Abstract
Chronic granulomatous disease (CGD) patients are susceptible to life-threatening infections by the Burkholderia cepacia complex. We used leukocytes from CGD and healthy donors and compared cell association, invasion, and cytokine induction by Burkholderia multivorans strains. A CGD isolate, CGD1, showed higher cell association than that of an environmental isolate, Env1, which correlated with cell entry. All B. multivorans strains associated significantly more with cells from CGD patients than with those from healthy donors. Similar findings were observed with another CGD pathogen, Serratia marcescens, but not with Escherichia coli. In a mouse model of CGD, strain CGD1 was virulent while Env1 was avirulent. B. multivorans organisms were found in the spleens of CGD1-infected mice at levels that were 1,000 times higher than those found in Env1-infected mice, which was coincident with higher levels of the proinflammatory cytokine interleukin-1β. Taken together, these results may shed light on the unique susceptibility of CGD patients to specific pathogens.
Chronic granulomatous disease (CGD) is a rare primary immunodeficiency resulting from genetic defects in the phagocyte NAPDH oxidase. It is characterized by life-threatening infections caused by specific bacteria and fungi, leading to pneumonias, tissue abscesses, and exuberant granuloma formation (38). The Burkholderia cepacia complex (Bcc) includes at least 10 distinct species and is a leading cause of bacterial infections in CGD (44). Patients with cystic fibrosis (CF) also develop Bcc infections with various outcomes, ranging from no change in clinical course to a more rapid deterioration of lung function to the dreadful cepacia syndrome, which is characterized by necrotizing pneumonia and sepsis (25, 45). Interestingly, Bcc rarely causes infection in healthy individuals, but it can infect patients undergoing bronchoscopies and other procedures (4).
Within the Bcc, Burkholderia cenocepacia and Burkholderia multivorans are commonly isolated from CF and non-CF patients (4, 32); the rate of B. multivorans infection now exceeds that of B. cenocepacia at several CF centers (15). In contrast to the high transmissibility of some CF B. cenocepacia strains (i.e., the epidemic lineage ET12) (24, 25), CF B. multivorans infections likely reflect independent acquisitions from unrelated sources (24). Curiously, unlike B. cenocepacia, B. multivorans has been recovered from environmental samples only rarely (1, 24), and it is the most frequently found species among CGD patients (16, 17).
The mechanisms by which the Bcc causes disease specifically in CF are not known. Bcc isolates can survive within macrophages (28, 33) and respiratory epithelial cells (5, 21) and can invade epithelial cells in vivo (8, 10) and persist in the lung (9, 10). Cell infection assays using monocytes, macrophages, and epithelial cells (10, 11, 29, 46) show great variability among individual Bcc strains, with no clear correlation between those isolated from CF patients and those isolated from the environment (22). For the most part, these studies have been carried out using tissue culture models (28, 29, 43) and, in some cases, CF human or CF mouse cell systems (34, 35).
Much less is known about the interaction between the Bcc and CGD despite the availability of animal models for the disease (20, 31). B. cenocepacia induced the necrosis of human CGD neutrophils but not normal controls (6). Similarly to healthy people, normal mice are resistant to the Bcc and usually show only transient infections upon inoculation (8, 37). On the other hand, CGD mice are highly susceptible to Bcc infection and show clinical signs that are similar to those of the human disease (20, 31, 37).
To address why B. multivorans is a pathogen in CGD, we initiated studies with strains isolated from CGD patients and CGD cells. Strains of B. multivorans differed in cell association and cell entry. We found a preferential association of bacteria with CGD instead of normal leukocytes as shown by microscopy and culture techniques. This preferential association is shared by another CGD pathogen, Serratia marcescens, but not by Escherichia coli. Finally, we demonstrate dramatic differences in virulence in B. multivorans strains in a mouse model of CGD.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Clinical strains used in this study were isolated from respiratory cultures of NIH patients with CGD (B. multivorans CGD1 and CGD3 and Serratia marcescens) or CF (B. multivorans CF2). Reference strains of B. multivorans used included the type strain ATCC BAA-247 (CF1, from a CF patient) and the environmental strain ATCC 17616 (Env1). Escherichia coli HB101 was used as the control. All strains were stored at −80°C. Prior to assays, bacteria were streaked onto tryptic soy agar (TSA) with 5% sheep blood, and colonies grown in Luria-Bertani broth at 37°C to logarithmic phase (optical density at 600 nm, ∼0.6).
Cell isolation and culture.
Purified monocytes from normal donors were obtained by counterflow centrifugal elutriation. Human peripheral blood mononuclear cells (PBMCs) were obtained through Ficoll-Hypaque density gradient centrifugation from normal volunteers (Department of Transfusion Medicine) and from CGD patients monitored at the NIH (Bethesda, MD). All subjects gave appropriate informed consent. Neutrophils were isolated through the cushion of Ficoll-Hypaque by sedimentation and the osmotic lysis of erythrocytes (3).
Cell viability was estimated by trypan blue exclusion (>99%), and the cells were seeded in culture on 12-mm coverslips on 24-well plates (Becton Dickinson) at 1 × 106 cells per well in complete RPMI medium (Invitrogen, Carlsbad, CA) containing 2 mM l-glutamine, 20 mM HEPES (HyClone, Logan, UT), and 5% human AB serum (Gemini Bio-Products, Woodland, CA) and allowed to adhere for at least 2 h.
