Abstract
Neospora caninum is an apicomplexan parasite closely related to Toxoplasma gondii. In nature this parasite is found especially in dogs and cattle, but it may also infect other livestock. The growth of N. caninum, which is an obligate intracellular parasite, is controlled mainly by the cell-mediated immune response. During infection the cytokine gamma interferon (IFN-γ) plays a prominent role in regulating the growth of N. caninum in natural and experimental disease. The present study showed that induction of the tryptophan-degrading enzyme indoleamine 2,3-dioxygenase (IDO) is responsible for the inhibition of parasite growth that is mediated by IFN-γ-activated bovine fibroblasts and endothelial cells. This antiparasite effect could be abrogated by addition of tryptophan, as well as by the IDO-specific inhibitor 1-l-methyltryptophan. In conclusion, our data show that human and bovine cells use the same effector mechanism to control the growth of N. caninum.
Toxoplasma gondii, a protozoan belonging to the apicomplexan phylum, is one of the most successful parasites on earth. This parasite is capable of infecting nearly all warm-blooded animals, including humans. T. gondii can be transmitted via tissue cysts, raw meat, and environmental-resistant oocysts derived from cat feces and is able to spread transplacentally from mother to fetus. Furthermore, another special feature of this evolutionarily successful parasite is the fact that it usually causes asymptomatic infections and in most cases does not kill immunocompetent hosts. However, without sufficient therapy, reactivation of T. gondii in immunocompromised individuals frequently results in death of the host (31, 26, 18).
In 1984 a T. gondii-like parasite was found in the cerebral tissue of dogs and described (5). This parasite was later detected in brain tissue from dogs which had clinical signs of neuromuscular disease and was named Neospora caninum (15). It took until 1998 to discover that dogs are not only intermediate hosts but also one of the definitive hosts of this parasite (29). In nature, dogs are frequently intermediate hosts of N. caninum, although canine neosporosis seems to be rare (2). N. caninum can also be isolated from cattle, and vertically transmitted N. caninum infection is considered an important cause of bovine abortion worldwide (17). In sheep N. caninum-associated abortion seems to be rare (16). This is in contrast to infections with T. gondii, which often cause abortion in sheep but seldom in cattle (14). Furthermore, so far, there is no evidence that N. caninum infection is zoonotic (16). It has been shown that under experimental conditions N. caninum is able to infect rhesus monkeys, indicating the zoonotic potential of this parasite. However, serologic studies with humans have shown that no or only small amounts of N. caninum-specific antibodies are detectable in some human sera, even sera from high-risk groups like farm workers (30, 16, 22). Despite the high levels of homology between T. gondii and N. caninum, many differences have also been detected. Both parasites can be transmitted via food, via oocysts in soil, and also transplacentally (23). Several species have been successfully infected experimentally with N. caninum, and in vitro N. caninum is capable of replicating in different types of cells derived from various animal species or humans.
The variability in the susceptibility to natural T. gondii or N. caninum infection among various host species might be due to differences in the immune responses. Different antiparasite effector mechanisms might, at least in part, be involved in the evolutionary success of both parasites. In support of this, workers have obtained some data showing that experimental infection with attenuated or apathogenic N. caninum strains can induce immunity to this parasite in mice and cattle (3). Furthermore, a lot of data indicate that the cellular immune response is necessary to control infection with N. caninum. In addition, it was found that gamma interferon (IFN-γ), a product of activated T cells and natural killer (NK) cells, is one of the main cytokines conferring resistance to N. caninum (21). So far, the IFN-γ-induced effector mechanism that is active against N. caninum in cattle has not been defined.
In this paper we provide evidence that induction of the tryptophan-degrading enzyme indoleamine 2,3-dioxygenase (IDO), which is the most prominent antiparasite effector mechanism active against T. gondii in human cells (28), is also effective for inhibiting N. caninum growth in tissue cells from humans and cattle.
MATERIALS AND METHODS
Cell lines and culture.
A549 human epithelial lung cells (American Type Culture Collection, Rockville, MD), human astrocytoma cell line 86HG39 (4), human foreskin fibroblasts (HFFs) (ATCC, LGC Standards, Wesel, Germany), bovine fibroblast-like cells (EBTr cells), and bovine endothelial cells (BAOEC cells) (European Collection of Cell Cultures, Salisbury, United Kingdom) were used. Human cells were cultivated in Iscove's modified Dulbecco's medium (IMDM) with 25 mM (wt/vol) HEPES (Cambrex, East Rutherford, NJ) containing 5% (vol/vol) fetal calf serum (FCS) (Cambrex, East Rutherford, NJ) in culture flasks (Costar, Cambridge, MA). Bovine fibroblasts were cultured in IMDM with 10% FCS, while bovine endothelial cells were cultured in Dulbecco's modified Eagle's medium (Gibco, Grand Island, NY) with 15% FCS. Depending on cell growth, cultures were split every 2 to 5 days using ratios of 1:3 to 1:10 and 0.25% trypsin-EDTA (Gibco, Grand Island, NY). For the toxicity assay, BAOEC cells (2 × 105 cells/per well) were cultured in six-well plates for 3 days, and then the cells were diluted in trypan blue and the number of cells was determined using a Neubauer chamber.
Reagents.
Recombinant human IFN-γ, human interleukin-1 (IL-1), human tumor necrosis factor alpha (TNF-α), and recombinant bovine IFN-γ were purchased from R&D Systems, Wiesbaden-Nordenstadt, Germany. l-Tryptophan, l-kynurenine, Griess reagent (0.3% naphthylenediamine dihydrochloride and 1% sulfanilamide), and 1-l-methyltryptophan (1-MT) were obtained from Sigma-Aldrich (Deisenhofen, Germany).
