neoformans strain H99 and three strains of C. gattii in two mouse strains. As described in Materials and Methods, mice were infected via intranasal inhalation with 5 × 104 fungal cells. Infected animals were observed until 58 and 38 days postinfection in C57BL/6 (A) and A/JCr (B) mice, respectively. The C. neoformans strain H99 and the C. gattii strain R265 were significantly more virulent than the C. gattii strains R272 and WM276 (P < 0.001) in both animal models. Statistical analysis was performed using the log-rank test to obtain P values (n = 10, except for the WM276 infection of C57BL/6 mice, where n = 9).
Histopathology of C. neoformans and C. gattii infections.
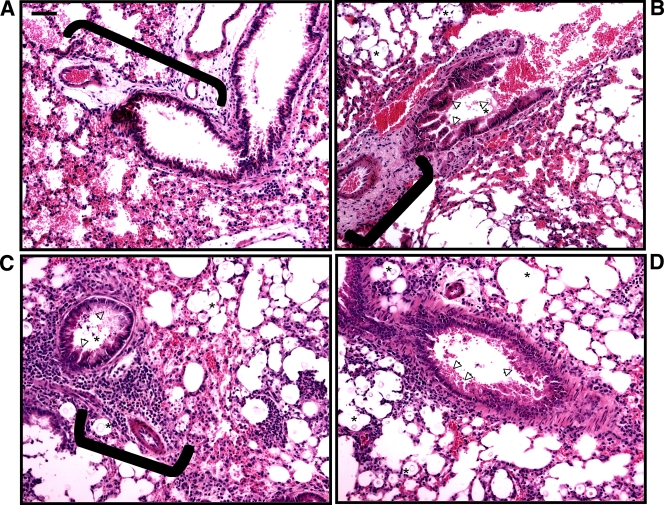
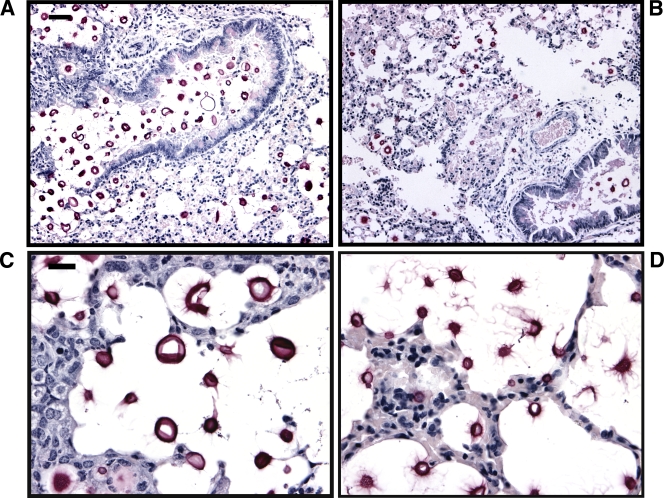
To initiate a comparison of the murine response to infection, we first performed a histopathological examination of the lungs of A/JCr mice infected with the C. neoformans strain H99 and the C. gattii strain R265 at 7 days postinfection. Strains H99 and R265 were selected because of their equivalent virulence (Fig. 2B). Lung sections were stained with H&E to visualize tissue structures and with MM to view the cryptococcal capsule (magenta color) (52). At 7 days postinfection, the lungs showed signs of differential inflammatory infiltrates for the two fungal strains. Specifically, the bronchovascular infiltrate surrounding small blood vessels and airways in the lungs of H99-infected mice (Fig. 3C and D) appeared to be densely packed with leukocytes. In contrast, the bronchovascular infiltrate in R265-infected mice (Fig. 3B) appeared more diffuse. These observations also were confirmed in C57BL/6 mice at 7 days postinfection (see Fig. S2 in the supplemental material). Diminished leukocyte infiltration and the presence of YM1 crystals have been associated with nonprotective Th2 immune responses to cryptococcal infections (2, 38). However, we did not observe the presence of YM1 crystals in the histopathology of our mice infected with C. gattii. We did note the presence of fungal cells and mucus development in the vicinity of the bronchovascular infiltrates (Fig. 3B to D). Furthermore, MM staining revealed cryptococci around the bronchiolar and alveolar airspaces with multiplication in the surrounding tissues (Fig. 4A and B). Interestingly, these cryptococci appeared to vary in size, and the C. neoformans H99 cells appeared to have more cell-associated capsular material (as indicated by MM staining) and significantly larger capsules (diameter, 19.71 ± 0.75 μm) (Fig. 4C) compared to those of the C. gattii R265 cells (diameter, 8.50 ± 0.22 μm) (Fig. 4D) (P < 0.0001; results are representative of two independent experiments). This significant difference in capsule size similarly was observed in the histology of C57BL/6 mice at 7 days postinfection; specifically, C. neoformans H99 capsule had a diameter of 20.26 ± 0.76 μm, whereas that for C. gattii strain R265 was 8.70 ± 0.31 μm (P < 0.0001).
FIG. 3.
