Abstract
Borrelia burgdorferi CspZ (BBH06/BbCRASP-2) binds the complement regulatory protein factor H (FH) and additional unidentified serum proteins. The goals of this study were to assess the ligand binding capability of CspZ orthologs derived from an extensive panel of human Lyme disease isolates and to further define the molecular basis of the interaction between FH and CspZ. While most B. burgdorferi CspZ orthologs analyzed bound FH, specific, naturally occurring polymorphisms, most of which clustered in a specific loop domain of CspZ, prevented FH binding in some orthologs. Sequence analyses also revealed the existence of CspZ phyletic groups that correlate with FH binding and with the relationships inferred from ribosomal spacer types (RSTs). CspZ type 1 (RST1) and type 3 (RST3) strains bind FH, while CspZ type 2 (RST2) strains do not. Antibody responses to CspZ were also assessed. Anti-CspZ antibodies were detected in mice by week 2 of infection, indicating that there was expression during early-stage infection. Analyses of sera collected from infected mice suggested that CspZ production continued over the course of long-term infection as the antibody titer increased over time. While antibody to CspZ was detected in several human Lyme disease serum samples, the response was not universal, and the titers were generally low. Vaccination studies with mice demonstrated that while CspZ is immunogenic, it does not elicit an antibody that is protective or that inhibits dissemination. The data presented here provide significant new insight into the interaction between CspZ and FH and suggest that there is a correlation between CspZ production and dissemination. However, in spite of its possible contributory role in pathogenesis, the immunological analyses indicated that CspZ is likely to have limited potential as a diagnostic marker and vaccine candidate for Lyme disease.
In mammals, complement is a key component of the innate immune system and represents one of the initial mechanisms of defense against pathogenic organisms (45, 46, 52). Several diverse pathogens, including bacteria, viruses, and parasites, have been demonstrated to bind negative regulators of the complement system to their surfaces as a means of evading complement-mediated destruction (for reviews, see references 29 and 52). Several Borrelia species, including those associated with Lyme disease and relapsing fever, bind members of the factor H (FH) protein family (13-16, 19, 37), which are key regulators of the alternate complement cascade. FH, an abundant ∼150-kDa serum protein, functions as a decay-accelerating factor of the C3 convertase complex and as a cofactor for the factor I-mediated cleavage of C3b (41, 42, 45). In terms of host-pathogen interactions, the binding of FH to the cell surface locally inhibits complement activation and increases the efficiency of C3b cleavage, thereby decreasing opsonophagocytosis (45). The Lyme disease spirochetes, Borrelia burgdorferi, Borrelia afzelii, and Borrelia garinii, differ in serum sensitivity, ranging from highly resistant to highly sensitive, respectively (6, 30, 44). In Borrelia species, serum resistance has been shown to directly correlate with the production of FH binding proteins (3, 7, 13, 40), and consistent with this, B. burgdorferi produces more FH binding proteins than B. afzelii or B. garinii (37). B. burgdorferi FH binding proteins include OspE paralogs (BBL39, BBN38, and BBP38/CRASPs3-5), CspA (BBA68/CRASP-1), and CspZ (BBH06/CRASP-2) (4, 16, 28, 35, 37). CspZ is the most recent of these proteins to be identified. While cspZ has been demonstrated for both B. burgdorferi and B. garinii, it is not clear if this gene is widely distributed among B. afzelii isolates (37, 39). In addition, although B. garinii produces CspZ, the protein lacks FH binding ability (39). However, CspZ appears to have other roles during infection, as suggested by its ability to bind to other, unidentified serum proteins (39). The sequence analyses conducted to date of representative B. burgdorferi and B. garinii cspZ genes have demonstrated that there are species-specific polymorphisms that influence ligand binding (26, 39).
The goals of this study were to assess the distribution, phylogeny, expression, and ligand binding properties of CspZ orthologs derived from human B. burgdorferi isolates and to determine if vaccination with CspZ elicits a protective response. The data demonstrate that for CspZ sequences there are distinct phyletic types that are associated with FH binding ability and that correlate with ribosomal spacer type (RST), a genetic marker of invasiveness and dissemination (22, 49). Analysis of the immune response to CspZ during experimental infection in mice revealed that CspZ-specific antibody was produced as early as week 2 of infection. However, the antibody response to CspZ in humans was variable. Vaccination of mice with two different recombinant CspZ (r-CspZ) orthologs also did not elicit a protective response or prevent dissemination.
MATERIALS AND METHODS
Bacterial isolates and cultivation.
Isolates were cultivated in BSK-H complete media (Sigma-Aldrich) at 33°C in sealed bottles under 5% CO2 and were harvested by centrifugation (14,000 × g, 4°C, 20 min). The isolates were obtained from human Lyme disease patients residing in New York and Maryland and have been characterized in previous studies (1, 11, 22, 23, 47). Brief descriptions of the isolates employed in this study and their genotypic properties and FH binding abilities are shown in Table 1.
TABLE 1.
Borrelia isolates used in this study
| Isolate | CspZ type | RSTa | FHb | Geographic origin | Source |
|---|---|---|---|---|---|
| B. burgdorferi isolates | |||||
| B31 | 1 | 1 | Y | New York | Ixodes scapularis |
| LDP60 | 1 | 1 | Y | Maryland | Human blood |
| LDP61 | 1 | 1 | Y | Maryland | Human blood |
| LDP73 | 1 | 1 | Y | Maryland | Human blood |
| LDS76 | 1 | 1 | Y | Maryland | Human skin |
| LDP80c | 1 | 1 | Y | Maryland | Human blood |
| LDP89 | 1 | NDd | Y | Maryland | Human blood |
| LDS106 | 1 | 1 | Y | Maryland | Human skin |
| BL206 | 1 | 1 | Y | New York | Human blood |
| B479 | 1 | 1 | Y | New York | Human skin |
| LDP74 | 2 | 2 | N | Maryland | Human blood |
| LDS79 | 2 | ND | N | Maryland | Human skin |
| LDP81c | 2 | 2 | N | Maryland | Human blood |
| LDS88 | 2 | 2 | N | Maryland | Human skin |
| LDS101 | 2 | ND | N | Maryland | Human skin |
| BL224 | 2 | 2 | N | New York | Human blood |
| B379 | 2 | 2 | N | New York | Human skin |
| LDP63 | 3 | ND | Y | Maryland | Human blood |
| LDP84 | 3 | 3 | Y | Maryland | Human blood |
| LDP120 | 3 | ND | Y | Maryland | Human blood |
| B331 | 3 | 3 | Y | New York | Human skin |
| B356c | 3 | 3 | Y | New York | Human skin |
| B408 | 3 | 3 | Y | New York | Human skin |
| LDP83 | ND | ND | Y | Maryland | Human blood |
| BL219 | ND | ND | ND | New York | Human blood |
| B. garinii FRG | 4 | NAe | N | Germany | Ixodes ricinus |
RSTs not determined in this study were determined by Iyer et al. (22).
Y, yes; N, no.
The CspZ group for this isolate has not been determined, but the isolate has been placed in the group indicated due to RST and FH binding characteristics.
ND, not determined.
NA, not applicable.
DNA isolation, PCR, and cloning procedures.
