Abstract
The lethal toxin (LeTx) of Bacillus anthracis plays a key role in the pathogenesis of anthrax. The protective antigen (PA) is a primary part of the anthrax toxin and forms LeTx by combination with lethal factor (LF). Phenylalanine-427 (F427) is crucial for PA function. This study was designed to discover potential novel therapeutic agents and vaccines for anthrax. This was done by screening PA mutants that were mutated at the F427 residue for a dominant-negative inhibitory (DNI) phenotype which was nontoxic but inhibited the toxicity of the wild-type LeTx. For this, PA residue F427 was first mutated to each of the other 19 naturally occurring amino acids. The cytotoxicity and DNI phenotypes of the mutated PA proteins were tested in the presence of 1 μg/ml LF in RAW264.7 cells and were shown to be dependent on the individual amino acid replacements. A total of 16 nontoxic mutants with various levels of DNI activity were identified in vitro. Among them, F427D and F427N mutants had the highest DNI activities in RAW264.7 cells. Both mutants inhibited LeTx intoxication in mice in a dose-dependent way. Furthermore, they induced a Th2-predominant immune response and protected mice against a challenge with five 50% lethal doses of LeTx. The protection was correlated mainly with a low level of interleukin-1β (IL-1β) and with high levels of PA-specific immunoglobulin G1, IL-6, and tumor necrosis factor alpha. Thus, PA DNI mutants, such as F427D and F427N mutants, may serve in the development of novel therapeutic agents and vaccines to fight B. anthracis infections.
Anthrax is a contagious zoonotic disease caused by Bacillus anthracis. B. anthracis is a rod-shaped, gram-positive, spore-forming bacterium. The spores are highly resistant to adverse environments, can survive for long periods, and are easy to produce. The bacteria initiate infection by the entry of spores into the host through abrasions in the skin, ingestion, or inhalation (7), and most B. anthracis infections are highly lethal. Naturally occurring human anthrax infection is rare, and it occurs primarily as a professional disease found in farmers and veterinarians through direct exposure to spores from infected animals or animal products such as hides or wool (28). However, the potential use of the spores as a biological weapon in terrorism and warfare poses a great threat to the public. Therefore, there is an urgent need for effective means of protection against anthrax (1, 5).
The virulence of B. anthracis is attributed primarily to two major factors, i.e., a tripartite toxin and the poly-γ-d-glutamic acid capsule (5, 7, 39). Anthrax toxins play a key role in the pathogenesis of anthrax, both in the early stages of disease and during disease progression. There are three toxins involved in anthrax, namely, protective antigen (PA), lethal factor (LF), and edema factor (EF). The combination of PA with LF results in lethal toxin (LeTx), while the combination of PA with EF forms edema toxin (19, 25).
The current standard of care for postexposure prophylaxis of inhalational anthrax is ciprofloxacin therapy twice daily for 60 days (8). Because of a demonstrated propensity to develop resistance and the ineffectiveness of antibiotic therapy during the late stage of B. anthracis infection, when the toxins are responsible for pathogenesis, vaccination may provide a novel approach to protection against B. anthracis infection (11, 26, 32, 36, 38, 42). PA is an essential component of the currently approved vaccines against anthrax (10). The antibodies to PA can neutralize anthrax toxin by blocking the adherence of PA to host cells, the binding of LF/EF to PA, or the assembly of the PA heptamer (1, 2).
However, it is dangerous to administer native PA as a vaccine either during or shortly after anthrax infection. As a functional part of anthrax toxin, PA (83 kDa) binds to the cell surface receptors, and furin or a furin-like protease will cleave it to an active, 63-kDa form (PA63). PA63 subsequently oligomerizes into a heptameric, receptor-bound prepore that can bind up to three molecules of EF and/or LF competitively. The resulting toxin-receptor complexes are endocytosed into endosomes, where the prepore experiences a conformational rearrangement under acidic pH to form a transmembrane pore. Subsequently, EF or LF is transported through the pore into the cell cytosol, where it exerts its enzymatic toxic actions (5).
It has been demonstrated that mutations of PA in domains 2 and 3 block translocation or self-assembly of the toxin specifically, but they do not seriously impair proteolytic activation and receptor binding (20, 35, 37). Some PA mutants, such as K397D, D425K, and F427A mutants or the domain II β2-β3 loop deletion mutant, inhibit toxin action strongly in cell cultures and provide immediate protection against anthrax toxins in vivo by coassembling with the wild-type protein (WPA) and blocking its ability to translocate the enzymatic moieties across membranes (35, 37). This is called the dominant-negative inhibitory (DNI) phenotype. In protection against anthrax, the induction of a DNI phenotype may be advantageous since a stronger immunogenic effect than that with WPA is obtained (1, 44). Therefore, postexposure use of PA DNI mutants may provide improved immunogenicity and therapeutic activity simultaneously.
Phenylalanine-427 (F427) of PA is a solvent-exposed residue in the lumen of the pore and is crucial for both steps, pore formation and protein translocation, in the overall transport function of PA (41). Sun et al. (41) replaced the PA F427 residue with 11 other amino acids and found that in vitro the mutants were affected in PA translocation, conformational transition of the prepore to the pore, or both, depending on the amino acid species (41). We therefore hypothesized that the activity of DNI mutants in vivo may also be dependent on amino acid substitution at F427. To the best of our knowledge, among all the mutants tested, only the F427A mutant has been shown to have the DNI phenotype and to have therapeutic activity in vivo (22).
In this study, we constructed a saturated point mutation library at residue F427 of PA and screened all 19 amino acid replacement mutants for the DNI phenotype in vitro. Two mutants, the F427N and F427D mutants, with the highest inhibitory activity were tested further for their therapeutic activity and immunogenicity in mice. In addition, the systemic release of proinflammatory cytokines in mice was studied to elucidate the underlying mechanism of protection against LeTx challenge, because proinflammatory cytokines have been reported previously to be a major factor in anthrax mortality (14, 34). The results of this study may be of significance in developing novel anthrax vaccines and antibacterial therapeutic agents.
MATERIALS AND METHODS
PA gene cloning and F427X mutation.
The entire PA gene (GenBank accession no. AF065404), excluding the portion that encodes the signal sequence, was PCR amplified from the noncapsulated attenuated strain of B. anthracis (Tecon Biotechnology Limited Company, Xinjiang, China) and cloned into the expression vector pGEX-KG, resulting in pGEX-KG-PA. The oligonucleotides used as forward and reverse primers for generating F427X mutations of PA in pGEX-KG-PA were as follows: F427X-f, 5′-CGCATTAAATGCACAAGACGATNNNAGTTCTACTCC-3′; and F427X-r, 5′-CATTGTAATTGGAGTAGAACTNNNATCGTCTTGTGC-3′ (N represents A, T, G, or C).