H&E staining of pulmonary tissue from infected mice reveals signs of differential inflammation for C. neoformans and C. gattii infections. A/JCr mice were infected with 5 × 104 fungal cells, and infected lungs were harvested 7 days postinfection for preparation as described in Materials and Methods. The photomicrographs (H&E; ×200 magnification; scale bar is 50 μm) present lung sections from uninfected mice (A), mice infected with the C. gattii strain R265 (B), and mice infected with the C. neoformans strain H99 (C and D). Note that at this time point, the bronchovascular infiltrate (brackets) in C. neoformans-infected mice appeared more densely packed with leukocytes than those in the uninfected or the C. gattii-infected mice. The cryptococci are indicated by an asterisk in the vicinity of the bronchovascular infiltrate, and mucus production in the airways is indicated by arrowheads.
FIG. 4.
MM staining of pulmonary tissue reveals that cells of C. neoformans strain H99 have a larger capsule than cells of C. gattii strain R265 in vivo. A/JCr mice were infected with 5 × 104 fungal cells, and infected lungs were harvested 7 days postinfection and prepared as described in Materials and Methods. The photomicrographs (MM staining; for panels A and B, ×200 magnification, scale bar is 50 μm; for panels C and D, ×630 magnification, scale bar is 10 μm) show lung sections from mice infected with C. neoformans strain H99 (A) and mice infected with the C. gattii strain R265 (B). The cryptococci are evident in the tissues around the bronchiolar and alveolar airspaces. Closer examination of the cryptococci showed that C. neoformans cells appear to have a larger capsule (C) than C. gattii cells (D).
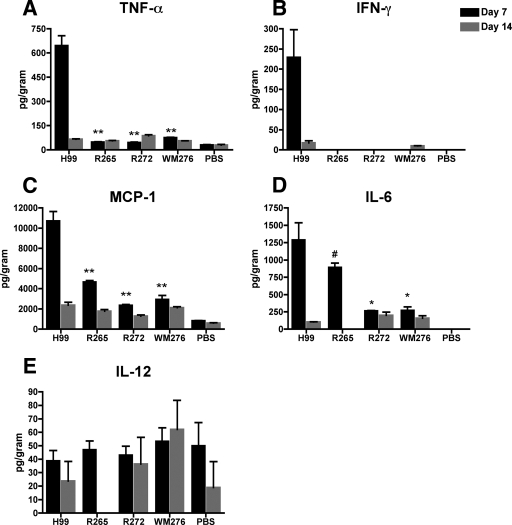
Cytokine profiles during pulmonary infection with C. neoformans and C. gattii.
Previous investigators suggested that C. gattii is less able to induce Th1-associated inflammation than C. neoformans, and that this difference contributes to the ability of C. gattii strains to cause disease in immunocompetent people (52). This idea has not yet been tested with approaches such as determining the cytokine profiles of C. gattii-infected mice or examining the infiltration of immune effector cells into sites of infection. Furthermore, there have not been any immunological studies of the C. gattii isolates from the British Columbia outbreak. As a first step to fill these gaps, we evaluated cytokine profiles in lung homogenates of infected C57BL/6 mice to determine whether the C. gattii isolates induce less protective immunity than C. neoformans strain H99 during infection. We found that there were no significant differences in cytokine expression among mice infected with the different strains at 24 h postinfection (data not shown). At 7 days postinfection, the C. gattii infections induced lower levels of protective cytokines than C. neoformans infection (Fig. 5). Specifically, mice infected with the C. gattii strains had significantly lower levels of TNF-α, (Fig. 5A) (P < 0.001), monocyte chemoattractant protein 1 (MCP-1) (Fig. 5C) (P < 0.001), and IL-6 (Fig. 5D) (P < 0.05) compared to those of mice infected with the C. neoformans strain. The levels of MCP-1 and IL-6 in mice infected with C. gattii isolates were slightly elevated at this time point compared to those of uninfected mice, especially in the case of R265, indicating that there was a response in these mice. In contrast, the levels of TNF-α in all of the C. gattii-infected mice were not significantly different from those in the uninfected mice, and the levels of IFN-γ (Fig. 5B) in these mice were below the limit of detection of the assay (2.5 pg/ml). This result suggests the absence of a significant immune response, especially compared to that of C. neoformans-infected mice. Furthermore, these observed differences in the cytokine responses were not due to differences in the pulmonary fungal load, because the CFU were similar in lung tissues from all infected mice (data not shown).
FIG. 5.
Cytokine expression in mice infected with C. neoformans or C. gattii. Lung homogenates from mice infected with 5 × 104 fungal cells of the indicated isolates were prepared by mechanical disruption and assayed for the production of the cytokines TNF-α (A), IFN-γ (B), MCP-1 (C), IL-6 (D), and IL-12 (E) at 7 and 14 days postinfection. The results are expressed as the means ± SEM (n = 3 mice/group/time point). The data are representative of three separate experiments. The symbols (**, P < 0.001; *, P < 0.01; #, P < 0.05) represent the statistical analysis based on comparisons to mice infected with C. neoformans strain H99.
We also detected very low levels of the Th1 cytokine IL-12 (Fig. 5E) in infected mice, although the levels were not significantly different from those in the uninfected mice at any of the time points tested. The absence of significantly elevated levels of IL-12 in the C. neoformans strain H99-infected mice suggests that the response in these mice was not due to a Th1-type immune response. After 14 days of infection, the levels of protective cytokines in the C. neoformans-infected mice decreased such that they were no longer significantly different from those in the C. gattii-infected mice, indicating a dampening effect. We did not detect the Th2 cytokine IL-10 in any samples at any of the time points; the lower limit of detection for this cytokine was 17.5 pg/ml under our assay conditions. Overall, our cytokine analysis indicated that immune responses to the specific C. gattii strains tested are consistent with a less protective profile than immune responses to the C. neoformans strain H99.