Cell infection assays were based on Bcc infection assays reported previously (5, 28). In short, coverslip monolayers of monocytes, PBMCs, or neutrophils were infected for 2 h with bacteria grown to mid-log phase and diluted in tissue culture medium (multiplicity of infection [MOI] = 4). Supernatants were collected for cytokine measurements as described below. For cell association experiments to detect attached and internalized bacteria, coverslips were washed extensively with RPMI medium, fixed with methanol, and then stained with Giemsa (EMD Chemicals Inc., Gibbstown, NJ). Stained coverslips were examined under light microscopy using ×40 and ×100 objectives for enumeration. A total number of 350 cells/sample in at least six random fields were counted. The percentage of infected cells was calculated using the following formula: (number of cells with associated bacteria/total number of cells counted) × 100. For quantitative microbial cultures, bacteria were released from coverslips by 0.5% Triton X-100 and serially diluted in Hanks balanced salt solution (HBSS). Quantitative microbial cultures were performed on TSA with 5% sheep blood plates for CFU analyses.
Confocal microscopy.
To aid the visualization of B. multivorans by confocal microscopy, plasmid pBHR1-GFP was introduced into bacteria by electroporation for the constitutive expression of green fluorescent protein (GFP) (41). GFP was excited with an argon laser at 488 nm using a Leica SP5 confocal microscope (Leica Microsystems, Exton, PA) with a ×40 oil immersion objective (numerical aperture, 1.4) and ×5 zoom. To assess internalized bacteria, differential interference contrast (DIC) images were collected simultaneously with the fluorescence images at the mid-plane of the cell nucleus using a transmitted light detector. Images were taken at several random fields for each sample and processed using Leica LAS-AF, version 1.6.3, and Adobe Photoshop.
Detection of cytokines.
Cytokines from culture supernatants or mice serum were assayed using multiplex bead-based assays (Bio-Rad Laboratories, Hercules, CA) and processed according to the manufacturer's instructions.
Animal model.
The virulence of selected B. multivorans strains was evaluated in autosomal-recessive CGD (p47phox−/−) C57BL/6 background mice (20). A total of 4 × 105 CFU of strain CGD1 or Env1 were inoculated intraperitoneally into groups of six to eight mice, and the number of moribund or dead mice was recorded (37). Wild-type C57BL/6 mice (Taconic) were used as controls. Mice were matched by age (2 to 4 months) and sex for each set of experiments.
In a different set of experiments, 3 days following bacterial inoculation serum was obtained by tail bleeding, and animals were sacrificed and spleens collected, homogenized, and serially diluted with PBS. Quantitative cultures were performed on TSA with 5% sheep blood plates for CFU analyses. The study was approved by the NIAID Animal Care and Use Committee.
Statistical analysis.
Values are expressed as the means ± standard errors of the means, and differences between study groups were assessed by the unpaired two-tailed t test using InStat/Prism software, version 5.0c (GraphPad Software, San Diego, CA), with a level of significance of P < 0.05. The log-rank (Mantel-Cox) test was applied to Kaplan-Meier survival data.
RESULTS
Burkholderia multivorans cell association.
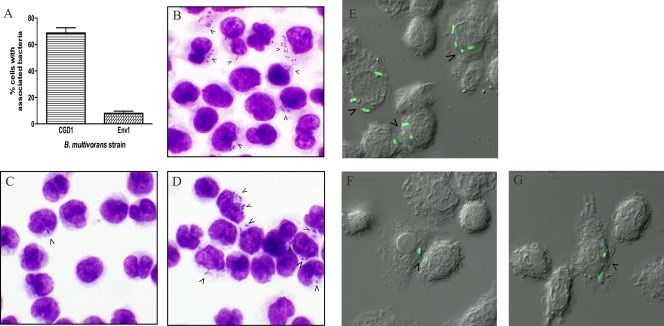
We assayed the ability of a B. multivorans strain from a CGD patient and an environmental strain to associate with normal human monocytes. Elutriated monocytes from healthy donors (n = 10) were infected in vitro with B. multivorans, and the percentage of cell association (% cells showing bound and/or ingested bacteria) was measured microscopically after the Giemsa staining of infected monolayers (MOI = 4 at 2 h of infection). The clinical isolate CGD1 had a very high cell association, with ∼70% of cells showing associated bacteria. In contrast, the environmental isolate Env1 showed only 8% cell association (Fig. 1A). The observed differences in cell association were preserved across monocyte samples from multiple donors and both at lower MOIs and in the absence of active complement by using heat-inactivated serum (not shown).
FIG. 1.
Cell association and cell invasion of B. multivorans with normal human monocytes. (A) Percent of normal human elutriated monocytes with associated bacteria. Cell monolayers on coverslips were exposed to bacteria for 2 h (MOI = 4). Coverslips were stained with Giemsa reagent and then examined at ×400 and ×1,000 magnifications to determine the percentages of cells with associated bacteria. These results were averaged from 10 independent experiments. P < 0.001. (B to D) Cell association was assessed by the Giemsa staining of monocytes infected with strain CGD1 (B) or Env1 (C and D); magnification, ×1,000. Cell invasion by GFP-expressing bacteria CGD1 (E) or Env1 (F and G) was assessed by DIC images collected simultaneously with the fluorescence images at the mid-plane of the cell nucleus. Arrows show cells with bacteria.