N. caninum and T. gondii culture and preparation.
T. gondii strain RH tachyzoites and N. caninum strain NS-1 tachyzoites were maintained in HFFs in IMDM containing 5% (vol/vol) FCS. Tachyzoites were usually harvested after 3 or 5 days of incubation, purified by differential centrifugation as described previously (9), resuspended in tryptophan-free RPMI 1640 medium (Gibco, Grand Island, NY), and then used for infection experiments.
IDO assay.
The activity of IDO correlates directly with the concentration of N-formyl-kynurenine in supernatants of tissue culture cells, and thus measurement of the kynurenine concentration can be used to determine IDO activity (10). The different types of cells described above were plated in 96-well flat-bottom microtiter plates (3 × 104 cells per well) in the appropriate media containing 0.6 mM l-tryptophan (0.08 mM in basic media, 0.52 mM added to simplify the measurement of kynurenine production using the Ehrlich reagent method). Cells were stimulated with human or bovine IFN-γ at concentrations ranging from 0 to 1,000 U/ml. The plates were incubated at 37°C for 72 h, after which 160 μl of the culture supernatant was removed from each well and transferred to a 96-well V-bottom plate. After addition of 10 μl 30% trichloroacetic acid to each well, the plates were incubated at 50°C for 30 min to hydrolyze the N-formyl-kynurenine to kynurenine. After centrifugation for 10 min at 600 × g, 100 μl of supernatant was transferred to 96-well flat-bottom plates, and 100 μl 1.2% (wt/vol) 4-(dimethylamino)benzaldehyde (Ehrlich reagent; Sigma-Aldrich, Deisenhofen, Germany) in glacial acetic acid was added. After incubation for 10 min at room temperature, the optical density was determined at 492 nm with a microplate reader (Tecan, Crailsheim, Germany). Data were expressed as mean optical densities for triplicate cultures. The concentration of kynurenine was calculated using a standard curve for l-kynurenine sulfate (Sigma-Aldrich, Deisenhofen, Germany). In some experiments 1-MT was used to inhibit IDO activity as described previously (19).
Proliferation of N. caninum and T. gondii.
After cytokine stimulation for 72 h at 37°C, cells were infected with 2 × 104 N. caninum tachyzoites per well or with 2 × 104 T. gondii tachyzoites per well. Parasite growth was measured by the [3H]uracil incorporation method, which could be used to determine the growth of T. gondii (35), as well as the growth of N. caninum (21). Forty-eight hours after infection 0.33 μCi [3H]uracil was added, and after an additional 24 h, host cells and parasites were lysed by freeze-thawing. [3H]uracil incorporation was measured using liquid scintillation spectrometry (1205 Betaplate; PerkinElmer, Jugesheim, Germany).
IDO inhibitor treatment.
The IDO inhibitor 1-MT was used at a final concentration of 1.5 mM. First, 1-MT was dissolved in 1 M NaOH to obtain a concentration of 1 M, and then it was diluted with IMDM to obtain a 20 mM stock solution. The inhibitor was always added at the beginning of the experiment. To exclude the possibility of direct toxicity of 1-MT or NaOH, the viability of cells was checked using the trypan blue method.
Statistical analysis.
All data are expressed as means and standard errors of the means for three or four independent experiments, and each experiment was performed in triplicate. For comparison of data Student's t test for unpaired groups was used, and the P value was calculated using GraphPad Prism software.
RESULTS
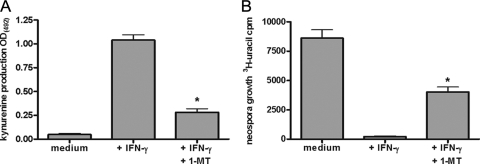
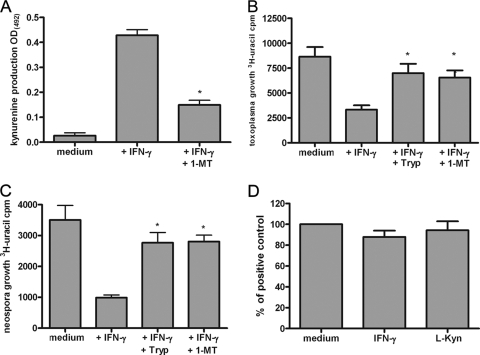