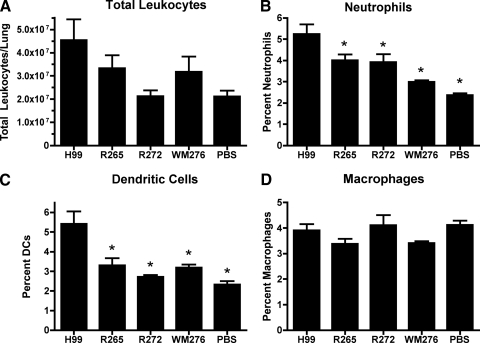
Pulmonary leukocyte infiltration during infection with C. neoformans and C. gattii.
Seven days postinfection previously has been shown to coincide with the onset of cell-mediated immunity in the C57BL/6 mouse model of cryptococcosis (51). Having observed differences in the histopathology and cytokine responses in C. neoformans- and C. gattii-infected mice at this time point, we next examined leukocyte subsets to determine if the lack of protective cytokine responses we observed in C. gattii-infected mice was mirrored in the population of cells in the pulmonary infiltrate. Previous studies have shown that a protective immune response to cryptococcal infection involves increased levels of neutrophils, classically activated macrophages, and lymphocytes in the lungs (12, 24). Our examination of leukocyte infiltration at 7 days postinfection revealed that total lung leukocyte levels (Fig. 6A) were lower in mice infected with C. gattii than in mice infected with the C. neoformans strain, but this result was not statistically significant. However, we did observe significantly lower levels of neutrophils (Fig. 6B) and DCs (Fig. 6C) in the lungs of mice infected with the C. gattii strains compared to those of mice infected with the C. neoformans H99 strain. These results are consistent with our cytokine analysis, indicating that immune responses to the C. gattii strains are of a less protective phenotype than the immune responses to the C. neoformans strain H99 at 7 days postinfection. Interestingly, we did not see a difference in total numbers of macrophages (Fig. 6D) for any of the infected mice or control mice, and it will be useful in future studies to determine whether differences in activation status exist for these cells. Given previous studies of neutrophils (17, 52), we focused on confirming the differential response of this cell type to C. gattii and C. neoformans.
FIG. 6.
Pulmonary leukocyte infiltration. Lungs of mice infected with 5 × 104 CFU of the indicated Cryptococcus isolates were digested enzymatically as described in Materials and Methods and analyzed for total leukocytes (A) by trypan blue staining. Neutrophils (B), DCs (C), and macrophages (D) were identified by immunofluorescent staining. Results are represented as the means ± SEM (n = 3 mice/group). The data are representative of three separate experiments, except in the cases of R272 and WM276, which are representative of two separate experiments. The symbol (*, P < 0.01) indicates the statistical differences compared to results for mice infected with C. neoformans strain H99.
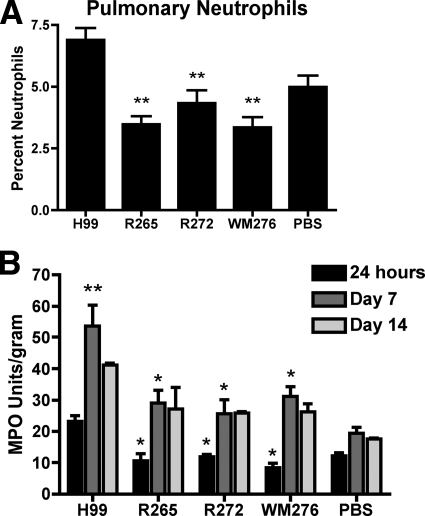
Neutrophil infiltration during pulmonary infection.
Neutrophils are important mediators of the protective immune response against C. neoformans (18, 35, 46), and as described above, our flow cytometry analysis revealed differences in neutrophil accumulation for the C. gattii and C. neoformans infections. To investigate this phenomenon in more detail, we measured neutrophil infiltration in the lungs of infected C57BL/6 mice by cytological and enzymatic methods. At the early time point of 24 h postinfection, there were significantly lower numbers of neutrophils in the lungs of all of the C. gattii-infected mice compared to those of the C. neoformans strain H99 infection (P < 0.001) (Fig. 7A). Furthermore, the levels of pulmonary neutrophils in mice infected with the C. gattii strains were not significantly different from those in uninfected mice, indicating that these C. gattii strains failed to provoke the migration of neutrophils into the sites of infection.
FIG. 7.
Pulmonary neutrophil infiltration in C. neoformans- or C. gattii-infected mice. (A) As described in Materials and Methods, lung leukocytes were isolated by the enzymatic digestion of tissue from mice at 24 h postinfection and cytospun onto glass slides for staining. Neutrophils were counted by microscopy, and frequencies are expressed as percentages of the total leukocytes present in the sample. The data are representative of two separate experiments. **, P < 0.001 for statistical comparisons to results for mice infected with C. neoformans strain H99. (B) MPO activity also was employed as a measure of neutrophil infiltration. Lungs were excised from mice at 24 h, 7 days, and 14 days postinfection, snap-frozen with liquid nitrogen, and measured for MPO activity as described in Materials and Methods. The asterisk (P < 0.01) represents statistical comparisons to results for mice infected with C. neoformans strain H99 at the respective time points. The double asterisk (P < 0.001) represents the statistical comparison of results for mice infected with C. neoformans H99 at 7 days postinfection to those of mice infected with H99 at 24 h postinfection and to those of uninfected mice at 7 days postinfection. The data are representative of three separate experiments. Results are expressed as the means ± SEM (n = 5 mice/group for A and n = 3 mice/group for B).