The number of bacteria that associated per cell differed among the bacterial isolates. Strain CGD1 showed an average of ∼6 rods/cell (Fig. 1B). In contrast, strain Env1 exhibited a distinct pattern of infection, with most of the infected cells showing 1 or 2 rods/cell (Fig. 1C) interspersed with occasional fields with cells showing multiple bacteria (Fig. 1D).
Correlation between cell association and invasion.
To see if differences in cell association correlated with differences in internalization, monocytes were infected with GFP-expressing bacteria and examined by confocal microscopy (MOI = 4 at 2 h of infection). To visualize internalized bacteria, DIC images were collected simultaneously with the fluorescence images at the mid-plane of the nucleus using a transmitted light detector.
Strain CGD1 invaded many human monocytes with several rods per cell (Fig. 1E). In contrast, strain Env1 most commonly showed 1 rod/cell (Fig. 1F); however, occasional cells with multiple rods were seen (Fig. 1G). Overall, these experiments indicate a correlation between associated and internalized bacteria for each strain: a higher bacterial association of CGD1 compared to that of Env1 by Giemsa staining correlated with more cells showing internalized CGD1 than Env1, as seen by the confocal microscopy of GFP fluorescent bacteria.
Cytokine induction in human monocytes by B. multivorans isolates.
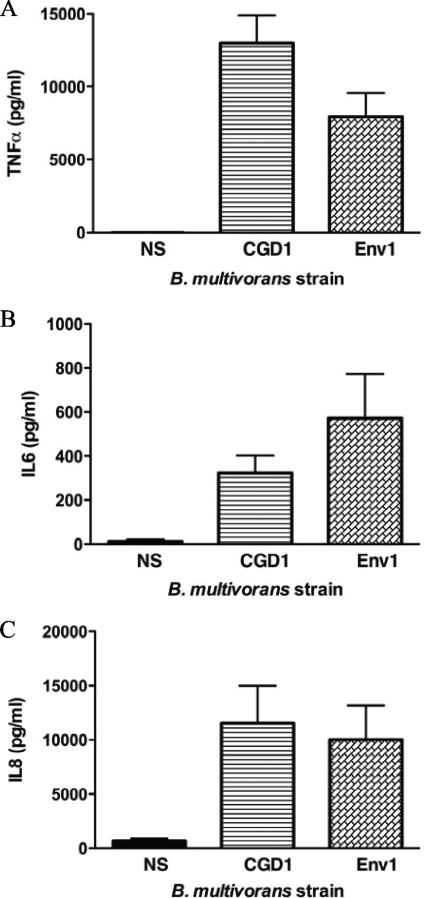
To compare the cytokine induction of B. multivorans isolates, monocyte culture supernatants were collected 2 h after infection. Both B. multivorans strains induced cytokines, predominantly tumor necrosis factor alpha (TNF-α) (Fig. 2A), interleukin-6 (IL-6) (Fig. 2B), and the chemokine IL-8 (Fig. 2C) to comparable levels (P > 0.05).
FIG. 2.
Cytokine production (TNF-α [A], IL-6 [B], and IL-8 [C]) by normal human elutriated monocytes infected with B. multivorans. Culture supernatants from monocytes exposed for 2 h to different strains (MOI = 4) were recovered and assayed through a multiplex bead-based assay (Bio-Rad Laboratories). NS, nonstimulated cells. Cytokines are expressed in picograms/milliliter. Results were averaged from 10 independent experiments. P > 0.05 for cytokine levels induced by CGD1 compared to those induced by Env1.
B. multivorans association with CGD and normal cells.
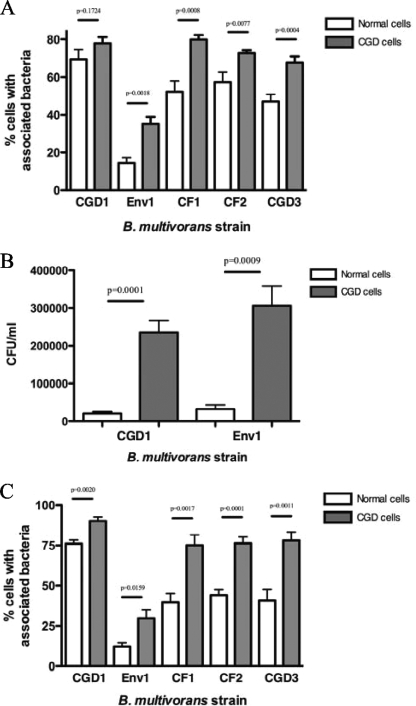
Having shown that the variability in terms of cell association and internalization was intrinsic to the strain of B. multivorans, we sought to explore whether the response of cells from CGD patients was different from that of healthy individuals. Therefore, we tested the cell association of B. multivorans with cells from CGD patients and compared it to that of cells from normal donors. For these experiments, three additional clinical strains of B. multivorans isolated from CF or CGD patients were used in addition to strains CGD1 and Env1. The percentage of normal PBMCs (n = 10 donors) with associated bacteria varied among the strains, with the highest values being for the clinical strain CGD1 and the lowest values being for the environmental strain Env1 (Fig. 3A). We found no significant differences in cell association between isolates from CF and CGD patients. We also tested the cell association of the five B. multivorans strains with PBMCs from CGD patients to study cell bacterial interactions in this disease. Notably, significantly more cells from CGD patients showed associated bacteria (Fig. 3A) than cells from normal volunteers independently of the strain source.
FIG. 3.
