Abstract
A heterologous prime-boost strategy using plasmid DNA, followed by replication-defective recombinant adenovirus 5, is being proposed as a powerful way to elicit CD4+ and CD8+ T-cell-mediated protective immunity against intracellular pathogens. We confirmed this concept and furthered existing research by providing evidence that the heterologous prime-boost regimen using the gene encoding amastigote surface protein 2 elicited CD4+ and CD8+ T-cell-mediated protective immunity (reduction of acute parasitemia and prolonged survival) against experimental infection with Trypanosoma cruzi. Protective immunity correlated with the presence of in vivo antigen-specific cytotoxic activity prior to challenge. Based on this, our second goal was to determine the outcome of infection after heterologous prime-boost immunization of perforin-deficient mice. These mice were highly susceptible to infection. A detailed analysis of the cell-mediated immune responses in immunized perforin-deficient mice showed an impaired gamma interferon (IFN-γ) secretion by immune spleen cells upon restimulation in vitro with soluble recombinant antigen. In spite of a normal numeric expansion, specific CD8+ T cells presented several functional defects detected in vivo (cytotoxicity) and in vitro (simultaneous expression of CD107a/IFN-γ or IFN-γ/tumor necrosis factor alpha) paralleled by a decreased expression of CD44 and KLRG-1. Our final goal was to determine the importance of IFN-γ in the presence of highly cytotoxic T cells. Vaccinated IFN-γ-deficient mice developed highly cytotoxic cells but failed to develop any protective immunity. Our study thus demonstrated a role for perforin and IFN-γ in a number of T-cell-mediated effector functions and in the antiparasitic immunity generated by a heterologous plasmid DNA prime-adenovirus boost vaccination strategy.
Genetic vaccination using naked DNA or recombinant viruses is being pursued as alternative vaccines. This strategy can be particularly important in the case of intracellular pathogens and neoplasic cells when the effectiveness relies heavily on the vaccination's capacity to elicit specific immune responses mediated by cytotoxic CD8+ T cells (reviewed in references 27, 48, and 49). One of the most prolific areas of genetic vaccination development is the strategy known as the heterologous prime-boost regimen. This consists in the use of two different vectors both carrying a gene that encodes the same antigenic protein for priming and boosting immunizations. This strategy was first proposed in the early 1990s using a combination of recombinant viruses (influenza and vaccinia viruses) to induce protective immunity against malaria (34, 50). Subsequently, this approach was extended and simplified by incorporating naked DNA priming, followed by a booster injection of a recombinant poxviral vector (i.e., modified vaccinia Ankara); this was also used to generate sterile protective immunity in rodent malaria (53, 54). Collectively, these studies demonstrated that the heterologous prime-boost regimen proved more effective than the repeated use of any of these vectors individually. In subsequent years, the heterologous plasmid DNA prime-poxvirus vector boost regimen was adopted worldwide as a powerful mean to elicit strong effector CD8+ Tc1-mediated immune responses against viral, parasitic, and neoplastic antigens in rodents and nonhuman primates (4; reviewed in references 29, 44, and 65). Based on the preclinical studies, a number of clinical human trials have also been initiated. However, to our knowledge, heterologous prime-boost regimens using plasmid DNA and recombinant poxviruses have not yet provided meaningful protective immunity to humans (21, 28, 42, 45).
Although there are a number of possible vector combinations that significantly improve cell-mediated immunity, particularly of specific CD8+ T cells, heterologous prime-boost vaccination using naked plasmid DNA for priming, followed by a booster injection of recombinant replication-deficient adenovirus 5 has recently received significant attention. This strategy has proved successful in some relevant experimental models such as simian immunodeficiency virus and malaria, providing considerable protective immunity (2, 13, 14, 26, 33, 41, 62).
The fact that protective CD8+ T cells could be induced in mice and nonhuman primates against both virus and parasites made it an interesting strategy against other microbial infections. We initially attempted to build on this strategy by generating protective CD4 Th1 and cytotoxic CD8+ T cells against the infection with a human intracellular protozoan parasite, Trypanosoma cruzi. Both CD4+ and CD8+ T cells were described as critical for acquired immunity against experimental infection with T. cruzi (38, 39, 56-59). Vaccination studies confirmed these observations by providing evidence that CD8+ T cells are particularly important to the development of protective immunity in mice immunized with recombinant plasmid DNA, proteins, or viruses (6, 19, 25, 31, 37, 43, 60). In addition to CD8+ T cells, CD4+ Th1 cells also play a role in immunity against T. cruzi after vaccination with plasmid DNA or recombinant protein (19, 30, 60).
In our previous studies, we showed that multiple immunizations of highly susceptible A/Sn mice with a gene or a recombinant protein based on the amastigote surface protein 2 (ASP-2) of T. cruzi generated protective immunity against a lethal challenge with this parasite (6, 17, 19, 55, 60). In both cases, vaccinated animals depleted of CD8+ T cells prior to challenge became highly susceptible to infection (6, 60). Protective CD8+ T cells were directed to the immunodominant epitope TEWETGQI located within amino acids 320 to 327 of ASP-2 (6, 19).
The present study was designed first to evaluate whether we could reduce the number of immunizing doses required to generate CD4+ and CD8+ T-cell-mediated protective immunity using a heterologous prime-boost regimen. A reduction in the number of doses may greatly improve the feasibility of human vaccination trials. The fact that mice immunized with recombinant AdASP-2 showed the highest levels of in vivo cytotoxicity mediated by CD8+ T cells prior to challenge, and some degree of protective immunity led us to study the role for perforin during vaccination. Subsequently, we determined whether the high susceptibility observed in perforin-deficient mice correlated with impaired effector T-cell immune responses and the expression of relevant activation markers by specific CD8+ T cells. Our final goal was to determine the importance of gamma interferon (IFN-γ), a critical mediator of adaptive immunity against T. cruzi infection, for protective immunity in the presence of highly cytotoxic T cells.
MATERIALS AND METHODS
Mice and parasites.
Female 5- to 8-week-old A/Sn, C57BL/6 wild-type (WT), C57BL/6 perforin-deficient (perforin KO), C57BL/6 CD4 deficient (CD4 KO), C57BL/6 CD8a-deficient (CD8 KO), or C57BL/6 IFN-γ-deficient (IFN-γ KO) mice were purchased from University of São Paulo, raised at the Instituto de Biofísica Carlos Chagas Filho or at the animal facilities of the Oswaldo Cruz Foundation. Parasites of the Y strain of T. cruzi were used here (10). Bloodstream trypomastigotes were obtained from mice infected 7 days earlier. The concentration of parasites was estimated and adjusted to 750 parasites/ml. Each A/Sn mouse was inoculated intraperitoneally (i.p.) with 0.2 ml. In the case of C57BL/6 or perforin KO mice, the concentration of parasites was estimated and adjusted to 5 × 104/ml, and 0.2 ml was injected i.p. (104 trypomastigotes/mouse). Parasite development was monitored by counting the number of bloodstream trypomastigotes in 5 μl of fresh blood collected from the tail vein (10). Hemocultures were performed with aliquots of 0.1 ml of blood collected and cultured at 28°C for 1 month in 5 ml of axenic liver infusion tryptose medium. Parasite growth was monitored microscopically every week. For PCR, DNA was extracted from 0.1 ml of blood and resuspended in a final volume of 100 μl. As controls, known numbers of T. cruzi Y strain blood trypomastigotes were diluted in 0.1 ml of blood obtained from naive mice (200, 100, 50, 25, 12.5, or 0 trypomastigotes/ml). Then, 5-μl portions were used for PCRs with Platinum Taq DNA polymerase (Invitrogen) and the T. cruzi kDNA primers S35 (5′-AAATAATGTACGGGGGAGATGCATGA-3′) and S36 (5′-GGGTTCGATTGGGGTTGGTGT-3′). The amplification cycles consisted of an initial denaturation of 5 min at 94°C, followed by 35 cycles of 30 s at 95°C (denaturation), 30 s at 62°C (primer annealing), and 1 min at 72°C (primer extension). We observed a 330-bp band in samples containing as few as 12.5 trypomastigotes/ml, but not in parasite-free blood DNA. Mouse survival was monitored daily. The use of animals and the experimental procedures were approved by the Ethics Committee for Animal Care of the Federal University of São Paulo.
Peptide synthesis.
Peptides VNHRFTLV and TEWETGQI were prepared by standard Nα[9-fluorenylmethyloxycarbonyl] on a PSSM8 multispecific peptide synthesizer (Shimadzu, Kyoto, Japan) by solid-phase synthesis with a scale of 30 μmol. Peptide was purified by high-pressure liquid chromatography in a Shimadzu system. Peptides were analyzed in a C18 Vydac column (10 by 250 mm, 5-μm particle diameter). The peptide purity was in a range of 80 to 90% purity. Their identities were confirmed by Q-TOF Micro equipped with an electrospray ionization source (Micromass, United Kingdom). Pentamer H-2Kb/VNHRFTLV was purchased from ProImmune, Inc. (Oxford, United Kingdom).
Recombinant plasmids and adenoviruses used for immunization.
Plasmid pIgSPclone9 was obtained by inserting into the commercial vector pcDNA3 (Invitrogen) the sequence encoding the signal peptide of mouse immunoglobulin kappa chain in-frame with the gene encoding T. cruzi ASP-2 (10). pAdCMV-ASP2 is an adenoviral transfer plasmid that contains a eukaryotic expression cassette formed by the cytomegalovirus immediate-early promoter and the simian virus 40 RNA polyadenylation sequences. Inside this cassette we cloned the DNA sequences encoding T. cruzi ASP-2 obtained by restriction digestion of plasmid pIgSPclone9 (AdASP-2 [37]). Viruses and plasmids were purified as described earlier, and they both lead to expression of the antigen as evaluated by in vitro transfections (10, 37). Mice were inoculated intramuscularly (i.m.) in each tibialis anterioris muscle with 50 μg of plasmid DNA. Twenty-one days later, these mice received in these same spots 50 μl of viral suspension containing 108 PFU of adenovirus. Immunological assays were performed 14 days after viral inoculation.