MPO is preferentially expressed in neutrophils and therefore is a useful indicator for neutrophil granulocyte sequestration (30). The measurement of MPO activity in the lung tissue at 24 h as well as 7 days postinfection showed that C57BL/6 mice infected with the C. gattii strains had significantly lower levels of pulmonary neutrophils compared to those of the C. neoformans-infected mice (P < 0.01) (Fig. 7B). Furthermore, neutrophil levels in the lungs of C. gattii-infected mice were not significantly different from those in uninfected mice at all three time points analyzed. These results therefore confirmed the cytology and flow cytometry analysis. Additionally, C. neoformans-infected mice showed a significant increase in neutrophil activity from 24 h to 7 days postinfection (P < 0.001); this activity also was significantly greater than that in uninfected mice at 7 days postinfection (P < 0.001), indicating the continued induction of the neutrophil response. By 14 days postinfection, neutrophil activity levels in C. neoformans-infected mice were slightly reduced compared to those at 7 days postinfection. Furthermore, we observed an increase in neutrophil activity in the C. gattii-infected mice from 24 h postinfection to 7 days postinfection, perhaps indicating some neutrophil infiltration. However, because the activities at 7 days postinfection were not significantly different from those of the uninfected mice, this increase may not be relevant. Overall, our findings demonstrate that there were significantly lower levels of neutrophil activity in mice infected with the C. gattii isolates, thus providing further evidence that these C. gattii infections induce less protective immune responses than infection with the C. neoformans strain H99.
DISCUSSION
Life-threatening disease caused by C. neoformans in people with AIDS occurs at a global rate of ∼1 million cases per year (39). In contrast, C. gattii infections are much less common in AIDS patients and generally occur in people regardless of their immune status. The occurrence of C. gattii infections in immunocompetent people is highlighted by the ongoing outbreak in British Columbia, where only 4 of 176 cases between 1999 and 2006 occurred in HIV-positive individuals (www.bccdc.ca/NR/rdonlyres/9A4F7B76-C7DA-4C32-A886-6DEECBBA3EDB/0/Epid_Stats_Research_CDAnnualReport_2006.pdf). In this report, we examined the immune responses elicited by selected C. gattii strains in a murine model of cryptococcosis to begin to understand how this species causes disease in immunocompetent people. This is the first study to examine the immune responses elicited by C. gattii strains from the outbreak, and we found that they induced a less protective inflammatory response in C57BL/6 mice than a strain of C. neoformans. Specifically, the C. gattii strains appeared to avoid triggering protective inflammation by inhibiting or failing to provoke the migration of neutrophils to sites of infection. Furthermore, the strains also failed to elicit the production of protective cytokines such as TNF-α, which did accumulate in C. neoformans-infected lung tissue.
We focused our analysis primarily on neutrophils, because the migration of these cells into lung tissue is important in the early protection of mice against progressive cryptococcosis (24). Furthermore, previous studies showed that C. gattii strains can inhibit neutrophil migration in vitro (17, 52). These previous studies suggested that C. gattii infections are not cleared by immunocompetent hosts because they do not induce protective inflammation during infection and, as a result, are not able to elicit adaptive Th1-type immune responses, whereas C. neoformans infections are. Our results provide experimental support for this conclusion, because we showed by histopathology, leukocyte enumeration, cell sorting, and a neutrophil activity assay that C. gattii strains inhibited or failed to provoke neutrophil migration in vivo. Specifically, neutrophil levels in the lungs of mice infected with the C. gattii strains were not significantly different from those of uninfected mice at 24 h as well as 7 and 14 days postinfection. It is likely that the lack of pulmonary neutrophil infiltration during the initial immune response contributed to the lack of protective inflammation observed in the C. gattii-infected mice. The inhibition of early neutrophil migration into infected lung tissues may delay the production of TNF-α, IFN-γ, and important chemokines, thus preventing the induction of an effective cellular immune response for the clearance of the pathogen (42). Our observations support this idea. Specifically, concurrently with the low levels of pulmonary neutrophil migration in these mice, we observed a minor increase in the levels of MCP-1 and IL-6 in the lungs of these C. gattii-infected mice at 7 days postinfection, a time that coincides with the onset of cell-mediated immunity in this mouse model (51). However, this immune response was quite weak, as demonstrated by the absence of IFN-γ and by the similarities between C. gattii-infected mice and uninfected mice in the levels of pulmonary TNF-α and DCs. Additionally, the histopathology of A/JCr and C57BL/6 mice infected with the C. gattii strain R265 showed a bronchovascular infiltrate that was diffuse compared to that in the C. neoformans-infected mice, further indicating weak inflammation at 7 days. Similar observations have been made by other groups in the analysis of mice producing ineffective immune responses during cryptococcosis (12, 38). Overall, it appears that the lack of early neutrophil infiltration into the lungs of C. gattii-infected mice failed to trigger an effective inflammatory response, including the absence of a clear Th1-type immune profile. We therefore also considered the possibility that C. gattii infection skews the immune response toward a Th2 profile. However, we did not detect the Th2 cytokine IL-10 in the mice at any of the time points tested, and we did not observe the formation of YM1/YM2 crystals, which are indicative of Th2 responses (2, 11, 13), in the histopathology of R265-infected mice at 7 days postinfection.