Cell association of bacteria with normal and CGD leukocytes. Shown are the associations of five B. multivorans strains with normal and CGD PBMCs (A) and neutrophils (C) and CFU of B. multivorans associated with normal or CGD PBMCs (B). Cell monolayers on coverslips were exposed to bacteria for 2 h (MOI = 4). Coverslips were washed extensively and either stained with Giemsa reagent (A and C) or treated with Triton X-100 and serially diluted with HBSS for microbial cultures (B). Stained coverslips were examined at a ×400 and ×1,000 magnification under light microscopy to determine the percentage of cells with associated bacteria. Quantitative microbial cultures were performed on TSA with 5% sheep blood plates.
To confirm the microscopically observed differences in the cell association of B. multivorans with CGD and normal PBMCs (Fig. 3A), quantitative cultures were performed following the release of bacteria from infected PBMCs (MOI = 4 at 2 h of infection). CGD cells had ∼10 times more associated bacteria (in CFU) than normal cells for both CGD1 and Env1 strains (Fig. 3B).
Since impaired oxidative burst production in neutrophils is thought to be the critical defect leading to the susceptibility of CGD to infection, we also compared the rates of bacterial association with neutrophils from CGD patients (n = 5) to that from normal donors (n = 5). Again, B. multivorans isolates associated significantly more with CGD neutrophils than with normal donor neutrophils (Fig. 3C).
Cell association of another CGD pathogen, S. marcescens, with CGD and normal leukocytes.
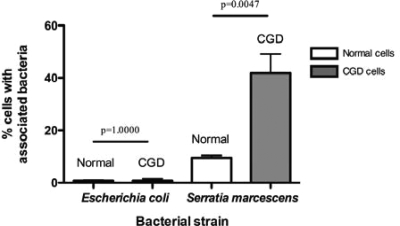
Having shown the preferential association of B. multivorans with PBMCs from CGD patients over healthy controls, we were interested to know whether this phenomenon was reproduced by other CGD pathogens. Therefore, we tested the cell association of a CGD clinical strain of S. marcescens with PBMCs from CGD patients and normal donors. Interestingly, S. marcescens associated significantly more with CGD PBMCs than healthy donor cells (Fig. 4).
FIG. 4.
Cell association of S. marcescens and E. coli with normal and CGD PBMCs. Cell monolayers on coverslips were exposed to bacteria for 2 h (MOI = 4). Coverslips were washed extensively, fixed with methanol and stained with Giemsa reagent, and then examined at ×400 and ×1,000 magnifications under light microscopy to determine the percentage of cells with associated bacteria.
To assess whether this higher bacterial association was a general feature of CGD cells (i.e., CGD cells are more sticky), we tested E. coli, an enteric organism that is related to S. marcescens. Interestingly, no differences in cell association between CGD and normal cells were seen for E. coli HB101 (Fig. 4) or a clinical isolate (not shown).
Cytokine induction by B. multivorans isolates in CGD and normal human cells.
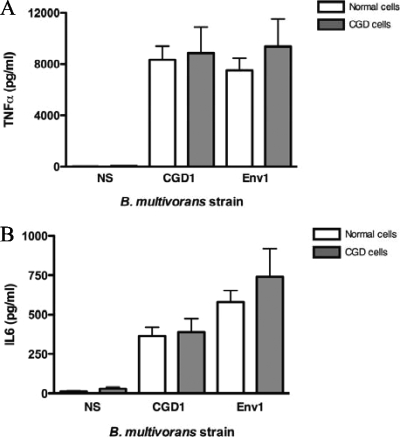
Cytokine secretion in PBMC infected with B. multivorans strains yielded predominantly TNF-α and IL-6. Comparisons between the induced TNF-α and IL-6 levels in CGD PBMCs and normal PBMCs showed a trend toward higher values with CGD PBMCs; however, the difference was not significant (P > 0.05) (Fig. 5).
FIG. 5.
TNF-α (A) and IL-6 (B) production by normal and CGD human PBMCs infected with B. multivorans strains. Culture supernatants from PBMCs exposed for 2 h to different strains (MOI = 4) were recovered and assayed through a multiplex bead-based assay (Bio-Rad Laboratories). NS, nonstimulated cells. Cytokines are expressed in picograms/milliliter. These results were averaged from 10 independent experiments. P > 0.05 for each pair of normal and CGD cells.
Virulence of three B. multivorans strains in a mouse model of CGD.
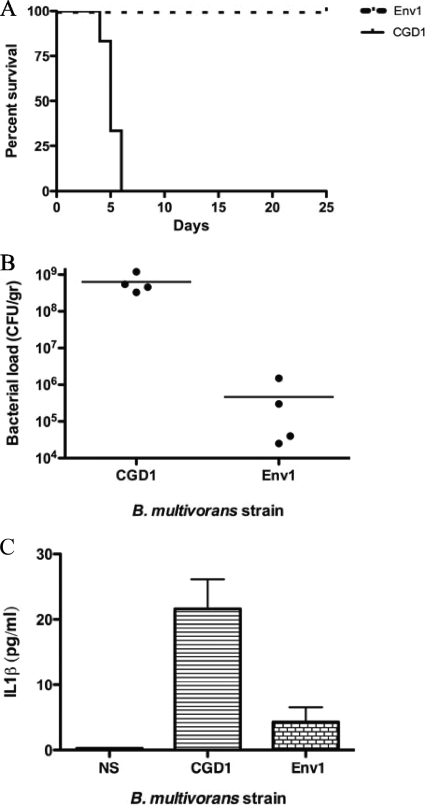
Given the variation among different bacterial strains with normal and CGD leukocytes in vitro, we sought to assess the virulence of these B. multivorans strains in vivo. Strains CGD1 and Env1 were inoculated (4 × 105 CFU intraperitoneally) into autosomal-recessive CGD mice (p47phox−/−) (20), and the number of moribund or dead animals was recorded. By day 6, all animals died or became moribund when inoculated with clinical strain CGD1, while none of the mice died or became moribund when inoculated with the environmental strain Env1 (Fig. 6A). No deaths were noted when wild-type C57BL/6 mice controls were inoculated with the same bacterial dose (not shown).