N. caninum is able to successfully infect and replicate within different human cells in vitro. Therefore, we first analyzed the growth of N. caninum in human fibroblasts (HFFs), in astrocytoma cells (86HG39 cells), and in lung alveolar cells (A549 cells). As shown in Fig. 1, the growth rates of N. caninum in the three lines of host cells were comparable. Moreover, we found that prestimulation of cells with about 500 U/ml IFN-γ allowed the different types of cells to mediate >90% inhibition of N. caninum growth. We observed slight differences in the amounts of IFN-γ necessary to mediate 50% inhibition of N. caninum growth. For example, more than 250 U/ml of IFN-γ had to be added to mediate 50% inhibition of N. caninum growth in A549 cells, while about 100 U/ml IFN-γ was sufficient to mediate quantitatively comparable inhibition of N. caninum growth in human fibroblasts. In addition to the antiparasite effect observed, we found that supplementation of cell cultures with excess amounts of l-tryptophan abrogated the IFN-γ-induced antiparasite effect. This indicates that, despite quantitative differences, the IFN-γ-inducible tryptophan-degrading enzyme IDO is responsible for the inhibition of parasite growth in all three types of human cells analyzed. In order to estimate the role of IDO in the antiparasite effect against N. caninum in more detail, 1-MT, an IDO-specific inhibitor, was used. Figure 2A shows that 1-MT is capable of inhibiting the production of kynurenine by IFN-γ-stimulated human fibroblasts significantly (P < 0.05). In parallel experiments we showed that 1-MT can block the IFN-γ-induced antineospora effect in human fibroblasts, at least partially (Fig. 2B). Thus, these data indicate that IDO can function as an antiparasite effector against N. caninum in human cells. Since humans are not natural hosts of N. caninum, we analyzed the capacity of bovine cells to restrict the growth of N. caninum, since cattle are the most important secondary host of N. caninum. In the first experiment, we analyzed the capacity of bovine fibroblast-like cells (EBTr cells) to express IDO activity. As shown in Fig. 3A, native bovine fibroblasts produced large amounts of kynurenine after stimulation with bovine IFN-γ. We detected 61.34 ± 6.616 μg/ml kynurenine (mean ± standard error of the mean) in the culture supernatant after stimulation with 100 U/ml IFN-γ (Fig. 3A). The amount of tryptophan degraded was fourfold greater than the physiologic tryptophan concentration in the serum. Having shown that bovine fibroblasts express IDO activity, we analyzed the capacity of these cells to restrict the growth of N. caninum in further experiments. As shown in Fig. 3B and 3C, we observed a strong IFN-γ-induced antiparasite effect mediated by bovine fibroblasts (EBTr cells) against T. gondii and N. caninum. This effect could be blocked, at least in part, by addition of excess tryptophan, indicating that IDO induction is the antiparasite effector mechanism involved. In order to explore the possible role of the inducible nitric oxide synthase (iNOS) in induction of an anti-N. caninum effect in bovine fibroblasts, we analyzed the production of nitric oxide by IFN-γ-stimulated bovine cells. We were unable to detect nitric oxide in the supernatants of IFN-γ-stimulated cells, even in the presence of IL-1 and TNF-α, using the Griess reagent method (13). Furthermore, NG-monomethyl-l-arginine (NGMMA), an iNOS inhibitor, did not influence the anti-N. caninum effect in the cells analyzed (data not shown).
FIG. 1.
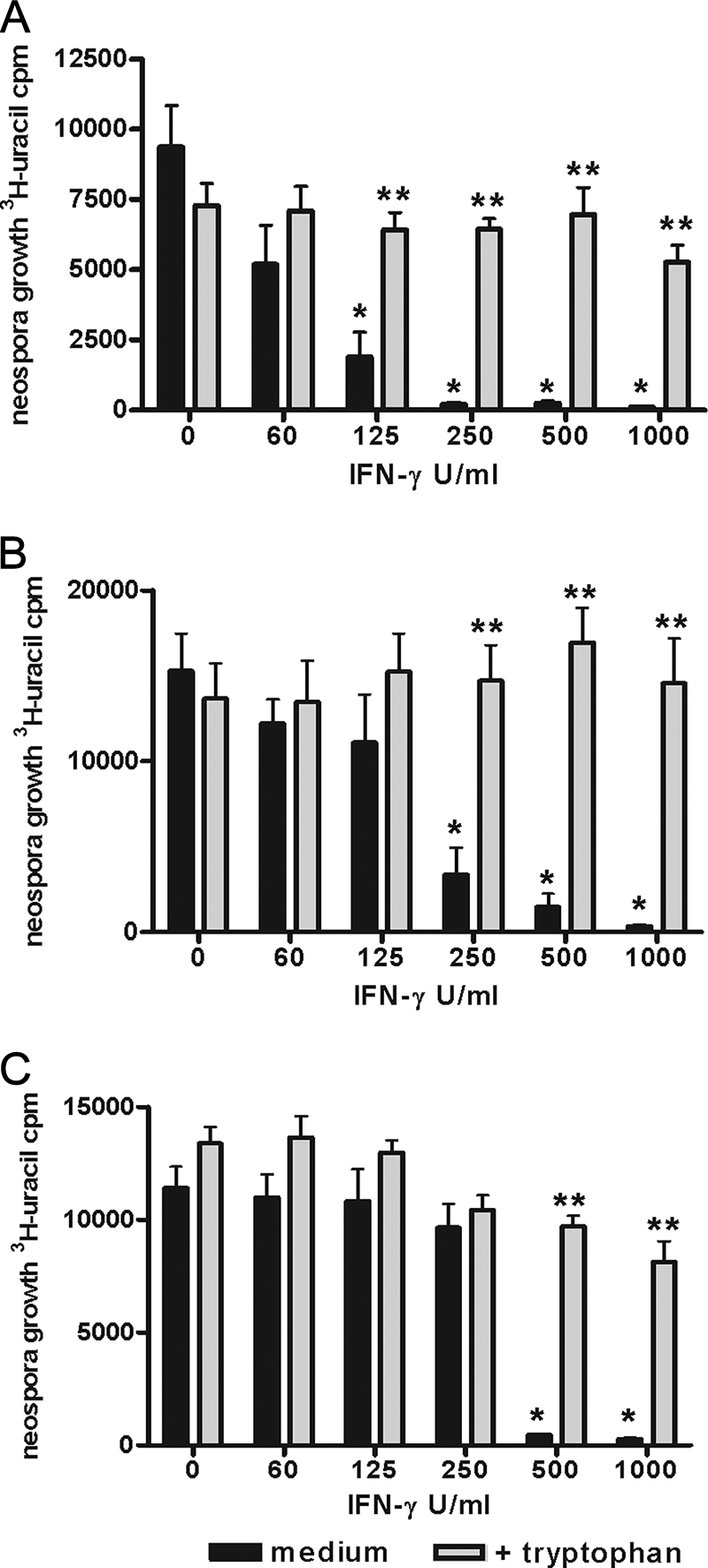
Inhibition of N. caninum growth in human cells and abrogation of inhibition by addition of tryptophan. A total of 3 × 104 HFFs (A), astrocytoma cells (86HG39 cells) (B), or human lung cells (A549 cells) (C) were stimulated with IFN-γ (0 to 1,000 U/ml) in the presence or absence of l-tryptophan (final concentration, 0.6 mM). After 3 days of incubation, N. caninum tachyzoites (2 × 104 tachyzoites per well) were added with or without l-tryptophan. Parasite growth was determined as described in Materials and Methods. The data are means and standard errors of the means of four independent experiments, each performed in triplicate. Significant inhibition of N. caninum growth by IFN-γ-activated cells is indicated by one asterisk, and significant abrogation of inhibition by l-tryptophan is indicated by two asterisks (P < 0.05).
