Abstract
The Myc transcription factor is a potent inducer of proliferation and is required for Wnt/β-catenin signaling in intestinal epithelium. Since deregulation of the Wnt/β-catenin pathway is a prerequisite for nonhereditary intestinal tumorigenesis, we asked whether activation of Myc recapitulates the tumorigenic changes that are driven by constitutive Wnt/β-catenin pathway signaling following adenomatous polyposis coli (APC) inactivation. Using mice in which expression of MycERTAM, a reversibly switchable form of Myc, is expressed transgenically in intestinal epithelium, we define the acute changes that follow Myc activation as well as subsequent deactivation. Myc activation reversibly recapitulates many, but not all, aspects of APC inactivation, including increased proliferation and apoptosis and loss of goblet cells. However, whereas APC inactivation induces redistribution of Paneth cells, direct Myc activation triggers their rapid attrition. Moreover, direct Myc activation engages the ARF/p53/p21cip1 tumor suppressor pathway, whereas deregulation of Wnt/β-catenin signaling does not. These observations illustrate key differences in oncogenic impact in intestinal epithelium of direct Myc activation and indirect Myc activation via the Wnt/β-catenin pathway. Furthermore, the in situ dedifferentiation of mature goblet cells that Myc induces indicates a novel cross talk between the Wnt/β-catenin and Notch signaling pathways.
Intestinal epithelium is a highly proliferative tissue that is constantly renewed through the processes of cell division, migration, and shedding. The epithelium is comprised of a crypt-villus unit that contains four differentiated cell types: enterocytes, enteroendothelial cells, goblet cells, and Paneth cells. These are all generated from pluripotent stem cell progenitors that reside at either the base (4, 5) or the +4 position (17, 39, 49) within each crypt and feed a CD133+ transit-amplifying cell population (56) that drives the 3- to 5-day-long upward migration of the enterocyte, enteroendothelial, and goblet cells to the villus tip, where they are eventually shed. Accumulating evidence suggests that the continuously proliferating lgr5+ crypt cell is the cell of origin in those intestinal tumors driven by aberrant signaling through the Wnt/β-catenin pathway (3), which constitute the great majority of colorectal cancers.
Two principal signaling pathways determine cell fate in the intestinal epithelium: the Wnt/β-catenin and Notch pathways. The secreted Wnt glycoproteins bind transmembrane receptors of the frizzled family, inhibiting the adenomatous polyposis coli (APC)/axin/CK1/glycogen synthase kinase 3b destruction complex that phosphorylates β-catenin and signals its degradation. β-Catenin then accumulates and translocates to the cell nucleus, where it forms an activating complex with the TCF-4 transcription factor and regulates expression of a characteristic set of targets (60) that includes the genes encoding the Myc oncoprotein (16), cyclin D1 (59), and the canonical intestinal crypt stem cell marker lgr5 (65). Signaling through the Wnt/β-catenin pathway, which is strongest in the crypt and declines along a gradient to the villus tip, is required for maintenance and proliferation of both the crypt stem cell and adjacent transit-amplifying compartments. Consistent with this, nuclear β-catenin is found only in the proliferative areas of the crypts in normal intestinal epithelium (25). The Wnt/β-catenin pathway is also critical for the spatial disposition of the various cell types within the crypt-villus unit, both Paneth cell maturation and retention at the crypt bottom and, in association with the ephrin receptors B2 and B3 (themselves encoded by β-catenin/TCF-4 target genes), distribution and migration of epithelial cells along the crypt-villus axis (6, 7). Inhibition of the Wnt/β-catenin pathway, whether through ectopic expression of the Wnt antagonist Dikkopf-1 (43), by genetic deletion (25), or by dominant negative inhibition of TCF-4 (61), leads to a diminished intestinal stem cell compartment and greatly reduced crypt proliferation. Conversely, constitutive signaling through the Wnt/β-catenin/TCF pathway effectively locks intestinal cells in a crypt progenitor-like phenotype, driving precocious proliferation and suppressing differentiation (61). Deregulation of Wnt/β-catenin signaling causally underlies almost all nonhereditary colorectal carcinomas. In most cases, deregulation arises through mutations that inactivate the APC tumor suppressor, a component of the obligate complex required to degrade β-catenin (45). However, a significant fraction of tumors instead inactivate Axin1 or harbor mutations in the serine residues of β-catenin that glycogen synthase kinase 3 phosphorylates to trigger β-catenin degradation (34). Whichever happens, the result is accumulation of nuclear β-catenin and deregulation of the β-catenin-TCF transcriptional program (26), blocking differentiation and driving the relentless cell expansion that underpins most colorectal cancers.
Both the Notch receptors and their Delta and Jagged ligands are transmembrane proteins. Upon ligation, Notch proteins are cleaved by γ-secretase to release an intracellular domain (NICD) that complexes with the DNA-binding protein RBP-J (58), displacing associated corepressors and activating expression of a set of target genes that include Hes1. Hes1 encodes a basic helix-loop-helix (bHLH) transcription factor that, in turn, represses expression of Math1, another bHLH protein that would otherwise direct differentiation away from the enterocyte lineage toward the three principal Paneth, goblet, and enteroendocrine secretory cell types. Selection out of these three terminal cell fates is determined by interactions with several other bHLH proteins, including neurogenin 3 (Ngn3) (18), Gfi1 (55), and NeuroD (38). When Notch signaling is inhibited, for example, by γ-secretase inhibitors, differentiation defaults to the Math1-dependent secretory lineage, rapidly resulting in a lethal intestinal goblet cell metaplasia (32). Conversely, Math1-deficient mutant mice lack all secretory cell intestinal lineages (66). Substantial evidence indicates that the Wnt/β-catenin and Notch signaling pathways act together to determine form and function along the crypt-villus axis (37).
Myc is a highly pleiotropic bHLHZip transcription factor that coordinates the diverse intracellular and extracellular transcriptional programs required for normal and tumor cell expansion. Myc expression is deregulated in most human cancers. However, preternaturally high levels of Myc trigger apoptosis and activation of ARF/p53, two intrinsic tumor suppressor programs that serve to constrain its oncogenic potential (29). It may be for this reason that Myc is directly activated by mutation (e.g., amplification and translocation) in only a small proportion of cancer types and, even then, usually only in more-aggressive variants that may already harbor deficits in apoptosis and/or the p53 pathway. More commonly, deregulated Myc expression in tumors appears to be a consequence of its relentless induction by the wide variety of upstream oncogenic signals that drive human tumorigenesis, including Wnt/β-catenin (61), Notch pathways (23), and receptor tyrosine kinase signaling via Ras. Myc is required both for the normal mitogenic actions of physiological Wnt/β-catenin signaling in promoting crypt proliferation in the intestine (35) and for the dramatic architectural disruption, precocious proliferation, and suppression of differentiation induced by Wnt/β-catenin deregulation (50). However, the surprisingly scant overlap between Myc (http://www.myccancergene.org/site/mycTargetDB.asp) and Wnt/β-catenin/TCF-4 (60; http://www-leland.stanford.edu/∼rnusse/wntwindow.html) target genes intimates that Myc, while necessary, cannot be the sole downstream transcriptional effector of the Wnt/β-catenin/TCF-4 pathway. Ascertaining the role played by Myc in intestinal homeostasis is also complicated by its likely role as a target of the Notch pathway, as has been shown for other epithelial (23), as well as hematopoietic (40, 53, 64), lineages. It is also clear that the intestinal Wnt/β-catenin, Notch, and Myc pathways do not function independently but are deeply intertwined. Not only do both Wnt and Notch pathways activate Myc, but Wnt signaling itself activates the Notch pathway in intestinal epithelium through induction of the Notch ligand Jagged 1 (48), while Myc has been shown to reinforce Wnt/β-catenin signaling by repressing the Wnt inhibitors DKK1 and SFRP1 (9) and inducing the Wnt receptor Fzd9 (27).