FIG. 6.
Kaplan-Meier survival curves of p47phox−/− mice after intraperitoneal (i.p.) challenge with 4 × 105 CFU of B. multivorans (A). Strain CGD1 or Env1 was inoculated into groups of six to eight animals. To assess in vivo bacterial loads (B) and serum levels of IL-1β (C), strain CGD1 or Env1 was inoculated in p47phox−/− mice as described for the survival experiments. Three days postinoculation, blood samples were obtained by tail bleeding right before mice were sacrificed, and spleens were collected and processed for CFU analyses. These data summarize three independent experiments (P = 0.0008, 0.0169, and 0.0281 for A, B, and C, respectively).
To assess the bacterial load in vivo, strains CGD1 and Env1 were inoculated into p47phox−/− mice as described for the survival experiments. Animals were sacrificed humanely 3 days postinoculation, and spleens were collected and processed for CFU analyses. The bacterial loads in the spleens from mice inoculated with strain CGD1 were 1,000 times higher than those in mice inoculated with strain Env1 (Fig. 6B). In addition, mice inoculated with strain CGD1 had significantly higher serum levels of the proinflammatory cytokine IL-1β than those exposed to strain Env1 (Fig. 6C).
DISCUSSION
CGD is a unique immunodeficiency caused by impaired phagocyte NAPDH oxidase activity. This defect leads to pneumonia and tissue abscesses from a narrow group of microorganisms, including the Bcc, Serratia marcescens, Staphylococcus aureus, Aspergillus spp., and Nocardia spp. (38). The Bcc also affects CF patients; however, little is known about why these two patient populations are particularly susceptible.
Earlier reports of Bcc from CGD or CF patients did not differentiate the Bcc at the species level; we now know that both B. cenocepacia and B. multivorans predominate in both patient populations, with B. multivorans being more frequently isolated. Bcc pathogenicity studies have focused on interactions of the Bcc (mostly B. cenocepacia) with normal and CF cells, with less research being done on the Bcc in CGD.
Data on a few B. multivorans strains show that it can attach, invade, and translocate across normal human epithelial cells (13, 22, 30) with different invasion routes (36). Using an infection system based on previously reported assays (5, 28), we compared the level and pattern of cellular association, invasion, and cytokine response of a respiratory B. multivorans clinical strain from a CGD patient to those of an environmental strain. We found remarkably high cell association and invasiveness of clinical isolate CGD1 in normal monocytes (Fig. 1). We also analyzed B. multivorans interactions with primary cells from CGD patients and healthy controls. Both strains showed similar cytokine induction on normal leukocytes (Fig. 2 and 5). There was no correlation between the levels of bacterium-cell association and cytokine induction. The latter suggests that the interaction of B. multivorans with the leukocyte surface (or a diffusible factor) mediates cell activation, and that bacterial uptake is not required for that induction, as described previously for another Burkholderia species (42).
CGD patients frequently suffer inflammatory complications, spurring a search for cellular correlates. CGD PBMCs have been reported to display a hyperinflammatory phenotype (7, 23) with increased TNF-α and IL-6 in response to Toll-like receptor agonists (7). In contrast, Hartl et al. showed lower levels of several Toll-like receptors on CGD neutrophils, resulting in decreased neutrophil activation (18). We observed a trend toward higher cytokine levels with CGD PBMCs; however, the difference from results for normal controls was not significant (P > 0.05) (Fig. 5).
Remarkably, we found significantly more association of B. multivorans with PBMCs and neutrophils of CGD patients than with normal controls (Fig. 3A and C). These microscopic observations were confirmed by CFU experiments showing ∼10 times more bacteria associated with CGD cells than with normal controls (Fig. 3B). Although the numbers of bacteria deduced from cell association experiments (Fig. 3A) do not equate with those obtained by CFU experiments (Fig. 3B), as they derive from very different techniques, they both show a similar pattern for each strain.
An aggregated pattern of infection of Env1, which is apparent in Fig. 1D and was described previously for this strain (13, 22, 30), may account for the CFU values observed for cell-associated Env1 (similarly to CGD1 in Fig. 3B) despite significant differences in the percentage of infected cells by both strains, as seen microscopically (Fig. 1A and 3A and C). Nevertheless, both microscopy and CFU experiments confirmed a preferential interaction of B. multivorans with CGD cells. We believe this difference has a net effect on the outcome of the infection, as we saw no differences in the viability of the two strains in the presence of cells (not shown).
To verify the specificity of this bacterial cell interaction, we showed that another CGD pathogen, Serratia marcescens, but not the related nonpathogenic Escherichia coli, bound preferentially to CGD PBMCs (Fig. 4). Similarly, the preferential adherence and ingestion of another typical CGD pathogen, S. aureus, was reported in neutrophils and monocytes of CGD patients (2, 19). These host- and pathogen-specific differences in physical interactions that presumably occur at the initial encounter of cells and bacteria likely reflect an important aspect of the remarkable selectivity of pathogens that infect CGD patients. The reported abnormal expression of several cellular receptors and neutrophils extracellular traps in CGD neutrophils (14, 18, 23) may be related to some of our findings.