In vivo depletion of CD4+ T cells were performed by treating vaccinated A/Sn or C57BL/6 mice with monoclonal antibody (MAb) GK1.5. At days 6, 4, and 2 before challenge with trypomastigotes, mice were injected i.p. with a dose of 0.5 mg of anti-CD4 or control rat immunoglobulin G (IgG). At 7 and 14 days postchallenge, each mouse received one more dose of 0.5 mg of anti-CD4 or rat IgG. As determined by fluorescence-activated cell sorting (FACS) analyses, the efficacy of depletion of CD4+ spleen cells before challenge was of 99.94% in anti-CD4 treated mice compared to rat IgG-treated ones. In vivo depletion of CD8+ T cells were performed by treating vaccinated A/Sn mice with MAb 53.6.7. At days 3 and 4 before challenge with trypomastigotes, mice were injected i.p. with a dose of 1 mg of anti-CD8 or control rat IgG. At 7 days after challenge, each mouse received one more dose of 1 mg of anti-CD8 or rat IgG. The efficacy of depletion of CD8+ spleen cells before challenge was more than 96% in anti-CD8-treated mice compared to rat IgG-treated ones.
Immunological assays.
For the in vivo cytotoxicity assays, splenocytes collected from naive A/Sn or C57BL/6 mice were treated with ACK buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM disodium EDTA [pH 7.4]) to lyse the red blood cells. These cells were divided into two populations and labeled with the fluorogenic dye carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) at a final concentration of 5 μM (CFSEhigh) or 0.5 μM (CFSElow). CFSEhigh cells were coated for 40 min at 37°C with a 1 or 2.5 μM concentration of the H-2Kb or H-2Kk ASP-2 peptides VNHRFTLV or TEWETGQI, respectively. CFSElow cells remained uncoated. Then, CFSEhigh cells were washed and mixed with equal numbers of CFSElow cells before 3 × 107 to 4 × 107 total cells per mouse were injected intravenously. Recipient animals were mice previously immunized with recombinant plasmids or adenoviruses or both. Spleen cells of recipient mice were collected 4 h or 20 h after transfer, as indicated in the figure legends, fixed with 1.0% paraformaldehyde, and analyzed by FACS using a FacsCanto flow cytometer (BD, Mountain View, CA). The percent specific lysis was determined by using the formula: [1 − (% CFSEhigh immunized/% CFSElow immunized)/(% CFSEhigh naive/% CFSElow naive)] × 100. IFN-γ secretion by cultured spleen cells and enzyme-linked immunospot (ELISPOT) assay for enumeration of IFN-γ producing cells was performed essentially as described previously (10, 50).
For flow cytometry analyses, we used mouse splenocytes treated with ACK buffer. Single-cell suspensions were washed in phosphate-buffered saline (PBS), stained for 10 min at room temperature with biotinylated major histocompatibility complex class I (MHC-I) multimer H-Kb/VNHRFTLV, and then stained 30 min at 4°C with avidin-phycoerythrin (PE)- and allophycocyanin (APC)-labeled anti-CD8 antibodies (both from BD Pharmingen). For the analyses of other cell surface markers, single cell suspension from spleens of mice were depleted of erythrocytes, and CD8+ T cells were purified by negative selection using a MACS separation system (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocols with magnetic LS columns. As estimated by FACS analysis, >85% of the cells were CD8+ T cells after enrichment. These cells were stained at room temperature with biotinylated MHC-I multimer H-Kb/VNHRFTLV and then stained 20 min at room temperature with avidin-PE- and APC-labeled anti-CD8 antibodies, as well as with fluorescein isothiocyanate (FITC)-labeled anti-CD11a, anti-CD43, anti-CD44, anti-CD62L, anti-CD127, and anti-KLRG-1 antibodies (all from BD Pharmingen). At least 100,000 cells were acquired on a FacsCanto flow cytometer and then analyzed with FlowJo (Tree Star) using a biexponential transform.
The expression of CD107a was evaluated after in vitro culture of splenocytes in the presence or absence of antigenic stimulus. Cells were washed three times in plain RPMI and resuspended in cell culture medium consisting of RPMI 1640 medium (pH 7.4) supplemented with 10 mM HEPES, 0.2% sodium bicarbonate, 59 mg of penicillin/liter, 133 mg of streptomycin/liter, and 10% HyClone fetal bovine serum (HyClone, Logan, UT). The viability of the cells was evaluated by using 0.2% trypan blue exclusion dye to discriminate between live and dead cells. The cell concentration was adjusted to 5 × 106 cells/ml in cell culture medium containing also anti-CD28 (2 μg/ml), brefeldin A (10 μg/ml), monensin (5 μg/ml), and the FITC-labeled anti-CD107a (2 μg/ml, all from BD Pharmingen). In half of the cultures, a final concentration of 10 μM VNHRFTLV peptide was added. The cells were cultivated in flat-bottom 96-well plates (Corning) in a final volume of 200 μl in duplicate at 37°C in a humid environment. After 12 h of incubation, cells were stained for surface marker with anti-CD8 on ice for 20 min. At least 100,000 cells were acquired on a FacsCanto flow cytometer and then analyzed with FlowJo.
To detect IFN-γ, tumor necrosis factor alpha (TNF-α), and interleukin-2 (IL-2) by intracellular staining (ICS), in vitro cultures of splenocytes were prepared as described above; however, the culture medium used contained neither monensin nor anti-CD107a. After 12 h of incubation, cells were stained for a surface marker with anti-CD8 for 20 min on ice. The cells were then washed twice in buffer containing PBS, 0.5% bovine serum albumin, and 2 mM EDTA; fixed in 4% PBS-paraformaldehyde solution for 10 min; and permeabilized for 15 min in a PBS-0.1% bovine serum albumin-0.1% saponin solution. After being washed twice, cells were stained for intracellular markers using APC-Cy7 anti-CD3, PE-Cy7 anti-IFN-γ, Alexa 488-anti-TNF-α, and APC-anti-IL-2 for 20 min on ice. Finally, the cells were washed twice and fixed in 1% PBS-paraformaldehyde. At least 300,000 cells were acquired on a FacsCanto flow cytometer and then analyzed with FlowJo.
Statistical analysis.
The values of parasitemia of each individual mouse were log transformed before being compared by one-way analysis of variance, followed by Tukey HSD tests (available at http://faculty.vassar.edu/lowry/VassarStats.html). The in vivo ELISPOT cytotoxicity assay results, the numbers of multimer-positive cells, and the percentages of cytokine- or CD107a-expressing cells are expressed as medians (bars) and individual sample values (dots) in the figures and were compared by using the nonparametric Mann-Whitney or Kruskall-Wallis tests. The log-rank test was used to compare mouse survival rate after challenge with T. cruzi. The differences were considered significant when the P value was <0.05.
RESULTS
Protective immunity of highly susceptible A/Sn mice after vaccination with replication-defective recombinant adenovirus type 5 expressing T. cruzi ASP-2.
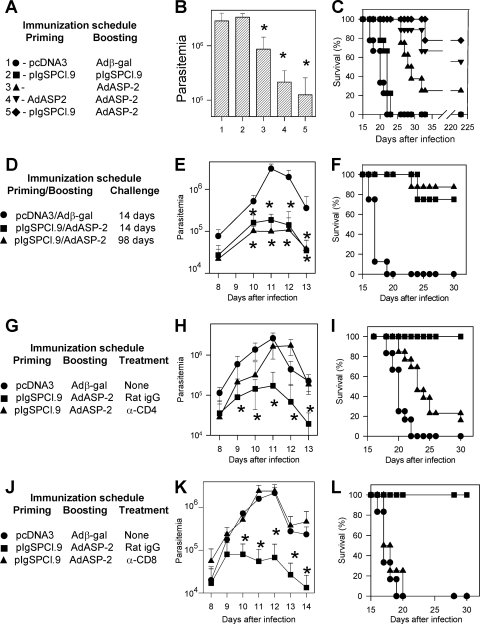
In earlier studies we observed that protective immunity, as measured by reduction in the peak parasitemia and delayed mouse mortality, required three doses of plasmid DNA or recombinant protein (6, 17, 19). To determine whether protective immunity could be elicited by two immunizing doses, we compared different protocols of vaccination using either homologous or heterologous prime-boosting regimens. The homologous protocols consisted of two immunizing doses of either plasmid DNA (pIgSPCl.9) or a recombinant replication-defective adenovirus type 5 (AdASP-2) containing the gene encoding ASP-2 of T. cruzi (6, 19, 60). The heterologous immunization regimen consisted of a priming immunization with pIgSPCl.9 followed by a booster injection of the AdASP-2. Simultaneously, a group of mice was injected with a single immunizing dose of AdASP-2.
Immunized mice were challenged with a lethal dose of T. cruzi bloodstream trypomastigotes after a short (14-day) or a long (98-day) period after the final immunizing dose. As shown in Fig. 1B, vaccination with two doses of pIgSPCl.9 failed to reduce the magnitude of the peak parasitemia (day 11) compared to control mice injected with pcDNA3 followed by recombinant Adβ-gal. Mice immunized with one or two doses of AdASP-2 displayed a peak parasitemia 3.34- or 13.61-fold lower than that of control mice (n = 6, P < 0.01 in both cases). The best reduction of the peak parasitemia was observed in the group of mice vaccinated with the heterologous DNA prime-recombinant adenovirus boost regimen (22.88-fold reduction compared to controls, n = 6, P < 0.01).
FIG. 1.