The four primers used to generate F427W and F427M mutations were as follows: F427W-f, 5′-CGCATTAAATGCACAAGACGATTGGAGTTCTACTCC-3′; F427W-r, 5′-CATTGTAATTGGAGTAGAACTCCAATCGTCTTGTGC-3′; F427M-f, 5′-CGCATTAAATGCACAAGACGATATGAGTTCTACTCC-3′; and F427M-r, 5′-CATTGTAATTGGAGTAGAACTCATATCGTCTTGTGC-3′ (mutated codons are shown in bold).
Long PCR was performed in 50-μl reaction volumes, using a DNA thermal cycler (Applied Biosystems). The reaction mix consisted of 20 ng of template DNA, a 0.2 mM concentration of each deoxynucleoside triphosphate, 0.1 nmol of each oligonucleotide, 1× PrimeSTAR polymerase buffer, and 1 unit of PrimeSTAR HS DNA polymerase (TaKaRa Biotechnology Co., Ltd.). PCR was performed under the following conditions: initial denaturation for 30 s at 98°C and 15 cycles of 10 s at 98°C, 15 s at 53°C, and 8 min at 68°C. After amplification, the reaction mix was incubated at 72°C for a further 5 min. The plasmid DNA was purified using a QIAquick PCR purification kit (Qiagen Co., Ltd.), subjected to treatment with 1 unit DpnI (NEB, Inc.) at 37°C for 1 h to eliminate any methylated template, and transformed into competent Escherichia coli DH5α cells. The amino acid replacements at position 427 of PA were identified by sequencing of the saturation libraries at Shanghai Sangon Biological Engineering Technology & Services Co., Ltd.
Protein expression and purification.
The proteins used were expressed and purified as described previously (4, 15). Briefly, the plasmids encoding PA, F427X mutant PAs (MPAs), and LF were transformed into BL21-CodonPlus (DE3)-RIL competent cells for expression. A single clone harboring the respective plasmid was grown at 37°C in 1 liter of Luria broth (LB) containing 100 μg/ml ampicillin and 35 μg/ml chloromycetin at 200 rpm. When the optical density at 600 nm reached 0.8, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the culture at a final concentration of 0.4 mM; the bacteria were then incubated at 28°C for a further 3 h. The culture was subjected to centrifugation at 8,000 × g for 5 min at 4°C, and the cell pellets were resuspended in 50 ml precooled phosphate-buffered saline (PBS) buffer and disrupted using a French cell press (Thermo Fisher Scientific Inc.). The glutathione S-transferase-PA protein in the supernatant was collected by centrifugation at 12,000 × g for 20 min at 4°C. The supernatant was added to a glutathione-Sepharose column, followed by washing with 100 ml PBS buffer to elute the unbound proteins. Subsequently, 20 units of thrombin (Sigma) was added directly to the resin-bound PA to remove the glutathione S-transferase tag according to the manufacturer's protocols. After the reaction, 15 ml PBS buffer was added to elute the protein of interest. The protein was then concentrated with a 30-kDa-cutoff Centriplus YM-30 concentrator (Millipore) and further purified with benzamidine-Sepharose (Amersham Pharmacia) to eliminate thrombin. The protein concentration was measured by using a bicinchoninic acid protein assay kit according to the manufacturers' instructions (Thermo Fisher Scientific Inc. and Pierce Biotechnology), with bovine serum albumin as the standard. The purity of the extracted proteins was analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Cytotoxicity and DNI assay of PA mutants in vitro.
WPA and the F427X MPAs were tested for susceptibility to cleavage by trypsin. The proteins (0.2 mg/ml) were incubated with trypsin (1:1,000 [wt/wt]) (Sigma) for 30 min at room temperature in PBS containing 25 mM HEPES, 1 mM CaCl2, and 0.5 mM EDTA (pH 7.5). The digestion reactions were stopped by adding phenylmethylsulfonyl fluoride to a final concentration of 1 mM. The samples were isolated and visualized by 12% SDS-PAGE.
The cytotoxicity of MPAs was tested on the LeTx-sensitive mouse macrophage cell line RAW264.7. The cells were grown in Dulbecco's modified Eagle's medium (Gibco, Invitrogen Corporation) containing 10% fetal bovine serum (Shanghai ExCell Biology, Inc.), 2 mM l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin. The cells were plated in 96-well plates (Greiner Bio-One) at a density of 3 × 104 cells/well. After 16 h of incubation, the medium was removed by gentle aspiration and replaced with 100 μl of fresh Dulbecco's modified Eagle's medium containing 1 μg/ml LF and various concentrations of WPA or F427X MPAs, which were twofold serially diluted from 20 μg/ml to 0.04 μg/ml. After 4 h of coincubation at 37°C, 10 μl (1:10 [vol/vol]) AlamarBlue dye (BioSource International, Inc.) was added as described previously (37). The cultures were incubated for a further 4 h at 37°C, and the fluorescence of each well was monitored with a multidetection microplate reader (BioTek) at an excitation wavelength of 530 nm and an emission wavelength of 590 nm for one cycle, with the gain set at 35. The viability of the cells was calculated based on the fluorescence intensity values at a wavelength of 590 nm (FI590) according to the manufacturer's instructions, as follows: % viability = (FI590 of test agent well − FI590 of negative control well)/(FI590 of positive control well − FI590 of negative control well) × 100. The negative control wells contained medium plus AlamarBlue but no cells, the positive control wells contained viable cells in medium plus AlamarBlue, and the test agent wells contained cells in medium, toxin proteins, and AlamarBlue.
For DNI assays, MPAs replaced WPA, with decreasing ratios of MPAs to WPA (wt/wt), i.e., 2:1, 1:1, 1:2, 1:3, 1:4, 1:6, 1:8, and 1:16, in the mixture of MPAs, WPA, and LF. For all groups, WPA and LF were kept at a constant concentration of 1 μg/ml, with previous experiments having established that these conditions induced 100% cell lysis. All of the experiments were performed in triplicate.
Toxicity of LeTx and the mutants in BALB/c mice.
Fifty-two female BALB/c mice (each 15 to 18 g) were randomly assigned to 13 groups, with four mice per group. Mice were injected intravenously with a mixture of purified LeTx (60 μg PA plus 25 μg LF), with or without the F427D or F427N mutant. The F427N and F427D mutants were injected in amounts of 60, 30, 15, 7.5, or 5 μg for different groups in a mixture containing LeTx in PBS buffer. At the same time, 60 μg WPA and 25 μg LF were injected separately, as the negative controls, or combined, as the positive control. The final volume of the dose injected into each mouse was 100 μl. After injection, the mice were kept under observation in the animal facility at Wuhan Ke Qian Biologicals Co. Ltd. for 10 days, and their survival was recorded.