FIG. 2.
IDO-specific inhibitor 1-MT antagonizes IFN-γ-induced inhibition of N. caninum growth. HFFs (3 × 104 cells per well) were stimulated with IFN-γ (250 U/ml) in the presence or absence of 1-MT (1.5 mM). (A) After 72 h of stimulation supernatants were harvested, and the kynurenine content was determined by use of the Ehrlich reagent. The data are means and standard errors of the means of four independent experiments performed in triplicate. Significant inhibition of IDO by 1-MT is indicated by an asterisk. OD(492), optical density at 492 nm. (B) After 72 h cultures were infected with N. caninum tachyzoites (2 × 104 tachyzoites per well). N. caninum growth was monitored using [3H]uracil, and the data are means and standard errors of the means of four independent experiments performed in triplicate. A significant inhibitory effect of 1-MT (P < 0.05) is indicated by an asterisk.
FIG. 3.
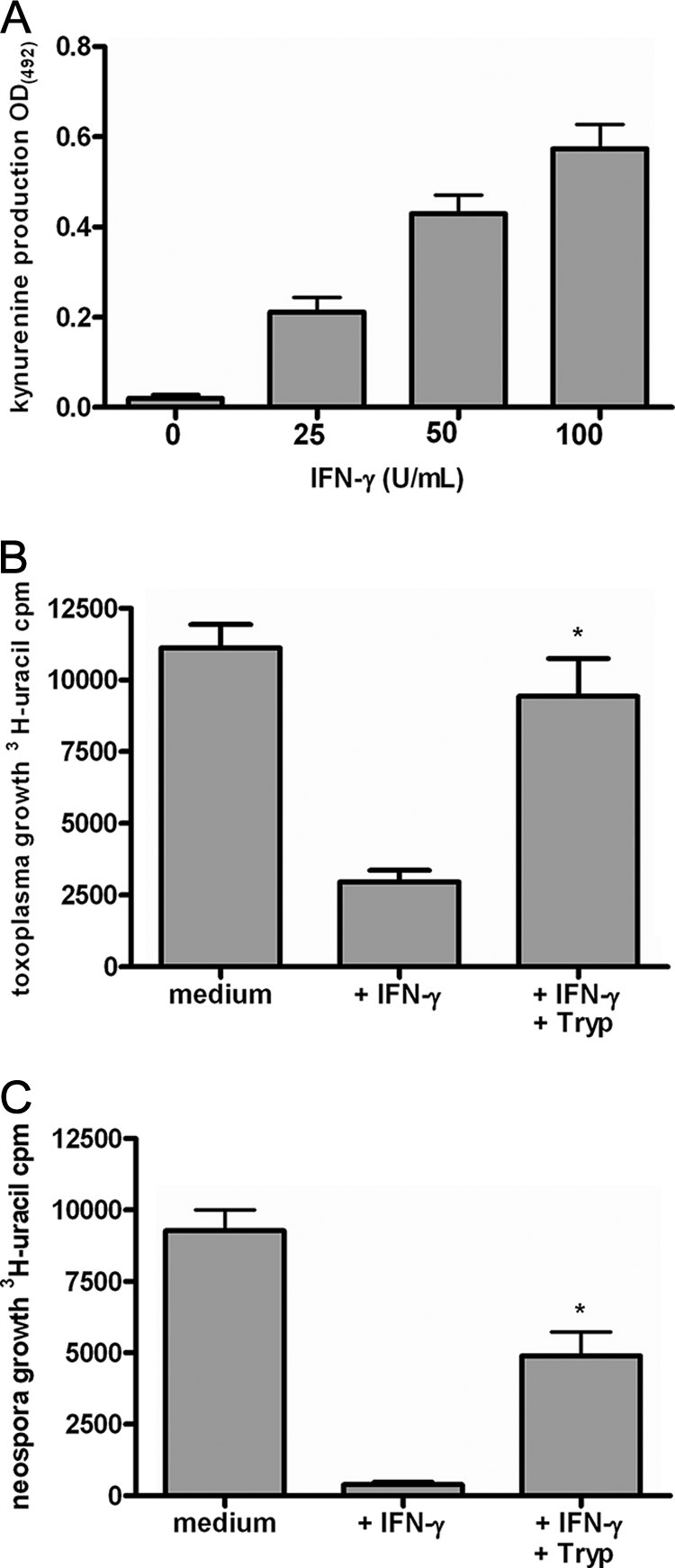
IDO induction as an antiparasite effector mechanism in bovine cells. Bovine fibroblasts (EBTr cells; 3 × 104 cells per well) were stimulated with bovine IFN-γ (0 to 100 U/ml) in the presence of 0.6 mM tryptophan. (A) After 72 h of stimulation supernatants were harvested, and the kynurenine content was determined by use of the Ehrlich reagent. The data are means and standard errors of the means of four independent experiments performed in triplicate. OD(492), optical density at 492 nm. (B) After 72 h cultures were infected with T. gondii tachyzoites (2 × 104 tachyzoites per well), and T. gondii growth was monitored using [3H]uracil. The data are means and standard errors of the means of three independent experiments performed in triplicate. A significant inhibitory effect of supplemental tryptophan (0.6 mM) on IFN-γ (50 U/ml)-induced inhibition of parasite growth is indicated by an asterisk. (C) At 72 h poststimulation, cells were infected with N. caninum tachyzoites (2 × 104 tachyzoites per well), and growth was monitored using [3H]uracil. The data are means and standard errors of the means of four independent experiments performed in triplicate. A significant inhibitory effect of supplemental tryptophan (0.6 mM) on IFN-γ (50 U/ml)-induced inhibition of parasite growth is indicated by an asterisk.