Trypomastigote-induced parasitemia and mortality in A/Sn mice immunized with different combinations of plasmid DNA and/or replication defective human adenovirus type 5 expressing the ASP-2 of T. cruzi. (A) A/Sn mice were immunized as described. Priming and boosting immunizations were performed with the indicated plasmid or adenovirus i.m. at 0 and 3 weeks, respectively. Two weeks after the final immunizing dose, mice were challenged i.p. with 150 bloodstream trypomastigotes. (B) The peak parasitemia for each mouse group is represented as the mean ± the standard deviation (SD) (n = 6). Asterisks denote that the parasitemia of mice from groups 3, 4, and 5 was significantly lower than that of mice from groups 1 and 2 (P < 0.01 in all cases). Mice from groups 4 and 5 had a significantly lower parasitemia than mice from group 3 (P < 0.05). (C) The graph shows the Kaplan-Meier curves for survival of the mice groups immunized and challenged as described above (n = 9). Pooled results from two experiments are shown. Mice from group 3 survived significantly longer than animals from group 1 and 2 (P < 0.0001 in both cases). Mice from groups 4 and 5 survived significantly longer than the other groups (P < 0.0001 in all cases). (D) A/Sn mice were immunized as described. Two weeks or 98 days after the final immunizing dose, mice were challenged i.p. with 150 bloodstream trypomastigotes. (E) The parasitemia for each mouse group is represented as the mean ± the SD (n = 6). Asterisks indicate that ASP-2-vaccinated mice had a significantly lower parasitemia (P < 0.01) than nonimmune animals. (F) The graph shows the Kaplan-Meier curves for survival of the mouse groups immunized and challenged as described above (n = 6). Mice from groups vaccinated with ASP-2 survived significantly longer than nonimmune control animals (P < 0.001). (G) A/Sn mice were immunized as described. Before and after challenge i.p. with 150 bloodstream trypomastigotes, mice were treated as described in Materials and Methods with rat IgG or anti-CD4 MAb. (H) The parasitemia for each mouse group is represented as the mean ± the SD (n = 12). Asterisks indicate that ASP-2-vaccinated mice treated with rat IgG had a significantly lower parasitemia (P < 0.01) than nonimmune animals or ASP-2-vaccinated mice treated with anti-CD4 MAb. (I) The graph shows the Kaplan-Meier curves for survival of mouse groups immunized and challenged as described above (n = 12). ASP-2-immunized mice treated with rat IgG survived significantly longer than nonimmune animals or ASP-2-immunized mice treated with anti-CD4 MAb (P < 0.0001 in both cases). ASP-2-immunized mice treated with anti-CD4 MAb survived longer than nonimmune animals. The results are pooled from two independent experiments. (J) A/Sn mice were immunized as described. Before and after challenge i.p. with 150 bloodstream trypomastigotes, mice were treated as described in Materials and Methods with rat IgG or anti-CD8 MAb. (K) The parasitemia for each mouse group is represented as the mean ± the SD (n = 6). Asterisks indicate that ASP-2-immunized mice treated with rat IgG had a significantly lower parasitemia (P < 0.01) than nonimmune animals or ASP-2-vaccinated mice treated with anti-CD8 MAb. (L) The graph shows the Kaplan-Meier curves for survival of mouse groups immunized and challenged as described above (n = 8 to 9). ASP-2-immunized mice treated with rat IgG survived significantly longer (P < 0.0001) than nonimmune animals or ASP-2-immunized mice treated with anti-CD8 MAb. The results are representative of two independent experiments.
When mouse survival was tracked after challenge (as shown on Fig. 1C), the results from the parasitemia were mainly confirmed. All three mouse groups immunized with AdASP-2 survived longer than control animals or mice immunized with two doses of pIgSPCl.9. However, mouse survival differed significantly among groups of mice immunized with two doses of AdASP-2 compared to animals injected only once (P < 0.001). Protective immunity in both groups of mice boosted with the recombinant AdASP-2 was high. No significant difference was observed among the mouse groups primed with pIgSPCl.9 or AdASP-2 (P > 0.05). Based on these results, we selected the heterologous prime-boost vaccination regimen to further research the protective cells.
Protective immunity elicited by the heterologous prime-boost regimen (pIgSPCl.9/AdASP-2) was stable at least until 98 days after the final immunizing dose. In ASP-2-immunized mice challenged 14 or 98 days after the final boost vaccination, the values of parasitemia were 16.70- or 31.41-fold lower than control mice, respectively (Fig. 1E, P < 0.01 in both cases). However, no difference was recorded during the peak parasitemia between the two groups of ASP-2-immunized mice (P > 0.05). Similarly, ASP-2-vaccinated mice challenged 14 or 98 days after infection survived longer than control animals (Fig. 1F, P < 0.001 in both cases). In addition, the mortality rates of the mice vaccinated with ASP-2 were not statistically different (P > 0.05).
To determine whether this protective immunity was dependent on CD4+ or CD8+ T cells, A/Sn mice vaccinated with the heterologous prime-boost regimen (pIgSPCl.9/AdASP-2) were treated with anti-CD4 or CD8 MAb prior to challenge with bloodstream trypomastigotes. Treatment with anti-CD4 MAb renders these mice more susceptible to infection. CD4-depleted mice presented parasitemia similar to control mice injected with pcDNA3/Adβ-gal (Fig. 1H). Although their mortality rate was high (80%), they lived longer than control mice (Fig. 1I, P < 0.001).
Treatment with anti-CD8 MAb renders these mice completely susceptible to infection. CD8-depleted mice presented parasitemia and survival rates similar to those of control mice injected with pcDNA3/Adβ-gal (Fig. 1K and L). In contrast, A/Sn mice vaccinated with a heterologous prime-boost regimen (pIgSPCl.9/AdASP-2) and treated with rat IgG had significantly lower parasitemia and survived the otherwise lethal challenge with T. cruzi.
The blood of AdASP-2-immunized animals were collected and added to hemocultures. Parasites did not grow in any of the samples, indicating a very low-grade parasitemia or the absence of parasites. PCR analysis revealed that in fact some mice had detectable parasite DNA. Comparing to known amounts of parasite DNA equivalents, the DNA detected ranged from <12.5 to 50 per ml of blood (n = 7, t = 150 days [data not shown]).
Perforin expression is required for maximal protective immunity following a heterologous prime-boost vaccination regimen in C57BL/6 mice.
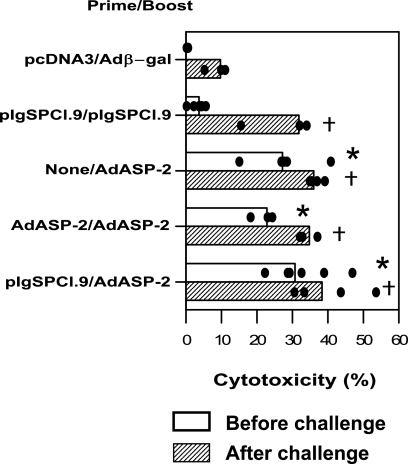
In order to determine the presence of ASP-2-specific cytotoxic T cells after immunization with the different protocols, we performed an in vivo cytotoxicity assay (57). For that reason, we used target cells coated with the peptide TEWETGQI, which we had previously identified as an immunodominant H-2Kk-restricted CD8 T-cell epitope (6, 19, 57, 58). The assay was performed in immunized A/Sn before or 13 days after challenge with bloodstream trypomastigotes. As shown in Fig. 2, groups of mice immunized with recombinant AdASP-2 presented in vivo cytotoxicity levels of >20% before challenge. In contrast, immunization with two doses of pIgSPCl.9 failed to elicit a significant degree of in vivo cytotoxicity.
FIG. 2.
In vivo cytotoxic activity against target cells coated with a peptide representing the CD8 epitope in mice immunized with different protocols before or after challenge with T. cruzi. A/Sn mice were injected with the different immunization protocols depicted at the side of the charter. The immunizing doses were injected i.m. at 0 and 3 weeks. Two weeks after the final immunizing dose, half of the mice were injected with splenic syngeneic cells labeled with CFSE and coated with peptide TEWETGQI. The remaining half of the animals was challenged i.p. with 150 blood forms of T. cruzi. Thirteen days later, these mice were also injected with splenic syngeneic cells labeled with CFSE and coated with the peptide TEWETGQI. The specific in vivo cytotoxic activity was estimated after 20 h as described in Materials and Methods. Results are expressed as medians (bars), and each dot represents an individual mouse. Symbols denote significantly higher (P < 0.01) specific in vivo cytotoxic activity compared to control naive or pcDNA-3/Adβ-gal injected mice prior to infection (asterisks) or after a challenge with T. cruzi (crosses). The results are representative of experiments performed twice with similar results.
After challenge with trypomastigotes, all mice groups immunized with the asp-2 gene (plasmid or adenovirus) showed a significant degree of in vivo cytotoxicity compared to control animals injected with pcDNA3/Adβ-gal or naive mice. Animals that received two doses of pIgSPCl.9 had slightly lower levels of in vivo cytotoxicity, although these did not vary significantly from the levels of the other groups.
These experiments were also performed in C57BL/6 mice because we planned to use genetically modified mice in subsequent experiments. In this mouse strain, we used target cells coated with the immunodominant H-2Kb-restricted epitope VNHRFTLV (57-59). The results essentially confirmed the observations described above except that the levels of the in vivo cytotoxicity were higher for this epitope (data not shown).
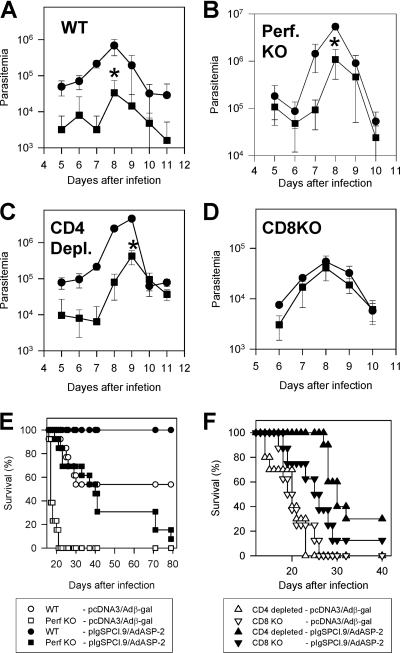
The fact that mice immunized with recombinant AdASP-2 showed the highest levels of in vivo cytotoxicity prior to challenge and some degree of protection (Fig. 1B and C) led us to pursue our second goal: whether the perforin could be critical to immunity elicited by vaccination.
In our earlier studies, we had observed that the in vivo cytotoxicity against the ASP-2-derived epitope (VNHRFTLV) was dependent on the presence of perforin since T. cruzi-infected or adenovirus-immunized perforin KO mice failed to eliminate peptide-coated cells in vivo (59; unpublished results). Based on these observations, we performed protective immunity experiments following heterologous prime-boost (pIgSPCl.9/AdASP-2) vaccination using perforin KO mice. Because these mice have a C57BL/6 genetic background, we had to change our experimental model to C57BL/6 WT mice. After the challenge with bloodstream trypomastigotes, ASP-2-vaccinated C57BL/6 WT mice had significantly lower peak parasitemia (20.47-fold reduction) than control mice injected with pcDNA3/Adβ-gal (Fig. 3A, P < 0.001). All of the C57BL/6 WT ASP-2-vaccinated mice survived, whereas 46% of the control mice died (Fig. 3E, P < 0.0001). After the challenge with trypomastigotes, the ASP-2-vaccinated perforin KO mice animals still had a significantly lower peak parasitemia (4.93-fold reduction) than control mice injected with pcDNA3/Adβ-gal (Fig. 3B, P < 0.001). Although vaccinated perforin KO mice survived longer than the control perforin KO mice (P < 0.0001), 92.3% of the challenged mice died (Fig. 3E). C57BL/6 WT ASP-2-vaccinated mice controlled better the peak parasitemia and survived longer than vaccinated perforin KO mice (P < 0.0001). These results indicated that perforin KO mice are capable of developing some degree of protective immunity, if assessed in terms of the reduction of peak parasitemia and delayed mortality. However, it confirmed our hypothesis that perforin expression is indeed critical for full protective immunity and mouse survival following heterologous prime-boost vaccination with pIgSPCl.9/AdASP-2.