On day 10 after injection of LeTx, the surviving mice were sacrificed. The spleens were collected, weighed immediately, and then fixed in 10% neutral buffered formalin. Paraffin-embedded sections were prepared, stained with hematoxylin and eosin according to conventional protocols, and examined under a light microscope.
Mouse immunization and LeTx challenge.
Forty-eight 5- to 6-week-old BALB/c mice were divided into six groups, with eight mice per group. The mice in the three immunization groups were injected with purified WPA or the F427N or F427D mutant, while the three negative control groups were mock immunized with PBS. For mouse immunization, 20 μg protein in 50 μl PBS buffer was injected intraperitoneally with an equal volume of Al(OH)3 containing 0.18 mg Al(OH)3 three times at intervals of 2 weeks. Blood samples were collected from the tail veins at 1 week postpriming and after each booster, and the sera from each group were collected, pooled, and stored at −80°C for antibody detection.
One week after the third immunization, the immunized mice and one group of negative control mice were challenged with LeTx, using a mixture of 60 μg WPA and 25 μg LF per mouse, representing five 50% lethal doses of LeTx, by tail vein injection. The other two PBS groups were challenged with either 60 μg WPA or 25 μg LF alone. Two hours after the challenge, four mice in each group were randomly selected and bled from the tail vein for cytokine detection because it has been shown that cytokine levels peak at this time point (18). Hemostasis was performed on the selected mice. All of the mice were kept under observation in the animal facility at Wuhan Ke Qian Biologicals Co. Ltd. for 10 days, and their survival was recorded.
Antibody detection.
PA-specific IgG titers were detected in all mice by end-point enzyme-linked immunosorbent assay (ELISA) (26, 32). Ninety-six-well plates were coated overnight at 4°C with 0.5 μg of purified PA in 100 μl of 50 mM sodium carbonate buffer (pH 9.6). Serum samples were twofold serially diluted, beginning at 1:100. After incubation of the samples for 1 h at 37°C, horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Southern Biotech) was used as the secondary antibody, and TMB/H2O2 was used as the HRP substrate to develop the reaction. The absorbance was determined at a wavelength of 630 nm. Titers of IgG subtypes were determined similarly, but HRP-conjugated goat anti-mouse IgG1 or IgG2a (Southern Biotech) replaced HRP-conjugated goat anti-mouse IgG as the secondary antibody. End-point titers were calculated as the reciprocal of the last serum dilution that gave a value two times greater than that for serum samples from preimmunized mice (26, 32).
Serum cytokine detection.
The levels of interleukin-6 (IL-6), IL-1β, tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and IL-18 in the sera were measured using commercial ELISA kits according to the manufacturer's protocol (R&D). Concentrations of cytokines were determined by comparison to standard curves produced using the purified cytokines provided in the kits.
Statistical analysis.
Analysis of variance or Student's unpaired t test was used to analyze the differences in IgG ELISA titers and cytokine levels of different groups. Statistical comparisons were made using a 5% level of significance.
RESULTS
Saturation mutagenesis at residue 427 of PA.
Sequencing and restriction digestion confirmed that the PA gene was cloned correctly into the expression vector pGEX-KG, resulting in pGEX-KG-PA. Fifty colonies were sent to be sequenced, and this confirmed that all colonies had experienced mutation at the expected sites, while the remaining sequence was unchanged. Among the colonies, 46 had amino acid replacements at residue 427 of wild-type PA, consisting of 17 naturally occurring amino acids. No tryptophan (W) or methionine (M) mutation was found. The other four colonies contained nonsense mutations. The F427M and F427W mutants were obtained by site-directed mutagenesis with specific primers. As a result, substitutions at the F427 residue of PA were obtained with all 19 naturally occurring amino acids.
Expression and purification of WPA, MPA, and LF.
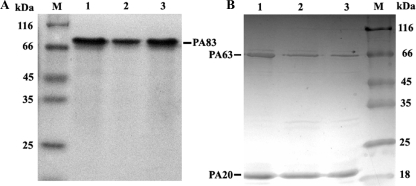
Soluble PA, MPAs, and LF were obtained after induction of the appropriate recombinant BL21-CodonPlus (DE3)-RIL strains at 28°C. SDS-PAGE demonstrated that the purified products were 96% pure. The average yield for PA or MPAs was 2 mg per liter of bacterial culture. Furthermore, trypsin could successfully cleave the WPA, as well as the F427X MPAs, from 83 kDa into two fragments, of 63 kDa and 20 kDa, as expected (Fig. 1).
FIG. 1.
SDS-PAGE analysis of purified WPA and MPAs (A) and trypsin cleavage of WPA and MPAs (B). Lanes 1, WPA; lanes 2, F427D mutant; lanes 3, F427N mutant; lanes M, reference protein marker. The proteins of 83 kDa were cleaved into fragments of 63 kDa and 20 kDa, as shown in panel B.
Cytotoxicity and DNI assay in vitro.
When WPA or LF was added to the cells separately at all tested concentrations, it was nontoxic to the RAW264.7 macrophage cell line, and all of the cells retained 100% viability. However, when WPA ranging from 0.63 μg/ml to 20 μg/ml was combined with LF at 1 μg/ml, all of the cells lost viability. Meanwhile, the F427X MPAs decreased cytotoxicity to different extents. Among the mutants, 16 had completely lost the ability to cause cytotoxicity, with cells having 100% viability at all concentrations tested, ranging from 0.04 to 20 μg/ml. Although the remaining three mutants, the F427W, F427L, and F427Y mutants, retained a small amount of cytotoxicity, they were significantly attenuated (P < 0.01). For example, at a concentration of 2.5 μg/ml for WPA or MPAs with LF at 1 μg/ml, the viabilities of the cells treated with WPA and the F427W, F427L, and F427Y mutants were −0.8% ± 5.0%, 33.0% ± 2.0%, 26.9% ± 1.4%, and 94.0% ± 6.5%, respectively (Fig. 2).
FIG. 2.
Cytotoxicity of WPA and partial MPAs. WPA at concentrations ranging from 0.63 μg/ml to 20 μg/ml was combined with LF at 1 μg/ml; all of the cells lost viability. However, cells incubated with the F427D and F427N mutants, like the case for the other 14 nontoxic MPAs, could maintain 100% cell viability. Although the remaining three mutants, the F427W, F427L, and F427Y mutants, retained some cytotoxicity, they were significantly attenuated.