In vivo studies showed a longer persistence of B. multivorans compared to that of B. cenocepacia in murine models of lung infection (9). On the other hand, mouse models of CGD allow the direct study of Bcc virulence in a target disease (20, 31). The Bcc is thought to be virulent in CGD due to its apparent intrinsic resistance to nonoxidative killing (37, 40); wild-type mice are known to be resistant to the Bcc (12, 20, 26). We compared the in vivo virulence of a clinical isolate (CGD1) to that of an environmental strain (Env1) in a mouse model of CGD following the in vitro assessment of cell association and cytokine induction characteristics using human cells.
Upon intraperitoneal inoculation into autosomal-recessive CGD mice (p47phox−/−), the clinical isolate was highly virulent while the environmental isolate was not (Fig. 6A). Under similar conditions, Segal et al. inoculated p47phox−/− mice with a CGD strain of the Bcc (later identified as B. cenocepacia) and showed lethality similar to what we observed with our clinical isolate (37). More recently, Sousa et al. used the intratracheal administration of a lower dose of bacteria (103 CFU) in a different CGD mouse model (X-linked CGD, gp91phox−/− mice) to compare different Bcc strains. Interestingly, while they observed 100% mortality with several isolates of B. cepacia and B. cenocepacia, the only B. multivorans strain they tested was avirulent (39). Even though Sousa et al. evaluated virulence of the Bcc in a mouse model of CGD, all of the strains that they tested were isolated from CF rather than CGD patients. In addition, these authors did not study the cellular interactions of the Bcc with cells from CGD patients.
Bcc variations in virulence are reflected by in vitro and in vivo qualitative and quantitative differences in bacterium-cell association, cytokine induction, and infection in susceptible mice. Despite the dissemination of both strains in our mouse model (Fig. 6B), the CGD clinical strain achieved much higher bacterial loads in spleen than the environmental strain at 2.5 days postinoculation (Fig. 6B), before animals were expected to start showing signs of distress or death (which can occur as early as day 3 postinoculation) (Fig. 6A). Mice inoculated with strain CGD1 also showed higher serum levels of the proinflammatory cytokine IL-1β than those inoculated with Env1 at 2.5 days postinoculation (Fig. 6C), which correlates with the observed differences in tissue bacterial load (Fig. 6B) and presumably with the induced tissue injury (27). Although Env1 is avirulent in CGD mice, we recognize that we cannot know if it would be virulent for CGD patients. The striking difference in virulence in CGD mice could be due to the presence of specific virulence factors in the CGD strain or to the presence of specific antivirulence factors in the environmental isolate.
Bcc virulence is complex and clearly involves both bacteria and host. The early preferential interactions of the CGD pathogens Burkholderia multivorans and Serratia marcescens with CGD leukocytes suggest that host susceptibility to specific bacteria in CGD begins at first sight.
Acknowledgments
We thank Frida Stock, Daniel P. Fedorko, Vee J. Gill, and Patrick R. Murray from the Microbiology Service, Department of Laboratory Medicine, Clinical Center, National Institutes of Health for providing the bacterial strains and for their scientific support. We thank Robert S. Munford for critically reviewing the manuscript.
This work was financially supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 27 July 2009.
REFERENCES
- 1.Baldwin, A., E. Mahenthiralingam, P. Drevinek, P. Vandamme, J. R. Govan, D. J. Waine, J. J. LiPuma, L. Chiarini, C. Dalmastri, D. A. Henry, D. P. Speert, D. Honeybourne, M. C. Maiden, and C. G. Dowson. 2007. Environmental Burkholderia cepacia complex isolates in human infections. Emerg. Infect. Dis. 13:458-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellinati-Pires, R., M. M. Salgado, I. P. Hypolito, A. S. Grumach, and M. M. Carneiro-Sampaio. 1995. Application of a fluorochrome-lysostaphin assay to the detection of phagocytic and bactericidal disturbances in human neutrophils and monocytes. J. Investig. Allergol. Clin. Immunol. 5:337-342. [PubMed] [Google Scholar]
- 3.Boyum, A. 1974. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens 4:269-274. [PubMed] [Google Scholar]
- 4.Bressler, A. M., K. S. Kaye, J. J. LiPuma, A. D. Alexander, C. M. Moore, L. B. Reller, and C. W. Woods. 2007. Risk factors for Burkholderia cepacia complex bacteremia among intensive care unit patients without cystic fibrosis: a case control study. Infect. Control Hosp. Epidemiol. 28:951-958. [DOI] [PubMed] [Google Scholar]
- 5.Burns, J. L., M. Jonas, E. Y. Chi, D. K. Clark, A. Berger, and A. Griffith. 1996. Invasion of respiratory epithelial cells by Burkholderia (Pseudomonas) cepacia. Infect. Immun. 64:4054-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bylund, J., P. A. Campsall, R. C. Ma, B. A. Conway, and D. P. Speert. 2005. Burkholderia cenocepacia induces neutrophil necrosis in chronic granulomatous disease. J. Immunol. 174:3562-3569. [DOI] [PubMed] [Google Scholar]