In vivo, fibroblasts might be host cells of N. caninum; however, especially during entry of this parasite into the brain or other tissues, endothelial cells also control the spread of the parasite. We therefore analyzed the capacity of native bovine endothelial cells (BAOEC cells) to express IDO activity and to inhibit the growth of N. caninum. As shown in Fig. 4, we found that N. caninum can replicate in bovine endothelial cells; however, the rate of proliferation of the parasite seems to be lower in endothelial cells than in fibroblasts. In addition, IFN-γ-activated bovine endothelial cells have a profound antiparasite capacity and restrict the growth of N. caninum and T. gondii. In these experiments the involvement of IDO in mediation of the antiparasite effect was shown by determination of IFN-γ-induced IDO activity (Fig. 4A) and by the antagonistic effect of the IDO-specific inhibitor 1-MT or supplemental tryptophan on the IFN-γ-induced antiparasite effect (Fig. 4B and 4C). In order to exclude the possibility of toxic effects of IFN-γ and l-kynurenine (41) on bovine endothelial cells, we determined that neither IFN-γ (100 U/ml) nor l-kynurenine (20 μg/ml) influenced the rate of proliferation or viability of BAOEC cells, as shown in Fig. 4D.
FIG. 4.
The IDO-specific inhibitor 1-MT antagonizes the IFN-γ-induced inhibition of N. caninum growth in bovine endothelial cells. Bovine endothelial cells (BAOEC cells; 3 × 104 cells per well) were stimulated with bovine IFN-γ (100 U/ml) in the presence or absence of 1-MT (1.5 mM). (A) After 72 h of stimulation supernatants were harvested, and the kynurenine content was determined by use of the Ehrlich reagent. The data are means and standard errors of the means of three independent experiments performed in triplicate. Significant inhibition of IDO by 1-MT is indicated by an asterisk. OD(492), optical density at 492 nm. (B) After 72 h cultures were infected with T. gondii tachyzoites (2 × 104 tachyzoites per well), and T. gondii growth was monitored using [3H]uracil. The data are means and standard errors of the means of three independent experiments performed in triplicate. Significant inhibitory effects of 1-MT (P < 0.05) or supplemental tryptophan (0.6 mM) are indicated by asterisks. (C) After 72 h cultures were infected with N. caninum tachyzoites (2 × 104 tachyzoites per well), and N. caninum growth was monitored using [3H]uracil. The data are means and standard errors of the means of three independent experiments performed in triplicate. Significant inhibitory effects of 1-MT (P < 0.05) or supplemental tryptophan (0.6 mM) are indicated by asterisks. (D) BAOEC cells (2 × 105 cells) were cultured in medium in the absence or in the presence of IFN-γ (100 U/ml) or l-kynurenine (20 μg/ml). After 3 days cells were harvested, and the number of cells was determined. The data are means and standard errors of the means of three independent experiments.
Thus, our data indicate that induction of the tryptophan-degrading enzyme IDO is involved in the control of N. caninum growth and that this effector mechanism is functional in human cells, as well as in bovine cells.
DISCUSSION
Neosporosis is a major cause of abortion in dairy and beef cattle (17). N. caninum seroprevalence is quite high in cattle in some countries (17). The risk of abortion in transplacentally infected seropositive animals is up to three- to sevenfold higher than that in seronegative animals (17). Therefore, significant economic loss is associated with this disease (17, 23). Thus, vaccination of cattle against N. caninum would be an interesting tool to avoid financial losses due to natural infections with this pathogen.