The DNI activities of 16 nontoxic mutants were tested further in RAW264.7 cells by mixing the individual MPAs with WPA at various ratios while both PA and LF were kept at 1 μg/ml. Generally speaking, the cytotoxicity of the MPA, WPA, and LF mixtures increased as the proportion of MPA decreased. The ratios (MPAs to WPA) of 2:1 and 1:1 provided complete protection of the cells for all 16 nontoxic mutants. The interference of MPAs with the cytotoxicity of WPA was dose dependent, and the average viabilities of the cells at MPA-to-WPA ratios of 2:1, 1:1, 1:2, 1:3, 1:4, 1:6, 1:8, 1:12, and 1:16 for all 16 mutants were 102.7% ± 4.6%, 98.6% ± 5.4%, 94.6% ± 5.3%, 88.0% ± 5.9%, 76.4% ± 4.7%, 58.2% ± 5.3%, 43.2% ± 4.76%, 23.63% ± 4.6%, and 11.2% ± 5.2%, respectively (Fig. 3).
FIG. 3.
DNI activity of F427X MPAs. RAW264.7 cells were incubated with MPAs at different ratios of MPA to WPA, from 2:1 to 1:16, while both WPA and LF were kept at 1 μg/ml in the mixture of WPA, LF, and MPA. The viability of the cells was calculated based on the FI590 values for AlamarBlue.
However, different mutants showed various DNI effects. The F427D and F427N mutants showed the strongest inhibitory activities in vitro. For example, at a ratio of 1:4, both the F427D and F427N mutants protected the cells completely from toxic effects, while the other mutants only partially inhibited the cytotoxicity of WPA, to different extents, with the viabilities of the cells falling below 93.60% ± 2.69%. Furthermore, the F427D mutant exerted a stronger inhibitory effect than that of the F427N mutant. The MPA-to-WPA ratio that gave the cells 100% protection was 1:6 for the F427D mutant, while it was 1:4 for the F427N mutant (Fig. 3).
Although the F427V mutant showed similar protection to that of the F427D and F427N mutants at high ratios of MPA to WPA, it showed a lower inhibitory ability at low ratios. For example, when the MPA-to-WPA ratio was 1:16, the viability of the treated cells with the F427V, F427D, and F427N mutants decreased to 3%, 31%, and 21%, respectively. Thus, we selected the F427D and F427N mutants for further investigation.
DNI assay in mice.
To verify the protective activity of the F427D and F427N mutants in vivo, BALB/c mice were injected with LeTx, with or without the F427D or F427N mutant. All of the mice injected with LeTx, containing a mixture of 25 μg LF and 60 μg WPA, died within 12 h. However, all of the mice survived the lethal challenge with LeTx when 7.5 to 60 μg of the F427D mutant or 15 to 60 μg of the F427N mutant was coinjected with LeTx at the same concentration as that described above (Table 1). The lowest ratios of MPAs to WPA that provided complete protection were 1:8 for the F427D mutant and 1:4 for the F427N mutant, respectively. The results confirmed that the F427D mutant had a stronger inhibitory effect in vivo, similar to the in vitro result.
TABLE 1.
PA F427D and F427N mutants inhibit LeTx toxicity in BALB/c mice
| Amt of WPA (μg) | Amt of added protein (μg)a
|
No. of survivors/no. of challenged mice | Degree of splenomegalyb | ||
|---|---|---|---|---|---|
| LF | F427D mutant | F427N mutant | |||
| 60 | 25 | 0 | 0 | 0/4 | NDc |
| 0 | 0 | 0 | 4/4 | − | |
| 25 | 60 | 0 | 4/4 | − | |
| 25 | 30 | 0 | 4/4 | + | |
| 25 | 15 | 0 | 4/4 | + | |
| 25 | 7.5 | 0 | 4/4 | +++ | |
| 25 | 5 | 0 | 2/4 | ++++ | |
| 25 | 0 | 60 | 4/4 | − | |
| 25 | 0 | 30 | 4/4 | + | |
| 25 | 0 | 15 | 4/4 | ++ | |
| 25 | 0 | 7.5 | 2/4 | ++++ | |
| 25 | 0 | 5 | 0/4 | NDd | |
| 0 | 25 | 0 | 0 | 4/4 | − |
Mixtures of constant amounts of WPA and LF with various amounts of MPAs were injected intravenously into BALB/c mice. WPA or LF was injected separately as a negative control, while LeTx injected alone was taken as a positive control. The mice were observed for 10 days.
The mice which survived for 10 days were sacrificed, and splenomegaly was determined by spleen weight.
Not determined. The mice died within 12 h, and spleen weights were in the normal range.
Not determined. The mice died within 72 h, and spleen weights were slightly increased.
The splenomegaly shown by surviving mice from different groups differed significantly at day 10 after LeTx injection, decreasing with increasing proportions of MPA in LeTx mixtures (Table 1 and Fig. 4). When 60 μg of the F427D or F427N mutant (MPA/WPA ratio = 1:1) was coinjected with LeTx (25 μg LF and 60 μg WPA), no splenomegaly was observed, and the spleens weighed 0.088 ± 0.017 g, similar to the negative control spleens (0.083 ± 0.013 g) (P = 0.688). However, the weight of the spleen increased significantly, to 0.113 ± 0.020 g (P = 0.053), 0.170 ± 0.026 g (P = 0.003), 0.255 ± 0.031 g (P = 0.0005), and 0.400 ± 0.081 g (P = 0.004), when the ratio of MPA to WPA decreased to 1:2, 1:4, 1:8, and 1:12, respectively.
FIG. 4.
Histopathologic examination of spleens and splenomegaly response of surviving mice on day 10 after the injection of a mixture of LF at 25 μg/ml, WPA at 60 μg/ml, and MPAs at different ratios of F427D mutant to WPA, from 1:1 to 1:12, or after injection of WPA alone as a negative control. Paraffin-embedded sections of the spleen were stained with hematoxylin and eosin and analyzed with a light microscope. (A) Mice injected with 60 μg WPA alone as a negative control. The spleen showed a normal structure. Magnification, ×400. (B) The spleen showed a normal structure and was associated with a significant immune response, with increased leukocytes and the appearance of germinal centers (indicated by the white arrow), in the mice coinjected with 60 μg F427D mutant, 60 μg WPA, and 25 μg LF. Magnification, ×400. (C) The spleen from a mouse coinjected with 5 μg F427D mutant, 60 μg WPA, and 25 μg LF experienced a marked reduction of splenocytes and disruption of the red and white pulp structure. Magnification, ×400. (D) Gross appearance of spleens from mice after injection of LeTx and the F427D mutant before they were sampled for paraffin-embedded section preparation as described for panels A to C. (E) Different ratios of F427D mutant to WPA in the mixture of 60 μg WPA and 25 μg LF. (F) Corresponding weight of spleens in panel D.