FIG. 3.
Trypomastigote-induced parasitemia and mortality in C57BL/6 WT, perforin KO, CD4-depleted, or CD8 KO mice immunized with the heterologous prime-boost regimen. C57BL/6 WT, perforin KO, CD4-depleted, or CD8 KO mice were immunized with pIgSPCl.9, followed by AdASP-2 both injected i.m., at 0 and 3 weeks, respectively. Control mice were injected with pcDNA3, followed by Adβ-gal both injected i.m., at 0 and 3. Two weeks after the last immunizing dose, mice were challenged i.p. with 104 bloodstream trypomastigotes. (A to D) The parasitemia for each mouse group is represented as the mean ± the SD (n = 5 to 8). Asterisks denote that parasitemia was significantly lower (P < 0.01) for ASP-2-vaccinated mice (▪) than for controls nonimmune mice (•). The results are representative of experiments performed twice with similar results. (E and F) The graphs show the Kaplan-Meier curves for survival of the mouse groups immunized and challenged as described above (A to D). For C57BL/6 WT and perforin KO mice, the total number of animals was 13. In the case of CD4-depleted or CD8 KO mice, the number of animals was 10 or 8, respectively. Statistical analysis showed that (i) ASP-2-vaccinated C57BL/6 WT mice survived longer than all of the other mouse groups (P < 0.0001 in all cases), (ii) ASP-2-vaccinated perforin KO mice survived longer than control nonimmune perforin KO mice or CD8 KO mice (P < 0.001 in both cases), (iii) ASP-2-vaccinated CD4-depleted mice survived longer than control nonimmune CD4-depleted mice (P < 0.001, and (iv) ASP-2-vaccinated CD8 KO mice survived longer than control nonimmune CD8 KO mice (P < 0.01).
To determine the participation of CD4+ and CD8+ T cells in this mouse model, we performed CD4 depletion or used CD8KO mice. We observed that animals vaccinated with pIgSPCl.9/AdASP-2 and depleted of CD4 T cells prior to infection still had a significantly lower peak parasitemia (10.94-fold reduction) compared to control animals injected with pcDNA3/Adβ-gal (Fig. 3C). However, only 30% of ASP-2-vaccinated CD4-depleted mice survived, whereas 100% of the control CD4-depleted animals died (Fig. 3F, P < 0.001).
CD8 KO mice vaccinated with ASP-2 did not show any specific reduction in the peak parasitemia (1.32-fold reduction) compared to control mice injected with pcDNA3/Adβ-gal (Fig. 3D). Although ASP-2-vaccinated CD8 KO mice survived longer than control CD8 KO mice (P < 0.05), 87.5% of the mice died (Fig. 3F). Compared to the CD4-depleted vaccinated mice, these mice were more susceptible (P = 0.001), suggesting that CD8+ T cells are a slightly more important during the effector phase.
Characterization of the cell-mediated immune responses in immunized C57BL/6 WT and perforin KO mice.
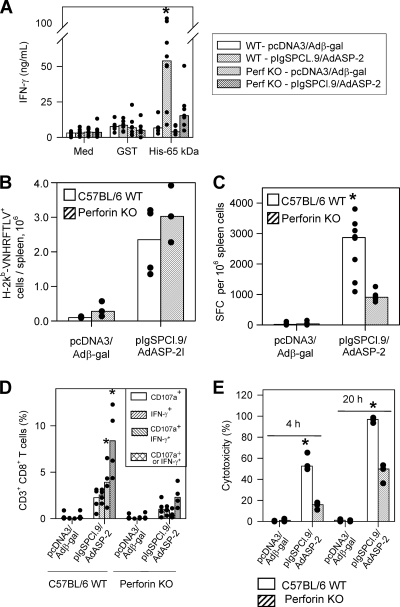
The fact that vaccinated perforin KO mice were highly susceptible to infection prompted us to further analyze the cell-mediated immune responses. When restimulated in vitro with the recombinant protein His-65 kDa (10), splenic cells from vaccinated WT mice secreted significantly higher amounts of IFN-γ than the perforin KO animals (Fig. 4A). This cytokine secretion upon stimulation with the soluble antigen reflects activation of CD4+ T cells because the presence of anti-CD4 MAb in culture inhibited IFN-γ secretion by ∼80% (reference 10 and data not shown).
FIG. 4.
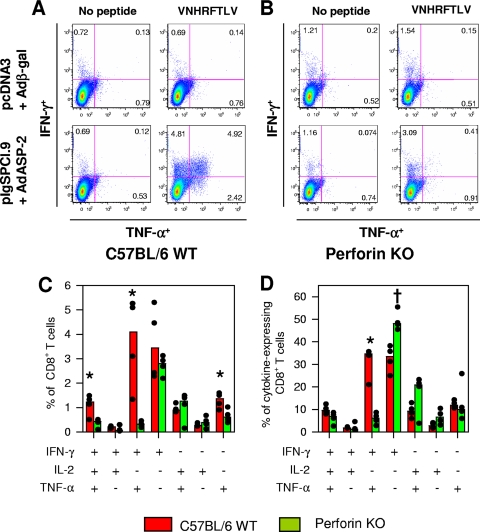
Cell mediated response of C57BL/6 WT or perforin KO mice after immunization with the heterologous prime-boost vaccination regimen. C57BL/6 WT or perforin KO mice were immunized as described in the legend of Fig. 3. Two weeks after the final immunizing dose, splenic cells were restimulated in vitro in the presence of medium (Med), recombinant glutathione S-transferase (GST; 10 μg/ml) or recombinant ASP-2 (His-65 kDa; 10 μg/ml [A]). When we compared all mouse groups, ASP-2-immunized WT spleens cells secreted more IFN-γ upon recombinant protein stimulation (asterisk, P < 0.05 in all cases). We estimated the frequency of specific splenic cells by staining with anti-CD8 and the multimer H-2Kb/VNHRFTLV (B) or the number of splenic IFN-γ spot-forming cells (SFC) by the ex vivo ELISPOT assay (C). When we compared groups of mice immunized with pIgSPCl.9/AdASP-2, immunized C57BL/6 WT mice had a higher frequency of SFC than the immunized perforin KO mice (asterisk, P < 0.001). Alternatively, these mice had their splenic cells cultured in the presence of anti-CD28 and FITC-labeled anti-CD107a, with or without the peptide VNHRFTLV. (D) After 12 h, cells were stained with APC-labeled anti-CD8, fixed, permeabilized, and stained with APC-Cy7-labeled anti-CD3, PE-Cy7-labeled anti-IFN-γ. When we compared groups of mice immunized with pIgSPCl.9/AdASP-2, immunized C57BL/6 WT mice had a higher frequency of CD3+CD8+ T cells expressing either IFN-γ or double-stained for CD107a/IFN-γ than the immunized perforin KO mice (asterisks, P < 0.01 in all cases). Finally, we estimated the in vivo cytotoxic activity by injecting immunized mice with CFSE-labeled splenic cells labeled with CFSE and coated with the peptide VNHRFTLV. (E) After 4 or 20 h, the in vivo cytotoxic activity was determined. When we compared groups of mice immunized with pIgSPCl.9/AdASP-2, immunized C57BL/6 WT mice displayed significantly higher in vivo cytotoxicity than perforin KO mice (asterisk, P < 0.01 in both cases). The results are expressed as medians (bars) and each individual mouse (dots) and are representative of experiments performed at least twice with similar results.
We then characterized several functional and phenotypic aspects of the specific CD8+ T cells. Staining of the specific CD8+ T cells with the multimer H-2Kb/VNHRFTLV showed that the frequency and total amounts of these cells in the spleen were the same for perforin KO and C57BL/6 WT mice vaccinated with the heterologous prime boost regimen (Fig. 4B).
Because specific CD8+ T cells secreted IFN-γ and granule-associated proteins upon ex vivo stimulation with peptides, we performed an ELISPOT assay and stained the splenic T cells with antibodies to CD3, CD8, CD107a, and IFN-γ after in vitro stimulation with peptide. The frequency of splenic IFN-γ-producing cells was 2.77 times higher in ASP-2-vaccinated C57BL/6 WT than in perforin KO mice (Fig. 4C, P < 0.001).
By FACS analysis, we found three populations of antigen-reactive CD3+ CD8+ T cells. These cells were positive for CD107a (a marker for T-cell degranulation and cytotoxicity [3]) or IFN-γ or both. The comparison of the amount of CD3+CD8+ T cells between vaccinated C57BL/6 WT and perforin KO mice showed a significantly higher number of cells expressing both markers or IFN-γ only in the first group (Fig. 4D, P < 0.05). This difference was also observed in CD3+ CD8+ T cells when we considered the total frequency of CD107a+ expressing or not IFN-γ (P < 0.01) but not CD107a only (P > 0.05). Considering that the total number of CD3+ CD8+ T cells stained with the multimer H-2Kb/VNHRFTLV were similar, we concluded that the generation and/or maturation of antigen-specific CD3+CD8+ T cells of perforin KO mice expressing both markers or IFN-γ+ was selectively impaired.
Similar results were observed when we estimated the in vivo cytotoxic activity against target cells coated with peptide VNHRFTLV. C57BL/6 WT mice displayed higher cytotoxic activity (two times or more) than vaccinated perforin KO mice (Fig. 4E, P < 0.01). This difference was slightly higher in short-term elimination (4 h) than in long-term elimination (20 h).