- 7.Bylund, J., K. L. Macdonald, K. L. Brown, P. Mydel, L. V. Collins, R. E. Hancock, and D. P. Speert. 2007. Enhanced inflammatory responses of chronic granulomatous disease leukocytes involve ROS-independent activation of NF-kappa B. Eur. J. Immunol. 37:1087-1096. [DOI] [PubMed] [Google Scholar]
- 8.Chiu, C. H., A. Ostry, and D. P. Speert. 2001. Invasion of murine respiratory epithelial cells in vivo by Burkholderia cepacia. J. Med. Microbiol. 50:594-601. [DOI] [PubMed] [Google Scholar]
- 9.Chu, K. K., D. J. Davidson, T. K. Halsey, J. W. Chung, and D. P. Speert. 2002. Differential persistence among genomovars of the Burkholderia cepacia complex in a murine model of pulmonary infection. Infect. Immun. 70:2715-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cieri, M. V., N. Mayer-Hamblett, A. Griffith, and J. L. Burns. 2002. Correlation between an in vitro invasion assay and a murine model of Burkholderia cepacia lung infection. Infect. Immun. 70:1081-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Soyza, A., C. D. Ellis, C. M. Khan, P. A. Corris, and R. Demarco de Hormaeche. 2004. Burkholderia cenocepacia lipopolysaccharide, lipid A, and proinflammatory activity. Am. J. Respir. Crit. Care Med. 170:70-77. [DOI] [PubMed] [Google Scholar]
- 12.Dinauer, M. C., M. A. Gifford, N. Pech, L. L. Li, and P. Emshwiller. 2001. Variable correction of host defense following gene transfer and bone marrow transplantation in murine X-linked chronic granulomatous disease. Blood 97:3738-3745. [DOI] [PubMed] [Google Scholar]
- 13.Duff, C., P. G. Murphy, M. Callaghan, and S. McClean. 2006. Differences in invasion and translocation of Burkholderia cepacia complex species in polarised lung epithelial cells in vitro. Microb. Pathog. 41:183-192. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs, T. A., U. Abed, C. Goosmann, R. Hurwitz, I. Schulze, V. Wahn, Y. Weinrauch, V. Brinkmann, and A. Zychlinsky. 2007. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176:231-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Govan, J. R., A. R. Brown, and A. M. Jones. 2007. Evolving epidemiology of Pseudomonas aeruginosa and the Burkholderia cepacia complex in cystic fibrosis lung infection. Future Microbiol. 2:153-164. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg, D. E., J. B. Goldberg, F. Stock, P. R. Murray, S. M. Holland, and J. J. LiPuma. 2009. Recurrent Burkholderia infection in patients with chronic granulomatous disease: 11-year experience at a large referral center. Clin. Infect. Dis. 48:1577-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guide, S. V., F. Stock, V. J. Gill, V. L. Anderson, H. L. Malech, J. I. Gallin, and S. M. Holland. 2003. Reinfection, rather than persistent infection, in patients with chronic granulomatous disease. J. Infect. Dis. 187:845-853. [DOI] [PubMed] [Google Scholar]
- 18.Hartl, D., N. Lehmann, F. Hoffmann, A. Jansson, A. Hector, G. Notheis, D. Roos, B. H. Belohradsky, and U. Wintergerst. 2008. Dysregulation of innate immune receptors on neutrophils in chronic granulomatous disease. J. Allergy Clin. Immunol. 121:375-382 e379. [DOI] [PubMed] [Google Scholar]
- 19.Hasui, M., Y. Hirabayashi, K. Hattori, and Y. Kobayashi. 1991. Increased phagocytic activity of polymorphonuclear leukocytes of chronic granulomatous disease as determined with flow cytometric assay. J. Lab. Clin. Med. 117:291-298. [PubMed] [Google Scholar]
- 20.Jackson, S. H., J. I. Gallin, and S. M. Holland. 1995. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 182:751-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keig, P. M., E. Ingham, and K. G. Kerr. 2001. Invasion of human type II pneumocytes by Burkholderia cepacia. Microb. Pathog. 30:167-170. [DOI] [PubMed] [Google Scholar]
- 22.Keig, P. M., E. Ingham, P. A. Vandamme, and K. G. Kerr. 2002. Differential invasion of respiratory epithelial cells by members of the Burkholderia cepacia complex. Clin. Microbiol. Infect. 8:47-49. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi, S. D., J. M. Voyich, K. R. Braughton, A. R. Whitney, W. M. Nauseef, H. L. Malech, and F. R. DeLeo. 2004. Gene expression profiling provides insight into the pathophysiology of chronic granulomatous disease. J. Immunol. 172:636-643. [DOI] [PubMed] [Google Scholar]
- 24.Lipuma, J. J. 2005. Update on the Burkholderia cepacia complex. Curr. Opin. Pulm. Med. 11:528-533. [DOI] [PubMed] [Google Scholar]
- 25.Mahenthiralingam, E., T. A. Urban, and J. B. Goldberg. 2005. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 3:144-156. [DOI] [PubMed] [Google Scholar]
- 26.Mardiney, M., III, S. H. Jackson, S. K. Spratt, F. Li, S. M. Holland, and H. L. Malech. 1997. Enhanced host defense after gene transfer in the murine p47phox-deficient model of chronic granulomatous disease. Blood 89:2268-2275. [PubMed] [Google Scholar]