The histopathological observations confirmed the above findings (Fig. 4). When the ratio of MPA to WPA was 1:12, the architecture of the spleen was disrupted, as shown by the disappearance of red and white pulp and a considerable loss of splenocytes. In contrast, for the groups given an MPA-to-WPA ratio of 1:1 (60 μg of F427D or F427N mutant and an equal amount of WPA in LeTx mixtures), the spleens showed normal histology, with a clear structure of red and white pulp and normal cell densities. In addition, the apparent germinal centers developed in experimental groups, as shown by light zones surrounded by dark zones in the white pulp, indicating that a humoral immune response was induced. This contrasted with the negative control group, where only dark zones with dense packing of lymphocytes in the white pulp were observed (Fig. 4).
Antibody response.
WPA and the F427D and F427N mutants were all highly immunogenic to BALB/c mice and induced high titers of IgG, as expected, while very low IgG titers were detected in mock (PBS)-immunized mice. At week 1 after the third immunization, the F427D and F427N mutants and WPA elicited IgG production, with titers of 1:512,000, 1:409,600 and 1:281,600, respectively, detected by ELISA (Fig. 5A). The IgG response to the mutants was stronger than that to WPA, but only the F427D mutant produced a statistically significantly higher titer of IgG than that with WPA (P = 0.012). Although WPA and the F427N and F427D mutants induced both IgG1 and IgG2a, the ratios of the mean IgG1 titer to the mean IgG2a titer for WPA, the F427N mutant, and the F427D mutant were 5, 8, and 5, respectively. Therefore IgG1, representing a Th2-type response, was predominant (Fig. 5B).
FIG. 5.
PA-specific antibody titers in immunized mice. The mice were immunized with WPA or the F427D or F427N mutant three times at 2-week intervals. Serum samples were collected 3 and 5 weeks after priming, and the IgG titers to PA were detected with end-point ELISA and are shown as dilutions. (A) Total IgG titers. (B) IgG1 and IgG2a titers at week 5 after priming. Both IgG1 and IgG2a were produced, but IgG1 was the predominant subtype.
Protection of immunized mice against LeTx challenge.
The immunized and mock (PBS)-inoculated mice were given LeTx containing 60 μg PA and 25 μg LF through the tail vein. Mice in the PBS group showed clinical signs, such as trembling and unkempt coats, and died within 12 h after LeTx challenge. In contrast, all 24 mice immunized with WPA or the F427N or F427D mutant exhibited no clinical signs, survived the lethal challenge, and remained healthy during the 10-day observation period.
Cytokine responses after LeTx challenge.
Serum samples from four mice in each group were assayed for the levels of TNF-α, IL-1β, IFN-γ, IL-18, and IL-6. The cytokine levels after LeTx challenge in mice from both immunized and negative control groups were significantly higher than those before the toxin challenge (P < 0.01) (Fig. 6). The average serum levels of IL-1β and IFN-γ in immunized mice, which were fully protected from LeTx challenge, were lower than those of the negative control mice that died from the LeTx challenge (Fig. 6A and B). However, only the mean IL-1β level of the immunized mice (276.36 ± 63.18 pg/ml) was statistically significantly lower than that of control mice (7,463.05 ± 532.23 pg/ml) (P = 0.0001). In contrast, the mean serum levels of IL-6, TNF-α, and IL-18 in immunized mice were higher than those of the negative control mice (Fig. 6C and D). Among these cytokines, the greatest statistically significant difference between the immunized and negative control mice was seen for mean IL-6 levels (10,923.38 ± 891.38 versus 9,163.15 ± 470.86 pg/ml; P = 0.0004), with a slight difference in the mean TNF-α levels (726.87 ± 56.98 versus 610.17 ± 81.02 pg/ml; P = 0.0553) and no statistically significant difference in the mean IL-18 levels (130.73 ± 15.29 versus 102.91 ± 25.10 pg/ml; P = 0.1089). Thus, a decrease in IL-1β secretion and an increase in the production of IL-6 may be significant contributors to the protective response to immunization. Challenge of PBS-inoculated mice with either PA or LF alone did not induce any detectable increase in cytokine production (data not shown).
FIG. 6.
Serum cytokine levels in immunized mice 2 h after LeTx challenge. Mice were vaccinated with WPA, the F427N mutant, the F427D mutant, or PBS buffer three times and then challenged with five 50% lethal doses of LeTx. Serum samples were collected from four mice from each group, and the cytokine levels were determined with commercial capture ELISA kits. The data were pooled according to the immunized and PBS groups. The error bars represent the standard errors for the groups.
DISCUSSION
There are three ways to tackle anthrax, including vaccination to prevent bacterial infection in the first place, antibiotics to cure infection, and antitoxin treatments to prevent the toxic effects of the bacterium during the late stage of infection (10).
Because PA is the central component of both anthrax LeTx and edema toxin, it has been studied extensively as a vaccine candidate for a long time. As demonstrated previously, PA is able to elicit neutralizing antibodies that play a major role in protective immunity against anthrax (1, 11, 12, 36, 38). However, it is dangerous to administer the native form of PA early after anthrax infection because PA serves as a portal for the entry of the enzymatic moieties of the toxin into the cytosol (13, 16, 21, 45). Therefore, nonfunctional PA DNI mutants may be an ideal substitute for WPA in a potential vaccine against anthrax. This theory was confirmed by evidence showing that double mutation, K397D and D425K, of PA not only rendered PA mutants nonfunctional but also enhanced their immunogenic potency in mice (1, 33, 35, 44). Phenylalanine-427 of PA is another candidate site for mutation because it is crucial for the transport function of PA, being involved in both pore formation and protein translocation (41). Furthermore, some F427 mutants have been proven to be DNI, with the PA F427A mutant having therapeutic activity in vivo (22). In this study, we replaced the F427 residue of PA with the other 19 naturally occurring amino acids by saturation mutagenesis and obtained each purified individual mutated PA protein. Trypsin cleavage in vitro demonstrated that the F427 mutations did not affect cleavage of 83-kDa PA into its active form, 63-kDa PA, probably because the enzyme site (RKKR) of PA is situated at residues 163 to 167, which are located far from the mutated site, residue 427. Additionally, the receptor binding domain, which is within domain 4 (residues 596 to 735), is also distant from the mutated F427 residue (3, 27, 33). This implies that the F427X MPAs may not affect the binding of PA to the cell surface receptors and/or cleavage by either furin or a furin-like protease on the cells. The findings in the current study confirmed the above hypothesis. All 16 nontoxic F427X MPAs displayed dose-dependent DNI effects in RAW264.7 cells. Among them, the F427D and F427N mutants exerted a therapeutic function in mice. This suggests that MPAs are able to complete the whole process of binding to cell surface receptors, being cleaved by furin or a furin-like protease, and co-oligomerizing into a heptameric, receptor-bound prepore in a competitive manner with WPA both in vitro and in vivo. One previous study has also lent support to the view that F427 mutation does not affect the binding of PA to the cell surface receptors (41). In this study, the loss of cytotoxicity to various degrees most likely resulted from deficiencies in the subsequent steps of conformational transition of the heptameric PA prepore to the pore and of protein translocation through the pore. This conclusion was also reached by Sun et al. (41), with the ability of F427X MPAs to inhibit pore formation or translocation being dependent on the side chain at residue 427. The replacement of F427 with R, D, or G strongly inhibited the acidic pH-induced conformational transition, whereas H, S, and T did not affect this step at all but inhibited translocation instead (16, 41). Moreover, the current study further confirmed previous findings by obtaining more PA mutants with amino acid replacements at residue 427 and determining that 16 of them were nontoxic, while the other three mutants were attenuated.