Subsequently, we used ICS to evaluate the cytokines expressed by specific CD8+ T cells after in vitro peptide stimulation. The staining of CD3+ CD8+ splenic cells stimulated with peptide in some cases was performed simultaneously with antibodies to IFN-γ, TNF-α, and IL-2. These cytokines are regularly used to identify multifunctional T cells (28). The number of peptide-specific T cells expressing any of these cytokines was higher in ASP-2-immunized C57BL/6 WT mice than in perforin KO mice. Most cytokine-producing CD3+ CD8+ T cells from ASP-2 immune C57BL/6 WT mice were stained for IFN-γ (∼87%) or TNF-α (58%) or both markers (∼45%). The frequency of CD3+ CD8+ T cells stained for IL-2 was only ∼7% and, for triple-stained cells, the frequency was less than ∼5% (Fig. 5A, B, and C). The frequency of CD3+ CD8+ T cells stained for either IFN-γ or TNF-α was reduced in splenic cells from ASP-2-vaccinated perforin KO mice compared to C57BL/6 WT (Fig. 5A to D, P < 0.05). However, the most significant difference was observed in the frequency of CD3+ CD8+ T cells simultaneously expressing IFN-γ and TNF-α after peptide stimulation. ASP-2 immune C57BL/6 WT mice had 19-fold more double-positive CD8+ T cells than perforin KO mice (P < 0.005). The frequency in the total of responder cells was significantly higher (a fivefold increase) in ASP-2 immune C57BL/6 WT mice than in perforin KO mice (P < 0.001). Again, considering that the total numbers of CD3+ CD8+ T cells stained with the multimer H-2Kb-VNHRFTLV were similar, we concluded that the generation and/or maturation of antigen-specific CD3+CD8+ T cells was severely impaired among ASP-2 immune perforin KO mice expressing either IFN-γ or TNF-α or both cytokines simultaneously. Comparison of the mean fluorescence intensities of the different cytokines with ICS failed to detect significant differences between the distinct CD8+ T cells subpopulations or between WT and KO mice (data not shown).
FIG. 5.
ICS of CD8 T cells from C57BL/6 WT or perforin KO mice immunized with the heterologous prime-boost vaccination regimen. C57BL/6 WT or perforin KO mice were immunized with pcDNA3/Adβ-gal or pIgSPCl.9/AdASP-2. Fourteen days after the final immunizing dose, these mice had their splenic cells cultured in the presence of anti-CD28 and brefeldin A, with or without the peptide VNHRFTLV. After 12 h, cells were stained with APC-labeled anti-CD8, fixed, permeabilized, and stained with APC-Cy7-labeled anti-CD3, PE-Cy7-labeled anti-IFN-γ, and Alexa 488-labeled anti-TNF-α. (A and B) Examples of splenic CD3+ CD8+ cells from immunized C57BL/6 WT or perforin KO mice stained for IFN-γ and TNF-α. (C) Frequency of each cell population. Asterisks indicate that C57BL/6 WT mice immunized with IgSPCl.9/AdASP-2 had a higher frequency of CD3+ CD8+ T cells expressing either IFN-γ or TNF-α or both cytokines than perforin KO mice immunized with pIgSPCl.9/AdASP-2 (P ≤ 0.01 in all cases). (D) We calculate the frequency of each cell population in relation to the total amount of cells expressing any cytokine. An asterisk indicates that C57BL/6 WT mice immunized with of pIgSPCl.9/AdASP-2 had higher frequencies of CD3+ CD8+ T cells expressing both IFN-γ and TNF-α than perforin KO mice immunized with pIgSPCl.9/AdASP-2 (P < 0.01 in all cases). A cross indicates that perforin KO mice immunized with IgSPCl.9/AdASP-2 had a higher frequency of CD3+ CD8+ T cells expressing only IFN-γ than C57BL/6 WT mice immunized with pIgSPCl.9/AdASP-2. The results are presented as medians (bars) and each individual mouse (dots) and are representative of experiments performed twice with similar results.
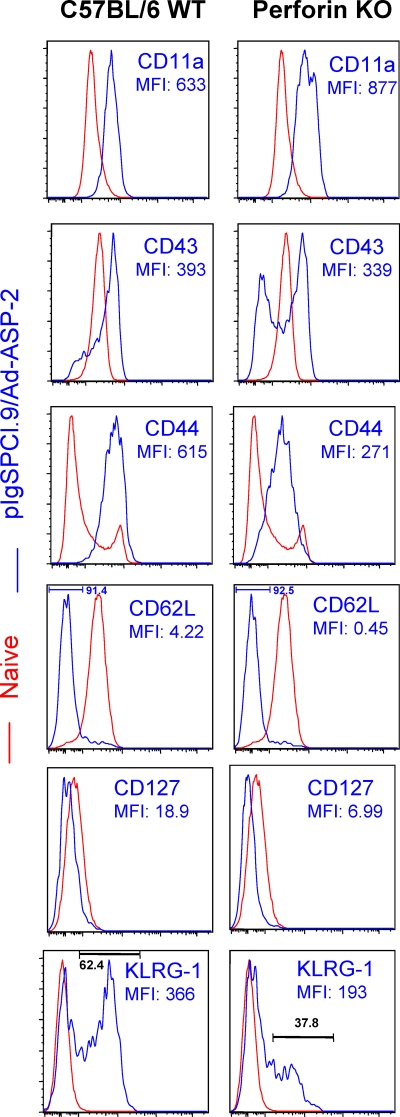
Finally, we compared the surface markers expressed on protective ASP-2-specific splenic CD8+ T lymphocytes elicited by the heterologous prime-boost regimen (pIgSPCl.9/AdASP-2). For that purpose, purified CD8+ T cells obtained from immune mice were triple stained with anti-CD8, multimer H-2Kb/VNHRFTLV, and one of several different activation markers. The fluorescence intensity was compared to the expression of these same activation markers on naive CD8+ T cells. As shown in Fig. 6, the phenotype of double-stained CD8+ H-2Kb/VNHRFTLV+ cells from immune C57BL/6 WT animals was CD11ahigh CD43high CD44high CD62Llow CD127low. The activation marker KLRG-1 reproducibly stained part of the cells (∼60%). On the other hand, ∼40% of the antigen-specific CD8+ T cells did not express this marker on the surface.
FIG. 6.
Phenotypic characterization of splenic specific CD8 T cells induced by immunization of C57BL/6 WT or perforin KO mice with the heterologous prime-boost vaccination regimen. C57BL/6 WT or perforin KO mice were immunized with pIgSPCl.9/AdASP-2. Fourteen days after the final immunizing dose, these mice had their CD8+ splenic cells purified and stained with APC-labeled anti-CD8, biotin-labeled H-2Kb-VNHRFTLV, and the indicated marker-specific antibody labeled with FITC prior to analysis by FACS. The histograms show the expression of the markers on CD8+ H-2Kb-VNHRFTLV+ T cells (blue lines) or control naive CD8+ spleen cells (red lines). Representative analyses from three or more mice studied are shown.
The pattern of expression of these surface markers on CD8+ specific T cells from ASP-2 immune perforin KO mice was generally similar. Only two significant differences were reproducibly noted: (i) the mean intensity fluorescence of CD44 was lower on epitope-specific CD8+ T cells from immune perforin KO mice, and (ii) the frequency of specific CD8+ T cells from immune perforin KO mice expressing the activation marker KLRG-1 was lower. The frequencies of specific CD8+ T expressing KLRG-1 marker in individual mice were 56.12 ± 11.84 and 31.67 ± 14.01%, respectively, in ASP-2 immune C57BL/6 WT mice and perforin KO mice (n = 4, P < 0.05).
IFN-γ KO mice immunized with a heterologous prime-boost vaccination regimen develop specific in vivo cytotoxicity but are highly susceptible to infection.
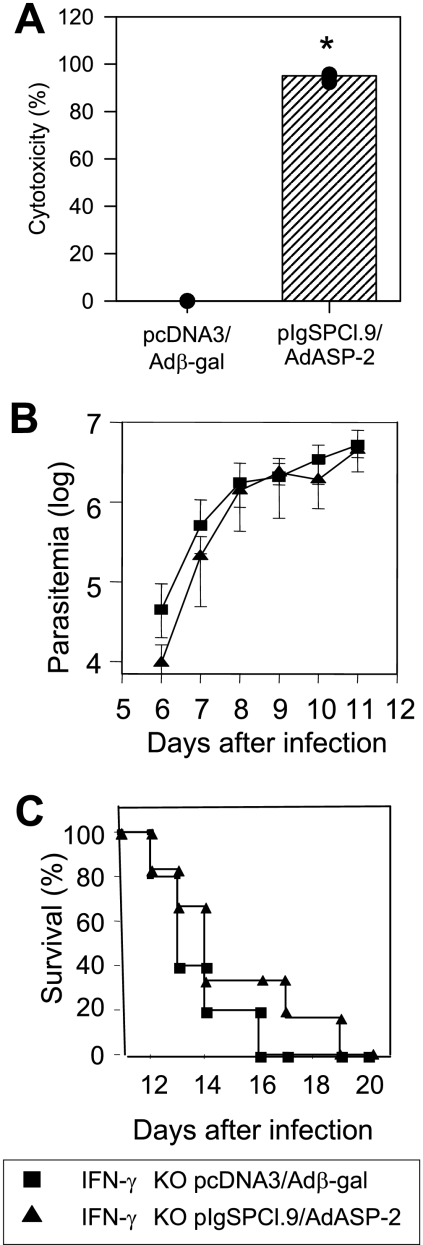
Our final goal was to determine whether specific cytotoxicity mediated by CD8+ T cells could be elicited by the heterologous prime-boost vaccination regimen in the absence of IFN-γ. If so, what would the impact of the absence of this critical mediator of adaptive immunity be on the protective immunity of vaccinated C57BL/6? ASP-2-vaccinated IFN-γ KO mice developed high levels of in vivo cytotoxicity (>95%, Fig. 7A). After challenge with bloodstream trypomastigotes, vaccinated IFN-γ KO mice had a parasitemia and a survival rate similar to that of control IFN-γ KO mice injected with pcDNA3/Adβ-gal (Fig. 7B and C, respectively). All vaccinated and control mice succumbed to challenge before the 20th day after challenge. These results demonstrated that, in the absence of IFN-γ, specific cytotoxicity mediated by CD8+ T cells was not sufficient to retard or reduce the parasitemia. Also, it did not delay mouse mortality, suggesting an interdependence between perforin and IFN-γ during protective immunity.
FIG. 7.