To ascertain the specific role played by Myc within the intestinal epithelium, as well as to establish the extent to which Myc can or cannot substitute for either Wnt or Notch signaling, we transgenically targeted expression of the reversibly switchable form of Myc, MycERTAM (28), to intestinal epithelium. Using this model, we have addressed the following questions. (i) Does acute activation of Myc recapitulate the phenotype observed upon acute loss of APC? (ii) Are the changes in differentiation observed upon Myc activation due to alterations in the status of terminally differentiated cells or due to changes at earlier stages of differentiation? (iii) What are the consequences of subsequent deactivation of Myc for intestinal epithelium that has already undergone Myc-driven changes?
MATERIALS AND METHODS
Transgenic mice.
The villin-MycERTAM transgene was generated by fusing a 9-kb fragment of the villin promoter (44) with the MycERTAM open reading frame (28) followed by a simian virus 40 polyA cassette. Vector DNA was microinjected into male-embryo pronuclei, and three lines positive for the transgene were isolated and propagated. MycERTAM was activated in intestinal epithelium by daily intraperitoneal injection of 1 mg tamoxifen dissolved in a peanut oil vehicle. All studies involving mice adhered to protocol number AN076148 and were approved by the University of California, San Francisco (UCSF), Institutional Animal Care and Use Committee.
Immunohistochemistry.
To identify cells in S phase, mice were injected intraperitoneally with 2 μmol bromodeoxyuridine (BrdU) 3.5 h prior to sacrifice. For histological analyses, tissue samples were harvested, immersed in Z-fix (Anatech Ltd., Battle Creek, MI) overnight, dehydrated, and embedded in paraffin and 5-μm sections were cut, which were then rehydrated and boiled in 10 mM sodium citrate (pH 6.0) at low power for 5 min to recover antigens. Following citrate retrieval (or EDTA retrieval, in the case of γ-H2.AX), primary antibodies—including rabbit polyclonal antibodies to serotonin (5-hydroxytryptamine [5-HT]), lysozyme (DakoCytomation), p53 (Vector), p21cip1 (BD Pharmingen), Muc2 (Abcam), and γ-H2.AX (Upstate)—were applied in blocking buffer (2.5% bovine serum albumin, 5% goat serum, 0.3% Triton X-100 in phosphate-buffered saline) for 2 to 16 h. Incorporated BrdU and apoptotic cells were detected with a BrdU detection kit II (Roche) and an Apoptag kit (Chemicon), respectively, according to the manufacturers' instructions. After each slide was washed, secondary antibodies (Molecular Probes) were applied in blocking buffer for 30 min. Fluorescent-antibody-labeled slides were rinsed in phosphate-buffered saline and mounted in Dako fluorescent mounting medium containing 1 μg/ml Hoechst stain. The terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) signal colocalized with Hoechst nuclear counterstain, although the latter was often irregular and weak due to pyknosis and nuclear disintegration. Alcian blue (Poly Scientific, Bayshore, NY) was used according to the manufacturer's instructions. Fluorescent images were collected in the Laboratory for Cell Analysis (UCSF Comprehensive Cancer Center) using an LSM510 confocal microscope (Zeiss) or an Axiovert 100 inverted microscope (Zeiss) equipped with a Hamamatsu Orca digital camera.
Quantitative real-time PCR.
Total RNA was extracted from mouse tissues by mechanical homogenization in TRIzol reagent (Invitrogen, Carlsbad, CA) followed by phenol-chloroform isolation, as per the manufacturer's protocol. cDNA was synthesized from 1 μg of DNase I (Invitrogen, Carlsbad, CA)-treated RNA using iScript (Bio-Rad, Hercules, CA). Real-time quantitative PCR was performed by the UCSF Comprehensive Cancer Center Genome Analysis core facility. The p19ARF primer/probe sets used to detect the p19ARF mRNA transcripts were as follows: 5′AGA GGA TCT TGA GAA GAG GGC C3′ (forward) and 5′GCA GTT CGA ATC TGC ACC3′ (reverse) for primers and 5′FAM-AAT CCT GGA CCA GGT GAT GAT GAT GGG-3′6TAMSp as the probe, where FAM is 6-carboxyfluorescein and 3′6TAMSp is tetramethyl-6-carboxyrhodamine (31).
RESULTS
Myc activation in intestinal epithelium leads to rapid and lethal changes in tissue morphology.
Elevated expression of the Myc proto-oncogene is observed in approximately 50 to 70% of all colon adenocarcinomas, in most instances as a direct result of precocious signaling through the Wnt/β-catenin/TCF pathway. To assess how aberrant activity of Myc directly contributes to the intestinal tumor phenotype, we generated mice expressing MycERTAM under the control of the villin promoter, a regulatory element active in all intestinal epithelial lineages. Immunofluorescence staining for the ERTAM moiety confirmed MycERTAM transgene expression throughout the intestinal epithelium (data not shown). Several independent villin-MycERTAM transgenic lines were generated, and all shared similar phenotypes. Hence, one was chosen for further study.
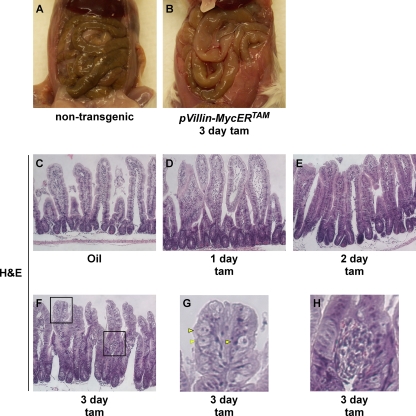
Sustained ectopic activation of MycERTAM in villin- MycERTAM mice induced lethargy, weight loss, poor grooming, and morbidity within 3 to 5 days. Analysis of blood chemistry after 3 days of Myc activation (data not shown) indicated a marked decrease in blood urea nitrogen and phosphate, consistent with starvation. Examination of the gastrointestinal tract revealed a full stomach but grossly swollen small intestines almost devoid of luminal content (Fig. 1A and B), indicating that starvation was (i) likely due to a failure of intestinal absorption rather than a failure to eat and (ii) consistent with the morbidity known to accompany perturbations in intestinal homeostasis (15, 66). Histological examination indicated that ectopic activation of Myc in intestinal epithelium rapidly elicits dramatic changes in tissue and cellular morphology. After only 1 day of Myc activation, the luminal spaces between villi were markedly reduced, a trend that progressed over time accompanied by an increase in overall cellular mass (Fig. 1C to F). Villi assumed a crypt-like morphology, similar to that observed following acute deletion of APC (51). After 3 days of Myc activation, the relative disposition of stromal and epithelial compartments was profoundly disrupted, with the frequent appearance of a stromal bulb (Fig. 1F to H) situated partway up the villus, indicating that the epithelial layer had outgrown its supporting stroma. We also observed frequent abnormal epithelial cell morphology, with some cells displaying a swollen cytoplasm that stained very weakly with eosin (Fig. 1G). We guess that these gross changes in intestinal structure underlie the impaired absorption and the starvation that Myc activation induces in these mice.