In contrast to our observations for C. gattii-infected mice, we found that C. neoformans strain H99 induced a strong inflammatory immune response that potentially contributes to protection. Specifically, in the C. neoformans-infected mice, we observed a significant influx of neutrophils early during the immune response, with a subsequent increase in the production of the protective cytokines TNF-α and IFN-γ and chemokines, as well as an increase in pulmonary neutrophils and DCs at 7 days postinfection. Furthermore, at this time point, histopathology staining revealed a dense bronchovascular infiltrate in A/JCr and C57BL/6 mice infected with the C. neoformans strain, which is indicative of protective inflammation (38). Although our analysis was limited to a single strain of C. neoformans, the results are consistent with previous studies that showed that H99 and other strains of C. neoformans elicit protective inflammatory immune responses in mice and other animal models (12, 38, 40, 51). However, in contrast to previous studies involving C. neoformans infection, we did not observe significant increases in the hallmark Th1 cytokine IL-12. The lack of IL-12 induction, together with the presence of pulmonary neutrophils and IL-6, suggests that an inflammatory Th17-type immune response was induced in the C. neoformans-infected mice. Other researchers have proposed an association between Th17-type responses and protection against cryptococcosis (37), and it will be interesting to determine in future studies if this is indeed the case.
We note that our observations are different from those of another study, which found that even though a C. gattii strain was able to inhibit neutrophil migration in vitro, neutrophil infiltration into the lungs of infected rats was similar in C. neoformans and C. gattii infections (52). It is possible that differences in the animal models, the fungal strains, and the methods account for the disparate results (44). Differences in mouse and rat models have been demonstrated, especially with regard to neutrophil infiltration during lung injury (4). Furthermore, rats and mice differ widely in their susceptibility to cryptococcal infection and have been shown to have differential immune responses (22, 41). Additionally, the specific methods to enumerate neutrophils in the lungs also might contribute to differences in the observed levels of infiltration.
Differences in capsule polysaccharide may contribute to the influence of C. gattii and C. neoformans strains on neutrophil infiltration and the cytokine response. Although differences in capsule size were not seen in vitro, the MM staining of capsular polysaccharide revealed more cell-associated capsular material present in tissues from C. neoformans-infected mice than in those of C. gattii-infected mice. Interestingly, others have described titan or giant cells in infections with the H99 strain of C. neoformans (K. Nielsen, personal communication). Encapsulated strains of C. neoformans can promote the secretion of protective cytokines from human neutrophils, and the magnitude of this response is dependent on capsule size (40). Thus, it is possible that the larger capsule/cell size observed in C. neoformans-infected mice at 7 days induce higher levels of protective cytokine secretion from neutrophils. Conversely, the lack of protective inflammation in C. gattii-infected mice may be due to a smaller capsule size in vivo. The capsule also may play an important role in interfering with neutrophil migration because, for example, 6-O-acetyl groups on the major component of the capsule, glucuronoxylmannan (GXM), are responsible for the inhibition of neutrophil migration in vitro (18, 20). Furthermore, free GXM has been shown to promote l-selectin shedding from neutrophils, and this may interfere with migration (17).
We also observed differences in the virulence of C. neoformans and C. gattii strains in the C57BL/6 and A/JCr mouse models of cryptococcosis. Specifically, the C. neoformans strain H99 and the C. gattii strain R265 were similar in virulence, and both were significantly more virulent than the C. gattii strains R272 and WM276. Interestingly, the A/JCr mice did not show a significant difference in susceptibility to the more virulent H99 and R265 strains, but they appeared to be significantly more susceptible to R272 and WM276 than were C57BL/6 mice. The host genetic background plays an important role in determining susceptibility to Cryptococcus infection (24, 53), and A/JCr mice may differ from C57BL/6 mice in one or more properties that cause increased susceptibility to R272 and WM276. For example, A/JCr mice are deficient in the complement component C5. It is interesting that these C. gattii isolates show different degrees of virulence in vivo, indicating that their ability to avoid protective inflammation is not the only factor involved in their pathogenicity.
Our findings differ slightly from those of a previous study that found that the C. gattii strain R265 was significantly more virulent than the C. neoformans strain H99 (19). The difference may be due to the use of different isolates of the strains or variation in the ages of mice. Our studies employed C57BL/6 and A/JCr mice at 12 to 14 weeks old, whereas the earlier study used A/JCr mice of an unspecified age (19). Several studies have shown that the outcome of cryptococcal infection varies greatly with respect to the age, strain, and source of mice, and this may explain differences in virulence assays in different laboratories (6, 12, 24, 53).
Although we observed differences in the virulence of strains R265 and R272 in the murine model, it is important to point out that both of these isolates caused clinically relevant disease in patients from British Columbia. The differences in virulence are interesting, given that these strains did not display any major differences in virulence factor production and provoked a similar immune response. It is likely that additional virulence factors remain to be discovered, and it is clear that genetic differences in even a single gene can influence disease progression. In fact, comparative genome hybridization studies revealed that there are several regions of difference (e.g., deletions in R272) between the R265 and R272 genomes (J. Kronstad, unpublished data). The further examination of these and other variable regions may help to explain differences in virulence for the C. gattii isolates.