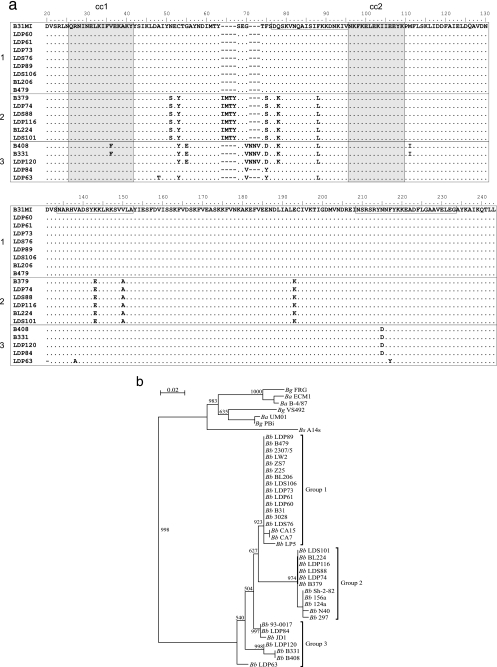
Bacterial DNA was isolated using a MasterPure DNA purification kit (Epicentre). To sequence cspZ from various isolates, this gene was amplified by PCR with primers designed based on the genome sequence of B. burgdorferi B31MI and the results of previous cspZ sequence analyses (12, 39). PCR was conducted using the Phusion high-fidelity DNA polymerase (Finnzymes). All primers had tail sequences that allowed ligase-independent cloning into the pET-46 Ek/LIC vector (Novagen). The resulting plasmids were propagated in Escherichia coli Novablue cells, and inserts of the purified plasmids were sequenced on a fee-for-service basis by MWG Biotech. For RST amplification, primers Pa and P42 were used (48). Following PCR amplification, each sequence was determined and compared to the RSTs determined previously for the New York isolates (48). RST sequences from Maryland isolates could be grouped into one of the three RSTs described by Wang et al. (47, 48), and no sequences resulting in unique clustering were recovered (Table 1). Sequences were aligned and a neighbor-joining tree was constructed using ClustalX. The dendrogram in Fig. 3b was generated with bootstrapping (n = 1,000) using NJplot.
FIG. 3.
(a) Sequence alignment of CspZ proteins from New York and Maryland human Lyme disease isolates. The cspZ genes from New York and Maryland isolates were sequenced and translated, and the protein sequences were aligned; the B. burgdorferi B31 CspZ sequence was used as the reference sequence. Dashes indicate gaps introduced during alignment, and periods indicate residues identical to residues in the reference sequence. The isolate from which each sequence was obtained is indicated on the left. Putative coiled-coil domains are indicated by shading, and boxes in the reference sequence indicate putative FH-FHL-1 binding regions as described by Siegel et al. (43). (b) Based on sequence similarity and dendrogram grouping, the B. burgdorferi CspZ sequences form three distinct phyletic groups (indicated on the right) designated groups 1, 2, and 3. Bg, B. garinii; Ba, B. afzelii; Bb, B. burgdorferi; Bs, speilmanii.
Expression of r-proteins and generation of antiserum.
To generate recombinant proteins (r-proteins), E. coli BL21(DE3) cells harboring the recombinant plasmids of interest were grown at 37°C with shaking (250 rpm) in LB broth supplemented with 50 μg ml−1 ampicillin. Protein expression was induced by addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h, and the cells were harvested by centrifugation (6,000 × g, 10 min). All r-proteins generated in this study harbor an N-terminal 1.7-kDa hexahistidine tag that was exploited for r-protein purification as previously described (39). Anti-CspZ antiserum was generated in C3H/HeJ mice (Jackson Labs) as previously described (39). Anti-CspA antiserum was generated as part of a previous study (32), and anti-OspA antiserum was kindly provided by Darrin Akins (University of Oklahoma Health Sciences Center). To generate infection serum, mice were infected with 104 B. burgdorferi B31MI cells by subcutaneous needle inoculation. Infection was confirmed by cultivation and quantitative PCR analysis of ear punch biopsies using established methods (50) that are described below. Serum was harvested from infected mice at weeks 0, 2, 6, and 10 postinoculation. Approval was obtained from the Virginia Commonwealth University Institutional Animal Care and Use Committee for all work involving animals.
SDS-PAGE, immunoblotting, and FH ALBI assays.
Purified r-proteins or Borrelia cell lysates were separated in 15% Criterion precast gels (Bio-Rad) by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (Millipore). Expression of r-proteins was confirmed by immunoblot analyses using mouse monoclonal anti-His Tag antibody (1:5,000; Novagen). To assess the production of OspA, CspA, and CspZ, immunoblots of Borrelia cell lysates were screened with rat anti-OspA (1:1,000,000), mouse anti-CspA (1:100,000), and mouse anti-CspZ (1:10,000) antisera. All antisera were diluted in blocking buffer (1× phosphate-buffered saline [PBS], 0.2% Tween, 5% nonfat dry milk), and secondary antibodies (Pierce) were used at a 1:40,000 dilution. Detection was performed using the Super Signal West Pico chemiluminescence substrate (Pierce) and exposure to X-ray film (Phenix). The FH binding protein profiles of different strains and FH binding abilities of r-CspZ proteins were assessed using the FH affinity ligand binding immunoblot (ALBI) assay as previously described (39). In brief, blots were incubated with purified human FH (5 ng μl−1; Calbiochem), and after washing, bound FH was detected using goat anti-human FH antiserum (1:1,000; Calbiochem). Human Lyme disease patient sera (provided by Allen Steere) were originally collected in strict accordance with all pertinent regulations. The sera were previously demonstrated to be seropositive for Lyme disease by an enzyme-linked immunosorbent assay (ELISA) and Western blotting (24).
ELISA.
ELISA analyses were conducted as previously described (39). In brief, r-protein was added to each well (1 ng μl−1 in carbonate buffer [pH 9.6], 16 h, 4°C), nonspecific binding was prevented using blocking buffer, and the wells were washed with PBS-T (PBS with 0.2% Tween). To verify that there was equivalent and consistent immobilization of the r-proteins in the wells, triplicate wells were screened with anti-His tag antibody (1:2,000). To assess FH binding, purified human FH was added (5 ng μl−1, 1 h), the wells were washed with PBS-T, and FH binding was detected with goat anti-human FH (1:1,000, 1 h) and rabbit anti-goat immunoglobulin (IgG) (1:10,000) horseradish peroxidase (HRP)-conjugated secondary antibodies. To screen for anti-CspZ antibodies in human or murine infection serum, serum (1:1,350) was added to wells harboring immobilized r-protein. Bound antibody was detected with HRP-conjugated goat anti-mouse IgG or goat anti-human IgG antibody (1:10,000), which was followed by addition of a chromagenic substrate [0.03% H2O2, 0.04% 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) (ABTS), 9.5 mM citric acid; pH 4.1]. The plates were read at A405 with an ELx 808 ELISA plate reader (Biotek). Both human and murine infection serum samples were confirmed to be positive for Lyme disease using the VlsE C6 peptide ELISA (Immunetics).
Vaccination studies and qPCR.