FIG. 1.
Myc activation in intestinal epithelium elicits rapid morphological changes. Nontransgenic control (A) and villin-MycERTAM (B) mice were treated with tamoxifen (tam) for 3 days and dissected (C to F). Intestinal swelling is clearly evident following 3 days of Myc activation. Hematoxylin and eosin (H&E) analysis of villin-MycERTAM mice shows that Myc activation drives villi into a more crypt-like morphology. Regions boxed in panel F are magnified in panels G and H. (G) Yellow arrowheads show morphologically abnormal epithelial cells. (H) An example of epithelial overgrowth and detachment from the underlying stroma is detailed.
Direct activation of Myc in intestinal epithelium induces increased proliferation, induction of apoptosis, and changes in secretory cell composition.
Myc drives multiple intracellular programs, including proliferation, dedifferentiation, apoptosis, and a shift to biosynthetic metabolism, as well as diverse extracellular programs associated with cell expansion, including angiogenesis, stromal remodeling, invasion, and inflammation. However, the net output of Myc activation is highly dependent upon cell type, context, and level (36). Hence, direct activation of Myc may not always recapitulate the effect of endogenous Myc indirectly activated by an upstream signaling pathway. Since Myc is the key effector responsible for aberrant intestinal epithelial proliferation in response to deregulated β-catenin signaling (51), we asked whether Myc activity alone is sufficient to elicit ectopic proliferation in intestinal epithelium. To do this, Myc was activated for 24 h in villin-MycERTAM mice, and its impact on the rate and extent of intestinal epithelial proliferation was assessed by BrdU incorporation. Myc activation significantly increased the percentage of crypt cells in S phase (BrdU positive), from 35% ± 3.7% to 71.2% ± 4.2% after 1 day and to 79.7% ± 4.3% after 2 days (Fig. 2A to C). Furthermore, the proliferative zone, as evidenced by both S-phase and mitotic cells, rapidly extended up along the villus shaft. Of note, a similar, although less dramatic, proliferative enhancement was observed in the large intestine, where the villin promoter is also active (data not shown).
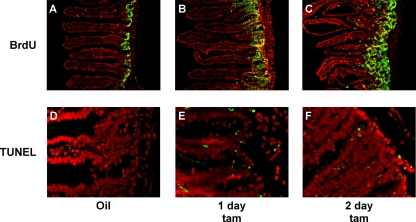
FIG. 2.
Myc activation induces rapid expansion of the proliferative crypt compartment together with increased crypt cell apoptosis. villin-MycERTAM mice were treated either with control vehicle for 2 days (A and D) or with tamoxifen (tam) for 1 (B and E) or 2 (C and F) days. (A to C) S-phase cells were labeled with BrdU administered systemically 3.5 h prior to sacrifice, and incorporated BrdU was detected by immunofluorescence (BrdU in green, Hoechst counterstain in red). Myc activation induces a dramatic increase in the number of S-phase cells in each crypt, expanding the proliferative zone. (D to F) Significant induction of apoptosis in the crypt was also observed following Myc activation, as indicated by TUNEL staining (TUNEL in green, Hoechst stain in red).
To assess induction of apoptosis by Myc in intestinal epithelium, we used TUNEL. Only occasional TUNEL-positive apoptotic cells were evident in control tissue, as expected (Fig. 2D). However, significant apoptosis was evident within 1 day of Myc activation (Fig. 2E) and thereafter maintained (Fig. 2F). Most apoptosis was confined to the crypts, overlapping with the most highly proliferative cells.
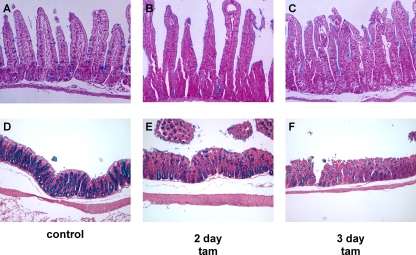
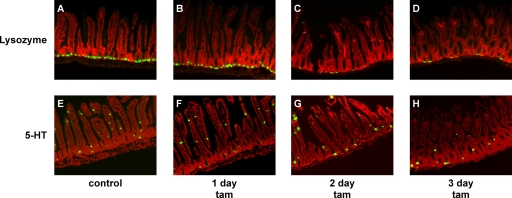
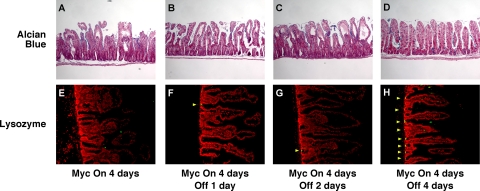
In various tissues, Myc is a downstream effector of both the Wnt and Notch pathways, each of which has distinct effects on differentiation within the intestinal epithelial compartment. To assess the direct impact of Myc activation on intestinal epithelial differentiation, we assessed the relative proportions and locations of each differentiated cell type. Alcian blue stains mucopolysaccharide-rich goblet cells, revealing the expected periodic distribution throughout the crypt-villus compartment in control tissue (Fig. 3A). Myc activation induced rapid, dramatic, and synchronous reductions in the number and intensity of Alcian blue-staining cells throughout the epithelial layer, with their effective disappearance within 2 days (Fig. 3B and C). Likewise, in the colon, where goblet cells are yet more prevalent, we observed rapid and synchronous loss of Alcian blue staining throughout the crypts (Fig. 3D to F). The synchrony of goblet cell loss, together with the fact that we never observed any goblet cell apoptosis (and, anyway, apoptosis is largely restricted to the crypts) suggests that Myc-induced loss of goblet cells is a consequence of in situ dedifferentiation or transdifferentiation, not cell death. This acute loss of goblet cells probably underlies the dramatic starvation phenotype induced by Myc activation in the villin-MycERTAM mice. Paneth cells reside at the base of the crypt and may be identified by antilysozyme staining. Activation of Myc induced the loss of lysozyme immunoreactivity (Fig. 4A to D), although more slowly and less completely than the loss of Alcian blue staining: even after 3 days of sustained Myc activation, strongly lysozyme-positive cells still remained in a few crypts (Fig. 4D). This sporadic disappearance of Paneth cells is most consistent with their loss by apoptosis, which occurs at significant rates within the crypt. Indeed, a detailed analysis revealed sporadic coincident staining for Paneth cell lysozyme and TUNEL following MycERTAM activation, confirming that Myc-dependent Paneth cell apoptosis does indeed occur (data not shown). Using 5-HT (Fig. 4E to H), chromogranin A, and synaptophysin (data not shown) staining to identify intestinal enteroendocrine cells, we observed no detectable change in the relative levels or distribution of enteroendocrine cells following Myc activation. Hence, direct activation of Myc affects the different secretory cell compartments of intestinal epithelium in distinct ways. Of note, the phenotype induced by direct Myc activation is clearly distinct from that induced by deregulated β-catenin signaling.
FIG. 3.