Although a loss of cytotoxicity is a prerequisite for the possible utilization of PA mutants in therapy and for vaccination, it is not directly correlated with the DNI phenotype. In the LeTx-sensitive cell line RAW264.7, the 16 nontoxic F427X MPAs exhibited different DNI effects. Among them, the F427D and F427N mutants had the strongest inhibitory activities. These activities may result from interference with pore formation, translocation, or both of these steps by individual amino acid replacements (41). The F427N mutant is a novel mutant with the DNI phenotype that was developed in this study.
To demonstrate further the potential of DNI F427X MPAs as novel antibacterial therapeutic agents, the F427D and F427N mutants were selected to be tested in mice. The mice were administered a background dose of purified LeTx (60 μg PA plus 25 μg LF) and five doses of the mutants, with WPA-to-MPA ratios of 1:1, 2:1, 4:1, 8:1, and 12:1. The evidence from the measured parameters, including number of surviving mice, splenomegaly, and histopathologic examination, demonstrated that both the F427D and F427N mutants possessed dose-dependent therapeutic activities. As seen in RAW264.7 cells, the F427D mutant exhibited stronger inhibitory effects than the F427N mutant in mice. In addition, an immune response was observed on spleen histology, as indicated by spleen histology for the surviving mice on day 10 after the lethal LeTx challenge. When mice were injected with 60 μg of F427N or F427D mutant with LeTx (60 μg PA plus 25 μg LF), the animals were protected from death and lymphocyte proliferation in their spleens was induced, as shown by the induction of germinal centers in the white pulp matter.
PA DNI mutants inhibit the toxicity of LeTx by replacing WPA to form hybrid PA heptamers in a competitive way, as discussed previously (35; this study). Theoretically, it is necessary for PA mutants to appear before the occurrence of LeTx (WPA and wild-type LF) to compete with WPA and display their inhibitory activity. Thus, the clinical therapeutic potential of these PA mutants for postexposure anthrax treatment might be exploited if the PA mutants are injected early, before bacterial entry into the body or at an early stage of infection when bacteria are in small numbers and secreting small amounts of toxin. In these circumstances, PA mutants could be used in combination with antibiotics for treatment.
Previous studies have demonstrated that the PA DNI phenotype is associated with a stronger immune response than that to WPA (1, 44), and the current study confirmed that both F427D and F427N PA mutants stimulated higher IgG titers than WPA did. However, only the F427D PA mutant stimulated statistically significantly higher IgG titers than those of WPA (P < 0.01). This result suggests that the immunogenicity of certain PA DNI mutants is related to the individual amino acid replacements in PA, in a similar manner to the cytotoxicity and DNI effects of the mutants, as mentioned above. Furthermore, the immunity to PA was a balance of Th1- and Th2-type responses, because both IgG1 and IgG2a were induced by WPA or MPA. However, the Th2-type response was predominant.
Based on the fact that systemic proinflammatory cytokines play a double role in anthrax, serving not only as mediators of lethality related to apoptotic cell death and inflammation but also as protectors against bacterial infection (14, 17, 18, 29-31), we explored whether the proinflammatory cytokine profile was changed in mice immunized with DNI mutants and protected from LeTx challenge. The cytokine profiles 2 h after LeTx challenge of either immunized or PBS-treated control mice, which later survived or died, respectively, were compared. The only statistically significant differences between the surviving and dead mice were in levels of IL-1β (P = 0.0001) and IL-6 (P = 0.0004).
As is well known, IL-1β is a major proinflammatory cytokine that contributes to LeTx-mediated cytotoxicity and apoptosis (17, 43). In vitro, macrophages that undergo membrane perturbation and cytolysis after LeTx treatment release IL-1β (6, 23, 43). In vivo, IL-1β accounts for the enhanced disease progression in LeTx-treated BALB/c mice and is considered to be a key modulator of host septic shock (6, 31), being responsible for fever, hypoperfusion, circulatory collapse, shock, metabolic acidosis, and cardiac dysfunction (46).
Because IL-1β is one of the substrates of caspase-1, the IL-1β converting enzyme, the mechanism for this cell death induced by IL-1β is related to activation of caspase-1. As reported previously, LeTx binds to the cell surface receptor, is internalized, and enters the endosomes. The acidification of the endosome mediates translocation of LF into the host cell cytosol. Catalytically active LF cleaves cytosolic substrates and activates caspase-1 by a mechanism that involves proteasome activity, potassium efflux, and the inflammasome adapter Nalp1 (24). The active caspase-1 cleaves its substrate, pro-IL-1β, into mature IL-1β and mediates a pathway of proinflammatory programmed cell death termed “pyroptosis.” Pyroptosis features DNA cleavage, cytokine activation, and ultimately cell lysis resulting from the formation of membrane pores and pathological ion fluxes (9, 23).
In the current study, a >20-fold decrease in IL-1β level was detected in the surviving mice compared to the dead mice. The most obvious reason for this phenomenon is that PA-specific antibodies effectively neutralized LeTx and significantly decreased the LeTx-mediated activation of caspase-1 and production of IL-1β. Therefore, the significantly lower level of IL-1β in serum may be a major reason for the protection of the immunized mice against LeTx-induced death.
Conversely, significantly higher levels of IL-6 were observed in immunized, surviving mice than in PBS-treated control (dead) mice after LeTx challenge. Given that Th2 cells secret IL-6, this cytokine profile was in agreement with an atmosphere dominated by Th2 cells in the immunized mice, which was confirmed by the IgG1 response observed in this study. The much higher level of IL-6 was likely another major reason that the immunized mice survived the LeTx challenge. This conclusion is supported by other studies showing that IL-6 contributes to resistance against B. anthracis in mice (31) and provides the principle mode of protection in the acute response of the liver to B. anthracis (40).