The differences that we observed in the neutrophil and cytokine responses to C. neoformans and C. gattii may help us understand how C. gattii isolates are able to cause disease in immunocompetent people. It is possible that C. gattii induces less protective inflammation in humans, as we have shown in mice, and this may contribute to persistence. A recently published case study suggests that our observations in mice are relevant for human cases of cryptococcosis. Specifically, a human immunodeficiency virus (HIV)-seronegative patient with C. gattii meningitis had reduced levels of the cytokines TNF-α, IFN-γ, and IL-6, which are associated with protective immune responses, compared to those of patients with HIV-related C. neoformans infections (7). Although this is only a single case, the observations suggest that the patient had a maladapted immune response to C. gattii. Of course, the situation may not be as simple as differences in the immune response, because C. neoformans and C. gattii have other phenotypic differences (e.g., metabolic capabilities). Taken together, our results provide important new insights into cryptococcal virulence and help focus attention on the identification of species-specific attributes that may influence leukocyte recruitment and the induction of a protective cytokine response. This information may be useful in understanding and mitigating the outbreak of C. gattii-mediated cryptococcosis in British Columbia.
Supplementary Material
Acknowledgments
We thank Joseph Heitman for providing strains, Lisa Thorson and Guntram Grassl for advice with cytokine assays, and Anastasia Nijnik and Robert Hancock for help with flow cytometry. We also thank Richard Stokes, Marie-Renee Blanchett, Gary Huffnagle, Pauline Johnson, and Kelly McNagny for advice and Gwo-Hsiao Chen and Michael Olszewski for help in interpreting histology results. Guanggan Hu and Joyce Wang assisted with the histopathology work.
This work was supported by the British Columbia Lung Association and the Canadian Institutes of Health Research. J.W.K. is a Burroughs Wellcome Fund Scholar in Molecular Pathogenic Mycology.
Editor: A. Casadevall
Footnotes
Published ahead of print on 27 July 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Aratani, Y., F. Kura, H. Watanabe, H. Akagawa, Y. Takano, A. Ishida-Okawara, K. Suzuki, N. Maeda, and H. Koyama. 2006. Contribution of the myeloperoxidase-dependent oxidative system to host defence against Cryptococcus neoformans. J. Med. Microbiol. 55:1291-1299. [DOI] [PubMed] [Google Scholar]
- 2.Arora, S., Y. Hernandez, J. R. Erb-Downward, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J. Immunol. 174:6346-6356. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett, K. H., S. E. Kidd, and J. W. Kronstad. 2008. The emergence of Cryptococcus gattii in British Columbia and the Pacific Northwest. Curr. Infect. Dis. Rep. 10:58-65. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez, E., J. B. Mangum, B. A. Wong, B. Asgharian, P. M. Hext, D. B. Warheit, and J. I. Everitt. 2004. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol. Sci. 77:347-357. [DOI] [PubMed] [Google Scholar]
- 5.Bicanic, T., and T. S. Harrison. 2004. Cryptococcal meningitis. Br. Med. Bull. 72:99-118. [DOI] [PubMed] [Google Scholar]
- 6.Blackstock, R., and J. W. Murphy. 2004. Age-related resistance of C57BL/6 mice to Cryptococcus neoformans is dependent on maturation of NKT cells. Infect. Immun. 72:5175-5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brouwer, A. E., A. A. Siddiqui, M. I. Kester, K. C. Sigaloff, A. Rajanuwong, S. Wannapasni, W. Chierakul, and T. S. Harrison. 2007. Immune dysfunction in HIV-seronegative, Cryptococcus gattii meningitis. J. Infect. 54:e165-e168. [DOI] [PubMed] [Google Scholar]
- 8.Byrnes, E. J., III, R. Bildfell, S. A. Frank, T. G. Mitchell, K. A. Marr, and J. Heitman. 2009. Molecular evidence that the Vancouver Island Cryptococcus gattii outbreak has expanded into the United States pacific northwest. J. Infect. Dis. 199:1081-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans. American Society for Microbiology Press, Washington, DC.