Myc activation induces rapid loss of goblet cells. villin-MycERTAM mice were treated either with control vehicle for 2 days (A and D) or with tamoxifen (tam) for 2 or 3 days (B, C, E, and F). (B and C) Goblet cell mucopolysaccharides were identified by Alcian blue staining, and cell nuclei were counterstained with fast red. Alcian blue-positive goblet cells disappeared rapidly and synchronously throughout the crypt-villus axis following MycERTAM activation. (E and F) Similar rapid and synchronous disappearance of Alcian blue-positive goblet cells was observed throughout the large intestine crypts.
FIG. 4.
Myc activation induces rapid loss of Paneth but not enteroendocrine cells. (A to D) Paneth cells, identified by immunofluorescence staining for lysozyme (in green; Hoechst counterstain in red), disappeared rapidly but sporadically during 3 days of Myc activation. (E to H) Enteroendocrine cells, identified by anti-5-HT immunostaining, were largely unaffected by Myc activation (5-HT staining in green, Hoechst counterstaining in red). tam, tamoxifen.
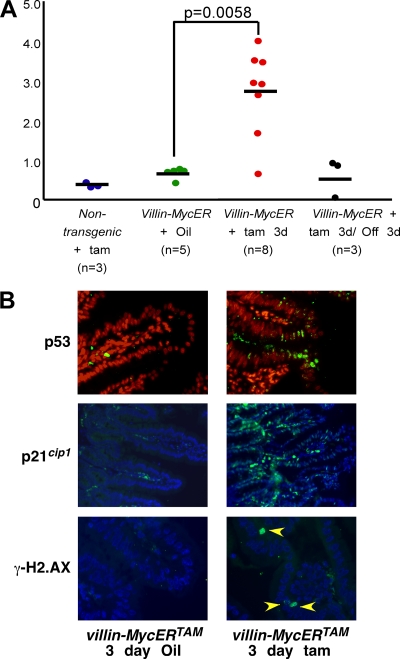
Activation of oncogenes typically triggers growth-suppressive pathways, an evolutionary adaptation to neoplastic risk dubbed intrinsic tumor suppression (29) that, in the case of Myc, appears to be expressed principally through the induction of apoptosis (2, 11, 41). How cells discriminate between normal and oncogenic signaling is unclear, although engagement of intrinsic tumor suppression appears to require significantly higher levels of Myc than does proliferation (36). Hence, one possible reason for the differing phenotypes induced by direct Myc activation versus its indirect activation via deregulated Wnt/β-catenin signaling could be different levels of Myc in each case engaging intrinsic tumor suppression with differing efficiencies. Thus, Myc represses expression of p21cip1 directly through complexes comprising Myc and Miz1 (52) or indirectly through induction of AP4 (19). Consistent with this, the mitogenic signal elicited by β-catenin suppresses p21cip1 expression (20, 61). However, high levels of Myc can also engage the ARF/p53 pathway (67), which potently induces expression of p21cip1 (21). We therefore asked which of these Myc outputs prevails following Myc activation in villin-MycERTAM mice. Acute activation of Myc strongly induced p19ARF mRNA in intestinal epithelium (Fig. 5A), and this was accompanied by accumulation of both p53 and p21cip1 proteins (Fig. 5B). Acute oncogenic stress has also been reported to activate p53 via the induction of DNA damage, at least in vitro. We therefore assessed induction of DNA damage foci following Myc activation by staining intestinal tissue sections for γ-H2.AX. However, Myc activation induced only a few, sporadic γ-H2.AX foci (Fig. 5B) that showed no correlation with the widespread stabilization of p53 and p21cip1. Hence, Myc activates the p53 pathway in villin-MycERTAM mice through direct induction of p19ARF and not indirectly by inducing DNA damage. To assess levels of expression of MycERTAM relative to that of endogenous c-myc in normal intestinal epithelia of villin-MycERTAM mice, we used quantitative real-time PCR. MycERTAM mRNA was expressed around 40-fold higher than endogenous c-myc mRNA (data not shown) and around 5-fold higher than the elevated level of endogenous Myc induced by the loss of APC (51). Evidently, MycERTAM is expressed at levels sufficiently high to engage ARF/p53, overriding any direct repression of the p21cip1 gene that Myc normally affords.
FIG. 5.
Activation of Myc in intestinal epithelium of villin-MycERTAM mice triggers the ARF/p53/p21cip1 tumor suppressor pathway. (A) Real-time quantitative PCR analysis of ARF (p19ARF mRNA) expression in intestinal epithelia of nontransgenic control mice treated with tamoxifen (tam) versus that in villin-MycERTAM mice treated with the vehicle control (oil) or tamoxifen for 3 days (tam 3d). Myc activation triggers strong induction of ARF, which is lost upon MycERTAM deactivation for 3 days (Off 3d). (B) Immunofluorescence staining for p53, p21cip1, and phospho-H2.AX (in green, Hoechst nuclear counterstain in red) in intestinal epithelia of villin-MycERTAM mice treated with either the vehicle control or tamoxifen for 3 days. Myc activation rapidly induced accumulation of both p53 and p21cip1. In contrast, anti-phospho-H2.AX staining (a marker of DNA damage) showed only very occasional foci following Myc activation (yellow arrowheads) that may be attributable merely to normal mitosis (14). Hence, widespread p53 and p21cip1 induction occurs without concomitant evidence of widespread DNA damage.
The biological effects of Myc overexpression and deregulation in the intestinal epithelia of villin-MycERTAM mice are rapidly reversed upon withdrawal of tamoxifen.
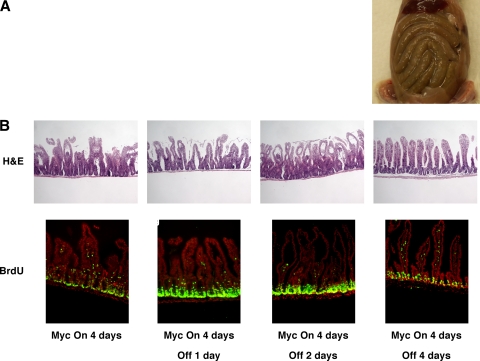
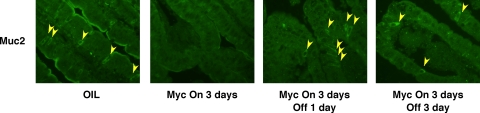
The high incidence of Myc overexpression in colonic adenocarcinoma (10, 13, 22, 30), together with its clear role as a mediator of β-catenin signaling, makes inhibition of Myc an attractive strategy for treating colorectal cancers. However, while inactivation of oncogenic Myc restores normal tissue architecture in some cases (41, 42), in some tissues its effects are irreversible, perhaps due to permanent perturbation of the stem cell compartment (1). To ascertain the consequences of subsequent deactivation of MycERTAM in intestinal epithelium, MycERTAM was activated for 4 days in villin-MycERTAM mice and then tamoxifen treatment was stopped, leading to MycERTAM deactivation after ∼24 h (41, 42, 54). Following tamoxifen surcease, mice rapidly regained weight. Dissection revealed refilling of intestines, indicating the resumption of normal digestive function (cf. Fig. 6A and Fig. 1A). By 2 days after tamoxifen withdrawal, intestinal architecture was still demonstrably aberrant (Fig. 6B), but by 4 days after tamoxifen withdrawal, crypt-villus units appeared normal, with normal cellular morphology and an epithelial layer once again properly attached to the underlying stroma (Fig. 6B). BrdU incorporation analysis indicated progressive shrinkage of the proliferative zone down toward the crypt (Fig. 6B). Myc deactivation was also accompanied by de-engagement of the ARF/p53/p21cip1 pathway, as evidenced by the loss of p21cip1 staining (data not shown) and p19ARF expression (Fig. 5A) and a significant decline in crypt cell apoptosis (data not shown). Myc deactivation triggered a rapid and synchronous reappearance of Alcian blue-positive cells, which were distributed sporadically throughout the crypt-villus axis (Fig. 7A to D). Likewise, cells positive for the goblet cell marker Muc2 reappeared throughout the intestinal layer, including high up the villus, within only 24 h of Myc deactivation (Fig. 8). Such rapid reemergence in the distal villus strongly supports the notion that the reappearance of goblet cells is due to redifferentiation of cells in situ rather than vectorial repopulation from the crypt base. Strongly lysozyme-positive Paneth cells also reappeared rapidly following Myc inactivation (Fig. 7E to H), although not in all crypts, with some remaining devoid of Paneth cells even after 4 days.