To determine if vaccination with r-CspZ elicits protective antibody, five mice each were inoculated and boosted twice at weeks 2 and 4 with 25 μg r-CspZ derived from either B. burgdorferi B31 or B. garinii FRG in Imject alum (Pierce) or with alum alone. At week 6, serum was collected, and levels of antibody to CspZ were determined. At week 7, mice were needle challenged with 103 spirochetes of either B. burgdorferi B31MI or B. garinii FRG. At week 4 postchallenge, the mice were sacrificed, and the bladder, ears, heart, joints, kidneys, and spleen of each mouse were recovered. The tissues were prepared for quantitative real-time PCR (qPCR) by treatment with 2 mg ml−1 collagenase in PBS (37°C, 16 h), followed by treatment with 0.2 mg ml−1 proteinase K (in 200 mM NaCl, 20 mM Tris-HCl [pH 8.0], 50 mM EDTA, 1% SDS) at 55°C for 16 h. To extract DNA, an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1) was added, and the mixture was vortexed vigorously and clarified by centrifugation (14,000 × g, 15 s). The aqueous phase was recovered, and the phenol-chloroform-isoamyl alcohol procedure was repeated. Then 0.1 volume of 3 M sodium acetate and 2.5 volumes of ice-cold 100% ethanol were added. The DNA was precipitated, washed with 75% ethanol, and suspended in H2O, and 10 ng was used as the template for qPCR. To determine the relative number of spirochetes in each organ, qPCR was performed using the SYBR green method (0.3 ng μl−1 DNA, 3.75 pmol μl−1 each primer) and the following cycling parameters: one cycle of 95°C for 10 min, followed by 40 cycles of 94°C for 15 s, 50°C for 30 s, and 72°C for 30 s. The PCR products were quantified and the data were analyzed using software provided by the thermocycler manufacturer (MJ Research). The following primers (5′ to 3′) were used: BbB31 flaB Fwd (TTCATGTTGGAGCAAACCAA), BbB31 flaB Rev (CTGAGCAGTTTGAGCTCCCT), BgFRG flaB Fwd (GAGTTCATGTAGGAGCAAATCAAATG), BgFRG flaB Rev (TCTGAGCAGCCTGAGATCCTT), nidogen Fwd (CCAGCCACAGAATACCATCC), and nidogen Rev (GGACATACTCTGCTGCAATC).
RESULTS
Analysis of CspZ and CspA production and of the FH binding profiles of human Lyme disease isolates from two geographic locations.
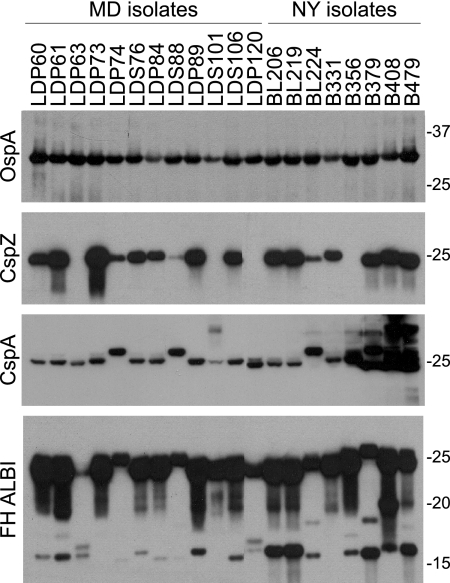
To assess CspZ production, human B. burgdorferi isolates collected from two distinct geographic locations (New York and Maryland) (Table 1) were analyzed by immunoblotting. Loading of cell lysate was standardized by Coomassie blue staining (not shown) and by immunoblot analysis with anti-OspA antiserum (Fig. 1). Identical blots were screened with anti-CspZ and anti-CspA antisera (Fig. 1). CspZ was found to be present at significant but different levels in 16 of 20 isolates analyzed. Based on the size of the detected protein, all B. burgdorferi isolates appear to lack the 60-amino-acid N-terminal domain that has been described for B. garinii CspZ orthologs (26, 39). B. burgdorferi produces a second FH binding protein designated CspA (25). CspA is a highly conserved protein with a molecular mass close to that of CspZ (32, 33). Since these proteins are difficult to differentiate based on migration position, immunoblot analyses were also conducted to assess production and thus facilitate interpretation of the FH binding analyses discussed below. The immunoblot analyses revealed that while all isolates produce CspA, there is some variation in the molecular mass, and some strains produced multiple proteins that were detected by the antiserum. CspA belongs to a paralogous protein family (family 54) which consists of 14 members for B. burgdorferi B31 (12). It is likely that the additional proteins detected in some strains (for example, isolates B379, B408, and B479) represent other members of protein family 54 with shared epitopes.
FIG. 1.
Analysis of CspZ production and FH binding in human isolates from two distinct geographic locations. Cell lysates of human Lyme disease isolates originating from patients in New York (NY) and Maryland (MD) were fractionated by SDS-PAGE and transferred to membranes. Identical blots were screened with anti-OspA, anti-CspA, and anti-CspZ antisera, as indicated on the left. FH binding protein profiles were determined using the FH ALBI assay as described in the text. The isolates used are indicated above the lanes, and the positions of molecular weight markers are indicated on the right.
To assess the possible correlation between the FH binding phenotype, CspZ production, and dissemination-associated genotypic markers, immunoblots of cell lysates were screened for FH binding using an FH ALBI assay. All isolates bound FH (Fig. 1) through either the OspE proteins (15 to 20 kDa), CspA (∼26.7 kDa), or CspZ (25 kDa). Due to the similar molecular masses of CspZ and CspA, it could not readily be determined if the strong FH binding observed with proteins in the 25- to 27-kDa size range indicated FH binding to CspZ, CspA, or both CspZ and CspA. As alluded to above, the additional CspA paralogs (family 54 members) detected in some strains with the anti-CspA antiserum did not bind FH. This is consistent with previous analyses which demonstrated that BBA69, the family 54 paralog most closely related to CspA, does not bind FH (33). The data indicate that while the FH binding phenotype is universal for the human Lyme disease isolates analyzed in this study, the expression levels and FH binding abilities of CspZ and CspA orthologs vary among strains.
Analysis of the FH binding potential of r-CspZ proteins derived from a panel of human Lyme disease isolates.
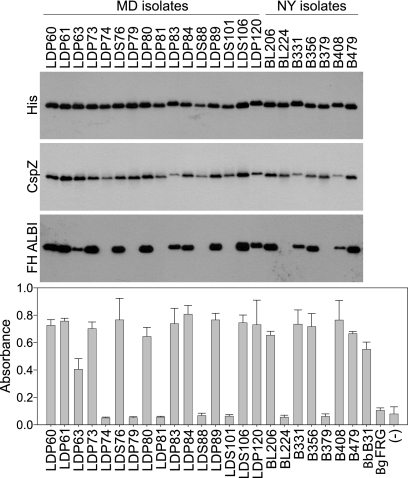
To directly assess the FH binding ability of r-CspZ proteins derived from human Lyme disease isolates, cspZ was PCR amplified from each strain (n = 23) and cloned, and r-protein was generated in E. coli. The proteins were purified, and equal loading in both immunoblot and ELISA analyses was verified using anti-His antibody and anti-CspZ antiserum (Fig. 2). For the ELISA, r-CspZ derived from B. garinii FRG served as a negative control as it has been shown to lack FH binding (39). An additional negative control consisted of an unrelated His-tagged protein. The FH ALBI and ELISA results obtained using the r-proteins were in good agreement. Sixteen of the 23 r-CspZ proteins bound FH, while 7 did not. Isolate B379 produces a CspZ protein during in vitro cultivation; however, the binding analyses with r-CspZ from this isolate indicated that it lacks FH binding ability. Isolate LDP63 encodes a CspZ protein that displayed an attenuated level of binding compared to other B. burgdorferi CspZ orthologs (∼50% of the highest level of binding). The molecular basis for the differential binding of FH was determined and is discussed below.