In this study, we constructed 19 PA F427X mutants. The cytotoxicity, DNI potential, and immunogenicity of the mutants were dependent on the individual amino acid replacements. A total of 16 nontoxic mutants were identified in a cell model. Among them, the PA F427D and F427N mutants were determined to have a DNI phenotype, both in RAW264.7 cells and in mice. These two PA DNI mutants possessed dose-dependent therapeutic activities against LeTx challenge in mice. They induced a predominantly Th2-type immune response and mediated protection against lethal LeTx challenge, which was correlated mainly with low levels of IL-1β and high levels of IL-6 and PA-specific IgG1. Therefore, the PA F427D and F427N DNI mutants have great potential as novel therapeutic agents and vaccines against B. anthracis.
Acknowledgments
We thank Paul R. Langford and Dieter M. Schifferli for critical readings of the manuscript.
This work was supported by the Key Project of the Chinese Ministry of Education (grant 306013).
Editor: S. R. Blanke
Footnotes
Published ahead of print on 20 July 2009.
REFERENCES
- 1.Aulinger, B. A., M. H. Roehrl, J. J. Mekalanos, R. J. Collier, and J. Y. Wang. 2005. Combining anthrax vaccine and therapy: a dominant-negative inhibitor of anthrax toxin is also a potent and safe immunogen for vaccines. Infect. Immun. 73:3408-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brey, R. N. 2005. Molecular basis for improved anthrax vaccines. Adv. Drug Deliv. Rev. 57:1266-1292. [DOI] [PubMed] [Google Scholar]
- 3.Brossier, F., J. C. Sirard, C. Guidi-Rontani, E. Duflot, and M. Mock. 1999. Functional analysis of the carboxy-terminal domain of Bacillus anthracis protective antigen. Infect. Immun. 67:964-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao, S., Z. Liu, A. Guo, Y. Li, C. Zhang, G. Wu, C. Feng, Y. Tan, and H. Chen. 2008. Efficient production and characterization of Bacillus anthracis lethal factor and a novel inactive mutant rLFm-Y236F. Protein Expr. Purif. 59:25-30. [DOI] [PubMed] [Google Scholar]
- 5.Collier, R. J., and J. A. T. Young. 2003. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 19:45-70. [DOI] [PubMed] [Google Scholar]
- 6.Cordoba-Rodriguez, R., H. Fang, C. S. Lankford, and D. M. Frucht. 2004. Anthrax lethal toxin rapidly activates caspase-1/ICE and induces extracellular release of interleukin (IL)-1beta and IL-18. J. Biol. Chem. 279:20563-20566. [DOI] [PubMed] [Google Scholar]
- 7.Dixon, T. C., M. Meselson, J. Guillemin, and P. C. Hanna. 1999. Anthrax. N. Engl. J. Med. 341:815-826. [DOI] [PubMed] [Google Scholar]
- 8.Drusano, G. L., O. O. Okusanya, A. Okusanya, B. V. Scoy, D. L. Brown, R. Kulawy, F. Sörgel, H. S. Heine, and A. Louie. 2008. Is 60 days of ciprofloxacin administration necessary for postexposure prophylaxis for Bacillus anthracis? Antimicrob. Agents Chemother. 52:3973-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fink, S. L., T. Bergsbaken, and B. T. Cookson. 2008. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc. Natl. Acad. Sci. USA 105:4312-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedlander, A. M. 2001. Tackling anthrax. Nature 414:160-161. [DOI] [PubMed] [Google Scholar]
- 11.Gaur, R., P. K. Gupta, A. C. Banerjea, and Y. Singh. 2002. Effect of nasal immunization with protective antigen of Bacillus anthracis on protective immune response against anthrax toxin. Vaccine 20:2836-2839. [DOI] [PubMed] [Google Scholar]
- 12.Grabenstein, J. D. 2008. Vaccines: countering anthrax: vaccines and immunoglobulins. Clin. Infect. Dis. 46:129-136. [DOI] [PubMed] [Google Scholar]
- 13.Gupta, P., A. Singh, V. Chauhan, and R. Bhatnagar. 2001. Involvement of residues 147VYYEIGK153 in binding of lethal factor to protective antigen of Bacillus anthracis. Biochem. Biophys. Res. Commun. 280:158-163. [DOI] [PubMed] [Google Scholar]
- 14.Hanna, P. C., D. Acosta, and R. J. Collier. 1993. On the role of macrophages in anthrax. Proc. Natl. Acad. Sci. USA 90:10198-10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, J., Y. M. Kim, B. S. Koo, Y. K. Chae, and M. Y. Yoon. 2003. Production and proteolytic assay of lethal factor from Bacillus anthracis. Protein Expr. Purif. 30:293-300. [DOI] [PubMed] [Google Scholar]
- 16.Melnyk, R. A., and R. J. Collier. 2006. A loop network within the anthrax toxin pore positions the phenylalanine clamp in an active conformation. Proc. Natl. Acad. Sci. USA 103:9802-9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miura, M., H. Zhu, R. Rotello, E. A. Hartwieg, and J. Yuan. 1993. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell 75:653-660. [DOI] [PubMed] [Google Scholar]
- 18.Moayeri, M., D. Haines, H. A. Young, and S. H. Leppla. 2003. Bacillus anthracis lethal toxin induces TNF-alpha-independent hypoxia-mediated toxicity in mice. J. Clin. Investig. 112:670-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mock, M., and A. Fouet. 2001. Anthrax. Annu. Rev. Microbiol. 55:647-671. [DOI] [PubMed] [Google Scholar]
- 20.Mogridge, J., M. Mourez, and R. J. Collier. 2001. Involvement of domain 3 in oligomerization by the protective antigen moiety of anthrax toxin. J. Bacteriol. 183:2111-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mourez, M., D. B. Lacy, K. Cunningham, R. Legmann, B. R. Sellman, J. Mogridge, and R. J. Collier. 2002. 2001: a year of major advances in anthrax toxin research. Trends Microbiol. 10:287-293. [DOI] [PubMed] [Google Scholar]
- 22.Mourez, M., M. Yan, D. B. Lacy, L. Dillon, L. Bentsen, A. Marpoe, C. Maurin, E. Hotze, D. Wigelsworth, R. A. Pimental, J. D. Ballard, R. J. Collier, and R. K. Tweten. 2003. Mapping dominant-negative mutations of anthrax protective antigen by scanning mutagenesis. Proc. Natl. Acad. Sci. USA 100:13803-13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muehlbauer, S. M., T. H. Evering, G. Bonuccelli, R. C. Squires, A. W. Ashton, S. A. Porcelli, M. P. Lisanti, and J. Brojatsch. 2007. Anthrax lethal toxin kills macrophages in a strain-specific manner by apoptosis or caspase-1-mediated necrosis. Cell Cycle 6:758-766. [DOI] [PubMed] [Google Scholar]