FIG. 6.
The Myc-induced phenotype in villin-MycERTAM mice is reversible following MycERTAM deactivation. Myc was activated for 4 days in villin-MycERTAM mice and tamoxifen, then withdrawn for 4 days to deactivate MycERTAM. MycERTAM deactivation triggered rapid refilling of the intestine (A) together with rapid return to normal intestinal architecture and shrinkage of the proliferative compartment back to its normal size and location in the intestinal crypts (B) (BrdU in green, Hoechst counterstaining in red). H&E, hematoxylin and eosin.
FIG. 7.
Myc deactivation in villin-MycERTAM mice induces rapid restoration of both goblet and Paneth cell compartments. villin-MycERTAM mice were treated with tamoxifen for 4 days to activate Myc, and then the treatment was withdrawn for 1 to 4 days, as indicated. (A to D) Sections of small intestine were stained with Alcian blue or antilysozyme antibody (lysozyme in green, Hoechst counterstain in red). Alcian blue-positive goblet cells reappear synchronously throughout the crypt-villus axis by 2 to 4 days after Myc deactivation. (E to H) Lysozyme-positive Paneth cells also reappeared over a similar time frame but more sporadically.
FIG. 8.
Myc deactivation in villin-MycERTAM mice induces rapid reappearance of goblet cells throughout the epithelium. villin-MycERTAM mice were treated with tamoxifen for 4 days to activate Myc and tamoxifen, and then the treatment was withdrawn for 3 days, as indicated. Sections of small intestine were stained for the intestinal mucin goblet cell marker Muc2. Muc2-positive goblet cells reappeared throughout the crypt-villus axis by 2 to 4 days after Myc deactivation.
DISCUSSION
Deregulation of the Wnt/β-catenin pathway is implicated in the majority of human colorectal cancers, where it appears to be critical in initiating the formation of polyps, the benign precursors of colorectal cancer. Studies indicate that deregulated Wnt/β-catenin locks crypt stem and progenitor cells in their proliferative and undifferentiated states, in great part through sustained actions of the TCF-4 transcription factor. More recently, the pleiotropic bHLHZip transcription factor Myc has been implicated as a key, obligate downstream effector of Wnt/β-catenin signaling. Myc is induced by Wnt/β-catenin signals, and its acute loss triggers abrupt growth arrest within the proliferating crypt compartment (35, 57). Moreover, acute Myc deletion forestalls the induction of the majority of Wnt/β-catenin target genes and abrogates the pathological and oncogenic impact of Wnt/β-catenin deregulation on intestinal epithelium (50). However, while endogenous Myc function is required for much of the biological output of Wnt-β-catenin signaling in intestinal epithelium, the extent to which Myc alone can substitute for Wnt/β-catenin signaling is unclear. To investigate this directly, we employed a transgenic approach in which the reversibly switchable variant of Myc, MycERTAM, was targeted to intestinal epithelium. Whereas excision of floxed APC (35, 50) is incomplete and irreversible and takes several days to achieve high-efficiency deletion of APC, systemic administration of tamoxifen activates MycERTAM rapidly (<2 h), synchronously, ubiquitously, and reversibly in target tissues (27). This makes it possible to ascertain the direct and immediate consequences of abrupt Myc activity in vivo. Direct activation of Myc in intestinal epithelium induced rapid phenotypic changes that overlapped with, but were not identical to, those induced by deregulation of β-catenin signaling. Thus, as with the acute loss of APC, acute Myc activation induced the loss of goblet cells. Of note, Myc-induced goblet cell loss was both rapid and synchronous, occurring throughout the epithelial layer irrespective of position within crypts or villi. Such synchronous loss is difficult to reconcile with the notion that goblet cell depletion results from failure to replenish these cells at the crypt together with their upward migration and loss at the villus tip. In the absence of any observable goblet cell apoptosis, in situ dedifferentiation seems the most likely mechanism, a notion confirmed by the similarly abrupt and synchronous reappearance of goblet cell markers throughout the epithelium shortly after Myc was deactivated. Terminal secretory cell fate in the intestinal epithelium is determined by Notch signaling, whereas the Wnt pathway regulates the emergence of cells from the cryptal stem cell and transit-amplifying compartment. Hitherto, the lack of differentiated cells caused by overactivity of the Wnt/β-catenin pathway was thought to be a consequence of retention of cells in a crypt-like state, preventing their emergence into the Notch-regulated compartment. However, the in situ dedifferentiation of goblet cells that we observed suggests that Myc can override and reverse established Notch-dependent terminal differentiation, indicating a novel potential cross talk between the Wnt/β-catenin and Notch pathways.
Wnt/β-catenin signaling is required for Paneth cell maturation (62), at least in part through induction of the Sox9 transcription factor (33), while another Wnt/β-catenin target, EphB3, is required for their localization to the bottom of the intestinal crypts (6, 7). Acute deregulation of Wnt/β-catenin signaling by APC loss induces mislocalization of Paneth cells within the crypts (51), probably as a consequence of gross expansion of the crypt progenitor compartment and perturbation of the interplay between EphB3 and another Wnt/β-catenin target, EphB2, which together govern migration and localization of cells along the crypt-villus axis. In contrast, direct Myc activation in intestinal epithelium caused rapid ablation of Paneth cells, apparently by apoptosis. This is perhaps surprising, given that Wnt/β-catenin-dependent induction of both EphB2 and EphB3 is dependent on endogenous Myc (50). On the other hand, Sox9, necessary for Paneth cell genesis, is one of the small number of Wnt/β-catenin targets whose induction is not dependent on endogenous Myc (50). Furthermore, while upregulation of EphB3 by APC loss may indeed depend on endogenous Myc, EphB3 is not itself a Myc target. Thus, Paneth cells are impacted by Myc very differently when it is activated directly from when it is activated indirectly in the context of Wnt/β-catenin signaling.
Identification of Myc as a key mediator of aberrant Wnt/β-catenin signaling underscores its importance as a conduit for oncogenic signaling in this tissue and indicates that the inhibition of Myc might be an effective strategy for therapeutic intervention, as it is for Ras-driven tumorigenesis in the lung (57). Nonetheless, the distinct impacts of constitutive Wnt/β-catenin signaling and directly activated Myc on the fate of Paneth cells indicate that a simple sequential model in which APC loss induces endogenous Myc, which is then solely responsible for the transcriptional impact of deregulated Wnt/β-catenin signaling, is overly simplistic.