FIG. 2.
Analysis of FH binding to r-CspZ orthologs derived from human Lyme disease isolates originating from New York (NY) and Maryland (MD). r-CspZ proteins were generated using the gene sequences from several Lyme disease isolates (as indicated above the lanes). The r-proteins were subjected to SDS-PAGE, transferred to membranes, and screened with anti-His and anti-CspZ antisera, as indicated on the left. The ability of each protein to bind FH was assessed using the ALBI assay. The lower panel shows the results of ELISA analyses in which the r-CspZ proteins were immobilized in wells, purified FH was added to each well, and binding was assessed as described in Materials and Methods. r-CspZ proteins from B. burgdorferi B31 and B. garinii FRG served as positive and negative controls, respectively. An unrelated His-tagged protein (r-Rrp1) also served as a negative control (indicated by a minus sign). The error bars indicate standard deviations for three independent experiments.
Sequence analysis of CspZ.
To determine if differences in FH binding by CspZ orthologs are due to specific sequence polymorphisms, the deduced amino acid sequences from multiple strains were aligned (Fig. 3a), and a dendrogram was constructed (Fig. 3b). Distinct phyletic clusters were delineated. B. burgdorferi sequences fall into two tightly defined clusters and a loosely defined third cluster (designated groups 1, 2,and 3) (Fig. 3 and Table 1). All group 1 sequences were identical to the CspZ sequence originally reported for B. burgdorferi B31MI (12). A strict correlation was observed between CspZ group and FH binding ability. CspZ proteins belonging to groups 1 and 3 bind FH, while group 2 CspZ proteins lack the ability to bind FH. B. garinii, B. afzelii, and Borrelia speilmanii CspZ sequences formed a fourth distinct phyletic group. As described above, proteins belonging to this cluster lack FH binding ability but bind to serum proteins whose identities are unknown (39). Alignment of the sequences suggested that polymorphisms between residues 63 and 71 (and possibly at residues 51 and 193) are responsible for differences in FH binding ability. Based on previous mutational analyses of CspZ, it can be concluded that the substitutions at positions 75, 143, and 150 are not factors that contribute to the loss of FH binding.
Temporal analysis of the anti-CspZ antibody response during experimental murine infection.
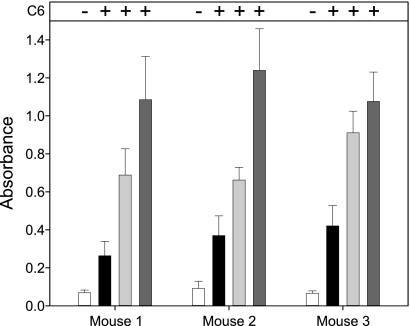
To assess the temporal nature of the anti-CspZ antibody response, three mice were experimentally infected with B. burgdorferi B31MI, and serum was collected at weeks 0, 2, 6, and 10. Each serum sample was tested against the VlsE C6 peptide by ELISA. Serum collected at weeks 2, 6, and 10 was strongly positive for Lyme disease, as shown in Fig. 4. The serum was then screened by ELISA using immobilized r-CspZ as the detection antigen. Specific antibody was detected as early as week 2, and the antibody levels increased through week 10 (Fig. 4). The early development of the anti-CspZ response suggests that CspZ is produced during the initial phases of infection and that expression continues at least through the first few weeks. This is consistent with previous assessments of cspZ transcript levels during infection in mammals (8).
FIG. 4.
Anti-CspZ antibody response during murine infection. To examine production of antibody to CspZ during experimental murine infection, sera were collected from three B. burgdorferi B31-infected mice at weeks 0, 2, 6, and 10 postinfection. To examine the murine sera for antibodies against CspZ, ELISA wells were coated with r-CspZ and incubated with the mouse serum. Bound antibody was detected using an HRP-conjugated secondary antibody. The error bars indicate standard deviations for three experiments. The plus and minus signs indicate the results of the VlsE C6 peptide ELISA for the corresponding serum samples.
Detection of CspZ antibodies during human Lyme disease infection.
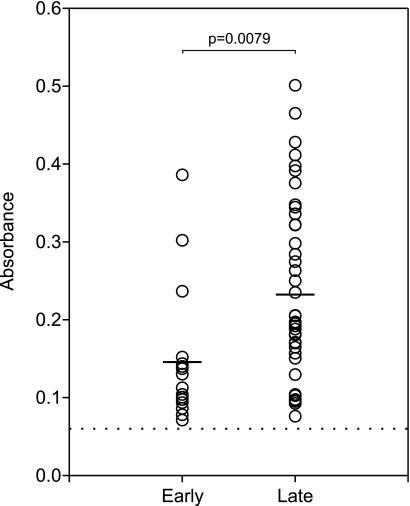
To determine if the anti-CspZ antibody response is a reliable indicator of human Lyme disease, banked seropositive serum samples from 58 human Lyme disease patients were screened by ELISA. Seropositivity was further confirmed using the VlsE C6 peptide ELISA (31) (data not shown). The negative control for these analyses consisted of pooled human serum from healthy individuals. Sixteen serum samples from patients with early Lyme disease and 42 serum samples from patients with late Lyme disease were tested. The serum samples were added to ELISA plate wells coated with r-CspZ derived from B. burgdorferi B31, and bound antibody was detected using an HRP-conjugated secondary antibody. The average absorbance value for the early Lyme disease serum samples was 0.14, while the average value for the late Lyme disease samples was 0.24 (Fig. 5). The difference between the two groups was compared statistically using analysis of variance. Although the difference was statistically significant (P = 0.0079), it remained relatively small. Anti-CspZ antibody levels varied dramatically among patients, indicating that there was significant variation in individual responses to CspZ. It is evident that while some patients mounted an anti-CspZ antibody response, such a response is not universal, indicating that CspZ is not a dominant antigen. Similar significant variations in antibody responses to CspZ in human Lyme disease patients from Europe and the United States (as inferred from the signal strength observed in line blot analyses) have also been reported previously (26).
FIG. 5.
Detection of CspZ antibodies in sera from human Lyme disease patients. To examine patient sera for antibodies against CspZ, r-CspZ was coated onto ELISA wells and incubated with human serum. Bound antibody was detected using an HRP-conjugated secondary antibody. Samples were run in duplicate, and each circle indicates the average for an individual serum sample. Prior to this analysis, all serum samples were confirmed positive for Lyme disease using the VlsE C6 peptide ELISA. The results for pooled serum from healthy donors that served as the negative control are indicated by the dotted line. Based on patient history, the patient samples were categorized as samples that originated from patients with early or late Lyme disease. The means for the early and late serum samples are indicated by bars. Statistical significance (P < 0.05) for the results for the early and late serum samples was determined using the analysis of variance test.