- 24.Nour, A. M., Y. G. Yeung, L. Santambrogio, E. D. Boyden, E. R. Stanley, and J. Brojatsch. 2009. Anthrax lethal toxin triggers the formation of a membrane-associated inflammasome complex in murine macrophages. Infect. Immun. 77:1262-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsnes, S., J. V. Kozlov, B. Deurs, and K. Sandvig. 1991. Bacterial protein toxins acting on intracellular targets. Semin. Cell Biol. 2:7-14. [PubMed] [Google Scholar]
- 26.Peachman, K. K., M. Rao, C. R. Alving, R. Burge, S. H. Leppla, V. B. Rao, and G. R. Matyas. 2006. Correlation between lethal toxin-neutralizing antibody titers and protection from intranasal challenge with Bacillus anthracis Ames strain spores in mice after transcutaneous immunization with recombinant anthrax protective antigen. Infect. Immun. 74:794-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petosa, C., R. J. Collier, K. R. Klimpel, S. H. Leppla, and R. C. Liddington. 1997. Crystal structure of the anthrax toxin protective antigen. Nature 385:833-838. [DOI] [PubMed] [Google Scholar]
- 28.Pilo, P., V. Perreten, and J. Frey. 2008. Molecular epidemiology of Bacillus anthracis: determining the correct origin. Appl. Environ. Microbiol. 74:2928-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popov, S. G., R. Villasmil, J. Bernardi, E. Grene, J. Cardwell, A. Wu, D. Alibek, C. Bailey, and K. Alibek. 2002. Lethal toxin of Bacillus anthracis causes apoptosis of macrophages. Biochem. Biophys. Res. Commun. 293:349-355. [DOI] [PubMed] [Google Scholar]
- 30.Popov, S. G., R. Villasmil, J. Bernardi, E. Grene, J. Cardwell, T. Popova, A. Wu, D. Alibek, C. Bailey, and K. Alibek. 2002. Effect of Bacillus anthracis lethal toxin on human peripheral blood mononuclear cells. FEBS Lett. 527:211-215. [DOI] [PubMed] [Google Scholar]
- 31.Popov, S. G., T. G. Popova, E. Grene, F. Klotz, J. Cardwell, C. Bradburne, Y. Jama, M. Maland, J. Wells, A. Nalca, T. Voss, C. Bailey, and K. Alibek. 2004. Systemic cytokine response in murine anthrax. Cell. Microbiol. 6:225-233. [DOI] [PubMed] [Google Scholar]
- 32.Price, B. M., A. L. Liner, S. Park, S. H. Leppla, A. Mateczun, and D. R. Galloway. 2001. Protection against anthrax lethal toxin challenge by genetic immunization with a plasmid encoding the lethal factor protein. Infect. Immun. 69:4509-4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosovitz, M. J., P. Schuck, M. Varughese, A. P. Chopra, V. Mehra, Y. Singh, L. M. McGinnis, and S. H. Leppla. 2003. Alanine-scanning mutations in domain 4 of anthrax toxin protective antigen reveal residues important for binding to the cellular receptor and to a neutralizing monoclonal antibody. J. Biol. Chem. 278:30936-30944. [DOI] [PubMed] [Google Scholar]
- 34.Schroder, K., P. J. Hertzog, T. Ravasi, and D. A. Hume. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75:163-189. [DOI] [PubMed] [Google Scholar]
- 35.Sellman, B. R., M. Mourez, and R. J. Collier. 2001. Dominant-negative mutants of a toxin subunit: an approach to therapy of anthrax. Science 292:695-697. [DOI] [PubMed] [Google Scholar]
- 36.Singh, Y., B. E. Ivins, and S. H. Leppla. 1998. Study of immunization against anthrax with the purified recombinant protective antigen of Bacillus anthracis. Infect. Immun. 66:3447-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh, Y., H. Khanna, A. P. Chopra, and V. Mehra. 2001. A dominant negative mutant of Bacillus anthracis protective antigen inhibits anthrax toxin action in vivo. J. Biol. Chem. 276:22090-22094. [DOI] [PubMed] [Google Scholar]
- 38.Sloat, B. R., and Z. Cui. 2006. Nasal immunization with anthrax protective antigen protein adjuvanted with polyriboinosinic-polyribocytidylic acid induced strong mucosal and systemic immunities. Pharm. Res. 23:1217-1226. [DOI] [PubMed] [Google Scholar]
- 39.Smith, H. 2000. Discovery of the anthrax toxin: the beginning of in vivo studies on pathogenic bacteria. Trends Microbiol. 8:199-200. [DOI] [PubMed] [Google Scholar]
- 40.Streetz, K. L., T. Wustefeld, C. Klein, M. P. Manns, and C. Trautwein. 2001. Mediators of inflammation and acute phase response in the liver. Cell. Mol. Biol. 47:661-673. [PubMed] [Google Scholar]
- 41.Sun, J., A. E. Lang, K. Aktories, and R. J. Collier. 2008. Phenylalanine-427 of anthrax protective antigen functions in both pore formation and protein translocation. Proc. Natl. Acad. Sci. USA 105:4346-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan, Y., N. R. Hackett, J. L. Boyer, and R. G. Crystal. 2003. Protective immunity evoked against anthrax lethal toxin after a single intramuscular administration of an adenovirus-based vaccine encoding humanized protective antigen. Hum. Gene Ther. 14:1673-1682. [DOI] [PubMed] [Google Scholar]
- 43.Wickliffe, K. E., S. H. Leppla, and M. Moayeri. 2008. Anthrax lethal toxin-induced inflammasome formation and caspase-1 activation are late events dependent on ion fluxes and the proteasome. Cell. Microbiol. 10:332-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan, M., M. H. Roehrl, E. Basar, and J. Y. Wang. 2008. Selection and evaluation of the immunogenicity of protective antigen mutants as anthrax vaccine candidates. Vaccine 26:947-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young, J. A., and R. J. Collier. 2007. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 76:243-265. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, X. H., R. M. Zhu, W. A. Xu, H. J. Wan, and H. Lu. 2007. Therapeutic effects of caspase-1 inhibitors on acute lung injury in experimental severe acute pancreatitis. World J. Gastroenterol. 13:623-627. [DOI] [PMC free article] [PubMed] [Google Scholar]