There are several plausible reasons why direct activation of Myc fails to substitute for deregulated Wnt/β-catenin signaling. First, as exemplified by Sox9 in intestine, some genes induced by Wnt/β-catenin are Myc independent (50) and mediate Myc-independent biological outputs. This is dramatically illustrated by what occurs in liver, where endogenous Myc appears completely dispensable for the hyperproliferative phenotype induced by APC inactivation (46). Second, while endogenous Myc activity may be needed for Wnt/β-catenin-dependent expression of some genes, this does not necessarily imply that Myc is the transcription factor directly responsible for their induction. Myc is a highly pleiotropic and context-dependent transcriptional modulator. By acting as a general modulator of global chromatin structure (24), Myc may act as a permissivity factor that dictates whether or not certain genes are available for induction by other transcription factors. Such a relationship is consistent with the remarkably scant overlap between known Myc targets and genes regulated by Wnt. Indeed, our own analysis of SOX17, Axin2, Tiam1, and Tcf1, four representative target genes that are induced by APC loss in intestine in a Myc-dependent manner (50), indicates that none is induced by direct Myc activation alone (not shown). Third, Myc is activated by many other pathways besides Wnt/β-catenin, including RTK/Ras signaling and Notch, that have the potential to modulate or even annul Wnt/β-catenin output. Last, certain attributes of Myc may be manifest only when Myc itself is directly activated, rendering it refractory to regulatory influences that limit endogenous Myc, even when driven by deregulated Wnt/β-catenin signals. For example, directly activated Myc has been shown to positively reinforce Wnt/β-catenin signaling in some tissues through suppression of the Wnt inhibitors DKK1 and SFRP1 (9) and induction of the Wnt ligand Fzd9 (27) (although this does not occur in intestinal epithelium; direct Myc activation induces no measurable translocation of β-catenin to the nucleus [data not shown]). Another outcome largely peculiar to directly activated Myc is engagement of the ARF/p53 pathway, which occurs only when Myc is expressed above certain threshold levels that engage the cell's intrinsic tumor surveillance programs (36). Expression of the Cdk inhibitor p21cip1 exemplifies two diametrically opposing possible outcomes of Myc activation in intestinal epithelium. Myc represses expression of p21cip1 through well- described mechanisms involving association with the transcriptional repressors Miz1 and Dnmt3 (8, 52, 63) and induction of AP4 (19). However, oncogenic Myc can also activate the p53 pathway, which potently induces p21cip (12, 67). When indirectly activated by deregulated Wnt/β-catenin signaling in intestine, Myc suppresses p21cip1 expression (50), presumably accounting for the negligible impact of p53 status on the aberrant proliferation induced by APC inactivation (47). In contrast, when directly activated in villin-MycERTAM mice, Myc engages the p53 pathway and potently induces p21cip1. The difference is most likely due to the significantly higher levels of Myc present in intestinal epithelial cells of villin-MycERTAM mice than those in APC-deficient epithelium, levels that breach the higher threshold required for p53 pathway activation (36).
Our data offer a compelling explanation for why it is that Myc, a potent oncogene and activator of multiple intra- and extracellular proliferative programs, is directly activated by gene amplification or rearrangement only relatively infrequently in patients with colorectal cancer and then only in more-advanced and metastatic stages of the disease. Colorectal tumorigenesis is initiated when deregulated Wnt/β-catenin signals lock crypt stem and progenitor cells in their proliferative and undifferentiated states. Although this requires Myc, Myc-independent functions of Wnt/β-catenin exist, and some of these may be critical for intestinal tumorigenesis. Furthermore, whereas direct activation of Myc triggers intrinsic tumor suppression, Myc activated indirectly by Wnt/β-catenin signaling fails to, allowing incipient intestinal tumors to evade tumor surveillance until such time as the p53 pathway has become eroded by happenstance and directly activated Myc is tolerable by the emerging colorectal tumor.
Acknowledgments
This study was supported by NIH grant RO1 CA98018, funds from Daiichi Corp., a Stewart Trust Cancer Research Award, and the Sandler Family Foundation (all to G.I.E.). M.R.J. is funded by an Enrique Cepero Fellowship of the Damon Runyon Cancer Research Foundation.
We are most grateful to Fanya Rostker for her technical assistance and to our colleagues in the Evan lab for their invaluable criticism and advice.
Footnotes
Published ahead of print on 27 July 2009.
REFERENCES
- 1.Arnold, I., and F. M. Watt. 2001. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr. Biol. 11:558-568. [DOI] [PubMed] [Google Scholar]
- 2.Askew, D. S., R. A. Ashmun, B. C. Simmons, and J. L. Cleveland. 1991. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene 6:1915-1922. [PubMed] [Google Scholar]
- 3.Barker, N., R. A. Ridgway, J. H. van Es, M. van de Wetering, H. Begthel, M. van den Born, E. Danenberg, A. R. Clarke, O. J. Sansom, and H. Clevers. 2009. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457:608-611. [DOI] [PubMed] [Google Scholar]
- 4.Barker, N., M. van de Wetering, and H. Clevers. 2008. The intestinal stem cell. Genes Dev. 22:1856-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker, N., J. H. van Es, J. Kuipers, P. Kujala, M. van den Born, M. Cozijnsen, A. Haegebarth, J. Korving, H. Begthel, P. J. Peters, and H. Clevers. 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449:1003-1007. [DOI] [PubMed] [Google Scholar]
- 6.Batlle, E., J. Bacani, H. Begthel, S. Jonkeer, A. Gregorieff, M. Van De Born, N. Malats, E. Sancho, E. Boon, T. Pawson, S. Gallinger, S. Pals, and H. Clevers. 2005. EphB receptor activity suppresses colorectal cancer progression. Nature 435:1126-1130. [DOI] [PubMed] [Google Scholar]
- 7.Batlle, E., J. T. Henderson, H. Beghtel, M. M. van den Born, E. Sancho, G. Huls, J. Meeldijk, J. Robertson, M. van de Wetering, T. Pawson, and H. Clevers. 2002. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111:251-263. [DOI] [PubMed] [Google Scholar]