Analysis of CspZ as a vaccinogen.
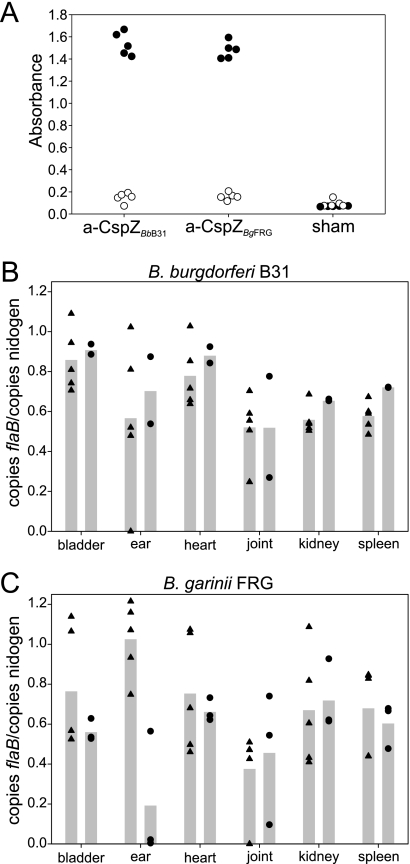
To determine if vaccination with CspZ elicits an antibody response that is protective or blocks dissemination, mice were vaccinated with r-CspZ derived from B. burgdorferi B31 or B. garinii FRG or with alum alone. Antibody responses were assessed by ELISA using r-CspZ as the test antigen (Fig. 6A). The vaccinated mice developed a high-titer, specific antibody response to both CspZ orthologs. The vaccinated mice were then challenged with 103 B. burgdorferi B31MI or B. garinii FRG cells. One thousand spirochetes is a relatively low dose, which is important to consider when the results of challenge experiments are interpreted. At week 4 postchallenge, the mice were sacrificed, and the organs and blood were harvested. The numbers of spirochetes at several sites were determined using qPCR (Fig. 6B and 6C). Nearly equivalent spirochete loads were detected in all organs, indicating that vaccination with CspZ does not provide protection or inhibit dissemination.
FIG. 6.
Analysis of CspZ as a potential vaccinogen. To assess the ability of CspZ to function as a vaccinogen, r-CspZ derived from B. burgdorferi B31 or B. garinii FRG was injected into mice as described in Materials and Methods. The antibody response to r-CspZ (filled circles) or to a negative control protein, r-Rrp1 (open circles), was assessed by ELISA (A). The mice were infected and sacrificed 4 weeks postinfection, and the bladders, ears, hearts, joints, kidneys, and spleens were harvested. DNA was harvested from the organs and analyzed by qPCR, and panels B and C show the results expressed as the ratio of the number of copies of the spirochete flaB gene to the number of copies of the mouse nidogen gene. In panels B and C, the triangles and circles show the results for individual vaccinated and control mice, respectively, and the bars indicate the averages.
DISCUSSION
Lyme disease is maintained in nature in an enzootic cycle involving ticks and mammals (for a review, see reference 5). The Lyme disease spirochetes cannot be transmitted from adult ticks to emerging larvae as transovarial transmission does not occur. Ticks can become infected only by feeding on infected mammals. Completion of the enzootic cycle and hence long-term population maintenance require that the Lyme disease spirochetes maintain persistent infections in mammals. In order to persist, the spirochetes must evade immune system-mediated destruction. Immune system evasion by the Lyme disease spirochetes involves multiple mechanisms and virulence factors that act synergistically (21, 27, 34, 51). Complement evasion is central to persistence. B. burgdorferi has been demonstrated to produce a diverse group of proteins that bind negative regulators of the complement system, including FH and possibly other members of the FH protein family (for a review, see reference 29). This study centered on CspZ, a linear plasmid-encoded lipoprotein produced by B. burgdorferi and B. garinii. It is not clear if cspZ is present in B. afzelii isolates. In a previous study in our laboratory, cspZ was PCR amplified from two of four B. afzelii strains, but the gene could not be detected by Southern blotting (39). The sequences of the apparent B. afzelii-derived amplicons were found to be identical to the B. garinii cspZ sequence, prompting us to speculate that the original isolates may have been mixed cultures in which a minor subpopulation of B. garinii was present. While it is clear that B. burgdorferi CspZ binds FH, there is disagreement regarding its ability to bind FHL-1 (14, 39). Other ligands for CspZ include a series of unidentified serum proteins; however, the specific ligand bound varies among CspZ orthologs (39). Previous analyses have demonstrated that subtle differences in the sequence and/or structure of spirochetal FH binding proteins can influence ligand binding ability (2, 18, 20, 32, 36, 38). Until this study, comparative FH binding by CspZ orthologs derived from Lyme disease patients had not been assessed. A detailed understanding of the sequence diversity and ligand binding properties of CspZ derived from isolates from humans is an essential step in deciphering the precise mechanisms associated with spirochetal persistence in these accidental hosts. In addition to assessing ligand binding properties in this study, we also sought to assess possible correlations between FH binding ability and RST, a genetic marker associated with dissemination potential (23, 48).
The Lyme disease isolates analyzed were collected from patients in two distinct geographic areas, New York and Maryland. The plasmid content, dissemination characteristics, and RST have been demonstrated previously for several of these strains (22, 23, 47, 48), while for other strains these parameters were determined as part of this study. To assess CspZ production by this panel of isolates, immunoblot analyses were conducted using anti-CspZ antiserum. Since the B. burgdorferi CspA protein also binds FH, since the size of CspA is similar to the size of CspZ, and since CspA cannot be easily distinguished from CspZ based on migration position alone, the production of CspA was also assessed by immunoblotting. CspA was detected in all strains, while CspZ was detected in 16 of 20 strains. The production of CspZ by in vitro-cultivated spirochetes is counter to the suggestion that cspZ is generally repressed under standard in vitro cultivation conditions (8). However, it is important to note that cspZ was amplified from some isolates that did not produce detectable levels of CspZ protein. This could indicate that there is transcriptional repression in some strains, as previously postulated (8). Alternatively, as discussed above for B. afzelii, the ability to amplify the gene could have been the result of a mixed population. It is probable that most human-derived isolates are not clonal. In any event, if cspZ transcriptional repression does occur during in vitro cultivation, the phenomenon may be strain specific and may not reflect general, environmentally controlled regulation. The transcriptional regulatory mechanisms associated with CspZ will be assessed in future analyses.
To further investigate CspZ-mediated FH binding by human Lyme disease isolates, the profile of FH binding proteins produced by each isolate was assessed using the FH ALBI assay. Consistent with the immunoblot analyses that indicated that most strains expressed both CspZ and CspA, all of the isolates analyzed produced one or more FH binding proteins in the 25- to 27-kDa size range. However, comparison of the proteins detected by immunoblotting with the proteins detected by the FH ALBI assay suggested that not all B. burgdorferi CspZ orthologs have FH binding ability. To directly assess FH binding, r-CspZ proteins derived from 23 human isolates were generated, and their FH binding was tested using both the ALBI and ELISA formats. Excellent concordance was observed between the results obtained by these two approaches. The data demonstrate that some natural CspZ orthologs, such as those produced by isolates LDP74, LDP79, LDP81, LDS88, LDS101, BL224, and B379, lack FH binding activity.