- 10.Chaturvedi, V., B. Wong, and S. L. Newman. 1996. Oxidative killing of Cryptococcus neoformans by human neutrophils. Evidence that fungal mannitol protects by scavenging reactive oxygen intermediates. J. Immunol. 156:3836-3840. [PubMed] [Google Scholar]
- 11.Chen, G. H., R. A. McDonald, J. C. Wells, G. B. Huffnagle, N. W. Lukacs, and G. B. Toews. 2005. The gamma interferon receptor is required for the protective pulmonary inflammatory response to Cryptococcus neoformans. Infect. Immun. 73:1788-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, G. H., D. A. McNamara, Y. Hernandez, G. B. Huffnagle, G. B. Toews, and M. A. Olszewski. 2008. Inheritance of immune polarization patterns is linked to resistance versus susceptibility to Cryptococcus neoformans in a mouse model. Infect. Immun. 76:2379-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, G. H., M. A. Olszewski, R. A. McDonald, J. C. Wells, R. Paine III, G. B. Huffnagle, and G. B. Toews. 2007. Role of granulocyte macrophage colony-stimulating factor in host defense against pulmonary Cryptococcus neoformans infection during murine allergic bronchopulmonary mycosis. Am. J. Pathol. 170:1028-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox, G. M., J. Mukherjee, G. T. Cole, A. Casadevall, and J. R. Perfect. 2000. Urease as a virulence factor in experimental cryptococcosis. Infect. Immun. 68:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craig, A., J. Mai, S. Cai, and S. Jeyaseelan. 2009. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect. Immun. 77:568-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixit, A., S. F. Carroll, and S. T. Qureshi. 2009. Cryptococcus gattii: an emerging cause of fungal disease in North America. Interdiscip. Perspect. Infect. Dis. 2009:840452. [DOI] [PMC free article] [PubMed]
- 17.Dong, Z. M., and J. W. Murphy. 1995. Effects of the two varieties of Cryptococcus neoformans cells and culture filtrate antigens on neutrophil locomotion. Infect. Immun. 63:2632-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellerbroek, P. M., D. J. Lefeber, R. van Veghel, J. Scharringa, E. Brouwer, G. J. Gerwig, G. Janbon, A. I. Hoepelman, and F. E. Coenjaerts. 2004. O-acetylation of cryptococcal capsular glucuronoxylomannan is essential for interference with neutrophil migration. J. Immunol. 173:7513-7520. [DOI] [PubMed] [Google Scholar]
- 19.Fraser, J. A., S. S. Giles, E. C. Wenink, S. G. Geunes-Boyer, J. R. Wright, S. Diezmann, A. Allen, J. E. Stajich, F. S. Dietrich, J. R. Perfect, and J. Heitman. 2005. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437:1360-1364. [DOI] [PubMed] [Google Scholar]
- 20.Fujihara, H., K. Kagaya, and Y. Fukazawa. 1997. Anti-chemotactic activity of capsular polysaccharide of Cryptococcus neoformans in vitro. Microbiol. Immunol. 41:657-664. [DOI] [PubMed] [Google Scholar]
- 21.Fyfe, M., L. MacDougall, M. Romney, M. Starr, M. Pearce, S. Mak, S. Mithani, and P. Kibsey. 2008. Cryptococcus gattii infections on Vancouver Island, British Columbia, Canada: emergence of a tropical fungus in a temperate environment. Can. Commun. Dis. Rep. 34:1-12. [PubMed] [Google Scholar]
- 22.Goldman, D., S. C. Lee, and A. Casadevall. 1994. Pathogenesis of pulmonary Cryptococcus neoformans infection in the rat. Infect. Immun. 62:4755-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Juarrero, M., T. S. Shim, A. Kipnis, A. P. Junqueira-Kipnis, and I. M. Orme. 2003. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J. Immunol. 171:3128-3135. [DOI] [PubMed] [Google Scholar]
- 24.Guillot, L., S. F. Carroll, R. Homer, and S. T. Qureshi. 2008. Enhanced innate immune responsiveness to pulmonary Cryptococcus neoformans infection is associated with resistance to progressive infection. Infect. Immun. 76:4745-4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu, G., P. Y. Cheng, A. Sham, J. R. Perfect, and J. W. Kronstad. 2008. Metabolic adaptation in Cryptococcus neoformans during early murine pulmonary infection. Mol. Microbiol. 69:1456-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Idnurm, A., Y. S. Bahn, K. Nielsen, X. Lin, J. A. Fraser, and J. Heitman. 2005. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat. Rev. Microbiol. 3:753-764. [DOI] [PubMed] [Google Scholar]
- 27.Jarvis, J. N., and T. S. Harrison. 2008. Pulmonary cryptococcosis. Semin. Respir. Crit. Care. Med. 29:141-150. [DOI] [PubMed] [Google Scholar]
- 28.Jung, W. H., A. Sham, T. Lian, A. Singh, D. J. Kosman, and J. W. Kronstad. 2008. Iron source preference and regulation of iron uptake in Cryptococcus neoformans. PLoS Pathog. 4:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kidd, S. E., F. Hagen, R. L. Tscharke, M. Huynh, K. H. Bartlett, M. Fyfe, L. Macdougall, T. Boekhout, K. J. Kwon-Chung, and W. Meyer. 2004. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc. Natl. Acad. Sci. USA 101:17258-17263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klebanoff, S. J. 2005. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 77:598-625. [DOI] [PubMed] [Google Scholar]
- 31.Koguchi, Y., and K. Kawakami. 2002. Cryptococcal infection and Th1-Th2 cytokine balance. Int. Rev. Immunol. 21:423-438. [DOI] [PubMed] [Google Scholar]
- 32.Lin, X., and J. Heitman. 2006. The biology of the Cryptococcus neoformans species complex. Annu. Rev. Microbiol. 60:69-105. [DOI] [PubMed] [Google Scholar]