A protective immune response against an experimental infection with N. caninum could be induced in mice by vaccination using several recombinant antigens (1, 7, 8, 34, 38, 43), as well as DNA vaccines (8). Vaccination using viable N. caninum has been used successfully in the mouse model (3), as well as in cattle (44). These vaccination studies showed the importance of the adaptive immune response in defense against N. caninum in different species. A type 1 T-helper-cell (TH1) response was found to protect against natural (27) and experimental N. caninum infections (22). During pregnancy, a shift of the immune response toward a TH2 response was described for mice (24, 25). In contrast, experimental infection of cattle with N. caninum early in gestation and also late in gestation results in profound activation of T cells. TH1 cytokines like IFN-γ and TH2 cytokines like IL-4 were produced by CD4-positive T cells irrespective of the gestation time (39), and this finding argues against the possibility of a polarized TH1 response in N. caninum-infected cattle.
So far, there is no doubt that IFN-γ is one of the most important cytokines involved in the control of N. caninum growth. For example, BALB/c mice were usually resistant to acute infection with N. caninum, but IFN-γ-deficient mice died within a few days after infection (33). Moreover, IFN-γ was found to be active against N. caninum in different experimental systems: Tanaka et al. (40) showed that IFN-γ-activated murine macrophages were able to restrict the growth of N. caninum. This anti-N. caninum effect was successfully blocked by NGMMA, a competitive inhibitor of iNOS. Furthermore, iNOS-deficient mice were more susceptible to experimental N. caninum infection than wild-type mice (40). Nitric oxide production is one of the most prominent antimicrobial effector mechanisms used by rodents; however, other species have evolved other defense mechanisms. For example, Pfefferkorn found that the IFN-γ-inducible tryptophan-degrading enzyme IDO is capable of blocking the growth of T. gondii (36). Proliferation of N. caninum in bovine fibroblasts was analyzed in a coculture system consisting of N. caninum-infected fibroblasts and bovine NK cells (6). Boysen et al. found that N. caninum-infected fibroblasts induce IFN-γ production in NK cells, which inhibit N. caninum growth by killing infected fibroblasts. In this study, we show for the first time that IFN-γ-activated bovine fibroblasts are capable of inhibiting N. caninum growth by an IDO-dependent mechanism. Therefore, human and bovine cells use the same antiparasite effector mechanism to defend themselves against N. caninum. In our experiments we used the inhibitory effects of 1-MT and of supplemental tryptophan on IFN-γ-induced inhibition of N. caninum growth to indicate the involvement of IDO. In bovine cells both of these compounds significantly, but not completely, block the IFN-γ-induced antiparasite effect. Despite the fact that 1-MT did not completely inhibit kynurenine production by IFN-γ-activated human and murine cells, we could not exclude the possibility of activity of other antiparasite compounds, like iNOS or GTPases. However, we found no evidence of iNOS induction in bovine cells, and as described previously, parallel iNOS induction and IDO induction would result in inhibition of IDO activity (12, 20, 42). Therefore, we suggest that it is unlikely that iNOS is involved in the observed antiparasite effect in bovine cells.
Pinheiro et al. (37) showed that native rat astrocytes can support proliferation of N. caninum and that an infection results in production of a proinflammatory reaction in astrocytes, indicated by production of nitric oxide and of TNF-α by these cells. These two compounds can be toxic for neurons and may contribute to the pathology of N. caninum in mice. Comparable experiments demonstrated that IFN-γ and TNF-α can activate primary bovine brain cells to restrict the growth of N. caninum. This antiparasite effect, mediated by bovine cells, was not blocked by NGMMA, indicating that iNOS is not involved in this process. Analogous to this finding, we observed that human astrocytoma cells are susceptible to N. caninum infection. However, these cells are unable to produce nitric oxide, as previously shown by us, but they are capable of restricting the growth of parasites due to an IDO-dependent mechanism (9, 11).
Defense against N. caninum during pregnancy is a problem since at this time point a semiallogeneic fetus is present in the uterus and immunosuppression is necessary to protect the fetus, while a proinflammatory TH1 response is necessary to mediate successful defense against N. caninum. Data obtained in animal experiments with mice indicated that during pregnancy immunosuppression or induction of tolerance of the fetal tissue is mediated by induction of IDO (32). In conclusion, in this paper, we show that IDO is a major antiparasite effector against N. caninum in bovine fibroblasts and endothelial cells. Therefore, a proinflammatory response, including IFN-γ production, might mediate immunosuppressive effects, as well as antiparasite effects.
Acknowledgments
This work was supported by grants from the German Federal Ministry of Education and Research (BMBF): “Zoonosis” (to G.S. and W.D.) and from the German Research Council (DFG) “Forschergruppe 729” (to W.D.).
We have no conflicting financial interests.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 20 July 2009.