The phylogenetic relationships among CspZ orthologs and the molecular basis of differential ligand binding were determined by DNA sequence analyses. The phylogenetic analyses revealed the existence of three distinct B. burgdorferi CspZ phyletic clusters (groups 1, 2, and 3) and one additional cluster consisting of B. garinii and other B. burgdorferi sensu lato strains. The phylogenetic relationships inferred from CspZ sequences reflect FH binding ability and are consistent with the genetic relationships inferred from RST, a marker for dissemination potential (Table 1) (23, 48, 49). CspZ type 1 and type 3 proteins, which bind FH, correlate with the invasive RST1 and RST3 genotypes, respectively (22). RST1 isolates are considered to be highly invasive, and RST3 isolates exhibit moderate invasiveness. CspZ type 2 sequences that lack FH binding ability correlate with the minimally invasive RST2 isolates. It is possible that the dissemination capability could in part be due to the inability of these strains to bind FH via CspZ. However, we do not suggest, and other analyses do not support the conclusion, that CspZ is required for or is the sole determinant of dissemination (9). Lastly, it is important to note that Kraiczy et al. also recently determined several cspZ gene sequences for B. burgdorferi isolates (26). Several of these previously determined sequences were included in the phylogenetic analyses conducted here. While the authors of the earlier study did not conduct a phylogenetic analysis per se, the comparative analyses conducted here indicate that several of the strains analyzed in their study (isolates 124a, 156a, 297, N40, and Sh-2-82) have type 2 CspZ sequences. Although FH binding to CspZ proteins from strains 124a, 156a, 297, N40, and Sh-2-82 was not directly assessed in this or previous studies, it is probable that these CspZ orthologs do not bind FH since their sequences are nearly identical to sequences of variants demonstrated here to lack this activity.
The cspZ sequence analyses conducted here provided a strong indication of the molecular basis associated with the inability of some orthologs to bind FH. The lack of FH binding strongly correlates with the presence of a 4-amino-acid insertion sequence within the N-terminal domain of the protein and possibly with amino acid sequence differences at positions 51, 150, and 193. The sequence of the insertion differs in B. burgdorferi and B. garinii. It is important to note that this insertion polymorphism is not in regions of CspZ that have previously been suggested to represent binding sites for FH and/or FHL-1 (Fig. 3a) (26, 43). Similarly, residues 51 and 193 also are outside the putative interaction sites for FH and or FHL-1. The fact that residues in separable parts of the CspZ linear sequence influence FH binding is not surprising and is consistent with numerous earlier studies that demonstrated that the FH interaction domains of most FH binding proteins are defined by discontinuous elements. The insertion is between two alpha helices with a high predicted probability of coiled-coil formation (39). In an earlier study, we demonstrated that site-directed mutations that decreased the coiled-coil formation probability of the CspZ alpha helices discussed above led to a decrease in FH binding (39). Based on this finding and the data described above, we postulate that an antiparallel hydrophobic interaction between CspZ alpha helices 1 and 2 may present a loop domain that serves as one of the critical interaction sites for FH. Similarly, FH binding loop domains dependent on coiled-coil interactions for their formation have been demonstrated in the Borrelia hermsii FH binding protein FhbA (17).
Previous studies demonstrated that CspZ is produced during infection in mice (8, 14, 26, 39), and the data described above support this finding. While it was previously demonstrated that anti-CspZ antibody can be detected at week 4 of infection, earlier time points and the temporal aspects of the antibody response have not been assessed (8, 14). To assess these factors, anti-CspZ antibody levels in experimentally infected mice were determined beginning at week 2 postinfection. CspZ stimulates an early antibody response that was readily detected at week 2, consistent with expression of CspZ during the initial stages of infection. The antibody titer continued to increase through week 10 (later infection time points for mice were not assessed), suggesting that expression occurs throughout infection. The antibody response to CspZ was also assessed in human Lyme disease patients diagnosed with either early or late Lyme disease. Consistent with the response seen in mice, most early Lyme disease patients had a detectable, but generally low, IgG response to CspZ. The anti-CspZ antibody titers were higher and responses were detected in a higher percentage of patients with late-stage disease. Nonetheless, it is evident that a significant percentage of confirmed Lyme disease patients develop only a weak IgG response to CspZ. A previous study suggested that the antibody response to CspZ may have diagnostic utility (26). In that study, anti-CspZ IgG antibody responses in patients from Europe and North America were assessed using a line blot approach. The authors reported that anti-CspZ responses were detectable in 100% of serum samples collected from patients with either erythema migrans, acrodermatitis chronicum atrophicans, or Lyme arthritis. Responses were detected in 80% of individuals with neuroborreliosis (26). However, titers were not determined, and the detection of responses in individuals reported to be infected for as little as 1 day suggests that the assay may not have been highly specific. We interpret the data presented here and data from earlier studies discussed above as suggesting that the anti-CspZ antibody response is not a reliable indicator of Lyme disease infection in humans.
It has been postulated by us and others that FH binding proteins may be attractive vaccine candidates. Antibody elicited to FH binding sites could conceivably elicit antibody-mediated killing while also rendering a cell more resistant to complement by blocking FH binding. The expression of CspZ during infection (8, 39), its presentation on the cell surface (14), and its inherent immunogenicity prompted us to investigate the potential of CspZ to elicit protective immunity and/or to block dissemination. The protective capabilities of two different CspZ orthologs, one derived from B. burgdorferi and one derived from B. garinii, in mice were assessed. While both r-CspZ proteins elicited a strong IgG response, the antibody responses did not protect against spirochetes administered by needle inoculation. Tick challenge experiments were not conducted as part of this study. To determine if vaccination prevents dissemination, organs were harvested from vaccinated and challenged mice, and qPCR was conducted. When vaccinated and control mice were compared, significant differences in spirochete numbers in different organs were not observed. It is possible that in order to realize the protective potential of borrelial FH binding proteins, it may be necessary to lipidate them. The protective role of OspA appears to be enhanced by lipidation (for a review, see reference 10). Although CspZ likely contributes to pathogenesis, it does not appear to be absolutely essential for infectivity and virulence (9). In spite of the potential and theoretical promise of vaccines based on FH binding proteins, this study, coupled with earlier analyses, does not elicit optimism regarding the utility of recombinant Borrelia FH binding proteins in development of a vaccine and/or diagnostic assay.
In summary, the analyses presented here demonstrate that there are significant differences in the FH binding abilities of naturally occurring variants of CspZ. Specific CspZ phyletic types were found to correlate with other markers of dissemination. However, an earlier study suggested that CspZ is not essential for infection and dissemination in mice (9). Since B. burgdorferi encodes several proteins with FH binding ability, it is not surprising that the absence or loss of any one protein in this functional class does not result in an avirulent phenotype. Several studies have suggested that the specific FH binding proteins can interact with multiple host-derived ligands, including FH, FHL-1, FHR-1, plasminogen, and possibly laminin. Each of these ligands has been shown to interact with several different Borrelia proteins, indicating that there is functional redundancy. It is becoming increasingly clear that Borrelia invasiveness and dissemination are most likely mediated by the collective action of several different Borrelia proteins.