In vivo cytotoxic activity against target cells coated with peptides representing the CD8 epitope and trypomastigote-induced parasitemia and mortality of IFN-γ KO mice immunized with heterologous prime-boost regimen. IFN-γ KO mice were immunized as described in the legend of Fig. 3. (A) Two weeks after the final immunizing dose, mice were injected with splenic syngeneic cells labeled with CFSE and coated with the peptide VNHRFTLV. The specific in vivo cytotoxic activity was estimated after 20 h as described in Materials and Methods. The results are expressed as medians (bars) and each individual mouse (dots, n = 3). An asterisk denotes significantly higher (P < 0.01) specific in vivo cytotoxic activity of ASP-2-immunized IFN-γ KO mice than control pcDNA-3/Adβ-gal-injected mice. (B) Two weeks after the final immunizing dose, mice were challenged i.p. with 104 bloodstream trypomastigotes. The parasitemia for each mouse group is represented as mean ± the SD (n = 6). (C) The graph shows the Kaplan-Meier curves for survival of the mice groups immunized and challenged as described above (n = 6). These experiments were performed twice with similar results.
DISCUSSION
As the initial aim of our study, we established an effective protocol of genetic vaccination with a heterologous prime-boost regimen using plasmid DNA and recombinant replication defective adenovirus both expressing the ASP-2 antigen of the human protozoan parasite Trypanosoma cruzi. This protocol was an improvement over others described earlier when we used at least three doses of either plasmid DNA or recombinant protein to achieve significant protective immunity (6, 17, 19, 60). The heterologous prime-boost vaccination was more effective than two doses of plasmid DNA or a single dose of recombinant adenovirus.
There was no significant improvement noted in the comparison of the heterologous prime-boost regimen with two doses of recombinant adenovirus, since both were highly effective in our model. Nevertheless, this regimen may have a number of advantages for the long-term development of genetic recombinant vaccines providing a simple solution to the problem of widespread immunity to the human adenoviral vector type 5 (1). Priming immunization with plasmid DNA seems to be sufficient for the subsequent expansion of the trans-gene specific CD8+ T cells in recombinant adenovirus-boosted animals (9). This expansion occurs even in animals with previous immunity to human adenovirus 5.
Hemocultures from these vaccinated mice were all negative denoting either very low or absent parasitemia. This result is an improvement compared to earlier studies in which we always observed parasites in hemocultures among a certain percentage of the vaccinated A/Sn mice (6, 60). Our results from this initial part of the study thus confirmed the findings and applied earlier studies of vaccination against experimental simian immunodeficiency virus and malaria infection to a human pathogen (2, 13, 14, 26, 33, 41).
Both CD4+ and CD8+ T cells accounted for nonredundant mechanisms of immunity since the depletion of each one prior to challenge renders the animals susceptible to infection. In vaccinated mice without CD8+ T cells, we observed a complete lack of control of parasitemia in both mouse strains (A/Sn and C57BL/6). Also, no delay in mouse mortality was seen in A/Sn mice, denoting a critical role for this subpopulation during immunity. In vaccinated C57BL/6 mice, we observed a slight delay in mouse mortality, suggesting the presence of effector non-CD8 immune cells. Whether they are in fact CD4+ remains to be investigated.
In CD4+ T-cell-depleted mice we still observed a significant delay in mouse mortality compared to nonimmune controls compatible with a CD8+ T-cel-mediated immunity. However, the control of parasitemia was significantly better in C57BL/6 mice. Finally, the fact that CD4+ and CD8+ T cells interact during the memory immune responses makes it more difficult to evaluate precisely the effector and/or helper function of CD4+ T cells during a complex process such as protective immunity in different experimental models. For that purpose more complex experimental systems will have to be developed.
In the second part of our study, we characterized the importance of perforin during protective immunity elicited by DNA prime adenovirus-boost vaccination. We observed that ASP-2-vaccinated perforin KO mice developed only limited immunity to the infection that did not prevent death.
Detailed analysis of the specific immune response revealed an impaired IFN-γ secretion by immune spleen cells. Although perforin deficiency did not impair the expansion of splenic specific CD8+ T cells, these cells had a significantly lower frequency of specific CD107a+/IFN-γ+ or IFN-γ+/TNF-α+ cells after in vitro restimulation. Also, the in vivo cytotoxicity was reproducibly reduced. Nevertheless, it is noteworthy that the in vivo cytotoxicity was present at certain levels in the perforin KO mice, indicating the presence of a perforin-independent mechanism(s) of lysis (TNF-α, FasL, etc.) yet to be identified. Finally, the pattern of expression of certain surface markers on CD8+ specific T cells from immune perforin KO mice were significantly different (CD44 and KLRG-1).
Earlier studies on the characteristics of specific CD8+ T cells in perforin-deficient mice indicated a dual function for this molecule during the homeostasis and effector phases of the immune response. In different reports (including ours), no significant modification was observed in the expansion of specific CD8+ T cells as estimated by the multimer staining (5, 15, 16). In contrast, an increase in the number of peptide-specific cytokine-expressing CD8+ T cells was noted among these KO mice in certain studies (7, 64). Those authors provided evidence that perforin-mediated lyses of antigen-presenting cells could account for a restriction in the expansion of specific CD8+ T cells (64). Equally important, it was also noted that during certain chronic viral infection, perforin-mediated downregulation of T-cell responses is critical to avoiding autoimmunity and immune-pathological damages (40). To date, the most predictable immunological impairment of the perforin KO mice has been the reduced level of cytotoxicity (in vivo or in vitro) reported by distinct groups, including ours (8, 15, 32, 59).
Based on other experimental human parasitic infections, it was not possible to predict that perforin expression would be in fact critical to the protective immunity against T. cruzi infection observed after DNA-prime adenovirus-boost vaccination. Compared to C57BL/6 WT mice, perforin KO mice are not more susceptible to infections with the intracellular protozoan parasites Toxoplasma gondii or Plasmodium berghei (20, 47). The fact that perforin-deficient CD8+ T cells efficiently eliminate liver stages of malaria parasites is very important (46). There is a single report stating that perforin deficiency abrogates protective immunity against Leishmania amazonensis infection elicited by vaccination with a recombinant protein (18). Therefore, we believe that the fact that perforin is critical to our system may help us to understand the role of this molecule in resistance to human parasitic infections.
The fact that perforin can be expressed in other cell types of the adaptive or innate immune system does not allow us to conclude that its expression on CD8+ T cells is the single restricting factor in our system. Other types of specific lymphocytes that may express perforin are CD4+ T cells (61, 63). We observed that in our vaccination strategy CD4+ T cells were important for T. cruzi immunity after infection of A/Sn and C57BL/6. CD8 KO mice were more susceptible than perforin KO mice, indicating the presence of a non-perforin-mediated mechanisms mediated by these cells.
Other cell types may play important roles in mice immunity against T. cruzi infection. Natural killer (NK) cells have been described as mediators of natural resistance to T. cruzi experimental infection. The depletion of NK cells by treatment with polyclonal anti-asialo GM1 renders animals more susceptible to infection (24). However, these cells are thought to act early, secreting IFN-γ (11, 52). A role for cytolysis mediated by perforin has been discarded during NK direct contact-mediated lysis of T. cruzi or T. cruzi-infected cells (35, 36). The role of NK cells in our system remains to be studied.
CD1d-restricted NKT cells also have been described as capable of improving the resistance to T. cruzi infection (22-24). Immunization of CD1d KO mice that fail to express NKT cells allowed us to test whether NKT cells are involved in immunity in our vaccination regimen. We found that following heterologous prime-boost immunization, these mice developed protective immunity similar to C57BL/6 WT mice (B. C. G. de Alencar and M. M. Rodrigues, unpublished data). Based on this result, these cells are clearly not involved in the development of protective immunity after vaccination. Finally, γδ T cells are also not clearly associated with protective immunity during experimental T. cruzi infection (12, 51).
Based on these observations and the fact that CD8+ T cells are critical for mouse survival after experimental vaccination and infection, we consider it plausible that these cells represent a major, but not the single, source of perforin in our system. Perforin mediates this function, allowing the full maturation of effector CD8+ T cells. The correlation between protective immunity and the presence of specific CD8+ T cells expressing CD107a+/IFN-γ+ or IFN-γ+/TNF-α+ simultaneously is important to defining the type of CD8+ T cells that should be generated during vaccination protocols. Up to now, vaccine studies aimed at determining immunity have relied heavily on the detection of the number of CD8+ T-cell using multimer staining or IFN-γ production (ELISPOT or ICS). However, as we established, these may not be the best criteria for determining the presence of immune protective CD8+ T cells. Although we can observe reproducible differences in the number of peptide-specific IFN-γ-producing cells by ELISPOT assay, we considered that the presence of double positive for IFN-γ/TNF-α or IFN-γ/CD107a was more accurate to estimate the protective immunity in our model of vaccination.
The final goal of our study was to define whether the in vivo cytotoxic activity prior to challenge would ensure protective immunity in the absence of IFN-γ, an important mediator of adaptive immunity during experimental T. cruzi infection. We concluded from experiments using IFN-γ KO mice that this cytokine is critical even in the presence of high levels of in vivo cytotoxicity. IFN-γ KO C57BL/6 mice were more susceptible than CD4-depleted or CD8 KO animals, indicating that IFN-γ most likely come from both sources.
A possible role for antibodies during the protective immunity response we observed is highly unlikely. ASP-2 is expressed only by intracellular amastigotes or it is not accessible to antibodies in the other forms of the parasite (10, 17). Also, immune sera or MAbs incubated with parasites are not able to neutralize their infectivity in vivo (M. M. Rodrigues, unpublished results).
In summary, we provide evidences that CD4+ and CD8+ T-cell mediated immunity elicited by the DNA-prime recombinant adenovirus-boost vaccination requires both perforin and IFN-γ. The implications are that in the case of T. cruzi infection, in addition to the number of specific cells (multimer staining) and IFN-γ production (ELISPOT assay), other parameters, such as the presence of double-positive IFN-γ/TNF-α or IFN-γ/CD107a or perforin-dependent cytotoxicity, might be crucial to determining whether immune protective T cells are present during infection in preclinical or clinical vaccination trials.