- 8.Brenner, C., R. Deplus, C. Didelot, A. Loriot, E. Vire, C. De Smet, A. Gutierrez, D. Danovi, D. Bernard, T. Boon, P. G. Pelicci, B. Amati, T. Kouzarides, Y. de Launoit, L. Di Croce, and F. Fuks. 2005. Myc represses transcription through recruitment of DNA methyltransferase corepressor. EMBO J. 24:336-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowling, V. H., C. M. D'Cruz, L. A. Chodosh, and M. D. Cole. 2007. c-Myc transforms human mammary epithelial cells through repression of the Wnt inhibitors DKK1 and SFRP1. Mol. Cell. Biol. 27:5135-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erisman, M. D., P. G. Rothberg, R. E. Diehl, C. C. Morse, J. M. Spandorfer, and S. M. Astrin. 1985. Deregulation of c-myc gene expression in human colon carcinoma is not accompanied by amplification or rearrangement of the gene. Mol. Cell. Biol. 5:1969-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evan, G. I., A. H. Wyllie, C. S. Gilbert, T. D. Littlewood, H. Land, M. Brooks, C. M. Waters, L. Z. Penn, and D. C. Hancock. 1992. Induction of apoptosis in fibroblasts by c-myc protein. Cell 69:119-128. [DOI] [PubMed] [Google Scholar]
- 12.Finch, A., J. Prescott, K. Shchors, A. Hunt, L. Soucek, T. B. Dansen, L. B. Swigart, and G. I. Evan. 2006. Bcl-xL gain of function and p19ARF loss of function cooperate oncogenically with Myc in vivo by distinct mechanisms. Cancer Cell 10:113-120. [DOI] [PubMed] [Google Scholar]
- 13.Finley, G. G., N. T. Schulz, S. A. Hill, J. R. Geiser, J. M. Pipas, and A. I. Meisler. 1989. Expression of the myc gene family in different stages of human colorectal cancer. Oncogene 4:963-971. [PubMed] [Google Scholar]
- 14.Forand, A., B. Dutrillaux, and J. Bernardino-Sgherri. 2004. Gamma-H2AX expression pattern in non-irradiated neonatal mouse germ cells and after low-dose gamma-radiation: relationships between chromatid breaks and DNA double-strand breaks. Biol. Reprod. 71:643-649. [DOI] [PubMed] [Google Scholar]
- 15.Fre, S., M. Huyghe, P. Mourikis, S. Robine, D. Louvard, and S. Artavanis-Tsakonas. 2005. Notch signals control the fate of immature progenitor cells in the intestine. Nature 435:964-968. [DOI] [PubMed] [Google Scholar]
- 16.He, T. C., A. B. Sparks, C. Rago, H. Hermeking, L. Zawel, L. T. da Costa, P. J. Morin, B. Vogelstein, and K. W. Kinzler. 1998. Identification of c-MYC as a target of the APC pathway. Science 281:1509-1512. [DOI] [PubMed] [Google Scholar]
- 17.He, X. C., T. Yin, J. C. Grindley, Q. Tian, T. Sato, W. A. Tao, R. Dirisina, K. S. Porter-Westpfahl, M. Hembree, T. Johnson, L. M. Wiedemann, T. A. Barrett, L. Hood, H. Wu, and L. Li. 2007. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat. Genet. 39:189-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenny, M., C. Uhl, C. Roche, I. Duluc, V. Guillermin, F. Guillemot, J. Jensen, M. Kedinger, and G. Gradwohl. 2002. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 21:6338-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung, P., A. Menssen, D. Mayr, and H. Hermeking. 2008. AP4 encodes a c-MYC-inducible repressor of p21. Proc. Natl. Acad. Sci. USA 105:15046-15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamei, J., T. Toyofuku, and M. Hori. 2003. Negative regulation of p21 by beta-catenin/TCF signaling: a novel mechanism by which cell adhesion molecules regulate cell proliferation. Biochem. Biophys. Res. Commun. 312:380-387. [DOI] [PubMed] [Google Scholar]
- 21.Kamijo, T., J. D. Weber, G. Zambetti, F. Zindy, M. F. Roussel, and C. J. Sherr. 1998. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc. Natl. Acad. Sci. USA 95:8292-8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klimpfinger, M., G. Zisser, C. Ruhri, B. Putz, P. Steindorfer, and H. Hofler. 1990. Expression of c-myc and c-fos mRNA in colorectal carcinoma in man. Virchows Arch. B 59:165-171. [DOI] [PubMed] [Google Scholar]
- 23.Klinakis, A., M. Szabolcs, K. Politi, H. Kiaris, S. Artavanis-Tsakonas, and A. Efstratiadis. 2006. Myc is a Notch1 transcriptional target and a requisite for Notch1-induced mammary tumorigenesis in mice. Proc. Natl. Acad. Sci. USA 103:9262-9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knoepfler, P. S., X. Y. Zhang, P. F. Cheng, P. R. Gafken, S. B. McMahon, and R. N. Eisenman. 2006. Myc influences global chromatin structure. EMBO J. 25:2723-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korinek, V., N. Barker, P. Moerer, E. van Donselaar, G. Huls, P. J. Peters, and H. Clevers. 1998. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 19:379-383. [DOI] [PubMed] [Google Scholar]
- 26.Korinek, V., N. Barker, P. J. Morin, D. van Wichen, R. de Weger, K. W. Kinzler, B. Vogelstein, and H. Clevers. 1997. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 275:1784-1787. [DOI] [PubMed] [Google Scholar]
- 27.Lawlor, E. R., L. Soucek, L. Brown-Swigart, K. Shchors, C. U. Bialucha, and G. I. Evan. 2006. Reversible kinetic analysis of Myc targets in vivo provides novel insights into Myc-mediated tumorigenesis. Cancer Res. 66:4591-4601. [DOI] [PubMed] [Google Scholar]
- 28.Littlewood, T. D., D. C. Hancock, P. S. Danielian, M. G. Parker, and G. I. Evan. 1995. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 23:1686-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowe, S. W., E. Cepero, and G. Evan. 2004. Intrinsic tumour suppression. Nature 432:307-315. [DOI] [PubMed] [Google Scholar]
- 30.Mariani-Costantini, R., C. Theillet, P. Hutzell, G. Merlo, J. Schlom, and R. Callahan. 1989. In situ detection of c-myc mRNA in adenocarcinomas, adenomas, and mucosa of human colon. J. Histochem. Cytochem. 37:293-298. [DOI] [PubMed] [Google Scholar]
- 31.Martins, C. P., L. Brown-Swigart, and G. I. Evan. 2006. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell 127:1323-1334. [DOI] [PubMed] [Google Scholar]
- 32.Milano, J. 2004. Modulation of notch processing by γ-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol. Sci. 82:341-358. [DOI] [PubMed] [Google Scholar]
- 33.Mori-Akiyama, Y., M. van den Born, J. H. van Es, S. R. Hamilton, H. P. Adams, J. Zhang, H. Clevers, and B. de Crombrugghe. 2007. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology 133:539-546. [DOI] [PubMed] [Google Scholar]
- 34.Morin, P. J., A. B. Sparks, V. Korinek, N. Barker, H. Clevers, B. Vogelstein, and K. W. Kinzler. 1997. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275:1787-1790. [DOI] [PubMed] [Google Scholar]
- 35.Muncan, V., O. J. Sansom, L. Tertoolen, T. J. Phesse, H. Begthel, E. Sancho, A. M. Cole, A. Gregorieff, I. M. De Alboran, H. Clevers, and A. R. Clarke. 2006. Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf-4 target gene c-Myc. Mol. Cell. Biol. 26:8418-8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy, D. J., M. R. Junttila, L. Pouyet, A. Karnezis, K. Shchors, D. A. Bui, L. Brown-Swigart, L. Johnson, and G. I. Evan. 2008. Distinct thresholds govern Myc's biological output in vivo. Cancer Cell 14:447-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura, T., K. Tsuchiya, and M. Watanabe. 2007. Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J. Gastroenterol. 42:705-710. [DOI] [PubMed] [Google Scholar]