Acknowledgments
We thank Allen Steere for providing the human serum samples and Chris Earnhart for testing them using the C6 peptide ELISA. We also thank Juni Sarkar for statistical analyses and Ira Schwartz and J. S. Dumler for providing isolates.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 20 July 2009.
REFERENCES
- 1.Alghaferi, M. Y., J. M. Anderson, J. Park, P. G. Auwaerter, J. N. Aucott, D. E. Norris, and J. S. Dumler. 2005. Borrelia burgdorferi ospC heterogeneity among human and murine isolates from a defined region of northern Maryland and southern Pennsylvania: lack of correlation with invasive and noninvasive genotypes. J. Clin. Microbiol. 43:1879-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alitalo, A., T. Meri, T. Chen, H. Lankinen, Z. Z. Cheng, T. S. Jokiranta, I. J. Seppala, P. Lahdenne, P. S. Hefty, D. R. Akins, and S. Meri. 2004. Lysine-dependent multipoint binding of the Borrelia burgdorferi virulence factor outer surface protein E to the C terminus of factor H. J. Immunol. 172:6195-6201. [DOI] [PubMed] [Google Scholar]
- 3.Alitalo, A., T. Meri, P. Comstedt, L. Jeffery, J. Tornberg, T. Strandin, H. Lankinen, S. Bergstrom, M. Cinco, S. R. Vuppala, D. R. Akins, and S. Meri. 2005. Expression of complement factor H binding immunoevasion proteins in Borrelia garinii isolated from patients with neuroborreliosis. Eur. J. Immunol. 35:3043-3053. [DOI] [PubMed] [Google Scholar]
- 4.Alitalo, A., M. T., H. Lankinen, I. Seppala, P. Lahdenne, P. S. Hefty, D. Akins, and S. Meri. 2002. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 169:3847-3853. [DOI] [PubMed] [Google Scholar]
- 5.Barbour, A. G., and S. F. Hayes. 1986. Biology of Borrelia species. Microbiol. Rev. 50:381-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhide, M. R., M. Travnicek, M. Levkutova, J. Culik, V. Revajova, and M. Levkut. 2005. Sensitivity of Borrelia genospecies to serum complement from different animals and human: a host-pathogen relationship. FEMS Immunol. Med. Microbiol. 43:165-172. [DOI] [PubMed] [Google Scholar]
- 7.Brooks, C. S., S. R. Vuppala, A. M. Jett, A. Alitalo, S. Meri, and D. R. Akins. 2005. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J. Immunol. 175:3299-3308. [DOI] [PubMed] [Google Scholar]
- 8.Bykowski, T., M. E. Woodman, A. E. Cooley, C. A. Brissette, V. Brade, R. Wallich, P. Kraiczy, and B. Stevenson. 2007. Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete's mammal-tick infection cycle. Infect. Immun. 75:4227-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman, A. S., X. Yang, M. Kumar, X. Zhang, K. Promnares, D. Shroder, M. R. Kenedy, J. F. Anderson, D. R. Akins, and U. Pal. 2008. Borrelia burgdorferi complement regulator-acquiring surface protein 2 does not contribute to complement resistance or host infectivity. PLoS ONE 3:3010e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earnhart, C., and R. T. Marconi. 2008. Lyme disease, p. 1031-1060. In A. D. Barrett and L. R. Stanberry (ed.), Vaccines for biodefense and emerging and neglected diseases. Elsevier, New York, NY.
- 11.Earnhart, C. G., E. L. Buckles, J. S. Dumler, and R. T. Marconi. 2005. Demonstration of OspC type diversity in invasive human Lyme disease isolates and identification of previously uncharacterized epitopes that define the specificity of the OspC murine antibody response. Infect. Immun. 73:7869-7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser, C., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischman, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 13.Grosskinsky, S., M. Schott, C. Brenner, S. J. Cutler, P. Kraiczy, P. F. Zipfel, M. M. Simon, and R. Wallich. 2009. Borrelia recurrentis employs a novel multifunctional surface protein with anti-complement, anti-opsonic and invasive potential to escape innate immunity. PLoS ONE 4:e4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartmann, K., C. Corvey, C. Skerka, M. Kirschfink, M. Karas, V. Brade, J. C. Miller, B. Stevenson, R. Wallich, P. F. Zipfel, and P. Kraiczy. 2006. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol. Microbiol. 61:1220-1236. [DOI] [PubMed] [Google Scholar]
- 15.Haupt, K., P. Kraiczy, R. Wallich, V. Brade, C. Skerka, and P. F. Zipfel. 2007. Binding of human factor H-related protein 1 to serum-resistant Borrelia burgdorferi is mediated by borrelial complement regulator-acquiring surface proteins. J. Infect. Dis. 196:124-133. [DOI] [PubMed] [Google Scholar]
- 16.Hellwage, J., T. Meri, T. Heikkila, A. Alitalo, J. Panelius, P. Lahdenne, I. J. T. Seppala, and S. Meri. 2001. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 17.Hovis, K. M., J. C. Freedman, H. Zhang, J. L. Forbes, and R. T. Marconi. 2008. Identification of an antiparallel coiled-coil/loop domain required for ligand binding by the Borrelia hermsii FhbA protein: additional evidence for the role of FhbA in the host-pathogen interaction. Infect. Immun. 76:2113-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hovis, K. M., J. P. Jones, T. Sadlon, G. Raval, D. L. Gordon, and R. T. Marconi. 2006. Molecular analyses of the interaction of Borrelia hermsii FhbA with the complement regulatory proteins factor H and factor H-like protein 1. Infect. Immun. 74:2007-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hovis, K. M., J. V. McDowell, L. Griffin, and R. T. Marconi. 2004. Identification and characterization of a linear-plasmid-encoded factor H-binding protein (FhbA) of the relapsing fever spirochete Borrelia hermsii. J. Bacteriol. 186:2612-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hovis, K. M., M. E. Schriefer, S. Bahlani, and R. T. Marconi. 2006. Immunological and molecular analyses of the Borrelia hermsii factor H and factor H-like protein 1 binding protein, FhbA: demonstration of its utility as a diagnostic marker and epidemiological tool for tick-borne relapsing fever. Infect. Immun. 74:4519-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hovius, J. W., T. J. Schuijt, K. A. de Groot, J. J. Roelofs, G. A. Oei, J. A. Marquart, R. de Beer, C. van't Veer, T. van der Poll, N. Ramamoorthi, E. Fikrig, and A. P. van Dam. 2008. Preferential protection of Borrelia burgdorferi sensu stricto by a Salp15 homologue in Ixodes ricinus saliva. J. Infect. Dis. 198:1189-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iyer, R., O. Kalu, J. Purser, S. Norris, B. Stevenson, and I. Schwartz. 2003. Linear and circular plasmid content in Borrelia burgdorferi clinical isolates. Infect. Immun. 71:3699-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iyer, R., D. Liveris, A. Adams, J. Nowakowski, D. McKenna, S. Bittker, D. Cooper, G. P. Wormser, and I. Schwartz. 2001. Characterization of Borrelia burgdorferi isolated from erythema migrans lesions: interrelationship of three molecular typing methods. J. Clin. Microbiol. 39:2954-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, K. L., L. J. Glickstein, N. Damle, V. K. Sikand, G. McHugh, and A. C. Steere. 2006. Borrelia burgdorferi genetic markers and disseminated disease in patients with early Lyme disease. J. Clin. Microbiol. 44:4407-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraiczy, P., J. Hellwage, C. Skerka, H. Becker, M. Kirschfink, M. S. Simon, V. Brade, P. F. Zipfel, and R. Wallich. 2004. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J. Biol. Chem. 279:2421-2429. [DOI] [PubMed] [Google Scholar]