REFERENCES
- 1.Alaeddine, F., N. Keller, A. Leepin, and A. Hemphill. 2005. Reduced infection and protection from clinical signs of cerebral neosporosis in C57BL/6 mice vaccinated with recombinant microneme antigen NcMIC1. J. Parasitol. 91:657-665. [DOI] [PubMed] [Google Scholar]
- 2.Barber, J. S., and A. J. Trees. 1998. Naturally occurring vertical transmission of Neospora caninum in dogs. Int. J. Parasitol. 28:57-64. [DOI] [PubMed] [Google Scholar]
- 3.Bartley, P. M., S. Wright, F. Chianini, D. Buxton, and E. A. Innes. 2008. Inoculation of Balb/c mice with live attenuated tachyzoites protects against a lethal challenge of Neospora caninum. Parasitology 135:13-21. [DOI] [PubMed] [Google Scholar]
- 4.Bilzer, T., D. Stavrou, E. Dahme, E. Keiditsch, K. F. Burrig, A. P. Anzil, and W. Wechsler. 1991. Morphological, immunocytochemical and growth characteristics of three human glioblastomas established in vitro. Virchows Arch. A Pathol. Anat. Histopathol. 418:281-293. [DOI] [PubMed] [Google Scholar]
- 5.Bjerkas, I., S. F. Mohn, and J. Presthus. 1984. Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Z. Parasitenkd. 70:271-274. [DOI] [PubMed] [Google Scholar]
- 6.Boysen, P., S. Klevar, I. Olsen, and A. K. Storset. 2006. The protozoan Neospora caninum directly triggers bovine NK cells to produce gamma interferon and to kill infected fibroblasts. Infect. Immun. 74:953-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannas, A., A. Naguleswaran, N. Müller, B. Gottstein, and A. Hemphill. 2003. Reduced cerebral infection of Neospora caninum-infected mice after vaccination with recombinant microneme protein NcMIC3 and ribi adjuvant. J. Parasitol. 89:44-50. [DOI] [PubMed] [Google Scholar]
- 8.Cannas, A., A. Naguleswaran, N. Müller, S. Eperon, B. Gottstein, and A. Hemphill. 2003. Vaccination of mice against experimental Neospora caninum infection using NcSAG1- and NcSRS2-based recombinant antigens and DNA vaccines. Parasitology 126:303-312. [DOI] [PubMed] [Google Scholar]
- 9.Däubener, W., K. Pilz, Z. S. Seghrouchni, T. Bilzer, H. G. Fischer, and U. Hadding. 1993. Induction of toxoplasmostasis in a human glioblastoma by interferon gamma. J. Neuroimmunol. 43:31-38. [DOI] [PubMed] [Google Scholar]
- 10.Däubener, W., N. Wanagat, K. Pilz, S. Seghrouchni, H. G. Fischer, and U. Hadding. 1994. A new, simple, bioassay for human IFN-gamma. J. Immunol. Methods 168:39-47. [DOI] [PubMed] [Google Scholar]
- 11.Däubener, W., C. Remscheid, S. Nockemann, K. Pilz, S. Seghrouchni, C. R. MacKenzie, and U. Hadding. 1996. Anti-parasitic effector mechanisms in human brain tumor cells: role of interferon-gamma and tumor necrosis factor-alpha. Eur. J. Immunol. 26:487-492. [DOI] [PubMed] [Google Scholar]
- 12.Däubener, W., V. Posdziech, U. Hadding, and C. R. MacKenzie. 1999. Inducible anti-parasitic effector mechanisms in human uroepithelial cells: tryptophan degradation vs. NO production. Med. Microbiol. Immunol. 187:143-147. [DOI] [PubMed] [Google Scholar]
- 13.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 14.Dubey, J. P. 1986. A review of toxoplasmosis in cattle. Vet. Parasitol. 22:177-202. [DOI] [PubMed] [Google Scholar]
- 15.Dubey, J. P., A. L. Hattel, D. S. Lindsay, and M. J. Topper. 1988. Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission. J. Am. Vet. Med. Assoc. 193:1259-1263. [PubMed] [Google Scholar]
- 16.Dubey, J. P. 2003. Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 41:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubey, J. P., G. Schares, and L. M. Ortega-Mora. 2007. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 20:323-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filisetti, D., and E. Candolfi. 2004. Immune response to Toxoplasma gondii. Ann. Ist. Super. Sanita 40:71-80. [PubMed] [Google Scholar]
- 19.Heseler, K., K. Spekker, S. K. Schmidt, C. R. MacKenzie, and W. Däubener. 2008. Antimicrobial and immunoregulatory effects mediated by human lung cells: role of IFN-gamma-induced tryptophan degradation. FEMS Immunol. Med. Microbiol. 52:273-281. [DOI] [PubMed] [Google Scholar]
- 20.Hucke, C., C. R. MacKenzie, K. D. Adjogble, O. Takikawa, and W. Däubener. 2004. Nitric oxide-mediated regulation of gamma interferon-induced bacteriostasis: inhibition and degradation of human indoleamine 2,3-dioxygenase. Infect. Immun. 72:2723-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Innes, E. A., W. R. Panton, J. Marks, A. J. Trees, J. Holmdahl, and D. Buxton. 1995. Interferon gamma inhibits the intracellular multiplication of Neospora caninum, as shown by incorporation of 3H uracil. J. Comp. Pathol. 113:95-100. [DOI] [PubMed] [Google Scholar]
- 22.Innes, E. A., S. Wright, P. Bartley, S. Maley, C. Macaldowie, I. Esteban-Redondo, and D. Buxton. 2005. The host-parasite relationship in bovine neosporosis. Vet. Immunol. Immunopathol. 108:29-36. [DOI] [PubMed] [Google Scholar]
- 23.Innes, E. A. 2007. The host-parasite relationship in pregnant cattle infected with Neospora caninum. Parasitology 134:1903-1910. [DOI] [PubMed] [Google Scholar]