- 33.Litvintseva, A. P., R. Thakur, R. Vilgalys, and T. G. Mitchell. 2006. Multilocus sequence typing reveals three genetic subpopulations of Cryptococcus neoformans var. grubii (serotype A), including a unique population in Botswana. Genetics 172:2223-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacDougall, L., S. E. Kidd, E. Galanis, S. Mak, M. J. Leslie, P. R. Cieslak, J. W. Kronstad, M. G. Morshed, and K. H. Bartlett. 2007. Spread of Cryptococcus gattii in British Columbia, Canada, and detection in the pacific northwest, USA. Emerg. Infect. Dis. 13:42-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mambula, S. S., E. R. Simons, R. Hastey, M. E. Selsted, and S. M. Levitz. 2000. Human neutrophil-mediated nonoxidative antifungal activity against Cryptococcus neoformans. Infect. Immun. 68:6257-6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milam, J. E., A. C. Herring-Palmer, R. Pandrangi, R. A. McDonald, G. B. Huffnagle, and G. B. Toews. 2007. Modulation of the pulmonary type 2 T-cell response to Cryptococcus neoformans by intratracheal delivery of a tumor necrosis factor alpha-expressing adenoviral vector. Infect. Immun. 75:4951-4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller, U., W. Stenzel, G. Kohler, C. Werner, T. Polte, G. Hansen, N. Schutze, R. K. Straubinger, M. Blessing, A. N. McKenzie, F. Brombacher, and G. Alber. 2007. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J. Immunol. 179:5367-5377. [DOI] [PubMed] [Google Scholar]
- 38.Osterholzer, J. J., J. L. Curtis, T. Polak, T. Ames, G. H. Chen, R. McDonald, G. B. Huffnagle, and G. B. Toews. 2008. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J. Immunol. 181:610-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park, B. J., K. A. Wannemuehler, B. J. Marston, N. Govender, P. G. Pappas, and T. M. Chiller. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525-530. [DOI] [PubMed] [Google Scholar]
- 40.Retini, C., A. Vecchiarelli, C. Monari, C. Tascini, F. Bistoni, and T. R. Kozel. 1996. Capsular polysaccharide of Cryptococcus neoformans induces proinflammatory cytokine release by human neutrophils. Infect. Immun. 64:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao, X., A. Mednick, M. Alvarez, N. van Rooijen, A. Casadevall, and D. L. Goldman. 2005. An innate immune system cell is a major determinant of species-related susceptibility differences to fungal pneumonia. J. Immunol. 175:3244-3251. [DOI] [PubMed] [Google Scholar]
- 42.Shoham, S., and S. M. Levitz. 2005. The immune response to fungal infections. Br. J. Haematol. 129:569-582. [DOI] [PubMed] [Google Scholar]
- 43.Sorrell, T. C. 2001. Cryptococcus neoformans variety gattii. Med. Mycol. 39:155-168. [PubMed] [Google Scholar]
- 44.Torda, A., R. K. Kumar, and P. D. Jones. 2001. The pathology of human and murine pulmonary infection with Cryptococcus neoformans var. gattii. Pathology 33:475-478. [DOI] [PubMed] [Google Scholar]
- 45.Upton, A., J. A. Fraser, S. E. Kidd, C. Bretz, K. H. Bartlett, J. Heitman, and K. A. Marr. 2007. First contemporary case of human infection with Cryptococcus gattii in Puget Sound: evidence for spread of the Vancouver island outbreak. J. Clin. Microbiol. 45:3086-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urban, C. F., S. Lourido, and A. Zychlinsky. 2006. How do microbes evade neutrophil killing? Cell. Microbiol. 8:1687-1696. [DOI] [PubMed] [Google Scholar]
- 47.Vecchiarelli, A., D. Pietrella, P. Lupo, F. Bistoni, D. C. McFadden, and A. Casadevall. 2003. The polysaccharide capsule of Cryptococcus neoformans interferes with human dendritic cell maturation and activation. J. Leukoc. Biol. 74:370-378. [DOI] [PubMed] [Google Scholar]
- 48.Vermaelen, K., and R. Pauwels. 2004. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A 61:170-177. [DOI] [PubMed] [Google Scholar]
- 49.Webert, K. E., J. Vanderzwan, M. Duggan, J. A. Scott, D. G. McCormack, J. F. Lewis, and S. Mehta. 2000. Effects of inhaled nitric oxide in a rat model of Pseudomonas aeruginosa pneumonia. Crit. Care Med. 28:2397-2405. [DOI] [PubMed] [Google Scholar]
- 50.Wickes, B. L., M. E. Mayorga, U. Edman, and J. C. Edman. 1996. Dimorphism and haploid fruiting in Cryptococcus neoformans: Association with the alpha-mating type. Proc. Natl. Acad. Sci. USA 93:7327-7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wormley, F. L., Jr., G. M. Cox, and J. R. Perfect. 2005. Evaluation of host immune responses to pulmonary cryptococcosis using a temperature-sensitive C. neoformans calcineurin A mutant strain. Microb. Pathog. 38:113-123. [DOI] [PubMed] [Google Scholar]
- 52.Wright, L., W. Bubb, J. Davidson, R. Santangelo, M. Krockenberger, U. Himmelreich, and T. Sorrell. 2002. Metabolites released by Cryptococcus neoformans var. neoformans and var. gattii differentially affect human neutrophil function. Microbes Infect. 4:1427-1438. [DOI] [PubMed] [Google Scholar]
- 53.Zaragoza, O., M. Alvarez, A. Telzak, J. Rivera, and A. Casadevall. 2007. The relative susceptibility of mouse strains to pulmonary Cryptococcus neoformans infection is associated with pleiotropic differences in the immune response. Infect. Immun. 75:2729-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.