Acknowledgments
This study was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (2006/1983-4), the National Institute for Vaccine Technology (INCTV-CNPq), the Millennium Institute for Vaccine Development and Technology (CNPq-420067/2005-1), the Millennium Institute for Gene Therapy, and FAPEMIG (EDT 24.000) (Brazil). P.M.P., A.V.M., R.T.G., O.B.-R., J.L.-V., and M.M.R. are recipients of fellowships from CNPq. B.C.G.D.A. and F.A.H. are recipients of a fellowship from FAPESP.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 3 August 2009.
REFERENCES
- 1.Abbi, P., A. A. Lemckert, B. A. Ewald, D. M. Lynch, M. Denholtz, S. Smits, L. Holterman, I. Damen, R. Vogels, A. R. Thorner, K. L. O'Brien, A. Carville, K. G. Mansfield, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acierno, P. M., J. E. Schmitz, D. A. Gorgone, Y. Sun, S. Santra, M. S. Seaman, M. H. Newberg, J. R. Mascola, G. J. Nabel, D. Panicali, and N. L. Letvin. 2006. Preservation of functional virus-specific memory CD8+ T lymphocytes in vaccinated, simian human immunodeficiency virus-infected rhesus monkeys. J. Immunol. 176:5338-5345. [DOI] [PubMed] [Google Scholar]
- 3.Aktas, E., U. C. Kucuksezer, S. Bilgic, G. Erten, and G. Deniz. 2009. Relationship between CD107a expression and cytotoxic activity. Cell. Immunol. 254:149-154. [DOI] [PubMed] [Google Scholar]
- 4.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. L. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 5.Andrews, D. M., C. E. Andoniou, P. Fleming, M. J. Smyth, and M. A. Degli-Esposti. 2008. The early kinetics of cytomegalovirus-specific CD8+ T-cell responses are not affected by antigen load or the absence of perforin or gamma interferon. J. Virol. 82:4931-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araujo, A. F., B. C. de Alencar, J. R. Vasconcelos, M. I. Hiyane, C. R. Marinho, M. L. Penido, S. B. Boscardin, D. F. Hoft, and R. T. Gazzinelli, and M. M. Rodrigues. 2005. CD8+-T-cell-dependent control of Trypanosoma cruzi infection in a highly susceptible mouse strain after immunization with recombinant proteins based on amastigote surface protein 2. Infect. Immun. 73:6017-6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badovinac, V. P., A. R. Tvinnereim, and J. T. Harty. 2000. Regulation of antigen-specific CD8 T cell homeostasis by perforin and interferon-γ. Science 290:1354-1357. [DOI] [PubMed] [Google Scholar]
- 8.Barber, D. L., E. J. Wherry, and R. Ahmed. 2003. Cutting edge: rapid in vivo killing by memory CD8 T cells. J. Immunol. 171:27-31. [DOI] [PubMed] [Google Scholar]
- 9.Barouch, D. H., P. F. McKay, S. M. Sumida, S. Santra, S. S. Jackson, D. A. Gorgone, M. A. Lifton, B. K. Chakrabarti, L. Xu, G. J. Nabel, and N. L. Letvin. 2003. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines. J. Virol. 77:8729-8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boscardin, S. B., S. S. Kinoshita, A. E. Fujimura, and M. M. Rodrigues. 2003. Immunization with cDNA expressed by amastigotes of Trypanosoma cruzi elicits protective immune response against experimental infection. Infect. Immun. 71:2744-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardillo, F., J. C. Voltarelli, S. G. Reed, and J. S. Silva. 1996. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect. Immun. 64:128-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardillo, F., A. Nomizo, and J. Mengel. 1998. The role of the thymus in modulating γδ T-cell suppressor activity during experimental Trypanosoma cruzi infection. Int. Immunol. 10:107-116. [DOI] [PubMed] [Google Scholar]
- 13.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casimiro, D. R., F. Wang, W. A. Schleif, X. Liang, Z. Q. Zhang, T. W. Tobery, M. E. Davies, A. B. McDermott, D. H. O'Connor, A. Fridman, A. Bagchi, L. G. Tussey, A. J. Bett, A. C. Finnefrock, T. M. Fu, A. Tang, K. A. Wilson, M. Chen, H. C. Perry, G. J. Heidecker, D. C. Freed, A. Carella, K. S. Punt, K. J. Sykes, L. Huang, V. I. Ausensi, M. Bachinsky, U. Sadasivan-Nair, D. I. Watkins, E. A. Emini, and J. W. Shiver. 2005. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J. Virol. 79:15547-15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, J., H. C. Hsu, A. J. Zajac, Q. Wu, P. Yang, X. Xu, S. A. McPherson, J. Li, D. T. Curiel, and J. D. Mountz. 2006. In vivo analysis of adenovirus-specific cytotoxic T lymphocyte response in mice deficient in CD28, fas ligand, and perforin. Hum. Gene Ther. 17:669-682. [DOI] [PubMed] [Google Scholar]
- 16.Christensen, J. E., D. Wodarz, J. P. Christensen, and A. R. Thomsen. 2004. Perforin and IFN-γ do not significantly regulate the virus-specific CD8+ T-cell response in the absence of antiviral effector activity. Eur. J. Immunol. 34:1389-1394. [DOI] [PubMed] [Google Scholar]
- 17.Claser, C., N. M. Espíndola, G. Sasso, A. J. Vaz, S. B. Boscardin, and M. M. Rodrigues. 2007. Immunologically relevant strain polymorphism in the amastigote surface protein 2 of Trypanosoma cruzi. Microbes Infect. 9:1011-1019. [DOI] [PubMed] [Google Scholar]
- 18.Colmenares, M., P. E. Kima, E. Samoff, L. Soong, and D. McMahon-Pratt. 2003. Perforin and gamma interferon are critical CD8+ T-cell-mediated responses in vaccine-induced immunity against Leishmania amazonensis infection. Infect. Immun. 71:3172-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Alencar, B. C., A. F. Araújo, M. L. Penido, R. T. Gazzinelli, and M. M. Rodrigues. 2007. Cross-priming of long-lived protective CD8+ T cells against Trypanosoma cruzi infection: importance of a TLR9 agonist and CD4+ T cells. Vaccine 25:6018-6027. [DOI] [PubMed] [Google Scholar]
- 20.Denkers, E. Y., G. Yap, T. Scharton-Kersten, H. Charest, B. A. Butcher, P. Caspar, S. Heiny, and A. Sher. 1997. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J. Immunol. 159:1903-1908. [PubMed] [Google Scholar]
- 21.Dunachie, S. J., M. Walther, J. E. Epstein, S. Keating, T. Berthoud, L. Andrews, R. F. Andersen, P. Bejon, N. Goonetilleke, I. Poulton, D. P. Webster, G. Butcher, K. Watkins, R. E. Sinden, G. L. Levine, T. L. Richie, J. Schneider, D. Kaslow, S. C. Gilbert, D. J. Carucci, and A. V. Hill. 2006. DNA prime-modified vaccinia virus ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect. Immun. 74:5933-5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duthie, M. S., M. Kahn, M. White, R. P. Kapur, and S. J. Kahn. 2005. Critical proinflammatory and anti-inflammatory functions of different subsets of CD1d-restricted natural killer T cells during Trypanosoma cruzi infection. Infect. Immun. 73:181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duthie, M. S., M. Wleklinski-Lee, S. Smith, T. Nakayama, M. Taniguchi, and S. J. Kahn. 2002. During Trypanosoma cruzi infection CD1d-restricted NK T cells limit parasitemia and augment the antibody response to a glycophosphoinositol-modified surface protein. Infect. Immun. 70:36-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duthie, M. S., and S. J. Kahn. 2006. During acute Trypanosoma cruzi infection highly susceptible mice deficient in natural killer cells are protected by a single alpha-galactosylceramide treatment. Immunology 119:355-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garg, N., and R. Tarleton. 2002. Genetic immunization elicits antigen-specific protective immune responses and decreases disease severity in Trypanosoma cruzi infection. Infect. Immun. 70:5547-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert, S. C., J. Schneider, C. M. Hannan, J. T. Hu, M. Plebanski, R. Sinden, and A. V. Hill. 2002. Enhanced CD8 T-cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime-boost immunization regimes. Vaccine 20:1039-1045. [DOI] [PubMed] [Google Scholar]
- 27.Gómez, C. E., J. L. Nájera, M. Krupa, and M. Esteban. 2008. The poxvirus vectors MVA and NYVAC as gene delivery systems for vaccination against infectious diseases and cancer. Curr. Gene Ther. 8:97-120. [DOI] [PubMed] [Google Scholar]
- 28.Goonetilleke, N., S. Moore, L. Dally, N. Winstone, I. Cebere, A. Mahmoud, S. Pinheiro, G. Gillespie, D. Brown, V. Loach, J. Roberts, A. Guimaraes-Walker, P. Hayes, K. Loughran, C. Smith, J. De Bont, C. Verlinde, D. Vooijs, S. C. Schmidt, M. Boaz, J. Gilmour, P. Fast, L. Dorrell, T. Hanke, and A. J. McMichael. 2006. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J. Virol. 80:4717-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanke, T., N. Goonetilleke, A. J. McMichael, and L. Dorrell. 2007. Clinical experience with plasmid DNA- and modified vaccinia virus Ankara-vectored human immunodeficiency virus type 1 clade A vaccine focusing on T-cell induction. J. Gen. Virol. 88:1-12. [DOI] [PubMed] [Google Scholar]
- 30.Hoft, D. F., and C. S. Eickhoff. 2005. Type 1 immunity provides both optimal mucosal and systemic protection against a mucosally invasive, intracellular pathogen. Infect. Immun. 73:4934-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoft, D. F., C. S. Eickhoff, O. K. Giddings, J. R. Vasconcelos, and M. M. Rodrigues. 2007. Trans-sialidase recombinant protein mixed with CpG motif-containing oligodeoxynucleotide induces protective mucosal and systemic Trypanosoma cruzi immunity involving CD8+ CTL and B cell-mediated cross-priming. J. Immunol. 179:6889-6900. [DOI] [PubMed] [Google Scholar]
- 32.Kagi, D., B. Ledermann, K. Burki, P. Seiler, B. Odermatt, K. J. Olsen, E. R. Podack, R. M. Zinkernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369:31-37. [DOI] [PubMed] [Google Scholar]