- 26.Kraiczy, P., A. Seling, C. A. Brissette, E. Rossmann, K. P. Hunfeld, T. Bykowski, L. H. Burns, M. J. Troese, A. E. Cooley, J. C. Miller, V. Brade, R. Wallich, S. Casjens, and B. Stevenson. 2008. Borrelia burgdorferi complement regulator-acquiring surface protein 2 (CspZ) as a serological marker of human Lyme disease. Clin. Vaccine Immunol. 15:484-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraiczy, P., C. Skerka, M. Kirschfink, P. F. Zipfel, and V. Brade. 2001. Mechanism of complement resistance of pathogenic Borrelia burgdorferi isolates. Int. Immunopharmacol. 1:3393-3401. [DOI] [PubMed] [Google Scholar]
- 28.Kraiczy, P., C. Skerka, and P. F. Zipfel. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 69:7800-7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraiczy, P., and R. Würzner. 2006. Complement escape of human pathogenic bacteria by acquisition of complement regulators. Mol. Immunol. 43:31-44. [DOI] [PubMed] [Google Scholar]
- 30.Kurtenbach, K., H. S. Sewell, N. H. Ogden, S. E. Randolph, and P. A. Nuttall. 1998. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect. Immun. 66:1248-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang, F. T., A. L. Alvarez, Y. Gu, J. M. Nowling, R. Ramamoorthy, and M. T. Philipp. 1999. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J. Immunol. 163:5566-5573. [PubMed] [Google Scholar]
- 32.McDowell, J. V., M. E. Harlin, E. A. Rogers, and R. T. Marconi. 2005. Putative coiled-coil structural elements of the BBA68 protein of Lyme disease spirochetes are required for formation of its factor H binding site. J. Bacteriol. 187:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDowell, J. V., K. M. Hovis, H. Zhang, E. Tran, J. Lankford, and R. T. Marconi. 2006. Evidence that the BBA68 protein (BbCRASP-1) of the Lyme disease spirochetes does not contribute to factor H-mediated immune evasion in humans and other animals. Infect. Immun. 74:3030-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDowell, J. V., S. Y. Sung, L. T. Hu, and R. T. Marconi. 2002. Evidence that the variable regions of the central domain of VlsE are antigenic during infection with Lyme disease spirochetes. Infect. Immun. 70:4196-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDowell, J. V., E. Tran, D. Hamilton, J. Wolfgang, K. Miller, and R. T. Marconi. 2003. Analysis of the ability of spirochete species associated with relapsing fever, avian borreliosis, and epizootic bovine abortion to bind factor H and cleave c3b. J. Clin. Microbiol. 41:3905-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDowell, J. V., J. Wolfgang, L. Senty, C. M. Sundy, M. J. Noto, and R. T. Marconi. 2004. Demonstration of the involvement of outer surface protein E coiled coil structural domains and higher order structural elements in the binding of infection-induced antibody and the complement-regulatory protein, factor H. J. Immunol. 173:7471-7480. [DOI] [PubMed] [Google Scholar]
- 37.McDowell, J. V., J. Wolfgang, E. Tran, M. S. Metts, D. Hamilton, and R. T. Marconi. 2003. Comprehensive analysis of the factor H binding capabilities of Borrelia species associated with Lyme disease: delineation of two distinct classes of factor H binding proteins. Infect. Immun. 71:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metts, M. S., J. V. McDowell, M. Theisen, P. R. Hansen, and R. T. Marconi. 2003. Analysis of the OspE determinants involved in binding of factor H and OspE-targeting antibodies elicited during Borrelia burgdorferi infection in mice. Infect. Immun. 71:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers, E. A., and R. T. Marconi. 2007. Delineation of species-specific binding properties of the CspZ protein (BBH06) of Lyme disease spirochetes: evidence for new contributions to the pathogenesis of Borrelia spp. Infect. Immun. 75:5272-5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogers, E. A., D. Terekhova, H. M. Zhang, K. M. Hovis, I. Schwartz, and R. T. Marconi. 2009. Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol. Microbiol. 71:1551-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruddy, S., and K. F. Austen. 1969. C3 inactivator of man. I. Hemolytic measurement by the inactivation of cell-bound C3. J. Immunol. 102:533-543. [PubMed] [Google Scholar]
- 42.Ruddy, S., and K. F. Austen. 1971. C3b inactivator of man. II. Fragments produced by C3b inactivator cleavage of cell-bound or fluid phase C3b. J. Immunol. 107:742-750. [PubMed] [Google Scholar]
- 43.Siegel, C., J. Schreiber, K. Haupt, C. Skerka, V. Brade, M. M. Simon, B. Stevenson, R. Wallich, P. F. Zipfel, and P. Kraiczy. 2008. Deciphering the ligand-binding sites in the Borrelia burgdorferi complement regulator-acquiring surface protein 2 (BbCRASP-2) required for interactions with the human immune regulators factor H and factor H-Like protein 1. J. Biol. Chem. 283:34855-34863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Dam, A. P., A. Oei, R. Jaspars, C. Fijen, B. Wilske, L. Spanjaard, and J. Dankert. 1997. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect. Immun. 65:1228-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walport, M. J. 2001. Complement—first of two parts. N. Engl. J. Med. 344:1058-1066. [DOI] [PubMed] [Google Scholar]
- 46.Walport, M. J. 2001. Complement—second of two parts. N. Engl. J. Med. 344:1140-1144. [DOI] [PubMed] [Google Scholar]
- 47.Wang, G., C. Ojaimi, R. Iyer, V. Saksenberg, S. A. McClain, G. P. Wormser, and I. Schwartz. 2001. Impact of genotypic variation of Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infect. Immun. 69:4303-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang, G., C. Ojaimi, H. Wu, V. Saksenberg, R. Iyer, D. Liveris, S. A. McClain, G. P. Wormser, and I. Schwartz. 2002. Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J. Infect. Dis. 186:782-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wormser, G. P., D. Brisson, D. Liveris, K. Hanincova, S. Sandigursky, J. Nowakowski, R. B. Nadelman, S. Ludin, and I. Schwartz. 2008. Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J. Infect. Dis. 198:1358-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, H., A. Raji, M. Theisen, P. R. Hansen, and R. T. Marconi. 2005. bdrF2 of Lyme disease spirochetes is coexpressed with a series of cytoplasmic proteins and is produced specifically during early infection. J. Bacteriol. 187:175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, J.-R., J. M. Hardham, A. G. Barbour, and A. G. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of vmp like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]
- 52.Zipfel, P. F., C. Skerka, J. Hellwage, S. T. Jokiranta, S. Meri, V. Brade, P. Kraiczy, M. Noris, and G. Remuzzi. 2002. Structure-function studies of the complement system. Biochem. Soc. Trans. 30:971-978. [DOI] [PubMed] [Google Scholar]