- 38.Naya, F. J., H. P. Huang, Y. Qiu, H. Mutoh, F. J. DeMayo, A. B. Leiter, and M. J. Tsai. 1997. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 11:2323-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ootani, A., X. Li, E. Sangiorgi, Q. T. Ho, H. Ueno, S. Toda, H. Sugihara, K. Fujimoto, I. L. Weissman, M. R. Capecchi, and C. J. Kuo. 2009. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 15:701-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palomero, T., W. K. Lim, D. T. Odom, M. L. Sulis, P. J. Real, A. Margolin, K. C. Barnes, J. O'Neil, D. Neuberg, A. P. Weng, J. C. Aster, F. Sigaux, J. Soulier, A. T. Look, R. A. Young, A. Califano, and A. A. Ferrando. 2006. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc. Natl. Acad. Sci. USA 103:18261-18266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelengaris, S., M. Khan, and G. I. Evan. 2002. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell 109:321-334. [DOI] [PubMed] [Google Scholar]
- 42.Pelengaris, S., T. Littlewood, M. Khan, G. Elia, and G. Evan. 1999. Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol. Cell 3:565-577. [DOI] [PubMed] [Google Scholar]
- 43.Pinto, D., A. Gregorieff, H. Begthel, and H. Clevers. 2003. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 17:1709-17013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinto, D., S. Robine, F. Jaisser, F. E. El Marjou, and D. Louvard. 1999. Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J. Biol. Chem. 274:6476-6482. [DOI] [PubMed] [Google Scholar]
- 45.Powell, S. M., N. Zilz, Y. Beazer-Barclay, T. M. Bryan, S. R. Hamilton, S. N. Thibodeau, B. Vogelstein, and K. W. Kinzler. 1992. APC mutations occur early during colorectal tumorigenesis. Nature 359:235-237. [DOI] [PubMed] [Google Scholar]
- 46.Reed, K. R., D. Athineos, V. S. Meniel, J. A. Wilkins, R. A. Ridgway, Z. D. Burke, V. Muncan, A. R. Clarke, and O. J. Sansom. 2008. β-Catenin deficiency, but not Myc deletion, suppresses the immediate phenotypes of APC loss in the liver. Proc. Natl. Acad. Sci. USA 105:18919-18923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reed, K. R., V. S. Meniel, V. Marsh, A. Cole, O. J. Sansom, and A. R. Clarke. 2008. A limited role for p53 in modulating the immediate phenotype of Apc loss in the intestine. BMC Cancer 8:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodilla, V., A. Villanueva, A. Obrador-Hevia, A. Robert-Moreno, V. Fernández-Majada, A. Grilli, N. López-Bigas, N. Bellora, M. M. Albà, F. Torres, M. Duñach, X. Sanjuan, S. Gonzalez, T. Gridley, G. Capella, A. Bigas, and L. Espinosa. 2009. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc. Natl. Acad. Sci. USA 106:6315-6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sangiorgi, E., and M. R. Capecchi. 2008. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 40:915-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sansom, O. J., V. S. Meniel, V. Muncan, T. J. Phesse, J. A. Wilkins, K. R. Reed, J. K. Vass, D. Athineos, H. Clevers, and A. R. Clarke. 2007. Myc deletion rescues Apc deficiency in the small intestine. Nature 446:676-679. [DOI] [PubMed] [Google Scholar]
- 51.Sansom, O. J., K. R. Reed, A. J. Hayes, H. Ireland, H. Brinkmann, I. P. Newton, E. Batlle, P. Simon-Assmann, H. Clevers, I. S. Nathke, A. R. Clarke, and D. J. Winton. 2004. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 18:1385-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seoane, J., H. V. Le, and J. Massague. 2002. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature 419:729-734. [DOI] [PubMed] [Google Scholar]
- 53.Sharma, V. M., J. A. Calvo, K. M. Draheim, L. A. Cunningham, N. Hermance, L. Beverly, V. Krishnamoorthy, M. Bhasin, A. J. Capobianco, and M. A. Kelliher. 2006. Notch1 contributes to mouse T-cell leukemia by directly inducing the expression of c-myc. Mol. Cell. Biol. 26:8022-8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shchors, K. 2006. The Myc-dependent angiogenic switch in tumors is mediated by interleukin 1beta. Genes Dev. 20:2527-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shroyer, N. F., D. Wallis, K. J. Venken, H. J. Bellen, and H. Y. Zoghbi. 2005. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 19:2412-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snippert, H. J., J. H. van Es, M. van den Born, H. Begthel, D. E. Stange, N. Barker, and H. Clevers. 2009. Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology 136:2187-2194. [DOI] [PubMed] [Google Scholar]
- 57.Soucek, L., J. Whitfield, C. P. Martins, A. J. Finch, D. J. Murphy, N. M. Sodir, A. N. Karnezis, L. B. Swigart, S. Nasi, and G. I. Evan. 2008. Modelling Myc inhibition as a cancer therapy. Nature 455:679-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamura, K., Y. Taniguchi, S. Minoguchi, T. Sakai, T. Tun, T. Furukawa, and T. Honjo. 1995. Physical interaction between a novel domain of the receptor Notch and the transcription factor RBP-J. kappa/Su(H). Curr. Biol. 5:1416-1423. [DOI] [PubMed] [Google Scholar]
- 59.Tetsu, O., and F. McCormick. 1999. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422-426. [DOI] [PubMed] [Google Scholar]
- 60.Van der Flier, L. G., J. Sabates-Bellver, I. Oving, A. Haegebarth, M. De Palo, M. Anti, M. E. Van Gijn, S. Suijkerbuijk, M. Van de Wetering, G. Marra, and H. Clevers. 2007. The intestinal Wnt/TCF signature. Gastroenterology 132:628-632. [DOI] [PubMed] [Google Scholar]
- 61.van de Wetering, M., E. Sancho, C. Verweij, W. de Lau, I. Oving, A. Hurlstone, K. van der Horn, E. Batlle, D. Coudreuse, A. P. Haramis, M. Tjon-Pon-Fong, P. Moerer, M. van den Born, G. Soete, S. Pals, M. Eilers, R. Medema, and H. Clevers. 2002. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111:241-250. [DOI] [PubMed] [Google Scholar]
- 62.van Es, J. H., P. Jay, A. Gregorieff, M. E. van Gijn, S. Jonkheer, P. Hatzis, A. Thiele, M. van den Born, H. Begthel, T. Brabletz, M. M. Taketo, and H. Clevers. 2005. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat. Cell Biol. 7:381-386. [DOI] [PubMed] [Google Scholar]
- 63.Vaque, J. P., J. Navascues, Y. Shiio, M. Laiho, N. Ajenjo, I. Mauleon, D. Matallanas, P. Crespo, and J. Leon. 2005. Myc antagonizes Ras-mediated growth arrest in leukemia cells through the inhibition of the Ras-ERK-p21Cip1 pathway. J. Biol. Chem. 280:1112-1122. [DOI] [PubMed] [Google Scholar]
- 64.Weng, A. 2006. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 20:2096-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamoto, Y., M. Sakamoto, G. Fujii, H. Tsuiji, K. Kenetaka, M. Asaka, and S. Hirohashi. 2003. Overexpression of orphan G-protein-coupled receptor, Gpr49, in human hepatocellular carcinomas with beta-catenin mutations. Hepatology 37:528-533. [DOI] [PubMed] [Google Scholar]
- 66.Yang, Q., N. A. Bermingham, M. J. Finegold, and H. Y. Zoghbi. 2001. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294:2155-2158. [DOI] [PubMed] [Google Scholar]
- 67.Zindy, F., C. M. Eischen, D. H. Randle, T. Kamijo, J. L. Cleveland, C. J. Sherr, and M. F. Roussel. 1998. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 12:2424-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]