- 24.Kano, R., Y. Masukata, Y. Omata, Y. Kobayashi, R. Maeda, and A. Saito. 2005. Relationship between type 1/type 2 immune responses and occurrence of vertical transmission in BALB/c mice infected with Neospora caninum. Vet. Parasitol. 129:159-164. [DOI] [PubMed] [Google Scholar]
- 25.Kano, R., A. Kudo, H. Kamiya, Y. Kobayashi, R. Maeda, and Y. Omata. 2007. C57BL/6 mice infected with Neospora caninum during administration of progesterone show bias toward type 2 immune response. J. Vet. Med. Sci. 69:1095-1097. [DOI] [PubMed] [Google Scholar]
- 26.Laliberte, J., and V. B. Carruthers. 2008. Host cell manipulation by the human pathogen Toxoplasma gondii. Cell. Mol. Life Sci. 65:1900-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Gatius, F., S. Almeria, G. Donofrio, C. Nogareda, I. Garcia-Ispierto, G. Bech-Sabat, P. Santolaria, J. L. Yaniz, M. Pabon, N. M. de Sousa, and J. F. Beckers. 2007. Protection against abortion linked to gamma interferon production in pregnant dairy cows naturally infected with Neospora caninum. Theriogenology 68:1067-1073. [DOI] [PubMed] [Google Scholar]
- 28.MacKenzie, C. R., K. Heseler, A. Müller, and W. Däubener. 2007. Role of indoleamine 2,3-dioxygenase in antimicrobial defence and immuno-regulation: tryptophan depletion versus production of toxic kynurenines. Curr. Drug Metab. 8:237-244. [DOI] [PubMed] [Google Scholar]
- 29.McAllister, M. M., J. P. Dubey, D. S. Lindsay, W. R. Jolley, R. A. Wills, and A. M. McGuire. 1998. Dogs are definitive hosts of Neospora caninum. Int. J. Parasitol. 28:1473-1478. [PubMed] [Google Scholar]
- 30.McCann, C. M., A. J. Vyse, R. L. Salmon, D. Thomas, D. J. Williams, J. W. McGarry, R. Pebody, and A. J. Trees. 2008. Lack of serologic evidence of Neospora caninum in humans, England. Emerg. Infect. Dis. 14:978-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montoya, J. G., and J. S. Remington. 2008. Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 47:554-566. [DOI] [PubMed] [Google Scholar]
- 32.Munn, D. H., M. Zhou, J. T. Attwood, I. Bondarev, S. J. Conway, B. Marshall, C. Brown, and A. L. Mellor. 1998. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281:1191-1193. [DOI] [PubMed] [Google Scholar]
- 33.Nishikawa, Y., K. Tragoolpua, N. Inoue, L. Makala, H. Nagasawa, H. Otsuka, and T. Mikami. 2001. In the absence of endogenous gamma interferon, mice acutely infected with Neospora caninum succumb to a lethal immune response characterized by inactivation of peritoneal macrophages. Clin. Diagn. Lab. Immunol. 8:811-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishikawa, Y., X. Xuan, H. Nagasawa, I. Igarashi, K. Fujisaki, H. Otsuka, and T. Mikami. 2001. Prevention of vertical transmission of Neospora caninum in BALB/c mice by recombinant vaccinia virus carrying NcSRS2 gene. Vaccine 19:1710-1716. [DOI] [PubMed] [Google Scholar]
- 35.Pfefferkorn, E. R., and L. C. Pfefferkorn. 1977. Specific labeling of intracellular Toxoplasma gondii with uracil. J. Protozool. 24:449-453. [DOI] [PubMed] [Google Scholar]
- 36.Pfefferkorn, E. R. 1984. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc. Natl. Acad. Sci. USA 81:908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinheiro, A. M., S. L. Costa, S. M. Freire, M. A. Almeida, M. Tardy, R. El Bacha, and M. F. Costa. 2006. Astroglial cells in primary culture: a valid model to study Neospora caninum infection in the CNS. Vet. Immunol. Immunopathol. 113:243-247. [DOI] [PubMed] [Google Scholar]
- 38.Pinitkiatisakul, S., J. G. Mattsson, and A. Lunden. 2008. Quantitative analysis of parasite DNA in the blood of immunized and naive mice after infection with Neospora caninum. Parasitology 135:175-182. [DOI] [PubMed] [Google Scholar]
- 39.Rosbottom, A., C. S. Guy, E. H. Gibney, R. F. Smith, J. F. Valarcher, G. Taylor, and D. J. Williams. 2007. Peripheral immune responses in pregnant cattle following Neospora caninum infection. Parasite Immunol. 29:219-228. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka, T., H. Nagasawa, K. Fujisaki, N. Suzuki, and T. Mikami. 2000. Growth-inhibitory effects of interferon-gamma on Neospora caninum in murine macrophages by a nitric oxide mechanism. Parasitol. Res. 86:768-771. [DOI] [PubMed] [Google Scholar]
- 41.Terness, P., T. M. Bauer, L. Röse, C. Dufter, A. Watzlik, H. Simon, and G. Opelz. 2002. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J. Exp. Med. 196:447-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas, S. R., D. Mohr, and R. Stocker. 1994. Nitric oxide inhibits indoleamine 2,3-dioxygenase activity in interferon-gamma primed mononuclear phagocytes. J. Biol. Chem. 269:14457-14464. [PubMed] [Google Scholar]
- 43.Vemulapalli, R., N. Sanakkayala, J. Gulani, G. G. Schurig, S. M. Boyle, D. S. Lindsay, and N. Sriranganathan. 2007. Reduced cerebral infection of Neospora caninum in BALB/c mice vaccinated with recombinant Brucella abortus RB51 strains expressing N. caninum SRS2 and GRA7 proteins. Vet. Parasitol. 148:219-230. [DOI] [PubMed] [Google Scholar]
- 44.Williams, D. J., C. S. Guy, R. F. Smith, J. Ellis, C. Bjorkman, M. P. Reichel, and A. J. Trees. 2007. Immunization of cattle with live tachyzoites of Neospora caninum confers protection against fetal death. Infect. Immun. 75:1343-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]