- 33.Letvin, N. L., J. R. Mascola, Y. Sun, D. A. Gorgone, A. P. Buzby, L. Xu, Z. Y. Yang, B. Chakrabarti, S. S. Rao, J. E. Schmitz, D. C. Montefiori, B. R. Barker, F. L. Bookstein, and G. J. Nabel. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, S., M. Rodrigues, D. Rodriguez, J. R. Rodriguez, M. Esteban, P. Palese, R. S. Nussenzweig, and F. Zavala. 1993. Priming with recombinant influenza virus followed by administration of recombinant vaccinia virus induces CD8+ T-cell-mediated protective immunity against malaria. Proc. Natl. Acad. Sci. USA 90:5214-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lieke, T., S. E. Graefe, U. Klauenberg, B. Fleischer, and T. Jacobs. 2004. NK cells contribute to the control of Trypanosoma cruzi infection by killing free parasites by perforin-independent mechanisms. Infect. Immun. 72:6817-6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieke, T., C. Steeg, S. E. Graefe, B. Fleischer, and T. Jacobs. 2006. Interaction of natural killer cells with Trypanosoma cruzi-infected fibroblasts. Clin. Exp. Immunol. 145:357-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Machado, A. V., J. E. Cardoso, C. Claser, M. M. Rodrigues, R. T. Gazzinelli, and O. Bruna-Romero. 2006. Long-term protective immunity induced against Trypanosoma cruzi infection after vaccination with recombinant adenoviruses encoding amastigote surface protein-2 and trans-sialidase. Hum. Gene Ther. 17:898-908. [DOI] [PubMed] [Google Scholar]
- 38.Martin, D. L., D. B. Weatherly, S. A. Laucella, M. A. Cabinian, M. T. Crim, S. Sullivan, M. Heiges, S. H. Craven, C. S. Rosenberg, M. H. Collins, A. Sette, M. Postan, and R. L. Tarleton. 2006. CD8+ T-cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS Pathog. 2:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin, D., and R. Tarleton. 2004. Generation, specificity, and function of CD8+ T cells in Trypanosoma cruzi infection. Immunol. Rev. 201:304-317. [DOI] [PubMed] [Google Scholar]
- 40.Matloubian, M., M. Suresh, A. Glass, M. Galvan, K. Chow, J. K. Whitmire, C. M. Walsh, W. R. Clark, and R. Ahmed. 1999. A role for perforin in downregulating T-cell responses during chronic viral infection. J. Virol. 73:2527-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mattapallil, J. J., D. C. Douek, A. Buckler-White, D. Montefiori, N. L. Letvin, G. J. Nabel, and M. Roederer. 2006. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J. Exp. Med. 203:1533-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McConkey, S. J., W. H. Reece, V. S. Moorthy, D. Webster, S. Dunachie, G. Butcher, J. M. Vuola, T. J. Blanchard, P. Gothard, K. Watkins, C. M. Hannan, S. Everaere, K. Brown, K. E. Kester, J. Cummings, J. Williams, D. G. Heppner, A. Pathan, K. Flanagan, N. Arulanantham, M. T. Roberts, M. Roy, G. L. Smith, J. Schneider, T. Peto, R. E. Sinden, S. C. Gilbert, and A. V. Hill. 2003. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat. Med. 9:729-735. [DOI] [PubMed] [Google Scholar]
- 43.Miyahira, Y., Y. Takashima, S. Kobayashi, Y. Matsumoto, T. Takeuchi, M. Ohyanagi-Hara, A. Yoshida, A. Ohwada, H. Akiba, H. Yagita, K. Okumura, and H. Ogawa. 2005. Immune responses against a single CD8+-T-cell epitope induced by virus vector vaccination can successfully control Trypanosoma cruzi infection. Infect. Immun. 73:7356-7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore, A. C., and A. V. Hill. 2004. Progress in DNA-based heterologous prime-boost immunization strategies for malaria. Immunol. Rev. 199:126-143. [DOI] [PubMed] [Google Scholar]
- 45.Moorthy, V. S., E. B. Imoukhuede, P. Milligan, K. Bojang, S. Keating, P. Kaye, M. Pinder, S. C. Gilbert, G. Walraven, S. M. Greenwood, and A. S. Hill. 2004. A randomised, double-blind, controlled vaccine efficacy trial of DNA/MVA ME-TRAP against malaria infection in Gambian adults. PLoS Med. 1:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrot, A., and F. Zavala. 2004. Effector and memory CD8+ T cells as seen in immunity to malaria. Immunol. Rev. 201:291-303. [DOI] [PubMed] [Google Scholar]
- 47.Renggli, J., M. Hahne, H. Matile, B. Betschart, J. Tschopp, and G. Corradin. 1997. Elimination of Plasmodium berghei liver stages is independent of Fas (CD95/Apo-I) or perforin-mediated cytotoxicity. Parasite Immunol. 19:145-148. [DOI] [PubMed] [Google Scholar]
- 48.Rice, J., C. H. Ottensmeier, and F. K. Stevenson. 2008. DNA vaccines: precision tools for activating effective immunity against cancer. Nat. Rev. Cancer 8:108-120. [DOI] [PubMed] [Google Scholar]
- 49.Robinson, H. L., and R. R. Amara. 2005. T cell vaccines for microbial infections. Nat. Med. 11:S25-S32. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigues, M., S. Li, K. Murata, D. Rodriguez, J. R. Rodriguez, I. Bacik, J. R. Bennink, J. W. Yewdell, A. Garcia-Sastre, R. S. Nussenzweig, M. Esteban, P. Palese, and F. Zavala. 1994. Influenza and vaccinia viruses expressing malaria CD8+ T and B cell epitopes: comparison of their immunogenicity and capacity to induce protective immunity. J. Immunol. 153:4636-4648. [PubMed] [Google Scholar]
- 51.Santos-Lima, E. C., and P. Minoprio. 1996. Chagas' disease is attenuated in mice lacking gamma delta T cells. Infect. Immun. 64:215-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sardinha, L. R., R. M. Elias, T. Mosca, K. R. Bastos, C. R. Marinho, M. R. D'Império Lima, and J. M. Alvarez. 2006. Contribution of NK, NK T, gamma delta T, and alpha beta T cells to the gamma interferon response required for liver protection against Trypanosoma cruzi. Infect. Immun. 74:2031-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T-cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 54.Sedegah, M., T. R. Jones, M. Kaur, R. Hedstrom, P. Hobart, J. A. Tine, and S. L. Hoffman. 1998. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 95:7648-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silveira, E. L., C. Claser, F. A. Haolla, L. G. Zanella, and M. M. Rodrigues. 2008. Novel protective antigens expressed by Trypanosoma cruzi amastigotes provide immunity to mice highly susceptible to Chagas' disease. Clin. Vaccine Immunol. 15:1292-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tarleton, R. L., J. Sun, L. Zhang, M. Postan, and L. Glimcher. 1996. Trypanosoma cruzi infection in MHC-deficient mice: further evidence for the role of both class I- and class II-restricted T cells in immune resistance and disease. Int. Immunol. 8:13-22. [DOI] [PubMed] [Google Scholar]
- 57.Tzelepis, F., B. C. de Alencar, M. L. Penido, R. T. Gazzinelli, P. M. Persechini, and M. M. Rodrigues. 2006. Distinct kinetics of effector CD8+ cytotoxic T cells after infection with Trypanosoma cruzi in naive or vaccinated mice. Infect. Immun. 74:2477-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tzelepis, F., B. C. de Alencar, M. L. Penido, C. Claser, A. V. Machado, O. Bruna-Romero, R. T. Gazzinelli, and M. M. Rodrigues. 2008. Infection with Trypanosoma cruzi restricts the repertoire of parasite-specific CD8+ T cells leading to immunodominance. J. Immunol. 180:1737-1748. [DOI] [PubMed] [Google Scholar]
- 59.Tzelepis, F., P. M. Persechini, and M. M. Rodrigues. 2007. Modulation of CD4+ T-cell-dependent specific cytotoxic CD8+ T cells differentiation and proliferation by the timing of increase in the pathogen load. PLoS ONE 2:e393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vasconcelos, J. R., M. I. Hiyane, C. R. F. Marinho, C. Claser, A. M. Vieira-Machado, R. T. Gazinelli, O. Bruña-Romero, J. M. Alvarez, and S. B. Boscardin, and M. M. Rodrigues. 2004. Protective immunity against Trypanosoma cruzi infection in a highly susceptible mouse strain following vaccination with genes encoding the amastigote surface protein-2 and trans-sialidase. Hum. Gene Ther. 15:878-886. [DOI] [PubMed] [Google Scholar]
- 61.Williams, N. S., and V. H. Engelhard. 1996. Identification of a population of CD4+ CTL that utilizes a perforin-rather than a Fas ligand-dependent cytotoxic mechanism. J. Immunol. 156:153-159. [PubMed] [Google Scholar]
- 62.Wilson, N. A., J. Reed, G. S. Napoe, S. Piaskowski, A. Szymanski, J. Furlott, E. J. Gonzalez, L. J. Yant, N. J. Maness, G. E. May, T. Soma, M. R. Reynolds, E. Rakasz, R. Rudersdorf, A. B. McDermott, D. H. O'Connor, T. C. Friedrich, D. B. Allison, A. Patki, L. J. Picker, D. R. Burton, J. Lin, L. Huang, D. Patel, G. Heindecker, J. Fan, M. Citron, M. Horton, F. Wang, X. Liang, J. W. Shiver, D. R. Casimiro, and D. I. Watkins. 2006. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 80:5875-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yana, F., E. Ishii, K. Kojima, A. Hasegawa, T. Azuma, S. Hirose, N. Suga, A. Mitsudome, M. Zaitsu, Y. Ishida, Y. Shirakata, K. Sayama, K. Hashimoto, and M. Yasukawa. 2003. Essential roles of perforin in antigen-specific cytotoxicity mediated by human CD4+ T lymphocytes: analysis using the combination of hereditary perforin-deficient effector cells and Fas-deficient target cells. J. Immunol. 170:2205-2213. [DOI] [PubMed] [Google Scholar]
- 64.Yang, J., S. P. Huck, S. Rebecca, S McHugh, I. F. Hermans, and F. Ronchese. 2006. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8 T cells in vivo. Proc. Natl. Acad. Sci. USA 103:147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zavala, F., M. Rodrigues, D. Rodriguez, J. R. Rodriguez, R. S. Nussenzweig, and M. Esteban. 2001. A striking property of recombinant poxviruses: efficient inducers of in vivo expansion of primed CD8+ T cells. Virology 280:155-159. [DOI] [PubMed] [Google Scholar]