- 27.Mariathasan, S., and D. M. Monack. 2007. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 7:31-40. [DOI] [PubMed] [Google Scholar]
- 28.Martin, D. W., and C. D. Mohr. 2000. Invasion and intracellular survival of Burkholderia cepacia. Infect. Immun. 68:24-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohr, C. D., M. Tomich, and C. A. Herfst. 2001. Cellular aspects of Burkholderia cepacia infection. Microbes Infect. 3:425-435. [DOI] [PubMed] [Google Scholar]
- 30.Moura, J. A., M. Cristina de Assis, G. C. Ventura, A. M. Saliba, L. Gonzaga, Jr., M. Si-Tahar, A. Marques Ede, and M. C. Plotkowski. 2008. Differential interaction of bacterial species from the Burkholderia cepacia complex with human airway epithelial cells. Microbes Infect. 10:52-59. [DOI] [PubMed] [Google Scholar]
- 31.Pollock, J. D., D. A. Williams, M. A. Gifford, L. L. Li, X. Du, J. Fisherman, S. H. Orkin, C. M. Doerschuk, and M. C. Dinauer. 1995. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 9:202-209. [DOI] [PubMed] [Google Scholar]
- 32.Reik, R., T. Spilker, and J. J. Lipuma. 2005. Distribution of Burkholderia cepacia complex species among isolates recovered from persons with or without cystic fibrosis. J. Clin. Microbiol. 43:2926-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saini, L. S., S. B. Galsworthy, M. A. John, and M. A. Valvano. 1999. Intracellular survival of Burkholderia cepacia complex isolates in the presence of macrophage cell activation. Microbiology 145:3465-3475. [DOI] [PubMed] [Google Scholar]
- 34.Sajjan, U., S. Keshavjee, and J. Forstner. 2004. Responses of well-differentiated airway epithelial cell cultures from healthy donors and patients with cystic fibrosis to Burkholderia cenocepacia infection. Infect. Immun. 72:4188-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sajjan, U., Y. Wu, G. Kent, and J. Forstner. 2000. Preferential adherence of cable-piliated Burkholderia cepacia to respiratory epithelia of CF knockout mice and human cystic fibrosis lung explants. J. Med. Microbiol. 49:875-885. [DOI] [PubMed] [Google Scholar]
- 36.Schwab, U. E., C. M. Ribeiro, H. Neubauer, and R. C. Boucher. 2003. Role of actin filament network in Burkholderia multivorans invasion in well-differentiated human airway epithelia. Infect. Immun. 71:6607-6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segal, B. H., L. Ding, and S. M. Holland. 2003. Phagocyte NADPH oxidase, but not inducible nitric oxide synthase, is essential for early control of Burkholderia cepacia and Chromobacterium violaceum infection in mice. Infect. Immun. 71:205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segal, B. H., T. L. Leto, J. I. Gallin, H. L. Malech, and S. M. Holland. 2000. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 79:170-200. [DOI] [PubMed] [Google Scholar]
- 39.Sousa, S. A., M. Ulrich, A. Bragonzi, M. Burke, D. Worlitzsch, J. H. Leitao, C. Meisner, L. Eberl, I. Sa-Correia, and G. Doring. 2007. Virulence of Burkholderia cepacia complex strains in gp91phox−/− mice. Cell Microbiol. 9:2817-2825. [DOI] [PubMed] [Google Scholar]
- 40.Speert, D. P., M. Bond, R. C. Woodman, and J. T. Curnutte. 1994. Infection with Pseudomonas cepacia in chronic granulomatous disease: role of nonoxidative killing by neutrophils in host defense. J. Infect. Dis. 170:1524-1531. [DOI] [PubMed] [Google Scholar]
- 41.Stevens, J. M., R. L. Ulrich, L. A. Taylor, M. W. Wood, D. Deshazer, M. P. Stevens, and E. E. Galyov. 2005. Actin-binding proteins from Burkholderia mallei and Burkholderia thailandensis can functionally compensate for the actin-based motility defect of a Burkholderia pseudomallei bimA mutant. J. Bacteriol. 187:7857-7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Utaisincharoen, P., N. Anuntagool, S. Arjcharoen, I. Lengwehasatit, K. Limposuwan, P. Chaisuriya, and S. Sirisinha. 2004. Burkholderia pseudomallei stimulates low interleukin-8 production in the human lung epithelial cell line A549. Clin. Exp. Immunol. 138:61-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valvano, M. A., K. E. Keith, and S. T. Cardona. 2005. Survival and persistence of opportunistic Burkholderia species in host cells. Curr. Opin. Microbiol. 8:99-105. [DOI] [PubMed] [Google Scholar]
- 44.Winkelstein, J. A., M. C. Marino, R. B. Johnston, Jr., J. Boyle, J. Curnutte, J. I. Gallin, H. L. Malech, S. M. Holland, H. Ochs, P. Quie, R. H. Buckley, C. B. Foster, S. J. Chanock, and H. Dickler. 2000. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 79:155-169. [DOI] [PubMed] [Google Scholar]
- 45.Zahariadis, G., M. H. Levy, and J. L. Burns. 2003. Cepacia-like syndrome caused by Burkholderia multivorans. Can. J. Infect. Dis. 14:123-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zughaier, S. M., H. C. Ryley, and S. K. Jackson. 1999. Lipopolysaccharide (LPS) from Burkholderia cepacia is more active than LPS from Pseudomonas aeruginosa and Stenotrophomonas maltophilia in stimulating tumor necrosis factor alpha from human monocytes. Infect. Immun. 67:1505-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]